Abstract
Liver ischemia reperfusion injury is associated with both local damage to the hepatic vasculature and systemic inflammatory responses. CD39 is the dominant vascular endothelial cell ectonucleotidase and rapidly hydrolyses both adenosine triphosphate (ATP) and adenosine diphosphate to adenosine monophosphate. These biochemical properties, in tandem with 5′-nucleotidases, generate adenosine and potentially illicit inflammatory vascular responses and thrombosis. We have evaluated the role of CD39 in total hepatic ischemia reperfusion injury (IRI). Wildtype mice, Cd39-hemizygous mice (+/−) and matched Cd39-null mice (−/−); (n = 25 per group) underwent 45 min of total warm ischemia with full inflow occlusion necessitating partial hepatectomy. Soluble nucleoside triphosphate diphosphohydrolase (NTPDases) or adenosine/amrinone were administered to wildtype (n = 6) and Cd39-null mice (n = 6) in order to study protective effects in vivo. Parameters of liver injury, systemic inflammation, hepatic ATP determinations by P31-NMR and parameters of lung injury were obtained. All wildtype mice survived up to 7 days with minimal biochemical disturbances and minor evidence for injury. In contrast, 64% of Cd39+/− and 84% of Cd39-null mice required euthanasia or died within 4 h post-reperfusion with liver damage and systemic inflammation associated with hypercytokinemia. Hepatic ATP depletion was pronounced in Cd39-null mice posthepatic IRI. Soluble NTPDase or adenosine administration protected Cd39-deficient mice from acute reperfusion injury. We conclude that CD39 is protective in hepatic IRI preventing local injury and systemic inflammation in an adenosine dependent manner. Our data indicate that vascular CD39 expression has an essential protective role in hepatic IRI.
Keywords: CD39, Hepatic ischemia reperfusion, Vascular endothelium
Introduction
Organ ischemia and the systemic inflammation after ischemia reperfusion injury (IRI) are a major cause of morbidity and mortality in hepatobiliary surgery and liver transplantation. Systemic inflammatory responses (SIRS) associated with IRI are characterized by vascular endothelial (EC) and neutrophil activation with elevated circulating cytokine levels and free oxygen radical release [1–4].
CD39/nucleoside triphosphate diphosphohydrolase (NTPDase) 1 is an important ectonucleotidase, which is expressed by both endothelium and leukocytes that degrades pro-inflammatory extracellular nucleotides (adenosine triphosphate (ATP) to adenosine diphosphate (ADP) and adenosine monophosphate (AMP)). The scavenging of these nucleotides inhibits proinflammatory platelet and cellular activation responses [5, 6]. Conversion of AMP to adenosine, which has anti-inflammatory and cytoprotective properties, is further mediated by endothelial associated 5′-nucleotidase (5′NT or CD73) that is expressed in tandem with CD39.
Modulation of purinergic signaling within the vasculature by CD39/NTPDase1 has the potential to downregulate acute inflammatory responses mediated by type-2 purinergic (P2) receptor activation and consequently facilitate protective adenosine receptor signaling. NTPDase1 has recently been linked to the regulation of inflammation and neutrophil chemotaxis by facilitating the hydrolysis of extracellular ATP [7]. In addition, vascular release of nitric oxide is also influenced by extracellular nucleotides [3, 8]. We also note that overexpression of CD39 ameliorates EC activation and apoptosis in vitro [9, 10]. Consequently, CD39 may be a critical regulatory element in the control of inflammatory responses and processes of vascular injury in the models of liver IRI.
We have previously shown that deletion of CD39 results in disordered purinergic signaling responses that compromise vascular thromboregulation, promote inflammatory responses, and impact hepatic metabolism [11–14].
Liver sinusoid endothelial cells (LSEC) are unique in their high endocytotic capacity as well as their fenestrations in the absence of a basal membrane, allowing an intensive interaction between the sinusoidal blood and the microvillous surface of the parenchymal cells [15]. CD39 is not expressed on resting LSEC or on hepatocytes which makes the liver unique among other organ systems such as the heart, kidney, and brain [16–18]. Curiously, after hepatic injury, CD39 expression by sinusoidal endothelial cells is highly upregulated in conjunction with hepato-protective effects and increased angiogenesis during regeneration [18]. This adaptive response is late and indeed we have shown that specific vascular NTPDase activity is decreased in the early phase of graft reperfusion in transplantation models [3, 19].
The studies presented here are the first ones to show the protective effects of the CD39 pathway in complete isothermic hepatic IRI. Our data suggest potential of vascular NTPDases in the maintenance of vascular integrity during hepatic IRI in vivo.
Materials and methods
Animals Cd39 gene deleted (C57BL6) mice have previously been characterized in detail [16]. All animals were housed in a pathogen-free facility accredited by the American Association for Accreditation of Laboratory Animal Care. Animals were maintained on a 12-h light/dark cycle and provided with commercially available rodent chow and tap water ad libitum. All interventions were fully compliant with the requirements of humane animal care as stipulated by the United States Department of Agriculture and the Department of Health and Human Services. The experimental animal protocols were approved by the Beth Israel Deaconess Medical Center Animal Care and Use Program.
Surgical procedures Prior to surgical intervention, all experimental animals were fasted overnight with unrestricted water access. Mice were anesthetized with Ketamine (100 mg/kg) and Xylazine (10 mg/kg). Ischemic preconditioning (IP) and partial hepatectomies followed by full inflow occlusion for 45 min were performed as previously described, using microsurgical vascular clamps [20]. After 45 min of ischemia, a second laparotomy was performed and all three clamps were removed [20]. Prior to removal of the clamps, the mice were injected intravenously with the respective test or control solutions. The abdomen was closed and the animals were allowed to recover with free access to food and water.For studies evaluating end-organ injury and systemic inflammation, wildtype mice, Cd39-hemizygous mice (+/−) or matched Cd39-null mice (−/−; n = 25 per group) underwent partial hepatectomies as described above, followed by 45 min total isothermic hepatic ischemia with full inflow occlusion. Euthanasia end points or death were followed and time points determined.
Parameters of liver injury Aspartate aminotransferase (AST) was measured in plasma using standard techniques [21].
Hematoxylin and eosin (H&E) staining Tissue specimens were fixed in neutral-buffered formaline and paraffin embedded. Sections for light microscopy were stained with hematoxylin and eosin.
Immunohistochemical staining for fibrin Formalin-fixed livers were embedded in paraffin blocks and sectioned (5 μm thickness). Sections were stained for fibrin deposition as previously described [22, 23].
Cell injury Paraffin-embedded tissues were sectioned and stained with H&E.
Apoptosis Apoptosis was analyzed with ApopTag Peroxidase Kit (Serologicals Corporation Norcross, GA cat# S7100). Tissue preparation and staining method was following the Apoptosis Detection Kit manual. The number of apoptosis-positive cells was determined by manual counting of six high-power fields per liver analyzed.
Serum cytokine levels Commercially available enzyme-linked immunosorbent assay kits for mouse interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF) were obtained from R&D Systems (Minneapolis, MN, USA) and performed according to manufacturer’s instructions.
31P NMR All experiments were performed at a 31P Frequency of 145.7 MHz (360 MHz for 1H) on a, 9-cm vertical bore, Bruker DRX (Burker Biospin Inc. Bellerica, MA) spectrometer. Mice were anesthetized with a mixture of Ketamine/Xylazine (100 mg and 10 mg/kg) and the liver was surgically exposed. The animal was placed in a steriotaxic holder and a custom build, 5 mm i.d., radiofrequency (RF) coil was positioned in close proximity to the median and left lateral lobes of the liver. The RF coil/animal holder ensemble was positioned vertically ensuring the region of interest in the liver remained relatively constant with respect to the coil and the magnet center. Repeated experiments of mounting the animal were performed before the actual study to ensure the reproducible positioning and survival of the mice during each of three time frames, pre-ischemic, ischemic, and reperfusion. Upon maximizing the RF coil performance for each mouse, the residual 1H NMR signal, detectable in the 31P RF coil, was used to optimize homogeneity of the magnetic field in the region of interest in the liver. Two sets of pre-ischemic base line 31P data sets were collected (spectral width = 10 khz, number of acquisitions = 512, date size = 2,048, repetition time = 1.5 s, total time per data set ~12 min). The animal was removed from the magnet and, while maintaining its position in stereotactic holder and coil, ischemia was induced and the cradle was repositioned and the performance of the RF coil optimized. A series of datasets were acquired during the 45 min ischemic time frame. Finally, the animal was removed again, the ischemic region reperfused, and the animal returned to the scanner. Another series of data sets were acquired for another 30 min during this third time frame.The data were processed and spectra depicting the effect of ischemia and reperfusion were generated to investigate variations between the wildtype and knockout mice. 31P spectra were displayed at the same vertical scale and calibrated so that chemical shifts of the other metabolites can be visualized with respect to the phosphocreatine peak
Soluble NTPDase treatment Mice that were randomly assigned to NTPDase treatment groups received a single intravenous injection of soluble grade VII NTPDase (0.2 units/g bodyweight) of a 20 units/ml stock solution in saline (apyrase, Sigma, St. Louis, MO, USA) or adenosine (Sigma, St. Louis, MO, USA) 1 mmol/kg/min and amrinone (Sigma, St. Louis, MO, USA) 0.05 mmol/kg/min for 60 min prior to reperfusion. Controls were injected with an equivalent volume of saline.
Statistical analysis All data are expressed as mean±SEM. Calculations were done using the SPSS software package (SPSS Inc., Chicago, IL, USA). For statistical analysis, Mann–Whitney U, Jonckheere–Terpstra, and Wilcoxon tests were used as appropriate. Survival rates were calculated according to the Kaplan–Meier method and compared using logrank tests.P values of <0.05 were considered statistically significant.
Results
IRI studies These studies were conducted in Cd39-hemizygous, Cd39-null and wildtype mice after 45 min of isothermic hepatic full inflow ischemia and consecutive reperfusion (n = 25 animals per group). In the hemizygous group, 16 out of 25 animals required euthanasia or had died within 4 h of reperfusion with a survival rate at 4 h of 36%. In the Cd39-null group, 21 out of 25 animals required euthanasia or had died within 4 h of reperfusion with a survival rate at 4 h of 16%. Twenty-three out of 25 wild-type animals survived 7 days (92%; Fig. 1).
Fig. 1.
Cd39-deficient (P < 0.001) and null (P < 0.05) mice exhibit significantly higher rates of mortality after 45 min of warm full hepatic inflow ischemia and consecutive reperfusion than the matched wildtype mice
Liver injury H&E staining revealed generalized sinusoidal congestion in the wildtype mouse livers subsequent to IRI (Fig. 2a). Cd39-null animals express massive sinusoidal hemorrhage and areas of necrosis (Fig. 2b) and apoptosis (f) after hepatic IRI. Fibrin staining was used as a marker of hepatic vascular injury. There were patchy areas of fibrin deposition in the wildtype mouse (Fig. 2c) with abundant fibrin deposition outside the vasculature and in the parenchyma of the Cd39-null mice (Fig. 2d) after hepatic IRI.
Fig. 2.
a Wild-type livers after 45 min of ischemia and 4 h reperfusion show patchy macroscopic hemorrhage. In contrast, the Cd39-deficient livers show overt areas of infarcted parenchyma from hepatic venous thrombosis (b). a Sinusoidal congestion is seen in the wildtype mouse post hepatic ischemia and reperfusion (IRI) (H&E staining). b The CD39-null mice show massive sinusoidal hemorrhagic necrosis post IRI (H&E staining). c Fibrin staining of non-infarcted hepatic parenchyma using T2G1 immunohistochemical staining showing patchy areas of fibrin deposition. d Abundant fibrin deposition in the hepatic parenchyma in the Cd39-deficient mice, suggesting hepatic parenchymal cell injury. TUNEL staining for apoptosis demonstrates minimal apoptosis in the wild-type animals (e), but large apoptotic areas in the CD39−/− mice (f)
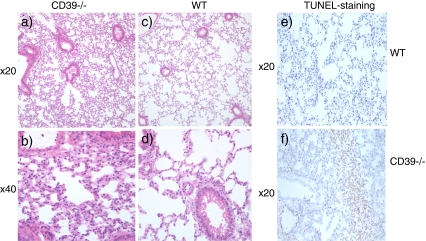
Lung injury H&E staining of wildtype and Cd39-null lung tissue revealed more edematous changes with cellular infiltration in the Cd39-null mice (Fig. 3a, b) when compared to the wildtype lung (Fig. 3c, d). In addition, Cd39-null animals expressed extensive areas of alveolar apoptosis (Fig. 3f) compared to the wildtype control (Fig. 3e) with minimal apoptotic changes.
Fig. 3.
H&E staining of lung tissue collected after 45 min of full hepatic ischemia and 4 h of reperfusion. It is clearly visible that the Cd39-null animals (a, b) show increased alveolar edema compared with the wildtype controls (c, d). TUNEL-staining indicative of apoptosis demonstrates areas of apoptosis in the Cd39-null (f) lung tissue after 45 min of full hepatic ischemia and 4 h of reperfusion, when compared to the wildtype control (e)
Plasma cytokines Cd39-deficient and -null mice exhibited significantly higher levels of plasma IL-1 than control wildtype animals at 4 h of reperfusion (Cd39 hemizygous p < 0.007, Cd39-null p < 0.005). Cd39-null mice also had significantly higher levels of plasma TNF and IL-6 than the wildtype controls after 4 h of reperfusion (TNF, p < 0.01, IL-6, p < 0.02; Fig. 4).
Fig. 4.
Systemic inflammation characterized by elevated pro inflammatory cytokines is noted in Cd39-hemizygous and null mice after 45 min of hepatic ischemia and 4 h of reperfusion. IL-6 (p < 0.02) and TNF (p < 0.01) are significantly increased in Cd39-null mice compared to the wildtype controls. Significant increases in IL-1 in the Cd39-hemizygous (p < 0.007) and Cd39-null mice (p < 0.005) compared to wildtype animals are evident
NMR Hepatic reperfusion injury: NMR–P31 spectra with ATP depletion in the wild-type mouse. 31P spectra from the left lateral mouse liver lobe were tested at three time points (top) pre-ischemia, (middle) 45 min of ischemia, and (bottom) after 30–60 min of reperfusion (Fig. 4). The peak associated with ATP is diminished during ischemia in both groups. There is clear reconstitution of hepatic ATP evident in the wild-type mice upon liver reperfusion. In contrast, the recovery of ATP energy charge in the Cd39-null mouse liver was very slow. Furthermore, there are increases in inorganic phosphate signals during the ischemic episode in both types of mice. This metabolite is more rapidly cleared with recovery in wild-type mice but remains elevated in Cd39 deficient mice after IRI (Fig. 5).
Fig. 5.
Hepatic reperfusion injury: NMR – P31 spectra with ATP depletion in the wild-type mouse. 31P spectra from the left lateral mouse liver lobe at the three different time frames (top) pre-ischemia, (middle) 45 min of ischemia, and (bottom) 30–60 min of reperfusion. The intensity of the three peaks due to the abg ATP is reduced during ischemia and recovery in the wild-type upon reperfusion. In contrast, the recovery of this metabolite in the Cd39-null mouse liver appears to be absent or comparatively slow. Furthermore, the increase in inorganic phosphate signal (Pi) due to anoxia/hypoxia during the ischemic episode in both types of mice, which recover in the wild type and remain elevated in the heterozygous even after 12reperfusion
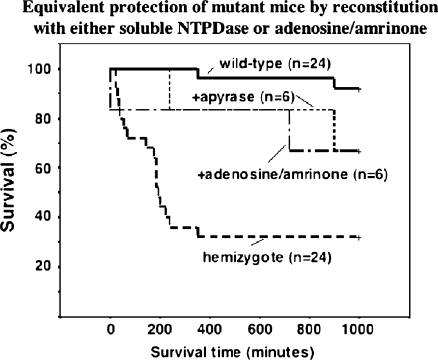
Reconstitution experiments and rescue Mice were administered apyrase, a soluble form of NTPDase. The survival rates in the Cd39 hemizygous group improved from 36% to 83% (five out of six animals survived at >4 h post reperfusion; Fig. 6). Adenosine, the ultimate catalytic product of nucleotide phosphohydrolysis, was administered with amrinone to maintain blood pressure in the post-IRI setting and had equivalent protective effects in Cd39-deficient mice (not shown). Times to euthanasia or death were unchanged in the Cd39-null mice by either of these interventions.
Fig. 6.
The administration of apyrase improves survival in Cd39-hemizygous mice when injected intravenously pre-reperfusion. Survival of Cd39-null animals could not be dramatically improved after apyrase injection (data not shown). The administration of adenosine also markedly improved Cd39-hemizygous survival after hepatic IRI
Plasma aspartate aminotransferase AST values, as a marker for severity of hepatic injury, were measured after hepatic IRI at 15 min, 1, 4, and 24 h of reperfusion in these studies. Cd39-deficient mice exhibited significant increases of transaminase levels at 15 min (p < 0.0005) and at 1 h (p < 0.0003) when compared to the wild-type animals. After administration of apyrase, AST levels were significantly decreased in the Cd39-deficient group when compared to Cd39-deficient mice without apyrase injection at 15 min (p < 0.003) and after1 h of reperfusion (p < 0.0004; Fig. 7).
Fig. 7.
AST levels after 45 min of hepatic ischemia and reperfusion. The Cd39-deficient mice show a significant increase of AST levels, when compared to wildtype mice at 15 min (p < 0.0005) and at 1 h of reperfusion (p < 0.0003). After administration of apyrase AST levels are significantly decreased in the Cd39-deficient group when compared to the Cd39-deficient group at 15 min (p < 0.003) and at 1 h of reperfusion (p < 0.0004)
Discussion
IRI has been increasingly recognized as an important source of morbidity and mortality in a number of clinical disorders, including hepatic and intestinal ischemia, acute renal injury, cardiac and cerebrovascular disease, shock and organ transplantation [24–28]. Previously, data has been published showing the deleterious effect of CD39 deletion on intestinal, myocardial, and renal ischemia as well as the crucial impact of CD39 on IP of these organs [24, 25, 28].
In this study, we show that the absence of CD39 results in increased mortality and an increased systemic inflammatory response in the setting of hepatic IRI. These effects appear secondary to decreased adenosine generation by lack of CD39 expression.
IRI is associated with systemic vascular inflammation and an SIRS that are frequently observed in clinical situations associated with hypoxic/IRI states [29, 30]. Our data suggests that CD39 plays a crucial role in the development of SIRS with acute lung injury developing after hepatic ischemia and reperfusion. Indeed, Cd39-null mice were found to have significantly higher levels of circulating cytokines (IL-1, IL-6, and TNF) as well as more acute lung injury than wildtype mice after hepatic IRI.
Hyman et al. have recently shown that CD39 on both endothelial cells and leukocytes reduces inflammatory cell trafficking and platelet reactivity following cerebral ischemia [31]. CD39 is also protective in cerebrovascular ischemia [32] and soluble CD39 infusion can restore postischemic cerebral perfusion [32]. Previous studies indicate that after IP of the heart, NTPDase transcripts can be rapidly induced in wildtype mice ultimately leading to protection during ischemia [24, 25]. Inhibiting NTPDase activity abolishes the beneficial effects of IP on the myocardium and increased infarct size in wildtype mice. Infusion of apyrase reinstituted cardioprotection from IP and reduced infarct size in Cd39-null mice. The same effects could be shown in murine kidneys during ischemia/reperfusion with clear induction of NTPDase activity after IP. Beneficial effects of apyrase infusion are also noted in Cd39-null mice on renal function post ischemia [24, 25]. Markedly decreased NTPDase activity has also been observed in rat kidneys subjected to ischemia-reperfusion injury [10].
Accumulating data suggest that hepatic reperfusion injury can be triggered by lymphocyte activation and that the activation of the adenosine A2A-receptor (A2AR) on bone marrow-derived cells mediates liver protection [33, 34]. In our model, the Cd39-null mice died rapidly after hepatic injury, most within 4 h, which suggests that a conventional CD4+ T lymphocyte pathway is unlikely given the rapid temporal relationship to the reperfusion injury. Lappas et al. have suggested that natural killer T (NKT) cells play a major role in the pathogenesis of hepatic IRI [35]. There is growing evidence that A2AR modulates inflammation and tissue injury inhibiting proinflammatory cytokine release from NKT cells in various inflammatory injury models [36–38]. Future work in progress requests the study of targeted deletions of CD39 on T-cell subsets (not shown).
Therapeutic strategies emerging from these studies could possibly lead to decreased morbidity and mortality after human liver injury, especially liver transplantation, with the prolonged periods of profound liver ischemia. Genetic polymorphisms in CD39 and potentially in P2 receptors could potentially explain the variable response of human livers to IRI [12, 39].
We conclude that systemic CD39 expression protects against hepatic cell death and decreases mortality from systemic inflammation after hepatic IRI. We further identify purinergic signaling as a potential target for therapeutic intervention in liver and systemic injury after global liver ischemia such as seen in liver transplantation.
Acknowledgments
Grant for this study: NIH P01 HL076540, NIH T32 GM007592-32
Footnotes
Xiaofeng Sun, Masato Imai, and Martina Nowak-Machen equally contributed to this paper.
References
- 1.Thiagarajan RR, Winn RK, Harlan JM. The role of leukocyte and endothelial adhesion molecules in ischemia-reperfusion injury. Thromb Haemost. 1997;78:310–314. [PubMed] [Google Scholar]
- 2.Milazzo VJ, Ferrante RJ, Sabido F, et al. Time course of leukocyte adhesion to endothelium in ischemia-reperfusion. J Surg Res. 1996;61:139–142. doi: 10.1006/jsre.1996.0094. [DOI] [PubMed] [Google Scholar]
- 3.Squadrito F, Altavilla D, Squadrito G, et al. The involvement of tumour necrosis factor-alpha in the protective effects of 17 beta oestradiol in splanchnic ischaemia-reperfusion injury. Br J Pharmacol. 1997;121:1782–1788. doi: 10.1038/sj.bjp.0701288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose S, Floyd RA, Eneff K, et al. Intestinal ischemia: reperfusion-mediated increase in hydroxyl free radical formation as reported by salicylate hydroxylation. Shock. 1994;1:452–456. doi: 10.1097/00024382-199406000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Kaczmarek E, Erb L, Koziak K, et al. Modulation of endothelial cell migration by extracellular nucleotides: involvement of focal adhesion kinase and phosphatidylinositol 3-kinase-mediated pathways. Thromb Haemost. 2005;93:735–742. doi: 10.1267/THRO05040735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcus AJ, Broekman MJ, Drosopoulos JH, et al. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corriden R, Chen Y, Inoue Y, et al. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J Biol Chem. 2008;283:28480–28486. doi: 10.1074/jbc.M800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Body SC. Platelet activation and interactions with the microvasculature. J Cardiovasc Pharmacol. 1996;27(Suppl 1):S13–S25. doi: 10.1097/00005344-199600001-00006. [DOI] [PubMed] [Google Scholar]
- 9.Goepfert C, Imai M, Brouard S, et al. CD39 modulates endothelial cell activation and apoptosis. Mol Med. 2000;6:591–603. [PMC free article] [PubMed] [Google Scholar]
- 10.Candinas D, Koyamada N, Miyatake T, et al. Loss of rat glomerular ATP diphosphohydrolase activity during reperfusion injury is associated with oxidative stress reactions. Thromb Haemost. 1996;76:807–812. [PubMed] [Google Scholar]
- 11.Enjyoji K, Sevigny J, Lin Y, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 12.Enjyoji K, Kotani K, Thukral C, et al. Deletion of cd39/entpd1 results in hepatic insulin resistance. Diabetes. 2008;57:2311–2320. doi: 10.2337/db07-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizumoto N, Kumamoto T, Robson SC, et al. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 14.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smedsrod B, Bleser PJ, Braet F, et al. Cell biology of liver endothelial and Kupffer cells. Gut. 1994;35:1509–1516. doi: 10.1136/gut.35.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beldi G, Wu Y, Banz Y, et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. 2008;48:841–852. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dranoff JA, Kruglov EA, Robson SC, et al. The ecto-nucleoside triphosphate diphosphohydrolase NTPDase2/CD39L1 is expressed in a novel functional compartment within the liver. Hepatology. 2002;36:1135–1144. doi: 10.1053/jhep.2002.36823. [DOI] [PubMed] [Google Scholar]
- 18.Beldi G, Wu Y, Sun X, et al. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology. 2008;135(5):1751–1760. doi: 10.1053/j.gastro.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai M, Takigami K, Guckelberger O, et al. Modulation of nucleoside [correction of nucleotide] triphosphate diphosphohydrolase-1 (NTPDase-1)cd39 in xenograft rejection. Mol Med. 1999;5:743–752. [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav SS, Gao W, Harland RC, Clavien PA. A new and simple technique of total hepatic ischemia in the mouse. Transplantation. 1998;65:1433–1436. doi: 10.1097/00007890-199806150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Dufour DR, Lott JA, Nolte FS, et al. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem. 2000;46:2050–2068. doi: 10.1093/clinchem/46.12.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallahan DE, Staba-Hogan MJ, Virudachalam S, Kolchinsky A. X-ray-induced P-selectin localization to the lumen of tumor blood vessels. Cancer Res. 1998;58:5216–5220. [PubMed] [Google Scholar]
- 23.Colpaert CG, Vermeulen PB, Benoy I, et al. Inflammatory breast cancer shows angiogenesis with high endothelial proliferation rate and strong E-cadherin expression. Br J Cancer. 2003;88:718–725. doi: 10.1038/sj.bjc.6600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grenz A, Zhang H, Hermes M, et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007;21:2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 25.Kohler D, Eckle T, Faigle M, et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 26.Pinsky DJ, Yan SF, Lawson C, et al. Hypoxia and modification of the endothelium: implications for regulation of vascular homeostatic properties. Semin Cell Biol. 1995;6:283–294. doi: 10.1006/scel.1995.0038. [DOI] [PubMed] [Google Scholar]
- 27.Dwyer KM, Robson SC, Nandurkar HH et al (2004) Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest 113:1440–1446 [DOI] [PMC free article] [PubMed]
- 28.Guckelberger O, Sun XF, Sevigny J, et al. Beneficial effects of CD39/ecto-nucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb Haemost. 2004;91:576–586. doi: 10.1160/TH03-06-0373. [DOI] [PubMed] [Google Scholar]
- 29.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dwyer KM, Deaglio S, Gao W, et al. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyman MC, Petrovic-Djergovic D, Visovatti SH, et al. Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39. J Clin Invest. 2009;119:1136–1149. doi: 10.1172/JCI36433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinsky DJ, Broekman MJ, Peschon JJ, et al. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J Clin Invest. 2002;109:1031–1040. doi: 10.1172/JCI10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Day YJ, Marshall MA, Huang L, et al. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol Gastrointest Liver Physiol. 2004;286:G285–G293. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- 34.Day YJ, Li Y, Rieger JM, et al. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J Immunol. 2005;174:5040–5046. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- 35.Lappas CM, Day YJ, Marshall MA, et al. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 37.Yu L, Huang Z, Mariani J, et al. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med. 2004;10:1081–1087. doi: 10.1038/nm1103. [DOI] [PubMed] [Google Scholar]
- 38.Sitkovsky MV, Lukashev D, Apasov S, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 39.Friedman DJ, Rennke HG, Csizmadia E, et al. The vascular ectonucleotidase ENTPD1 is a novel renoprotective factor in diabetic nephropathy. Diabetes. 2007;56:2371–2379. doi: 10.2337/db06-1593. [DOI] [PubMed] [Google Scholar]