Abstract
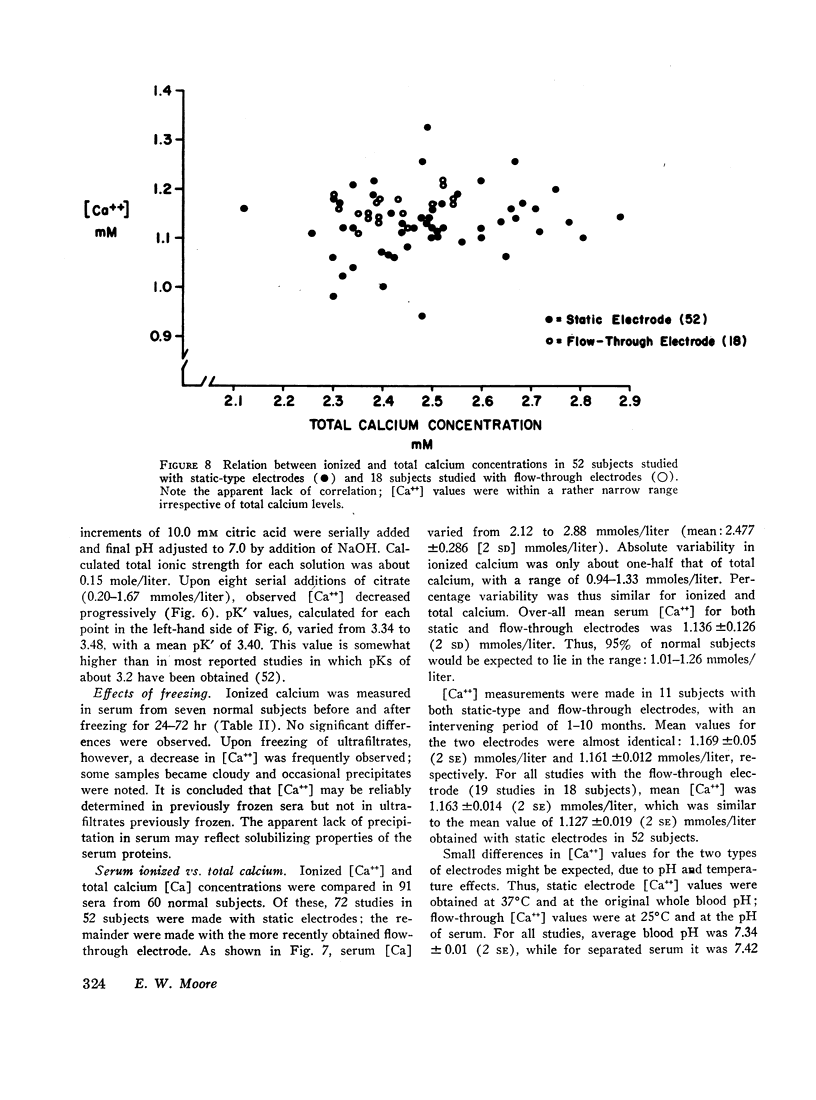
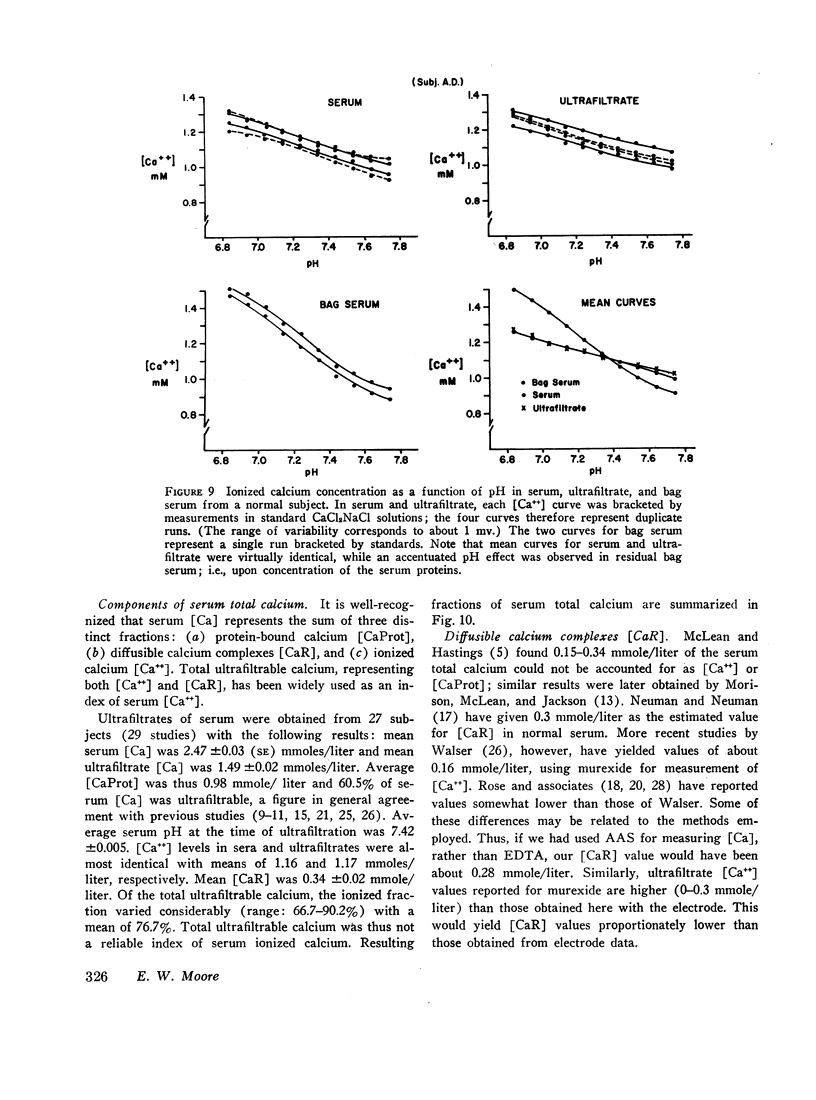
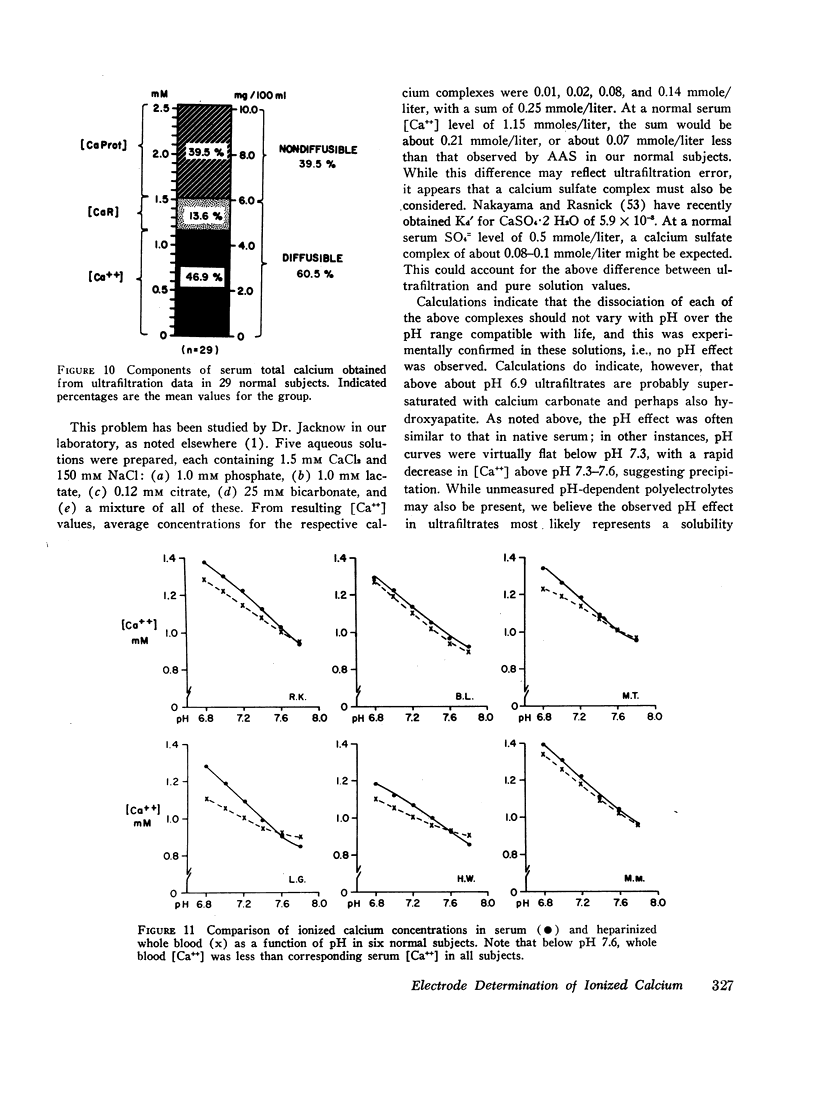
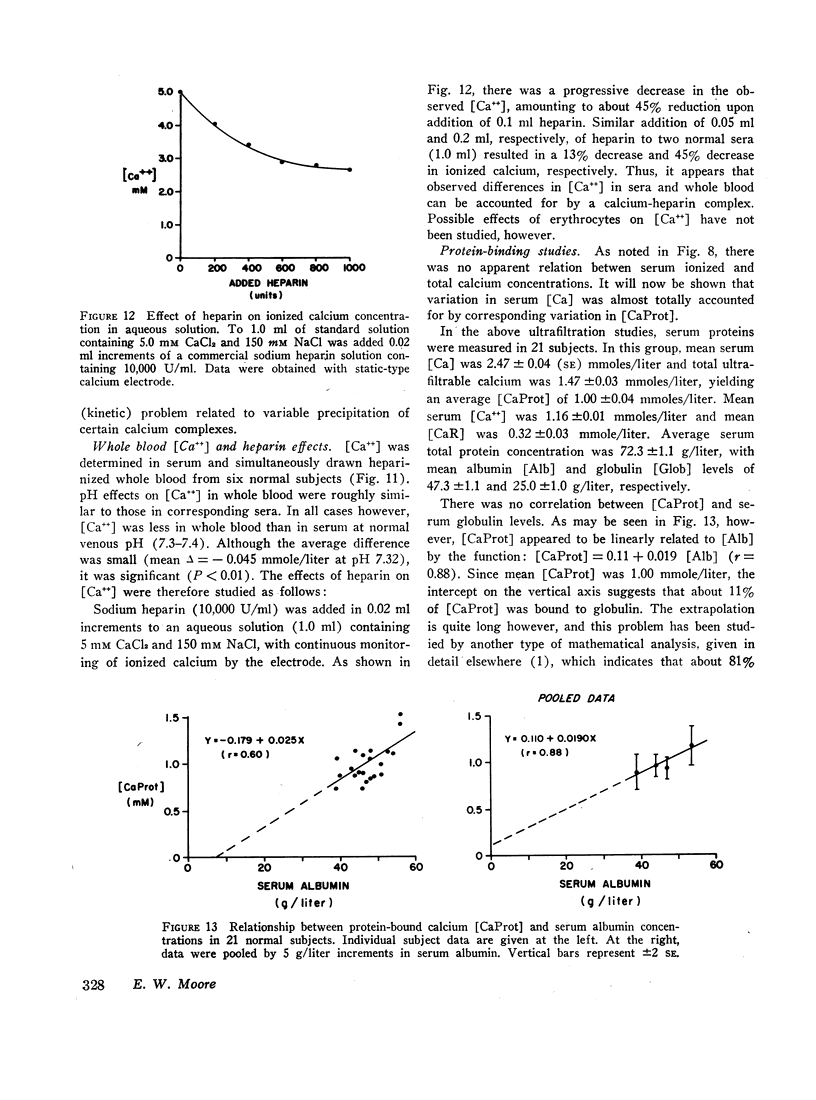
Ion-exchange calcium electrodes represent the first practical method for the direct measurement of ionized calcium [Ca++] in biologic fluids. Using both “static” and “flow-through” electrodes, serum [Ca++] was within a rather narrow range: 0.94-1.33 mmoles/liter (mean, 1.14 mmoles/liter). Within a given individual, [Ca++] varied only about 6% over a several month period. Consistent pH effects on [Ca++] were observed in serum and whole blood, [Ca++] varying inversely with pH. Less consistent pH effects were also noted in ultrafiltrates, believed to largely represent precipitation of certain calcium complexes from a supersaturated solution. Heparinized whole blood [Ca++] was significantly less than in corresponding serum at normal blood pH, related to the formation of a calcium-heparin complex. [Ca++] in ultrafiltrates represented a variable fraction (66.7-90.2%) of total diffusible calcium. There was no apparent correlation between serum ionized and total calcium concentrations. Thus, neither serum total calcium nor total ultrafiltrable calcium provided a reliable index of serum [Ca++]. Change in serum total calcium was almost totally accounted for by corresponding change in protein-bound calcium [CaProt]. About 81% of [CaProt] was estimated to be bound to albumin and about 19% to globulins. From observed pH, serum protein, and [CaProt] data, a nomogram was developed for estimating [CaProt] without ultrafiltration. Data presented elsewhere indicate that calcium binding by serum proteins obeys the mass-law equation for a monoligand association. This was indicated in the present studies by a close correspondence of observed serum [Ca++] values with those predicted by the McLean-Hastings nomogram. While these electrodes allow study of numerous problems not possible previously, they have not been perfected to the same degree of reliability obtainable with current pH electrodes. The commercial (Orion flow-through) electrode is: (a) expensive. (b) requires periodic replacement of membranes, and (c) has not yet been thermostated. As with blood pH measurements. (d) electrode response is logarithmic, i.e. small potential errors generate rather large [Ca++] errors. (e) loss of CO2 should be prevented, and (f) errors due to other cations must be considered under certain conditions. Despite these limitations, we believe the electrode represents a major advance in calcium metabolism.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACHRA B. N., DAUER A., SOBEL A. E. The complexometric titration of micro and ultramicro quantities of calcium in blood serum, urine, and inorganic salt solutions. Clin Chem. 1958 Apr;4(2):107–119. [PubMed] [Google Scholar]
- EISENMAN G., RUDIN D. O., CASBY J. U. Glass electrode for measuring sodium ion. Science. 1957 Oct 25;126(3278):831–834. doi: 10.1126/science.126.3278.831. [DOI] [PubMed] [Google Scholar]
- ETTORI J., SCOGGAN S. M. Ionized calcium in biological media. Nature. 1959 Oct 24;184(Suppl 17):1315–1316. doi: 10.1038/1841315b0. [DOI] [PubMed] [Google Scholar]
- FANCONI A., ROSE G. A. The ionized, complexed, and protein-bound fractions of calcium in plasma; an investigation of patients with various diseases which affect calcium metabolism, with an additional study of the role of calcium ions in the prevention of tetany. Q J Med. 1958 Oct;27(108):463–494. [PubMed] [Google Scholar]
- HOPKINS T., HOWARD J. E., EISENBERG H. Ultrafiltration studies on calcium and phosphorus in human serum. Bull Johns Hopkins Hosp. 1952 Jul;91(1):1–21. [PubMed] [Google Scholar]
- LLOYD H. M., ROSE G. A. Ionised, protein-bound, and complexed calcium in the plasma in primary hyperparathyroidism. Lancet. 1958 Dec 13;2(7059):1258–1261. doi: 10.1016/s0140-6736(58)91390-4. [DOI] [PubMed] [Google Scholar]
- LLOYD H. M., ROSE G. A., SMEENK D. The ability of plasma proteins to bind calcium in normal subjects, in patients with primary hyperparathyroidism both pre- and post-operatively, and in other hypercalcaemic conditions. Clin Sci. 1962 Jun;22:353–362. [PubMed] [Google Scholar]
- LOKEN H. F., HAVEL R. J., GORDAN G. S., WHITTINGTON S. L. Ultracentrifugal analysis of protein-bound and free calcium in human serum. J Biol Chem. 1960 Dec;235:3654–3658. [PubMed] [Google Scholar]
- Marrack J., Thacker G. The State of Calcium in Body Fluids. Biochem J. 1926;20(3):580–594. doi: 10.1042/bj0200580. [DOI] [PMC free article] [PubMed] [Google Scholar]
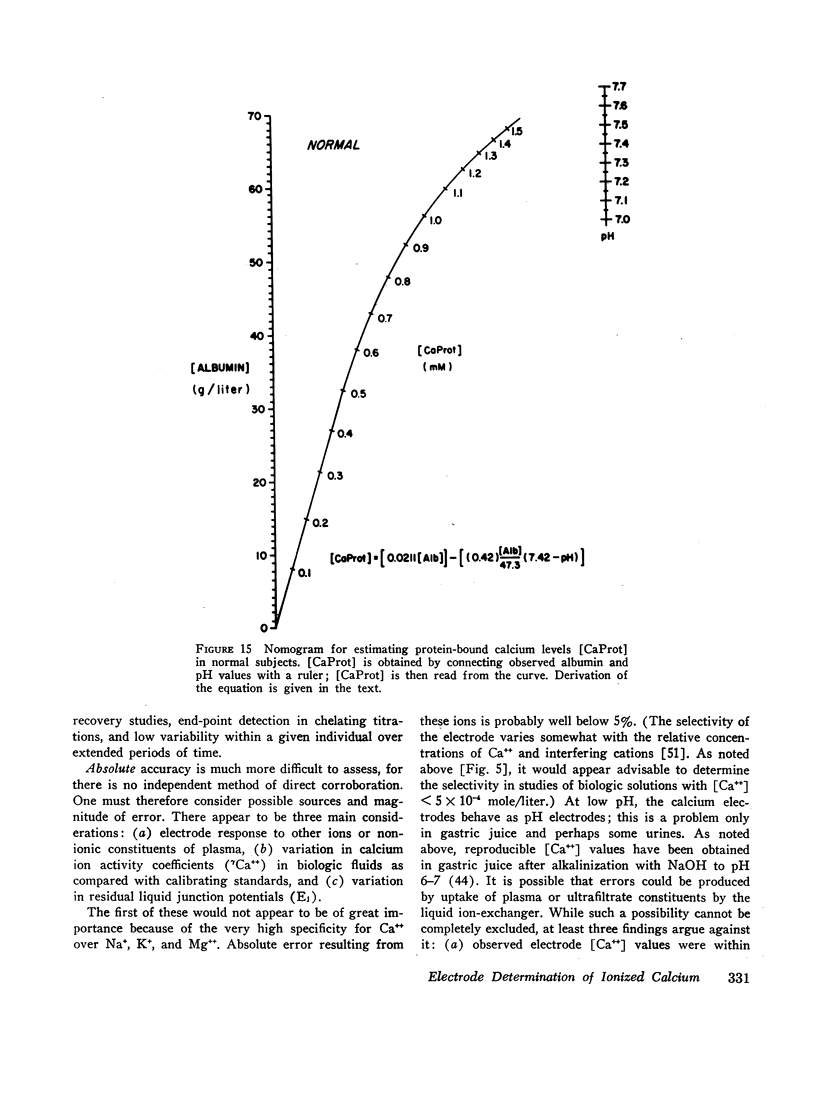
- Moore E. W., Makhlouf G. M. Calcium in normal human gastric juice. A four-component model with speculation on the relation of calcium to pepsin secretion. Gastroenterology. 1968 Oct;55(4):465–480. [PubMed] [Google Scholar]
- Moore E. W., Wilson D. W. THE DETERMINATION OF SODIUM IN BODY FLUIDS BY THE GLASS ELECTRODE. J Clin Invest. 1963 Mar;42(3):293–304. doi: 10.1172/JCI104716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRASAD A. S., FLINK E. B. The base binding property of the serum proteins with respect to calcium. J Lab Clin Med. 1958 Mar;51(3):345–350. [PubMed] [Google Scholar]
- PRASAD A. S. Studies on ultrafiltrable calcium. Arch Intern Med. 1960 Apr;105:560–573. doi: 10.1001/archinte.1960.00270160058008. [DOI] [PubMed] [Google Scholar]
- ROSE G. A. Determination of the ionised and ultrafilterable calcium of normal human plasma. Clin Chim Acta. 1957 Jun;2(3):227–236. doi: 10.1016/0009-8981(57)90107-9. [DOI] [PubMed] [Google Scholar]
- Ross J. W. Calcium-selective electrode with liquid ion exchanger. Science. 1967 Jun 9;156(3780):1378–1379. doi: 10.1126/science.156.3780.1378. [DOI] [PubMed] [Google Scholar]
- SOLLNER K., NEIHOF R. Strong-acid type of permselective membrane. Arch Biochem Biophys. 1951 Aug;33(1):166–168. doi: 10.1016/0003-9861(51)90091-4. [DOI] [PubMed] [Google Scholar]
- TORIBARA T. Y., TEREPKA A. R., DEWEY P. A. The ultrafiltrable calcium of human serum. I. Ultrafiltration methods and normal values. J Clin Invest. 1957 May;36(5):738–748. doi: 10.1172/JCI103477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSER M. Ion association. I. The effect of multivalent anions on the protein-bound and complexed calcium in serum. J Cell Comp Physiol. 1960 Jun;55:245–250. doi: 10.1002/jcp.1030550306. [DOI] [PubMed] [Google Scholar]
- WALSER M. Ion association. VI. Interactions between calcium, magnesium, inorganic phosphate, citrate and protein in normal human plasma. J Clin Invest. 1961 Apr;40:723–730. doi: 10.1172/JCI104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSER M. The separate effects of hyperparathyroidism, hypercalcemia of malignancy, renal failure, and acidosis on the state of calcium, phosphate, and other ions in plasma. J Clin Invest. 1962 Jul;41:1454–1471. doi: 10.1172/JCI104601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchorn E., McCance R. A. Inorganic constituents of cerebrospinal fluid: The ultrafiltration of calcium and magnesium from human sera. Biochem J. 1932;26(1):54–64. doi: 10.1042/bj0260054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YENDT E. R., CONNOR T. B., HOWARD J. E. In vitro calcification of rachitic rat cartilage in normal and pathological human sera with some observations on the pathogenesis of renal rickets. Bull Johns Hopkins Hosp. 1955 Jan;96(1):1–19. [PubMed] [Google Scholar]


