Abstract
Background
Rice transcription regulator OsWRKY13 influences the functioning of more than 500 genes in multiple signalling pathways, with roles in disease resistance, redox homeostasis, abiotic stress responses, and development.
Results
To determine the putative transcriptional regulation mechanism of OsWRKY13, the putative cis-acting elements of OsWRKY13-influenced genes were analyzed using the whole genome expression profiling of OsWRKY13-activated plants generated with the Affymetrix GeneChip Rice Genome Array. At least 39 transcription factor genes were influenced by OsWRKY13, and 30 of them were downregulated. The promoters of OsWRKY13-upregulated genes were overrepresented with W-boxes for WRKY protein binding, whereas the promoters of OsWRKY13-downregulated genes were enriched with cis-elements putatively for binding of MYB and AP2/EREBP types of transcription factors. Consistent with the distinctive distribution of these cis-elements in up- and downregulated genes, nine WRKY genes were influenced by OsWRKY13 and the promoters of five of them were bound by OsWRKY13 in vitro; all seven differentially expressed AP2/EREBP genes and six of the seven differentially expressed MYB genes were suppressed by in OsWRKY13-activated plants. A subset of OsWRKY13-influenced WRKY genes were involved in host-pathogen interactions.
Conclusion
These results suggest that OsWRKY13-mediated signalling pathways are partitioned by different transcription factors. WRKY proteins may play important roles in the monitoring of OsWRKY13-upregulated genes and genes involved in pathogen-induced defence responses, whereas MYB and AP2/EREBP proteins may contribute most to the control of OsWRKY13-downregulated genes.
Background
WRKY genes, which encode proteins binding to the cis-acting element W-box, have been isolated from many plant species [1,2]. During the past decade, numerous reports have indicated that WRKY genes are involved in defence responses (Arabidopsis AtWRKY6, [3]; AtWRKY18, [4]; AtWRKY70, [5]; AtWRKY33, [6]; and rice OsWRKY03, [7]; OsWRKY71, [8]; OsWRKY13, [9]; OsWRKY45, [10]), development (TRANSPARENT TESTA GLABRA2, [11]; MINI3, [12]), hormone regulation (OsWRKY51 and OsWRKY71, [13,14]), as well as sugar signalling and sesquiterpene and benzylisoquinoline alkaloid biosynthesis (SUSIBA2, [15]; GaWRKY1, [16]; CjWRKY1, [17]).
The most stringent definition for a WRKY binding site, a W-box, is a hexamer of TTGAC(C/T), which is found in the promoter regions of many pathogenesis-related genes [18]. Based on the core sequence (TTGAC) of a W-box, there are variant W-boxes, TTTGACA, TTTGAC(C/T), TTGACTT, TTGAC(A/C), TTGAC(A/C)A, and TTGAC(A/C)(C/G/T), and a W-box like cis-element, TGAC(C/T) [18-21]. Recently, another variant W-box, TTGACG, which carried a minimum cis-element as-1 (TGACG) for the TGA transcription factor, was reported to be bound by rice OsWRKY13 transcription factor in vitro [9]. Furthermore, another novel WRKY binding site PRE4 (TACTGCGCTTAGT), which was identified in the promoter of OsWRKY13, participates in the self-regulation of OsWRKY13 [22]. Previously, barley WRKY protein SUSIBA2 was reported to specifically bind to the sugar responsive cis-element (SURE) in addition to a W-box [15]. Tobacco NtWRKY12 can bind to a WK-box (TTTTCCAC) in the PR-1a promoter, which deviated significantly from the consensus sequence of a W-box [23]. These results suggest that the cis-elements for the action of WRKY proteins are variable.
Computational methods that define relationships between gene expression levels and putative regulatory sequences in the promoter regions of differentially expressed genes based on large-scale microarray data and genome sequence screening are increasingly being used to establish a signal transduction network [18,24,25]. Evidence from microarray studies revealed an overrepresentation of W-box elements within the promoters of a cluster of genes that are coexpressed during systemic acquired resistance [18]. Transgenic AtWRKY70 microarray experiments showed that W-box elements are similarly enriched in both up- and downregulated clusters predicted by a bootstrapping program [20]. Thus, the potential relationship between different genes, including WRKY genes, may be obtained by integrating the knowledge of WRKY or other transcription factors and their related regulatory elements.
Rice OsWRKY13 is a potentially important transcriptional regulator involved in multiple physiological processes. It mediates disease resistance to bacterial blight caused by Xanthomonas oryzae pv. oryzae (Xoo) and fungal blast caused by Magnaporthe grisea through activation of salicylic acid (SA)-dependent pathways and suppression of jasmonic acid (JA)-dependent pathways; OsWRKY13 can bind to the W-box and W-box like cis-elements that are present in the promoters of some pathogen-induced defence-responsive genes [9,22]. Furthermore, genomewide analyses of the expression profiling of OsWRKY13-activated lines reveal that OsWRKY13 directly or indirectly regulates the expression of more than 500 genes [26]. OsWRKY13 is also a potential regulator of other physiological processes during pathogen infection. It activates redox homeostasis by the glutathione/glutaredoxin system as well as the flavonoid biosynthesis pathway, which may enhance the biosynthesis of antimicrobial flavonoid phytoalexins [26]. OsWRKY13 inhibits the SNAC1-mediated abiotic stress defence pathway and terpenoid metabolism pathway to suppress salt and cold defence responses as well as to putatively retard rice growth and development [26]. Compared to the large number of differentially expressed genes in OsWRKY13-activated plants, however, most OsWRKY13-regulated pathways have yet to be elucidated.
To understand the transcriptional regulation of OsWRKY13, the types of transcription factors and conserved motifs in the promoter regions of the genes differentially expressed in OsWRKY13-activated plants were analyzed. The results suggest that the actions of OsWRKY13 on the expression of more than 500 genes are partitioned by different types of transcription factors through binding to distinctly distributed cis-acting elements in the promoters of OsWRKY13-upregulated and -downregulated genes. Furthermore, OsWRKY13 appears to bind preferentially to the promoters of downregulated genes in vitro, suggesting that it may function more as a negative transcriptional regulator.
Methods
Microarray data
The microarray data, generated using Affymetrix GeneChip Rice Genome Arrays, were from our previous report [26] and the data were released under accession number GSE8380 of the Gene Expression Omnibus (GEO) database http://www.ncbi.nlm.nih.gov/geo. The data were generated from the leaves of a pool of 20 4-week-old wild-type Mudanjiang 8 (Oryza sativa ssp. japonica) plants and OsWRKY13-overexpressing independent homozygous transgenic lines, D11UM1-1 and D11UM7-2 [9]. D11UM1-1 and D11UM7-1 carry two and one copies of the transgene, respectively, and the two lines have more than 20-fold higher OsWRKY13 transcript levels than wild type with or without pathogen infection [26].
Promoter analysis
The rice genomic sequence was obtained from TIGR (The Institute for Genomic Research, http://rice.tigr.org) Rice Genome Annotation version 4.0. The 2-kb sequence upstream of the known or predicted coding region of rice genes that are differentially expressed on the microarray chip were identified with a 'present' call using the MAS5 method (version 5 edition, Affymetrix, Inc.) and their annotation was extracted. In total, 18,362 promoter sequences were filtered for further analysis. To search for overrepresented motifs within the promoter sequences of these genes, we performed one modified Perl script according to the enumerate methods of one- through 10-mer in the coregulated set of promoters (Sift program; [27]). The number of occurrences of each motif was compared with an expected value derived from the frequency of that element in the whole microarray (18,362 promoter sequences as baseline control). The overrepresented motifs in up- and downregulated genes were confirmed using the binomial distribution [27]. Only the motif with P value < 1e-5 (e = 10, 1e-5 = 1 × 10-5) was considered significant and selected for further analysis. Comparison of the detected overrepresented motifs with known cis-elements was performed using the PLACE http://www.dna.affrc.go.jp/PLACE/signalscan.html[28] and PlantCARE http://bioinformatics.psb.ugent.be/webtools/plantcare/html[29] databases and literature searches.
Rice transformation
To construct an RNA interference (RNAi) vector of OsWRKY13, a 900 bp cDNA fragment of OsWRKY13, obtained by PCR amplification from OsWRKY13 cDNA clone EI12I1 [GenBank: BF108309] [9] using primers WRKY12F (5'-GGGGACAAGTTTGTACAAAAAAGCAGGCTGTGATGGCGGCAGGAGAG-3') contained attB1 site (in bold) and WRKY12R (5'-GGGGACCACTTTGTACAAGAAAGCTGGGTTGAACACGACGGCGCACTC-3') contained attB2 site (in bold), was inserted into the pHELLSGATE2 vector by BP and LR reactions (Gateway Kit, Invitrogen, USA). Agrobacterium-mediated transformation was performed using calli derived from mature embryos of rice variety Minghui 63 (O. sativa ssp. indica) according to the protocol of Lin and Zhang [30]
Pathogen inoculation
Plants were inoculated with Xoo strain PXO61 at the booting stage by the leaf clipping method [31]. Rice variety Mudanjiang 8 was susceptible to PXO61 and variety Minghui 63 (O. sativa ssp. indica) was moderately resistant to PXO61. Mock-inoculated (control) plants were treated under the same condition except that the pathogen suspension was replaced with water.
Quantitative reverse transcription-PCR
For RNA isolation, 5- to 6-cm leaf segments located below the inoculation cutting sites were obtained. The RNA sample for OsWRKY13-activated line was a mixture isolated from eight leaves of four plants of a T2 family (D11UM7-2), and the RNA sample for the wild-type control was a mixture isolated from eight leaves of four Mudanjiang 8 plants. The RNA samples for OsWRKY13-suppressed plants were a mixture isolated from 4–6 leaves each plant at booting stage, and the RNA sample for the wild-type control was a mixture isolated from six leaves of three Minghui 63 plants. Total RNA was treated for 30 min with DNase I (Invitrogen) to remove contaminating DNA and used for quantitative reverse transcription (qRT)-PCR analysis. The qRT-PCR was conducted as described previously [32]. PCR primers for qRT-PCR are listed in Additional file 1. The expression level of actin gene was used to standardize the RNA sample for each qRT-PCR. Each qRT-PCR assay was repeated at least twice, with each repetition having three replicates; similar results were obtained in repeated experiments.
Yeast one-hybrid assay
The interaction of OsWRKY13 protein with the DNA regulatory element was assayed by yeast one-hybrid assay according to the manufacturer's protocol (Clontech Yeast Protocols Handbook, BD Biosciences Clontech, Mountain View, CA, USA). In brief, the full-length cDNA of OsWRKY13 was obtained by RT-PCR using primers WRKY16F (5'-ATGAATTCGGAGTGGTGGTGGTGATG-3') harbouring a digestion site of enzyme EcoRI (in bold) and WRKY13R (5'-ATAGGATCCAGGAGCACGGCGCGGTGGC-3') harbouring a digestion site of enzyme BamHI (in bold). The PCR product was ligated into the EcoRI/BamHI cloning site of vector pGADT7-Rec2 containing a GAL4 activation domain. The target cis-acting DNA fragments harbouring W-box or W-box like elements were obtained by PCR amplification of the promoter regions of a series of genes using promoter-specific primers (Additional file 2). The PCR products were ligated into the EcoRI, SacI, or EcoRI/SacI cloning site of vector pHIS2. The negative control DNA fragment (W17, Additional file 2) without a W-box from the promoter region of OsWRKY13 was ligated into the EcoRI/SacI cloning site of vector pHIS2. The yeast strain Y187 was cotransformed with pGADT7-Rec2/OsWRKY13 and pHIS2/target promoter or pHIS2/control. Positive interactions were verified by growing yeast cells on SD-Leu-Trp-His agar medium.
Results
A group of transcription factors was influenced by OsWRKY13
Analysis of the rice whole genome microarray data, generated using Affymetrix GeneChip Rice Genome Arrays [26], indicated that 32 transcription factor genes were differentially expressed after activation of OsWRKY13 (Additional file 3). Twenty-four (75%) of the differentially expressed genes were downregulated and eight (25%) of them were upregulated. Sixteen of these transcription factor genes belong to AP2/EREBP (seven), Myb (seven), and MADS (two) type transcription factors, which generally relate to the regulation of plant growth and development [33]. All of AP2/EREBP type genes were downregulated in OsWRKY13-activated lines. These genes appear to be involved in JA-mediated signalling pathways and/or the terpenoid metabolism pathway [26]. Furthermore, six of the seven Myb type genes and one of the two MADS type genes were also downregulated in OsWRKY13-activated plants. In addition, three of the four NAC type (NAM, ATAF, and CUC) genes and the two WRKY type genes were downregulated (Additional file 3). Among the downregulated NAC type genes, SNAC1, which is involved in abiotic stress responses [34], was also negatively regulated by OsWRKY13 during pathogen-induced defence responses [26]. The transcription factor gene with the greatest expressional change, Os08g44830, is putatively connected to OsWRKY13 within the flavonoid biosynthesis pathway [26]. Thus, OsWRKY13 influences the expression of a subset of genes that control some key physiological processes via interaction with W-box or W-box like cis-elements [9,26]. OsWRKY13 may have further effects on additional genes through other transcription factors.
W-boxes overrepresented in the promoter regions of OsWRKY13-upregulated genes
Functional cis-elements on plant promoters are typically found within a 2-kb range upstream of the translation start site [18,35]. To predict the genes that are directly regulated by WRKY proteins, promoter sequences comprising the 2 kb upstream of the translation start site (ATG) were analyzed. Our previous study identified 236 upregulated and 273 downregulated genes in OsWRKY13-activated lines [26]. Only the promoter regions of 211 upregulated and 257 downregulated genes had transcription unit information annotated by TIGR, however, and were analyzed in this study. Using the method applied in this study to find conserved sequences on both strands of these promoters, a wide distribution of W-boxes [TTGAC, TTGAC(C/T), TTTGAC(C/T), and TTGACA] in both up- and downregulated genes was identified, but the TTGAC, TTGAC(C/T) and TTGACT elements were overrepresented in 207, 190 and 149 upregulated genes, respectively (Table 1, [19,21,36-44]). Furthermore, two conserved motifs, GTTGAC(C/T) (P = 4.68e-06) and TTGACCTC, were significantly enriched in both strands of the promoters of upregulated genes (Table 2, [18,19,21,22,45-55]). The two motifs contain the typical W-box TTGAC(C/T) [18,19,21]. Thus, they were considered as variant W-boxes. The GTTGACC (P = 1.20e-06) was more enriched than GTTGACT (P = 9.05e-06) in both strands of the promoters. The GTTGAC motif containing the core of a W-box (TTGAC) was also enriched in both strands of the upregulated gene promoters. These results suggest that WRKY transcription factor(s) may play important roles in the regulation of the differentially expressed genes, especially the upregulated genes in OsWRKY13-activated lines, but it is unknown whether these upregulated genes are directly monitored by OsWRKY13 and/or other WRKY proteins.
Table 1.
Frequency of occurrence of known cis-elements in OsWRKY13-regulated genesa
| Cis-Element type | Type of transcription factor | Motif sequence | Observed occurrenceb | Expected occurrence | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Up | Down | Total | Up | Down | Total | ||||
| W-box (core) | WRKY | TTGAC | 922* | 999 | 1921* | 780 | 950 | 1729 | 21 |
| W-box | WRKY | TTGAC(C/T) | 509* | 529 | 1038* | 409 | 498 | 907 | 21 |
| W-box | WRKY | TTGACC | 211 | 243 | 454 | 177 | 216 | 393 | 21 |
| W-box | WRKY | TTGACT | 298* | 287 | 585* | 232 | 283 | 515 | 21 |
| W-box | WRKY | TTTGAC(C/T) | 194 | 238 | 432 | 181 | 220 | 401 | 19 |
| W-box | WRKY | TTGACA | 269 | 303 | 572 | 249 | 304 | 553 | 21 |
| G-box | bZIP, GBF, bHLH | CACGTG | 89 | 113 | 202 | 90 | 109 | 199 | 36 |
| as-1 | bZIP, TGA-type | TGACG | 498 | 637 | 1135 | 482 | 587 | 1069 | 37 |
| DRE | AP2/EREBP, DREB | ACCGACA | 26 | 25 | 51 | 30 | 36 | 66 | 38 |
| CRT | AP2/EREBP, CBF | (A/G)CCGAC | 270 | 290 | 560 | 275 | 336 | 611 | 39 |
| GCC-box | AP2/EREBP, AP2 | GCCGCC | 543* | 461* | 1004* | 623 | 759 | 1383 | 40 |
| MADS | MADS | CC(A/T)6CG | 30 | 29 | 59 | 27 | 33 | 61 | 41 |
| NACRS | NAC | CATGTG | 287 | 378 | 665 | 294 | 358 | 651 | 42 |
| Myb1 | MYB | (A/C)TCC(A/T)ACC | 58 | 72 | 130 | 62 | 75 | 137 | 37 |
| Myb2 | MYB | TAAC(G/C)GTT | 13 | 18 | 31 | 13 | 16 | 28 | 37 |
| Myb3 | MYB | TAACTAAC | 19 | 35* | 54* | 13 | 16 | 29 | 37 |
| Myb4 | MYB | A(A/C)C(A/T)A(A/C)C | 511 | 659 | 1170 | 515 | 627 | 1142 | 37 |
| EIN3/EIL | EIN3/EIL | GGATGTA | 39 | 33 | 72 | 39 | 47 | 85 | 43 |
| CCAATBOX1 | heat shock element | CCAAT | 858 | 1015 | 1873 | 858 | 1045 | 1903 | 44 |
aOnly the cis-elements putatively bound by the transcription factor types or related ones (Additional file 3), whose expression was regulated by OsWRKY13, were analyzed.
bP-values < 0.05 (chi-square test and corrected for multiple comparisons using the Bonferroni correction) in each category are indicated with an asterisk.
Table 2.
Enumerative selection of overrepresented motifs harbouring known cis-elements in the promoters of OsWRKY13-regulated genes
| Overrepresented motif sequencea | Strandb | P-valuec | Known homologous cis-element | |||
|---|---|---|---|---|---|---|
| Elementd | Transcription factor type | Potential signalling pathway | Reference | |||
| Upregulated | ||||||
| GTTGACC | bs | 1.20e-06 | W-box | WRKY | biotic/abiotic response, development | 18, 19, 21 |
| GTTGACT | bs | 9.05e-06 | W-box | WRKY | biotic/abiotic response, development | 18, 19, 21 |
| GTTGAC | bs | 1.21e-06 | W-box (core) | WRKY | biotic/abiotic response, development | 18, 19, 21 |
| TTGACCTC | bs | 3.80e-06 | W-box | WRKY | biotic/abiotic response, development | 18, 19, 21 |
| TGCTGCCGC | ss | 7.52e-06 | PRE2 | Rad51-like | biotic response | 22 |
| ATGGTGAA | ss/bs | 4.99e-06 | GTGA motif | unknown | pollen development | 45 |
| GTGCAGAAAT | ss | 3.92e-06 | POLLEN1LELAT52 | unknown | pollen development | 46 |
| ATTCTGTCAG | bs | 6.82e-06 | BIHD1OS | homeodomain | defence response | 47 |
| Downregulated | ||||||
| CGTACG | bs/ss | 2.13e-06 | CURECORECR | SBP domain family | copper response | 48 |
| GTACGTAC | bs/ss | 3.05e-06 | ACGTATERD1, ACGTABOX | AP2/EREBP, bZIP | dehydration response, seed development | 49, 50 |
| A(C/G)AGTGAC | bs/ss | 3.81e-06 | GTGA motif | unknown | pollen development | 45 |
| GAAAGTCCGG | bs/ss | 6.06e-06 | DOFCOREZM/EECCRCAH1 (-) | ZF-DOF | carbon metabolism | 51 |
| GGTTAGTTA | bs | 7.19e-07 | Myb1 | MYB | defence response | 40 |
| TATTGGTTGT | ss | 2.91e-06 | REALPHALGLHCB21 (-), AREOSREP1 | unknown | Phytochrome regulation gibberellin response | 52, 53 |
| CTACTGGC | bs | 4.27e-06 | CACTFTPPCA1 | unknown | carbon metabolism | 54 |
| GTGCAATTAT | ss | 6.69e-06 | CAATBOX1 | unknown | tissue-specific response | 55 |
a The known cis-elements in the overrepresented sequences are in bold.
b The letters "bs" or "ss" designate whether the element was detected as overrepresented on both strands (bs) or just on the sense strand (ss); "bs/ss" refers to consensus sequence from bs and ss with priority on both strands and "ss/bs" with priority on the sense strand.
c The P-value of motif with bs/ss or ss/bs annotation was calculated by average of the P-values for bb and ss.
d Dash indicates that the complementary sequence of the known cis-element is harboured by the conserved motif.
A subset of WRKY family members were influenced by OsWRKY13
To examine whether the other rice WRKY family members are directly monitored by WRKY proteins, the expression profiling of WRKY family members in OsWRKY13-activated lines was analyzed using the microarray data (GEO database accession number GSE8380). In total, 98 WRKY family members in rice were identified from the TIGR database and the literature [56]. Analysis of the promoters of these WRKY genes showed overrepresentation of different W-boxes (P < 0.05, Additional file 4), suggesting that self-regulation by the WRKY family plays an important role. However, only 42 WRKY members, including overexpressed OsWRKY13 and downregulated OsWRKY14 and OsWRKY42 (Additional file 3), produced a hybridization signal (P < 0.05) in the rice whole genome microarray chip. The 42 WRKY genes were classified into two groups based on a comparison of their expression patterns in two OsWRKY13-activated lines and wild type (Additional file 5). Twenty-seven of the 42 WRKY genes were clustered into the downregulated group and 15 into the upregulated group, although most of the genes were not significantly differentially expressed in the chip based on the 2-fold change threshold.
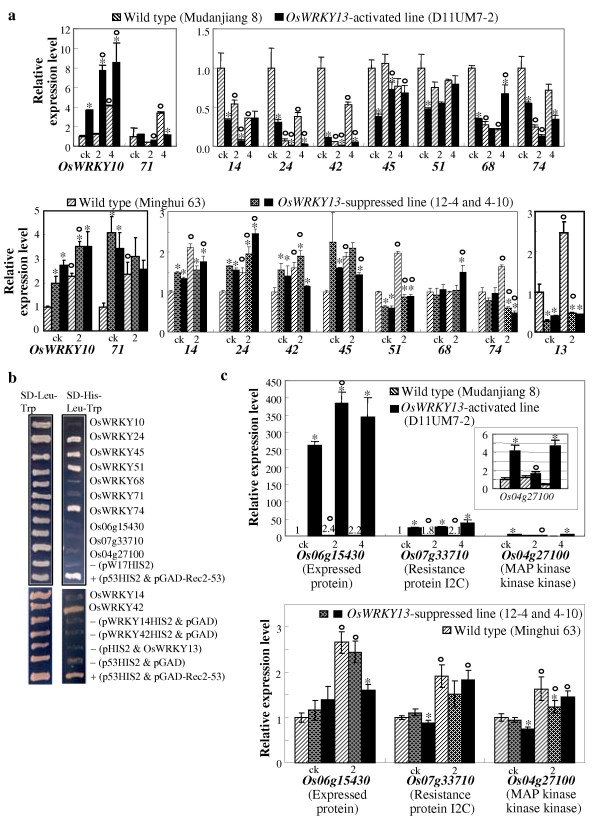
Consistent with the classification in the microarray data (Additional file 5), using qRT-PCR analyses we confirmed that other WRKY genes also showed differential expression after activation of OsWRKY13 when free of pathogen infection. These include the upregulation of OsWRKY10 and the downregulation of OsWRKY14, OsWRKY24, OsWRKY42, OsWRKY45, OsWRKY51, OsWRKY68, and OsWRKY74 (Figure 1a). The analyses also showed that the expression levels of OsWRKY10 and OsWRKY68 in OsWRKY13-activated plants were significantly higher than that in wild type and the expression levels of OsWRKY14, OsWRKY24, OsWRKY42, OsWRKY45, and OsWRKY71 in OsWRKY13-activated plants were significantly lower than that in wild type on at least one time point after pathogen infection. Furthermore, pathogen infection significantly induced the expression of OsWRKY10 and OsWRKY71 and suppressed the expression of OsWRKY14, OsWRKY24, OsWRKY42, OsWRKY68, and OsWRKY74 in wild-type plants; pathogen infection also significantly induced OsWRKY10, OsWRKY45, and OsWRKY68 and suppressed OsWRKY71, OsWRKY14, OsWRKY24, OsWRKY42, and OsWRKY74 in OsWRKY13-activated plants (Figure 1a).
Figure 1.
Analyses of rice WRKY gene expression and OsWRKY13 DNA-binding activity. (a) and (c). Expression patterns of WRKY and OsWRKY13-activated genes genes after inoculation of Xoo strain PXO61 at booting stage. Samples were obtained before (ck) and at 2 and 4 d after pathogen inoculation. The expression level of each gene in transgenic plants was calculated relative to that in non-inoculated wild-type plants. Circle indicates a significant difference (P < 0.05) between non-inoculated and inoculated plants and asterisk indicates a significant difference (P < 0.05) between the transgenic plant and corresponding wild type within the same treatment. Bars represent mean (three replicates) ± standard deviation. (b) Yeast one-hybrid assay using OsWRKY13 as target protein and target DNA fragments from the promoters of rice WRKY genes and three other genes as baits. +, positive control; -, negative control; pW17HIS2, OsWRKY13 promoter fragment without W-box. All experiments were performed twice with similar results.
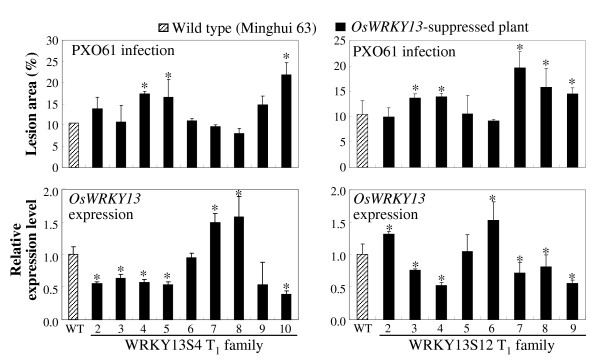
To examine whether the differential expression of these WRKY genes was due to the non-physiologic overexpression of OsWRKY13, RNAi strategy was used to generate OsWRKY13-suppressed plants. Twenty-one independent transformants were obtained. These plants were inoculated with Xoo strain PXO61 at booting stage. Ten plants showed significantly increased susceptibility (P < 0.05) compared to wild-type Minghui 63 (data not shown). Four T1 families from four T0 plants, WRKY13S2, WRKY13S4, WRKY13S5, and WRKY13S12 that showed increased susceptibility or suppressed OsWRKY13 expression, were further analyzed for their resistance to PXO61 and OsWRKY13 transcript level. The increased susceptibility cosegregated with the reduced OsWRKY13 transcripts in the four families (Figure 2 for two families and Additional file 6 for another two families). Two independent OsWRKY13-suppressed T1 plants (WRKY13S4-10 and WRKY13S12-4), which showed increased susceptibility and suppressed OsWRKY13 expression, were used to analyze the expression of these WRKY genes after pathogen infection. The expression patterns of OsWRKY71, OsWRKY14, OsWRKY24, OsWRKY42, OsWRKY45, OsWRKY68, and OsWRKY74 in OsWRKY13-suppressed lines were complementary to those in OsWRKY13-activated plants in at least one time point examined (Figure 1b). Suppression of OsWRKY13 also influenced the expression of OsWRKY10 and OsWRKY51. However, the expression patterns of OsWRKY10 and OsWRKY51 in OsWRKY13-suppressed lines were similar as those in OsWRKY13-activated plants (Figure 1a). These results suggest that these WRKY genes regulated directly or indirectly by OsWRKY13 may be also involved in pathogen-induced defence responses and OsWRKY10 and OsWRKY51 may be also regulated by other transcription factor(s) that was influenced by OsWRKY13.
Figure 2.
The increased susceptibility cosegregated with suppressed expression of OsWRKY13 in two OsWRKY13-suppressed T1 families. Disease was scored at 14 d after infection of Xoo strain PXO61. RNA samples were obtained after disease scoring. The expression level of OsWRKY13 in OsWRKY13-suppressed plants was calculated relative to that in wild-type (WT) Minghui 63. Bars represent mean (three leaves for lesion area and three replicates for expression level) ± standard deviation. Asterisk indicates a significant difference (P < 0.05) from wild-type Minghui 63.
The nine WRKY genes analyzed (Figure 1a) all harbour W-boxes in their promoters. To evaluate whether these WRKY genes were directly influenced by OsWRKY13, yeast one-hybrid assays were performed. Detection of protein-DNA binding activity by growth performance on SD-His-Leu-Trp agar medium showed that OsWRKY13 possessed specific DNA-binding ability to the promoters of OsWRKY24, OsWRKY42, OsWRKY45, OsWRKY51, and OsWRKY74, but not to those of OsWRKY10, OsWRKY14, OsWRKY68, and OsWRKY71 (Figure 1b). The expression of all the genes whose promoters were bound by OsWRKY13 was suppressed in OsWRKY13-activated plants, suggesting that OsWRKY13 may bind preferentially to the promoters of downregulated genes in vitro. To examine this hypothesis, we randomly analyzed OsWRKY13 binding activity to the promoters of Os06g15430, Os07g33710, and Os04g27100, which showed markedly induced expression in OsWRKY13-activated plants and a tendency of reduced expression in OsWRKY13-suppressed lines (Figure 1c; [26]) and their promoters also harbour W-boxes. Yeast one-hybrid assay showed that OsWRKY13 did not bind to the promoters of the three genes (Figure 1b). Thus, OsWRKY13 appears to bind preferentially to the promoters of those genes whose expression was suppressed in OsWRKY13-activated plants.
The promoters of OsWRKY13-influenced genes contain multiple types of other known cis-elements
In addition to W-boxes, other cis-elements required for binding of different types of transcription factors (including some of the types listed in Additional file 3: bHLH, AP2/EREBP, MADS, NAC, MYB, EIL, and CCAAT-binding protein) were identified in OsWRKY13-influenced genes (Table 1). Among these cis-elements, Myb3 for binding of MYB type transcription factors was overrepresented in the promoters of downregulated genes. GCC-box for binding of AP2/EREBP type transcription factors was underrepresented from both up- and downregulated genes (Table 1).
Conserved motifs harbouring known cis-elements were also identified in the promoters of OsWRKY13-influenced genes, but only a few of the known cis-elements are putatively bound by the types of transcription factors regulated by OsWRKY13 (Table 2). The GGTTAGTTA element enriched in the promoters of OsWRKY13-downregulated genes harboured the Myb1 element (GTTAGTT, [40]), putatively for MYB protein binding. The GTACGTAC motif, harbouring the ACGTATERD1 and ACGTABOX elements for binding of AP2/EREBP or bZIP types of proteins, was also enriched in OsWRKY13-downregulated genes. The other conserved motifs harbour known cis-elements, which are involved in biotic/abiotic responses, pollen development, and hormone responses and bound by proteins not classified among the transcription factors listed in Additional file 3 or by unknown proteins (Table 2).
The OsWRKY13-influenced genes are enriched with novel elements in their promoters
Twelve novel elements, which were not included in the PLACE and PlantCARE databases or reported in the literature, were overrepresented in the promoters of OsWRKY13-influenced genes (Table 3). Seven of the 12 elements were located in both strands of the promoters, and the remaining five elements were strand-dependent. Novel elements 6 and 7, enriched in the promoters of OsWRKY13-downregulated genes, each comprise two four-nucleotide repeats, CGAT and AGCT, respectively. Novel element 8 (TATATATA), overrepresented in the promoters of downregulated genes, is similar to a TATA-box (CTATAAATAC) in rice [57]. These results suggest that the OsWRKY13-regulated genes also may be monitored by WRKY or other types of transcription factors through novel cis-elements.
Table 3.
Enumerative selection of novel motifs overrepresented in the promoters of OsWRKY13-regulated genes
| Gene cluster | Motif | Consensus sequence | Stranda | P-valueb |
|---|---|---|---|---|
| Upregulated | novel 1 | TCTCGGGCAA | ss | 4.07e-06 |
| novel 2 | GCACGGCA | bs | 4.51e-06 | |
| novel 3 | ACAGGACTTA | bs | 5.14e-06 | |
| novel 4 | CTATTTCGCA | ss | 6.31e-06 | |
| novel 5 | GCTTGCGA | ss | 8.33e-06 | |
| Downregulated | novel 6 | CGATCGAT | ss/bs | 1.40e-06 |
| novel 7 | CAGCTAGCT | bs/ss | 2.65e-06 | |
| novel 8 | TATATATA | bs/ss | 4.31e-06 | |
| novel 9 | TGTGTGTGGTT | bs/ss | 6.17e-06 | |
| novel 10 | TGCTTTT | ss | 1.71e-06 | |
| novel 11 | TGGCCTAGAA | bs | 5.35e-06 | |
| novel 12 | ACATGCCTG | ss | 8.58e-06 |
aThe letters "bs" or "ss" designate whether an element was detected as overrepresented on both strands (bs) or on the sense strand (ss); "bs/ss" refers to consensus sequence from bs and ss with priority on both strands and "ss/bs" with priority on the sense strand.
bThe P-value of motif with bs/ss or ss/bs annotation was calculated by average of the P-values for bb and ss.
Discussion
Although OsWRKY13 is potentially involved in multiple physiological processes, including disease resistance, redox homeostasis, abiotic stress responses, and development [9,26], the signalling pathways related to these processes remain to be elucidated. Our present exploration of known and putative cis-acting elements involved in transcriptional regulation provides a better understanding of the signal transduction from OsWRKY13 to its downstream genes.
OsWRKY13-mediated signalling pathways are partitioned by different transcription factors
The overrepresentation of W-boxes in the promoters of upregulated genes in OsWRKY13-activated plants suggests that WRKY proteins may play important roles in the regulation of this cluster of genes. The evidence that at least nine WRKY genes are influenced by OsWRKY13 supports this hypothesis. However, the expression of eight of the nine WRKY genes was suppressed after activation of OsWRKY13 with or without pathogen infection, suggesting that some of the WRKY proteins might be expressional inhibitors of the upregulated genes in OsWRKY13-activated plants. The expression of all the nine WRKY genes influenced by OsWRKY13 was pathogen-responsive in OsWRKY13-activated, OsWRKY13-suppressed, and/or wild-type plants, indicating that they are also involved in host-pathogen interactions. The present results also suggest that OsWRKY13-mediated signalling pathways may be directly partitioned by some WRKY proteins, such as OsWRKY24, OsWRKY45, OsWRKY51, and OsWRKY74, whose promoters could be bound by and expression influenced by OsWRKY13. OsWRKY24, OsWRKY45, OsWRKY51, and OsWRKY74 appeared to be involved in defence pathways, because their expression was pathogen-responsive in at least one of the two wild-type plants and overexpressing OsWRKY45 enhances rice resistance to fungal blast [10]. Overexpressing OsWRKY71 enhances rice resistance to bacterial blight [8]. However, the expression of OsWRKY45 and OsWRKY71 was suppressed by OsWRKY13, an activator of disease resistance, suggesting that OsWRKY45 and OsWRKY71 may play roles other than biotic responses when OsWRKY13 is activated. This hypothesis is supported by the evidence that OsWRKY45 and OsWRKY24 repress abscisic acid (ABA) induction of the ABA-inducible HVA22 promoter [56]. OsWRKY51 interacts with OsWRKY71 and results in enhanced binding affinity of OsWRKY71 to the promoter of the alpha-amylase gene and suppressed expression of the gene [13].
Consistent with suppressed expression of a subset of AP2/EREBP and MYB types of transcription factors, the promoters of the downregulated genes in OsWRKY13-activated plants are enriched with elements harbouring ACGTATERD1, Myb1, and Myb3 cis-elements for putative binding of AP2/EREBP and MYB types of proteins. The ACGTATERD1 element is water-stress responsive [49]. Myb1 and Myb3 elements are enriched in the promoters of cold- and pathogen-inducible genes [37,40]. Activation of OsWRKY13 results in plants being more sensitive to abiotic stresses, including dehydration and cold stresses, in addition to exhibiting enhanced disease resistance [26]. Thus, the AP2/EREBP and MYB types of transcription factors may play important roles in directly monitoring the expression of OsWRKY13-downregulated genes.
A group of novel and variant known cis-acting elements appear to be involved in OsWRKY13-mediated transcriptional regulation
OsWRKY13 and Arabidopsis AtWRKY70 are functional homologues in pathogen-induced defence responses, as each serves as a node of the antagonistic crosstalk between SA- and JA-dependent pathways [5,9]. However, the transcriptional regulatory mechanisms mediated by the two WRKY proteins differ. The present results show that W-boxes are only enriched in the promoters of upregulated gene in OsWRKY13-activated plants, but both up- and downregulated genes by AtWRKY70 are enriched with W-boxes [20]. The W-box like TTGAC(A/C)A and TTGAC(A/C)(C/G/T) motifs are mostly enriched in the promoters of down- and upregulated clusters by AtWRKY70, respectively [20]. The promoters of the upregulated genes by OsWRKY13 are mostly enriched with GTTGAC(C/T) and TTGACCTC motifs that harbours the typical W-box (in bold). The W-box consensus alone is insufficient for the binding of WRKY proteins and additional neighbouring nucleotides or space between adjacent W-box elements also contribute to determining high-affinity binding in vitro [58]. Thus, it appears that the 5'-residue G in the consensus GTTGAC(C/T) motif and 3'-residues TC in the TTGACCTC motif may be related to specific or high-affinity binding of certain WRKY protein(s) to the promoters of OsWRKY13-influenced genes. Ciolkowski et al. [58] reported that Arabidopsis AtWRKY6 and AtWRKY11 bind well to W-boxes that have a G residue directly 5' adjacent to the element, whereas AtWRKY26, AtWRKY38, and AtWRKY43 bind to the same motif if the 5'-residue is a T, C, or A. Furthermore, bacterial challenge changed the binding intensity of proteins to W-boxes [9]. Therefore, WRKY proteins may regulate the expression of the downstream genes by pathogen-induced modification such as phosphorylation or binding to diversified W-boxes.
The variant PRE2, ACGTATERD1, and Myb1 cis-elements for putative binding of Rad51-like, AP2/EREBP, and MYB proteins, respectively, also may be related to binding of specific proteins or function status-modified proteins. Due to the limited knowledge of cis-acting elements, the roles of the 12 novel conserved motifs identified in the promoter regions of OsWRKY13-influenced genes remains to be elucidated. However, overrepresentation of these motifs in the promoters of OsWRKY13-targeted genes suggests that they may play roles in OsWRKY13-mediated transcriptional regulation.
OsWRKY13 might bind preferentially to the promoters of downregulated genes
The bindings of OsWRKY13 to the W-box-containing promoters of 18 OsWRKY13-influenced genes, including eight up- and 10 downregulated genes, have been examined in vitro. The present results showed that OsWRKY13 bound to the promoters of five of the eight downregulated genes examined, but could not bind to the promoters of any of the four upregulated genes examined (Figure 1b). Our previous study showed that OsWRKY13 bound specifically to the promoters of two downregulated genes, OsAOS2 and OsLOX, involved in JA synthesis in defence response and one upregulated gene, PR1a, functioning in SA-dependent pathway, but OsWRKY13 could not bind to the promoters of three upregulated defence-responsive genes, OsICS1, NH1, and OsPAD4 [9]. Furthermore, OsWRKY13 can bind to its own promoter, as revealed by gel mobility shift assays [9,22]. Self-regulation of WRKY genes by their own proteins has been reported in both negative and positive feedback control [3,4,59]. The results suggest that OsWRKY13 may function more as a negative transcriptional regulator.
Conclusion
As a potential important transcriptional regulator of disease resistance, redox homeostasis, abiotic stress responses, and development, OsWRKY13-mediated signalling pathways are partitioned by different transcription factors through binding to distinctly distributed cis-acting elements in the promoters of more 500 genes. A group of novel and variant known cis-acting elements may contribute to OsWRKY13-mediated transcriptional regulation. WRKY proteins appear to play important roles in the monitoring of OsWRKY13-upregulated genes and genes involved in pathogen-induced defence responses, whereas MYB and AP2/EREBP proteins may contribute most to the control of OsWRKY13-downregulated genes. As some of the results were based only on the ectopic expression of OsWRKY13, some of the differentially expressed genes in OsWRKY13-activated plants may not really function in the downstream of OsWRKY13 in physiological condition. Although the actual transcriptional activation or suppression capability of OsWRKY13 remains to be determined, the present results certainly provide large amount of information for further targeted analyses of direct signal transduction from OsWRKY13 to its putatively downstream genes.
Authors' contributions
DQ performed microarray data, promoter, gene expression, and protein-DNA interaction analyses, and drafted the manuscript. JX generated the RNAi plants and performed cosegregating analysis, and protein-DNA interaction analyses. WX carried out promoter analysis. HC analyzed protein-DNA interaction and gene expression. XL provided biochemical and molecular analysis supports. SW contributed to data interpretation and to writing the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Primers for quantitative RT-PCR analysis. The table lists the primers sequence used for quantitative RT-PCR analysis and related GenBank accession number of each gene.
PCR primers for amplifying promoter fragments harbouring W-box or W-box like cis-elements. The table lists the primer sequences used for yeast one-hybrid assays.
Differentially expressed transcription factor genes in OsWRKY13-activated lines. The table lists the TIGR ID, fold changes, and function annotations of differentially expressed transcription factor genes in OsWRKY13-activated lines.
The statistical distribution of different W-boxes in the promoters of 98 WRKY genes. The table lists the statistical distribution of different W-boxes in the promoters of 98 WRKY genes.
Hierarchical clustering display of expression profile of rice WRKY family genes in OsWRKY13-activated lines. The figure shows the expression profile of rice WRKY family genes in OsWRKY13-activated lines. (A) transgenic line D11UM1-1; (B) transgenic line D11UM7-2; M, wild-type Mudanjiang 8; 1, 2, and 3, replication 1, 2, and 3. The fold changes of expressional differences of these genes were log2 transformed, clustered using the Cluster 3.0 program, and visualized by the Treeview program (Eisen et al., 1998. Proc. Natl. Acad. Sci. USA 95:14863–14868). Vertical lines on the right side indicate the genes that were further analyzed (see Figure 1).
The increased susceptibility cosegregated with suppressed expression of OsWRKY13 in two OsWRKY13-suppressed T1 families. The figure shows the cosegregating analysis of another two OsWRKY13-suppressed T1 families. Disease was scored at 14 d after infection of Xoo strain PXO61. RNA samples were obtained after disease scoring. The expression level of OsWRKY13 in OsWRKY13-suppressed plants was calculated relative to that in wild-type (WT) Minghui 63. Bars represent mean (three leaves for lesion area and three replicates for expression level) ± standard deviation. Asterisk indicates a significant difference (P < 0.05) from wild-type Minghui 63.
Contributor Information
Deyun Qiu, Email: qiudeyun@hotmail.com.
Jun Xiao, Email: shawn@webmail.hzau.edu.cn.
Weibo Xie, Email: xwbcn@webmail.hzau.edu.cn.
Hongtao Cheng, Email: chenghongtao@webmail.hzau.edu.cn.
Xianghua Li, Email: xhli@mail.hzau.edu.cn.
Shiping Wang, Email: swang@mail.hzau.edu.cn.
Acknowledgements
This work was supported by grants from the National Program of High Technology Development of China, the National Program on the Development of Basic Research in China, and the National Natural Science Foundation of China.
References
- Ulker B, Somssich IE. WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol. 2004;7:491–498. doi: 10.1016/j.pbi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang L. The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evol Biol. 2005;5:1–12. doi: 10.1186/1471-2148-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S, Somssich IE. Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev. 2002;16:1139–1149. doi: 10.1101/gad.222702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen Z. Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol. 2002;129:706–716. doi: 10.1104/pp.001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Brader G, Palva ET. The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell. 2004;16:319–331. doi: 10.1105/tpc.016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Qamar SA, Chen Z, Mengiste T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006;48:592–605. doi: 10.1111/j.1365-313X.2006.02901.x. [DOI] [PubMed] [Google Scholar]
- Liu XQ, Bai XQ, Qian Q, Wang XJ, Chen MS, Chu CC. OsWRKY03, a rice transcriptional activator that functions in defense signaling pathway upstream of OsNPR1. Cell Res. 2005;15:593–603. doi: 10.1038/sj.cr.7290329. [DOI] [PubMed] [Google Scholar]
- Liu X, Bai X, Wang X, Chu C. OsWRKY71, a rice transcription factor, is involved in rice defense response. J Plant Physiol. 2007;164:969–979. doi: 10.1016/j.jplph.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Qiu D, Xiao J, Ding X, Xiong M, Cai M, Cao Y, Li X, Xu C, Wang S. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact. 2007;20:492–499. doi: 10.1094/MPMI-20-5-0492. [DOI] [PubMed] [Google Scholar]
- Shimono M, Sugano S, Nakayama A, Jiang CJ, Ono K, Toki S, Takatsuji H. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell. 2007;19:2064–2076. doi: 10.1105/tpc.106.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CS, Kolevski B, Smyth DR. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell. 2002;14:1359–1375. doi: 10.1105/tpc.001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Dennis ES, Berger F, Peacock WJ, Chaudhury A. MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc Natl Acad Sci USA. 2005;102:17531–17536. doi: 10.1073/pnas.0508418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Zhang ZL, Zou X, Yang G, Komatsu S, Shen QJ. Interactions of two abscisic-acid induced WRKY genes in repressing gibberellin signaling in aleurone cells. Plant J. 2006;46:231–242. doi: 10.1111/j.1365-313X.2006.02694.x. [DOI] [PubMed] [Google Scholar]
- Zhang ZL, Xie Z, Zou X, Casaretto J, Ho TH, Shen QJ. A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol. 2004;134:1500–1513. doi: 10.1104/pp.103.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Palmqvist S, Olsson H, Boren M, Ahlandsberg S, Jansson C. A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell. 2003;15:2076–2092. doi: 10.1105/tpc.014597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wang J, Wang S, Wang J, Chen X. Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-delta-cadinene synthase-A. Plant Physiol. 2004;135:507–515. doi: 10.1104/pp.104.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N, Dubouzet E, Kokabu Y, Yoshida S, Taniguchi Y, Dubouzet JG, Yazaki K, Sato F. Identification of a WRKY protein as a transcriptional regulator of benzylisoquinoline alkaloid biosynthesis in Coptis japonica. Plant Cell Physiol. 2007;48:8–18. doi: 10.1093/pcp/pcl041. [DOI] [PubMed] [Google Scholar]
- Maleck K, Levine A, Eulgem T, Morgan A, Schmid J, Lawton KA, Dangl JL, Dietrich RA. The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nature Genet. 2000;26:403–410. doi: 10.1038/82521. [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Scie. 2000;5:199–206. doi: 10.1016/S1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- Kankainen M, Holm L. POBO, transcription factor binding site verification with bootstrapping. Nucl Acids Res. 2004;32:W222–W229. doi: 10.1093/nar/gkh463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Zipfel C, Rowland O, Keller I, Robatzek S, Boller T, Jones JD. The transcriptional innate immune response to flg22: interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol. 2004;135:1113–1128. doi: 10.1104/pp.103.036749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Qiu D, Yuan T, Ding X, Li H, Duan L, Xu C, Li X, Wang S. Identification of novel pathogen-responsive cis-elements and their binding proteins in the promoter of OsWRKY13, a gene regulating rice disease resistance. Plant Cell Environ. 2008;31:86–96. doi: 10.1111/j.1365-3040.2008.01773.x. [DOI] [PubMed] [Google Scholar]
- van Verk MC, Pappaioannou D, Neeleman L, Bol JF, Linthorst HJ. A novel WRKY transcription factor is required for induction of PR-1a gene expression by salicylic acid and bacterial elicitors. Plant Physiol. 2008;146:1983–1995. doi: 10.1104/pp.107.112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Geisler M, Trygg J, Huner N, Hurry V. Consensus by democracy: using meta-analyses of microarray and genomic data to model the cold acclimation signaling pathway in Arabidopsis. Plant Physiol. 2006;141:1219–1232. doi: 10.1104/pp.106.083527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lee KK, Walsh S, Smith C, Hadingham S, Sorefan K, Cawley G, Bevan MW. Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a relevance vector machine. Genome Res. 2006;16:414–427. doi: 10.1101/gr.4237406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu D, Xiao J, Xie W, Liu H, Li X, Xiong L, Wang S. Rice gene network inferred from expression profiling of plants overexpressing OsWRKY13, a positive regulator of disease resistance. Mol Plant. 2008;1:538–551. doi: 10.1093/mp/ssn012. [DOI] [PubMed] [Google Scholar]
- Hudson ME, Quail PH. Identification of promoter motifs involved in the network of phytochrome A-regulated gene expression by combined analysis of genomic sequence and microarray data. Plant Physiol. 2003;133:1605–1616. doi: 10.1104/pp.103.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucl Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescot M, Déhais P, Moreau Y, De-Moor B, Rouzé P, Rombauts S. PlantCARE: a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucl Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YJ, Zhang Q. Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep. 2005;23:540–547. doi: 10.1007/s00299-004-0843-6. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang S, Zhang Q. New gene for bacterial blight resistance in rice located on chromosome 12 identified from Minghui 63, an elite restorer line. Phytopathology. 2002;92:750–754. doi: 10.1094/PHYTO.2002.92.7.750. [DOI] [PubMed] [Google Scholar]
- Wang G, Ding X, Yuan M, Qiu D, Li X, Xu C, Wang S. Dual function of rice OsDR8 gene in disease resistance and thiamine accumulation. Plant Mol Biol. 2006;60:437–449. doi: 10.1007/s11103-005-4770-x. [DOI] [PubMed] [Google Scholar]
- Qu LJ, Zhu YX. Transcription factor families in Arabidopsis: major progress and outstanding issues for future research. Curr Opin Plant Biol. 2006;9:544–549. doi: 10.1016/j.pbi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci USA. 2006;103:12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombauts S, Florquin K, Lescot M, Marchal K, Rouzé P, Peer Y van de. Computational approaches to identify promoters and cis-regulatory elements in plant genomes. Plant Physiol. 2003;132:1162–1176. doi: 10.1104/pp.102.017715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler U, Beckmann H, Cashmore AR. TGA1 and G-box binding factors: two distinct classes of Arabidopsis leucine zipper proteins compete for the G-box-like element TGACGTGG. Plant Cell. 1992;4:1309–1319. doi: 10.1105/tpc.4.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, Mauch F, Luan S, Zou G, Whitham SA, Budworth PR, Tao Y, Xie Z, Chen X, Lam S, Kreps JA, Harper JF, Si-Ammour A, Mauch-Mani B, Heinlein M, Kobayashi K, Hohn T, Dangl JL, Wang X, Zhu T. Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell. 2002;14:559–574. doi: 10.1105/tpc.010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003;33:751–763. doi: 10.1046/j.1365-313X.2003.01661.x. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Sakuma Y, Kasuga M, Ito Y, Seki M, Goda H, Shimada Y, Yoshida S, Shinozaki K, Yamaguchi-Shinozaki K. Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant J. 2004;38:982–993. doi: 10.1111/j.1365-313X.2004.02100.x. [DOI] [PubMed] [Google Scholar]
- Chakravarthy S, Tuori RP, D'Ascenzo MD, Fobert PR, Despres C, Martin GB. The tomato transcription factor Pti4 regulates defence-related gene expression via GCC box and non-GCC box cis elements. Plant Cell. 2003;15:3033–3050. doi: 10.1105/tpc.017574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann JL, Wang M, Meyerowitz EM. DNA-binding properties of Arabidopsis MADS domain homeotic proteins APETALA1, APETALA3, PISTILLATA and AGAMOUS. Nucl Acids Res. 1996;24:3134. doi: 10.1093/nar/24.16.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran LS, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell. 2004;16:2481–2498. doi: 10.1105/tpc.104.022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Gene Dev. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralampidis K, Milioni D, Rigas S, Hatzopoulos P. Combinatorial interaction of cis elements specifies the expression of the Arabidopsis AtHsp90-1 gene. Plant Physiol. 2002;129:1138–1149. doi: 10.1104/pp.004044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers HJ, Bate N, Combe J, Sullivan J, Sweetman J, Swan C, Lonsdale DM, Twell D. Functional analysis of cis-regulatory elements within the promoter of the tobacco late pollen gene g10. Plant Mol Biol. 2001;45:577–585. doi: 10.1023/A:1010695226241. [DOI] [PubMed] [Google Scholar]
- Filichkin SA, Leonard JM, Monteros A, Liu PP, Nonogaki H. A novel endo-beta-mannanase gene in tomato LeMAN5 is associated with anther and pollen development. Plant Physiol. 2004;134:1080–1087. doi: 10.1104/pp.103.035998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Song F, Goodman RM, Zheng Z. Up-regulation of OsBIHD1, a rice gene encoding BELL homeodomain transcriptional factor, in disease resistance responses. Plant Biol (Stuttg) 2005;7:459–468. doi: 10.1055/s-2005-865851. [DOI] [PubMed] [Google Scholar]
- Kropat J, Tottey S, Birkenbihl RP, Depège N, Huijser P, Merchant S. A regulator of nutritional copper signaling in Chlamydomonas is an SBP domain protein that recognizes the GTAC core of copper response element. Proc Natl Acad Sci USA. 2005;102:18730–18735. doi: 10.1073/pnas.0507693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SD, Nakashima K, Narusaka Y, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J. 2003;33:259–270. doi: 10.1046/j.1365-313X.2003.01624.x. [DOI] [PubMed] [Google Scholar]
- Izawa T, Foster R, Nakajima M, Shimamoto K, Chua NH. The rice bZIP transcriptional activator RITA-1 is highly expressed during seed development. Plant Cell. 1994;6:1277–1287. doi: 10.1105/tpc.6.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S, Schmidt RJ. Diversity and similarity among recognition sequences of Dof transcription factors. Plant J. 1999;17:209–214. doi: 10.1046/j.1365-313X.1999.00363.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Tobin EM. A DNA binding activity for one of two closely defined phytochrome regulatory elements in an Lhcb promoter is more abundant in etiolated than in green plants. Plant Cell. 1996;8:31–41. doi: 10.1105/tpc.8.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoh K, Yamauchi D. Two cis-acting elements necessary and sufficient for gibberellin-upregulated proteinase expression in rice seeds. Plant J. 2003;34:635–645. doi: 10.1046/j.1365-313X.2003.01753.x. [DOI] [PubMed] [Google Scholar]
- Gowik U, Burscheidt J, Akyildiz M, Schlue U, Koczor M, Streubel M, Westhoff P. cis-Regulatory elements for mesophyll-specific gene expression in the C4 plant Flaveria trinervia, the promoter of the C4 phosphoenolpyruvate carboxylase gene. Plant Cell. 2004;16:1077–1090. doi: 10.1105/tpc.019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirsat A, Wilford N, Croy R, Boulter D. Sequences responsible for the tissue-specific promoter activity of a pea legumin gene in tobacco. Mol Gen Genet. 1989;215:326–331. doi: 10.1007/BF00339737. [DOI] [PubMed] [Google Scholar]
- Xie Z, Zhang ZL, Zou X, Huang J, Ruas P, Thompson D, Shen QJ. Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol. 2005;137:176–189. doi: 10.1104/pp.104.054312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace ML, Chandrasekharan MB, Hall TC, Crowe AJ. Sequence and spacing of TATA box elements are critical for accurate initiation from the beta-phaseolin promoter. J Biol Chem. 2004;279:8102–8110. doi: 10.1074/jbc.M309376200. [DOI] [PubMed] [Google Scholar]
- Ciolkowski I, Wanke D, Birkenbihl RP, Somssich IE. Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Mol Biol. 2008;68:81–92. doi: 10.1007/s11103-008-9353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers for quantitative RT-PCR analysis. The table lists the primers sequence used for quantitative RT-PCR analysis and related GenBank accession number of each gene.
PCR primers for amplifying promoter fragments harbouring W-box or W-box like cis-elements. The table lists the primer sequences used for yeast one-hybrid assays.
Differentially expressed transcription factor genes in OsWRKY13-activated lines. The table lists the TIGR ID, fold changes, and function annotations of differentially expressed transcription factor genes in OsWRKY13-activated lines.
The statistical distribution of different W-boxes in the promoters of 98 WRKY genes. The table lists the statistical distribution of different W-boxes in the promoters of 98 WRKY genes.
Hierarchical clustering display of expression profile of rice WRKY family genes in OsWRKY13-activated lines. The figure shows the expression profile of rice WRKY family genes in OsWRKY13-activated lines. (A) transgenic line D11UM1-1; (B) transgenic line D11UM7-2; M, wild-type Mudanjiang 8; 1, 2, and 3, replication 1, 2, and 3. The fold changes of expressional differences of these genes were log2 transformed, clustered using the Cluster 3.0 program, and visualized by the Treeview program (Eisen et al., 1998. Proc. Natl. Acad. Sci. USA 95:14863–14868). Vertical lines on the right side indicate the genes that were further analyzed (see Figure 1).
The increased susceptibility cosegregated with suppressed expression of OsWRKY13 in two OsWRKY13-suppressed T1 families. The figure shows the cosegregating analysis of another two OsWRKY13-suppressed T1 families. Disease was scored at 14 d after infection of Xoo strain PXO61. RNA samples were obtained after disease scoring. The expression level of OsWRKY13 in OsWRKY13-suppressed plants was calculated relative to that in wild-type (WT) Minghui 63. Bars represent mean (three leaves for lesion area and three replicates for expression level) ± standard deviation. Asterisk indicates a significant difference (P < 0.05) from wild-type Minghui 63.