Abstract
Epidemiological studies have implicated stress (psychosocial and physical) as a trigger of first onset or exacerbation of irritable bowel syndrome (IBS) symptoms of which visceral pain is an integrant landmark. A number of experimental acute or chronic exteroceptive or interoceptive stressors induce visceral hyperalgesia in rodents although recent evidence also points to stress-related visceral analgesia as established in the somatic pain field. Underlying mechanisms of stress-related visceral hypersensitivity may involve a combination of sensitization of primary afferents, central sensitization in response to input from the viscera and dysregulation of descending pathways that modulate spinal nociceptive transmission or analgesic response. Biochemical coding of stress involves the recruitment of corticotropin releasing factor (CRF) signaling pathways. Experimental studies established that activation of brain and peripheral CRF receptor subtype 1 plays a primary role in the development of stress-related delayed visceral hyperalgesia while subtype 2 activation induces analgesic response. In line with stress pathways playing a role in IBS, non-pharmacologic and pharmacologic treatment modalities aimed at reducing stress perception using a broad range of evidence-based mind-body interventions and centrally-targeted medications to reduce anxiety impact on brain patterns activated by visceral stimuli and dampen visceral pain.
Keywords: colorectal distension, CRF receptor, irritable bowel syndrome, mast cells, stress, visceral pain
Introduction
Visceral hypersensitivity reflected by enhanced perception of physiological signals from the gut and/or enhanced perception of experimental visceral stimuli along with hypervigilance to those, is commonly considered to play a major role in the pathophysiology of irritable bowel syndrome (IBS) (Choung et al., 2009; Elsenbruch et al., 2010a; Elsenbruch et al., 2010b; Lackner et al., 2010; Mayer et al., 2008; Posserud et al., 2004; Shen et al., 2009). Epidemiological studies have implicated stress (psychosocial and physical) as a trigger of first onset or exacerbation of IBS symptoms (Blanchard et al., 2008; Dufton et al., 2008; Mayer et al., 2001). Over the past 15 years, various animal models have been developed to get insight into the underlying mechanisms of visceral hypersensitivity and the influence of stress on visceral pain pathways (Barreau et al., 2007b; Larauche et al., 2009a; Mayer et al., 2008; Qin et al., 2011; Yarushkina, 2008). In this review we will outline the recent development in stress pathways and mediators, how they contribute to stress modulation of visceral pain mechanisms, and the clinical relevance of these preclinical studies to unravel potential molecular targets to alleviate pain symptoms in IBS.
Stress: pathways and mediators
The term “stress” was first coined by the endocrinologist Hans Selye more than 70 years ago to define the physiological adaptive responses to emotional or physical threats (“stressors”) to the organism, whether real or perceived (Selye, 1936). When exposed to an acute threatening challenge, the body engages a “fight or flight” response as described originally by W. Cannon (Cannon, 1915) in 1915 which is driven by sympathetic activation leading to rapid heart rate and respiration, increased arousal, alertness, and inhibition of acutely non adaptive vegetative functions related to feeding, digestion, growth and reproduction (Selye, 1936). Concurrently, a negative feedback is activated to limit the stress response and bring the body back to a state of homeostasis or eustasis (Chrousos, 2009) through activation of neural, neuroendocrine and immune mechanisms, a process called allostasis (McEwen, 1998) or “stability through changes” (McEwen, 1998; Sterling and Eyer, 1988). However, if the stressor persists and becomes chronic, the body enters a resistance phase and tries to adapt to the strains and demands of the environment by engaging coping mechanisms. When the severity and/or chronicity of the stressors are exceeding the limits, and the adaptive system becomes defective or excessive, the organism is no longer brought back to basal homeostasis leading to a state of allostatic load (McEwen, 1998; McEwen and Stellar, 1993) recently also named “cacostasis” (Chrousos, 2009). This state is harmful to the organism and lies at the origin of a variety of stress-related diseases that develop in the context of a vulnerable background (genetic, epigenetic and/or constitutional) (Chrousos, 2009). The pathogenesis of stress-induced disorders affects the whole body, including the viscera of which the gastrointestinal (GI) tract is a sensitive target (Chrousos, 2009; Stengel and Taché, 2010).
In recent decades, the biochemical coding of the stress response was unraveled through the identification of the 41 amino acid peptide, corticotropin releasing factor (CRF), and related peptides, urocortin 1, urocortin 2 and urocortin 3 along with the characterization of CRF receptors CRF1 and CRF2 which display specific affinity for CRF and related agonists (Hauger et al., 2003). Activation of CRF receptors underly the various biological components of the stress response (Bale and Vale, 2004; Koob and Heinrichs, 1999; Stengel and Taché, 2010). Indeed, hypothalamic CRF was established to play a pivotal role in the activation of the hypothalamic-pituitary-adrenal (HPA) axis. When a stressor is perceived, a convergence of stimulatory inputs from different brain regions (amygdala, prefrontal cortex, pons, medulla) activates the paraventricular nucleus of the hypothalamus which releases CRF and arginine-vasopressin into the hypophyseal portal system. There, CRF binds to CRF1 receptors located on corticotrope cells of the pituitary gland to release the adrenocorticotropic hormone while arginine-vasopressin interacts with vasopressin1b pituitary receptor to potentiate adrenocorticotropic hormone release leading to glucocorticoids secretion from the adrenal glands. Corticosterone (rodent)/cortisol (human) exert a negative feedback on the paraventricular nucleus of the hypothalamus and pituitary gland ultimately contributing to the termination of the response (Turnbull and Rivier, 1997). Far beyond an exclusive neuroendocrine role, CRF, which is widely distributed outside of the hypothalamus (De Souza and Grigoriadis, 2002), also acts as a neurotransmitter/ neuromodulator to coordinate the behavioral, autonomic, immune, and visceral efferent limbs of the stress response (Bale and Vale, 2004; Caso et al., 2008; Friedman and Irwin, 1995; Taché et al., 2001). For instance brain CRF activates the sympathetic nervous system inducing the systemic release of catecholamines (adrenaline and noradrenaline) involved in the ”fight or flight” response (Tsatsanis et al., 2007; Usui et al., 2009; Yorimitsu et al., 2008). The locus coeruleus is also activated and its noradrenergic projections to the forebrain provide an additional source of noradrenaline which contributes to the arousal and alertness (Valentino et al., 1993). Convergent preclinical evidence has accumulated over the years suggesting that stress-related alterations of colonic motor and visceral functions are primarily mediated by the activation of brain CRF/CRF1 signaling pathway, while CRF2 receptor activation may exert a counteracting influence (Fukudo, 2007; Million et al., 2005; Million et al., 2006; Taché and Brunnhuber, 2008). However, recent experimental and clinical studies point to an equally important contribution of the peripheral CRF/CRF1 signaling locally expressed in the gut to the GI stress response (Larauche et al., 2009a; Larauche et al., 2009b).
Visceral pain pathways
The perception of pain in peripheral tissues depends on the transmission of a signal from the site of pain origin to a number of distinct regions in the cerebral cortex. This pain signal is subject to modulation at every step of its travel to and from the central nervous system (CNS) (Anand et al., 2007). In the periphery, pain is transmitted by the activation of nociceptors (receptors activated by noxious stimuli) (Basbaum et al., 2009), located in two sets of primary afferent fibers innervating the viscera that project to distinct regions in the CNS (Sengupta, 2009). The GI tract innervation from the esophagus to the transverse colon is provided by vagal afferent fibers originating in the nodose ganglia and projecting centrally to the nucleus of the solitary tract. The remaining part of the large bowel (descending and sigmoid colon, rectum) is innervated by pelvic nerve afferent fibers, originating in the lumbosacral dorsal root ganglia, and projecting centrally to the sacral spinal cord. Due to their anatomical association with parasympathetic nerves, those are often qualified of parasympathetic afferents. The entire GI tract is also innervated by afferent fibers in the splanchnic nerves projecting to the thoracic 5- lumbar 2 segments of the spinal cord, which are qualified of sympathetic afferents due to their anatomical association with sympathetic nerves (Robinson and Gebhart, 2008). Visceral afferents which constitute only 10% of all afferents are present in the mucosal epithelium, serosa, muscles and myenteric ganglia, and are able to monitor changes in the gut milieu and participate in the transmission of visceral sensory information (Blackshaw et al., 2007; Grundy, 2002). While vagal afferents do not encode painful stimuli, it is well established that changes in their activity can modulate nociceptive processing in the spinal cord and the brain (Grundy, 2002; Ness et al., 2000; Randich and Gebhart, 1992).
Upon entering the dorsal horn, visceral afferents terminate in spinal cord laminae I, II V and X (Sugiura et al., 1993). Visceral information carried out by the pelvic and splanchnic nerves converges onto spinal neurons in the lumbosacral segments and thoracolumbar segments respectively. Lumbosacral segments appear sufficient to process reflex responses to acute visceral pain. In contrast, the involvement of thoracolumbar segments in normal visceral sensation is uncertain (Wang et al., 2005). Both segments process inflammatory stimuli (Wang et al., 2005). Interestingly, activity in pelvic nerve colonic afferents inhibits thoracolumbar dorsal horn neuron processing of the same colonic stimulus through a supraspinal loop: homovisceral descending modulation (Wang et al., 2007). In addition to the well known ascending pathways involved in the transmission of nociceptive information including the spinothalamic tract, the dorsal column pathway was recently found to have a major role in relaying visceral nociceptive information (Al-Chaer et al., 1996; Palecek, 2004; Willis, Jr. and Westlund, 2001).
Subpopulations of neurons within the dorsal horn project to discrete nuclei within the thalamus (i.e. ventral posterior lateral thalamus) as well as other structures in the brain stem (parabrachial nucleus, periaqueductal gray). From the thalamus, the information is conveyed to cortical areas involved in sensory processing (such as the somatosensory cortex) or those involved in processing emotional or affective information (such as the anterior cingulate gyrus and insular cortex) (Basbaum et al., 2009; Price, 2002).
In addition to the ascending system, which enables pain perception described above, there is evidence of neural circuits originating from supraspinal sites that influence nociceptive activity in the spinal cord and in primary afferents, a system referred to as descending pathways (Heinricher et al., 2009). There are two types of descending control pathways: inhibitory and facilitatory. Descending inhibitory control pathways recruited to inhibit the pain response and produce analgesia include the periaqueductal gray and the locus coeruleus (Tsuruoka et al., 2010). Descending facilitatory control pathways include the rostroventral medulla and its two populations of neurons: one that stops firing immediately before the initiation of a nociceptive reflex - “OFF cells” and is involved in antinociception and another that begins firing immediately prior to the initiation of a nociceptive reflex - “ON cells” leading to facilitation of pain (Heinricher et al., 2009; Martenson et al., 2009).
Lastly, there is also critical evidence that the modulation of the nociceptive response can occur at afferent peripheral terminals and involves the sympathoadrenal and hypothalamic-pituitary-adrenal axes. Antinociception is provided by inhibitory peptides such as β-endorphin and enkephalin released by the pituitary and the adrenal medulla as well as immune cells (Busch-Dienstfertig and Stein, 2010; Stein et al., 1995). In contrast, an increase in nociception is mediated by adrenaline released from the adrenal medulla (Khasar et al., 2005; Khasar et al., 2009).
Clinical evidence of stress-related visceral pain modulation
Stress and IBS – impact on symptoms
A predominant role of stress in the pathophysiology, presentation and treatment outcome in clinical pain states, in particular functional gastrointestinal disorders such as IBS, has been well documented (Bennett et al., 1998; Gwee et al., 1999; Kearney and Brown-Chang, 2008; Monnikes et al., 2001). Epidemiological data have shown that a history of early adverse life events in the form of emotional, sexual, or physical abuse is a major predisposing factor for the development of IBS later in life (Chitkara et al., 2008; Videlock et al., 2009). Childhood trauma, especially in genetically predisposed individuals, is thought to induce persistence changes in the central response system, including the HPA axis (Videlock et al., 2009). Additionally, it may cause epigenetic programming of glucocorticoid receptor expression that affects behavioral adaptation and susceptibility to stress-related disorders (Chrousos, 2009). In adult IBS patients, the illness experience, health care-seeking behavior, and treatment outcome are adversely associated with acute stress episodes, chronic social stress, anxiety disorders, and maladaptive coping style (Leserman and Drossman, 2007; Videlock et al., 2009). Stress-related psychosocial factors like somatization, neuroticism, and hypochondriasis are also important predictors in the development of postinfectious IBS (Gwee et al., 1999; Spiller and Garsed, 2009a).
Autonomic disturbances
At the autonomic nervous system level, stress-related disturbances characterized by the decreased parasympathetic activity and increased sympathetic outflow frequently occur in IBS patients (Jarrett et al., 2003; Spaziani et al., 2008). Increased sympathetic tone has been shown to raise the level of perception to gastrointestinal stimuli (Azpiroz, 2002). Stress can affect different aspects of visceral pain including referred pain area, as well as accompanying motor and autonomic reflexes (Cervero, 2009). In fact, in IBS patients aberrant viscerosomatic referral has been reported (Mertz et al., 1995). Alterations in the somatic nociceptive flexion (RIII) reflex indicating the presence of hyperexcitability of spinal nociceptive processes have been also shown in a large subgroup of IBS patients undergoing rectal distension (Coffin et al., 2004). Autonomic dysfunction, in particular lower parasympathetic activity, has been also suggested to represent the physiological pathways accounting for many extra-intestinal symptoms occurring in IBS patients and the frequent overlap with other chronic pain disorders such as fibromyalgia, chronic pelvic pain, chronic fatigue syndrome, migraine headache as well as psychiatric disorders (Jarrett et al., 2003; Mayer and Tillisch, 2011; Mulak and Bonaz, 2004; Palsson and Drossman, 2005; Warnock and Clayton, 2003; Whitehead et al., 2002).
Central mechanisms
The processing and evaluation of sensory information, particularly of noxious stimuli, has been confirmed to have important cognitive, motivational and emotional components, all of which are likely affected by psychological stress (Mayer et al., 2001; Van Oudenhove et al., 2007). In IBS patients hypervigilance expressed as an increased psychological tendency to report pain, which is in turn associated with psychological distress, is considered as an essential contributor to visceral hypersensitivity (Naliboff et al., 2006). Additionally, expectation of pain and perceived controllability of pain can modify reported unpleasantness of stimuli (Salomons et al., 2007; Sawamoto et al., 2000). Functional brain imaging studies in IBS patients have provided evidence of an exaggerated activation of a vigilance network (i.e., the prefrontal cortex) and a failure in activation of regions involved in pain inhibition (e.g., anterior cingulate cortex) (Chang, 2005; Derbyshire, 2003; Piche et al., 2010). The influence of cognitive aspects and emotions on the processing of sensory information is mediated by extensive neuro-anatomical network with a pivotal role of the insular cortex and anterior cingulate cortex (Seminowicz et al., 2004). Additionally, functional brain imaging studies in IBS patients revealed that negative emotions of anxiety, anger and stress correlated negatively with anticipatory downregulation within the dorsal pons, amygdala and anterior cingulate cortex during visceral pain (Berman et al., 2008). It has been also shown that anxiety and depression are associated with the subjective response to painful visceral stimuli and are correlated with brain activation during painful rectal distension (Elsenbruch et al., 2010a; Elsenbruch et al., 2010b).
Recently, a concept of “central sensitivity syndromes” has been proposed based on many common abnormalities shared by chronic pain conditions (Yunus, 2008). This concept corresponds well to the “top-down” pathophysiological model of stress-related disorders, according to which alterations in the central stress circuits in susceptible individuals play a primary role in the pathogenesis of symptoms (Chang, 2005; Mayer et al., 2000). Two main disturbances in “central sensitivity syndromes” include alterations in serotonergic system and dysregulation of the HPA-axis. Abnormalities in the level of other neurotransmitters have also been suggested such as noradrenaline, dopamine, endocannabinoid deficiency, and the increase of substance P (SP) in the spinal fluid (Binder and Nemeroff, 2010; Warnock and Clayton, 2003). Once the CNS hyper-reactivity becomes expressed, any or all the effector arms (e.g., increased visceral nociception, neurogenic inflammation, neuroendocrine disturbances and autonomic dysfunction) can be involved (Warnock and Clayton, 2003)
Experimental stress models and visceral pain
Based on stress-related modulation of visceral pain in IBS patients as well as in healthy subjects (Mayer et al., 2001; Rosenberger et al., 2009), experimental models using exposure to various clinically relevant stressors have been developed to recapture features of IBS symptoms, of which lowered pain threshold and hyperalgesia to sigmoid distensions are hallmarks (Bouin et al., 2002; Elsenbruch, 2011). Importantly, animal models provide ways to explore hypotheses regarding the pathophysiological mechanisms underlying stress-related pain modulation. The validity of these models however should take into consideration three aspects: 1) face validity - how closely they mimic clinical pain features in IBS patients; 2) construct validity - how consistent the animal models are with the hypothetical pathogenesis; and 3) predictive validity - how well the models are responsive to treatments showing some efficacy in alleviating visceral pain in IBS patients and therefore can predict treatment responses to specific drugs or non pharmacological interventions in humans (Mayer and Collins, 2002).
Monitoring of visceral pain in rodents
The assessment of pseudoaffective reflex responses (and to a lesser degree of behavioral responses) to controlled isobaric distensions of the distal colon has become the primary readout and the standard assay for the measurement of visceral pain in rodents since its development in 1988 by Ness and Gebhart (Ness and Gebhart, 1988). When applied to rats, colorectal distension (CRD) produces a range of autonomic and behavioral pseudoaffective reflexes (changes in arterial pressure and heart rate, passive avoidance behaviors, and contraction of abdominal musculature). The monitoring of contraction of abdominal muscles or visceromotor response (VMR) is the most commonly used index of visceral pain response in rats and mice. In conscious animals, it can be recorded as a measure of electromyographic (EMG) signals via implantation of recording electrodes which are either externalized through the skin (abdomen, neck) (Bradesi et al., 2005; Christianson and Gebhart, 2007; Larsson et al., 2003) or connected to radiotelemetric implants in the abdominal cavity (Nijsen et al., 2003; Welting et al., 2005), but also as manometric changes in the pressure of the balloon inserted into the distal colon (Arvidsson et al., 2006; Tammpere et al., 2005) or changes in pressure inside the colonic lumen (Larauche et al., 2009a; Larauche et al., 2010a). Additional indirect approaches such as abdominal withdrawal reflex monitoring (Al-Chaer et al., 2000), operant behavioral assays (Ness and Gebhart, 1988) or functional brain imaging of integrated brain responses to nociceptive stimuli (Johnson et al., 2010; Wang et al., 2008b) have also been used in some studies.
Stress-induced modulation of visceral pain: animal models
Stressors are conventionally categorized into exteroceptive (psychological or neurogenic) and interoceptive (physical or systemic) classes (Herman and Cullinan, 1997; Sawchenko et al., 2000). Exteroceptive stressors are limbic-sensitive and dependent upon the forebrain cognitive circuits mediating the endocrine and autonomic responses to these types of stressors. The limbic-sensitive neural network encompasses namely the cortex (lateral, medial prefrontal, vendromedial, and infragenual cingulate), bed nucleus of the stria terminalis, lateral septum, hippocampus, amygdala, hypothalamus (mainly paraventricular nucleus) and periaqueductal gray. Interoceptive stressors in contrast are considered limbic-insensitive, the cognitive processing is bypassed and brainstem/pontine nuclei such as the lateral parabrachial nucleus, nucleus tractus solitarius, brainstem/pontine catecholaminergic neurons in the ventrolateral medulla and the locus coeruleus, respectively, receive sensory visceral input (Herman and Cullinan, 1997). The development of animal models to study the relationship between visceral pain and stress has relied on the use of those two classes of stressors based on their primary pathogenic mechanisms: those initiated by a CNS-directed (psychosocial) stressor and those initiated by a gut-directed (physical) stressor (gut inflammation, infection) (Mayer and Collins, 2002). Of note, because of the bidirectional brain-gut interactions, secondary changes in the gut are likely to occur even if the primary target may be directed at the brain and vice versa.
Two types of visceral pain responses have been described in rodents with exteroceptive stressors models: visceral hyperalgesia and visceral analgesia. Though less described and studied the latter bears very relevant implications in the understanding of visceral pain-associated pathologies. In contrast, interoceptive stressors have been most inevitably associated with the development of stress-induced hyperalgesia.
Exteroceptive stress-induced visceral hyperalgesia
• Acute stress
Exposure to water avoidance stress (WAS) that entails rodents to stand on a small platform to avoid the aversive environment of surrounding water, was originally developed to assess stress-related alterations of gut motor function (Bonaz and Taché, 1994; Enck et al., 1989). Since then, this stressor has been largely used as a form of psychological stressor to assess modulation of visceral pain (Larauche et al. 2010). Reports indicate that male Wistar exposed to WAS for 1h develop a delayed visceral hyperalgesia to CRD, appearing 24h later (Schwetz et al., 2004a). Interestingly, exposure to partial restraint stress for 2h, a stressor with stronger psychological component than WAS, was found to induce an immediate hyperalgesia to CRD in male (Gué et al., 1997) and female Wistar rats (Rosztoczy et al., 2003), even though this hyperalgesia was not reproduced in male Wistar rats in a more recent work by the same group indicative of sex difference in the response (Rosztoczy et al., 2003). Transient stressors generally trigger adaptive responses and this model has good face validity in mimicking stress-related hypersensitivity to CRD as reported in IBS patients. However it does not show good construct validity, as in clinical settings, it is most commonly the exaggerated or chronic activation of the stress system, particularly in vulnerable individuals, which is responsible for the detrimental effects on the body, including the viscera (Chrousos, 2009).
• Chronic mild stress (CMS) model
Convergent reports established that daily chronic stress predicts the intensity and severity of subsequent symptoms in IBS patients (Bennett et al., 1998; Chang, 2004; Choung et al., 2009; Elsenbruch et al., 2010b; Elsenbruch, 2011; Lackner et al., 2010). Therefore to better mimic this feature, a variety of rodent models involving chronic intermittent exposure to stress have been recently developed. One of the first chronic stress models to be adapted to the study of visceral hypersensitivity was the repeated exposure to psychological stress of WAS (Söderholm et al., 2002; Yang et al., 2006). Initial studies using this model indicate that male Wistar rats exposed to 10 consecutive days of WAS for 1 h daily developed visceral hypersensitivity to CRD (Bradesi et al., 2005; Hong et al., 2009) lasting up to 30 days after the end of the stress. In these studies, the visceral pain response was monitored using EMG recording that entails surgical implantation of electrodes and subsequent single housing of animals (Bradesi et al., 2005). However when naïve male and female Wistar rats were exposed to a similar WAS schedule, they developed visceral analgesia to CRD as monitored by intraluminal colonic solid-state manometry (Larauche et al., 2010b). Similarly, in C57Bl/6 mice, chronic WAS has been described to induce visceral hyperalgesia (Larauche et al., 2010a), visceral analgesia (Larauche et al., 2010a) or to have no influence on the VMR (Larsson et al., 2009) depending upon preconditions (surgery, housing conditions) associated with the method of recording of VMR (Larauche et al., 2010a). Therefore, the impact of repeated mild stress such as WAS in modulating visceral pain response is largely influenced by the basal state condition of the animals before applying the repeated stressor [see Exteroceptive stress-induced visceral analgesia and (Larauche et al., 2010a)]. Furthermore, exposure to homotypic stressors leads to habituation associated with the recruitment of endogenous mediators such as oxytocin (Zheng et al., 2010) and endocannabinoids (Patel and Hillard, 2008) which have been found to modulate visceral sensitivity (Black et al., 2009; Sanson et al., 2006). In that context, heterotypic stress models using different and alternating types of stressors have been more recently developed. Male Wistar rats exposed intermittently to a combination of cold restraint stress, WAS or forced swimming (one stressor per day for 9 consecutive days) were found to develop visceral hypersensitivity at 8h but not at 24h or 7 days after the end of the last stressor (Winston et al., 2010). The use of more complex and long lasting heterotypic stressors including 2–3 stressors per day for up to 35–40 days (Seo et al., 2010; Tagliari et al., 2010) causes behavioral changes in rodents that parallel symptoms of depression (Gamaro et al. 2008; Ni et al. 2008; Wilner 2005; Katz and Hersh 1981). This later CMS model may display strong construct validity in the context of subset of IBS patients presenting depression symptoms (Folks, 2004) when sustained alterations in visceral sensitivity will be established in these models. Of note, the chronic mild stress model can be difficult to be characterized and be reproducible as a number of pre-existing variables may affect the response (Willner, 1997). Specifically, the strain and sex of the animal, the extent of single housing before the beginning of the procedure or even diurnal variation in sensitivity to stress seem to have major influence on the impact of CMS on animals exposed to it and the end point parameters measured (Willner, 1997). Variety within the CMS schedule appears critical to prevent or delay the habituation of rodents to the stressor, which has been shown to occur rapidly when an homotypic stressor is presented repeatedly (Babygirija et al., 2010; Griffiths et al., 1992; Muscat and Willner, 1992; Zheng et al., 2010). All of these aspects could also potentially affect the influence of CMS on the visceral pain response, as recently evaluated (Larauche et al., 2010a).
• Neonatal maternal separation stress model
Early life events and childhood trauma by biopsychosocial factors (neglect, abuse, loss of caregiver or life threatening situation) enhance the vulnerability of individuals later in life to develop affective disorders (depression, anxiety, emotional distress) and put them at a greater risk for development of IBS (Elsenbruch, 2011; Videlock et al., 2009). Early stress/childhood trauma is mimicked in rodents by isolating the pups from the dam for 2–3 hours per day during the first 2 weeks after birth from postnatal day 1–2 to postnatal day 14 (Barreau et al., 2007b; O'Mahony et al., 2011; Plotsky and Meaney, 1993; Rosztoczy et al., 2003). Maternal care in rodents affects the function of the HPA axis, and the development of both cognitive and emotional functions (Fish et al., 2004). By disrupting the normal pup-mother interaction and thereby affecting the quality of the maternal care, neonatal maternal separation stress results in permanent changes in pups' CNS, documented at the level of gene expression, neurochemistry, electrophysiology, and morphology (Szyf et al., 2007). At adulthood, rats previously subjected to neonatal maternal separation exhibit visceral hypersensitivity to CRD in basal conditions which is further exacerbated by exposure to an acute stressor (WAS; 1h) (Barreau et al., 2004; Coutinho et al., 2002). Interestingly, the protocol of separation, removal of all pups from home cage (type M) or separation of half of littermates (type P) was found to differentially affect the visceromotor responses of pups at adulthood in a sex-dependent manner (Rosztoczy et al., 2003). Under basal conditions, male exposed to type M and females exposed to both type M and P developed visceral allodynia and hyperalgesia to CRD (Rosztoczy et al., 2003). In males, an additional acute stress did not modify the VMR to CRD, while females exposed to acute WAS (1h) exhibited an exacerbated VMR (Rosztoczy et al., 2003). This model which has both face (visceral hypersensitivity) and construct (early life events induce long term changes in gut function) validity is a relevant model in the context of subset of IBS patients with history of early life traumatic events.
• Genetic models of chronic stress
Anxiety is a well established co-morbid condition in a subgroup of IBS patients (Folks, 2004; Lee et al., 2009; Thijssen et al., 2010). A study used three strains of rats known to have varying levels of baseline anxiety as determined by the acoustic startle response and open-arm exploration in the elevated plus-maze assay: high-anxiety Wistar–Kyoto rats and low-anxiety Sprague-Dawley and Fisher-344. They demonstrated that high-anxiety rats had increased VMR to CRD compared to low-anxiety animals suggesting a direct link between anxiety and visceral hypersensitivity (Gunter et al., 2000). In addition, compared to low-anxiety rats, the sensitivity of high-anxiety rats was highly exacerbated by peripheral sensitization of the colon with a small dose of acetic acid (Gunter et al., 2000). Genetic models that blocked chronically the stress pathways by deleting CRF1 receptors showed a decrease in anxiety and colonic sensitivity to colorectal distention (Trimble et al., 2007). Chronic stress relying on alterations of the CRF system such as CRF over-expressing mice (Million et al., 2007b) are available and could be useful to study IBS-like manifestations, but the visceral sensitivity of those transgenic animals has not been assessed yet. New promising genetic models with more selective conditional and/or region-targeted genetic manipulations including RNAi gene silencing technology to modify CRF-related genes are continuously developed (Bakshi and Kalin, 2000; Delic et al., 2008 Deussing and Wurst, 2005; Kimura et al., 2010; Kolber et al., 2010; Lu et al., 2008). These models will be suitable to explore specific stress circuitries in the context of chronic stress-related visceral pain modulation which so far is lagging behind.
• Post-traumatic stress disorder model
There is evidence of increased prevalence of GI symptoms and IBS in post-traumatic stress disorder sufferers including women veterans (Cohen et al., 2006; Drossman et al., 1990; Irwin et al., 1996; Savas et al., 2009; White et al., 2010). Clinical and experimental studies indicate that exposure to an uncontrollable stressor can induce an alteration in the behavioral, autonomic, and neuroendocrine responses to subsequent stressors. In rats, treatment with a relatively short-lasting session of shocks or a social confrontation with a predator or aggressive conspecific animals induces long-lasting (weeks–months) conditioned fear-responses to trauma-related cues, and a generalized behavioral sensitization to novel stressful stimuli that persists or grows stronger over time (Rau et al., 2005; Stam et al., 1997; Stam, 2007; Wang et al., 2008a). Repetitive balloon distention of the distal colon causes increased cardiovascular $$`pseudoaffective' reflexes in pre-shocked rats compared to controls, 2 weeks after a single session of foot shocks (Stam, 2007). Interestingly, female rats appear to show a different pattern of sensitized behavioral responsiveness to the same challenge, possibly pointing to differential alterations in the neuronal substrates involved (Stam et al., 2002). Whether these sex differences in behavior translate into sex differences in visceral sensitivity has not been assessed yet. These rodent PTSD models mimic clinical features (stress-related visceral hypersensitivity) of IBS and have a good construct validity, relevant for a subset of IBS patients with post-traumatic stress disorder. As the visceral pain alterations observed in this paradigm of uncontrollable stress model have been mainly characterized by one group of investigator, the reliability of the model however needs further independent confirmation.
Exteroceptive stress-induced visceral analgesia
While extensively described in somatic pain (Butler and Finn, 2009), activation of descending inhibitory pathways in stress-related visceral responses has only been reported in a few studies. Opioids have been implicated in descending inhibition of visceral sensitivity following stress as evidenced by the fact that naloxone unmasked WAS-induced hyperalgesia to repeated phasic CRD in normal rats and exacerbated the pain response to CRD in maternally-separated rats (Coutinho et al., 2002). In another study, a visceral analgesic response was observed 6h after exposure to an acute session of WAS in wild-type mice and Sprague-Dawley rats, with males exhibiting stronger analgesia than females (Gui et al., 2004). This visceral analgesia was shown to be dependent on the activation of neurotensin signaling pathways, as it is no longer observed in neurotensin deficient mice. Likewise using pharmacologic approach, the intravenous administration of a neurotensin receptor antagonist unmasked the visceral hyperalgesia in male rats and to a much greater extent in female rats (Gui et al., 2004). In another experimental model, a daily short period (15 min) of separation from post natal day 2 to 14 decreased VMR to phasic CRD performed immediately after WAS as well as prevented the development of hyperalgesia 24h after WAS in adult male Long-Evans rats (Schwetz et al., 2005). These data suggest a potential alteration of endogenous pain-modulatory systems by this mild maternal separation stress (Schwetz et al., 2005). Similar findings in adult rats have been recently reported, such that chronically handled rats develop visceral hypoalgesia in response to CRD that becomes significant 7 days after the last handling (Winston et al., 2010). However, the underlying mechanisms and potential implication of this visceral analgesia were not discussed by the authors (Winston et al., 2010).
Interestingly, we recently demonstrated that mice repeatedly exposed to WAS (1h/day) for 10 consecutive days exhibit a differential profile of VMR to CRD 24h after the last session of stress depending on the basal status of the animal before WAS, in particular the context in which they are housed and method used to monitor visceral pain. Mice that had undergone surgery for the placement of EMG electrodes on abdominal wall and were subsequently single housed to avoid deterioration of implanted electrodes by cage-mate, developed visceral hyperalgesia in response to repeated WAS while mice tested for visceral pain using the non-invasive solid-state intraluminal pressure recording and kept group housed developed a strong visceral analgesia under otherwise similar conditions of chronic WAS (Larauche et al., 2010a). Recent reports suggest that previous injury or exposure to opioids in male rats can switch stress influence on pain responses from analgesia to hyperalgesia (Rivat et al., 2007). Furthermore, paw incision in male Sprague-Dawley rats was shown to induce a long lasting visceral hyperalgesia (Cameron et al., 2008). Together these data point to the state of animals (naïve vs surgery), its social environment (group housing vs single housing, cage enrichment or not), the handling by the investigator, the methods used to record its visceromotor responses (EMG requiring surgery vs manometry not requiring surgery), as well as its sex can significantly affect the response to exteroceptive stressors and should be carefully considered and weighed in the design, conduct and interpretations of the studies addressing the influence of stress on visceral sensitivity in experimental animals. Based on recent clinical findings demonstrating that IBS patients have compromised engagement of the inhibitory descending pain modulation systems (Berman et al., 2008; Coffin et al., 2004; Piche et al., 2010; Song et al., 2006; Wilder-Smith et al., 2004), gaining a deeper understanding of the mechanisms involved in the expression of stress-induced visceral analgesia or lack thereof are promising avenues to explore that may lead to new therapeutic targets for IBS. Therefore the use of non-invasive methods of monitoring VMR that alleviates the surgical and housing impact on repeated stress modulation of visceral pain represents a step forward to gain insight into the underlying mechanisms in particular the neural substrates and neurochemistry of stress-related analgesia as established in the somatic field (Butler and Finn, 2009).
Interoceptive stressors and visceral hyperalgesia
• Neonatal inflammation/neonatal pain models
The newborn's gut may be exposed to a variety of factors resulting in mucosal inflammation and tissue irritation. Daily colon irritation during the neonatal period (days 8–21) either in the form of daily noxious colorectal distention (CRD) (two 60 mmHg-60 s distensions separated by 30 min of rest) or by performing daily intracolonic injection of mustard oil (5%), increases pain behavior to CRD from postnatal week 5 up to postnatal week 12 (Al-Chaer et al., 2000; Lin and Al-Chaer, 2003). These findings suggest that two different perturbations of the intestinal homeostasis in early life (mechanical or chemical irritation) can result in permanent central sensitization initiated and partly maintained by peripheral afferent input sensitized by neonatal events (Al-Chaer et al., 2000; Lin and Al-Chaer, 2003). The model has both face (visceral hypersensitivity) and construct validity (long lasting, permanent sensitization of dorsal horn neurons) and could apply to a subset of IBS patients, even though no longitudinal studies exist to show that gut irritation in early life is a risk factor for IBS development at adulthood (Mayer and Collins, 2002).
• Intestinal infection: post-infectious IBS model
In approximately 10% of patients with IBS, the onset of symptoms began with an intestinal infectious illness (Collins et al., 1999). Prospective studies have shown that 3% to 36% of enteric infections lead to persistent new IBS symptoms depending on the infecting organism. In addition, the co-existence of adverse psychological factors at time of infection is also an important determinant to the susceptibility to develop post-infectious IBS (Spiller and Garsed, 2009b). While viral gastroenteritis seems to have only short-term effects, bacterial enteritis and protozoan and helminth infections are followed by prolonged post-infectious IBS (Spiller and Garsed, 2009b). Long-lasting visceral hyperalgesia has been observed in mice after transient intestinal inflammation induced by Trichinella spiralis infection (Bercik et al., 2004; Long et al., 2010) or in rats infected by Nippostrongylus brasiliensis (McLean et al., 1997). Although the vast majority of human post-inflammatory hypersensitivity symptoms are observed after bacterial infection (Campylobacter, Shigella, Salmonella or Escherichia coli infections), no animal model of hypersensitivity postbacterial infection has been developed yet. For instance mice infected with Citrobacter rodentium, an attaching-effacing murine enteropathogen similar in its mechanisms of infection to enteropathogenic E. coli, do not spontaneously develop visceral hypersensitivity symptoms (Vergnolle, 2008). These models of nematode parasites in rodents have however good construct and face validity in that they show that long-term immune modulation of smooth muscle and enteric nervous system can result in persistent altered gut motility and visceral hypersensitivity.
• Noninfectious intestinal inflammation: post-inflammatory IBS model
Despite some controversies on the origin of the symptoms (Keohane et al., 2010; Long and Drossman, 2010), “IBS-like” symptoms appear to be common in patients in remission from ulcerative colitis (Spiller and Garsed, 2009a). Several chemical irritants have been used to produce colonic inflammation resulting in visceral hyperalgesia in rodents. In rats, acetic acid (Burton and Gebhart, 1995), mustard oil (Ji et al., 2005; Palecek and Willis, 2003), and zymosan (Coutinho et al., 1996; Traub and Murphy, 2002) evoke short-term hyperalgesia associated with transmural tissue damage/colonic inflammation. Intracolonic trinitrobenzene sulfonic acid induces a severe colonic transmural inflammation and visceral hypersensitivity that develops at 4–5 days with the disappearance of symptoms by 14 days (Adam et al., 2006; Gschossmann et al., 2004). Interestingly, in 24% of rats there is reoccurrence of visceral hyperalgesia 16 weeks after the induction of inflammation, whereas no evidence of microscopic inflammation is observed in rat colonic tissues at that time point (Adam et al., 2006; Zhou et al., 2008). Mild non-specific colitis and acute dextran sodium sulfate (DSS)-induced colitis were associated with increased responsiveness to CRD on days 5 or 60 after the induction of colitis in male Balb/c mice while chronic DSS colitis was not (Verma-Gandhu et al., 2007). These results are in contrast with another study showing that DSS colitis failed to cause the development of visceral hypersensitivity in response to CRD at any of the observed time points after the induction of inflammation (days 5, 12, 16, 20, 30, 40 or 51 after the induction of colitis) in C57Bl/6 or Balb/c mice (Larsson et al., 2006). A number of factors may have contributed to these conflicting results among which the protocol of colitis induction (three 5 days DSS with a 15 days rest between cycles vs 5–7 days DSS-rest), the dose of DSS used (5% vs 4%) or even the sex of the animals, which was surprisingly not even mentioned in the latter study. While it is difficult to make any assumptions on the reasons for these differences, these results suggest that inflammation alone may not always lead to visceral hypersensitivity and that the type of inflammatory insult and severity determine whether this will result in the development of postinflammatory hypersensitivity (Adam et al., 2006). In most, but not all the studies (Larsson et al., 2009), previous exposure of rats and mice to psychological or psycho-social stress was shown to enhance their susceptibility to colitis and to aggravate their colonic inflammatory response (Reber et al., 2006; Reber et al., 2008; Veenema et al., 2008) as well as to precipitate the reactivation of colonic inflammation in animals in which colitis had healed (Melgar et al., 2008; Saunders et al., 2006). Likewise, previous colitis was found to render the colon more susceptible to the effects of stress on enteric nerve function and to increase some parameters of inflammation in response to stress (Collins et al., 1996). Nevertheless, the relationship between stress, colitis and visceral pain remains controversial as stress was found to exacerbate post-inflammatory visceral hyperalgesia in rats (Liebregts et al., 2007) or either to have no effect in mice (Larsson et al., 2009).
Bile salt malabsorption underlies some forms of postinfectious IBS (Spiller, 2003). A rodent model was developed in which a bile acid deoxycholic acid instilled intracolonically daily for 3 days induces a mild, transient colonic inflammation within 3 days of administration that resolves within 3 weeks. Adult male Sprague-Dawley rats exposed to this regimen of bile acid develop a persistent visceral hyperalgesia starting after 1 week and lasting up to 4 weeks (Traub et al., 2008).
Animal models: predictive validity
Some commonly used animal models showed the modulation of visceral pain to CRD by acute or chronic stressors presenting good face and construct validity, however, their predictive validity in humans is either unknown or has been unsatisfying in clinical trials (Bradesi and Mayer, 2009; Holschneider et al., 2011; Mayer et al., 2008). The use of novel non-invasive techniques to assess visceral pain such as intraluminal colonic pressure (Larauche et al., 2009a; Larauche et al., 2010a) by allowing for the monitoring of stress-induced visceral analgesia, may open new venues to deepen our understanding of stress-related analgesic pain pathways in the viscera. Novel strategies such as identification and characterization of endophenotypes in IBS patients, followed by reverse translation of these endophenotypes for pharmacological studies in rodents, have also been recently suggested (Bradesi and Mayer, 2009; Holschneider et al., 2011; Mayer et al., 2008).
Mechanisms involved in stress-induced modulation of visceral pain
Pathological pain refers to conditions characterized by hyperalgesia and allodynia, in which maladaptive neuroplastic changes lead to persistent increased perception and responsiveness to noxious stimuli, or response to normally non-noxious stimuli. Such neuroplastic changes can occur in primary afferent terminals (peripheral sensitization) but also in the spinal cord (central sensitization) and in the brain (supraspinal pain modulation) or in descending pathways that modulate spinal nociceptive transmission. Such alterations in the processing of sensory information are all considered as possible mechanisms of visceral hypersensitivity in IBS patients (Azpiroz et al., 2007; Sengupta, 2009).
Stress and peripheral sensitization: a role for the CRF system, mast cells, gut microbiota and ion channels
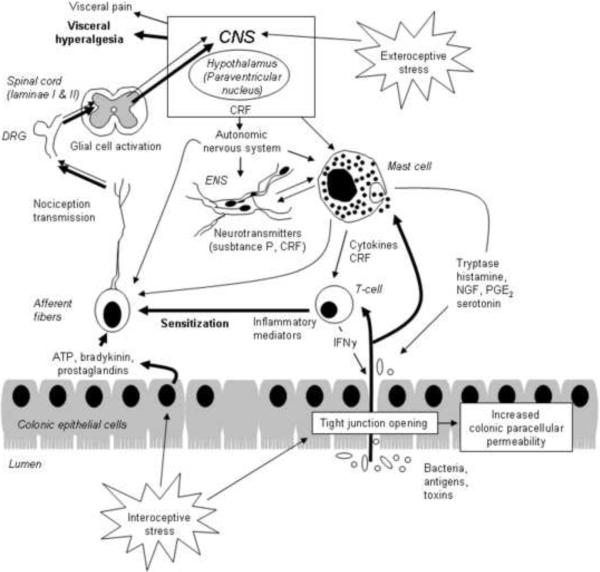
A key role for peripheral CRF signaling in the development and expression of visceral pain is well documented by several reports in both humans and rodents (La et al., 2008; Larauche et al., 2009a; Lembo et al., 1996; Nozu and Kudaira, 2006; Sagami et al., 2004; Taché and Brunnhuber, 2008; Tayama et al., 2007). Converging evidence support the involvement of peripheral CRF1 receptors in these effects. Peripheral injection of CRF induces visceral hypersensitivity to CRD (La et al., 2008), an effect reproduced by the intraperitoneal administration of the selective CRF1 agonist, cortagine in rats and mice (Larauche et al., 2009a) while intraperitoneal injection of selective (urocortin 2) or preferential (sauvagine) CRF2 agonists, reduced visceromotor response to CRD in rats (Million et al., 2005; Million et al., 2006). In addition, the visceral hyperalgesia induced by peripheral injection of cortagine in rats is abolished by peripheral, but not central, administration of the non selective CRF receptor antagonist astressin (Larauche et al., 2009a). Likewise under conditions of repeated exposure to WAS for 10 days, we found that peripheral administration of the peptide CRF1/CRF2 receptor antagonist astressin before each stress session prevented the development of visceral hyperalgesia supporting the participation of a peripheral component to the development of visceral hypersensitivity (Larauche et al., 2008). Of translational relevance intravenous administration of a non-selective and peripherally-restricted CRF receptors antagonists, α-helical CRF9–41 or astressin was reported to reduce visceral hyperalgesia in diarrhea-predominant IBS patients subjected to colonic electrical stimulation (Sagami et al., 2004; Tayama et al., 2007).
Stress and peripheral administration of CRF induce mast cells degranulation in the colon in experimental animals and humans (Barreau et al., 2007a; Wallon et al., 2008)which can in turn lead to the development of visceral hypersensitivity via the release of several preformed or newly generated mediators namely histamine (Barbara et al., 2007; Cenac et al., 2007; van den Wijngaard et al., 2010), tryptase (Cenac et al., 2007), prostaglandin E2 (Gold et al., 2002), nerve growth factor (NGF) (Barreau et al., 2004) that can activate or sensitize sensory afferents (Sengupta, 2009; van den Wijngaard et al., 2009). It is now well established that stress can disrupt the intestinal epithelial barrier which may increase the penetration of soluble factors (antigens) into the lamina propria, and lead to nociceptors sensitization (Barbara et al., 2007; Piche et al., 2009). Increased intestinal permeability appears as a prerequisite for the development of visceral hypersensitivity in both humans and rodents (Ait-Belgnaoui et al., 2005; Piche et al., 2009; Zhou et al., 2009). Alterations of epithelial permeability following stress involves the activation of the peripheral CRF system and may (Demaude et al., 2006; Santos et al., 2001; Söderholm et al., 2002; Vicario et al., 2010; Yu and Perdue, 2001) or may not be dependent from mast cell activation (Demaude et al., 2006; van den Wijngaard et al., 2009) in a time-dependent manner.
Lastly, there is mounting evidence that stress can affect the intestinal and fecal microbiota of rodents which display changes in composition, diversity and number of gut microorganisms (Bailey et al., 2011; Bailey et al., 2010; O'Mahony et al., 2009). These alterations in the microbiota can in turn have significant impact on the host and affect his behavior, visceral sensitivity and inflammatory susceptibility (Bercik, 2011; Collins and Bercik, 2009; Cryan and O'Mahony, 2011; Heijtz et al., 2011; Rhee et al., 2009).
Stress-induced intestinal epithelial permeability or intestinal injury or inflammation can lead to the sensitization of peripheral terminals. Three mechanisms can account for this sensitization: change in tissue properties, change in transduction process (increased release of mediators) or changes in the properties of protein/protein complexes underlying stimulus transduction (receptors sensitivity or number). In response to an injury such as inflammation, the intestinal mucosa leads to the release of chemical mediators (ATP, bradykinin, prostaglandins) which can directly stimulate afferent neuron terminals but also promote the release of algogenic substances (proteases, histamine, SP), serotonin, NGF and prostaglandins), leading to the amplification of the afferent chemical or mechanical stimulus (Blackshaw et al., 2007; Chung et al., 2009; Lin and Al-Chaer, 2003; Mantyh, 2002; Robinson and Gebhart, 2008; Vergnolle, 2010; Winston et al., 2010). This peripheral sensitization is mediated by a number of ion channels widely expressed in colonic afferents (Cregg et al., 2010; Holzer, 2001; Jones, III et al., 2005; McRoberts et al., 2001). Among these, the N-methyl-D-aspartate receptor (NMDA) (McRoberts et al., 2001), proteinase-activated receptor, PAR2 (Cenac et al., 2007), transient receptor potential vanilloid 1 (TRPV1) (Ravnefjord et al., 2009; Winston et al., 2007; Yu et al., 2010) and TRPV4 (Cenac et al., 2010), transient receptor potential ankyrin 1 (TRPA1) (Cattaruzza et al., 2010; Yu et al., 2010), purinergic receptor P2X (Holzer, 2001), neurokinin receptor 1 (NK1) (Greenwood-Van Meerveld et al., 2003) and acid-sensing ion channels (ASICs) (Page et al., 2004) have all been found to be associated with visceral hypersensitivity.
Spinal cord plasticity and glia activation: role in the central processing of peripheral pain perception
Once peripheral sensitization has developed, it can in turn activates the release of spinal cord mediators. Among them, ASIC1A was recently shown to be increased in sensory neurons of rats exhibiting hypersensitivity in response to repeated colonic butyrate enemas (Matricon et al., 2010). Similarly, NK1 receptors were found to be upregulated in the spinal cord of chronically stressed rats and to mediate the development of visceral hypersensitivity (Bradesi et al., 2009b; Bradesi et al., 2006; Gaudreau and Plourde, 2003). In conscious animals subjected to psychological chronic stress, the decrease in the somatic mechanical nociceptive threshold was shown to be related to the activation of cholecystokinin (CCK)-dependent descending facilitatory nociceptive pathways (Rivat et al., 2007), a phenomenon which could also explain visceral hypersensitivity (Friedrich and Gebhart, 2000; Friedrich and Gebhart, 2003). Increase in growth factors such as NGF (Chung et al., 2007) or brain-derived neurotrophic factor (BDNF) (Chung et al., 2009) as a result of stress exposure in rodents has also been established to play a key role in mediating visceral hyperalgesia. Phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK) in the spinal cord induced by the binding of neurotrophins to their specific tyrosine kinase receptors or by neuronal activity leading to glutamate release and binding to its ionotropic and metabotropic receptors (Cruz and Cruz, 2007), are known to be nociceptive-specific signaling pathways and their upregulation is associated with visceral hyperalgesia (Million et al., 2006; Zhang et al., 2009).
Very recently, another potential mechanism through which spinal sensitization may occur in response to stress has been suggested in the form of spinal cord glia activation. While this phenomenon is now considered an important component in the development and maintenance of allodynia and hyperalgesia in various models of chronic pain, including neuropathic pain and pain associated with peripheral inflammation, it is only recently that the possibility of spinal cord glia changes in relation with visceral hypersensitivity and stress-induced visceral hypersensitivity have been addressed (Bradesi, 2010). One study using a model of visceral hypersensitivity induced by neonatal colonic irritation showed an increase spinal immuno-reactivity for OX42 (indicating microglia proliferation) in sensitized rats compared to controls (Saab et al., 2004). Visceral hypersensitivity to CRD in these animals was blocked by an acute treatment with minocycline, a microglia inhibitor. Likewise, chronic WAS in male Wistar rats increases p38 phosphorylation in OX42 positive cells, and this effect was blocked by treatment with minocycline (Bradesi et al., 2009a). The concomitant visceral hyperalgesia exhibited by those rats was blocked by intrathecal treatment with minocycline or the p38 inhibitor SB203580, supporting a functional role of spinal microglia activation in the development of visceral hypersensitivity (Bradesi et al., 2009a). The modulatory influence exerted by spinal microglia on visceral nociception was further supported by the findings that spinal injection of the microglia activator fractalkine in naïve rats, induces visceral hyperalgesia (Bradesi et al., 2009a; Saab et al., 2004). Candidate molecules involved in glia activation signaling include neurotransmitters such as SP or glutamate, but also purinergic agents, opioids, chemokines and glucocorticoids [for review see (Bradesi, 2010)].
Glutamate uptake through spinal glutamate transporters is critical for maintaining normal sensory transmission under physiologic conditions (Liaw et al., 2005; Lin et al., 2009). A potential deficiency in glutamate reuptake by astrocytes associated with the activation of spinal cord glia (Svensson et al., 2003) has been recently suggested to play a role in the spinal sensitization and the development of visceral hypersensitivity in rats (Gosselin et al., 2010).Together, these data strongly support the concept that transmission of visceral nociceptive signals may be enhanced in various conditions of spinal microglia activation (Tjong et al., 2010).
Supraspinal pain modulation: a fine-tuning between pain facilitation and inhibition
Various supraspinal sites are involved in the modulation of visceral pain signals. Rectosigmoid distension in humans activates sensory (insula, somatosensory cortex), and limbic and paralimbic regions (including anterior cingulate cortex and amygdala) (Tillisch et al., 2011). Many of these regions were also found to be significantly affected by colorectal distension in rats (Wang et al., 2008b; Zhang et al., 2011).
The anterior cingulate cortex mediates key emotional-aversive aspects of pain and may also have a mnemonic role in which it allows transient storage of information during pain processing (Chang, 2005; Mayer and Tillisch, 2011). The cingulate cortex contains specific subareas for integrating visceral sensation (Ladabaum et al., 2000) and has direct connections to the amygdala, periaqueductal grey, and orbitofrontal cortex (Devinsky et al., 1995). Wistar-Kyoto rats, high-anxiety rats exhibiting visceral hypersensitivity (Gunter et al., 2000) have greater prefrontal cortex activation in response to CRD compared to Sprague-Dawley (Gibney et al., 2010).
Another key limbic system structure that has been implicated in the affective component of pain is the central amygdala. It is involved in the processing of visceral information, attention, emotion and integrating the physical and psychological components of the stress response (Dayas et al., 2001). Additionally, the central amygdala plays a crucial role in the generation and development of fear and anxiety (LeDoux et al., 1988; Rosen and Schulkin, 1998). Specifically, the central nucleus of the amygdala (CeA) has been shown to facilitate the activation of the HPA axis in response to stress and increase the release of CRF, adrenocorticotropic hormone, and corticosterone (Feldman and Weidenfeld, 1998; Shepard et al., 2003). Increased glucocorticoid levels in turn upregulates CRF gene expression in neurons within the CeA and implants of corticosterone in the CeA have been found to induce visceral hypersensitivity in rodents (Greenwood-Van Meerveld et al., 2001; Myers et al., 2007; Myers and Greenwood-Van Meerveld, 2007). The interaction between the CRF signaling system in the CeA and visceral nociception has been recently reported by showing that noxious CRD increases CRF expression and p-ERK in the CeA and fos in the spinal cord (Kim et al., 2010).
The locus coeruleus is a well established target of stress, expressing CRF1 receptors, which receives CRF innervation from nearby Barrington nucleus and increases firing in response to CRD in the same neurons responsive to central injection of CRF (Curtis et al., 1995; Kosoyan et al., 2005; Lechner et al., 1997; Reyes et al., 2007; Reyes et al., 2008; Rouzade-Dominguez et al., 2001; Valentino et al., 1993). Electrophysiological studies in anesthetized rats showed that the CRF1/2 antagonists, [DPhe12]CRF12–41, administered into the lateral brain ventricle or microinfused into the LC, or astressin injected into the cisterna magna, and selective CRF1 antagonist crossing the blood brain barrier, NBI 35965 given intravenously prevented LC neuronal activation and bursting activity in response to central injection of CRF and CRD at submaximal distention (40 mmHg) (Kosoyan et al., 2005; Lechner et al., 1997). In addition, peripheral injection of CRF1 antagonist which enters into the brain, JTC-017, reduced the rise in noradrenaline levels in the hippocampus induced by CRD in rats. Bursting activity in the LC is associated with the release of noradrenaline in the cortical and limbic rostral efferent projections of the LC leading to arousal, hypervigilance and anxiogenic response (Koob, 1999; Koob and Heinrichs, 1999). Therefore these pontine sites are well positioned to coordinate gut-brain interaction with visceral information from the gut impacting on cortical and limbic activities under stress conditions and may modulate visceral pain responses (Tsuruoka et al., 2010; Valentino et al., 1999).
Thalamic relay nuclei have a key role in gating, filtering and processing sensory information en route to the cerebral cortex and are subject to similar activity-induced plasticity processes as the spinal cord (Kuner, 2010). Two modes of firing of thalamocortical neurons, tonic and burst firing, are believed to reflect the divergent states of sensory signal transmission from the thalamus to the cortex and have been shown to modulate visceral pain (Cheong et al., 2008; Ren et al., 2009). Upregulation of CRF1 receptor in the thalamus is associated with visceral hyperalgesia in the rat model of NMS (Tjong et al., 2010).
Lastly, spinal visceral nociceptive reflexes are subject to facilitatory modulation from the RVM, providing the basis for a mechanism by which visceral sensations can be enhanced from supraspinal sites (Sanoja et al., 2010; Zhuo and Gebhart, 2002) under stress conditions associated with development of visceral hyperalgesia (Martenson et al., 2009). Compromised engagement of descending pain inhibitory pathways as observed in maternally-stressed rats may also contribute to increasing the visceral pain responses in those animals (Coutinho et al., 2002).
Sex differences in stress-induced alterations of visceral sensitivity
Female predominance in IBS patients (women to men ratio about 2:1) is consistent with an observation that women are more susceptible to stress-related disorders (Adeyemo et al., 2010; Heitkemper and Chang, 2009). A number of clinical studies have documented sex differences in the stress response and stress-induced pain modulation (Fillingim et al., 2009). However, although there is a dearth of preclinical studies assessing the effect of sex on stress-induced modulation of visceral sensitivity, most preclinical studies addressing stress-related modulation of visceral pain have been conducted in male rodents (Gui et al., 2004; Taché et al., 2005).
There is convergent evidence that sex hormones, in particular estrogens, strongly modulate the HPA axis regulation, for example estrogen receptors α and β have been shown to increase CRF gene expression (Chen et al., 2008; Mulak and Taché, 2010). Fluctuations in estrogen levels during ovarian cycle cause also changes in the serotonergic system (e.g. estrogens enhance serotonergic postsynaptic responsiveness in the brain) and interactions between the serotonergic and reproductive endocrine systems have implication in the etiology as well as treatment of stress-related disorders (Heitkemper and Chang, 2009; Linthorst, 2005; Lu et al., 2009; Mulak and Bonaz, 2004; Warnock and Clayton, 2003). Sex hormones have been shown to significantly affect visceral sensitivity in rodents (Aloisi et al., 2010; Holdcroft et al., 2000; Ji et al., 2008; Sapsed-Byrne et al., 1996; Taché et al., 2005). Addressing the influence of sex in the modulation of visceral pain by stress appears critical to find novel therapies (Adeyemo et al., 2010; Ouyang and Wrzos, 2006).
Therapeutic interventions targeting stress reduction: outcome in IBS
The modulatory role of stress-related psychological factors, emotional state, and established brain-gut interactions in the pathophysiology of IBS (Mulak and Bonaz, 2004; Mayer and Tillisch, 2011), has been confirmed by the encouraging outcome of nonpharmacological approaches by treatment modalities using a broad range of evidence-based mind-body interventions such as psychotherapy, cognitive behavioral therapy, hypnotherapy, relaxation exercises or mindfulness mediation (Kearney and Brown-Chang, 2008; Palsson and Drossman, 2005; Whorwell, 2009). Different forms of therapies are focused on teaching better stress coping strategies, both at a cognitive level (catastrophic or self-defeating thoughts) and at a behavioral level (problem solving, especially interpersonal problems) (Blanchard et al., 2007; Whorwell, 2009). The symptomatic improvement seems to result from the modulation of stress response, restoration of the autonomic system balance, and changes in the brain activation pattern in response to visceral stimuli. Several well-designed IBS studies of hypnotherapy, including randomized, controlled trials, have shown substantial long-term improvement not only of gastrointestinal symptoms but also of anxiety, depression, quality of life, disability and excess healthcare (Kearney and Brown-Chang, 2008). However, a few recent large-scale randomized controlled trials of cognitive behavioral therapy have failed to show significant advantages in reduction of gastrointestinal symptoms in comparison of psychological control conditions (Blanchard et al., 2007; Boyce et al., 2003; Drossman et al., 2003). The results of study by Blanchard et al. (Blanchard et al., 2008) suggest that there is rather a reciprocal than a causal relation between stress and symptoms. Thus, as described by Miller and Whorwell (Miller and Whorwell, 2009), more direct targetting to gastrointestinal symptoms including abdominal pain, such as with gut-directed hypnotherapy might be more effective.
In addition to psychological mind-body interventions, clinical trials confirm the effectiveness of centrally acting pharmacological interventions such as with antidepressants, and anxiolytics, or combination of drugs from both groups in the treatment of chronic pain disorders (Palsson and Drossman, 2005; Verdu et al., 2008; Warnock and Clayton, 2003). Tricyclic antidepressant amitriptyline has been shown to reduce brain activation during rectal distension in IBS patients, but only during mental stress (Morgan et al., 2005; Thoua et al., 2009). Based on the reduced activation in cognitive and affective cortical regions, it was postulated that amitriptyline improves symptoms in IBS due to a CNS effect rather than a peripheral one, most likely reducing the affective and hypervigilance component to pain or stress-related exacerbation of symptoms (Morgan et al., 2005). Another antidepressant – fluoxetine (selective serotonin reuptake inhibitor) reduces abdominal pain in IBS, but only in patients displaying hypersensitivity to rectal distension, thus adequate patients selection would determine treatment effectiveness (Kuiken et al., 2003) (Table 1).
Table 1.
| Drug class | Compounds | Effect on visceral sensation | References |
|---|---|---|---|
|
| |||
| Tricyclic antidepressant | Amitriptyline | Reduces brain activation in cognitive and affective cortical regions during rectal distension in IBS | (Morgan et al., 2005; Thoua et al., 2009) |
|
| |||
| Antidepressant SSRI | Fluoxetine | Reduces abdominal pain but only in patients with hypersensitivity to rectal distension | (Kuiken et al., 2003) |
| Citalopram | Reduces abdominal pain in IBS patients with coexisting depression | (Ford et al., 2009) | |
|
| |||
| Antidepressant SNRI | Venlafaxine | Increases compliance, decreases tone and sensation in healthy humans | (Chial et al., 2003) |
|
| |||
| 5-HT1 agonist | Buspirone | Induces analgesic effect in the CRD model in rats | (Sivarao et al., 2004) |
| Not significantly alters colonic compliance, tone, or sensation in healthy humans compared to placebo | (Chial et al., 2003) | ||
|
| |||
| Robalzotan tartrate monohydrate (AZD7371) | Induces analgesic effect in the CRD model in rats | (Lindström et al., 2009) | |
| Ineffective in clinical trial in IBS | (Drossman et al., 2008) | ||
|
| |||
| 5-HT3 antagonist | Alosetron | Inhibits stress-induced visceral hyperalgesia in a rat model | (Bradesi et al., 2007) |
| Increases colonic compliance in IBS patients | (Delvaux et al., 1998) | ||
| No significant effect on gastrointestinal transit or rectal sensory and motor function in IBS | (Thumshirn et al., 2000) | ||
| Reduces symptom ratings, emotional stimulus ratings as well as regional blood flow in various limbic structure including the amygdala | (Mayer et al., 2002) | ||
|
| |||
| GABAB receptor agonist | Baclofen and CGP7930 (positive allosteric modulator) | Exert anxiolytic-like action on stress-related behavior and inhibit visceromotor response to CRD in rats | (Brusberg et al., 2009b) |
|
| |||
| GABA analogue | Gabapentin | Reduces rectal mechanosensitivity and increases rectal compliance in diarrhea-predominant IBS patients Reduces central sensitization? | (Lee et al., 2005) |
|
| |||
| α2δ ligand | Pregabalin | Reduces the viscerosomatic and autonomic responses associated with CRD-induced visceral pain and increased colonic compliance in rats | (Million et al., 2007a; Ravnefjord et al., 2008) |
| Reduces visceral sensation in hypersensitive IBS patients Reduces central sensitization? | (Houghton et al., 2007b) | ||
|
| |||
| 2,3-benzodiazepine receptor modulator | Dextofisopam | Reduces visceral pain in the CRD animal model but not in preliminary study in IBS patients | (Leventer et al., 2008) |
|
| |||
| α2-adrenergic receptor agonist | Clonidine | Inhibits visceromotor response to CRD in rats | (Brusberg et al., 2008) |
| Induces sedative and analgesic response during CRD in humans | (Bharucha et al., 1997b) | ||
|
| |||
| Nonselective CRF receptor antagonist | α-helical CRF | Reduces colonic distension-induced sensitivity in IBS patients | (Sagami et al., 2004) |
| Astressin | Attenuates post-inflammatory visceral hypersensitivity | (La et al 2008) | |
|
| |||
| CRF1 receptor antagonist | Antalarmin NBI 35965 JTC-017 CP-154,526 | Blocks stress-induced visceral sensitivity in rats | (Greenwood-Van Meerveld et al., 2005; Million et al., 2003; Million et al., 2005) |
|
| |||
| GW876008 | Not effective in IBS patients regarding global improvement and pain/discomfort | (Dukes et al., 2009) | |
|
| |||
| μ-opioid agonist | Fentanyl | Prevents the sensitizing response to repetitive CRD in mice | (Arvidsson et al., 2006) |
|
| |||
| κ1-opioid agonist | Fedotozine | Reduces visceral hypersensitivity in a model of colonic irritation Increases thresholds of perception during CRD in IBS patients | (Delvaux et al., 1999; Langlois et al., 1997) |
| Asimadoline | Not effective in reducing severity of abdominal pain in IBS, but has a tendency to reduce the anxiety score | (Szarka et al., 2007) | |
|
| |||
| Nociceptin receptor (ORL-1) agonist | Nociceptin/Orphanin FQ | Injected peripherally, reduces visceral hypersensitivity triggered by inflammation or stress in rats | (Agostini et al., 2009) |
|
| |||
| CB1 receptor agonist | SAB-378 | Inhibits pain-related responses to repetitive noxious CRD in rats | (Brusberg et al., 2009a) |
|
| |||
| CB1/CB2 receptor agonist | WIN55,212-2 | Inhibits pain-related responses to repetitive noxious CRD in rats | (Brusberg et al., 2009a; Hong et al., 2009) |
| Dronabinol | Failed to reduce visceral perception to CRD in healthy subjects and IBS patients | (Klooker et al., 2011) | |
|
| |||
| NK1 receptor antagonist | SR140333 | Diminishes the enhanced visceromotor reflex to CRD in rats | (Bradesi et al., 2006) |
| Ezlopitant (CJ-11974) | Reduces the emotional response to rectosigmoid distension but does not decrease rectal sensitivity in IBS patients | (Oh-Young et al., 2000) | |
|
| |||
| NK3 receptor antagonist | Talnetant | Reduces nociception associated with CRD and hypersensitivity induced by stress but not inflammation | (Fioramonti et al., 2003) |
| No benefit in clinical trial in IBS | (Houghton et al., 2007a) | ||
|
| |||
| CCK2 receptor antagonist | Cl-988 | Blocks anxiety-induced hyperalgesia in rats | (Rivat et al., 2010) |
|
| |||
| Somatostatin receptor agonist | Octreotide (sst2,3,5 agonist) | Exerts antinociceptive effect on responses to CRD at the central level in rats | (Su et al., 2001) |
| Normalizes visceral perception thresholds in IBS patients | (Schwetz et al., 2004b) | ||
| Failed to improve IBS symptoms in a long-term treatment | (Klooker et al., 2007) | ||
| sst2 agonist | Inhibits mesenteric afferents involved in nociceptive transmission and induce anxiolytic-like and antidepressant-like behavior effect in rats | (Booth et al., 2001; Engin and Treit, 2009) | |
|
| |||
| Melatonin | Melatonin | Decreases abdominal pain and improve overall IBS symptom scores in patients | (Mozaffari et al., 2010) |
| Exerts antinociceptive effect in a rat model of post-inflammatory visceral hyperalgesia via a centrally mediated process | (Mickle et al., 2010) | ||
| Neu-P11 (melatonin agonist) | Exerts antidepressant and anxiolytic activities in rodent models - effect on visceral sensitivity not studied yet | (Tian et al., 2010) | |
|
| |||
| Antibiotics | Rifaximin, neomycin | Inhibit stress-induced visceral hyperalgesia in animal models and alleviate symptoms in IBS patients modulating also stress-related behavioral changes via the brain-gut-enteric microbiota axis | (Pimentel et al., 2006; Pimentel et al., 2011b; Pimentel et al., 2011a) |
|
| |||
| Probiotics | VSL#3 Lactobacilus spp. Bifidobacterium spp. | (Collins et al., 2009a; McKernan et al., 2010; Messaoudi et al., 2011; Moayyedi et al., 2010; Verdu et al., 2006) | |
|
| |||
| Prebiotics | Enzyme-treated rice fiber Transgalactooligosaccharide | (Kanauchi et al., 2010; Silk et al., 2009) | |
As serotonin plays a critical role not only in stress-related alterations of gut motility, visceral sensitivity, and intestinal secretion (ligands of 5-HT3 and 5-HT4 receptors belong to the most effective treatments in IBS), but also in the pathophysiology of many extra-intestinal stress-related disorders frequently associated with IBS such as anxiety, depression or chronic pain syndromes (e.g., migraine headaches) and therefore points to the development of efficacious and safe serotonergic agents to alleviate IBS symptoms (De Ponti and Tonini, 2001; Maneerattanaporn et al., 2011). For example, buspirone, a partial 5-HT1 receptor agonist, with anxiolytic activity displays analgesic effect in the CRD-induced visceral pain model in rats (Sivarao et al., 2004). Similarly, robalzotan tartrate monohydrate (AZD7371), a selective competitive 5-HT1A receptor antagonist, initially developed as a potential treatment for depression and anxiety disorders significantly reduces the visceromotor response to CRD in rats (Lindström et al., 2009). However, the clinical development of AZD7371 has been discontinued due to severe adverse events including hallucinations, and the inability to demonstrate significant efficacy in IBS patients compared with placebo (Drossman et al., 2008). Recently, a new piperazinylpyridine derivative, TZB-20810 with mixed 5-HT1A agonistic and 5-HT3 antagonistic activities has been proposed as a promising drug in the treatment of IBS (Asagarasu et al., 2009).
Data from preclinical and clinical studies on triptans, 5-HT1B/D agonists used for migraine treatment revealed that, although they do not exert anxiolytic effect, can influence the perception of colorectal and gastric distension (Mulak and Paradowski, 2006; Tack et al., 2000; Vera-Portocarrero et al., 2008). Furthermore, based on experimental data, the role of 5-HT2B receptor antagonist (RS-127445), probably involved in the descending pain modulatory pathway, has been suggested as a potential therapeutic target in IBS (O'Mahony et al., 2010).
Apart from serotonergic agents, some other compounds including GABAB receptor agonist, baclofen and the positive allosteric modulator CGP7930 (Brusberg et al., 2009b) as well as the α2-receptor agonist, clonidine (Bharucha et al., 1997a; Brusberg et al., 2008), reveal sedative or anxiolytic-like action on stress-related behavior along with analgesic effect on visceral pain. Preliminary data suggest that anxiolytic activity of γ-aminobutyric acid-ergic agent (gabapentin) and α2δ ligand (pregabalin) may be also efficient in reducing central sensitization in hyperalgesia (Camilleri and Andresen, 2009) as shown in experimental model (Million et al., 2007a). New centrally acting agents providing analgesic effects include dextofisopam (2,3-benzodoazepine receptor modulator) and quetiapine (atypical antipsychotic agent ameliorating anxiety, sleep disturbances and augmenting the effect of antidepressants) (Camilleri, 2010).
The central as well as peripheral role of CRF1 receptors in stress-related visceral pain response has been intensively studied over the past decade based on convergent preclinical data and phase I clinical studies showing blockade of acute and chronic stress-related visceral hyperalgesia using selective small molecule CRF1 antagonists that cross the blood brain barrier such as antalarmin, NBI 35965, JTC-017, CP-154,526 or GW876008 (Martinez and Taché, 2006; Taché and Brunnhuber, 2008)(Table 1). However, despite encouraging data from preclinical animal models of IBS, recently published results from a randomized, double blind, placebo controlled, crossover study did not confirm the effectiveness of GW876008 in IBS patients regarding the global improvement scale, daily selfassessment of pain/discomfort or individual lower gastrointestinal symptoms (Dukes et al., 2009). Currently, there are several CRF1 receptor antagonists undergoing clinical trials (Phase II/III) for depression (GSK561679, GW876008 and pexacerfont), IBS and anxiety (GSK561679 and GW876008) (Zorrilla and Koob, 2010). The results of these trials should provide definitive conclusion as to whether this is still a viable target.
Growing evidence has accumulated suggesting that the endogenous cannabinoid system is also an important signaling system playing a key role in mediating and/or modulating behavioral, neurochemical, neuroendocrine, neuroimmune and molecular responses to stress (Finn, 2010). Activation of cannabinoid receptors (CB1, CB2, and G protein-coupled receptor GPR55) induces analgesic effect in several experimental models, including visceral pain originating from the gastrointestinal tract (Izzo and Sharkey, 2010; Sanson et al., 2006). The allodynic and hyperalgesic responses induced by a selective CB1 receptor antagonist suggest the existence of endogenous cannabinoid tone and the activation of CB1 receptors during noxious CRD in rodents (Brusberg et al., 2009a). However, in a recent clinical trial the mixed CB1/CB2 receptor agonist delta-9-tetrahydrocannabinol (dronabinol) has failed to reduce visceral perception to rectal distension in healthy volunteers and IBS patients (Klooker et al., 2011). These initial data dampen the potential use of centrally acting cannabinoid agonists as therapeutic agents to treat IBS.
While opioids may also exert antidepressant- and anxiolytic-like effects, their clinical use for gastrointestinal conditions has been limited by CNS side effects. A new generation of peripheral opioid receptor ligands free of these side effects has been developed (Healy, 2009; Moultry et al., 2011). Interestingly, in a recent study an on-demand dosing schedule of asimadoline, a peripheral kappaopioid agonist, was not effective in reducing the severity of abdominal pain in IBS, but modestly reduced the anxiety score (p = .053) (Szarka et al., 2007).
Tachykinin neuropeptides including SP, neurokinin A, neurokinin B and their receptors (NK1, NK2, NK3) localized in brain areas known to be implicated in stress-mechanisms, mood/anxiety regulation, emotion-processing and pain modulation and also modulating the CRF system may present another potential therapeutic targets in stress-related disorders such as IBS (Ebner et al., 2009; Frisch et al., 2010). However, although in a pilot study in IBS patients, the NK1 receptor antagonist, ezlopitant, reduced the emotional response to rectosigmoid distension, it did not significantly decreased rectal sensitivity (Oh-Young et al., 2000).
Recent studies have pointed to CCK as being involved in stress-induced sensory hypersensitivity, through neuroinflammation in the dorsal horn of the spinal cord (Rivat et al., 2007). Whether these preclinical observations will translate as potential good therapeutic candidate in the context of anxietyinduced hyperalgesia remains to be established. The potential role of other peptide signaling pathways such as somatostatin or melatonin in the modulation of visceral pain in the context of their anxiolytic and antidepressant properties requires further studies (see Table 1).
The better understanding of a recently developed concept of the brain-gut-enteric microbiota axis and the critical interdependence between the composition and stability of the microbiota and sensorymotor function of the gut as well as stress-related behavioral changes indicate a novel approach for IBS with a use of probiotics, prebiotics, and antibiotics (Collins et al., 2009b; Rhee et al., 2009). Whether the uprising new line of investigations related to specific modulation of the enteric microbiota will open new promising strategy for stress-related disorders, in particular co-morbid aspects of functional gastrointestinal disorders such as IBS (Cryan and O'Mahony, 2011) is still to be defined.
Figure 1. Putative mechanisms involved in exteroceptive and interoceptive stress-induced visceral hyperalgesia.
Exteroceptive stress when perceived by the CNS, induces the activation of the paraventricular nucleus of the hypothalamus and the release of CRF centrally leading to the activation of the pituitary-adrenal axis and the release of corticosterone and catecholamines, and peripherally stimulating the ENS to release peptides (SP, CRF). These peptides stimulate mast cells degranulation and release of preformed and newly synthesized mediators (tryptase, histamine, NGF, prostaglandins, cytokines, serotonin) leading to T-cell activation and increased colonic paracellular permeability. This in turn, increases the transfer of bacteria and antigens into the mucosa, further activating mast cells and T-cells, with an increased release of inflammatory mediators that sensitize the afferent fibers to mechanical stimuli leading to the development of visceral hypersensitivity. Interoceptive stressors can induce the release of inflammatory mediators by epithelial cells (ATP, bradykinin, prostaglandins) which can directly sensitize afferent fibers or induce the additional release of algogenic substances (histamine, SP, NGF, serotonin). Sensitization of afferent nerves can lead to long term alterations in the spinal cord and hyperactivation of spinal glial cells, which once the peripheral insult or stressor is resolved can perpetuate the visceral hypersensitivity. ATP: adenosine triphosphate, CNS: central nervous system, CRF: corticotropin-releasing factor, DRG: dorsal root ganglia, ENS: enteric nervous system, IFN: interferon, NGF: nerve growth factor, PGE2: prostaglandin E2, SP: substance P.
Acknowledgements
This review is part of studies supported by the VA Research Career Scientist Award, NIH grants R01 DK-57238 and DK 33061 and P50 DK-64539 (YT). The authors thank Miss E. Hu for reviewing the manuscript.
Abbreviations
- ASICs
acid-sensing ion channels
- BDNF
brain-derived neutrophic factor
- ATP
adenosine triphosphate
- CB
cannabinoid
- CeA
central amygdala
- CNS
central nervous system
- CRD
colorectal distension
- CRF
corticotropin releasing factor
- CRF1
CRF receptor subtype 1
- CRF2
CRF receptor subtype 2
- DRG
dorsal root ganglia
- DSS
dextran sodium sulfate
- EMG
electromyography
- ENS
enteric nervous system
- ERK
extracellular signal-regulated kinases
- GABA
gamma-amino butyric acid
- GI
gastrointestinal
- GPR
G-protein coupled receptor
- 5-HT
5-hydroxytryptamine
- HPA
hypothalamic-pituitary-adrenal
- IBS
irritable bowel syndrome
- IFN
interferon
- NK
neurokinin
- NMDA
N-methyl-D-aspartate receptor
- P2X
purinergic receptor 2X
- PAR2
proteinase-activated receptor 2
- PGE2
prostaglandin E2
- PTSD
post-traumatic stress disorder
- SNRI
serotoninnoradrenaline reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
- SP
substance P
- TRPA
transient receptor potential ankyrin
- TRPV
transient receptor potential vanilloid
- VMR
visceromotor response
- WAS
water avoidance stress
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam B, Liebregts T, Gschossmann JM, Krippner C, Scholl F, Ruwe M, Holtmann G. Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain. 2006;123:179–186. doi: 10.1016/j.pain.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Adeyemo MA, Spiegel BM, Chang L. Meta-analysis: do irritable bowel syndrome symptoms vary between men and women? Aliment.Pharmacol.Ther. 2010;32:738–755. doi: 10.1111/j.1365-2036.2010.04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini S, Eutamène H, Broccardo M, Improta G, Petrella C, Theodorou V, Buéno L. Peripheral anti-nociceptive effect of nociceptin/orphanin FQ in inflammation and stress-induced colonic hyperalgesia in rats. Pain. 2009;141:292–299. doi: 10.1016/j.pain.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Bradesi S, Fioramonti J, Theodorou V, Buéno L. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain. 2005;113:141–147. doi: 10.1016/j.pain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J.Neurophysiol. 1996;76:2661–2674. doi: 10.1152/jn.1996.76.4.2661. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Affaitati G, Ceccarelli I, Fiorenzani P, Lerza R, Rossi C, Pace MC, Chiefari M, Aurilio C, Giamberardino MA. Estradiol and testosterone differently affect visceral pain-related behavioural responses in male and female rats. Eur.J.Pain. 2010;14:602–607. doi: 10.1016/j.ejpain.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Anand P, Aziz Q, Willert R, van OL. Peripheral and central mechanisms of visceral sensitization in man. Neurogastroenterol.Motil. 2007;19:29–46. doi: 10.1111/j.1365-2982.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- Arvidsson S, Larsson M, Larsson H, Lindström E, Martinez V. Assessment of visceral pain-related pseudo-affective responses to colorectal distension in mice by intracolonic manometric recordings. J.Pain. 2006;7:108–118. doi: 10.1016/j.jpain.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Asagarasu A, Matsui T, Hayashi H, Tamaoki S, Yamauchi Y, Sato M. Design and synthesis of piperazinylpyridine derivatives as novel 5-HT1A agonists/5-HT3 antagonists for the treatment of irritable bowel syndrome (IBS) Chem.Pharm.Bull.(Tokyo) 2009;57:34–42. doi: 10.1248/cpb.57.34. [DOI] [PubMed] [Google Scholar]
- Azpiroz F. Gastrointestinal perception: pathophysiological implications. Neurogastroenterol.Motil. 2002;14:229–239. doi: 10.1046/j.1365-2982.2002.00324.x. [DOI] [PubMed] [Google Scholar]
- Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, Spiller RC. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol.Motil. 2007;19:62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- Babygirija R, Zheng J, Ludwig K, Takahashi T. Central oxytocin is involved in restoring impaired gastric motility following chronic repeated stress in mice. Am.J.Physiol Regul.Integr.Comp Physiol. 2010;298:R157–R165. doi: 10.1152/ajpregu.00328.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav.Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect.Immun. 2010;78:1509–1519. doi: 10.1128/IAI.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi VP, Kalin NH. Corticotropin-releasing hormone and animal models of anxiety: geneenvironment interactions. Biol.Psychiatry. 2000;48:1175–1198. doi: 10.1016/s0006-3223(00)01082-9. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu.Rev.Pharmacol.Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Barbara G, Wang B, Stanghellini V, De GR, Cremon C, Di NG, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Barreau F, Cartier C, Ferrier L, Fioramonti J, Buéno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. 2004;127:524–534. doi: 10.1053/j.gastro.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Barreau F, Cartier C, Levêque M, Ferrier L, Moriez R, Laroute V, Rosztoczy A, Fioramonti J, Buéno L. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J.Physiol. 2007a;580:347–356. doi: 10.1113/jphysiol.2006.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Buéno L. New insights in the etiology and pathophysiology of irritable bowel syndrome: contribution of neonatal stress models. Pediatr.Res. 2007b;62:240–245. doi: 10.1203/PDR.0b013e3180db2949. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett EJ, Tennant CC, Piesse C, Badcock CA, Kellow JE. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut. 1998;43:256–261. doi: 10.1136/gut.43.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P. The microbiota-gut-brain axis: learning from intestinal bacteria? Gut. 2011;60:288–289. doi: 10.1136/gut.2010.226779. [DOI] [PubMed] [Google Scholar]
- Bercik P, Wang L, Verdu EF, Mao YK, Blennerhassett P, Khan WI, Kean I, Tougas G, Collins SM. Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology. 2004;127:179–187. doi: 10.1053/j.gastro.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J.Neurosci. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha AE, Camilleri M, Zinsmeister AR, Hanson RB. Adrenergic modulation of human colonic motor and sensory function. Am.J.Physiol. 1997a;273:G997–1006. doi: 10.1152/ajpgi.1997.273.5.G997. [DOI] [PubMed] [Google Scholar]
- Bharucha AE, Novak V, Camilleri M, Zinsmeister AR, Hanson RB, Low PA. Alpha 2-adrenergic modulation of colonic tone during hyperventilation. Am.J.Physiol. 1997b;273:G1135–G1140. doi: 10.1152/ajpgi.1997.273.5.G1135. [DOI] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol.Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black LV, Ness TJ, Robbins MT. Effects of oxytocin and prolactin on stress-induced bladder hypersensitivity in female rats. J.Pain. 2009;10:1065–1072. doi: 10.1016/j.jpain.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol.Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Lackner JM, Jaccard J, Rowell D, Carosella AM, Powell C, Sanders K, Krasner S, Kuhn E. The role of stress in symptom exacerbation among IBS patients. J.Psychosom.Res. 2008;64:119–128. doi: 10.1016/j.jpsychores.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Lackner JM, Sanders K, Krasner S, Keefer L, Payne A, Gudleski GD, Katz L, Rowell D, Sykes M, Kuhn E, Gusmano R, Carosella AM, Firth R, Dulgar-Tulloch L. A controlled evaluation of group cognitive therapy in the treatment of irritable bowel syndrome. Behav.Res.Ther. 2007;45:633–648. doi: 10.1016/j.brat.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Taché Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res. 1994;641:21–28. doi: 10.1016/0006-8993(94)91810-4. [DOI] [PubMed] [Google Scholar]
- Booth CE, Kirkup AJ, Hicks GA, Humphrey PP, Grundy D. Somatostatin sst(2) receptor-mediated inhibition of mesenteric afferent nerves of the jejunum in the anesthetized rat. Gastroenterology. 2001;121:358–369. doi: 10.1053/gast.2001.26335. [DOI] [PubMed] [Google Scholar]
- Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganière M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–1777. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- Boyce PM, Talley NJ, Balaam B, Koloski NA, Truman G. A randomized controlled trial of cognitive behavior therapy, relaxation training, and routine clinical care for the irritable bowel syndrome. Am.J.Gastroenterol. 2003;98:2209–2218. doi: 10.1111/j.1572-0241.2003.07716.x. [DOI] [PubMed] [Google Scholar]
- Bradesi S. Role of spinal cord glia in the central processing of peripheral pain perception. Neurogastroenterol.Motil. 2010;22:499–511. doi: 10.1111/j.1365-2982.2010.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradesi S, Kokkotou E, Simeonidis S, Patierno S, Ennes HS, Mittal Y, McRoberts JA, Ohning G, McLean P, Marvizon JC, Sternini C, Pothoulakis C, Mayer EA. The role of neurokinin 1 receptors in the maintenance of visceral hyperalgesia induced by repeated stress in rats. Gastroenterology. 2006;130:1729–1742. doi: 10.1053/j.gastro.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Bradesi S, Lao L, McLean PG, Winchester WJ, Lee K, Hicks GA, Mayer EA. Dual role of 5-HT3 receptors in a rat model of delayed stress-induced visceral hyperalgesia. Pain. 2007;130:56–65. doi: 10.1016/j.pain.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Bradesi S, Mayer EA. Experimental models of stress and pain: do they help to develop new therapies? Dig.Dis. 2009;27(Suppl 1):55–67. doi: 10.1159/000268122. [DOI] [PubMed] [Google Scholar]
- Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am.J.Physiol Gastrointest.Liver Physiol. 2005;289:G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- Bradesi S, Svensson CI, Steinauer J, Pothoulakis C, Yaksh TL, Mayer EA. Role of spinal microglia activation in visceral hyperalgesia following chronic psychological stress in Wistar rats. Gastroenterology. 2009a;136:1339–1348. doi: 10.1053/j.gastro.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradesi S, Svensson CI, Steinauer J, Pothoulakis C, Yaksh TL, Mayer EA. Role of spinal microglia in visceral hyperalgesia and NK1R up-regulation in a rat model of chronic stress. Gastroenterology. 2009b;136:1339–2. doi: 10.1053/j.gastro.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusberg M, Arvidsson S, Kang D, Larsson H, Lindström E, Martinez V. CB1 receptors mediate the analgesic effects of cannabinoids on colorectal distension-induced visceral pain in rodents. J.Neurosci. 2009a;29:1554–1564. doi: 10.1523/JNEUROSCI.5166-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusberg M, Ravnefjord A, Lindgreen M, Larsson H, Lindström E, Martinez V. Oral clonidine inhibits visceral pain-related viscerosomatic and cardiovascular responses to colorectal distension in rats. Eur.J.Pharmacol. 2008;591:243–251. doi: 10.1016/j.ejphar.2008.06.056. [DOI] [PubMed] [Google Scholar]
- Brusberg M, Ravnefjord A, Martinsson R, Larsson H, Martinez V, Lindström E. The GABA(B) receptor agonist, baclofen, and the positive allosteric modulator, CGP7930, inhibit visceral pain-related responses to colorectal distension in rats. Neuropharmacology. 2009b;56:362–367. doi: 10.1016/j.neuropharm.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Burton MB, Gebhart GF. Effects of intracolonic acetic acid on responses to colorectal distension in the rat. Brain Res. 1995;672:77–82. doi: 10.1016/0006-8993(94)01382-r. [DOI] [PubMed] [Google Scholar]
- Busch-Dienstfertig M, Stein C. Opioid receptors and opioid peptide-producing leukocytes in inflammatory pain--basic and therapeutic aspects. Brain Behav.Immun. 2010;24:683–694. doi: 10.1016/j.bbi.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Butler RK, Finn DP. Stress-induced analgesia. Prog.Neurobiol. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Cameron DM, Brennan TJ, Gebhart GF. Hind paw incision in the rat produces long-lasting colon hypersensitivity. J.Pain. 2008;9:246–253. doi: 10.1016/j.jpain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Review article: new receptor targets for medical therapy in irritable bowel syndrome. Aliment.Pharmacol.Ther. 2010;31:35–46. doi: 10.1111/j.1365-2036.2009.04153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, Andresen V. Current and novel therapeutic options for irritable bowel syndrome management. Dig.Liver Dis. 2009;41:854–862. doi: 10.1016/j.dld.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage: An Account of Recent Researches into the Function of Emotional Excitement D. Appleton and company; New York and London: 1915. [Google Scholar]
- Caso JR, Leza JC, Menchen L. The effects of physical and psychological stress on the gastro-intestinal tract: lessons from animal models. Curr.Mol.Med. 2008;8:299–312. doi: 10.2174/156652408784533751. [DOI] [PubMed] [Google Scholar]
- Cattaruzza F, Spreadbury I, Miranda-Morales M, Grady EF, Vanner S, Bunnett NW. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am.J.Physiol Gastrointest.Liver Physiol. 2010;298:G81–G91. doi: 10.1152/ajpgi.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N, Altier C, Motta JP, d'Aldebert E, Galeano S, Zamponi GW, Vergnolle N. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut. 2010;59:481–488. doi: 10.1136/gut.2009.192567. [DOI] [PubMed] [Google Scholar]
- Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N. Role for protease activity in visceral pain in irritable bowel syndrome. J.Clin.Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F. Visceral versus somatic pain: similarities and differences. Dig.Dis. 2009;27(Suppl 1):3–10. doi: 10.1159/000268115. [DOI] [PubMed] [Google Scholar]
- Chang L. Brain responses to visceral and somatic stimuli in irritable bowel syndrome: a central nervous system disorder? Gastroenterol.Clin.North Am. 2005;34:271–279. doi: 10.1016/j.gtc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Chang L. Review article: epidemiology and quality of life in functional gastrointestinal disorders. Aliment.Pharmacol.Ther. 2004;20(Suppl 7):31–39. doi: 10.1111/j.1365-2036.2004.02183.x. [DOI] [PubMed] [Google Scholar]
- Chen XN, Zhu H, Meng QY, Zhou JN. Estrogen receptor-alpha and -beta regulate the human corticotropin-releasing hormone gene through similar pathways. Brain Res. 2008;1223:1–10. doi: 10.1016/j.brainres.2008.05.043. [DOI] [PubMed] [Google Scholar]
- Cheong E, Lee S, Choi BJ, Sun M, Lee CJ, Shin HS. Tuning thalamic firing modes via simultaneous modulation of T- and L-type Ca2+ channels controls pain sensory gating in the thalamus. J.Neurosci. 2008;28:13331–13340. doi: 10.1523/JNEUROSCI.3013-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chial HJ, Camilleri M, Ferber I, Delgado-Aros S, Burton D, McKinzie S, Zinsmeister AR. Effects of venlafaxine, buspirone, and placebo on colonic sensorimotor functions in healthy humans. Clin.Gastroenterol.Hepatol. 2003;1:211–218. doi: 10.1053/jcgh.2003.50031. [DOI] [PubMed] [Google Scholar]
- Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am.J.Gastroenterol. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choung RS, Locke GR, III, Zinsmeister AR, Schleck CD, Talley NJ. Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: a psychological component is the rule. Am.J.Gastroenterol. 2009;104:1772–1779. doi: 10.1038/ajg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nat.Protoc. 2007;2:2624–2631. doi: 10.1038/nprot.2007.392. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat.Rev.Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Chung EK, Bian ZX, Xu HX, Sung JJ. Neonatal maternal separation increases brain-derived neurotrophic factor and tyrosine kinase receptor B expression in the descending pain modulatory system. Neurosignals. 2009;17:213–221. doi: 10.1159/000224631. [DOI] [PubMed] [Google Scholar]
- Chung EK, Zhang XJ, Xu HX, Sung JJ, Bian ZX. Visceral hyperalgesia induced by neonatal maternal separation is associated with nerve growth factor-mediated central neuronal plasticity in rat spinal cord. Neuroscience. 2007;149:685–695. doi: 10.1016/j.neuroscience.2007.07.055. [DOI] [PubMed] [Google Scholar]
- Coffin B, Bouhassira D, Sabate JM, Barbe L, Jian R. Alteration of the spinal modulation of nociceptive processing in patients with irritable bowel syndrome. Gut. 2004;53:1465–1470. doi: 10.1136/gut.2003.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Jotkowitz A, Buskila D, Pelles-Avraham S, Kaplan Z, Neumann L, Sperber AD. Post-traumatic stress disorder and other co-morbidities in a sample population of patients with irritable bowel syndrome. Eur.J.Intern.Med. 2006;17:567–571. doi: 10.1016/j.ejim.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Collins S, Verdu E, Denou E, Bercik P. The role of pathogenic microbes and commensal bacteria in irritable bowel syndrome. Dig.Dis. 2009a;27(Suppl 1):85–89. doi: 10.1159/000268126. [DOI] [PubMed] [Google Scholar]
- Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- Collins SM, Denou E, Verdu EF, Bercik P. The putative role of the intestinal microbiota in the irritable bowel syndrome. Dig.Liver Dis. 2009b;41:850–853. doi: 10.1016/j.dld.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Collins SM, McHugh K, Jacobson K, Khan I, Riddell R, Murase K, Weingarten HP. Previous inflammation alters the response of the rat colon to stress. Gastroenterology. 1996;111:1509–1515. doi: 10.1016/s0016-5085(96)70012-4. [DOI] [PubMed] [Google Scholar]
- Collins SM, Vallance B, Barbara G, Borgaonkar M. Putative inflammatory and immunological mechanisms in functional bowel disorders. Baillieres Best.Pract.Res.Clin.Gastroenterol. 1999;13:429–436. doi: 10.1053/bega.1999.0037. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Meller ST, Gebhart GF. Intracolonic zymosan produces visceral hyperalgesia in the rat that is mediated by spinal NMDA and non-NMDA receptors. Brain Res. 1996;736:7–15. doi: 10.1016/0006-8993(96)00661-0. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am.J.Physiol Gastrointest.Liver Physiol. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- Cregg R, Momin A, Rugiero F, Wood JN, Zhao J. Pain channelopathies. J.Physiol. 2010;588:1897–1904. doi: 10.1113/jphysiol.2010.187807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz CD, Cruz F. The ERK 1 and 2 pathway in the nervous system: from basic aspects to possible clinical applications in pain and visceral dysfunction. Curr.Neuropharmacol. 2007;5:244–252. doi: 10.2174/157015907782793630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, O'Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol.Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Grigoriadis DE, Valentino RJ. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–550. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur.J.Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- De Ponti F, Tonini M. Irritable bowel syndrome: new agents targeting serotonin receptor subtypes. Drugs. 2001;61:317–332. doi: 10.2165/00003495-200161030-00001. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Grigoriadis DE. Corticotropin-Releasing Factor: Physiology, Pharmacology and Role in Central Nervous System Disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff CB, editors. Psychopharmacology: the Fifth Generation of Progress American College of Neuropsychopharmacology. 2002. [Google Scholar]
- Delic S, Streif S, Deussing JM, Weber P, Ueffing M, Holter SM, Wurst W, Kuhn R. Genetic mouse models for behavioral analysis through transgenic RNAi technology. Genes Brain Behav. 2008;7:821–830. doi: 10.1111/j.1601-183X.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- Delvaux M, Louvel D, Lagier E, Scherrer B, Abitbol JL, Frexinos J. The kappa agonist fedotozine relieves hypersensitivity to colonic distention in patients with irritable bowel syndrome. Gastroenterology. 1999;116:38–45. doi: 10.1016/s0016-5085(99)70226-x. [DOI] [PubMed] [Google Scholar]
- Delvaux M, Louvel D, Mamet JP, Campos-Oriola R, Frexinos J. Effect of alosetron on responses to colonic distension in patients with irritable bowel syndrome. Aliment.Pharmacol.Ther. 1998;12:849–855. doi: 10.1046/j.1365-2036.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- Demaude J, Salvador-Cartier C, Fioramonti J, Ferrier L, Buéno L. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut. 2006;55:655–661. doi: 10.1136/gut.2005.078675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SW. A systematic review of neuroimaging data during visceral stimulation. Am.J.Gastroenterol. 2003;98:12–20. doi: 10.1111/j.1572-0241.2003.07168.x. [DOI] [PubMed] [Google Scholar]
- Deussing JM, Wurst W. Dissecting the genetic effect of the CRH system on anxiety and stress-related behaviour. C.R.Biol. 2005;328:199–212. doi: 10.1016/j.crvi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Danilewitz M, Naesdal J, Hwang C, Adler J, Silberg DG. Randomized, double-blind, placebo-controlled trial of the 5-HT1A receptor antagonist AZD7371 tartrate monohydrate (robalzotan tartrate monohydrate) in patients with irritable bowel syndrome. Am.J.Gastroenterol. 2008;103:2562–2569. doi: 10.1111/j.1572-0241.2008.02115.x. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Leserman J, Nachman G, Li ZM, Gluck H, Toomey TC, Mitchell CM. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann.Intern.Med. 1990;113:828–833. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, Emmott S, Proffitt V, Akman D, Frusciante K, Le T, Meyer K, Bradshaw B, Mikula K, Morris CB, Blackman CJ, Hu Y, Jia H, Li JZ, Koch GG, Bangdiwala SI. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- Dufton LM, Konik B, Colletti R, Stanger C, Boyer M, Morrow S, Compas BE. Effects of stress on pain threshold and tolerance in children with recurrent abdominal pain. Pain. 2008;136:38–43. doi: 10.1016/j.pain.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Dukes GE, Mayer EA, Kelleher DL, Hicks KJ, Boardley RL, Alpers DH. A randomized, double blind, placebo (PLA) controlled, crossover study to evaluate the efficacy and safety of the corticotropin releasing factor 1 (CRF1) receptor antagonist (RA) GW876008 in irritable bowel syndrome (IBS) patients (Pts) Neurogastroenterol. Motil. 2009;21:84. abstract. [Google Scholar]
- Ebner K, Sartori SB, Singewald N. Tachykinin receptors as therapeutic targets in stress-related disorders. Curr.Pharm.Des. 2009;15:1647–1674. doi: 10.2174/138161209788168074. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S. Abdominal Pain in Irritable Bowel Syndrome: A Review of Putative Psychological, Neural and Neuro-Immune Mechanisms. Brain Behav.Immun. 2011;25:386–394. doi: 10.1016/j.bbi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010a;139:1310–1319. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2010b;59:489–495. doi: 10.1136/gut.2008.175000. [DOI] [PubMed] [Google Scholar]
- Enck P, Merlin V, Erckenbrecht JF, Wienbeck M. Stress effects on gastrointestinal transit in the rat. Gut. 1989;30:455–459. doi: 10.1136/gut.30.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Treit D. Anxiolytic and antidepressant actions of somatostatin: the role of sst2 and sst3 receptors. Psychopharmacology (Berl) 2009;206:281–289. doi: 10.1007/s00213-009-1605-5. [DOI] [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J. The excitatory effects of the amygdala on hypothalamo-pituitary-adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain Res.Bull. 1998;45:389–393. doi: 10.1016/s0361-9230(97)00384-5. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: a review of recent clinical and experimental findings. J.Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DP. Endocannabinoid-mediated modulation of stress responses: physiological and pathophysiological significance. Immunobiology. 2010;215:629–646. doi: 10.1016/j.imbio.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Fioramonti J, Gaultier E, Toulouse M, Sanger GJ, Buéno L. Intestinal anti-nociceptive behaviour of NK3 receptor antagonism in conscious rats: evidence to support a peripheral mechanism of action. Neurogastroenterol.Motil. 2003;15:363–369. doi: 10.1046/j.1365-2982.2003.00420.x. [DOI] [PubMed] [Google Scholar]
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic programming of stress responses through variations in maternal care. Ann.N.Y.Acad.Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- Folks DG. The interface of psychiatry and irritable bowel syndrome. Curr.Psychiatry Rep. 2004;6:210–215. doi: 10.1007/s11920-004-0066-0. [DOI] [PubMed] [Google Scholar]
- Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58:367–378. doi: 10.1136/gut.2008.163162. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Irwin MR. A role for CRH and the sympathetic nervous system in stress-induced immunosuppression. Ann.N.Y.Acad.Sci. 1995;771:396–418. doi: 10.1111/j.1749-6632.1995.tb44698.x. [DOI] [PubMed] [Google Scholar]
- Friedrich AE, Gebhart GF. Effects of spinal cholecystokinin receptor antagonists on morphine antinociception in a model of visceral pain in the rat. J.Pharmacol.Exp.Ther. 2000;292:538–544. [PubMed] [Google Scholar]
- Friedrich AE, Gebhart GF. Modulation of visceral hyperalgesia by morphine and cholecystokinin from the rat rostroventral medial medulla. Pain. 2003;104:93–101. doi: 10.1016/s0304-3959(02)00469-4. [DOI] [PubMed] [Google Scholar]
- Frisch P, Bilkei-Gorzo A, Racz I, Zimmer A. Modulation of the CRH system by substance P/NKA in an animal model of depression. Behav.Brain Res. 2010;213:103–108. doi: 10.1016/j.bbr.2010.04.044. [DOI] [PubMed] [Google Scholar]
- Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J.Gastroenterol. 2007;42(Suppl 17):48–51. doi: 10.1007/s00535-006-1942-7. [DOI] [PubMed] [Google Scholar]
- Gaudreau GA, Plourde V. Role of tachykinin NK1, NK2 and NK3 receptors in the modulation of visceral hypersensitivity in the rat. Neurosci.Lett. 2003;351:59–62. doi: 10.1016/s0304-3940(03)00414-2. [DOI] [PubMed] [Google Scholar]
- Gibney SM, Gosselin RD, Dinan TG, Cryan JF. Colorectal distension-induced prefrontal cortex activation in the Wistar-Kyoto rat: implications for irritable bowel syndrome. Neuroscience. 2010;165:675–683. doi: 10.1016/j.neuroscience.2009.08.076. [DOI] [PubMed] [Google Scholar]
- Gold MS, Zhang L, Wrigley DL, Traub RJ. Prostaglandin E(2) modulates TTX-R I(Na) in rat colonic sensory neurons. J.Neurophysiol. 2002;88:1512–1522. doi: 10.1152/jn.2002.88.3.1512. [DOI] [PubMed] [Google Scholar]
- Gosselin RD, O'Connor RM, Tramullas M, Julio-Pieper M, Dinan TG, Cryan JF. Riluzole normalizes early-life stress-induced visceral hypersensitivity in rats: role of spinal glutamate reuptake mechanisms. Gastroenterology. 2010;138:2418–2425. doi: 10.1053/j.gastro.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res. 2001;893:135–142. doi: 10.1016/s0006-8993(00)03305-9. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Gibson MS, Johnson AC, Venkova K, Sutkowski-Markmann D. NK1 receptor-mediated mechanisms regulate colonic hypersensitivity in the guinea pig. Pharmacol.Biochem.Behav. 2003;74:1005–1013. doi: 10.1016/s0091-3057(03)00032-7. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Johnson AC, Cochrane S, Schulkin J, Myers DA. Corticotropin-releasing factor 1 receptor-mediated mechanisms inhibit colonic hypersensitivity in rats. Neurogastroenterol.Motil. 2005;17:415–422. doi: 10.1111/j.1365-2982.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Griffiths J, Shanks N, Anisman H. Strain-specific alterations in consumption of a palatable diet following repeated stressor exposure. Pharmacol.Biochem.Behav. 1992;42:219–227. doi: 10.1016/0091-3057(92)90519-l. [DOI] [PubMed] [Google Scholar]
- Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51(Suppl 1):i2–i5. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschossmann JM, Liebregts T, Adam B, Buenger L, Ruwe M, Gerken G, Holtmann G. Long-term effects of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Dig.Dis.Sci. 2004;49:96–101. doi: 10.1023/b:ddas.0000011609.68882.3a. [DOI] [PubMed] [Google Scholar]
- Gué M, Del Rio-Lachèze C, Eutamène H, Theodorou V, Fioramonti J, Buéno L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol.Motil. 1997;9:271–279. doi: 10.1046/j.1365-2982.1997.d01-63.x. [DOI] [PubMed] [Google Scholar]
- Gui X, Carraway RE, Dobner PR. Endogenous neurotensin facilitates visceral nociception and is required for stress-induced antinociception in mice and rats. Neuroscience. 2004;126:1023–1032. doi: 10.1016/j.neuroscience.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Gunter WD, Shepard JD, Foreman RD, Myers DA, Greenwood-Van Meerveld B. Evidence for visceral hypersensitivity in high-anxiety rats. Physiol Behav. 2000;69:379–382. doi: 10.1016/s0031-9384(99)00254-1. [DOI] [PubMed] [Google Scholar]
- Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, Underwood JE, Read NW. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol.Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- Healy R. Effectiveness of two opioid antagonists in treating opioid-induced constipation. Br.J.Nurs. 2009;18:998–1002. doi: 10.12968/bjon.2009.18.16.43969. [DOI] [PubMed] [Google Scholar]
- Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc.Natl.Acad.Sci.U.S.A. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res.Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkemper MM, Chang L. Do fluctuations in ovarian hormones affect gastrointestinal symptoms in women with irritable bowel syndrome? Gend.Med. 2009;6(Suppl 2):152–167. doi: 10.1016/j.genm.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Holdcroft A, Sapsed-Byrne S, Ma D, Hammal D, Forsling ML. Sex and oestrous cycle differences in visceromotor responses and vasopressin release in response to colonic distension in male and female rats anaesthetized with halothane. Br.J.Anaesth. 2000;85:907–910. doi: 10.1093/bja/85.6.907. [DOI] [PubMed] [Google Scholar]
- Holschneider DP, Bradesi S, Mayer EA. The role of experimental models in developing new treatments for irritable bowel syndrome. Expert.Rev.Gastroenterol.Hepatol. 2011;5:43–57. doi: 10.1586/egh.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Gastrointestinal afferents as targets of novel drugs for the treatment of functional bowel disorders and visceral pain. Eur.J.Pharmacol. 2001;429:177–193. doi: 10.1016/s0014-2999(01)01319-x. [DOI] [PubMed] [Google Scholar]
- Hong S, Fan J, Kemmerer ES, Evans S, Li Y, Wiley JW. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut. 2009;58:202–210. doi: 10.1136/gut.2008.157594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton LA, Cremonini F, Camilleri M, Busciglio I, Fell C, Cox V, Alpers DH, Dewit OE, Dukes GE, Gray E, Lea R, Zinsmeister AR, Whorwell PJ. Effect of the NK(3) receptor antagonist, talnetant, on rectal sensory function and compliance in healthy humans. Neurogastroenterol.Motil. 2007a;19:732–743. doi: 10.1111/j.1365-2982.2007.00934.x. [DOI] [PubMed] [Google Scholar]
- Houghton LA, Fell C, Whorwell PJ, Jones I, Sudworth DP, Gale JD. Effect of a second-generation alpha2delta ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut. 2007b;56:1218–1225. doi: 10.1136/gut.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin C, Falsetti SA, Lydiard RB, Ballenger JC, Brock CD, Brener W. Comorbidity of posttraumatic stress disorder and irritable bowel syndrome. J.Clin.Psychiatry. 1996;57:576–578. doi: 10.4088/jcp.v57n1204. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Sharkey KA. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol.Ther. 2010;126:21–38. doi: 10.1016/j.pharmthera.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Jarrett ME, Burr RL, Cain KC, Hertig V, Weisman P, Heitkemper MM. Anxiety and depression are related to autonomic nervous system function in women with irritable bowel syndrome. Dig.Dis.Sci. 2003;48:386–394. doi: 10.1023/a:1021904216312. [DOI] [PubMed] [Google Scholar]
- Ji Y, Tang B, Traub RJ. Modulatory effects of estrogen and progesterone on colorectal hyperalgesia in the rat. Pain. 2005;117:433–442. doi: 10.1016/j.pain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience. 2008;154:1562–1567. doi: 10.1016/j.neuroscience.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Myers B, Lazovic J, Towner R, Greenwood-Van Meerveld B. Brain activation in response to visceral stimulation in rats with amygdala implants of corticosterone: an FMRI study. PLoS.ONE. 2010;5:e8573. doi: 10.1371/journal.pone.0008573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RC, III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J.Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanauchi O, Mitsuyama K, Komiyama Y, Yagi M, Andoh A, Sata M. Preventive effects of enzyme-treated rice fiber in a restraint stress-induced irritable bowel syndrome model. Int.J.Mol.Med. 2010;25:547–555. doi: 10.3892/ijmm_00000376. [DOI] [PubMed] [Google Scholar]
- Kearney DJ, Brown-Chang J. Complementary and alternative medicine for IBS in adults: mind-body interventions. Nat.Clin.Pract.Gastroenterol.Hepatol. 2008;5:624–636. doi: 10.1038/ncpgasthep1257. [DOI] [PubMed] [Google Scholar]
- Keohane J, O'Mahony C, O'Mahony L, O'Mahony S, Quigley EM, Shanahan F. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? Am.J.Gastroenterol. 2010;105:1788, 1789–1788, 1794. doi: 10.1038/ajg.2010.156. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Dina OA, Green PG, Levine JD. Estrogen regulates adrenal medullary function producing sexual dimorphism in nociceptive threshold and beta-adrenergic receptor-mediated hyperalgesia in the rat. Eur.J.Neurosci. 2005;21:3379–3386. doi: 10.1111/j.1460-9568.2005.04158.x. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Dina OA, Green PG, Levine JD. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J.Pain. 2009;10:1073–1077. doi: 10.1016/j.jpain.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Han JE, Hwang S, Oh DH. The expression of corticotropin-releasing factor in the central nucleus of the amygdala, induced by colorectal distension, is attenuated by general anesthesia. J.Korean Med.Sci. 2010;25:1646–1651. doi: 10.3346/jkms.2010.25.11.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Muller-Preuss P, Lu A, Wiesner E, Flachskamm C, Wurst W, Holsboer F, Deussing JM. Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep. Mol.Psychiatry. 2010;15:154–165. doi: 10.1038/mp.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klooker TK, Kuiken SD, Lei A, Boeckxstaens GE. Effect of long-term treatment with octreotide on rectal sensitivity in patients with non-constipated irritable bowel syndrome. Aliment.Pharmacol.Ther. 2007;26:605–615. doi: 10.1111/j.1365-2036.2007.03398.x. [DOI] [PubMed] [Google Scholar]
- Klooker TK, Leliefeld KE, van den Wijngaard RM, Boeckxstaens GE. The cannabinoid receptor agonist delta-9-tetrahydrocannabinol does not affect visceral sensitivity to rectal distension in healthy volunteers and IBS patients. Neurogastroenterol.Motil. 2011;23:30–5. e2. doi: 10.1111/j.1365-2982.2010.01587.x. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Boyle MP, Wieczorek L, Kelley CL, Onwuzurike CC, Nettles SA, Vogt SK, Muglia LJ. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J.Neurosci. 2010;30:2571–2581. doi: 10.1523/JNEUROSCI.4470-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol.Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Kosoyan HP, Grigoriadis DE, Taché Y. The CRF(1) receptor antagonist, NBI-35965, abolished the activation of locus coeruleus neurons induced by colorectal distension and intracisternal CRF in rats. Brain Res. 2005;1056:85–96. doi: 10.1016/j.brainres.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Kuiken SD, Tytgat GN, Boeckxstaens GE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: a double blind, randomized, placebo-controlled study. Clin.Gastroenterol.Hepatol. 2003;1:219–228. doi: 10.1053/cgh.2003.50032. [DOI] [PubMed] [Google Scholar]
- Kuner R. Central mechanisms of pathological pain. Nat.Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- La JH, Sung TS, Kim HJ, Kim TW, Kang TM, Yang IS. Peripheral corticotropin releasing hormone mediates post-inflammatory visceral hypersensitivity in rats. World J.Gastroenterol. 2008;14:731–736. doi: 10.3748/wjg.14.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JM, Brasel AM, Quigley BM, Keefer L, Krasner SS, Powell C, Katz LA, Sitrin MD. The ties that bind: perceived social support, stress, and IBS in severely affected patients. Neurogastroenterol.Motil. 2010;22:893–900. doi: 10.1111/j.1365-2982.2010.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladabaum U, Minoshima S, Owyang C. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications V. Central nervous system processing of somatic and visceral sensory signals. Am.J.Physiol Gastrointest.Liver Physiol. 2000;279:G1–G6. doi: 10.1152/ajpgi.2000.279.1.G1. [DOI] [PubMed] [Google Scholar]
- Langlois A, Diop L, Friese N, Pascaud X, Junien JL, Dahl SG, Rivière PJ. Fedotozine blocks hypersensitive visceral pain in conscious rats: action at peripheral kappa-opioid receptors. Eur.J.Pharmacol. 1997;324:211–217. doi: 10.1016/s0014-2999(97)00089-7. [DOI] [PubMed] [Google Scholar]
- Larauche M, Bradesi S, Million M, McLean P, Taché Y, Mayer EA, McRoberts JA. Corticotropin-releasing factor type 1 receptors mediate the visceral hyperalgesia induced by repeated psychological stress in rats. Am.J.Physiol Gastrointest.Liver Physiol. 2008;294:G1033–G1040. doi: 10.1152/ajpgi.00507.2007. [DOI] [PubMed] [Google Scholar]
- Larauche M, Gourcerol G, Million M, Adelson DW, Taché Y. Repeated psychological stress-induced alterations of visceral sensitivity and colonic motor functions in mice: Influence of surgery and postoperative single housing on visceromotor responses. Stress. 2010a;13:343–354. doi: 10.3109/10253891003664166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larauche M, Gourcerol G, Wang L, Pambukchian K, Brunnhuber S, Adelson DW, Rivier J, Million M, Taché Y. Cortagine, a CRF1 agonist, induces stresslike alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am.J.Physiol Gastrointest.Liver Physiol. 2009a;297:G215–G227. doi: 10.1152/ajpgi.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larauche M, Kiank C, Taché Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J.Physiol Pharmacol. 2009b;60(Suppl 7):33–46. [PMC free article] [PubMed] [Google Scholar]
- Larauche M, Mulak A, Kim YS, Million M, Taché Y. Sex differences in visceral sensitivity induced by repeated psychological stress in rats: differential role of opioid pathway. Gut. 2010b;59:A104. [Google Scholar]
- Larsson M, Arvidsson S, Ekman C, Bayati A. A model for chronic quantitative studies of colorectal sensitivity using balloon distension in conscious mice -- effects of opioid receptor agonists. Neurogastroenterol.Motil. 2003;15:371–381. doi: 10.1046/j.1365-2982.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- Larsson MH, Miketa A, Martinez V. Lack of interaction between psychological stress and DSS-induced colitis affecting colonic sensitivity during colorectal distension in mice. Stress. 2009;12:434–444. doi: 10.1080/10253890802626603. [DOI] [PubMed] [Google Scholar]
- Larsson MH, Rapp L, Lindström E. Effect of DSS-induced colitis on visceral sensitivity to colorectal distension in mice. Neurogastroenterol.Motil. 2006;18:144–152. doi: 10.1111/j.1365-2982.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- Lechner SM, Curtis AL, Brons R, Valentino RJ. Locus coeruleus activation by colon distention: role of corticotropin-releasing factor and excitatory amino acids. Brain Res. 1997;756:114–124. doi: 10.1016/s0006-8993(97)00116-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J.Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Kim JH, Cho SW. Gabapentin reduces rectal mechanosensitivity and increases rectal compliance in patients with diarrhoea-predominant irritable bowel syndrome. Aliment.Pharmacol.Ther. 2005;22:981–988. doi: 10.1111/j.1365-2036.2005.02685.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Wu J, Ma YL, Tsang A, Guo WJ, Sung J. Irritable bowel syndrome is strongly associated with generalized anxiety disorder: a community study. Aliment.Pharmacol.Ther. 2009;30:643–651. doi: 10.1111/j.1365-2036.2009.04074.x. [DOI] [PubMed] [Google Scholar]
- Lembo T, Plourde V, Shui Z, Fullerton S, Mertz H, Taché Y, Sytnik B, Munakata J, Mayer E. Effects of the corticotropin-releasing factor (CRF) on rectal afferent nerves in humans. Neurogastroenterol.Motil. 1996;8:9–18. doi: 10.1111/j.1365-2982.1996.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Leserman J, Drossman DA. Relationship of abuse history to functional gastrointestinal disorders and symptoms: some possible mediating mechanisms. Trauma Violence Abuse. 2007;8:331–343. doi: 10.1177/1524838007303240. [DOI] [PubMed] [Google Scholar]
- Leventer SM, Raudibaugh K, Frissora CL, Kassem N, Keogh JC, Phillips J, Mangel AW. Clinical trial: dextofisopam in the treatment of patients with diarrhoea-predominant or alternating irritable bowel syndrome. Aliment.Pharmacol.Ther. 2008;27:197–206. doi: 10.1111/j.1365-2036.2007.03566.x. [DOI] [PubMed] [Google Scholar]
- Liaw WJ, Stephens RL, Jr., Binns BC, Chu Y, Sepkuty JP, Johns RA, Rothstein JD, Tao YX. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain. 2005;115:60–70. doi: 10.1016/j.pain.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Liebregts T, Adam B, Bertel A, Lackner C, Neumann J, Talley NJ, Gerken G, Holtmann G. Psychological stress and the severity of post-inflammatory visceral hyperalgesia. Eur.J.Pain. 2007;11:216–222. doi: 10.1016/j.ejpain.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73–82. doi: 10.1016/s0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- Lin Y, Tian G, Roman K, Handy C, Travers JB, Lin CL, Stephens RL., Jr. Increased glial glutamate transporter EAAT2 expression reduces visceral nociceptive response in mice. Am.J.Physiol Gastrointest.Liver Physiol. 2009;296:G129–G134. doi: 10.1152/ajpgi.90556.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström E, Ravnefjord A, Brusberg M, Hjorth S, Larsson H, Martinez V. The selective 5-hydroxytryptamine 1A antagonist, AZD7371 [3(R)-(N,N-dicyclobutylamino)-8-fluoro-3,4-dihydro-2H-1-benzopyran-5-carboxamide (R,R)-tartrate monohydrate] (robalzotan tartrate monohydrate), inhibits visceral pain-related visceromotor, but not autonomic cardiovascular, responses to colorectal distension in rats. J.Pharmacol.Exp.Ther. 2009;329:1048–1055. doi: 10.1124/jpet.109.152330. [DOI] [PubMed] [Google Scholar]
- Linthorst AC. Interactions between corticotropin-releasing hormone and serotonin: implications for the aetiology and treatment of anxiety disorders. Handb.Exp.Pharmacol. 2005:181–204. doi: 10.1007/3-540-28082-0_7. [DOI] [PubMed] [Google Scholar]
- Long MD, Drossman DA. Inflammatory bowel disease, irritable bowel syndrome, or what?: A challenge to the functional-organic dichotomy. Am.J.Gastroenterol. 2010;105:1796–1798. doi: 10.1038/ajg.2010.162. [DOI] [PubMed] [Google Scholar]
- Long Y, Liu Y, Tong J, Qian W, Hou X. Effectiveness of trimebutine maleate on modulating intestinal hypercontractility in a mouse model of postinfectious irritable bowel syndrome. Eur.J.Pharmacol. 2010;636:159–165. doi: 10.1016/j.ejphar.2010.03.037. [DOI] [PubMed] [Google Scholar]
- Lu A, Steiner MA, Whittle N, Vogl AM, Walser SM, Ableitner M, Refojo D, Ekker M, Rubenstein JL, Stalla GK, Singewald N, Holsboer F, Wotjak CT, Wurst W, Deussing JM. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol.Psychiatry. 2008;13:1028–1042. doi: 10.1038/mp.2008.51. [DOI] [PubMed] [Google Scholar]
- Lu CL, Hsieh JC, Dun NJ, Oprea TI, Wang PS, Luo JC, Lin HC, Chang FY, Lee SD. Estrogen rapidly modulates 5-hydroxytrytophan-induced visceral hypersensitivity via GPR30 in rats. Gastroenterology. 2009;137:1040–1050. doi: 10.1053/j.gastro.2009.03.047. [DOI] [PubMed] [Google Scholar]
- Maneerattanaporn M, Chang L, Chey WD. Emerging pharmacological therapies for the irritable bowel syndrome. Gastroenterol.Clin.North Am. 2011;40:223–243. doi: 10.1016/j.gtc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. Neurobiology of substance P and the NK1 receptor. J.Clin.Psychiatry. 2002;63(Suppl 11):6–10. [PubMed] [Google Scholar]
- Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain. 2009;142:236–244. doi: 10.1016/j.pain.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Taché Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr.Pharm.Des. 2006;12:4071–4088. doi: 10.2174/138161206778743637. [DOI] [PubMed] [Google Scholar]
- Matricon J, Gelot A, Etienne M, Lazdunski M, Muller E, Ardid D. Spinal cord plasticity and acid-sensing ion channels involvement in a rodent model of irritable bowel syndrome. Eur.J.Pain. 2010 doi: 10.1016/j.ejpain.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Berman S, Derbyshire SW, Suyenobu B, Chang L, Fitzgerald L, Mandelkern M, Hamm L, Vogt B, Naliboff BD. The effect of the 5-HT3 receptor antagonist, alosetron, on brain responses to visceral stimulation in irritable bowel syndrome patients. Aliment.Pharmacol.Ther. 2002;16:1357–1366. doi: 10.1046/j.1365-2036.2002.01287.x. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Bradesi S, Chang L, Spiegel BM, Bueller JA, Naliboff BD. Functional GI disorders: from animal models to drug development. Gut. 2008;57:384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122:2032–2048. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Derbyshire S, Naliboff BD. Cerebral activation in irritable bowel syndrome. Gastroenterology. 2000;119:1418–1420. doi: 10.1053/gast.2000.20116. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am.J.Physiol Gastrointest.Liver Physiol. 2001;280:G519–G524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K. The Brain-Gut Axis in Abdominal Pain Syndromes. Annu.Rev.Med. 2011;62:381–396. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann.N.Y.Acad.Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch.Intern.Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McKernan DP, Fitzgerald P, Dinan TG, Cryan JF. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol.Motil. 2010;22:1029–35. e268. doi: 10.1111/j.1365-2982.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- McLean PG, Picard C, Garcia-Villar R, More J, Fioramonti J, Buéno L. Effects of nematode infection on sensitivity to intestinal distension: role of tachykinin NK2 receptors. Eur.J.Pharmacol. 1997;337:279–282. doi: 10.1016/s0014-2999(97)01275-2. [DOI] [PubMed] [Google Scholar]
- McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Geppetti P, Bunnett NW, Mayer EA. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- Melgar S, Engström K, Jagervall A, Martinez V. Psychological stress reactivates dextran sulfate sodium-induced chronic colitis in mice. Stress. 2008;11:348–362. doi: 10.1080/10253890701820166. [DOI] [PubMed] [Google Scholar]
- Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br.J.Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- Mickle A, Sood M, Zhang Z, Shahmohammadi G, Sengupta JN, Miranda A. Antinociceptive effects of melatonin in a rat model of post-inflammatory visceral hyperalgesia: a centrally mediated process. Pain. 2010;149:555–564. doi: 10.1016/j.pain.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V, Whorwell PJ. Hypnotherapy for functional gastrointestinal disorders: a review. Int.J.Clin.Exp.Hypn. 2009;57:279–292. doi: 10.1080/00207140902881098. [DOI] [PubMed] [Google Scholar]
- Million M, Grigoriadis DE, Sullivan S, Crowe PD, McRoberts JA, Zhou H, Saunders PR, Maillot C, Mayer EA, Taché Y. A novel water-soluble selective CRF1 receptor antagonist, NBI 35965, blunts stress-induced visceral hyperalgesia and colonic motor function in rats. Brain Res. 2003;985:32–42. doi: 10.1016/s0006-8993(03)03027-0. [DOI] [PubMed] [Google Scholar]
- Million M, Maillot C, Adelson DA, Nozu T, Gauthier A, Rivier J, Chrousos GP, Bayati A, Mattsson H, Taché Y. Peripheral injection of sauvagine prevents repeated colorectal distension-induced visceral pain in female rats. Peptides. 2005;26:1188–1195. doi: 10.1016/j.peptides.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Million M, Wang L, Adelson DW, Roman F, Diop L, Taché Y. Pregabalin decreases visceral pain and prevents spinal neuronal activation in rats. Gut. 2007a;56:1482–1484. doi: 10.1136/gut.2007.129304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million M, Wang L, Stenzel-Poore MP, Coste SC, Yuan PQ, Lamy C, Rivier J, Buffington T, Taché Y. Enhanced pelvic responses to stressors in female CRF-overexpressing mice. Am.J.Physiol Regul.Integr.Comp Physiol. 2007b;292:R1429–R1438. doi: 10.1152/ajpregu.00626.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million M, Wang L, Wang Y, Adelson DW, Yuan PQ, Maillot C, Coutinho SV, McRoberts JA, Bayati A, Mattsson H, Wu V, Wei JY, Rivier J, Vale W, Mayer EA, Taché Y. CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut. 2006;55:172–181. doi: 10.1136/gut.2004.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- Mönnikes H, Tebbe JJ, Hildebrandt M, Arck P, Osmanoglou E, Rose M, Klapp B, Wiedenmann B, Heymann-Mönnikes I. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig.Dis. 2001;19:201–211. doi: 10.1159/000050681. [DOI] [PubMed] [Google Scholar]
- Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601–607. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffari S, Rahimi R, Abdollahi M. Implications of melatonin therapy in irritable bowel syndrome; a systematic review. Curr.Pharm.Des. 2010;16:3646–3655. doi: 10.2174/138161210794079254. [DOI] [PubMed] [Google Scholar]
- Mulak A, Bonaz B. Irritable bowel syndrome: a model of the brain-gut interactions. Med.Sci.Monit. 2004;10:RA55–RA62. [PubMed] [Google Scholar]
- Mulak A, Paradowski L. Effect of 5-HT1 agonist (sumatriptan) on anorectal function in irritable bowel syndrome patients. World J.Gastroenterol. 2006;12:1591–1596. doi: 10.3748/wjg.v12.i10.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulak A, Taché Y. Sex difference in irritable bowel syndrome: do gonadal hormones play a role? Gastroenterol.Pol. 2010;17:89–97. [PMC free article] [PubMed] [Google Scholar]
- Muscat R, Willner P. Suppression of sucrose drinking by chronic mild unpredictable stress: a methodological analysis. Neurosci.Biobehav.Rev. 1992;16:507–517. doi: 10.1016/s0149-7634(05)80192-7. [DOI] [PubMed] [Google Scholar]
- Myers B, Dittmeyer K, Greenwood-Van Meerveld B. Involvement of amygdaloid corticosterone in altered visceral and somatic sensation. Behav.Brain Res. 2007;181:163–167. doi: 10.1016/j.bbr.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B. Corticosteroid receptor-mediated mechanisms in the amygdala regulate anxiety and colonic sensitivity. Am.J.Physiol Gastrointest.Liver Physiol. 2007;292:G1622–G1629. doi: 10.1152/ajpgi.00080.2007. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, Mandelkern MA, Mayer EA. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Fillingim RB, Randich A, Backensto EM, Faught E. Low intensity vagal nerve stimulation lowers human thermal pain thresholds. Pain. 2000;86:81–85. doi: 10.1016/s0304-3959(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Nijsen MJ, Ongenae NG, Coulie B, Meulemans AL. Telemetric animal model to evaluate visceral pain in the freely moving rat. Pain. 2003;105:115–123. doi: 10.1016/s0304-3959(03)00170-2. [DOI] [PubMed] [Google Scholar]
- Nozu T, Kudaira M. Corticotropin-releasing factor induces rectal hypersensitivity after repetitive painful rectal distention in healthy humans. J.Gastroenterol. 2006;41:740–744. doi: 10.1007/s00535-006-1848-4. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Bulmer DC, Coelho AM, Fitzgerald P, Bongiovanni C, Lee K, Winchester W, Dinan TG, Cryan JF. 5-HT(2B) receptors modulate visceral hypersensitivity in a stress-sensitive animal model of brain-gut axis dysfunction. Neurogastroenterol.Motil. 2010;22:573–8. e124. doi: 10.1111/j.1365-2982.2009.01432.x. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology (Berl) 2011;214:71–88. doi: 10.1007/s00213-010-2010-9. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Marchesi JR, Scully P, Codling C, Coelho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol.Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Oh-Young L, Manakata J, Naliboff B. A double-blind, parallel group pilot study of the effect of CJ-11974 and placebo on perceptual and emotional responses to rectosigmoid distension in IBS patients. Gastroenterology. 2000;118:A846. abstract. [Google Scholar]
- Ouyang A, Wrzos HF. Contribution of gender to pathophysiology and clinical presentation of IBS: should management be different in women? Am.J.Gastroenterol. 2006;101:S602–S609. doi: 10.1111/j.1572-0241.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Martinez-Salgado C, Wemmie JA, Brennan TJ, Symonds E, Omari T, Lewin GR, Welsh MJ, Blackshaw LA. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127:1739–1747. doi: 10.1053/j.gastro.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Palecek J. The role of dorsal columns pathway in visceral pain. Physiol Res. 2004;53(Suppl 1):S125–S130. [PubMed] [Google Scholar]
- Palecek J, Willis WD. The dorsal column pathway facilitates visceromotor responses to colorectal distention after colon inflammation in rats. Pain. 2003;104:501–507. doi: 10.1016/S0304-3959(03)00075-7. [DOI] [PubMed] [Google Scholar]
- Palsson OS, Drossman DA. Psychiatric and psychological dysfunction in irritable bowel syndrome and the role of psychological treatments. Gastroenterol.Clin.North Am. 2005;34:281–303. doi: 10.1016/j.gtc.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Adaptations in endocannabinoid signaling in response to repeated homotypic stress: a novel mechanism for stress habituation. Eur.J.Neurosci. 2008;27:2821–2829. doi: 10.1111/j.1460-9568.2008.06266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piche M, Arsenault M, Poitras P, Rainville P, Bouin M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain. 2010;148:49–58. doi: 10.1016/j.pain.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Piche T, Barbara G, Aubert P, Bruley des Varannes S,V, Dainese R, Nano JL, Cremon C, Stanghellini V, De GR, Galmiche JP, Neunlist M. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- Pimentel M, Chatterjee S, Chow EJ, Park S, Kong Y. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: subanalysis of a double-blind randomized controlled study. Dig.Dis.Sci. 2006;51:1297–1301. doi: 10.1007/s10620-006-9104-6. [DOI] [PubMed] [Google Scholar]
- Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N.Engl.J.Med. 2011a;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- Pimentel M, Morales W, Jee SR, Low K, Hwang L, Pokkunuri V, Mirocha J, Conklin J, Chang C. Antibiotic Prophylaxis Prevents the Development of a Post-Infectious Phenotype in a New Rat Model of Post-Infectious IBS. Dig.Dis.Sci. 2011b doi: 10.1007/s10620-010-1548-z. DOI: 10.1007/s10620-010-1548-z. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res.Mol.Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–1108. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD. Central neural mechanisms that interrelate sensory and affective dimensions of pain. Mol.Interv. 2002;2:392–403. 339. doi: 10.1124/mi.2.6.392. [DOI] [PubMed] [Google Scholar]
- Qin HY, Wu JC, Tong XD, Sung JJ, Xu HX, Bian ZX. Systematic review of animal models of post-infectious/post-inflammatory irritable bowel syndrome. J.Gastroenterol. 2011;46:164–174. doi: 10.1007/s00535-010-0321-6. [DOI] [PubMed] [Google Scholar]
- Randich A, Gebhart GF. Vagal afferent modulation of nociception. Brain Res.Brain Res.Rev. 1992;17:77–99. doi: 10.1016/0165-0173(92)90009-b. [DOI] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci.Biobehav.Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Ravnefjord A, Brusberg M, Kang D, Bauer U, Larsson H, Lindström E, Martinez V. Involvement of the transient receptor potential vanilloid 1 (TRPV1) in the development of acute visceral hyperalgesia during colorectal distension in rats. Eur.J.Pharmacol. 2009;611:85–91. doi: 10.1016/j.ejphar.2009.03.058. [DOI] [PubMed] [Google Scholar]
- Ravnefjord A, Brusberg M, Larsson H, Lindström E, Martinez V. Effects of pregabalin on visceral pain responses and colonic compliance in rats. Br.J.Pharmacol. 2008;155:407–416. doi: 10.1038/bjp.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber SO, Obermeier F, Straub RH, Falk W, Neumann ID. Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology. 2006;147:4968–4976. doi: 10.1210/en.2006-0347. [DOI] [PubMed] [Google Scholar]
- Reber SO, Obermeier F, Straub RH, Veenema AH, Neumann ID. Aggravation of DSS-induced colitis after chronic subordinate colony (CSC) housing is partially mediated by adrenal mechanisms. Stress. 2008;11:225–234. doi: 10.1080/10253890701733351. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zhang L, Lu Y, Yang H, Westlund KN. Central lateral thalamic neurons receive noxious visceral mechanical and chemical input in rats. J.Neurophysiol. 2009;102:244–258. doi: 10.1152/jn.90985.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Glaser JD, Van Bockstaele EJ. Ultrastructural evidence for co-localization of corticotropin-releasing factor receptor and mu-opioid receptor in the rat nucleus locus coeruleus. Neurosci.Lett. 2007;413:216–221. doi: 10.1016/j.neulet.2006.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gutenteric microbiota axis. Nat.Rev.Gastroenterol.Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivat C, Becker C, Blugeot A, Zeau B, Mauborgne A, Pohl M, Benoliel JJ. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain. 2010;150:358–368. doi: 10.1016/j.pain.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Rivat C, Laboureyras E, Laulin JP, Le RC, Richebe P, Simonnet G. Non-nociceptive environmental stress induces hyperalgesia, not analgesia, in pain and opioid-experienced rats. Neuropsychopharmacology. 2007;32:2217–2228. doi: 10.1038/sj.npp.1301340. [DOI] [PubMed] [Google Scholar]
- Robinson DR, Gebhart GF. Inside information: the unique features of visceral sensation. Mol.Interv. 2008;8:242–253. doi: 10.1124/mi.8.5.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol.Rev. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Rosenberger C, Elsenbruch S, Scholle A, de GA, Schedlowski M, Forsting M, Gizewski ER. Effects of psychological stress on the cerebral processing of visceral stimuli in healthy women. Neurogastroenterol.Motil. 2009;21:740–e45. doi: 10.1111/j.1365-2982.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- Rosztoczy A, Fioramonti J, Jarmay K, Barreau F, Wittmann T, Buéno L. Influence of sex and experimental protocol on the effect of maternal deprivation on rectal sensitivity to distension in the adult rat. Neurogastroenterol.Motil. 2003;15:679–686. doi: 10.1046/j.1350-1925.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- Rouzade-Dominguez ML, Curtis AL, Valentino RJ. Role of Barrington's nucleus in the activation of rat locus coeruleus neurons by colonic distension. Brain Res. 2001;917:206–218. doi: 10.1016/s0006-8993(01)02917-1. [DOI] [PubMed] [Google Scholar]
- Saab CY, Park YC, Al-Chaer ED. Thalamic modulation of visceral nociceptive processing in adult rats with neonatal colon irritation. Brain Res. 2004;1008:186–192. doi: 10.1016/j.brainres.2004.01.083. [DOI] [PubMed] [Google Scholar]
- Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–964. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Shackman AJ, Davidson RJ. Individual differences in the effects of perceived controllability on pain perception: critical role of the prefrontal cortex. J.Cogn Neurosci. 2007;19:993–1003. doi: 10.1162/jocn.2007.19.6.993. [DOI] [PubMed] [Google Scholar]
- Sanoja R, Tortorici V, Fernandez C, Price TJ, Cervero F. Role of RVM neurons in capsaicin-evoked visceral nociception and referred hyperalgesia. Eur.J.Pain. 2010;14:120–129. doi: 10.1016/j.ejpain.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson M, Buéno L, Fioramonti J. Involvement of cannabinoid receptors in inflammatory hypersensitivity to colonic distension in rats. Neurogastroenterol.Motil. 2006;18:949–956. doi: 10.1111/j.1365-2982.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- Santos J, Yang PC, Söderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48:630–636. doi: 10.1136/gut.48.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapsed-Byrne S, Ma D, Ridout D, Holdcroft A. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res. 1996;742:10–16. doi: 10.1016/s0006-8993(96)00989-4. [DOI] [PubMed] [Google Scholar]
- Saunders PR, Miceli P, Vallance BA, Wang L, Pinto S, Tougas G, Kamath M, Jacobson K. Noradrenergic and cholinergic neural pathways mediate stress-induced reactivation of colitis in the rat. Auton.Neurosci. 2006;124:56–68. doi: 10.1016/j.autneu.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Savas LS, White DL, Wieman M, Daci K, Fitzgerald S, Laday SS, Tan G, Graham DP, Cully JA, El-Serag HB. Irritable bowel syndrome and dyspepsia among women veterans: prevalence and association with psychological distress. Aliment.Pharmacol.Ther. 2009;29:115–125. doi: 10.1111/j.1365-2036.2008.03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J.Neurosci. 2000;20:7438–7445. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog.Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- Schwetz I, Bradesi S, McRoberts JA, Sablad M, Miller JC, Zhou H, Ohning G, Mayer EA. Delayed stress-induced colonic hypersensitivity in male Wistar rats: role of neurokinin-1 and corticotropin-releasing factor-1 receptors. Am.J.Physiol Gastrointest.Liver Physiol. 2004a;286:G683–G691. doi: 10.1152/ajpgi.00358.2003. [DOI] [PubMed] [Google Scholar]
- Schwetz I, McRoberts JA, Coutinho SV, Bradesi S, Gale G, Fanselow M, Million M, Ohning G, Taché Y, Plotsky PM, Mayer EA. Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. Am.J.Physiol Gastrointest.Liver Physiol. 2005;289:G704–G712. doi: 10.1152/ajpgi.00498.2004. [DOI] [PubMed] [Google Scholar]
- Schwetz I, Naliboff B, Munakata J, Lembo T, Chang L, Matin K, Ohning G, Mayer EA. Anti-hyperalgesic effect of octreotide in patients with irritable bowel syndrome. Aliment.Pharmacol.Ther. 2004b;19:123–131. doi: 10.1111/j.1365-2036.2004.01774.x. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mikulis DJ, Davis KD. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain. 2004;112:48–58. doi: 10.1016/j.pain.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Sengupta JN. Visceral pain: the neurophysiological mechanism. Handb.Exp.Pharmacol. 2009:31–74. doi: 10.1007/978-3-540-79090-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HJ, Ham HD, Jin HY, Lee WH, Hwang HS, Park SA, Kim YS, Choi SC, Lee S, Oh KJ, Kim BS, Park BR, Lee MY. Chronic Administration of Monosodium Glutamate under Chronic Variable Stress Impaired Hypothalamic-Pituitary-Adrenal Axis Function in Rats. Korean J.Physiol Pharmacol. 2010;14:213–221. doi: 10.4196/kjpp.2010.14.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Kong H, Hou X. Prevalence of irritable bowel syndrome and its relationship with psychological stress status in Chinese university students. J.Gastroenterol.Hepatol. 2009;24:1885–1890. doi: 10.1111/j.1440-1746.2009.05943.x. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Stereotaxic localization of corticosterone to the amygdala enhances hypothalamo-pituitary-adrenal responses to behavioral stress. Brain Res. 2003;963:203–213. doi: 10.1016/s0006-8993(02)03978-1. [DOI] [PubMed] [Google Scholar]
- Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: the effects of a transgalactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment.Pharmacol.Ther. 2009;29:508–518. doi: 10.1111/j.1365-2036.2008.03911.x. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Newberry K, Lodge NJ. Effect of the 5HT1A receptor partial agonist buspirone on colorectal distension-induced pseudoaffective and behavioral responses in the female Wistar rat. Eur.J.Pharmacol. 2004;494:23–29. doi: 10.1016/j.ejphar.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Söderholm JD, Yang PC, Ceponis P, Vohra A, Riddell R, Sherman PM, Perdue MH. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123:1099–1108. doi: 10.1053/gast.2002.36019. [DOI] [PubMed] [Google Scholar]
- Song GH, Venkatraman V, Ho KY, Chee MW, Yeoh KG, Wilder-Smith CH. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain. 2006;126:79–90. doi: 10.1016/j.pain.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Spaziani R, Bayati A, Redmond K, Bajaj H, Mazzadi S, Bienenstock J, Collins SM, Kamath MV. Vagal dysfunction in irritable bowel syndrome assessed by rectal distension and baroreceptor sensitivity. Neurogastroenterol.Motil. 2008;20:336–342. doi: 10.1111/j.1365-2982.2007.01042.x. [DOI] [PubMed] [Google Scholar]
- Spiller R, Garsed K. Infection, inflammation, and the irritable bowel syndrome. Dig.Liver Dis. 2009a;41:844–849. doi: 10.1016/j.dld.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009b;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662–1671. doi: 10.1016/s0016-5085(03)00324-x. [DOI] [PubMed] [Google Scholar]
- Stam R. PTSD and stress sensitisation: a tale of brain and body Part 2: animal models. Neurosci.Biobehav.Rev. 2007;31:558–584. doi: 10.1016/j.neubiorev.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Stam R, Akkermans LM, Wiegant VM. Trauma and the gut: interactions between stressful experience and intestinal function. Gut. 1997;40:704–709. doi: 10.1136/gut.40.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam R, van Laar TJ, Akkermans LM, Wiegant VM. Variability factors in the expression of stress-induced behavioural sensitisation. Behav.Brain Res. 2002;132:69–76. doi: 10.1016/s0166-4328(01)00387-4. [DOI] [PubMed] [Google Scholar]
- Stein C, Schafer M, Hassan AH. Peripheral opioid receptors. Ann.Med. 1995;27:219–221. doi: 10.3109/07853899509031962. [DOI] [PubMed] [Google Scholar]
- Stengel A, Taché Y. Corticotropin-releasing factor signaling and visceral response to stress. Exp.Biol.Med.(Maywood.) 2010;235:1168–1178. doi: 10.1258/ebm.2010.009347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress. Cognition and Health Wiley; New York: 1988. pp. 629–649. [Google Scholar]
- Su X, Burton MB, Gebhart GF. Effects of octreotide on responses to colorectal distension in the rat. Gut. 2001;48:676–682. doi: 10.1136/gut.48.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Terui N, Hosoya Y, Tonosaki Y, Nishiyama K, Honda T. Quantitative analysis of central terminal projections of visceral and somatic unmyelinated (C) primary afferent fibers in the guinea pig. J.Comp Neurol. 1993;332:315–325. doi: 10.1002/cne.903320305. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Hua XY, Protter AA, Powell HC, Yaksh TL. Spinal p38 MAP kinase is necessary for NMDA-induced spinal PGE(2) release and thermal hyperalgesia. Neuroreport. 2003;14:1153–1157. doi: 10.1097/00001756-200306110-00010. [DOI] [PubMed] [Google Scholar]
- Szarka LA, Camilleri M, Burton D, Fox JC, McKinzie S, Stanislav T, Simonson J, Sullivan N, Zinsmeister AR. Efficacy of on-demand asimadoline, a peripheral kappa-opioid agonist, in females with irritable bowel syndrome. Clin.Gastroenterol.Hepatol. 2007;5:1268–1275. doi: 10.1016/j.cgh.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M, Weaver I, Meaney M. Maternal care, the epigenome and phenotypic differences in behavior. Reprod.Toxicol. 2007;24:9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Taché Y, Brunnhuber S. From Hans Selye's discovery of biological stress to the identification of corticotropin-releasing factor signaling pathways: implication in stress-related functional bowel diseases. Ann.N.Y.Acad.Sci. 2008;1148:29–41. doi: 10.1196/annals.1410.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taché Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am.J.Physiol Gastrointest.Liver Physiol. 2001;280:G173–G177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- Taché Y, Million M, Nelson AG, Lamy C, Wang L. Role of corticotropin-releasing factor pathways in stress-related alterations of colonic motor function and viscerosensibility in female rodents. Gend.Med. 2005;2:146–154. doi: 10.1016/s1550-8579(05)80043-9. [DOI] [PubMed] [Google Scholar]
- Tack J, Coulie B, Wilmer A, Andrioli A, Janssens J. Influence of sumatriptan on gastric fundus tone and on the perception of gastric distension in man. Gut. 2000;46:468–473. doi: 10.1136/gut.46.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliari B, dos Santos TM, Cunha AA, Lima DD, Delwing D, Sitta A, Vargas CR, Dalmaz C, Wyse AT. Chronic variable stress induces oxidative stress and decreases butyrylcholinesterase activity in blood of rats. J.Neural Transm. 2010;117:1067–1076. doi: 10.1007/s00702-010-0445-0. [DOI] [PubMed] [Google Scholar]
- Tammpere A, Brusberg M, Axenborg J, Hirsch I, Larsson H, Lindström E. Evaluation of pseudo-affective responses to noxious colorectal distension in rats by manometric recordings. Pain. 2005;116:220–226. doi: 10.1016/j.pain.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Tayama J, Sagami Y, Shimada Y, Hongo M, Fukudo S. Effect of alpha-helical CRH on quantitative electroencephalogram in patients with irritable bowel syndrome. Neurogastroenterol.Motil. 2007;19:471–483. doi: 10.1111/j.1365-2982.2007.00903.x. [DOI] [PubMed] [Google Scholar]
- Thijssen AY, Jonkers DM, Leue C, van d., V, Vidakovic-Vukic M, van Rood YR, Clemens CH, Masclee AA. Dysfunctional cognitions, anxiety and depression in irritable bowel syndrome. J.Clin.Gastroenterol. 2010;44:e236–e241. doi: 10.1097/MCG.0b013e3181eed5d8. [DOI] [PubMed] [Google Scholar]
- Thoua NM, Murray CD, Winchester WJ, Roy AJ, Pitcher MC, Kamm MA, Emmanuel AV. Amitriptyline modifies the visceral hypersensitivity response to acute stress in the irritable bowel syndrome. Aliment.Pharmacol.Ther. 2009;29:552–560. doi: 10.1111/j.1365-2036.2008.03918.x. [DOI] [PubMed] [Google Scholar]
- Thumshirn M, Coulie B, Camilleri M, Zinsmeister AR, Burton DD, Van DC. Effects of alosetron on gastrointestinal transit time and rectal sensation in patients with irritable bowel syndrome. Aliment.Pharmacol.Ther. 2000;14:869–878. doi: 10.1046/j.1365-2036.2000.00786.x. [DOI] [PubMed] [Google Scholar]
- Tian SW, Laudon M, Han L, Gao J, Huang FL, Yang YF, Deng HF. Antidepressant- and anxiolytic effects of the novel melatonin agonist Neu-P11 in rodent models. Acta Pharmacol.Sin. 2010;31:775–783. doi: 10.1038/aps.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjong YW, Ip SP, Lao L, Wu J, Fong HH, Sung JJ, Berman B, Che CT. Neonatal maternal separation elevates thalamic corticotrophin releasing factor type 1 receptor expression response to colonic distension in rat. Neuro.Endocrinol.Lett. 2010;31:215–220. [PubMed] [Google Scholar]
- Traub RJ, Murphy A. Colonic inflammation induces fos expression in the thoracolumbar spinal cord increasing activity in the spinoparabrachial pathway. Pain. 2002;95:93–102. doi: 10.1016/s0304-3959(01)00381-5. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Tang B, Ji Y, Pandya S, Yfantis H, Sun Y. A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology. 2008;135:2075–2083. doi: 10.1053/j.gastro.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble N, Johnson AC, Foster A, Greenwood-Van Meerveld B. Corticotropin-releasing factor receptor 1-deficient mice show decreased anxiety and colonic sensitivity. Neurogastroenterol.Motil. 2007;19:754–760. doi: 10.1111/j.1365-2982.2007.00951.x. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C, Dermitzaki E, Venihaki M, Chatzaki E, Minas V, Gravanis A, Margioris AN. The corticotropin-releasing factor (CRF) family of peptides as local modulators of adrenal function. Cell Mol.Life Sci. 2007;64:1638–1655. doi: 10.1007/s00018-007-6555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruoka M, Wang D, Tamaki J, Inoue T. Descending influence from the nucleus locus coeruleus/subcoeruleus on visceral nociceptive transmission in the rat spinal cord. Neuroscience. 2010;165:1019–1024. doi: 10.1016/j.neuroscience.2009.11.055. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proc.Soc.Exp.Biol.Med. 1997;215:1–10. doi: 10.3181/00379727-215-44108. [DOI] [PubMed] [Google Scholar]
- Usui D, Yamaguchi-Shima N, Okada S, Shimizu T, Wakiguchi H, Yokotani K. Selective activation of the sympathetic ganglia by centrally administered corticotropin-releasing factor in rats. Auton.Neurosci. 2009;146:111–114. doi: 10.1016/j.autneu.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann.N.Y.Acad.Sci. 1993;697:173–188. doi: 10.1111/j.1749-6632.1993.tb49931.x. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Miselis RR, Pavcovich LA. Pontine regulation of pelvic viscera: pharmacological target for pelvic visceral dysfunctions. Trends Pharmacol.Sci. 1999;20:253–260. doi: 10.1016/s0165-6147(99)01332-2. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard RM, Klooker TK, de Jonge WJ, Boeckxstaens GE. Peripheral relays in stress-induced activation of visceral afferents in the gut. Auton.Neurosci. 2010;153:99–105. doi: 10.1016/j.autneu.2009.07.004. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard RM, Klooker TK, Welting O, Stanisor OI, Wouters MM, van der CD, Bulmer DC, Peeters PJ, Aerssens J, De HR, Lee K, de Jonge WJ, Boeckxstaens GE. Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterol.Motil. 2009;21:1107–1e94. doi: 10.1111/j.1365-2982.2009.01339.x. [DOI] [PubMed] [Google Scholar]
- Van Oudenhove L, Coen SJ, Aziz Q. Functional brain imaging of gastrointestinal sensation in health and disease. World J.Gastroenterol. 2007;13:3438–3445. doi: 10.3748/wjg.v13.i25.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Reber SO, Selch S, Obermeier F, Neumann ID. Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology. 2008;149:2727–2736. doi: 10.1210/en.2007-1469. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Ossipov MH, King T, Porreca F. Reversal of inflammatory and noninflammatory visceral pain by central or peripheral actions of sumatriptan. Gastroenterology. 2008;135:1369–1378. doi: 10.1053/j.gastro.2008.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdu B, Decosterd I, Buclin T, Stiefel F, Berney A. Antidepressants for the treatment of chronic pain. Drugs. 2008;68:2611–2632. doi: 10.2165/0003495-200868180-00007. [DOI] [PubMed] [Google Scholar]
- Verdu EF, Bercik P, Verma-Gandhu M, Huang XX, Blennerhassett P, Jackson W, Mao Y, Wang L, Rochat F, Collins SM. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55:182–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N. Visceral afferents: what role in post-inflammatory pain? Auton.Neurosci. 2010;153:79–83. doi: 10.1016/j.autneu.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Vergnolle N. Postinflammatory visceral sensitivity and pain mechanisms. Neurogastroenterol.Motil. 2008;20(Suppl 1):73–80. doi: 10.1111/j.1365-2982.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- Verma-Gandhu M, Verdu EF, Bercik P, Blennerhassett PA, Al-Mutawaly N, Ghia JE, Collins SM. Visceral pain perception is determined by the duration of colitis and associated neuropeptide expression in the mouse. Gut. 2007;56:358–364. doi: 10.1136/gut.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario M, Guilarte M, Alonso C, Yang P, Martinez C, Ramos L, Lobo B, Gonzalez A, Guila M, Pigrau M, Saperas E, Azpiroz F, Santos J. Chronological assessment of mast cell-mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress. Brain Behav.Immun. 2010;24:1166–1175. doi: 10.1016/j.bbi.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Videlock EJ, Adeyemo M, Licudine A, Hirano M, Ohning G, Mayer M, Mayer EA, Chang L. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137:1954–1962. doi: 10.1053/j.gastro.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallon C, Yang PC, Keita AV, Ericson AC, McKay DM, Sherman PM, Perdue MH, Söderholm JD. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57:50–58. doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- Wang G, Tang B, Traub RJ. Pelvic nerve input mediates descending modulation of homovisceral processing in the thoracolumbar spinal cord of the rat. Gastroenterology. 2007;133:1544–1553. doi: 10.1053/j.gastro.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Tang B, Traub RJ. Differential processing of noxious colonic input by thoracolumbar and lumbosacral dorsal horn neurons in the rat. J.Neurophysiol. 2005;94:3788–3794. doi: 10.1152/jn.00230.2005. [DOI] [PubMed] [Google Scholar]
- Wang W, Liu Y, Zheng H, Wang HN, Jin X, Chen YC, Zheng LN, Luo XX, Tan QR. A modified single-prolonged stress model for post-traumatic stress disorder. Neurosci.Lett. 2008a;441:237–241. doi: 10.1016/j.neulet.2008.06.031. [DOI] [PubMed] [Google Scholar]
- Wang Z, Bradesi S, Maarek JM, Lee K, Winchester WJ, Mayer EA, Holschneider DP. Regional brain activation in conscious, nonrestrained rats in response to noxious visceral stimulation. Pain. 2008b;138:233–243. doi: 10.1016/j.pain.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock JK, Clayton AH. Chronic episodic disorders in women. Psychiatr.Clin.North Am. 2003;26:725–740. doi: 10.1016/s0193-953x(03)00042-x. [DOI] [PubMed] [Google Scholar]
- Welting O, van den Wijngaard RM, de Jonge WJ, Holman R, Boeckxstaens GE. Assessment of visceral sensitivity using radio telemetry in a rat model of maternal separation. Neurogastroenterol.Motil. 2005;17:838–845. doi: 10.1111/j.1365-2982.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- White DL, Savas LS, Daci K, Elserag R, Graham DP, Fitzgerald SJ, Smith SL, Tan G, El-Serag HB. Trauma history and risk of the irritable bowel syndrome in women veterans. Aliment.Pharmacol.Ther. 2010;32:551–561. doi: 10.1111/j.1365-2036.2010.04387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- Whorwell PJ. Behavioral therapy for IBS. Nat.Clin.Pract.Gastroenterol.Hepatol. 2009;6:148–149. doi: 10.1038/ncpgasthep1361. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Jr., Westlund KN. The role of the dorsal column pathway in visceral nociception. Curr. Pain Headache Rep. 2001;5:20–26. doi: 10.1007/s11916-001-0006-1. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology. 2010;138:294–304. doi: 10.1053/j.gastro.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PC, Jury J, Söderholm JD, Sherman PM, McKay DM, Perdue MH. Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am. J. Pathol. 2006;168:104–114. doi: 10.2353/ajpath.2006.050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarushkina NI. The role of hypothalamo-hypophyseal-adrenocortical system hormones in controlling pain sensitivity. Neurosci. Behav. Physiol. 2008;38:759–766. doi: 10.1007/s11055-008-9044-z. [DOI] [PubMed] [Google Scholar]
- Yorimitsu M, Okada S, Yamaguchi-Shima N, Shimizu T, Arai J, Yokotani K. Role of brain adrenoceptors in the corticortopin-releasing factor-induced central activation of sympathoadrenomedullary outflow in rats. Life Sci. 2008;82:487–494. doi: 10.1016/j.lfs.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Yu LC, Perdue MH. Role of mast cells in intestinal mucosal function: studies in models of hypersensitivity and stress. Immunol. Rev. 2001;179:61–73. doi: 10.1034/j.1600-065x.2001.790107.x. [DOI] [PubMed] [Google Scholar]
- Yu YB, Yang J, Zuo XL, Gao LJ, Wang P, Li YQ. Transient receptor potential vanilloid-1 (TRPV1) and ankyrin-1 (TRPA1) participate in visceral hyperalgesia in chronic water avoidance stress rat model. Neurochem. Res. 2010;35:797–803. doi: 10.1007/s11064-010-0137-z. [DOI] [PubMed] [Google Scholar]
- Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin. Arthritis Rheum. 2008;37:339–352. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zou N, Li J, Lv H, Wei J, Fang XC, Qian JM. Elevated expression of c-fos in central nervous system correlates with visceral hypersensitivity in irritable bowel syndrome (IBS): a new target for IBS treatment. Int. J. Colorectal Dis. 2011 doi: 10.1007/s00384-011-1153-4. DOI: 10.1007/s00384-011-1153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XJ, Li Z, Chung EK, Zhang HQ, Xu HX, Sung JJ, Bian ZX. Activation of extracellular signal-regulated protein kinase is associated with colorectal distension-induced spinal and supraspinal neuronal response and neonatal maternal separation-induced visceral hyperalgesia in rats. J. Mol. Neurosci. 2009;37:274–287. doi: 10.1007/s12031-008-9134-y. [DOI] [PubMed] [Google Scholar]
- Zheng J, Babygirija R, Bulbul M, Cerjak D, Ludwig K, Takahashi T. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am. J. Physiol Gastrointest. Liver Physiol. 2010;299:G946–G953. doi: 10.1152/ajpgi.00483.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain. 2008;134:9–15. doi: 10.1016/j.pain.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41–46. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Facilitation and attenuation of a visceral nociceptive reflex from the rostroventral medulla in the rat. Gastroenterology. 2002;122:1007–1019. doi: 10.1053/gast.2002.32389. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov. Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Web References
- Moultry AM, Godley CB, Wanami M. A review of peripherally acting mu-opioid receptor antagonists. 2011 Modern Medicine. http://www.modernmedicine.com/modernmedicine/Clinical+News/A-review-of-peripherally-acting-mu-opioid-receptor/ArticleStandard/Article/detail/705809.