Abstract
Benign prostatic hypertrophy has been related with glandular ischemia processes and adenosine is a potent vasodilator agent. This study investigates the mechanisms underlying the adenosine-induced vasorelaxation in pig prostatic small arteries. Adenosine receptors expression was determined by Western blot and immunohistochemistry, and rings were mounted in myographs for isometric force recording. A2A and A3 receptor expression was observed in the arterial wall and A2A-immunoreactivity was identified in the adventitia–media junction and endothelium. A1 and A2B receptor expression was not obtained. On noradrenaline-precontracted rings, P1 receptor agonists produced concentration-dependent relaxations with the following order of potency: 5′-N-ethylcarboxamidoadenosine (NECA) = CGS21680 > 2-Cl-IB-MECA = 2-Cl-cyclopentyladenosine = adenosine. Adenosine reuptake inhibition potentiated both NECA and adenosine relaxations. Endothelium removal and ZM241385, an A2A antagonist, reduced NECA relaxations that were not modified by A1, A2B, and A3 receptor antagonists. Neuronal voltage-gated Ca2+ channels and nitric oxide (NO) synthase blockade, and adenylyl cyclase activation enhanced these responses, which were reduced by protein kinase A inhibition and by blockade of the intermediate (IKCa)- and small (SKCa)-conductance Ca2+-activated K+ channels. Inhibition of cyclooxygenase (COX), large-conductance Ca2+-activated-, ATP-dependent-, and voltage-gated-K+ channel failed to modify these responses. These results suggest that adenosine induces endothelium-dependent relaxations in the pig prostatic arteries via A2A purinoceptors. The adenosine vasorelaxation, which is prejunctionally modulated, is produced via NO- and COX-independent mechanisms that involve activation of IKCa and SKCa channels and stimulation of adenylyl cyclase. Endothelium-derived NO playing a regulatory role under conditions in which EDHF is non-functional is also suggested. Adenosine-induced vasodilatation could be useful to prevent prostatic ischemia.
Keywords: Pig prostatic small arteries, Endothelial A2A purinoceptors, A2A expression, NECA, Vasorelaxation, Adenylyl cyclase, IKCa channels, SKCa channels
Introduction
Adenosine is an autacoid produced by the action of ecto-5′-nucleotidase on extracellular ATP that mediates different biological actions via membrane receptors denoted P1 purinoceptors [1]. Four subtypes of P1 receptors (A1, A2A, A2B, and A3) have been cloned and characterized based on their molecular structure, pharmacology, and mechanisms of G protein-mediated signaling mechanisms [2–4]. Adenosine A1 and A3 receptors are coupled to Gi/Go/Gq proteins and inhibit the activity of adenylyl cyclase, while adenosine A2A and A2B receptors are coupled to Gs ones and activate adenylyl cyclase, leading to cAMP accumulation and subsequent activation of protein kinase A (PKA) [3, 4]. Although all four adenosine receptors subtypes are found in vascular smooth muscle cells, only A2A and A2B have been shown to be present on endothelial cells [5–7]. A3 purinoceptors have also been localized on endothelial cells in mouse aorta producing smooth muscle contraction, via cyclooxygenase-1 [8].
Purinergic signaling is involved both in short-term control of vascular tone and in longer-term control of cell proliferation, migration and death involved in vascular remodeling. There is a dual control of the vascular tone by ATP released from perivascular nerves and by ATP released from endothelial cells in response to changes in blood flow (shear stress) and hypoxia [9]. Both ATP and adenosine regulate smooth muscle and endothelial cell proliferation, these regulatory mechanisms being important under conditions associated with vascular endothelial dysfunction such as hypertension and atherosclerosis. These pathologies are risk factors for benign prostatic hypertrophy, thus opening the possibility for a pathophysiological role for purines under this disturbance [9–12]. In addition, adenosine receptor agonists have been proposed as anticancer agents, since the adenosine purinoceptor agonist 2-chloroadenosine modulates PAR-1 and IL-23 gene expression, thus suggesting a modulation of cancer metastasis and immune system activity [13].
Knowing the physiological mechanisms controlling prostatic vasculature is essential in order to a basic knowledge as well as to understand the prostate pathophysiology. Our group has recently characterized the mechanisms involved in the vasoconstriction of pig prostatic small arteries, and both endothelin 1, acting through constrictor muscle ETA and relaxant endothelial ETB receptors [14], and neurally released noradrenaline (NA), that activates postjunctional α1L-adrenoceptors and prejunctional inhibitory autoreceptors [15], play a main role. The mechanisms involved in the relaxation of prostatic small arteries are relatively unknown [16], and there is no information about the role played by adenosine in the reactivity of the prostatic small arteries responsible for deep gland vascularization. Therefore, the aim of the present study was to investigate the mechanisms involved in the vasoactive effects induced by adenosine in the pig prostatic small arteries.
Material and methods
Collection and tissue dissection
Prostates were obtained from adult pigs at the local slaughterhouse immediately after the animals were killed, and placed in cold (4°C) physiological saline solution (PSS). The gland was opened, the adjacent connective and fatty tissues were removed and second-order branches of the prostatic artery, with an internal lumen diameter of 220–350 μm, which are distributed in the deep of the glandular parenchyma, were carefully dissected.
Western blotting
Prostatic arterial rings were homogenized in 50 mM Tris–HCl, pH 7.5, containing 125 mM NaCl, 10 mM sodium pyrophosphate, 5 mM sodium fluoride, 1 mM ethylenediaminetetraacetic acid (EDTA; all from Sigma, USA), 1% Nonidet P 40 (Fluka Chemie, Buchs, Switzerland) and 1% Halt Protease Inhibitor (Thermo Scientific, Rockford, IL, USA). After centrifugation at 12,000 ×g for 5 min, the supernatant was collected and submitted to protein determination (BCA Protein Assay Kit, Rockford, IL, USA). Twenty micrograms protein samples were then separated by SDS-PAGE on a discontinuous (7.5% and 12%) acrylamide gel and transferred to a polyvinylidene fluoride membrane (Amersham, GE Healthcare, Buckinghamshire, UK). Blots were blocked with 1% casein in phosphate-buffered saline (PBS), pH 7.4, containing 0.1% Tween 20 (PBST, Bio-Rad, Hercules, CA, USA), for 1 h. The primary antibodies used were as follows: A1 receptor, polyclonal rabbit antibody raised against rat A1 receptor (Lifespan Biosciences, Seattle, WA, USA). A2A receptor, monoclonal mouse anti-human A2A receptor antibody (Novus Biologicals, Cambridge, UK). A2B receptor, polyclonal goat anti-human A2B receptor antibody (Abnova, Walnut, CA, USA). A3 receptor, polyclonal rabbit antibody raised against human A3 receptor (MBL, Woburn, MA, USA). Blots were incubated with the corresponding receptor antibody, at a 1/100, 1/500, 1/100, or 1/200 dilution for the anti-A1, anti-A2A, anti-A2B, or anti-A3 receptor, respectively. Primary antibodies were incubated in PBST, at 4°C, overnight. Blots were then washed for 10 min with PBST, and incubated with a peroxydase-labeled anti-mouse (1/5,000 dilution, Amersham, GE Healthcare, Buckinghamshire, UK) or anti-goat (1/1,500 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibody, at room temperature, for 1 h. Enhanced chemiluminescence (ECL) was performed with an ECL Western blot detection kit (Amersham, GE Healthcare, Buckinghamshire, UK) according to the manufacturer’s instructions. Blots were exposed to Hyperfilm ECL (Amersham, GE Healthcare, Buckinghamshire, UK), for 1 min. For determination of the molecular mass, a SDS-PAGE standard set (Amersham, GE Healthcare, Buckinghamshire, UK) was used.
Immunohistochemistry
Prostatic artery segments were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 24 to 48 h at 4°C, and subsequently placed in 30% sucrose in 0.1 M phosphate buffer for cryoprotection. The tissue was frozen in CO2 and stored at −80°C until sectioning. Transversal sections of 10 μm were obtained by means of a cryostat. The sections were processed for immunohistochemistry following the avidin–biotin–peroxidase complex (ABC) method. Samples were preincubated in 10% normal goat serum (NGS) in PB containing 0.3% Triton-X-100 for 2–3 h, an then incubated in the presence of A2A receptor antibody (5 μg/ml concentration) in 2% NGS in PB containing 0.3% Triton-X-100, 4°C for 48 h. The sections were reacted with a biotinylated goat anti-mouse secondary serum (Chemicon International Inc, 1:400 dilution), for 2 h at room temperature. The specimens were incubated with ABC (Vector, 1:100 dilution), for 90 min at room temperature. The immunocomplex was visualized with 0.05% 3,3′diaminobenzidine and 0.001% H2O2 in PB. No immunoreactivity could be detected in sections incubated in the absence of the primary antisera.
Microvascular myograph
Arterial rings (2 mm long) were mounted on two 40-μm wires in a double microvascular myograph, by fixing one of the wires to a force transducer for isometric tension recordings and the second wire to a length displacement device. The small arteries were allowed to equilibrate in PSS at 37°C, pH 7.4, for 30 min. The relation between resting wall tension and internal circumference L100 corresponding to a transmural pressure of 100 mmHg for a relaxed vessel in situ was calculated. The arteries were set to an internal circumference L1, given by L1 = 0.9 × L100. Preliminary experiments showed that the force development was close to maximal at this internal circumference in the present study.
The contractile ability of the strips was determined by exposing them to K+-rich (124 mM) PSS (KPSS). Intact endothelium was evaluated by inducing a stable contraction with NA (1 μM), and then adding 10 μM acetylcholine. Relaxations larger than 80% were taken as evidence for the presence of endothelium. Relaxations to purinergic receptor agonists were carried out on 1 μM NA-induced tone. Responses to purinoceptor agonists were reproducible in at least two consecutive concentration–response curves (CRC). Thus, a first control curve was performed and after washing the preparation in PSS every 20 min during a total period of 80 min, the vessel was incubated with the specific treatment for 30 min, and then a new CRC was constructed. The NA-induced control level in arteries incubated with specific blockers was matched to that exhibited in control preparations. Neither of the used pharmacological treatments or mechanical removal of endothelium modify the initial basal tone. Control curves were run in parallel.
Calculations and statistics
For each relaxation CRC to adenosine receptor agonists, the drug concentration required to give 50% (EC50) relaxation of the NA-induced contraction was estimated by computerized non-linear regression analysis (GraphPad Prism, San Diego, CA, USA). The sensitivity of the drugs is expressed in terms of pD2, where pD2 is defined as the negative logarithms of EC50 (pD2 = −log EC50 [M]). Results are expressed as mean ± s.e.m. of n (number of arteries). Statistical significance of the differences was studied by Student’s t test for paired observations and by analysis of variance (ANOVA) and an a posteriori Bonferroni method for multiple comparisons. Differences were considered significant with a probability level of P < 0.05. P values are shown in the table and figure legends.
Drugs and solutions
The following drugs were used: adenosine, 4-aminopyridine (4-AP), apamin, atropine, NG-nitro-l-arginine (l-NOARG) and NA all from Sigma (USA). [1-2-chloro-6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-b-d-ribofuranuronamide (2-Cl-IB-MECA), 2-chloro-N6-cyclopentyladenosine (2-Cl-cyclopentyladenosine), ω-conotoxin GVIA (ω-CgTX), dipyridamole, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), [4-2[[6-amino-9-(N-ethyl-b-d-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl] benzene propanoic acid hydrochloride (CGS21680), forskolin, glibenclamide, iberiotoxin (IbTX), (9R,10S,12S)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1, 6]benzodiazocine-10-carboxylic acid (KT5720), N-[9-chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-yl]benzene acetamide (MRS1220), 5′-N-ethylcarboxamidoadenosine (NECA), 4-(2,3,6,7-tetrahydro-2,6-dioxo-1-propyl-1H-purin-8-yl)-benzenesulfonic acid potassium salt (PSB1115), 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM 34) and 4-(-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM241385) all from Tocris (UK). CGS 21680, 2-Cl-IB-MECA, 2-Cl-cyclopentyladenosine, dipyridamole DPCPX, forskolin, glibenclamide, KT5720, MRS1220, NECA, PSB1115, 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM34), and ZM241385 were dissolved in 1% dimethyl sulfoxide. The other drugs were dissolved in distilled water. The solvents, at the final concentration used in the bath, had no effect on the reactivity of the vessels. The composition of PSS was (in millimolar): NaCl 119, KCl 4.6, MgCl2 1.2, NaHCO3 24.9, glucose 11, CaCl2 1.5, KH2PO4 1.2, EDTA 0.027. The solution was maintained at 37°C and continuously gassed with 95% O2 and 5% CO2 to maintain pH at 7.4. KPSS was PSS with KCl exchanged for NaCl on an equimolar basis. Stock solutions were prepared daily in distilled water.
Results
In normalized prostatic arterial rings, the amplitude of the 124 mM KPSS response amounted to 16.9 ± 4.9 mN (n = 106 preparations). NA (1 μM) induced a sustained contraction above basal tension of 14.3 ± 2.8 mN (n = 106).
Relaxations induced by adenosine receptor agonists
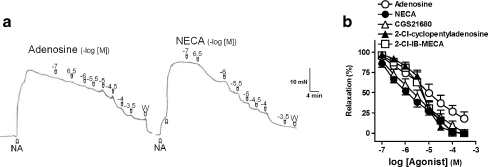
Adenosine and P1 receptor agonists induced relaxations in a concentration-dependent manner, with the following order of potency: NECA = CGS 21680 > 2-Cl-IB-MECA = 2-Cl-cyclopentyladenosine = adenosine (Fig. 1a and b and Table 1).
Fig. 1.
a Isometric force recordings showing the relaxations evoked by adenosine (0.1–300 μM) and 5′-N-ethylcarboxamidoadenosine (NECA, 0.1–300 μM), on 1 μM noradrenaline (NA)-precontracted pig prostatic small arterial rings. Vertical bar shows tension in millinewtons and horizontal bar time in minutes. b Log concentration–response relaxation curves to adenosine (open circle), NECA (closed circle), [4-2[[6-amino-9-(N-ethyl-b-d-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzene propanoic acid hydrochloride 2-chloro-N6-cyclopentyladenosine (CGS 21680, open triangle), 2-chloro-N6-cyclopentyladenosine (2-Cl-cyclopentyladenosine, closed triangle) and [1-2-chloro-6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-b-d-ribofuranuronamide (2-Cl-IB-MECA, open square). Results are expressed as a percentage of the NA-induced contraction and represent mean ± s.e.m. of six to eight arteries
Table 1.
Relaxation induced by adenosine receptor agonists in the pig prostatic small arteries
| NECA | ||||
|---|---|---|---|---|
| n | It (mN) | pD2 | Emax (%) | |
| Adenosine | 8 | 5.9 ± 2.2 | 5.2 ± 0.1 | 81.7 ± 8.4 |
| NECA | 8 | 8.1 ± 1.0 | 5.9 ± 0.1* | 100 ± 0* |
| CGS21680 | 6 | 5.0 ± 1.0 | 5.8 ± 0.1* | 99.4 ± 0.5* |
| 2-Cl-cyclopentyladenosine | 6 | 6.7 ± 1.6 | 5.2 ± 0.1 | 100 ± 0* |
| 2-Cl-IB-MECA | 6 | 6.9 ± 1.3 | 5.3 ± 0.1 | 99.8 ± 0.1* |
Results are expressed as mean ± s.e.m. of n arteries. pD2 = −log EC50, where EC50 is the concentration of agonist producing 50% of the Emax
It initial tension; Emax is the maximal relaxation, expressed as a percentage of the noradrenaline-induced contraction
*P < 0.05 versus adenosine (ANOVA following Bonferroni t test)
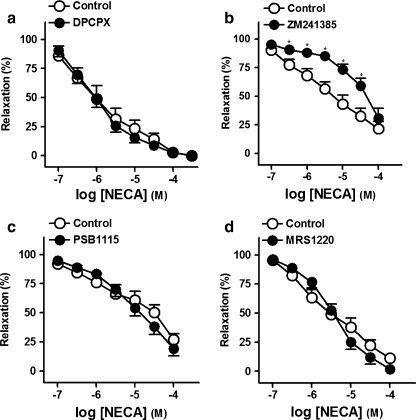
Effects of A1, A2A, A2B, and A3 receptor antagonists on the relaxations to NECA
ZM241385 (0.1 μM; Fig. 2b and Table 2), an A2A receptor selective antagonist, reduced relaxations to NECA, whereas DPCPX (0.1 μM; Fig. 2a), PSB1115 (0.1 μM; Fig. 2c) and MRS1220 (10 μM) (Fig. 2d), antagonists of the A1, A2B, and A3 receptors, respectively, failed to modify these responses (Table 2).
Fig. 2.
Log concentration–response relaxation curves to 5′-N-ethylcarboxamidoadenosine (NECA), in the absence (open circle) and presence (closed circle) of 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, 0.1 μM) (a), 4-(-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl) phenol (ZM241385, 0.1 μM) (b), 4-(2,3,6,7-tetrahydro-2,6-dioxo-1-propyl-1H-purin-8-yl)-benzenesulfonic acid potassium salt (PSB1115, 0.1 μM) (c) or N-[9-chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-yl]benzene acetamide (MRS1220, 10 μM) (d), on 1 μM noradrenaline (NA)-precontracted pig prostatic small arteries. Results are expressed as a percentage of the NA-induced contraction and represent mean ± s.e.m. of six to eight arteries. *P < 0.05 versus control value (paired t test)
Table 2.
Effects of blockers of adenosine A1, A2A, A2B, and A3 receptors on relaxations evoked by 5′-N-ethylcarboxamidoadenosine (NECA, 0.1–300 μM) in the pig prostatic small arteries
| NECA | ||||
|---|---|---|---|---|
| n | It (mN) | pD2 | Emax (%) | |
| Control | 8 | 5.6 ± 1.1 | 6.0 ± 0.1 | 100 ± 0 |
| DPCPX (0.1 μM) | 8 | 4.9 ± 1.1 | 6.0 ± 0.1 | 100 ± 0 |
| Control | 8 | 6.3 ± 1.0 | 5.2 ± 0.1 | 78.4 ± 6.5 |
| ZM241385 (0.1 μM) | 8 | 7.0 ± 1.2 | 4.3 ± 0.1** | 69.1 ± 8.8 |
| Control | 6 | 6.2 ± 1.3 | 4.6 ± 0.1 | 72.8 ± 5.0 |
| PSB1115 (0.1 μM) | 6 | 6.5 ± 1.7 | 4.8 ± 0.1 | 80.8 ± 5.9 |
| Control | 6 | 2.7 ± 1.2 | 5.5 ± 0.2 | 90.8 ± 3.1 |
| MRS1220 (10 μM) | 6 | 2.3 ± 1.0 | 5.4 ± 0.2 | 98.1 ± 1.4 |
Results are expressed as mean ± s.e.m. of n arteries. pD2 = −log EC50, where EC50 is the concentration of agonist producing 50% of the Emax
It initial tension; Emax is the maximal relaxation, expressed as a percentage of the noradrenaline-induced contraction
**P < 0.01 versus control (paired t test)
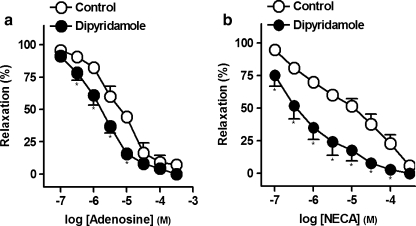
Effect of adenosine reuptake inhibition on the relaxations to adenosine or NECA
Dipyridamole (10 μM), an inhibitor of the adenosine reuptake, potently enhanced the relaxations to adenosine (pD2 and Emax values being 5.2 ± 0.1 and 5.7 ± 0.1* and 92.7 ± 4.3% and 100 ± 0%, in control and in the presence of dipyridamole, respectively, *P < 0.05, paired t test versus control, n = 6, Fig. 3a) or to NECA (pD2 and Emax values being 5.1 ± 0.1 and 6.3 ± 0.1* and 94.3 ± 2.8% and 100 ± 0%, in control and in the presence of dipyridamole, respectively, *P < 0.05, paired t test versus control, n = 6, Fig. 3b).
Fig. 3.
Log concentration–response relaxation curves to adenosine (a) or 5′-N-ethylcarboxamido adenosine (NECA) (b), in the absence (open circle) and presence (closed circle) of dipyridamole (10 μM), on 1 μM noradrenaline (NA)-precontracted pig prostatic small arteries. Results are expressed as a percentage of the NA-induced contraction and represent mean ± s.e.m. of six arteries. *P < 0.05 versus control value (paired t test)
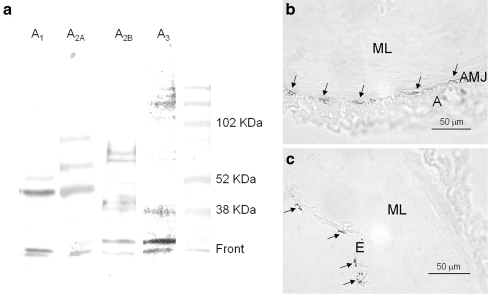
Western blot of the A1, A2A, A2B and A3 receptors and immunoreactivity of the A2A receptor
In western blots of prostatic small arteries, antibodies directed against the A2A or the A3 receptors recognized several bands. For the A2A receptor blot, one of the bands was located at approximately 43 kDa (Fig. 4a), while the A3 receptor blot showed a 39-kDa band (Fig. 4a); both bands corresponding to the expected molecular weights for its respective receptor. Western blots using antibodies directed against the A1 or the A2B receptor showed several bands, neither of them matching the expected molecular weight (38 and 36 kDa for the A1 or the A2B receptor, respectively). Immunohistochemical studies revealed the presence of small “patches” of A2A-immunoreactives (MsIgG2A-IR) at the boundary between muscular layer and adventitia (Fig. 4b) and in the endothelium (Fig. 4c).
Fig. 4.
a Western blot of pig prostatic small arteries membranes incubated with A1, A2A, A2B, and A3 receptor antibodies. The A2A and the A3 receptor antibodies show major bands of 43 and 39 kDa, respectively, compatible with the presence of these purinoceptor subtypes. b, c Immunohistochemical staining demonstrating the existence of A2A-immunoreactivity (A2A-IR) in the arterial wall. Small “patches” of A2A-IR (arrows) on the border between the muscularis and adventitia layer (b) and in the endothelium (c) (A adventitia, AMJ adventitia–media junction, ML muscular layer, E endothelium)
Effect of endothelium mechanical removal and of NO synthase and cyclooxygenases inhibitors on the relaxations to NECA
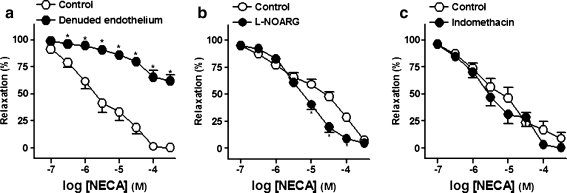
Mechanical removal of the endothelium potently reduced the relaxations to NECA (Fig. 5a and Table 3). l-NOARG (100 μM; Fig. 5b), blocker of nitric oxide (NO) synthase, potentiated the relaxations to NECA whereas, indomethacin (3 μM; Fig. 5c), a non-selective cyclooxygenases (COX) inhibitor, failed to modify these responses (Table 3).
Fig. 5.
Log concentration–response relaxation curves to 5′-N-ethylcarboxamidoadenosine (NECA), in the intact (control, open circle) and endothelium-denuded (closed circle) rings (a) and in the absence (open circle) and presence (closed circle) of NG-nitro-l-arginine (l-NOARG, 100 μM) (b) or indomethacin (3 μM) (c), on 1 μM noradrenaline (NA)-precontracted pig prostatic small arteries. Results are expressed as a percentage of the NA-induced contraction and represent mean ± s.e.m. of six to eight arteries. *P < 0.05 versus control value (paired t test)
Table 3.
Effects of endothelium mechanical removal and of blockers of nitric oxide synthase and cyclooxygenases on relaxations evoked by 5′-N-ethylcarboxamido adenosine (NECA, 0.1–300 μM) in the pig prostatic small arteries
| NECA | ||||
|---|---|---|---|---|
| n | It (mN) | pD2 | Emax(%) | |
| Endothelium-intact (Control) | 7 | 7.4 ± 1.4 | 5.6 ± 0.1 | 100 ± 0 |
| Endothelium-denuded | 7 | 7.2 ± 2.0 | – | 37.9 ± 5.7*** |
| Control | 8 | 4.8 ± 1.2 | 4.8 ± 0.1 | 92.8 ± 2.5 |
| l-NOARG (100 μM) | 8 | 5.7 ± 1.6 | 5.2 ± 0.1* | 95.3 ± 2.4 |
| Control | 6 | 5.1 ± 0.9 | 5.2 ± 0.1 | 91.1 ± 5.5 |
| Indomethacin (3 μM) | 6 | 4.8 ± 1.4 | 5.4 ± 0.1 | 99.8 ± 0.2 |
Results are expressed as mean ± s.e.m. of n arteries. pD2 = −log EC50, where EC50 is the concentration of agonist producing 50% of the Emax
It initial tension; Emax is the maximal relaxation, expressed as a percentage of the noradrenaline-induced contraction
*P < 0.05 and ***P < 0.001 versus control (paired t test)
Role of the PKA pathway and of the K+ channels on the relaxations to NECA
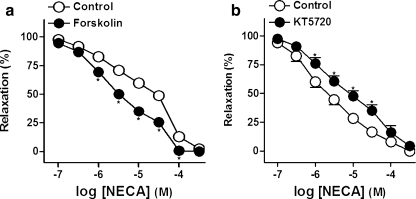
A threshold concentration (30 nM) of the adenylyl cyclase activator forskolin evoked a leftward displacement of the relaxation CRC to NECA (Fig. 6a and Table 4). The PKA inhibitor KT 5720 (3 μM) induced a rightwards displacement of the relaxation CRC to NECA (Fig. 6b and Table 4).
Fig. 6.
Log concentration–response relaxation curves to 5′-N-ethylcarboxamidoadenosine (NECA), in the absence (open circle) and presence (closed circle) of forskolin (30 nM) (a) or KT 5720 (3 μM) (b), on 1 μM noradrenaline (NA)-precontracted pig prostatic small arteries. Results are expressed as a percentage of the NA-induced contraction and represent mean ± s.e.m. of six to seven arteries. *P < 0.05 versus control value (paired t test)
Table 4.
Effects of adenylyl cyclase activation and of protein kinase A inhibition on relaxations evoked by 5′-N-ethylcarboxamidoadenosine (NECA, 0.1–300 μM) in the pig prostatic small arteries
| NECA | ||||
|---|---|---|---|---|
| n | It (mN) | pD2 | Emax (%) | |
| Control | 7 | 7.3 ± 0.8 | 4.8 ± 0.1 | 97.7 ± 1.7 |
| Forskolin (30 nM) | 7 | 6.0 ± 1.0 | 5.4 ± 0.1** | 100 ± 0 |
| Control | 6 | 8.0 ± 3.0 | 5.6 ± 0.1 | 100 ± 0 |
| KT5720 (3 μM) | 6 | 6.0 ± 1.6 | 5.1 ± 0.1* | 95.5 ± 1.9 |
Results are expressed as mean ± s.e.m. of n arteries. pD2 = −log EC50, where EC50 is the concentration of agonist producing 50% of the Emax
It initial tension; Emax is the maximal relaxation, expressed as a percentage of the noradrenaline-induced contraction
*P < 0.05 and **P < 0.01 versus control (paired t test)
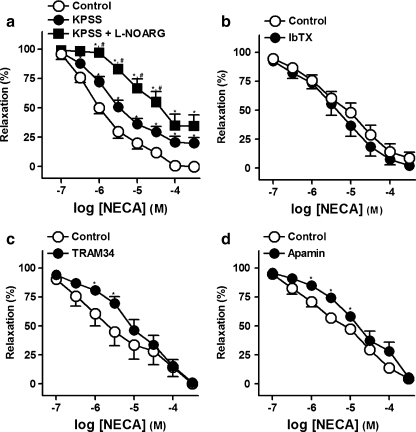
Raising extracellular K+ to 60 mM induced a sustained tone of 11.1 ± 1.2 mN (n = 6). NECA produced a concentration-dependent relaxation on 60 mM KPSS-precontracted arteries was lower than that obtained on NA-contracted rings (Fig. 7a, Table 5). In KPSS-precontracted rings, a situation in which the endothelial-dependent hyperpolarizing factor (EDHF) is inoperative, l-NOARG (100 μM) caused a profound reduction of the NECA relaxations (Fig. 7a, Table 5).
Fig. 7.
a Log concentration–response relaxation curves to 5′-N-ethylcarboxamidoadenosine (NECA), on 1 μM noradrenaline (NA)- (control, open circle) or 60 mM K+ rich physiological saline solution (KPSS)-precontracted pig prostatic small arteries, in the absence (closed circle) and presence (closed square) of NG-nitro-l-arginine (l-NOARG, 100 μM). Concentration–response relaxation curves to NECA in the absence (open circle) and presence (closed circle) of iberiotoxin (IbTX, 100 nM) (b), 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM 34, 20 nM) (c), or apamin (0.5 μM) (d), on 1 μM NA-precontracted rings. Results are expressed as a percentage of the NA-induced contraction and represent mean ± s.e.m. of six to seven arteries. *,#P < 0.05 versus control and KPSS, respectively (paired t test for paired observations and analysis of variance and an a posteriori Bonferroni method for multiple comparisons)
Table 5.
Effects of extracellular K+ raising and of blockers of NO synthase, large-, intermediate-, small-conductance Ca2+-activated-, ATP-dependent-, and voltage-gated-K+ channels on relaxations evoked by 5′-N-ethylcarboxamidoadenosine (NECA, 0.1–300 μM) in the pig prostatic small arteries
| NECA | ||||
|---|---|---|---|---|
| N | It (mN) | pD2 | Emax (%) | |
| Control (on NA-induced contraction) | 6 | 5.4 ± 1.7 | 5.9 ± 0.1 | 100 ± 0 |
| Control (on KPSS-induced contraction) | 6 | 4.7 ± 1.7 | 5.2 ± 0.1* | 79.9 ± 4.8* |
| l-NOARG (100 μM) (on KPSS-induced contraction) | 6 | 4.8 ± 1.5 | 4.3 ± 0.1*,** | 65.4 ± 9.2* |
| On NA-induced contraction | ||||
| Control | 6 | 10.2 ± 2.1 | 5.3 ± 0.1 | 97.7 ± 1.7 |
| IbTX (100 nM) | 6 | 10.3 ± 3.1 | 5.2 ± 0.1 | 91.2 ± 5.1 |
| Control | 7 | 7.3 ± 1.5 | 5.6 ± 0.1 | 99.4 ± 0.3 |
| TRAM34 (20 nM) | 7 | 5.9 ± 1.3 | 5.0 ± 0.1* | 98.7 ± 0.8 |
| Control | 6 | 6.3 ± 1.3 | 5.2 ± 0.1 | 95.5 ± 2.3 |
| Apamin (0.5 μM) | 6 | 5.7 ± 1.9 | 4.7 ± 0.1* | 94.3 ± 2.7 |
| Control | 6 | 5.7 ± 0.7 | 5.7 ± 0.2 | 99.7 ± 0.3 |
| Glibenclamide (1 μM) | 6 | 4.9 ± 1.1 | 5.7 ± 0.2 | 100 ± 0 |
| Control | 6 | 7.2 ± 1.6 | 5.6 ± 0.2 | 98.6 ± 0.8 |
| 4-AP (1 mM) | 6 | 6.0 ± 1.2 | 5.7 ± 0.1 | 95.2 ± 1.1 |
Results are expressed as mean ± s.e.m. of n arteries. pD2 = −log EC50, where EC50 is the concentration of agonist producing 50% of the Emax
It initial tension, Emax is the maximal relaxation, expressed as a percentage of the 1 μM noradrenaline (NA)- or 60 mM KPSS-induced contraction
*,**P < 0.05 versus control and potassium-enriched (60 mM) saline solution (KPSS), respectively (paired t test for paired observations and analysis of variance and an a posteriori Bonferroni method for multiple comparisons)
TRAM34 (20 nM; Fig. 7c) and apamin (0.5 μM; Fig. 7d), blockers of intermediate (IKCa)- and small (SKCa)-conductance Ca2+-activated K+ channels, respectively, reduced the relaxations to NECA (Table 5). However, IbTX (100 nM; Fig. 7b), glibenclamide (1 μM) and 4-AP (1 mM), inhibitors of large-conductance Ca2+-activated K+ channels (BKCa), ATP-dependent K+ (KATP) and voltage-gated K+ channels (Kv) channels, respectively, failed to modify the relaxations to NECA (Table 5).
Effect of the inhibitor of neuronal voltage-gated Ca2+ channels on the relaxations to NECA
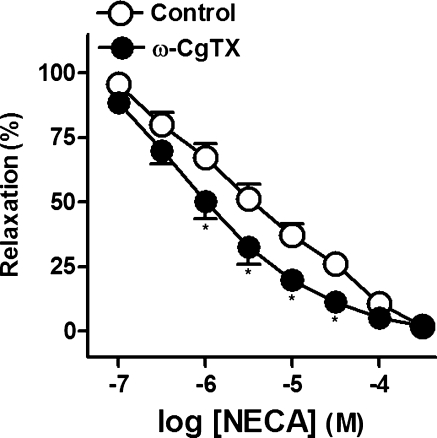
ω-CgTX (1 μM; Fig. 8), a blocker of the neuronal voltage-gated Ca2+ channels, potentiated the relaxations to NECA (pD2 and Emax values being 5.4 ± 0.1 and 5.9 ± 0.1* and 97.8 ± 1.0% and 97.9 ± 0.6%, in control and in the presence of ω-CgTX, respectively, *P < 0.05, paired t test versus control, n = 6).
Fig. 8.
Log concentration–response relaxation curves to 5′-N-ethylcarboxamidoadenosine (NECA), in the absence (open circle) and presence (closed circle) of ω-conotoxin GVIA (CgTX, 1 μM), on 1 μM noradrenaline (NA)-precontracted pig prostatic small arteries. Results are expressed as a percentage of the NA-induced contraction and represent mean ± s.e.m. of six arteries. *P < 0.05 versus control value (paired t test)
Discussion
The present study was designed to investigate the involved mechanisms in the vasoactive effect of adenosine in the pig prostatic small arteries. Our results suggest that adenosine produces vasodilatation of the pig prostatic arteries through endothelial A2A purinoceptors. Such relaxation is produced via NO- and COX-independent mechanisms that involve activation of IKCa and SKCa channels and stimulation of adenylyl cyclase. A prejunctional modulation of the adenosine-induced relaxation is also suggested.
Adenosine or its analogs are mostly directed toward adenosine-mediated effects on the cardiovascular system, such as the treatment of supraventricular arrhythmia, congestive heart failure, controlling blood pressure, attenuating reperfusion injury following regional myocardial infarction, reducing infarct size and incidence of arrhythmias, and improving post-ischemic cardiac function [17, 18]. Due to adenosine receptors differential coupling to either Gs (A2A and A2B receptors) or Gi (A1 and A3 receptors) proteins, along with the variable tissue distribution of adenosine receptor subtypes, adenosine elicits both relaxation (A2A- and A2B-mediated) and constriction (A1- and A3-mediated) in the peripheral and coronary vasculature. Vasodilatation is mainly due to the A2A receptor [19]. A1 and A3 receptors inhibit coronary vasodilatation induced by A2A and/or A2B receptor activation [20].
Adenosine A1 receptors cause porcine coronary arteries vasorelaxation, via activation of K+ channels, as well as vasoconstriction [21, 22]. A negative modulatory role has been suggested for A1 in A2A- and A2B-mediated coronary flow in isolated mouse hearts [20]. In fact, A1 receptors protect against the injury caused by myocardial ischemia and reperfusion, by inhibiting adenylyl cyclase and by activating KATP channels [23]. The A1 receptor-mediated contractile effect has been reported in mouse aorta and coronary arteries [24, 25]. A role for the phospholipase C (PLC)–protein kinase C (PKC) system has been suggested in adenosine A1 receptor-mediated contraction of coronary vascular smooth muscle has also been demonstrated [25]. Other studies have shown that adenosine A1 receptor enhances PKC expression in porcine coronary arteries [26]. These findings suggest that the PLC–PKC pathway has a major role in adenosine A1 receptor-mediated vascular contraction of the coronary arteries and the aorta. In pig prostatic arteries, the low relaxant potency shown by the A1 purinoceptor selective agonist 2-Cl-cyclopentyladenosine, and the lack of inhibitory effect shown by DPCPX, an adenosine A1 receptor selective antagonist, on NECA relaxations, seem to rule out the mediation of an A1 receptor in the purinergic relaxation mediation.
Adenosine A3 receptors have previously been reported to induce a dual (contraction/relaxation) effect on vascular smooth muscle. Thus, A3 receptors mediate in vivo vasoconstriction by stimulation of mast cells and subsequent release of histamine and thromboxane [27] while a coronary vasodilator effect has also been demonstrated in rat [28]. In our study, the low relaxant potency shown by the A3 receptor agonist 2-Cl-IB-MECA and the lack of inhibitory effect of the A3 receptor selective antagonist MRS 1220 on NECA-induced relaxations seems to rule out the mediation of these receptors in adenosine responses. The fact that A3 receptors were expressed in pig prostatic arteries agrees with the enigmatic role previously described for these receptors, whose function is not fully known [29]. However, A3 selective ligands might be useful for the treatment of pathologies such as glaucoma, asthma, arthritis or cancer, in which inflammation is present [29].
Adenosine A2 receptor subtypes have been involved in the vasorelaxation of the coronary and mesenteric vascular bed of several species, including humans. Thus, adenosine A2A receptors have been described in coronary vessels from rat mesenteric arteries [30], while adenosine A2B receptors mediate vasorelaxation of human coronary arteries [31]. It has recently been suggested that there is a compensatory upregulation of the adenosine A2B receptor in an adenosine A2A receptor knock-out mouse model [32]. In pig prostatic arteries, CGS21680, an A2A receptor selective agonist, produced a significant vascular smooth muscle relaxation. This fact, together with the agonist potency order in producing relaxation (NECA = CGS21680 > 2-Cl-IB-MECA 2-Cl-cyclopentyladenosine = adenosine), suggests an A2A receptor subtype mediation [33]. Agonist-based purinoceptors characterization requires, however, some caution, since the rank order of agonist potency may substantially be influenced by the different susceptibilities of purine analogs to degradation by ecto-nucleoside triphosphate diphosphohydrolase [34]. The involvement of the A2A subtype in the NECA-induced vasorelaxation is confirmed by the inhibition produced by the adenosine A2A receptor selective antagonist, ZM241385. A2A receptors participation in pig prostatic arteries was also confirmed by adenosine A2A receptor expression studies. Western blot assays showed a band at about 43 kDa, compatible with that expected for the A2A receptor, and immunostaining of prostatic arteries samples with an A2A antibody revealed a labeling of small A2A-IR “patches” in the adventitia–media junction and in the intima layer. The fact that NECA acts not only on postsynaptic endothelial receptors but also on presynaptic ones located at nerve endings in the arterial preparations can support the variability observed in NECA-induced relaxations [15]. The fact that the non-selective adenosine analog NECA produced relaxation with a potency similar to that of the A2A-selective CGS 21680 suggests the mediation of another adenosine receptor subtype (possibly the A2B receptor) contributing to this relaxation. However, the lack of effect produced by the A2B receptor selective antagonist PSB1115, as well as the absence, in Western blot studies, of a band compatible with the A2B subtype, seems to exclude the expression of these receptors in the pig prostatic small arteries. On the other hand, dipyridimole, an inhibitor of the adenosine reuptake, potentiated the NECA- or adenosine-induced relaxation. This result could be explained on the basis that increased adenosine levels would preferentially activate receptors with a high affinity for adenosine, such as the A1 subtype [3]. Western blot assays, however, did not show A1 receptor expression, and an A1 receptor selective antagonist had no effect on NECA relaxations, which seems to discard an A1 receptor participation in the purinergic relaxations. The A3 receptor, while being expressed in our preparation, neither seems to be involved in adenosine or NECA relaxations, as they were not modified by the A3 receptor selective antagonist MRS1220. Purinergic relaxations in our preparation, therefore, seem to be due to the A2A subtype present in the endothelium of the prostatic small arteries. These results agree with those obtained in rabbit corpora cavernosa, where dipyridamole enhances the A2A mediated adenosine-induced relaxation [35]. Since adenosine plays an essential role in the vasorelaxation of the prostatic vascular bed, compounds that enhance adenosine levels, such as dipyridamole, may be useful to prevent prostatic glandular ischemia.
Both adenosine A2 receptor subtypes can mediate hyperpolarization of smooth muscle and NO release from vascular endothelium [6]. Cell culture studies have demonstrated an adenosine A2A- and A2B-receptor-mediated NO release in porcine and human coronary endothelial cells [5, 6]. However, very few functional studies have demonstrated that NO release is responsible for adenosine A2A- or A2B-mediated coronary vasodilatation. It has been suggested that endogenously released adenosine and prostanoids induce NO- and/or KATP channel-dependent vasorelaxation and thereby modulate basal coronary tone [36]. Whereas adenosine A2A receptor plays a significant role in background NO release, thus affecting basal coronary tone [32], the role played by adenosine A2B receptors in NO release remains to be determined. In addition, both adenosine A2A and A2B receptors have also been implicated in endothelium-independent relaxation of coronary artery smooth muscle [37]. In our study, mechanical removal of the endothelium potently reduced the relaxations to NECA, thus indicating that adenosine relaxes pig prostatic arteries through adenosine receptors located on endothelial cells. On the basis of the reduction produced by endothelium removal, several mediators such as NO, prostanoids and/or EDHF could be expected to be the mediators released by the vascular endothelium. However, NO synthase blockade enhanced the relaxations to NECA, thus ruling out the involvement of NO released from vascular endothelium in the responses to adenosine. The potentiation produced ω-CgTX, a blocker of the neuronal voltage-gated Ca2+ channels, on the NECA relaxations suggests the involvement of neuronal mechanisms in the adenosine-induced vasodilatation. The fact that the potentiation produced by l-NOARG was observed at high concentrations could be ascribed to the mediation of prejunctional mechanisms, such as that found in lamb small coronary arteries, where the blockade of NO synthase enhanced the relaxations to ATP, suggesting a nerve modulation of purinergic relaxations [38].
In addition to NO, the COX pathway has also been involved in the vasorelaxations to adenosine [39]. In pig prostatic arteries, indomethacin, a non-selective COX inhibitor, failed to modify the relaxations to NECA, thus excluding a possible role of prostanoids in these responses. However, our results suggest the mediation of EDHF, on the basis of the l-NOARG-resistant NECA relaxations obtained in 60 mM KPSS-precontracted rings. The fact that endothelium-derived NO plays a regulatory role under conditions in which EDHF is non-functional suggests that endothelium-derived relaxing (EDRF) and EDHF factors can act via independent signaling pathways. Thus, acetylcholine produces vasorelaxation in guinea-pig basilar artery [40] and vasodilation in the rat kidney in vivo [41] by causing release of both EDRF and EDHF but through different mechanisms.
Adenosine A2A receptor-induced vasodilatation is positively coupled to adenylyl cyclase, leading to a cytoplasmic cAMP elevation [42]. cAMP-dependent relaxations in vascular smooth muscle are generally mediated by PKA activation. However, other second-messenger systems, such as those of phosphatidylinositol 3-kinase, tyrosine kinase and PLC, may also be activated by adenosine receptors [20], even their roles in mediating adenosine vasoactive effects have not clearly been defined. In addition, crosstalk between the cAMP/PKA pathway and the PLC/PKC pathway has also been reported [43]. In the current study, the potentiation by the adenylyl cyclase activator forskolin of the NECA relaxations and their reduction by the PKA inhibitor KT 5720 suggest that NECA produces relaxation of pig prostatic arteries in part via activation of adenosine receptors linked to the PKA pathway.
K+ channels have been reported to be involved in the vasorelaxations to adenosine. Thus, BKCa channel modulation is involved in cAMP-mediated relaxation in adenosine-induced canine coronary vasodilatation [44]. In vascular smooth muscle, an IKCa channel upregulation seems to compensate a deficient activity of SKCa channels in the arteries of spontaneously hypertensive rats [45]. IKCa and SKCa channels are important for the regulation of a variety of neuronal and non-neuronal functions. Their presence in human prostate suggests that these channels are likely to have different biological functions and could be specifically targeted for human diseases, such as prostatism [46]. In the current study, the lower NECA relaxation obtained as a consequence of the elevation of extracellular K+, the reduction produced by TRAM 34 and by apamin, inhibitors of the IKCa and SKCa channels, respectively, and the lack of effect shown by IbTX, a blocker of BKCa channels, suggest the activation of IKCa and SKCa channels in the NECA vasorelaxations. Both KATP and KCa are involved in adenosine A2A and A2B receptors-mediated hyperpolarization, which also leads to NO release in coronary endothelial cells [5]. In addition to these channels, Kv channels are involved in adenosine-mediated relaxation of coronary arterioles from pig males [47]. Kv channels of the of the Kv1.3 family have also been reported in rat prostate epithelial cells, and a significant increase in Kv channel expression, particularly of the Kv1 and Kv2 subfamily members, has been shown to be involved in the proliferation of prostate carcinoma [48, 49]. In pig prostatic arteries, glibenclamide and 4-AP, blockers of the KATP and Kv channels, respectively, failed to modify the NECA relaxations thus ruling out the involvement of these channels.
Conclusion
Our results suggest that adenosine induces vasorelaxation of the pig prostatic small arteries through endothelial A2A purinoceptors. Such relaxation, which is prejunctionally modulated, is produced via NO- and COX-independent mechanisms that involve activation of IKCa and SKCa channels and stimulation of adenylyl cyclase. In these arteries, the possibility that EDRF and EDHF act via independent mechanisms is also suggested. Adenosine-induced vasodilatation could be useful to prevent prostatic ischemia.
Acknowledgments
The authors wish to thank to Ms. Carmen Azuara, Mr. Francisco Puente and Mr. Manuel Perales for their expert technical assistance. They also thank to Industrias Cárnicas Vaquero slaughterhouse (Madrid) for kindly donating the prostates. This work was supported by a grant from the Medical Foundation of Mutua Madrileña, Inc. (FMM, 2006/09, Madrid, Spain).
Conflict of interest statement None.
Glossary
- CGS21680
[4-2[[6-amino-9-(N-ethyl-b-d-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzene propanoic acid hydrochloride
- 2-Cl-cyclopentyladenosine
2-chloro-N6-cyclopentyladenosine
- 2-Cl-IB-MECA
[1-2-chloro-6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-b-d-ribofuranuronamide
- ω-CgTX
ω-Conotoxin GVIA
- COX
Cyclooxygenases
- DPCPX
8-Cyclopentyl-1,3-dipropylxanthine
- KATP
ATP-dependent K+ channels
- BKCa
Large-conductance Ca2+-activated K+ channels
- IKCa
Intermediate-conductance Ca2+-activated K+ channels
- SKCa
Small-conductance Ca2+-activated K+ channels
- Kv
Voltage-gated K+ channels
- KT5720
(9R,10S,12S)-2,3,9,10,11,12-Hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, hexyl ester
- l-NOARG
NG-nitro-l-arginine
- MRS1220
N-[9-chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-yl]benzene acetamide
- NA
Noradrenaline
- NECA
5′-N-ethylcarboxamidoadenosine
- NO
Nitric oxide
- PKA
Protein kinase A
- PSB1115
4-(2,3,6,7-tetrahydro-2,6-dioxo-1-propyl-1H-purin-8-yl)-benzenesulfonic acid potassium salt
- TRAM34
1-[(2-Chlorophenyl)diphenylmethyl]-1H-pyrazole
- ZM241385
4-(-[7-amino-2-(2-furyl)[1,2,4] triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl) phenol
References
- 1.Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis L, editors. Cell membrane receptors for drugs and hormones: a multidisciplinary approach. New York: Raven; 1978. pp. 107–118. [Google Scholar]
- 2.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 3.Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olanrewaju HA, Qin W, Feoktistov I, Scemama JL, Mustafa SJ. Adenosine A(2A) and A(2B) receptors in cultured human and porcine coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279:H650–H656. doi: 10.1152/ajpheart.2000.279.2.H650. [DOI] [PubMed] [Google Scholar]
- 6.Olanrewaju HA, Gafurov BS, Lieberman EM. Involvement of K + channels in adenosine A2A and A2B receptor-mediated hyperpolarization of porcine coronary artery endothelial cells. J Cardiovasc Pharmacol. 2002;40:43–49. doi: 10.1097/00005344-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. Control of vascular tone by purines and pyrimidines. Br J Pharmacol. 2010;161:527–529. doi: 10.1111/j.1476-5381.2010.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ansari HR, Nadeem A, Tilley SL, Mustafa SJ. Involvement of COX-1 in A3 adenosine receptor-mediated contraction through endothelium in mice aorta. Am J Physiol Heart Circ Physiol. 2007;293:H3448–H3455. doi: 10.1152/ajpheart.00764.2007. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G. Purinergic regulation of vascular tone and remodelling. Auton Autacoid Pharmacol. 2009;29:63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- 10.Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- 11.Kiowski W, Linder L, Stoschitzky K, Pfisterer M, Burckhardt D, Burkart F, Bühler FR. Diminished vascular response to inhibition of endothelium-derived nitric oxide and enhanced vasoconstriction to exogenous administered endothelin-1 in clinically healthy smokers. Circulation. 1994;90:27–34. doi: 10.1161/01.cir.90.1.27. [DOI] [PubMed] [Google Scholar]
- 12.Golomb E, Rosenzweig N, Eilam R, Abramovici A. Spontaneous hyperplasia of the ventral lobe of the prostate in aging genetically hypertensive rats. J Androl. 2000;21:58–64. [PubMed] [Google Scholar]
- 13.Bellezza I, Tucci A, Minelli A. 2-Chloroadenosine and human prostate cancer cells. Anticancer Agents Med Chem. 2008;8:783–789. doi: 10.2174/187152008785914725. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez A, Recio P, Orensanz LM, Bustamante S, Navarro-Dorado J, Climent B, Benedito S, García-Sacristán A, Prieto D, Hernández M. Mechanisms involved in the effects of endothelin-1 in pig prostatic small arteries. Eur J Pharmacol. 2010;640:190–196. doi: 10.1016/j.ejphar.2010.04.059. [DOI] [PubMed] [Google Scholar]
- 15.Recio P, Orensanz LM, Martínez MP, Navarro-Dorado J, Bustamante S, García-Sacristán A, Prieto D, Hernández M. Noradrenergic vasoconstriction of pig prostatic small arteries. Naunyn Schmiedeberg’s Arch Pharmacol. 2008;376:397–406. doi: 10.1007/s00210-007-0227-x. [DOI] [PubMed] [Google Scholar]
- 16.Navarro-Dorado J, Orensanz LM, Recio P, Bustamante S, Benedito S, Martínez AC, García-Sacristán A, Prieto D, Hernández M. Mechanisms involved in testosterone-induced vasodilatation in pig prostatic small arteries. Life Sci. 2008;83:569–573. doi: 10.1016/j.lfs.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 18.Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol Ther. 2007;114:208–221. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Frøbert O, Haink G, Simonsen U, Gravholt CH, Levin M, Deussen A. Adenosine concentration in the porcine coronary artery wall and A2A receptor involvement in hypoxia-induced vasodilatation. J Physiol. 2006;570:375–384. doi: 10.1113/jphysiol.2005.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tawfik HE, Teng B, Morrison RR, Schnermann J, Mustafa SJ. Role of A1 adenosine receptor in the regulation of coronary flow. Am J Physiol Heart Circ Physiol. 2006;291:H467–H472. doi: 10.1152/ajpheart.01319.2005. [DOI] [PubMed] [Google Scholar]
- 21.Dart C, Standen NB. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. J Physiol. 1993;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merkel LA, Lappe RW, Rivera LM, Cox BF, Perrone MH. Demonstration of vasorelaxant activity with an A1-selective adenosine agonist in porcine coronary artery: involvement of potassium channels. J Pharmacol Exp Ther. 1992;260:437–443. [PubMed] [Google Scholar]
- 23.Morita Y, Murakami T, Iwase T, Nagai K, Nawada R, Kouchi I, Akao M, Sasayama S. K(ATP) channels contribute to the cardioprotection of preconditioning independent of anaesthetics in rabbit hearts. J Mol Cell Cardiol. 1997;29:1267–1276. doi: 10.1006/jmcc.1996.0364. [DOI] [PubMed] [Google Scholar]
- 24.Prentice DJ, Boon K, Hourani SMO. Relaxation of mouse isolated aorta to adenosine and its analogues does not involve adenosine A1, A2 or A3 receptors. Eur J Pharmacol. 2001;415:251–255. doi: 10.1016/S0014-2999(01)00841-X. [DOI] [PubMed] [Google Scholar]
- 25.Ansari HR, Teng B, Nadeem A, Roush KP, Martin KH, Schnermann J, Mustafa SJ. A(1) adenosine receptor-mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2009;297:H1032–H1039. doi: 10.1152/ajpheart.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marala RB, Mustafa SJ. Adenosine analogues prevent phorbol ester-induced PKC depletion in porcine coronary artery via A1 receptor. Am J Physiol. 1995;268:H271–H277. doi: 10.1152/ajpheart.1995.268.1.H271. [DOI] [PubMed] [Google Scholar]
- 27.Shepherd RK, Linden J, Duling BR. Adenosine-induced vasoconstriction in vivo. Role of the mast cell and A3 adenosine receptor. Circ Res. 1996;78:627–634. doi: 10.1161/01.res.78.4.627. [DOI] [PubMed] [Google Scholar]
- 28.Hinschen AK, Rose’Meyer RB, Headrick JP. Adenosine receptor subtypes mediating coronary vasodilation in Rat Hearts. J Cardiovasc Pharmacol. 2003;41:73–80. doi: 10.1097/00005344-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA. The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther. 2008;117:123–140. doi: 10.1016/j.pharmthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Zhang Y, Wier WG, Yu X, Zhao M, Hu H, Sun L, He X, Wang Y, Wang B, Zang W. Role of store-operated Ca(2+) entry in adenosine-induced vasodilatation of rat small mesenteric artery. Am J Physiol Heart Circ Physiol. 2009;297:H347–H354. doi: 10.1152/ajpheart.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemp BK, Cocks TM. Adenosine mediates relaxation of human small resistance-like coronary arteries via A2B receptors. Br J Pharmacol. 1999;126:1796–1800. doi: 10.1038/sj.bjp.0702462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teng B, Ledent C, Mustafa SJ. Up-regulation of A2B adenosine receptor in A2A adenosine receptor knockout mouse coronary artery. J Mol Cell Cardiol. 2008;44:905–914. doi: 10.1016/j.yjmcc.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feoktistov I, Biaggioni I. Characterization of adenosine receptors in human erythroleukemia cells and platelets: further evidence for heterogeneity of adenosine A2 receptor subtypes. Mol Pharmacol. 1993;43:909–914. [PubMed] [Google Scholar]
- 34.Evans RJ, Kennedy C. Characterization of P2-purinoceptors in the smooth muscle of the rat tail artery: a comparison between contractile and electrophysiological responses. Br J Pharmacol. 1994;113:853–860. doi: 10.1111/j.1476-5381.1994.tb17071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantelli L, Amerini S, Ledda F, Forti G, Maggi M. The potent relaxant effect of adenosine in rabbit corpora cavernosa is nitric oxide independent and mediated by A2 receptors. J Androl. 1995;16:312–317. [PubMed] [Google Scholar]
- 36.Zatta AJ, Headrick JP. Mediators of coronary reactive hyperaemia in isolated mouse heart. Br J Pharmacol. 2005;144:576–587. doi: 10.1038/sj.bjp.0706099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teng B, Qin W, Ansari HR, Mustafa SJ. Involvement of p38-mitogen-activated protein kinase in adenosine receptor-mediated relaxation of coronary artery. Am J Physiol Heart Circ Physiol. 2005;288:H2574–H2580. doi: 10.1152/ajpheart.00912.2004. [DOI] [PubMed] [Google Scholar]
- 38.Simonsen U, García-Sacristán A, Prieto D. Involvement of ATP in the non-adrenergic non-cholinergic inhibitory neurotransmission of lamb isolated coronary small arteries. Br J Pharmacol. 1997;120:411–420. doi: 10.1038/sj.bjp.0700918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kedzior K, Szczepańska R, Kocić I. Contribution of NO, ATP-sensitive K + channels and prostaglandins to adenosine receptor agonists-induced relaxation of the rat tail artery. Pharmacol Rep. 2009;61:330–334. doi: 10.1016/s1734-1140(09)70040-4. [DOI] [PubMed] [Google Scholar]
- 40.Nishiye E, Nakao K, Itoh T, Kuriyama H. Factors inducing endothelium-dependent relaxation in the guinea-pig basilar artery as estimated from the actions of haemoglobin. Br J Pharmacol. 1989;96:645–655. doi: 10.1111/j.1476-5381.1989.tb11864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edgley AJ, Tare M, Evans RG, Skordilis C, Parkington HC. In vivo regulation of endothelium-dependent vasodilation in the rat renal circulation and the effect of streptozotocin-induced diabetes. Am J Physiol Regul Integr Comp Physiol. 2008;295:R829–R839. doi: 10.1152/ajpregu.00861.2007. [DOI] [PubMed] [Google Scholar]
- 42.Rekik M, Mustafa JS. Modulation of A2A adenosine receptors and associated Galphas proteins by ZM 241385 treatment of porcine coronary artery. J Cardiovasc Pharmacol. 2003;42:736–744. doi: 10.1097/00005344-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Germack R, Dickenson JM. Characterization of ERK1/2 signalling pathways induced by adenosine receptor subtypes in newborn rat cardiomyocytes. Br J Pharmacol. 2004;141:329–339. doi: 10.1038/sj.bjp.0705614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabell F, Weiss DS, Price JM. Inhibition of adenosine-induced coronary vasodilation by block of large-conductance Ca(2+)-activated K + channels. Am J Physiol. 1994;267:H1455–H1460. doi: 10.1152/ajpheart.1994.267.4.H1455. [DOI] [PubMed] [Google Scholar]
- 45.Giachini FR, Carneiro FS, Lima VV, Carneiro ZN, Dorrance A, Webb RC, Tostes RC. Upregulation of intermediate calcium-activated potassium channels counterbalance the impaired endothelium-dependent vasodilation in stroke-prone spontaneously hypertensive rats. Transl Res. 2009;154:183–193. doi: 10.1016/j.trsl.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen MX, Gorman SA, Benson B, Singh K, Hieble JP, Michel MC, Tate SN, Trezise DJ. Small and intermediate conductance Ca(2+)-activated K + channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedeberg’s Arch Pharmacol. 2004;369:602–615. doi: 10.1007/s00210-004-0934-5. [DOI] [PubMed] [Google Scholar]
- 47.Heaps CL, Bowles DK. Gender-specific K(+)-channel contribution to adenosine-induced relaxation in coronary arterioles. J Appl Physiol. 2002;92:550–558. doi: 10.1152/japplphysiol.00566.2001. [DOI] [PubMed] [Google Scholar]
- 48.Ouadid-Ahidouch H, Coppenolle F, Bourhis X, Belhaj A, Prevarskaya N. Potassium channels in rat prostate epithelial cells. FEBS Lett. 1999;459:15–21. doi: 10.1016/S0014-5793(99)01121-7. [DOI] [PubMed] [Google Scholar]
- 49.O’Grady SM, Lee SY. Molecular diversity and function of voltage-gated (Kv) potassium channels in epithelial cells. Int J Biochem Cell Biol. 2005;37:1578–1594. doi: 10.1016/j.biocel.2005.04.002. [DOI] [PubMed] [Google Scholar]