Abstract
In this work, an innovative and non-radioactive functional cAMP assay was validated at the GPR17 receptor. This assay provides a simple and powerful new system to monitor G protein-coupled receptor activity through change in the intracellular cAMP concentration by using a mutant form of Photinus pyralis luciferase into which a cAMP-binding protein moiety has been inserted. Results, expressed as EC50 or IC50 values for agonists and antagonists, respectively, showed a strong correlation with those obtained with [35S]GTPγS binding assay, thus confirming the validity of this approach in the study of new ligands for GPR17. Moreover, this method allowed confirming that GPR17 is coupled with a Gαi.
Keywords: GPR17, cAMP assay, GloSensor, Luciferine–luciferase system
Introduction
Purinergic signalling is now recognized to be involved in a wide range of activities: extracellular adenine and uracil nucleotides mediate short-term (acute) signalling functions in neurotransmission, secretion and vasodilatation, and long-term (chronic) signalling functions in development, regeneration, proliferation and cell death [1]. In addition, P2 receptors play an important role at respiratory system and in correlated pathologies [2].
Cysteinyl leukotrienes (cys-LTs) (LTC4, LTD4, and LTE4) are potent proinflammatory mediators which increase vascular permeability and contract smooth muscles. They are implicated in several pathologies with a clear role in respiratory diseases such as asthma and allergic rhinitis and are implicated in other inflammatory conditions including cardiovascular, gastrointestinal, skin and immune disorders [3].
Evidences suggest that among purinergic and cys-LTs signalling molecules and their receptors there are important functional interactions. The P2Y family is composed of eight receptors, which can be subdivided into two groups based on their coupling to specific G proteins. The P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors couple to Gαq, to activate PLC, and the P2Y12, P2Y13, and P2Y14 receptors couple to Gαi, to inhibit adenylyl cyclase [4].
To date, pharmacological studies identified two receptors for cys-LTs, named CysLT1 and CysLT2, whose characteristics were then largely confirmed by molecular biology studies that allowed the cloning of two genes for the human CysLT1 and CysLT2 receptors [5].
The G protein-coupled receptor (GPCR) GPR17 is a “dual” receptor specifically activated by two unrelated families of endogenous ligands: uracil nucleotides and cys-LTs [6].
GPR17 is highly expressed in organs typically undergoing ischemic damage like the brain, heart and kidney, and its in vivo early knock down, in a rat focal brain ischemia model, with either pharmacological or specific antisense strategies, reduces the progression of cerebral ischemic damage, highlighting GPR17 as novel therapeutic target for ischemia [7]. In addition, recent data have shown that GPR17 has an important role in the development and post-injury repair of damage in the brain and in the spinal cord [6]. At very early times after injury, GPR17 seems to mediate cell death and, at later stages, GPR17 may even participate in the repair mechanisms [8]. These data further highlight that this receptor could be an attractive new target for drug discovery, and the use of GPR17 selective ligands could represent a therapy strategy to contrast damage evolution in stroke.
Although a number of assay formats already exist for monitoring GPCR functions and for pharmacologically characterizing their ligands, the development of new technologies that enable increased sensitivity and of novel experimental readouts will undoubtedly advance efforts both in academic research and drug discovery.
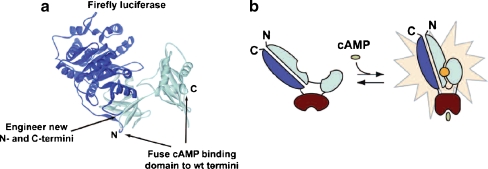
Promega recently developed a biosensor technology called GloSensor™ cAMP assay, which provides a simple and powerful new approach to monitor GPCR activity through change in the intracellular cAMP concentration. This assay uses a mutant form of Photinus pyralis luciferase into which a cAMP-binding protein moiety has been inserted (Fig. 1). Upon binding of cAMP, conformational change is induced leading to increased light output that allows evaluating the activity of ligands at the receptor under study. Following pre-equilibration with a substrate, cells stably expressing a biosensor variant can be used to evaluate GPCR function enabling easy kinetic measurements of cAMP accumulation or turnover in living cells.
Fig. 1.
Overview of the GloSensor cAMP assay. a Schematic representation of the biosensor used in the GloSensor cAMP assay. b Conformational change upon binding to cAMP that produces an increase of light output
Materials and methods
Cell culture
Human embryonic kidney (HEK293) L9-2 cells, as gift from Promega Corporation (Promega, Madison, WI), were grown adherently in Dulbecco’s Modified Eagle Medium high glucose supplemented with 10% foetal bovine serum (FBS), 20 mM l-glutamine, 1% sodium pyruvate, 1% penicillin and streptomycin, 1% amphotericin B and 0.2 mg/mL hygromycin and incubated at 37°C in an atmosphere of 5% CO2, 95% O2.
Transient transfection
Cells were seeded in a six-well plate and transfected at a confluence of about 80% by Arrest-In reagent. In particular, 3 μg of hgpr17 and, separately, 15 μl of Arrest-In were diluted in serum-free medium. Afterwards, the DNA was added to the Arrest-In reagent, mixed rapidly then incubated for 20 min at room temperature according to the manual protocol. The DNA–Arrest-In complex mixture was added into the cells and incubated for 6 h in the CO2 incubator at 37°C [9]. After the incubation, the transfection complex was removed and fresh medium containing 10% of FBS was replaced in each well. After 48 h of incubation, the cells were used to perform a GloSensor cAMP assay.
GloSensor cAMP assay
Cells were harvested in CO2-independent medium and were counted in a Neubauer chamber. The desiderate cell number was incubated in equilibration medium containing a 3% v/v GloSensor cAMP reagent stock solution, 10% FBS and 87% CO2 independent medium. After 2 h of incubation, the cells were dispensed in wells of 384-well plate and, when a steady-state basal signal was obtained, agonists, at different concentrations, were added. The antagonist profile of the compounds was evaluated by assessing their ability to counteract an agonist-induced decrease of cAMP accumulation. The cells were incubated in the reaction medium (10 min at room temperature) with different antagonist concentrations and then treated with agonists. Forskolin (FSK) 10 μM was added 10 min after the agonists and various luminescence reads were performed at different incubation times.
Statistical analysis
Responses were expressed as percentage of the maximal relative luminescence units (RLU) and referred to UDP taken as 100%. Concentration–response curves were fitted by a non-linear regression using a Prism 4.0 programme (GraphPAD Software, San Diego, CA, USA). To quantify agonist potency, EC50 values were calculated. EC50 is the concentration of agonists required to produce 50% of the maximum effect. To evaluate the antagonist profile, IC50 values were determined. IC50 is the concentration of antagonists that produces 50% inhibition of the agonist effect. Each concentration was tested five times in triplicate, and the values are given as mean ± standard error (SE).
Results and discussion
Initially, this work was focused to quantify cAMP after GPR17 stimulation by using two different immune-competitive assays, but no result was obtained. For this reason, the new sensitive and non-radioactive GloSensor cAMP assay was considered.
The assay optimization process and its validation are essential to use it as a method to screen GPR17 ligands. For this reason, at the beginning, the first step was aimed at individuating the specific crucial features of the assay that are strictly correlated to its technology itself. In particular, the number of cells, concentration of GloSensor cAMP reagent to incubate with the cells, equilibration time, and temperature were considered.
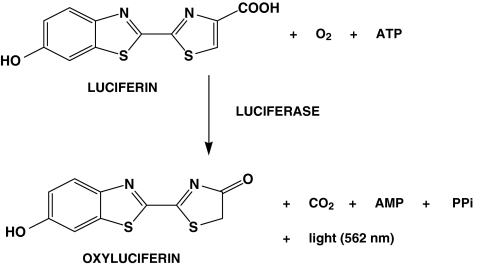
The GloSensor cAMP reagent is mainly composed of Luciferin. Luciferin is the luciferase substrate that works in the presence of cAMP produced after receptor activation. The biosensor, becoming active, interacts with its substrate luciferin converting it in oxyluciferin and emitting light at 562 nm, as reported in Fig. 2.
Fig. 2.
Schematic representation of luciferase reaction. Oxidation of luciferin to oxyluciferin
The equilibration time required for the GloSensor cAMP reagent will vary depending on the cell number, concentration used in the assay, and incubation temperature. During this process, the temperature should be constant, and it was noticed that at room temperature (20–25°C), the results obtained were better than those obtained at 37°C. Only when the steady-state basal signal is reached, usually after 120 min, the cells are stimulated with the agonist and luminescence measured. Generally, the luminescent kinetic of each response shows a rapid initial increase, and it remains relatively stable for 30–40 min. So, within a window of 30–40 min, luminescent signals are red. Moreover, this is a no-lytic assay that works well without the incubation with phosphodiesterase inhibitors such as rolipram suggesting the high response and system stability. The increase of cAMP accumulation by addition of UDP at HEK293 cells, stably transfected with the L9-2 plasmid expressing a genetically modified form of firefly luciferase and transiently transfected with human GPR17 (hGPR17) plasmid, was determined. HEK293 cells do not constitutively express P2Y receptors [6], and for this reason they can be utilized to characterize the hGPR17 P2Y nature.
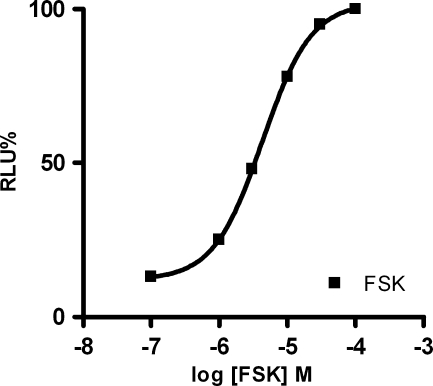
The experiments were performed after 48 h of hGPR17 transfection utilizing UDP, UDP galactose, and UDP glucose as referent agonists. In order to establish the concentration of FSK that produce 80% of the maximum response, the concentration–response curve was constructed (Fig. 3). Results showed that the best concentration of FSK was 10 μM. Initially, the experiments were carried out in the presence or in the absence of FSK in order to evaluate if GPR17 was coupled to Gαi or Gαs. Results showed that in the absence of FSK, UDP did not produce the concentration–response curve, but in the presence of it, a good concentration–response curve was obtained, confirming that this receptor is coupled with a Gαi [10]. Experiments for cell number optimization were achieved. Results showed that an RLU decrease was noticed when the number of cells as well as UDP doses, compared to the control (non-stimulated cell), were increased. The number of cells necessary to get a good luminescent signal at HEK293 L9-2 cells, transiently transfected with hGPR17, was 80,000.
Fig. 3.
FSK dose–response curve at HEK293 L9-2 cells transiently transfected with hGPR17
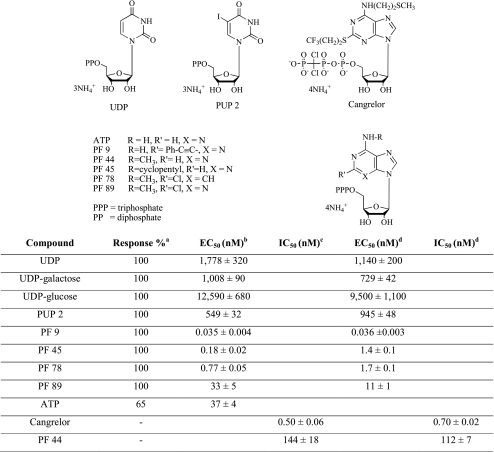
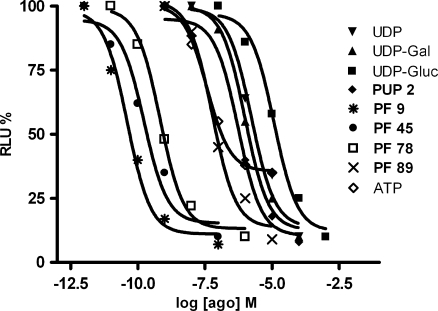
In order to validate this method, a series of compounds previously evaluated with [35S]GTPγS binding assay, based on the ability of agonists to increase the binding of radioactive GTP to the activated GPCR, was considered [11]. In particular, the UDP analogue PUP 2 and the ATP derivatives Cangrelor, PF 9, PF 44, PF 45, PF 78 and PF 89, differently modified in N6 and in 2-position, were selected (see Table 1). All the chosen compounds resulted to behave as agonists in the [35S]GTPγS binding assay with the exception of Cangrelor and PF 44, which proved to be antagonists. In addition, also ATP behaviour was considered. The concentration–response curves of the tested compounds, in comparison with UDP, UDP-galactose, and UDP-glucose, are shown in Fig. 4.
Table 1.
Biological activity and response of the studied compounds at HEK293 L9-2 cells transiently transfected with hGPR17 using the GloSensor cAMP assay in comparison with [35S]GTPγS binding assay
aResponse is expressed as percentage of the maximal relative luminescence units and is referenced against UDP, set at 100%
bConcentration of agonist required to produce 50% of the maximum effect
cConcentration of antagonists that produces 50% inhibition of agonist effect. Each concentration was tested five times in triplicate, and values are given as the mean ± SE
Fig. 4.
Concentration–response curves in GloSensor cAMP functional assays at HEK293 L9-2 cells transiently transfected with hGPR17 of considered compounds. Each point represents the mean of five experiments performed in triplicate with a maximum SE lower than ±10
The results reported in Table 1 revealed that compounds PF 9, PF 45, PF 78, PF 89, and PUP 2 are more potent than the reference agonists, with EC50 values very close to those obtained with the other method reported in literature [11]. In fact, the most active of the series was PF 9 with an EC50 value of 0.035 nM obtained with GloSensor cAMP method vs 0.036 nM reported with [35S]GTPγS binding assay. In addition, the less active compound was confirmed to be PUP 2 with an EC50 value of 549 vs 945 nM. Moreover, the correspondence of other compounds EC50 values was elevated. All agonists showed full agonist behaviour with the exception of ATP that resulted to be a partial agonist. In this cell system, no response was ever observed when cells were transfected with a corresponding empty vector.
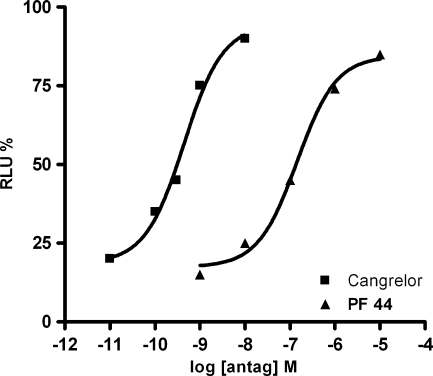
The ability of the known purinergic antagonist Cangrelor and of PF 44 to counteract an agonist-induced decrease of cAMP accumulation in HEK293 L9-2 cells expressing the hGPR17 were also assessed. Both Cangrelor and PF 44 were able to inhibit, in a concentration-dependent manner, the decrease of cAMP induced by 50 μM UDP (Fig. 5) and by 0.5 nM PF 9 (data not shown). Results obtained with these two agonists are very similar, confirming that they interact with the same receptor site.
Fig. 5.
Effect of Cangrelor and compound PF 44 on UDP mediated cAMP accumulation (vs forskolin, set to 100%) in HEK293 L9-2 cells transiently transfected with hGPR17. Each point represents the mean of five experiments performed in triplicate with a maximum SE lower than ±10
A strong correlation was found by comparing these results, expressed as EC50 or IC50 values for agonists and antagonists, respectively, with the data obtained with [35S]GTPγS binding assay, thus confirming the validity of this approach in the study of new potential ligands for GPR17.
Conclusions
In this work, a new alternative assay to pharmacologically characterize new and known receptors was validated. Results obtained in terms of EC50 and IC50 values, reported and summarized in Table 1, are similar to the data reported in literature and obtained by using [35S]GTPγS binding assay. Hence, our experiments proved that this innovative and non-radioactive assay is a valid, reproducible and robust alternative to the more traditional methods, and for this reason, it may be used to screen new synthesized GPR17 ligands.
Acknowledgements
This work was supported by grants from the Italian Ministry for University and Research (PRIN2008) and the Ministry for Health (Progetto Ordinario Neurolesi, RF-CNM-2007-662855).
Footnotes
Michela Buccioni and Gabriella Marucci equally contributed to this work.
References
- 1.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Wegner CD. P2 receptors in the respiratory system. Handb Exp Pharmacol. 2001;151/II:281–300. doi: 10.1007/978-3-642-56921-0_8. [DOI] [Google Scholar]
- 3.Capra V. Molecular and functional aspects of human cysteinyl leukotriene receptors. Pharmacol Res. 2004;50:1–11. doi: 10.1016/j.phrs.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mellor EA, Maekawa A, Austen KF, Boyce JA. Cysteinyl leukotriene receptor 1 is also a pyrimidinergic receptor and is expressed by human mastcells. Proc Natl Acad Sci USA. 2001;98:7964–7969. doi: 10.1073/pnas.141221498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lecca D, Trincavelli ML, Gelosa P, Sironi L, Ciana P, Fumagalli M, Villa G, Verderio C, Grumelli C, Guerrini U, Tremoli E, Rosa P, Cuboni S, Martini C, Buffo A, Cimino M, Abbracchio MP. The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS One. 2008;3:e3579. doi: 10.1371/journal.pone.0003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceruti S, Villa G, Genovese T, Mazzon E, Longhi R, Rosa P, Bramanti P, Cuzzocrea S, Abbracchio MP. The P2Y-like receptor GPR17 as a sensor of damage and a new potential target in spinal cord injury. Brain. 2009;132:2206–2218. doi: 10.1093/brain/awp147. [DOI] [PubMed] [Google Scholar]
- 9.Marucci G, Lammi C, Buccioni M, Dal Ben D, Lambertucci C, Amantini C, Santoni G, Kandhavelu M, Abbracchio MP, Lecca D, Volpini R, Cristalli G. Comparison and optimization of transient transfection methods at human astrocytoma cell line 1321N1. Anal Biochem. 2011;414:300–302. doi: 10.1016/j.ab.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Benned-Jensen T, Rosenkilde MM. Distinct expression and ligand-binding profiles of two constitutively active GPR17 splice variants. BJP. 2010;159:1092–1105. doi: 10.1111/j.1476-5381.2009.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calleri E, Ceruti S, Cristalli G, Martini C, Temporini C, Parravicini C, Volpini R, Daniele S, Caccialanza G, Lecca D, Lambertucci C, Trincavelli ML, Marucci G, Wainer IW, Ranghino G, Fantucci P, Abbracchio MP, Massolini G. Frontal affinity chromatography-mass spectrometry useful for characterization of new ligands for GPR17 receptor. J Med Chem. 2010;53:3489–3501. doi: 10.1021/jm901691y. [DOI] [PMC free article] [PubMed] [Google Scholar]