Abstract
Pain is a major problem after burns. Procedural pain evoked by burn dressing changes is common in patients, and its management is a critical part of treatment in acute burn injuries. Burn pain is very likely the most difficult form of acute pain to treat. ATP contributes to inflammation, and ATP is implicated in peripheral pain signaling via actions upon P2X3 receptors. Puerarin is extracted from a traditional Chinese medicine and may act on P2X3 receptor mechanisms. The Visual Analogue Scale (VAS) has been shown to be a sensitive indicator of pain intensity and treatment effects. Peripheral blood mononuclear cells (PBMCs) are involved in nociception or pain after burn injury. Burn patients were randomly divided into normal saline (NS) group (salt solution is saline) and puerarin-treated group and pain (Visual Analogue Scale scores) and inflammation (PBMCs) measured. Burn pain produces a stress response, so blood glucose, insulin, and cortisol levels in burn patients were determined. Furthermore, the expression of P2X3 protein and mRNA in PBMCs was detected. The VAS scores in the puerarin-treated group were lower than those in NS group. The blood glucose, insulin, and cortisol levels in the puerarin-treated group at post-dressing changes were significantly decreased in comparison with those in NS group. The expression levels of P2X3 protein and mRNA in PBMCs of burn patients in NS group were significantly increased in comparison with those in the puerarin-treated group. Puerarin can antagonize inflammatory factors (such as ATP) and decrease the upregulated expressions of P2X3 protein and mRNA in PBMCs after burns to decrease VAS. Thus, puerarin had an analgesic effect on procedural pain in dressing changes of burn patients related to P2X3 receptors.
Keywords: P2X3 receptors, Burn, Puerarin
Introduction
Burn injuries are frequent and disabling problems in most areas of the world [14]. Pain is a major problem after burns [49] and is one of the patients’ most serious and persisting complaints. Adequate pain control is therefore of utmost importance. Burn pain includes procedural pain, background pain, and breakthrough pain [23]. There are two main features of burn pain, one is its long-lasting course which frequently exceeds healing time, and the other is the repetition of highly nociceptive procedures which can lead to severe psychological disturbances if pain control is inappropriate [28]. Procedural pain evoked by burn dressing changes is more common and painful in patients, and its management is a critical part of treatment in acute burn injuries. Burn pain is very likely the most difficult form of acute pain to treat [41]. Management of burn pain is still a challenge in clinical settings, although several treatment options have been suggested [61]. It is necessary to combine antinociceptive treatment with antineuropathic treatment for the management of burn pain [45]. Our objective is to explore a safe, effective, and feasible analgesic drug and a new mechanism of pain formation.
P2 purinoceptors responding to ATP are divided into ionotropic receptors (P2X, seven types; P2X1–7) coupled to ion channels and metabotropic receptors (P2Y, eight types; P2Y1,2,4,6,11,12,13,14) coupled to intracellular second-messenger systems through heterotrimeric G-proteins [6]. P2 receptors expressed in blood cells regulate responses such as cell proliferation, differentiation, chemotaxis, cytokine release, and immune and inflammatory responses [8, 11]. ATP acts on P2X receptors on nociceptive sensory neurons and participate in the transmission of pain signals from the periphery to the spinal cord. ATP can activate peripheral nociceptors and produces intense pain when skin is inflamed [10, 21, 22]. ATP is implicated in peripheral pain signaling via actions upon P2X3 receptors [4, 7]. P2X3 knock-down animals show noticeable reductions in chronic inflammation-induced thermal and mechanical hyperalgesia, and spinal nerve ligation-induced mechanical allodynia [37]. A role for the P2X3 receptors in peripheral pain mechanisms, such as chronic inflammatory pain and some features of neuropathic pain has been suggested.
Puerarin (C21H20O9) is an isoflavone isolated from a traditional Chinese medicine Ge-gen (Radix Puerariae) [19]. It seems to be a vasodilator similar to papaverine. Puerarin is widely used in clinical treatment of myocardial infarction [19], cerebral ischemia [15], and diabetes [52] in China. Puerarin protects the heart from arrhythmias induced by BaCl2 and aconitine in rats [19]. Puerarin given intravenously to patients is reported to relieve angina, lower blood pressure, and slow heart rate [15, 19, 52]. Puerarin can significantly increase blood supply of cardiac patients, improve microcirculation, and protect ischemic myocardium. Puerarin reduces blood sugar and cholesterol [52]. It has been reported that puerarin possesses protective effects, at least in part, which are related to its ability to increase superoxide dismutase activity, decrease lipid peroxidation, and enhance fibrinolysis [19]. Puerarin is a scavenger of oxygen-free radicals and is capable of prophylactically antagonizing against the oxidative injury by H2O2 and superoxide anion [60]. Recent studies demonstrate that puerarin may inhibit oxidative stress induced by acute alcoholism [59], reducing the production of PGE2, TNF, and IL-6 [42] and act as an anti-inflammatory agent by blocking NF–kappa B signaling, so it may possibly be developed as a useful agent for the chemoprevention of atherosclerosis [55]. In preclinical studies, after burn injury in the rats, puerarin could reduce the nociceptive transmission of burn injury pain mediated by P2X3 receptors and alleviate hyperalgesia induced by P2X3 receptor activation [54]. The purpose of this study is to investigate the relationship between burn pain and the expression of P2X3 receptors on peripheral blood mononuclear cells (PBMCs) and the effects of puerarin on burn pain in humans.
Methods
Patients
The study was conducted in the Burn Center of the First Affiliated Hospital of Nanchang University, China. Following local ethics committee approval, written informed consent was obtained from 40 patients of either sex, aged between 25 to 50 years with second- and third-degree burns of 10–55% of total body surface area (TBSA) and less than 19% of TBSA to third degree. Patients weighting 50 to 80 kg were enrolled. They were admitted to hospital within 24 h after burns and received no medication pre-hospital. The time of patients’ each dressing change was no less than 8 min and Visual Analogue Scale (VAS) score was ≧5.
Patients who were confused, who had a history of substance abuse, who had injury too severe to allow normal use of intravenously drip and multi-function ECG monitor, or who had compromised airway, or pregnancy and lactation were excluded from the study. Other patients excluded were those who required inotropic support or mechanical ventilatory support, those with arterial oxygen saturation (SpO2) of <90% on room air, those with significant renal or hepatic insufficiency, and those with a known hypersensitivity to puerarin.
Patient groups
Patients were randomly divided into normal saline (NS) group (A group) and puerarin-treated group (B group). “A” group was applied with 100 ml 0.9% sodium chloride injection and “B” group with 100 ml puerarin glucose injection (Yangtze River Pharmaceutical Group, SFDA Licence Number H20020450, including puerarin 200 mg). Both administrations by intravenously drip (30–40 drops/min, within 30–40 min) lasted for 3 days before dressing changes after the burn shock. Other routine clinic treatment was the same. There was no difference in sex, age, weight, and burned body surface (% body surface area burned = BSA) between two groups before the study (p > 0.05; Table 1).
Table 1.
Basic comparison of patients in each group
| A group | B group | |
|---|---|---|
| Sex (female/male) | 6/12 | 8/14 |
| Age, years | 39.69 ± 12.57 | 42.29 ± 17.53 |
| Weight, kg | 60.53 ± 8.77 | 63.59 ± 9.52 |
| BSA, % | 27.63 ± 11.04 | 29.38 ± 10.55 |
A group: normal saline (NS)-treated group; B group: puerarin (PUE)-treated group. There was no difference in sex, age, weight, and burned body surface (% body surface area burned = BSA) between two groups before the study (p > 0.05)
Visual Analogue Scale
The VAS is one of the most commonly used pain assessment instruments and is regarded as the ‘gold standard’ in research and clinical practice [36, 38, 56]. The VAS consists of a 100-mm horizontal or vertical straight line with anchors indicating, for example, ‘no pain’ and ‘worst pain imaginable’. The pain experience is recorded by marking the appropriate point on the line [38, 39]. Patients considered suitable for the study were instructed in the use of VAS for assessment of pain intensity. After administration, VAS was applied at pre-, mid-, and post-dressing changes. Six time points respectively were at 10 min pre-dressing changes, 1 min and 8 min mid-dressing, 10 min, 30 min, and 60 min post-dressing changes.
The determination of blood glucose, insulin, and cortisol
Peripheral blood samples (n = 40) were collected from patients at three time points, when pre-treated on the first day and post-treated on the second and third day after dressing changes. Eligible participants included ten healthy volunteers who did not undergo any treatment. Blood in ten healthy volunteers was collected and transported in an identical manner compared with that in patients. Approximately 6–8 ml peripheral blood was collected in vacuum blood collection tubes at each time point. About 2 ml peripheral blood was transported to the clinical laboratory of the first affiliated hospital of Nanchang University for the analysis of blood glucose, insulin, and cortisol and others preserved on ice sent to physiology laboratory. Reference values of blood glucose, insulin, and cortisol were 3.8 ~ 6.1 mmol/L, 5 ~ 10 mIU/L, and 4 ~ 20 μg/dl, respectively.
Isolation of PBMCs
Approximately 4–6 ml blood samples at each time point were diluted (V ratio1:1) with 0.1 M phosphate-buffered saline (PBS) and subjected to density gradient centrifugation using Ficoll (Beijing Broad-Wright Science and Technology, LTS1077) at 2,200 rpm for 25 min. PBMCs were isolated and washed twice in sterile 0.1 M PBS solution at 1,500 rpm for 5 min. After washing, 2–3 × 106 cells were collected in two centrifuge tubes with each one about 1 × 106 cells. In one tube, 4% paraformaldehyde (0.5 ml) was added and chilled on 4°C for 1 day, and then used for immunohistochemistry. Other cells were used to extract the total RNA.
Immunohistochemistry
Isolated PBMCs were fixed and embedded in OCT (tissue-freezing medium, Sakura Finetek USA, Inc.). Preparations were cut into 8 μm thick on the cryostat. Sections were first rinsed for 3 × 5 min in 0.1 M PBS and then blocked for endogenous peroxidase activity with 0.3% hydrogen peroxide for 10 min at room temperature, then next with 10% goat serum for 30 min at room temperature to block non-specific binding, and followed by an incubation overnight at 4°C with a rabbit anti-P2X3 (1:2,000 diluted in PBS; CHEMICON International, Inc., USA). On the second day, after 3 × 5 min rinse in PBS, the sections were incubated with biotinylated goat anti-rabbit secondary antibody (Beijing Zhongshan Goldenbridge Biotechnology Co. Ltd, SP-9001 kit) for 40 min at 37°C. Finally, streptavidin–horseradish peroxidase (Beijing Zhongshan Goldenbridge Biotechnology Co. Ltd, SP-9001 kit) was added, and slides were incubated for 20 min at 37°C. Diaminobenzidine was used as the chromogen, and color development was stopped by gently dipping slides into distilled water. Image scanning analysis system (HMIV-2000, Wuhan) was used to analyze the changes in stain values (integrated optical density) of P2X3 in isolated cells. Background was determined by averaging the optical density of ten random areas.
Reverse transcription polymerase chain reaction (RT-PCR)
The total RNA was extracted from the isolated PBMCs using TRIZOL reagent according to the manufacturer’s instructions (TIANGEN Co.). TRIZOL (1 ml) was added to 1 × 106 cells to extract the total RNA. It was reverse-transcribed into complementary DNA (cDNA) with Revert Aid First Strand cDNA Synthesis Kit (Fermentas Co.). cDNA was amplified in 50 μl of polymerase chain reaction (PCR) mixture (TIANGEN Co.). The samples were amplified for 35 cycles. Each cycle consisted of denaturation at 94°C for 45 s, annealing at 48°C for 45 s, and extension at 72°C for 1 min. The primers used were: human P2X3 receptors (Gen Bank TM accession number NM_002559): sense 5-CTCCTTCCCAACCTGACA-3 and antisense 5-CTCATTCACCTCCTCAAACT-3. β-actin message was used to normalize the cDNA amount to be used. The electrophoresis of PCR products was made on 1.5% agarose gel and visualized by ethidium bromide staining.
Statistical analysis
Statistical analyses of the data were performed by SPSS 11.5. All results were expressed as mean±SEM. Statistical significance was determined by one-factor analysis of variance followed by the Fisher post hoc test for multiple comparisons. Student’s t test was used for other data analysis. p < 0.05 was considered significant.
Results
Effects of puerarin on Visual Analogue Scale
Analysis of VAS responses was given by each patient at six time points after administration. There was no statistical significance between NS-treated group (A group, n = 18) and puerarin (PUE)-treated group (B group, n = 22) before dressing changes (p > 0.05). During the dressing change procedure, mean VAS scores of A group at mid- and post-dressing changes were higher than those at pre-dressing changes (p < 0.05) from the first day to third day. The lower values in B group were presented in comparison with those in A group at 8 min mid-dressing and every time point at post-dressing from the first day to third day after the burn shock (p < 0.05). Especially at 8 min mid-dressing on the third day, mean VAS scores in B group was decreased by 32%, from 4.67 ± 0.80 to 3.17 ± 0.60. At 30 and 60 min post-dressing, mean VAS scores in B group came back to the basic and even lower than those at pre-dressing (Table 2).
Table 2.
Effects of puerarin on Visual Analogue Scale
| Group | Day | 10 min pre-dressing | 1 min mid | 8 min mid | 10 min post | 30 min post | 60 min post |
|---|---|---|---|---|---|---|---|
| A group (n = 18) | 1 | 1.75 ± 0.48# | 4.50 ± 1.04 | 5.00 ± 1.08# | 3.50 ± 1.19# | 2.75 ± 1.11# | 2.50 ± 1.19# |
| 2 | 1.50 ± 0.28# | 5.00 ± 1.08 | 5.50 ± 1.56# | 3.50 ± 0.96# | 1.75 ± 0.47# | 1.75 ± 0.48# | |
| 3 | 1.75 ± 0.25# | 4.00 ± 0.81 | 4.50 ± 1.26# | 3.00 ± 1.0# | 2.00 ± 0.70# | 1.50 ± 0.29# | |
| B group (n = 22) | 1 | 1.83 ± 0.31 | 5.83 ± 0.91 | 4.67 ± 0.80* | 2.50 ± 0.43* | 1.67 ± 0.21* | 1.17 ± 0.17* |
| 2 | 1.67 ± 0.21 | 5.17 ± 1.05 | 5.00 ± 0.68* | 3.17 ± 0.60* | 2.00 ± 0.45* | 1.33 ± 0.21* | |
| 3 | 1.50 ± 0.22 | 4.50 ± 0.76 | 3.17 ± 0.60* | 1.67 ± 0.21* | 1.33 ± 0.21* | 1.17 ± 0.17* |
A group: normal saline (NS)-treated group; B group: puerarin (PUE)-treated group. The VAS scores of A group at mid- and post-dressing changes were higher than those at pre-dressing changes #(p < 0.05) from the first day to third day. The lower values in B group were presented in comparison with those in A group at 8 min mid-dressing and every time point at post-dressing from the first day to third day after the burn shock *(p < 0.05)
Effects of puerarin on blood glucose, insulin,, and cortisol
Ten healthy volunteers were used as a control group (C group). The values of blood glucose, insulin, and cortisol in burn patients of NS-treated group (A group, n = 18) from the first day to third day were not different (p > 0.05), but were significantly higher than those in C group (p < 0.05). The values of blood glucose and insulin in burn patients of PUE-treated group (B group, n = 22) on the second and third days were lower than those on the first day (p < 0.05) but were also significantly decreased in comparison with those in burn patients of A group at post-dressing on the second day and third day (p < 0.01; Table 3).
Table 3.
Effects of puerarin on blood glucose, insulin, and cortisol
| Healthy | NS d1 | NS d2 | NS d3 | PUE d1 | PUE d2 | PUE d3 | |
|---|---|---|---|---|---|---|---|
| Blood glucose, mmol/L | 5.18 ± 0.52 | 7.83 ± 1.27# | 7.47 ± 2.11# | 7.43 ± 2.48# | 7.55 ± 1.27 | 5.72 ± 1.13*## | 5.60 ± 1.14*## |
| Insulin, mIU/L | 7.42 ± 1.40 | 21.77 ± 4.90# | 23.23 ± 7.72# | 21.00 ± 9.90# | 24.75 ± 6.08 | 20.00 ± 11.34*## | 13.67 ± 1.04*## |
| Cortisol, μg/dl | 11.45 ± 2.68 | 18.80 ± 3.76# | 21.48 ± 3.71# | 18.00 ± 3.46# | 11.28 ± 2.12 | 14.03 ± 2.74*## | 10.1 ± 4.84*## |
A group: normal saline (NS)-treated group; B group: puerarin (PUE)-treated group; C group: healthy volunteers (control). The values of blood glucose, insulin, and cortisol of A group from the first day to third day were significantly higher than those in C group #(p < 0.05), and the values of B group on the second and third days were lower than those on the first day *(p < 0.05), but were also significantly decreased in comparison with those in A group at post-dressing on the second day and third days ##(p < 0.01)
Effects of puerarin on the expression of P2X3 immunoreactivity in PBMCs
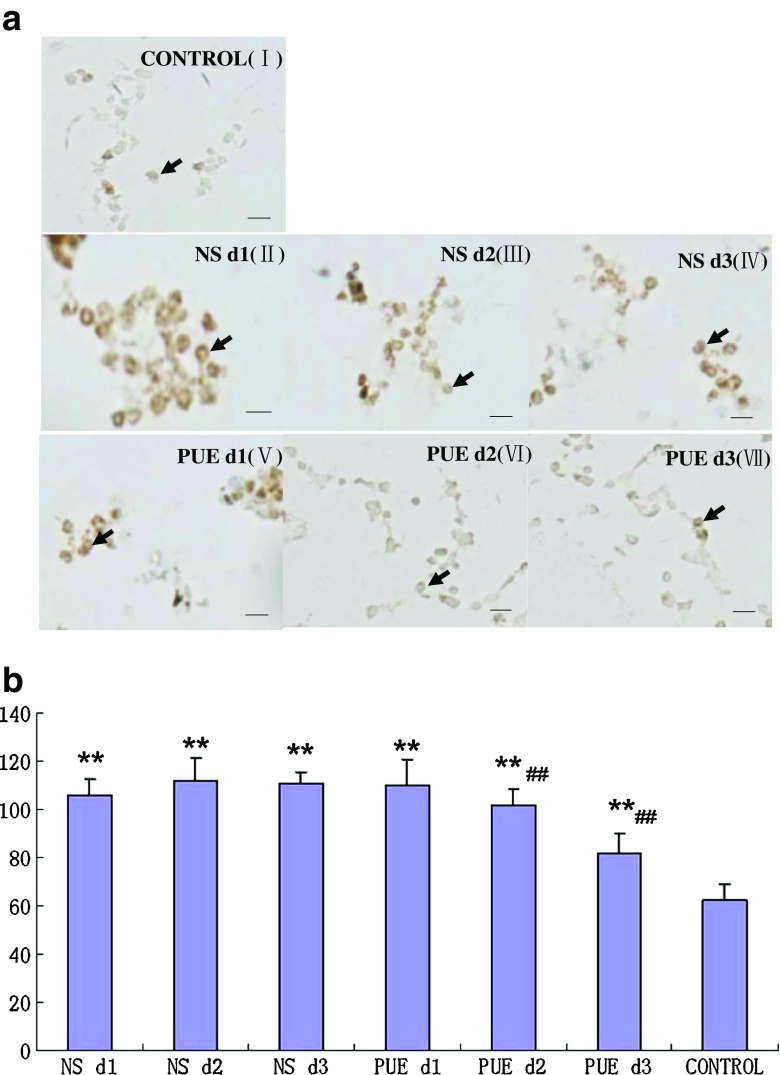
P2X3 receptor immunoreactivity in the PBMCs was detected using immunohistochemistry. The stain values (integrated optical density) of P2X3 expression in C group (healthy volunteers) from the first to third day were not different (p > 0.05). The stain value of P2X3 expression in C group was 62.4 ± 6.33. The stain values of P2X3 expression from the first to third day in burn patients of A group (NS-treated group) were 105.9 ± 6.48, 111.8 ± 9.18, and 110.6 ± 4.48, respectively. And those in burn patients of B group (PUE-treated group) were 110.1 ± 10.56, 102.0 ± 6.25, and 81.6 ± 8.41, respectively.
The expression values of P2X3 immunoreactivity from the first to third days in A and B group were significantly higher than those in C group (p < 0.01), and no difference was found between A and B group before dressing changes (p > 0.05). The integrated optical density of P2X3 expression in B group on the second day was lower than that on the first day (p < 0.05) but also lower than that in A group on the second day (p < 0.01) and third day (p < 0.05). The stain values of P2X3 expression in B group on the second day and third day were sharply decreased in comparison with those in A group (p < 0.01) but still higher than those in C group (p < 0.01; Fig. 1).
Fig. 1.
Effect of puerarin on the expression of P2X3 receptors in PBMCs by immunohistochemistry. a I: healthy volunteers(control); II: NS-treated group on the first day; III: NS-treated group on the second day; IV: NS-treated group on the third day; V: PUE-treated group on the first day; VI: PUE-treated group on the second day; V II: PUE-treated on the third day. (Arrows indicate the immunostaining of PBMCs; scale bars, 20 μm). b **p < 0.01, compared with Healthy group(control); ## p < 0.01, compared with NS-treated group in histogram
Effects of puerarin on the expression of P2X3 mRNA in PBMCs
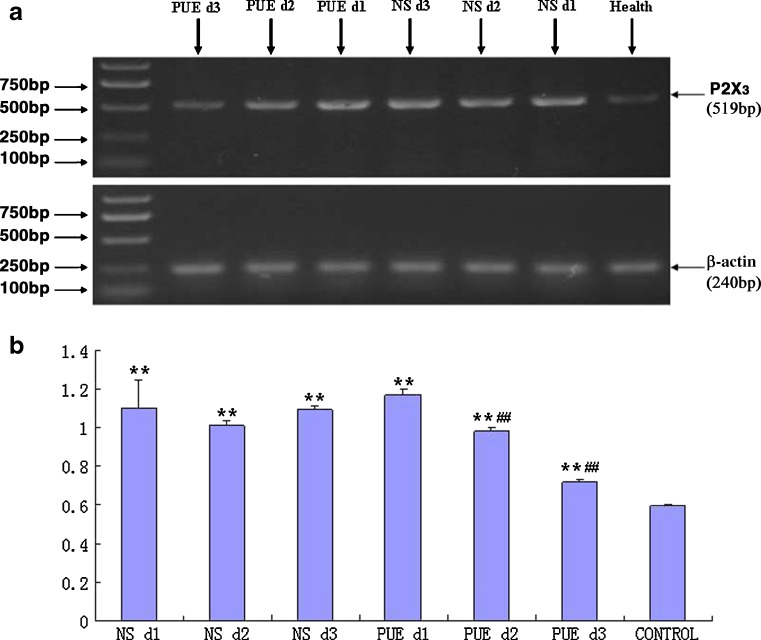
The expression level of P2X3 mRNA in PBMCs was detected by RT-PCR. The gray scale values of P2X3/β-actin in C group (healthy volunteers) from the first to third day were not different (p > 0.05). The expression value of P2X3 mRNA in C group was 0.5935 ± 0.0080. The stain values of P2X3 mRNA expression from the first to third days in burn patients of A group (NS-treated group) were 1.1001 ± 0.1476, 1.0143 ± 0.0221, and 1.0923 ± 0.0197, respectively. The expression values of P2X3 mRNA from the first to third days in burn patients of B group (PUE-treated group) were 1.1715 ± 0.0308, 0.9848 ± 0.0145, and 0.7188 ± 0.0086, respectively.
The expression values of P2X3 mRNA from the first to third days in A and B groups were significantly higher than those in the C group (p < 0.01). The expression levels of P2X3 mRNA in B group on the second and third days were not only significantly lower than those on the first day (p < 0.01) but also lower than those in A group from the first to third days (p < 0.01; Fig. 2).
Fig. 2.
Effects of puerarin on the expression of P2X3 mRNA in PBMCs were detected by RT-PCR. a The expression of P2X3 receptors mRNA in NS and PUE group was higher than that in the healthy control group (p < 0.01). The expression of P2X3 mRNA in group PUE d2 and PUE d3 was significantly lower than that in PUE d1 (p < 0.01) and significantly lower than that in NS group(p < 0.01), although still higher than that in the healthy control group (p < 0.01). b **p < 0.01, compared with Healthy group (control); ## p < 0.01, compared with NS-treated group in histogram
Discussion
Acute pain following burn is due to the stimulation of skin nociceptors. Burns will immediately prompt an intense inflammatory response and the release of chemical mediators that sensitize the active nociceptors at the site of injury. This will lead to the wound being sensitive to mechanical stimuli such as touch, rubbing, or debridement, as well as chemical stimuli such as antiseptics or other topical applications, which is called primary hyperalgesia [41, 43]. Our results show that the VAS pain scores in the puerarin-treated group were lower compared with those in the saline group at 8 min mid-dressing and at each time point post-dressing from the first to third days after the burn shock. Especially at 8 min mid-dressing on the third day, the VAS scores in the puerarin-treated group were significantly decreased. The VAS has been shown to be a sensitive indicator of pain intensity and treatment effects [36, 56], and the degree of pain relief is significantly correlated with a decrease in VAS [36, 56]. It appears that the treatment with puerarin can relieve the procedural pain involved in dressing changes of burn patients.
Pain accompanies burns and involves emotion and physical stress and dysregulation of the hypothalamic–pituitary–adrenocortical axis and immune system [14, 40]. This rapid response is brought about by sudden increases in sympathetic nervous system activity and endogenous stress hormone levels [14, 50]. It has been reported that the secretions of plasma cortisol, adrenocorticotropic hormone, and aldosterone are significantly increased immediately after severe burns, and the extent of this is associated with intensity of stress [14, 40]. Furthermore, the levels of blood glucose, insulin, and cortisol in burn patients are enhanced. In patients with large areas of burn, particularly third-degree burns, lasting hyperglycemia and hypermetabolism promote secretion from Islets of Langerhans to accelerate glycogenolysis, which elevate blood glucose [14, 25, 40, 51]. Increased levels of cortisols are further associated with enhanced hepatic gluconeogenesis and a reduced insulin-mediated glucose uptake into skeletal muscle and adipose tissue [20, 44]. These phenomena may lead to elevated blood glucose levels in association with normal or elevated serum insulin concentrations [53, 57]. The blood glucose, insulin, and cortisol levels of burn patients in the puerarin-treated group at post-dressing change times were not only lower than those at pre-dressing but were also significantly decreased in comparison with those in the saline group at post-dressing change times. After treatment with puerarin, the decrease in VAS correlated with the changes of blood glucose, insulin, and cortisol levels. The levels of blood glucose, insulin, and cortisol in burn patients were enhanced after stress response-induced by burn pain. The pain relief in dressing changes of burn patients followed the reduction of blood glucose, insulin, and cortisol levels. These observations further indicate that puerarin has an analgesic effect on procedural pain in burn dressing changes.
It has been reported that puerarin could decrease the sensitization of P2X3 receptors involved in hyperalgesia after burn injury in the rats [54]. Does the mechanism of puerarin inhibiting procedural pain in burn dressing changes of patients also affect P2X3 receptors?
ATP is involved in peripheral pain signaling by acting upon P2X3 receptors [3–5, 7, 24], and P2X3 receptors are crucial in mediating both acute pain and chronic pain [7, 12, 16–18, 29–34, 46–48, 58]. The P2X2/3 knockouts display significant reduction in pain reception in response to ATP [9]. Neurons expressing P2X3 receptors are dramatically more responsive to ATP with even small increases in temperature [13]. P2X3 expression in sensory neurons was increased after burn injury in the rats [18, 54], and P2X3 receptors are relevant with burn injury pain [18, 54]. PBMCs are a key link in inflammatory response [35]. There are strong immunoreactivities for β-endorphin, EM-1, and EM-2 in leukocytes of inflamed subcutaneous paw tissue at 4 days after intraplantar injection of complete Freund’s adjuvant [27]. These leukocytes had morphological appearances consistent with mononuclear cells and granulocytes. The pain of tissue damage is conveyed by the release of cytosolic ATP onto receptors expressed by nociceptive sensory neurons (nociceptors) [1, 2, 26]. ATP can also be released by macrophages in response to cytokines, osmotic stress, and mechanical stimulation. Puerarin may decrease the release of ATP at the site of burn injury and inhibit the intense inflammatory response to relieve the burn-related procedural pain evoked by dressing changes.
We investigated whether P2X3 receptors are involved in puerarin actions. Our results show that the expression of P2X3 protein and mRNA in PBMCs of burn patients is significantly increased. P2X3 receptors on PBMCs appear to be involved in nociception or pain after burn injury. The expression of P2X3 protein and mRNA in PBMCs of burn patients was significantly decreased in the puerarin-treated group at post-dressing times. The effect of puerarin on P2X3 receptor expression of PBMCs in humans is in agreement with previous studies that show downregulation of the expression of P2X3 receptors following in burn rats [18, 54]. The effect mechanisms of puerarin on burn pain may include antagonism of inflammatory factors (such as ATP) by downregulated expression of P2X3 receptors in PBMCs of burn patients.
Conclusions
In summary, P2X3 receptors are involved in procedural pain in burn dressing changes. Puerarin can decrease the stress response to burn pain, antagonize the inflammatory response mediated by ATP, and decreases the upregulated expression of P2X3 protein and mRNA in PBMCs after burns to decrease VAS. These actions may mediate the analgesic effect of puerarin on procedural pain in dressing changes of burn patients related to P2X3 receptors.
Acknowledgments
This work was supported by the grant (nos. 31060139, 30860086, 30860333, 30660048, and 30460040) from National Natural Science Foundation of China, the grant (no. 20070403007) from Doctoral Fund of Ministry of Education of China, and the grant (nos. 0640042 and 2008GZY0029) from Natural Science Foundation of Jiangxi Province; the grant (no. 2010BSA09500) from Technology Pedestal and Society Development Project of Jiangxi Province; the grant (nos. 2007–60 and GJJ08049) from the Educational Department of Jiangxi Province; and the grant (YBP08A01) from Jiangxi Province Excellent PhD Students Foundation.
Footnotes
Xin Li, Jun Zhang, Yun Gao, and Yang Yang are jointly first authors.
Change history
9/26/2020
Due to the authors��� carelessness, we used mistakenly PBMCs isolated from same patient in Fig. 1a for P2X3 immunoreactivity in VI: PUE-treated group on the second day (Third row middle graph) and VII: PUE-treated on the third day (Third row right- side graph).
References
- 1.Bleehen T, Keele CA. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 1996;347:1604–1605. doi: 10.1016/S0140-6736(96)91082-X. [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G. P2X receptors in sensory neurones. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110:433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 9.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 10.Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- 11.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.V97.3.587. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly-Roberts D, McGaraughty S, Shieh CC, Honore P, Jarvis MF. Painful purinergic receptors. J Pharmacol Exp Ther. 2008;324:409–415. doi: 10.1124/jpet.106.105890. [DOI] [PubMed] [Google Scholar]
- 13.Dussor G, Koerber HR, Oaklander AL, Rice FL, Molliver DC. Nucleotide signaling and cutaneous mechanisms of pain transduction. Brain Res Rev. 2009;60:24–35. doi: 10.1016/j.brainresrev.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fauerbach JA, McKibben J, Bienvenu OJ, Magyar-Russel G, Smith MT, Holavanahalli R, Patterson DR, Wiechman SA, Blakeney P, Lezotte D. Psychological distress after major burn injury. Psychosom Med. 2007;69:473–482. doi: 10.1097/psy.0b013e31806bf393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao L, Ji X, Song J, Liu P, Yan F, Gong W, Dang S, Luo Y. Puerarin protects against ischemic brain injury in a rat model of transient focal ischemia. Neurol Res. 2009;31:402–406. doi: 10.1179/174313209X444017. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Liu H, Deng LB, Zhu GH, Xu CS, Li GL, Liu SM, Xie JY, Liu J, Kong FJ, Wu RP, Li GD, Linag SD. Effect of emodin on neuropathic pain transmission mediated by P2X2/3 receptor of primary sensory neurons. Brain Res Bull. 2011;84:406–413. doi: 10.1016/j.brainresbull.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Xu CS, Liang SD, Zhang AX, Mu SN, Wang YX, Wan F. Effect of tetramethylpyrazine on primary afferent transmission mediated by P2X3 receptor in neuropathic pain states. Brain Res Bull. 2008;77:27–32. doi: 10.1016/j.brainresbull.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Xu CS, Yu KH, Li GL, Wan F, Liu SM, Lin JR, Liu H, Zhang J, Li X, Liang SD. Effect of tetramethylpyrazine on DRG neuron P2X3 receptor involved in transmitting pain after burn. Burns. 2010;36:127–134. doi: 10.1016/j.burns.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 19.Gao Q, Yang B, Ye ZG, Wang J, Bruce IC, Xia Q. Opening the calcium-activated potassium channel participates in the cardioprotective effect of puerarin. Eur J Pharmacol. 2007;574:179–184. doi: 10.1016/j.ejphar.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Gearhart MM, Parbhoo SK. Hyperglycemia in the critically ill patient. AACN Clin Issues. 2006;17:50–55. doi: 10.1097/00044067-200601000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton SG, McMahon SB, Lewin GR. Selective activation of nociceptors by P2X receptor agonists in normal and inflamed rat skin. J Physiol. 2001;534:437–445. doi: 10.1111/j.1469-7793.2001.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton SG, Warburton J, Bhattacharjee A, Ward J, McMahon SB. ATP in human skin elicits a dose-related pain response which is potentiated under conditions of hyperalgesia. Brain. 2000;123:1238–1246. doi: 10.1093/brain/123.6.1238. [DOI] [PubMed] [Google Scholar]
- 23.Hanafiah Z, Potparic O, Fernandez T. Addressing pain in burn injury. Curr Anaesth Crit Care. 2008;19:287–292. doi: 10.1016/j.cacc.2008.09.010. [DOI] [Google Scholar]
- 24.Jarvis MF, Kowaluk EA. Pharmacological characterization of P2X3 homomeric and heteromeric channels in nociceptive signaling and behavior. Drug Dev Res. 2001;52:220–231. doi: 10.1002/ddr.1119. [DOI] [Google Scholar]
- 25.Jeffries MK, Vance ML. Growth hormone and cortisol secretion in patients with burn injury. J Burn Care Rehabil. 1992;13:391–395. doi: 10.1097/00004630-199207000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Khmyz V, Maximyuk O, Teslenko V, Verkhratsky A, Krishtal O. P2X3 receptor gating near normal body temperature. Pflugers Arch. 2008;456:339–347. doi: 10.1007/s00424-007-0376-2. [DOI] [PubMed] [Google Scholar]
- 27.Labuz D, Berger S, Mousa SA, Zőllner C, Rittner HL, Shaqura MA, Segovia-Silvestre T, Przewlocka B, Stein C, Machelska H. Peripheral antinociceptive effects of exogenous and immune cell-derived endomorphins in prolonged inflammatory pain. J Neurosci. 2006;26:4350–4358. doi: 10.1523/JNEUROSCI.4349-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latarjet J, Choinère M. Pain in burn patients. Burns. 1995;21:344–348. doi: 10.1016/0305-4179(95)00003-8. [DOI] [PubMed] [Google Scholar]
- 29.Li GL, Liu SM, Yang Y, Xie JY, Liu J, Kong FJ, Tu GH, Wu RP, Li GD, Liang SD. Effects of oxymatrine on sympathoexcitatory reflex induced by myocardial ischemic signaling mediated by P2X3 receptors in rat SCG and DRG. Brain Res Bull. 2011;84:419–424. doi: 10.1016/j.brainresbull.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Li GL, Liu SM, Zhang J, Yu KH, Xu CS, Lin JR, Li X, Liang SD. Increased sympathoexcitatory reflex induced by myocardial ischemic nociceptive signaling via P2X2/3 receptor in rat superior cervical ganglia. Neurochem Int. 2010;56:984–990. doi: 10.1016/j.neuint.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Liang SD, Gao Y, Xu CS, Xu BH, Mu SN. Effect of tetramethylpyrazine on acute nociception mediated by signaling of P2X receptor activation in rat. Brain Res. 2004;995:247–252. doi: 10.1016/j.brainres.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 32.Liang SD, Xu CS, Li GL, Gao Y. P2X receptors and modulation of pain transmission: focus on effects of drugs and compounds used in traditional Chinese medicine. Neurochem Int. 2010;57:705–712. doi: 10.1016/j.neuint.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Liang SD, Xu CS, Zhou T, Liu HQ, Gao Y, Li GL. Tetramethylpyrazine inhibits ATP-activated currents in rat dorsal root ganglion neurons. Brain Res. 2005;1040:92–97. doi: 10.1016/j.brainres.2005.01.076. [DOI] [PubMed] [Google Scholar]
- 34.Lin JR, Li GL, Den XR, Xu CS, Liu SM, Gao Y, Liu H, Zhang J, Li X, Liang SD. VEGF and its receptor-2 involved in neuropathic pain transmission mediated by P2X2(/)3 receptor of primary sensory neurons. Brain Res Bull. 2010;83:284–291. doi: 10.1016/j.brainresbull.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Mousa SA, Machelska H, Schäfer M, Stein C. Immunohistochemical localization of endomorphin-1 and endomorphin-2 in immune cells and spinal cord in a model of inflammatory pain. J Neuroimmunol. 2002;126:5–15. doi: 10.1016/S0165-5728(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 36.Myles PS, Urquhart N. The linearity of the Visual Analogue Scale in patients with severe acute pain. Anaesth Intensive Care. 2005;33:54–58. doi: 10.1177/0310057X0503300108. [DOI] [PubMed] [Google Scholar]
- 37.North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol. 2004;554:301–308. doi: 10.1113/jphysiol.2003.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen S, Nolan MF, Kori S. Pain measurement. An overview of two commonly used methods. Anesthesiol Rev. 1992;19:11–15. [PubMed] [Google Scholar]
- 39.Pal SK, Cortiella J, Herndon D. Adjunctive methods of pain control in burns. Burns. 1997;23:404–412. doi: 10.1016/S0305-4179(97)00029-6. [DOI] [PubMed] [Google Scholar]
- 40.Patterson DR, Everett JJ, Bombardier CH, Questad KA, Lee VK, Marvin JA. Psychological effects of severe burn injuries. Psychol Bull. 1993;113:362–378. doi: 10.1037/0033-2909.113.2.362. [DOI] [PubMed] [Google Scholar]
- 41.Patterson DR, Hofland HW, Espey K, Sharar S. Pain management. Burns. 2004;30:A10–A15. doi: 10.1016/j.burns.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Qu ZW, Wen CY, Wang AP, Ju WB, Ren AH, Liu MY, Zhou Y. Protective effect of puerarin on rats with alcoholic hepatitis. World Chin J Digestol. 2009;17:657–661. [Google Scholar]
- 43.Richardson P, Mustard L. The management of pain in the burns unit. Burns. 2009;35:921–936. doi: 10.1016/j.burns.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Robinson LE, van Soeren MH. Insulin resistance and hyperglycemia in critical illness: role of insulin in glycemic control. AACN Clin Issues. 2004;15:45–62. doi: 10.1097/00044067-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Schneider JC, Harris NL, El Shami A, Sheridan RL, Schulz JT, 3rd, Bilodeau ML, Ryan CM. A descriptive review of neuropathic-like pain after burn injury. J Burn Care Res. 2006;7:524–528. doi: 10.1097/01.BCR.0000226019.76946.5D. [DOI] [PubMed] [Google Scholar]
- 46.Wan F, Li GL, Liu SM, Zhu GH, Xu CS, Lin JR, Zhang J, Li X, Liang SD. P2X(2/3) receptor activity of rat nodose ganglion neurons contributing to myocardial ischemic nociceptive signaling. Auton Neurosci Basic Clin. 2010;158:58–64. doi: 10.1016/j.autneu.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Wang YX, Li GL, Liang SD, Zhang AX, Xu CS, Gao Y, Zhang CP, Wan F. Role of P2X3 receptor in myocardial ischemia injury and nociceptive sensory transmission. Autonomic Neuroscience: Basic and Clinical. 2008;139:30–37. doi: 10.1016/j.autneu.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Wang YX, Li GL, Yu KH, Liang SD, Wan F, Xu CS, Gao Y, Liu SM, Lin JR. Expression of P2X2 and P2X3 receptors in rat nodose neurons after myocardial ischemia injury. Auton Neurosci Basic Clical. 2009;145:71–75. doi: 10.1016/j.autneu.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Wassermann D. Evaluation and first aid of burned patients. Rev Prat. 2002;52:2228–2233. [PubMed] [Google Scholar]
- 50.Wilmore DW. Pathophysiology of the hypermetabolic response to burn injury. J Trauma. 1990;30:S4–S6. doi: 10.1097/00005373-199012001-00003. [DOI] [PubMed] [Google Scholar]
- 51.Woolf PD. Hormonal responses to trauma. Crit Care Med. 1992;20:216–226. doi: 10.1097/00003246-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Wu H, Zhang RY, Li QX, Wang CY, He JL, Zhong HJ, Wen GB. Effect of puerarin on the expression of fibronectin in streptozotocin-induced diabetic nephropathy rats. Pharmacol Clin Chin Mater Med. 2007;23:18–21. [Google Scholar]
- 53.Xin-Long C, Zhao-Fan X, Dao-Feng B, Jian-Guang T, Duo W. Insulin resistance following thermal injury: an animal study. Burns. 2007;33:480–483. doi: 10.1016/j.burns.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 54.Xu C, Li G, Gao Y, Liu S, Lin J, Zhang J, Li X, Liu H, Liang S. Effect of puerarin on P2X3 receptor involved in hyperalgesia after burn injury in the rat. Brain Res Bull. 2009;80:341–346. doi: 10.1016/j.brainresbull.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 55.Yang X, Hu W, Zhang Q, Wang Y, Sun L. Puerarin inhibits C-reactive protein expression via suppression of nuclear factor kappaB activation in lipopolysaccharide-induced peripheral blood mononuclear cells of patients with stable angina pectoris. Basic Clin Pharmacol Toxicol. 2010;107:637–642. doi: 10.1111/j.1742-7843.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- 56.Yarnitsky D, Sprecher E, Zaslansky R, Hemli JA. Multiple session experimental pain measurement. Pain. 1996;67:327–333. doi: 10.1016/0304-3959(96)03110-7. [DOI] [PubMed] [Google Scholar]
- 57.Zauner A, Nimmerrichter P, Anderwald C, Bischof M, Schiefermeier M, Ratheiser K, Schneeweiss B, Zauner C. Severity of insulin resistance in critically ill medical patients. Metabolism. 2007;56:1–5. doi: 10.1016/j.metabol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 58.Zhang AX, Gao Y, Zhong XQ, Xu CS, Li GL, Liu SM, Lin JR, Li X, Zhang Y, Liu H, Liang SD. Effect of sodium ferulate on the hyperalgesia mediated by P2X3 receptor in the neuropathic pain rats. Brain Res. 2010;1313:215–221. doi: 10.1016/j.brainres.2009.11.067. [DOI] [PubMed] [Google Scholar]
- 59.Zhao M, Du YQ, Yuan L, Wang NN. Protective effect of puerarin on acute alcoholic liver injury. Am J Chin Med. 2010;38:241–249. doi: 10.1142/S0192415X10007816. [DOI] [PubMed] [Google Scholar]
- 60.Zhu QL, He AX, Lu XR. Effects of puerarin on the scavenge of oxygen free radicals and the antagonism against oxidative injury. Pharmaceutical j Chin People’s Liberation Army. 2001;17:1–3. [Google Scholar]
- 61.Zor F, Ozturk S, Bilgin F, Isik S, Cosar A. Pain relief during dressing changes of major adult burns: ideal analgesic combination with ketamine. Burns. 2010;36:501–505. doi: 10.1016/j.burns.2009.08.007. [DOI] [PubMed] [Google Scholar]