Abstract
It is proposed that ATP is released from both neurons and glia during electroconvulsive therapy (ECT) and that this leads to reduction of depressive behaviour via complex stimulation of neurons and glia directly via P2X and P2Y receptors and also via P1 receptors after extracellular breakdown of ATP to adenosine. In particular, A1 adenosine receptors inhibit release of excitatory transmitters, and A2A and P2Y receptors may modulate the release of dopamine. Sequential ECT may lead to changes in purinoceptor expression in mesolimbic and mesocortical regions of the brain implicated in depression and other mood disorders. In particular, increased expression of P2X7 receptors on glial cells would lead to increased release of cytokines, chemokines and neurotrophins. In summary, we suggest that ATP release following ECT involves neurons, glial cells and neuron–glial interactions acting via both P2 and after breakdown to adenosine via P1 receptors. We suggest that ecto-nucleotidase inhibitors (increasing available amounts of ATP) and purinoceptor agonists may enhance the anti-depressive effect of ECT.
Keywords: ATP, Depression, Electroconvulsive therapy, Mood, Puringeric signalling
Introduction
Electroconvulsive therapy (ECT), the therapeutic application of electricity to the scalp, has been used in the treatment of psychiatric disorders for more than 60 years [1]. It is now well established and considered one of the most effective methods for treating major depression [2] and catatonic schizophrenia [3]. Major depression is a debilitating condition that includes a range of symptoms and results from the contribution of multiple genetic and environmental factors [4]. It has been claimed that a depressive state can be characterized by abnormalities in the functions of monoaminergic neurotransmission, of the hypothalamic-pituitary-adrenocortical system or of neurotrophin and cytokine activities in genetically predisposed individuals that have endured the impact of stress or infection [5]. There have been numerous studies to elucidate the precise mechanism of action of ECT thought to include generalized seizures, normalization of neuroendocrine dysfunction and increased hippocampal neurogenesis and synaptogenesis [6], but a definitive candidate remains to be found. A multitude of neurotrophic factors, hormones, neuropeptides [7–9] and neurotransmitters and their receptors [10] have all been implicated. The use of ECT has been questioned, due to the experience of side effects in some patients (e.g. retrograde and anterograde amnesia), and its efficacy may depend on the aetiology of the depression and also the placement of the electrodes. We hypothesize that purines, and their receptors may be central to the clinical results observed as a result of ECT. Following the characterization of adenosine (ADO) and adenosine 5′-triphosphate (ATP) receptors, the field of purinergic signalling has become well established [11]. Recent findings suggest that ATP, its breakdown products and receptors are key players in pathological states both in the central nervous system (CNS) [10] and elsewhere [11]. There is also emerging evidence that purines are involved in mood and motivation [10].
Supporting evidence
Modulation of synaptic transmission by ATP and its metabolites
The concept of purinergic neurotransmission was introduced over 40 years ago when ATP was shown to be a transmitter in non-adrenergic, non-cholinergic inhibitory nerves in the guinea pig taenia coli [12]. It has since been shown to be a co-transmitter in both the peripheral and central nervous systems [11]. ATP acts as a fast excitatory neurotransmitter [13] and is taken up by and stored possibly in all secretory and synaptic vesicles [14]. It is known to be stored and co-released with γ-aminobutyric acid, glutamate, noradrenaline and dopamine within the CNS [14].
ATP and its metabolites act upon two classes of receptors that are expressed throughout the CNS. ADO, a breakdown product of ATP, acts upon P1 membrane receptors that are further sub-classified into A1, A2A, A2B and A3 receptors. ATP acts upon P2 receptors, which are further divided into ionotropic P2X and metabotropic P2Y receptors [11]. There are seven P2X ligand-gated ion channel receptor subtypes and eight P2Y G protein-coupled receptor subtypes [11]. ADO activates P1 receptors leading to activation of mitogen-activated protein kinases and can modulate neuronal and glial signalling [15]. P2X subunits are heterogeneously expressed throughout the CNS [14], and synaptic currents, mediated through activation of P2X receptors, are present in CNS regions, such as the cortex and the hippocampus [11, 14].
In addition to direct and paired release from neurons, there is increasing evidence that purines play a significant role in glial neurotransmission [gliotransmission] [16]. Immunocytochemical studies have demonstrated that vesicular ATP and glutamate are contained within astrocytes [16]. Release of ATP from astrocytes, for example from in vitro culture preparations [17] and acute slices [18], results in neuronal excitation through activation of P2X receptors (for review, see [19]). Activation of P2X7 receptors on these neurons can also cause an enhancement of AMPA receptor surface expression, and a resulting increase in miniature excitatory post-synaptic currents [16]. ADO, produced as a result of hydrolysis from ATP, can give rise to an inhibitory response through activation of A1 receptors [17] (see Fig. 1). Interestingly, the synthesis and release of neurotrophins, cytokines and chemokines in glial cells is also controlled by purine receptors. All four ADO (P1) receptor subtypes and P2X7 and P2Y receptors have been shown to be expressed on glial cells [11, 19]. These receptors have varying affinities to their ligands, and it is possible that different receptor subtypes are selectively activated in a concentration-dependent manner. Varying concentrations of extracellular purines can therefore precisely regulate the release of different neurotrophins or chemokines from glial cells.
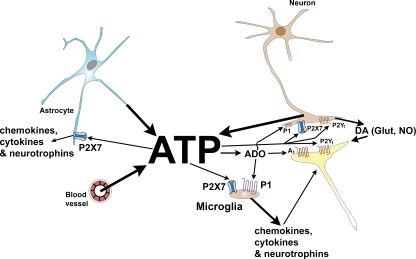
Fig. 1.
Schematic hypothesis of purinergic signalling in electroconvulsive therapy. During electroconvulsive therapy, ATP is released from astrocytes, neurons and vascular endothelial cells. The ATP acts upon P2X7, P2Y1 receptors and also P1 receptors after the extracellular breakdown of ATP to ADO. Activation of P2X7 and ADO receptors on glial cells results in increased release of cytokines, chemokines and neurotrophins, which act upon neurons in regions involved in the control of mood and motivation. Stimulation of P2Y receptors may modulate the release of dopamine (DA), glutamate (Glut) and nitric oxide (NO) all implicated in control of mood
Purinergic signalling in mood and motivation
ATP
The stimulation of P2X and P2Y receptors has been implicated in sensitization, reward and motivation [20]. Stimulation of P2 receptors in rats has demonstrated extended periods of novelty-induced locomotion indicative of an anxiolytic effect [21]. Intracerebroventricular injections of a P2Y1 receptor agonist into rats has demonstrated an anxiolytic effect, which is in close relationship with the ensuing formation and release of dopamine [21] and nitric oxide [22], known to modulate noradrenaline, serotonin, dopamine and glutamate, the major neurotransmitters involved in the neurobiology of major depression [23]. It has been shown that P2Y1 receptor agonists induce an antidepressant effect in a rat model of depression, which was reversed by P2Y1 receptor antagonists [24]. The authors showed further that P2Y1 receptor knockout mice displayed decreased depression-like behaviour. P2 receptors of the mesolimbic-mesocortical system, probably of the P2Y1 subtype, are involved in the release of transmitters such as dopamine and glutamate, which are responsible for the generation and pattern behaviour following motivation-related stimuli [25]. P2X7 receptor knockout mice exhibited an antidepressant-like profile [26]. Additionally, gene studies in patients with recurrent major depressive illness [27] and bipolar-effective disorder [28] have demonstrated a specific single nucleotide polymorphism involving the P2X7 receptor. Changes in the expression of the P2X7 receptor are not unique to disorders of mood; there is a large body of evidence to suggest increased expression in microglia and other cell types in multiple CNS disorders [29–31]. Depression is considered to be associated with central inflammation [32] and P2X7 receptor occupation leads to release of inflammatory cytokines [33]. The role of the P2X7 receptor in depression and its involvement with central inflammation requires further study.
Adenosine
Adenosingeric activity has been implicated in mania, aggression and panic disorder [34, 35]. ADO is also thought to interact with other potent mood regulators: the psychotomimetic phencyclidine and alcohol [10]. ADO and dopamine receptors share an extensive co-localisation within the forebrain regions implicated in mood and motivational processes, and there is considerable evidence that A2A receptor activation is able to influence dopaminergic function [36] and hence mediate goal-directed behaviour [37]. There is attenuation of psychostimulant-induced behavioural responses in mice lacking A2A receptors [38]. A2A and dopamine receptor knockout mice exhibit decreased preference and consumption of ethanol and saccharin [37], more so than those of D1 knockout mice. In A1 receptor knockout mice, there is an increased state of anxiety [39]. Indeed, selective stimulation of the A1 receptor in rats impairs the acquisition of fear conditioning [40]. ADO has been demonstrated to act as an antidepressant when either given by intraperitoneal or intracerebroventricular routes in mouse models of depression [41], acting via A1 and A2A receptors, and high doses of caffeine, a non-selective ADO antagonist, can produce anxiety, irritability and agitation [42]. There is conflicting evidence to suggest that both A2A receptor agonists [41] and antagonists [43] have an antidepressant effect in mouse models of depression; this conflict may reflect that where A2A receptors are claimed to have an antidepressant effect, this is believed to occur only following an interaction between A1 and A2A receptors [41]. Indirect evidence for the role of ADO in major depression is also suggested by the finding that serum activity of adenosine deaminase (a T-cell-associated enzyme) was decreased in patients with major depression, with an inverse relationship between the enzyme activity and the severity of the depression [44]. This supports a role for ADO having a depressant effect, since less enzyme results in greater ADO concentrations. These results also suggest that decreased enzyme activities might reflect that depressed patients may have a greater tendency to immune dysfunction.
Putative role of ECT on purinergic signalling in mood
We hypothesize that high levels of ATP are released following ECT, from microglia [19], neurons [45] and vascular endothelial cells [11], resulting in improvement in depressive symptoms through direct stimulation of pre- and post-synaptic purinoceptors that modulate neuronal and glial signalling (see Fig. 1). Pronounced increases in brain ADO levels in mice following electroconvulsive shock in rats has been shown [46], which would occur following breakdown of released ATP. In cases where ECT does not alleviate depression, this may reflect different aetiologies of the depression and also the placement of the electrodes and the amount of ATP released following the seizure. Sequential ECT may herald changes in pre- and post-synaptic purinergic receptor profiles, and it is these changes that are fundamental to the long-term benefits of ECT in major depressive illness. For instance, P2Y1 receptors inhibit long-term depression of rat prefrontal cortex neurons [47], and synaptic plasticity may underlie the long-term benefits of ECT in depression. An important aspect of the physiological effect of ECT is the induction of a seizure-like state, and it is recognized that seizures give rise to increased levels of extracellular ATP [2, 11, 48, 49]. We suggest that this released pool of ATP following ECT is derived, in part, from astrocytes through connexin hemichannels [50] and directly from neurons throughout the brain. ATP is also released from astrocytes via connexin or pannexin hemichannels, ABC transporters, maxi ion channels or by vesicular release [19, 51, 52]. There is also evidence that ATP may be released from vascular endothelial cells in addition to neurons and glial cells [53]. Once released into the extracellular space, ATP and its breakdown product ADO have a multitude of targets (see Fig. 1), which can improve mood and motivation. Extracellular ATP is co-released in conjunction with dopamine [54, 55] within the mesolimbic-mesocortical system, and ATP may reduce anxiety and depression through stimulation of P2X7 receptors, the gene for which has been associated with major depressive disorder [27]. ECT is associated with an increase of ADO (after breakdown of ATP) and up-regulation of ADO A1 receptors in the brain [56]. Activation of pre- and post-synaptic A1 receptors by ADO can cause inhibition of NMDA receptor activation [57], which in turn has been shown to exert an antidepressant action in both pre-clinical and clinical studies[58]. At the simpler level, increased concentrations of ADO as a result of ATP hydrolysis may relieve depression [44]. Released ATP may also stimulate glial cells to release neurotransmitters, neurotrophins, cytokines and chemokines [59–61] (see Fig. 1), which can give rise to long term changes in neuronal circuitry, such as synaptic plasticity (long-term potentiation/long-term depression) and up-regulation/down-regulation of receptors [62, 63] (i.e. more long-term effects of ECT). The use of ecto-nucleotidase inhibitors (e.g. ARL 67156 (Sigma)) to increase extracellular ATP may be a potential novel therapeutic strategy to supplement ECTs long-term effects [64, 65]. P2X7 receptor agonists, such as the potent agonist 2′,3′-O-(benzoyl-4-benzoyl)-ATP, may also offer therapeutic potential.
Future developments
The effect of ATP release following ECT is multifaceted; neuronal, glial and neuronal-glial signalling is implicated. The proposed hypothesis for this mechanism has the advantage that the tools are available so that every step can be tested both in vivo and in vitro. Very sensitive ATP assay techniques are now available, as well as some selective purinoceptor subtype agonists and antagonists. Increase of extracellular ATP during ECT by the use of ecto-nucleotidase inhibitors as well as purinoceptor agonists may be worth consideration to enhance the anti-depressive effects of ECT.
Acknowledgments
Conflict of interest The authors have no competing interests that might be perceived to influence the results and discussion reported in this paper.
Contributor Information
Ahmed-Ramadan Sadek, Email: a.sadek@soton.ac.uk.
Geoffrey Burnstock, Phone: +44-207-8302948, Email: g.burnstock@ucl.ac.uk.
References
- 1.Mackinon AL. Electric shock therapy. Can Med Assoc J. 1948;58:478–483. [PMC free article] [PubMed] [Google Scholar]
- 2.Busnello JV, Oses JP, Silva RS, Feier G, Barichello T, Quevedo J, Böhmer AE, Kapczinski F, Souza DO, Sarkis JJ, Portela LV. Peripheral nucleotide hydrolysis in rats submitted to a model of electroconvulsive therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1829–1833. doi: 10.1016/j.pnpbp.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Zervas IM, Theleritis C, Soldatos CR (2011) Using ECT in schizophrenia: a review from a clinical perspective. World J Biol Psychiatry. doi:10.3109/15622975.2011.564653 [DOI] [PubMed]
- 4.Soleimani L, Lapidus KA, Iosifescu DV. Diagnosis and treatment of major depressive disorder. Neurol Clin. 2011;29:177–193. doi: 10.1016/j.ncl.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Mann JJ, Currier DM. Stress, genetics and epigenetic effects on the neurobiology of suicidal behavior and depression. Eur Psychiatry. 2010;25:268–271. doi: 10.1016/j.eurpsy.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolwig TG. How does electroconvulsive therapy work? Theories on its mechanism. Can J Psychiatry. 2011;56:13–18. doi: 10.1177/070674371105600104. [DOI] [PubMed] [Google Scholar]
- 7.Taylor SM. Electroconvulsive therapy, brain-derived neurotrophic factor, and possible neurorestorative benefit of the clinical application of electroconvulsive therapy. J ECT. 2008;24:160–165. doi: 10.1097/YCT.0b013e3181571ad0. [DOI] [PubMed] [Google Scholar]
- 8.Yatham LN, Liddle PF, Lam RW, Zis AP, Stoessl AJ, Sossi V, Adam MJ, Ruth TJ. Effect of electroconvulsive therapy on brain 5-HT2 receptors in major depression. Br J Psychiatry. 2010;196:474–479. doi: 10.1192/bjp.bp.109.069567. [DOI] [PubMed] [Google Scholar]
- 9.Nikisch G, Mathe AA. CSF monoamine metabolites and neuropeptides in depressed patients before and after electroconvulsive therapy. Eur Psychiatry. 2008;23:356–359. doi: 10.1016/j.eurpsy.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 11.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 12.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 13.Zimmerman H. Nucleotide signalling in nervous system development. Pflugers Arch. 2006;453:573–588. doi: 10.1007/s00424-006-0067-4. [DOI] [PubMed] [Google Scholar]
- 14.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Dare E, Schulte G, Karovic O, Hammarberg C, Fredholm BB. Modulation of glial cell functions by adenosine receptors. Physiol Behav. 2007;92:15–20. doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57:343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowser DN, Khakh BS. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J Gen Physiol. 2007;129:485–491. doi: 10.1085/jgp.200709780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- 19.Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors in neuroglia. Mol Neurobiol. 2009;39:190–208. doi: 10.1007/s12035-009-8063-2. [DOI] [PubMed] [Google Scholar]
- 20.Kittner H, Krügel U, Illes P. The purinergic P2 receptor antagonist pyridoxalphosphate-6-azophenyl-2′4′-disulphonic acid prevents both the acute locomotor effects of amphetamine and the behavioural sensitization caused by repeated amphetamine injections in rats. Neuroscience. 2001;102:241–243. doi: 10.1016/S0306-4522(00)00555-8. [DOI] [PubMed] [Google Scholar]
- 21.Kittner H, Krugel U, Hoffmann E, Illes P. Effects of intra-accumbens injection of 2-methylthio ATP: a combined open field and electroencephalographic study in rats. Psychopharmacology (Berl) 2000;150:123–131. doi: 10.1007/s002130000403. [DOI] [PubMed] [Google Scholar]
- 22.Kittner H, Franke H, Fischer W, Schultheis N, Krügel U, Illes P. Stimulation of P2Y1 receptors causes anxiolytic-like effects in the rat elevated plus-maze: implications for the involvement of P2Y1 receptor-mediated nitric oxide production. Neuropsychopharmacology. 2003;28:435–444. doi: 10.1038/sj.npp.1300043. [DOI] [PubMed] [Google Scholar]
- 23.Dhir A, Kulkarni SK. Nitric oxide and major depression. Nitric Oxide. 2011;24:125–131. doi: 10.1016/j.niox.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Taayedi R, Köhler C, Drendel V, Seidel B, Illes P, Krügel U. Purinergic mechanisms mediate depression-like responses to chronic stress. J Neurochem. 2007;102:288. [Google Scholar]
- 25.Krügel U, Spies O, Regenthal R, Illes P, Kittner H. P2 receptors are involved in the mediation of motivation-related behavior. Purinergic Signal. 2004;1:21–29. doi: 10.1007/s11302-004-4745-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basso AM, Bratcher NA, Harris RR, Jarvis MF, Decker MW, Rueter LE. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav Brain Res. 2009;198:83–90. doi: 10.1016/j.bbr.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Lucae S, Salyakina D, Barden N, Harvey M, Gagne B, Labbe M, Binder EB, Uhr M, Paez-Pereda M, Sillaber I, Ising M, Bruckl T, Lieb R, Holsboer F, Muller-Myhsok B. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum Mol Genet. 2006;15:2438–2445. doi: 10.1093/hmg/ddl166. [DOI] [PubMed] [Google Scholar]
- 28.Barden N, Harvey M, Gagne B, Shink E, Tremblay M, Raymond C, Labbe M, Villeneuve A, Rochette D, Bordeleau L, Stadler H, Holsboer F, Muller-Myhsok B. Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:374–382. doi: 10.1002/ajmg.b.30303. [DOI] [PubMed] [Google Scholar]
- 29.Franke H, Gunther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63:686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- 30.Melani A, Amadio S, Gianfriddo M, Vannucchi MG, Volonté C, Bernardi G, Pedata F, Sancesario G. P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 2006;26:974–982. doi: 10.1038/sj.jcbfm.9600250. [DOI] [PubMed] [Google Scholar]
- 31.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Hashioka S (2011) Anti-neuroinflammatory effects of psychopharmaceuticals: further than monoamine modulators. Mini Rev Med Chem, in press [DOI] [PubMed]
- 33.Virgilio F. Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Machado-Vieira R, Lara DR, Souza DO, Kapczinski F. Purinergic dysfunction in mania: an integrative model. Med Hypotheses. 2002;58:297–304. doi: 10.1054/mehy.2001.1543. [DOI] [PubMed] [Google Scholar]
- 35.Lam P, Hong CJ, Tsai SJ. Association study of A2a adenosine receptor genetic polymorphism in panic disorder. Neurosci Lett. 2005;378:98–101. doi: 10.1016/j.neulet.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Ferre S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/S0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- 37.Short JL, Ledent C, Drago J, Lawrence AJ. Receptor crosstalk: characterization of mice deficient in dopamine D1 and adenosine A2A receptors. Neuropsychopharmacology. 2006;31:525–534. doi: 10.1038/sj.npp.1300852. [DOI] [PubMed] [Google Scholar]
- 38.Chen JF, Beilstein M, Xu YH, Turner TJ, Moratalla R, Standaert DG, Aloyo VJ, Fink JS, Schwarzschild MA. Selective attenuation of psychostimulant-induced behavioral responses in mice lacking A2A adenosine receptors. Neuroscience. 2000;97:195–204. doi: 10.1016/S0306-4522(99)00604-1. [DOI] [PubMed] [Google Scholar]
- 39.Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hardemark A, Betsholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corodimas KP, Tomita H. Adenosine A1 receptor activation selectively impairs the acquisition of contextual fear conditioning in rats. Behav Neurosci. 2001;115:1283–1290. doi: 10.1037/0735-7044.115.6.1283. [DOI] [PubMed] [Google Scholar]
- 41.Kaster MP, Rosa AO, Rosso MM, Goulart EC, Santos AR, Rodrigues AL. Adenosine administration produces an antidepressant-like effect in mice: evidence for the involvement of A1 and A2A receptors. Neurosci Lett. 2004;355:21–22. doi: 10.1016/j.neulet.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 42.Nehlig A. Is caffeine a cognitive enhancer? J Alzheimers Dis. 2010;20(Suppl 1):S85–S94. doi: 10.3233/JAD-2010-091315. [DOI] [PubMed] [Google Scholar]
- 43.El Yacoubi M, Ledent C, Parmentier M, Bertorelli R, Ongini E, Costentin J, Vaugeois JM. Adenosine A2A receptor antagonists are potential antidepressants: evidence based on pharmacology and A2A receptor knockout mice. Br J Pharmacol. 2001;134:68–77. doi: 10.1038/sj.bjp.0704240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elgun S, Keskinege A, Kumbasar H. Dipeptidyl peptidase IV and adenosine deaminase activity. Decrease in depression. Psychoneuroendocrinology. 1999;24:823–832. doi: 10.1016/S0306-4530(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 45.Burnstock G, Verkhratsky A, Fredholm B. Adenosine and ATP receptors in the brain. Curr Top Med Chem. 2011;11:973–1011. doi: 10.2174/156802611795347627. [DOI] [PubMed] [Google Scholar]
- 46.Lewin E, Bleck V. Electroshock seizures in mice: effect on brain adenosine and its metabolites. Epilepsia. 1981;22:577–581. doi: 10.1111/j.1528-1157.1981.tb04129.x. [DOI] [PubMed] [Google Scholar]
- 47.Guzman SJ, Schmidt H, Franke H, Krügel U, Eilers J, Illes P, Gerevich Z. P2Y1 receptors inhibit long-term depression in the prefrontal cortex. Neuropharmacology. 2010;59:406–415. doi: 10.1016/j.neuropharm.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 48.Wieraszko A, Goldsmith G, Seyfried TN. Stimulation-dependent release of adenosine triphosphate from hippocampal slices. Brain Res. 1989;485:244–250. doi: 10.1016/0006-8993(89)90567-2. [DOI] [PubMed] [Google Scholar]
- 49.Dale N, Frenguelli BG. Release of adenosine and ATP during ischemia and epilepsy. Curr Neuropharmacol. 2009;7:160–179. doi: 10.2174/157015909789152146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signalling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 51.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kreft M, Potokar M, Stenovec M, Pangrsic T, Zorec R. Regulated exocytosis and vesicle trafficking in astrocytes. Ann N Y Acad Sci. 2009;1152:30–42. doi: 10.1111/j.1749-6632.2008.04005.x. [DOI] [PubMed] [Google Scholar]
- 53.Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krügel U, Kittner H, Franke H, Illes P. Purinergic modulation of neuronal activity in the mesolimbic dopaminergic system in vivo. Synapse. 2003;47:134–142. doi: 10.1002/syn.10162. [DOI] [PubMed] [Google Scholar]
- 55.Fischer W, Krügel U. P2Y receptors: focus on structural, pharmacological and functional aspects in the brain. Curr Med Chem. 2007;14:2429–2455. doi: 10.2174/092986707782023695. [DOI] [PubMed] [Google Scholar]
- 56.Calker D, Biber K. The role of glial adenosine receptors in neural resilience and the neurobiology of mood disorders. Neurochem Res. 2005;30:1205–1217. doi: 10.1007/s11064-005-8792-1. [DOI] [PubMed] [Google Scholar]
- 57.Mendonça A, Sebastião AM, Ribeiro JA. Adenosine: does it have a neuroprotective role after all? Brain Res Brain Res Rev. 2000;33:258–274. doi: 10.1016/S0165-0173(00)00033-3. [DOI] [PubMed] [Google Scholar]
- 58.Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- 59.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 60.Sperlágh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signalling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cunha RA, Vizi ES, Ribeiro JA, Sebastiao AM. Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J Neurochem. 1996;67:2180–2187. doi: 10.1046/j.1471-4159.1996.67052180.x. [DOI] [PubMed] [Google Scholar]
- 63.Wieraszko A. Extracellular ATP as a neurotransmitter: its role in synaptic plasticity in the hippocampus. Acta Neurobiol Exp (Warsz) 1996;56:637–648. doi: 10.55782/ane-1996-1168. [DOI] [PubMed] [Google Scholar]
- 64.Randall PA, Nunes EJ, Janniere SL, Stopper CM, Farrar AM, Sager TN, Baqi Y, Hockemeyer J, Müller CE, Salamone JD (2011) Stimulant effects of adenosine antagonists on operant behavior: differential actions of selective A2A and A1 antagonists. Psychopharmacology (Berl). doi:10.1007/s00213-011-2198-3 [DOI] [PMC free article] [PubMed]
- 65.Baqi Y, Hausmann R, Rosefort C, Rettinger J, Schmalzing G, Muller CE (2011) Discovery of potent competitive antagonists and positive modulators of the P2X2 receptor. J Med Chem 54(3):817–830 [DOI] [PubMed]