Abstract
Acacetin (5,7-dihydroxy-4′-methoxyflavone) is a flavone compound, some of which have anti-cancerous effects. Vascular endothelial growth factor (VEGF) plays an important role in angiogenesis and tumor growth. In this study, we found that acacetin decreased the steady level of VEGF mRNA level and inhibited VEGF transcriptional activation. To further determine the potential mechanism of acacetin in inhibiting VEGF expression, we showed that acacetin inhibited HIF-1α expression and AKT activation. Over-expression of HIF-1α or AKT restored acacetin-decreasing VEGF transcriptional activation, indicating that AKT and HIF-1 are the essential downstream targets of acacetin for inhibiting VEGF expression in the cells. Moreover, acacetin significantly inhibited ovarian cancer cell-induced angiogenesis and tumor growth in vivo through inhibiting HIF-1α and VEGF expression. Acacetin did not change HIF-1α mRNA level, but inhibited HIF-1α protein level through increasing its degradation and decreasing its stability. These results indicate that acacetin may be a useful natural compound for ovarian cancer prevention and treatment.
Keywords: acacetin, VEGF, angiogenesis, HIF-1, AKT
1. Introduction
Ovarian cancer has leading fatalities in all gynecological cancers [1]. Although many cancers respond to chemotherapy at the beginning of the treatment, the ability of cancer cells to become resistant to chemotherapeutic drugs remains a significant impediment to successful chemotherapy [2]. In addition, many of the present chemotherapeutic agents have strong side effects on the normal cells. Thus, it is important to continue our efforts to discover new treatments.
Angiogenesis is the process by which new blood capillaries are generated from the pre-existing vasculature, and plays an important role in including tumor growth and progression. Vascular endothelial growth factor (VEGF) is a potent inducer of angiogenesis and tumor growth. Hypoxia-inducible factor 1 (HIF-1) is a heterodimeric transcription factor composed of HIF-1α and HIF-1β subunits, and is a major regulator of VEGF expression in response to hypoxia [3,4]. HIF-1 is a key factor in carcinogenesis, tumor angiogenesis, tumor growth, invasion, and metastasis and can be induced by hypoxia, growth factors [5]. HIF-1α is often upregulated in human cancers to regulate VEGF expression by binding to the hypoxia responsive element of VEGF promoter [3,6]. It has been well demonstrated that AKT is one of the important upstream regulators of HIF-1α and this signaling pathway is one of the major pathways in regulating tumor angiogenesis and tumor growth in ovarian, prostate, and lung cancer cells [7–9].
Flavonoids are present in fruits, vegetables, seeds, and medicinal herbs. More and more evidence has showed that flavonoids exert their anti-cancerous effects through multiple levels: scavenging reactive species induced by carcinogens, inhibiting the activation of pro-carcinogens, suppressing the proliferation of cancer cells, inducing selective apoptosis of cancer cells, inhibiting tumor metastasis and angiogenesis, activating immune responses against cancer cells, and reversing drug resistance against chemotherapy [10,11]. Up to now, many kinds of flavonoids such as apigenin, genistein, green tea polyphenol (−)-epigallocatechin-3-gallate, chrysin, curcumin, quercetin, and luteolin have shown the cancer prevention effects in vitro and in vivo [12–16]. Our previous studies have shown that apigenin and its analogs can suppress angiogenesis and tumor growth through inhibiting the expression of HIF-1α and VEGF, indicating the high pharmacological potency of these natural compounds [17–20].
Acacetin (5,7-dihydroxy-4′-methoxyflavone) is a flavonoid compound commonly present in several plants, seeds, and flowers [21]. It has been reported that acacetin exhibits anti-cancerous effect by inhibiting cell proliferation and cell cycle progression in human cancer cells [22,23], suppressing invasion and migration of cancer cells [24–26], but the role of acacetin in regulating tumor growth and angiogenesis remains to be elucidated. In this study, we want to investigate that 1) whether acacetin inhibits VEGF expression; 2) whether acacetin inhibits HIF-1α expression; 3) which signaling pathway is involved in acacetin-inhibited VEGF expression; 4) whether acacetin inhibits angiogenesis and tumor growth in vivo; and 5) how acacetin affects HIF-1α protein expression. These studies will help to understand the role and mechanism of acacetin in inhibiting angiogenesis and tumor growth in human ovarian cancer cells.
2. Methods
2.2. Cell culture and reagents
Mouse epidermal cell line JB6clone 41 (Cl 41) stably transfected with VEGF reporter was maintained in MEM medium supplemented with 5% fetal bovine serum, 100 units/ml penicillin, 100 mg/ml streptomycin, 5% CO2 at 37oC [27]. OVCAR-3 and A2780 ovarian cancer cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics. Acacetin was from Sigma (St. Louis, MO), dissolved in dimethyl sulphoxide (DMSO), and stored at −20°C. Antibodies against HIF-1α and HIF-1β were from BD Biosciences (Franklin Lakes, NJ). Antibodies against phospho-AKT (Thr473) and total AKT were from Cell Signaling (Beverly, MA). The growth-factor-reduced phenol red-free Matrigel was purchased from BD Biosciences (Bedford, MA). Lipofectamine was from Invitrogen (Carlsbad, CA). Reporter lysis buffer, luciferase (Luc) assay system, and reverse transcriptase AMV were from Promega (Madison, WI). TRIzol was purchased from Invitrogen (Carlsbad, CA). High Capacity RNA-to cDNA Kit and Power SYBR Green PCR Master Mix for real-time RT-PCR were from ABI (Froster City, CA).
2.2. VEGF activity assay
To test the effect of acacetin on VEGF transcriptional activation, JB6 cells carrying VEGF reporter were trypsinized and seeded into 12-well plate. After the cell density reached80% to 90%, various concentrations of acacetin (10, 20, and 30 μM) were added to the cells. The cells treated by DMSO were used as negative control. Total proteins were assayed by the Protein Assay Kit (Bio-Rad, Hercules, CA) and used as an internal control. For ovarian cancer cells, transient transfection and Luc activity assay in OVCAR-3 and A2780 cells were performed and measured as we previously described [28]. The relative Luc activity was calculated by the ratio of luc/β-gal activity, and normalized to that of the control.
2.3. Real-time reverse transcription-polymerase chain reaction (RT-PCR)
OVCAR-3 cells were treated with various doses of acacetin (0, 10, and 20 μM) for 12 h. Total RNAs were extracted by TRIzol, and cDNAs were synthesized and obtained by using High Capacity RNA-to cDNA Kit according to the introduction. The PCR reactions were performed by using Power SYBR Green PCR Master Mix and StepOne Real-time PCR Systems (ABI, Froster City, CA) per the manufacturer’s instruction. The primers used for real-time PCR are follows:
VEGF forward primer: 5’-CGAGGGCCTGGAGTGTGT-3’;
VEGF reverse primer: 5’-CCGCATAATCTGCATGGTGAT-3’;
GAPDH forward primer: 5’-ATGGGTGTGAACCATGAGAAGTATG-3’;
GAPDH reverse primer: 5’-GGTGCAGGAGGCATTGCT-3’. The PCR procedure is:
95°C for 10 min, followed by 40 cycles of 95°C 15 sec and 60°C 60 sec. A melt curve was generated at the end of each run to verify specificity.
2.4. Western blotting
Western blotting was performed as described previously [20]. In brief, OVCAR-3 cells were seeded in 60 mm dishes and cultured to 70–80% confluence. After treatment with acacetin, the cells were harvested and lysed. Aliquots of proteins were resolved on SDS-PAGE, and transferred onto nitro-cellulose membrane. Proteins of interest were detected by Western blotting using specific antibodies as indicated.
2.5. Tumor angiogenesis and tumor growth assay
Fertilized white Leghorn chicken eggs were incubated at 37°C with 70% humidity for 8 days. An artificial air sac was created as previously described [9]. To test tumor angiogenesis, the OVCAR-3 cells were suspended in serum-free medium containing 50% Matrigel (BD Biosciences, Bedford, MA) with acacetin at 10 μM. Treatment with equal volume of solvent DMSO was used as a negative control. Aliquots (3×106 cells) of the mixture were then applied onto the chicken chorioallantoic membrane (CAM). After 96 h, the area around the implanted Matrigel was photographed and the number of blood vessels was obtained by counting the branching of blood vessels. The experiments were performed using 8 chicken embryos for each treatment. For tumor growth assay, similar treatment was performed. After the implantation of cancer cells for 9 days, tumors were trimmed out, photographed, and weighed. Part of tissue samples were ground in liquid nitrogen and used to test HIF-1and VEGF expression by Western blotting and RT-PCR, respectively.
2.6. Statistical analysis
The data represent mean ± SE from independent experiments as indicated in figure legends. Statistical analysis was performed by Student’s t test at a significance level of P<0.05.
3. Results
3.1. Acacetin inhibited VEGF expression under the nomoxia and hypoxia conditions
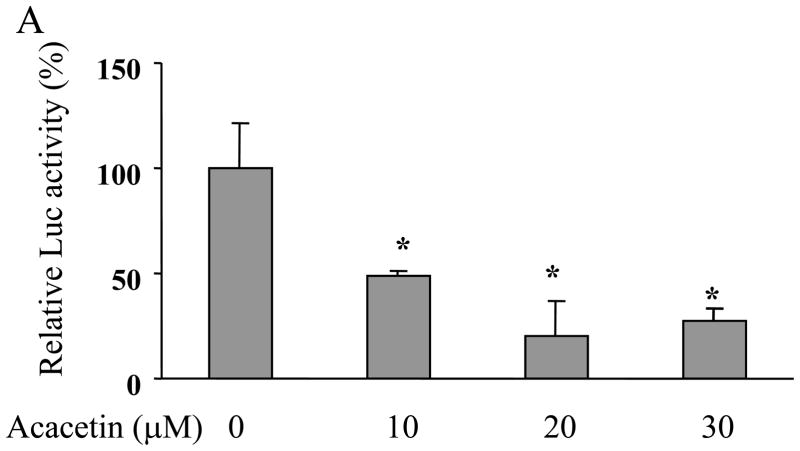
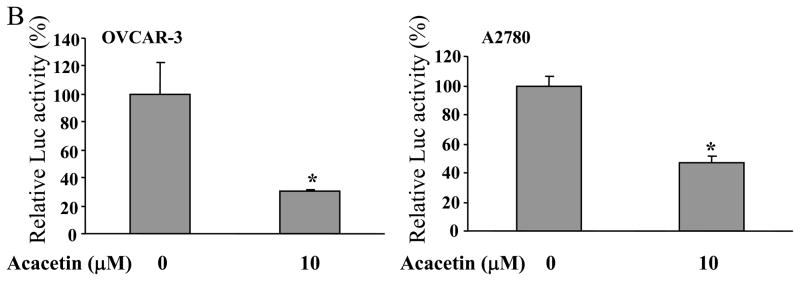
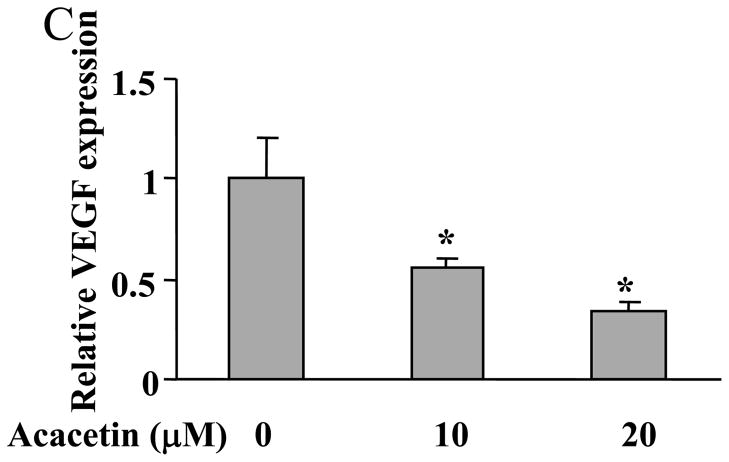
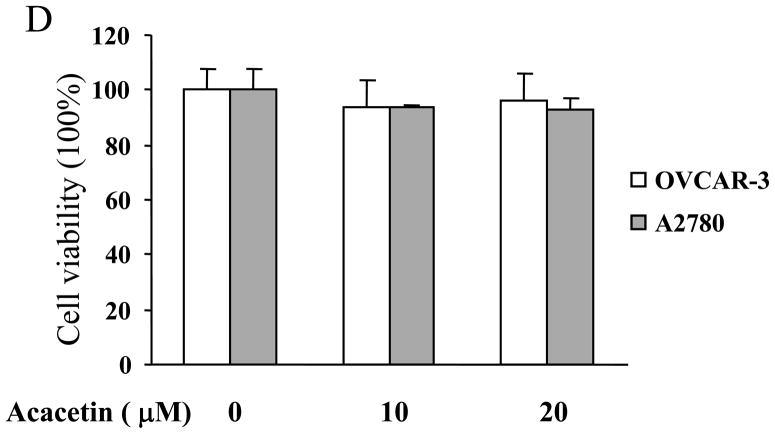
To determine whether acacetin regulates VEGF transcriptional activation, JB6 cells stably transfected with VEGF reporter were treated with acacetin. VEGF Luc activity showed acacetin at 10 μM inhibited more than 50% of VEGF transcriptional activation, with stronger inhibitory effect at higher concentrations (Fig. 1A). To further test whether acacetin inhibits VEGF transcriptional activation in human ovarian cancer cells, OVCAR-3 cells and A2780 cells were transiently transfected with VEGF reporter and β-gal plasmids, and treated without or with 10 μM of acacetin. Acacetin decreased VEGF transcriptional activation to 40% and 50% in OVCAR-3 and A2780 cells, respectively, suggesting that this compound has a general effect to inhibit VEGF transcriptional activation in ovarian cancer cells (Fig. 1B). Consistent with this result, acacetin at 10 μM and 20 μM greatly inhibited VEGF expression in OVCAR-3 cells (Fig. 1C). Cell viability assay indicated that the inhibition of VEGF transcriptional expression was not due to the toxicity of acacetin in the cells (Fig. 1D).
Fig. 1.
Acacetin inhibited VEGF expression at transcriptional level. (A) JB6 cells stably transfected with VEGF reporter were seeded into 12-well plates and cultured to 90% confluence. The cells were then treated with various concentrations of acacetin (0, 10, 20, and 30 μM) for 24 h. The cells treated with solvent DMSO were used as control. Cells were lysed and assayed for luciferase (Luc) activity. Relative Luc activities were the ratio of Luc activity/protein concentration normalized to the value of the control. * indicates significant decrease compared to the control, P<0.05. (B) OVCAR-3 and A2780 cells were seeded into a 12-well plate. When cells were 70% confluence, cells were co-tansfected with VEGF reporter (0.4 μg) and β-gal plasmid (0.2 μg), and cultured overnight. Then cells were treated with varying concentrations of acacetin (0 and 10 μM) for 24 h. A control group was treated with solvent DMSO alone. Cells were then lysed and assayed for Luc and β-gal activity. The ratio of Luc/β-gal activity was normalized to that of the solvent DMSO control. * indicates significant decrease compared to the control, P<0.05. (C) OVCAR-3 cells were cultured to 80–90% confluence and treated with acacetin (0, 10, and 20 μM) for 12 h. Total RNAs were extracted and subjected to real-time RT- PCR analysis for VEGF expression. Relative VEGF levels were the ratio of VEGF/GAPDH expression normalized to the value of the control. The data are mean ± SE from 3 replicates. * indicates significantly decrease compared to the control, P<0.05. (D) OVCAR-3 and A2780 cells were seeded into 12-well plate and cultured as above. Various doses of acacetin (0, 10 and 20 μM) were added to the cells and cultured for 12 h. Cell viability was determined by the trypan blue dye exclusion method.
3.2. Acacetin inhibited VEGF transcriptional activation through HIF-1α expression
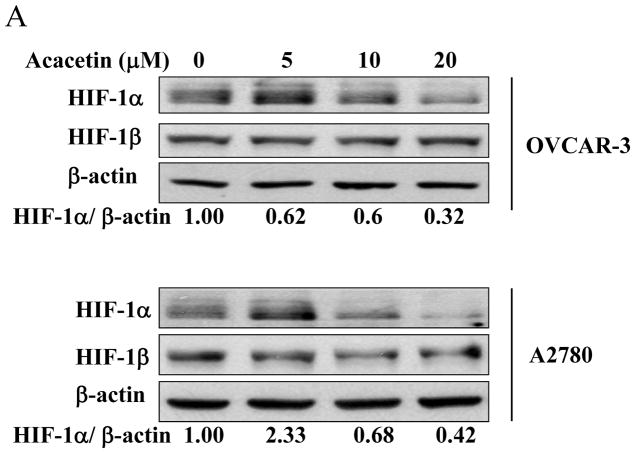
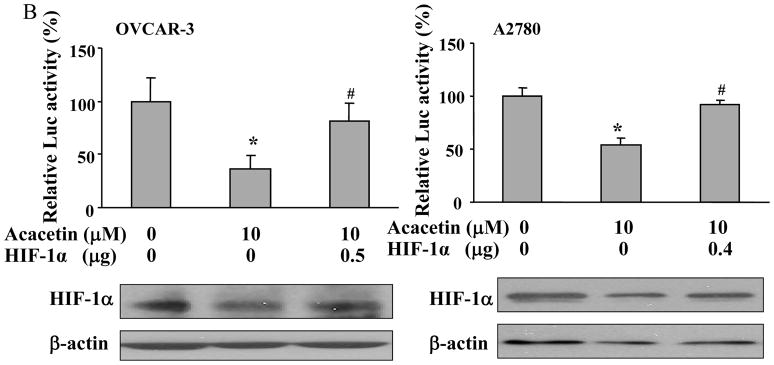
HIF-1 belongs to the basic helix-loop-helix-Per-ARNT-Sim-proteins. To determine whether acacetin affects HIF-1 expression, we found acacetin treatment at 10 and 20 μM decreased HIF-1α, but not HIF-1β expression in OVCAR-3 cells and A2780 cells (Fig. 2A). To further study whether acacetin inhibits VEGF transcriptional activation through regulating HIF-1α expression, we found forced expression of HIF-1α was sufficient to abolish acacetin-inhibiting VEGF transcriptional activation, suggesting that HIF-1α is a downstream target of acacetin for regulating VEGF expression (Fig. 2B). These results suggest acacetin inhibits VEGF transcriptional activation through decreasing HIF-1α expression.
Fig. 2.
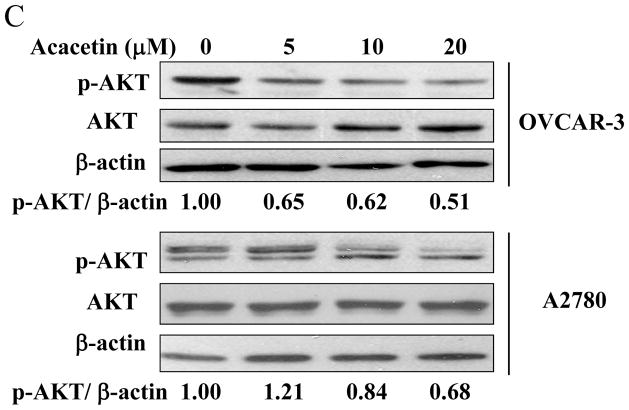
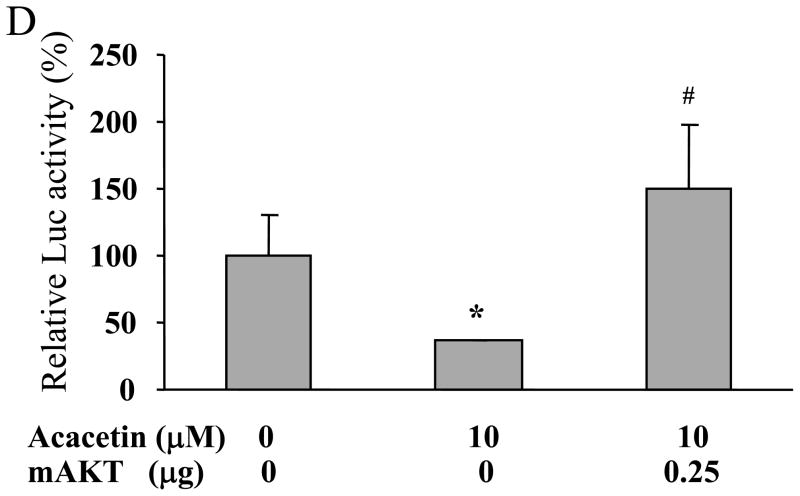
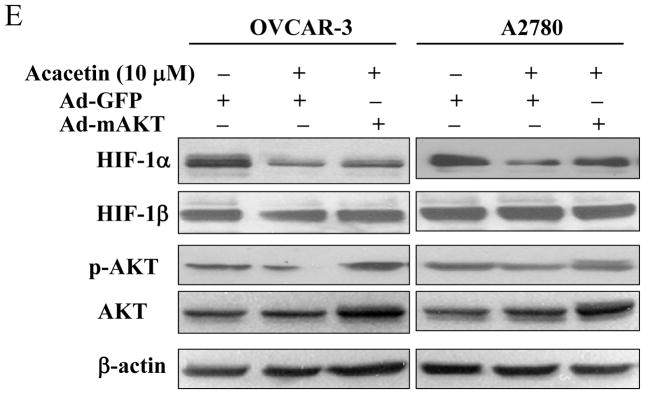
Acacetin inhibited VEGF transcriptional activation through HIF-1α expression and AKT activation. (A) OVCAR-3 and A2780 cells were seeded into 60 mm dishes and cultured overnight. Various concentrations of acacetin (0, 5, 10, and 20 μM) were then administered for 6 h. Total proteins were subjected to immunoblotting analysis for HIF-1α, HIF-1β, and β-actin levels. Relative expression level of HIF-1α was determined by the ratio of density of HIF-1α/βactin using ImageQuant 5.2 software. (B) OVCAR-3 and A2780 cells were seeded into 12-well plates, and allowed to grow to 70% confluence. Cells were then transfected with 0.4 μg of pGL-StuI VEGF reporter, 0.2 μg of β-gal plasmid and empty vector or the vector carrying HIF-1α plasmid as indicated. The cells were cultured overnight, then treated with acacetin (10 μM) for 24 h. Cells were then lysed, and the supernatant was subjected to Luc and β-gal activity assay, and immunoblotting analysis. The ratio of Luc/β-gal activity was normalized to that of the DMSO control. * indicates significant difference compared to the control, P<0.05. # indicates significant difference compared to the acacetin treatment group, P<0.05. (C) OVCAR-3 and A2780 cells were seeded into 60 mm dishes and cultured overnight. Various concentrations of acacetin (0, 5, 10, and 20 μM) were then administered for 2 h. Total proteins were subjected to immunoblotting analysis for phospho-AKT (p-AKT), total AKT, and β-actin levels. (D) OVCAR-3 cells were co-transfected with VEGF reporter, β-gal plasmid, and 0.25 μg of empty vector or the vector carrying Myr-AKT (mAKT) plasmid. Then 10 μM of acacetin was added for 24 h. The relative Luc activity was obtained by the ratio of Luc/β-gal activity, and normalized to that of the control group. * indicates significant difference compared to the control, P<0.05. # indicates significant difference compared to the acacetin treatment group, P<0.05. (E) OVCAR-3 and A2780 cells were infected with adenovirus carrying GFP or mAKT at 50 multiplicity of infection (MOI) for 48 h. Then cells were treated with 10 μM of acacetin for 6 h. Cells treated with equal volume of DMSO were used as a negative control. Cells were lysed, and total proteins were subjected to immunoblotting analysis for HIF-1α, HIF-1β, p-AKT, total AKT, and β-actin levels.
3.3. Acacetin inhibited VEGF expression through AKT activation
AKT (or protein kinase B), a serine/threonine protein kinase, plays a central role in regulating cell survival, proliferation, tumor growth and angiogenesis [7]. Consistent with the effect of acacetin on HIF-1α expression, the levels of phospho-AKT were inhibited by acacetin in a dose-dependent manner (Fig. 2C). To further test whether AKT is the upstream molecule in regulating VEGF transcriptional activation, we found that over-expression of AKT (Myr-AKT) completely abolished acacetin-inhibited VEGF transcriptional activation in OVCAR-3 cells (Fig. 2D), demonstrating that acacetin inhibited VEGF transcriptional activation through AKT signaling pathway. We found that over-expression of AKT by infecting ovarian cancer cells using adenovirus carrying AKT did restore HIF-1α expression inhibited by acacetin (Fig. 2E). This result is consistent with previous studies demonstrating that HIF-1α is one of the downstream targets of AKT [8,9], suggesting that acacetin inhibits VEGF expression through AKT activation and HIF-1 expression.
3.4. Acacetin inhibited HIF-1α expression by affecting its degradation
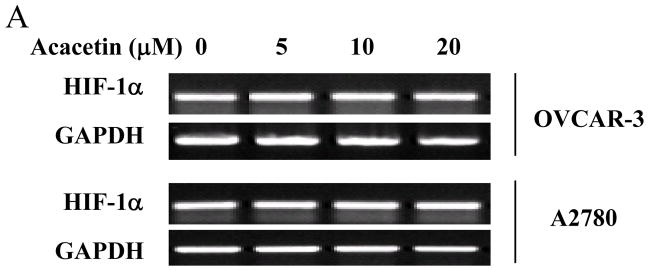
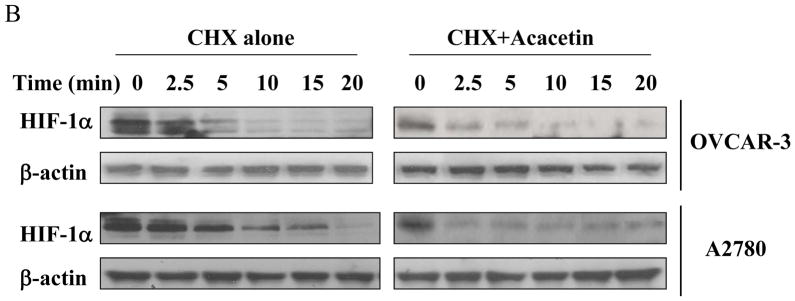
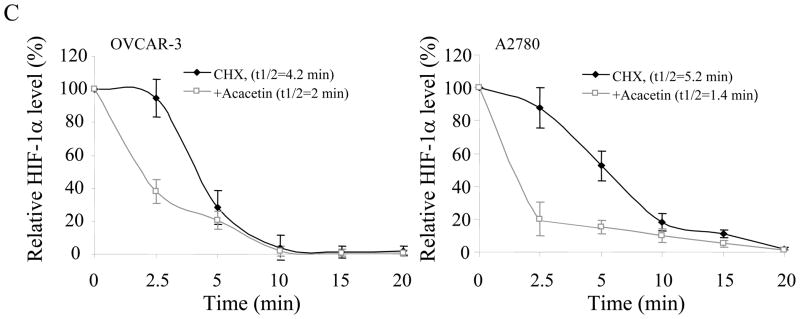
To determine whether acacetin inhibits HIF-1α expression at transcriptional level, OVCAR-3 and A2780 cells were treated with different doses of acacetin for 6 h and HIF-1α mRNA was tested by RT-PCR. As shown in Fig. 3A, acacetin treatment did not decrease HIF-1α mRNA levels, indicating that acacetin did not inhibit HIF-1α expression at transcriptional level. We next determined the effect of acacetin on the stability of HIF-1α protein by using cycloheximide (CHX) treatment to inhibit new protein synthesis in the cells. OVCAR-3 and A2780cells were treated with CHX or CHX plus acacetin for a different time period. The levels of HIF-1α protein were detected by immunoblotting, and normalized to those of β-actin in the cells (Fig. 3B). The relative half-life of HIF-1α protein in the cells was calculated. The half-life of HIF-1α was 4.2 min and 5.2 min in OVCAR-3 and A2780 cells, respectively, in the presence of CHX alone, and was decreased to 2 min and 1.4 min, respectively with the treatment of acacetin, suggesting that acacetin treatment significantly increased HIF-1α protein degradation (Fig. 3C).
Fig. 3.
Acacetin inhibited HIF-1α expression by affecting its degradation. (A) OVCAR-3 and A2780 cells were treated with acacetin (0, 5, 10, and 20 μM) for 6 h. Total RNAs were extracted, and HIF-1α level was assayed using semi-quantitative RT-PCR. (B) Acacetin increased HIF-1α protein degradation. OVCAR-3 and A2780 cells were pretreated with solvent or 40 μM of acacetin for 30 min, followed by addition of CHX (100 μM). The cells were then harvested at different times as indicated. HIF-1α expression was detected by immunoblotting with β-actin as an internal control. (C) Levels of HIF-1α protein were determined by measuring the density of HIF-1α protein band, and normalized to that of β-actin. The relative HIF-1α protein level at time zero was defined as 100%. The experiments were performed 3 times. * indicates significant difference compared to the treatment of CHX alone (P<0.05).
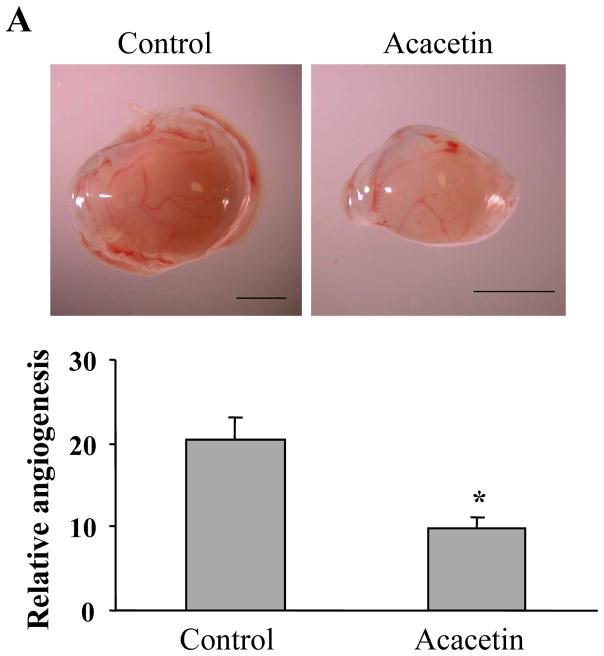
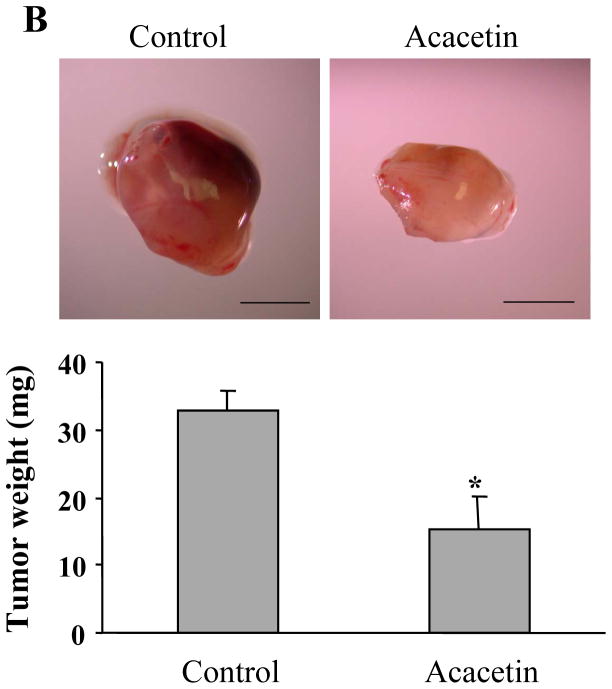
3.5. Acacetin inhibited ovarian tumor angiogenesis, tumor growth, and HIF-1α and VEGF expression in vivo
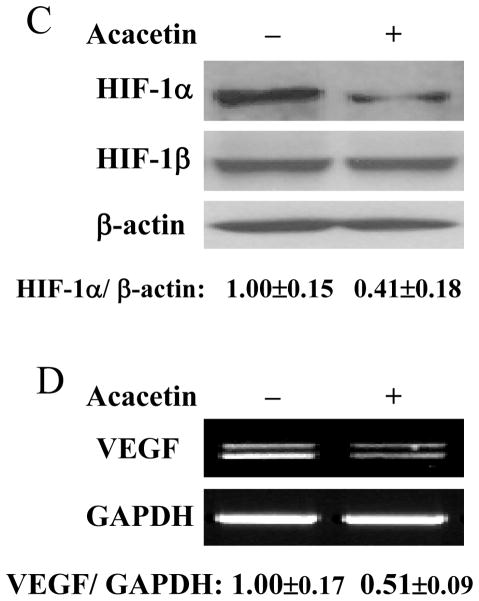
The results above showed that acacetin inhibited VEGF and HIF-1α expression. Given the key roles of VEGF and HIF-1α in regulating tumor growth and angiogenesis, we used chicken chorioallantoic membrane (CAM) model to test the effect of acacetin on tumor angiogenesis. The results showed that acacetin treatment greatly inhibited tumor angiogenesis. The micro-vessel density was decreased by acacetin treatment to 50% of the control (Fig. 4A), showing that acacetin inhibited ovarian cancer cells-induced angiogenesis in vivo. To further test whether acacetin inhibited tumor growth, OVCAR-3 cells were implanted on the CAM in the absence or presence of acacetin to grow tumors for 9 days. As shown in Fig. 4B, acacetin treatment inhibited tumor growth with 50% decrease of tumor weight when compared to that from the control group, indicating that acacetin suppresses tumor growth through impeding the angiogenesis. Consistent with the results of in vitro studies, acacetin inhibited the levels of HIF-1α and VEGF expression in tumor tissue samples (Figs. 4C and D). These results suggest that acacetin has strong effect to inhibit tumor growth and angiogenesis.
Fig. 4.
Acacetin inhibited OVCAR-3 cell-induced angiogenesis and tumor growth. (A) OVCAR-3 cells were trypsinized and suspended in serum-free medium. Then the cells (2×106 cells, 15 μl) were mixed with 15 μl Matrixgel without or with acacetin (10 μM), and implanted onto the CAM of a 9-day old chicken embryo. After 4 days of implantation, the tumors were excited, and photographed by stereomicroscope. Representative plugs treated by solvent DMSO or acacetin (upper panel, Bar, 2 mm). The number of blood vessels was counted from replicate experiments, and normalized to that of the negative control group as relative angiogenesis (n=5). * indicates significant difference compared to that of control group, P<0.05. (B) The cancer cell implantation and treatment were performed as described above. After the implantation for 9 days, the tumors were trimmed out and photographed by stereomicroscope. Bar, 2 mm (upper panel). The tumor weights were presented as mean ± SE (n=5). * indicates the significant difference compared to the control group, P<0.05. (C) Two tumor tissue samples from the control and acacetin treatment group were ground in liquid nitrogen in RIPA buffer, and total proteins were subjected to immunoblotting analysis for HIF-1α and HIF-1β expression. (D) Two tumor tissue samples from the control, and acacetin treatment group were ground in liquid nitrogen in Trizol solution, and total RNAs were subjected to RT-PCR analysis for VEGF and GAPDH levels.
4. Discussion
VEGF is the most important inducer of tumor angiogenesis. The increased level of VEGF is correlated with angiogenesis and poor prognosis in cancer, showing the vital role of VEGF in tumor angiogenesis and development. Tumor growth and metastasis require angiogenesis when the tumor reaches 1–2 mm in diameter. Inhibition of angiogenesis specifically suppresses tumor growth and invasion without affecting the normal mature vessels in human body. Thus, there are growing interests in developing anti-angiogenesis approaches for human cancer therapy.
Acacetin shows inhibitory effect on cell proliferation, cell cycle progression, induces cell apoptosis in vitro [22,23,29,30], and suppresses invasion and migration of cancer cells [24–26]. It also suppresses LPS-induced up-expression of iNOS and COX-2 in murine macrophages and TPA-induced tumor promotion in mice [31]. In this study, acacetin decreased VEGF transcriptional activation in both JB6 cells and ovarian cancer cells. It inhibited VEGF mRNA expression in OVCAR-3 cells. AKT transmits survival signals from growth factors, and regulates cell survival, migration, proliferation, metabolism, and tumor growth. To identify the relative signaling pathway, we also found that acacetin inhibited AKT activation. Overexpression of HIF-1α or AKT reversed acacetin-inhibited VEGF transcriptional activation, indicating that HIF-1α and AKT are the upstream molecules of VEGF, which is inhibited by acacetin. Overexpression of active form of AKT by adenovirus reversed acacetin-suppressed HIF-1α expression, suggesting that acacetin inhibited HIF-1 through AKT activaton. Acacetin also inhibited tumor angiogenesis and tumor growth by suppressing HIF-1α and VEGF expression by using CAM model.
In most cases, HIF-1α protein levels are constitutively expressed, but rapidly degraded by the ubiquitin-proteasome pathway under normoxia. The von Hippel-Lindau tumor suppressor gene product, pVHL, functions as the substrate recognition component of an E3-ubiquitin ligase, which targets the oxygen-sensitive HIF-1α subunit for rapid proteasomal degradation under normoxic conditions. To study whether acacetin inhibits HIF-1α protein level at transcriptional level, RT-PCR results indicated that HIF-1α mRNA was not be inhibited by acacetin. The regulation of HIF-1α stability is the major factor in controlling HIF-1α protein levels. We found that acacetin greatly shortened the half-life of HIF-1α in both OVCAR-3 and A2780 cells, suggesting that acacetin inhibited HIF-1α expression through decreasing its stability.
In summary, this study demonstrated that acacetin inhibited tumor growth and angiogenesis via suppressing AKT/HIF-1 signaling pathway to inhibit VEGF expression. These results help to understand molecular basis of acacetin in ovarian tumor growth and angiogenesis, which may be useful for rational design for cancer prevention and therapy in the future.
Acknowledgments
This work was supported by grants CA109460 and CA123675 from National Cancer Institute, NIH.
References
- 1.Rapkiewicz AV, Espina V, Petricoin EF, III, Liotta LA. Biomarkers of ovarian tumours. Eur J Cancer. 2004;40:2604–2612. doi: 10.1016/j.ejca.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Hadaschik BA, Sowery RD, Gleave ME. Novel targets and approaches in advanced prostate cancer. Curr Opin Urol. 2007;17:182–187. doi: 10.1097/MOU.0b013e3280dd8a4f. [DOI] [PubMed] [Google Scholar]
- 3.Forsythe JA, Hiang B, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 6.Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- 7.Jiang BH, Liu LZ. AKT signaling in regulating angiogenesis. Curr Cancer Drug Targets. 2008;8:19–26. doi: 10.2174/156800908783497122. [DOI] [PubMed] [Google Scholar]
- 8.Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322–3331. [PubMed] [Google Scholar]
- 9.Xia C, Meng Q, Cao Z, Shi X, Jiang BH. Regulation of angiogenesis and tumor growth by p110 alpha and AKT1 via VEGF expression. J Cell Physiol. 2006;209:56–66. doi: 10.1002/jcp.20707. [DOI] [PubMed] [Google Scholar]
- 10.Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983;32:1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 11.Kale A, Gawande S, Kotwal S. Cancer phytotherapeutics: role for flavonoids at the cellular level. Phytother Res. 2008;22:567–577. doi: 10.1002/ptr.2283. [DOI] [PubMed] [Google Scholar]
- 12.Constantinou A, Kiguchi K, Huberman E. Induction of differentiation and DNA strand breakage in human HL-60 and K-562 leukemia cells by genistein. Cancer Res. 1990;50:2618–2624. [PubMed] [Google Scholar]
- 13.Katiyar SK, Agarwal R, Wang ZY, Bhatia AK, Mukhtar H. (-)-Epigallocatechin-3-gallate in Camellia sinensis leaves from Himalayan region of Sikkim: inhibitory effects against biochemical events and tumor initiation in Sencar mouse skin. Nutr Cancer. 1992;18:73–83. doi: 10.1080/01635589209514207. [DOI] [PubMed] [Google Scholar]
- 14.Santel T, Pflug G, Hemdan NY, Schafer A, Hollenbach M, Buchold M, Hintersdorf A, Lindner I, Otto A, Bigl M, Oerlecke I, Hutschenreuter A, Sack U, Huse K, Groth M, Birkemeyer C, Schellenberger W, Gebhardt R, Platzer M, Weiss T, Vijayalakshmi MA, Kruger M, Birkenmeier G. Curcumin inhibits glyoxalase 1: a possible link to its anti-inflammatory and anti-tumor activity. PLoSONE. 2008;3:e3508. doi: 10.1371/journal.pone.0003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uhl M, Ecker S, Kassie F, Lhoste E, Chakraborty A, Mohn G, Knasmuller S. Effect of chrysin, a flavonoid compound, on the mutagenic activity of 2-amino-1-methyl-6-phenylimidazo[4,5- b]pyridine (PhIP) and benzo(a)pyrene (B(a)P) in bacterial and human hepatoma (HepG2) cells. Arch Toxicol. 2003;77:477–484. doi: 10.1007/s00204-003-0469-4. [DOI] [PubMed] [Google Scholar]
- 16.Wei H, Tye L, Bresnick E, Birt DF. Inhibitory effect of apigenin, a plant flavonoid, on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res. 1990;50:499–502. [PubMed] [Google Scholar]
- 17.Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang BH. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19:342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- 18.Fang J, Zhou Q, Shi XL, Jiang BH. Luteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cells. Carcinogenesis. 2007;28:713–723. doi: 10.1093/carcin/bgl189. [DOI] [PubMed] [Google Scholar]
- 19.Fu B, Xue J, Li Z, Shi X, Jiang BH, Fang J. Chrysin inhibits expression of hypoxia-inducible factor-1alpha through reducing hypoxia-inducible factor-1alpha stability and inhibiting its protein synthesis. Mol Cancer Ther. 2007;6:220–226. doi: 10.1158/1535-7163.MCT-06-0526. [DOI] [PubMed] [Google Scholar]
- 20.Liu LZ, Fang J, Zhou Q, Hu X, Shi X, Jiang BH. Apigenin inhibits expression of vascular endothelial growth factor and angiogenesis in human lung cancer cells: implication of chemoprevention of lung cancer. Mol Pharmacol. 2005;68:635–643. doi: 10.1124/mol.105.011254. [DOI] [PubMed] [Google Scholar]
- 21.Yim SH, Kim HJ, Lee IS. A polyacetylene and flavonoids from Cirsium rhinoceros. Arch Pharm Res. 2003;26:128–131. doi: 10.1007/BF02976657. [DOI] [PubMed] [Google Scholar]
- 22.Hsu YL, Kuo PL, Liu CF, Lin CC. Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer Lett. 2004;212:53–60. doi: 10.1016/j.canlet.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Singh RP, Agrawal P, Yim D, Agarwal C, Agarwal R. Acacetin inhibits cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells: structure-activity relationship with linarin and linarin acetate. Carcinogenesis. 2005;26:845–854. doi: 10.1093/carcin/bgi014. [DOI] [PubMed] [Google Scholar]
- 24.Chien ST, Lin SS, Wang CK, Lee YB, Chen KS, Fong Y, Shih YW. Acacetin inhibits the invasion and migration of human non-small cell lung cancer A549 cells by suppressing the p38alpha MAPK signaling pathway. Mol Cell Biochem. 2011;350:135–148. doi: 10.1007/s11010-010-0692-2. [DOI] [PubMed] [Google Scholar]
- 25.Fong Y, Shen KH, Chiang TA, Shih YW. Acacetin inhibits TPA-induced MMP-2 and u-PA expressions of human lung cancer cells through inactivating JNK signaling pathway and reducing binding activities of NF-kappaB and AP-1. J Food Sci. 2010;75:H30–H38. doi: 10.1111/j.1750-3841.2009.01438.x. [DOI] [PubMed] [Google Scholar]
- 26.Shen KH, Hung SH, Yin LT, Huang CS, Chao CH, Liu CL, Shih YW. Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer DU145 cells via inactivation of the p38 MAPK signaling pathway. Mol Cell Biochem. 2010;333:279–291. doi: 10.1007/s11010-009-0229-8. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Li J, Song L, Zhang D, Tong Q, Ding M, Bowman L, Aziz R, Stoner GD. Black raspberry extracts inhibit benzo(a)pyrene diol-epoxide-induced activator protein 1 activation and VEGF transcription by targeting the phosphotidylinositol 3-kinase/Akt pathway. Cancer Res. 2006;66:581–587. doi: 10.1158/0008-5472.CAN-05-1951. [DOI] [PubMed] [Google Scholar]
- 28.Liu LZ, Hu XW, Xia C, He J, Zhou Q, Shi X, Fang J, Jiang BH. Reactive oxygen species regulate epidermal growth factor-induced vascular endothelial growth factor and hypoxia-inducible factor-1alpha expression through activation of AKT and P70S6K1 in human ovarian cancer cells. Free Radic Biol Med. 2006;41:1521–1533. doi: 10.1016/j.freeradbiomed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Hsu YL, Kuo PL, Lin CC. Acacetin inhibits the proliferation of Hep G2 by blocking cell cycle progression and inducing apoptosis. Biochem Pharmacol. 2004;67:823–829. doi: 10.1016/j.bcp.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 30.Pan MH, Lai CS, Hsu PC, Wang YJ. Acacetin induces apoptosis in human gastric carcinoma cells accompanied by activation of caspase cascades and production of reactive oxygen species. J Agric Food Chem. 2005;53:620–630. doi: 10.1021/jf048430m. [DOI] [PubMed] [Google Scholar]
- 31.Pan MH, Lai CS, Wang YJ, Ho CT. Acacetin suppressed LPS-induced up-expression of iNOS and COX-2 in murine macrophages and TPA-induced tumor promotion in mice. Biochem Pharmacol. 2006;72:1293–1303. doi: 10.1016/j.bcp.2006.07.039. [DOI] [PubMed] [Google Scholar]