Abstract
Objective
To determine the association of fetuin-A with subclinical CVD in community-living individuals.
Background
Fetuin-A is a hepatic secretory protein that inhibits arterial calcium deposition in vitro. Lower fetuin-A levels are associated with arterial calcification and death in end-stage renal disease populations. The association of fetuin-A with subclinical cardiovascular disease (CVD) in the general population is unknown.
Methods
Among 1,375 community-living individuals without prevalent clinical CVD, we measured plasma fetuin-A concentrations measured by ELISA. Peripheral arterial disease (PAD) was defined by ankle brachial index (ABI) < 0.90, coronary artery calcification (CAC) was measured by computed tomography, and common and internal intima media thickness (cIMT) were measured by carotid ultrasound. PAD was measured concurrent with fetuin-A, and CAC and cIMT was measured 4.6 years (mean) later.
Results
Mean age was 70 ± 11 years and 64% were female. Fetuin-A levels were inversely associated with CAC severity. When evaluated as CAC categories (0, 1–100, 101–300, > 300) using ordinal logistic regression, each standard deviation higher fetuin-A was associated with a 31% lower odds of CAC severity (proportional odds ratio [POR] 0.69; 95% confidence interval [CI] 0.46, 0.92; p=0.008) in models adjusted for demographics, lifestyle factors, traditional CVD risk factors and kidney function. In contrast, no association of fetuin-A was observed with PAD or high common or internal cIMT in adjusted models.
Conclusions
Lower fetuin-A levels are independently associated with greater CAC severity, but not PAD or cIMT. If confirmed, fetuin-A may mark calcium deposition within the vasculature, but not atherosclerosis per se.
Keywords: Fetuin-A, Cardiovascular Disease, Coronary Artery Calcification
INTRODUCTION
Fetuin-A is a protein secreted from the liver that inhibits arterial calcium deposition in vitro.(1) In serum, it interacts with calcium and phosphorus, increasing their solubility, and inhibiting calcium crystal growth and precipitation, reminiscent of mechanisms by which lipoproteins solubilize lipids. Consistent with this function, fetuin-A knock-out mice develop greater soft tissue calcification compared to wild-type control mice when challenged with diets enriched in vitamin D or phosphorus.(2,3) Fetuin-A inhibits arterial calcification within the blood stream, raising the possibility that blood levels may provide a useful marker of the burden of arterial calcification.
Studies in end-stage renal disease (ESRD) populations have consistently shown that lower fetuin-A levels are associated with CVD events and all-cause mortality.(4–7) Most,(8–11) but not all,(12,13) studies in ESRD have also reported that low fetuin-A levels are associated with coronary or abdominal aortic calcification. However, the associations of fetuin-A with subclinical CVD events in the general population is much less clear. Prior studies in individuals with known or clinically suspected CVD have shown that lower fetuin-A levels are associated with coronary artery calcification (CAC)(14) and cardiac valve calcification,(15) and one prior study in patients with type 2 diabetes reported that lower fetuin-A levels are associated with peripheral arterial disease (PAD).(16) However, two other small studies (n=90 and 315, respectively) observed associations in the opposite direction, reporting that higher fetuin-A levels were associated with greater carotid intima media thickness (cIMT).(17,18) Enrollment criteria required known atherosclerosis in one,(18) and obesity, insulin resistance, or family history of diabetes in the other.(17) Comparing these studies is difficult not only because of seemingly conflicting directions of associations, but also because they uniformly studied select populations with either prevalent CVD, diabetes, or diabetic risk factors.(14–16,19,20) These conditions are marked by high CVD risk and extensive arterial calcification burden. Thus, the association of fetuin-A with subclinical CVD in a community-dwelling population remains unexplored.
To our knowledge, no study has evaluated the association of fetuin-A with subclinical CVD in a single community-dwelling population not selected on the basis of prevalent disease or risk factors for disease. We therefore sought to determine the association of fetuin-A with subclinical CVD in community-dwelling individuals without known clinical CVD. We hypothesized that lower fetuin-A levels would be associated with each marker of subclinical CVD, independent of traditional CVD risk factors or kidney function.
METHODS
Study Participants
The Rancho Bernardo Study is a prospective study of older community-dwelling individuals designed to investigate the epidemiology of chronic diseases in older adults. Between 1972–74, all community-dwelling residents living in Rancho Bernardo, a community in Southern California, and aged 30–79 years were invited to participate in a study of heart disease risk factors, and 82% (n=5,052) enrolled. Nearly all were Caucasian, middle to upper-middle class, and relatively well educated. Since then, sequential study visits have been conducted approximately every 4 years. The present analysis included individuals who participated in the 1992–96 study visit (n=1,781). Of these, 39 had insufficient stored blood specimens for fetuin-A measurement, 349 were excluded due to known prevalent clinical CVD (history of myocardial infarction, coronary artery bypass graft, or stroke), and 18 had missing covariate data, resulting in a final analytic sample of 1,375 individuals. All participants gave written informed consent; the study protocol was approved by the human research protection program at the University of California San Diego.
Fetuin-A
Fetuin-A was measured in EDTA plasma collected at the 1992–96 study visit using a human enzyme linked immunosorbent assay kit (Epitope Diagnostics, San Diego, CA). Samples were stored at −70° Celcius until assayed in 2010. The assay uses a 2-site “sandwich” technique with polyclonal antibodies that bind different epitopes of human fetuin-A. Plasma samples were measured twice in each participant, and results were averaged. Intra- and inter-assay coefficients of variation (CV) were 2.4–4.7%, and 9.5–9.9%, respectively.
Subclinical Cardiovascular Disease
Coronary Artery Calcification
At the follow-up visit subsequent to when blood samples for fetuin-A measurements were obtained (1998–2002), surviving participants were invited to have chest electron beam computed tomography (EBCT) scans for CAC. To be invited, participants must (1) have been evaluated by our research team between 1997 and 1999, (2) be age 55 to 80, (3) be postmenopausal (more than 1 year without menses) if female, and (4) be free of clinically manifest CHD (no physician-diagnosed angina, myocardial infarction, or coronary artery revascularization). An Imatron C-150 ultrafast CT scanner that produced contiguous thin-section sections was used. Scans were electrocardiographically triggered to the R-R interval, and images were obtained at end-diastole during a single breath hold; CAC was scored according to the Agatston method.(21) Of the 525 individuals who were invited, 2 were deceased, 15 were ineligible due to prevalent CHD, 57 declined the invitation, 47 had moved from the local area or were not seen due to scheduling problems, and 22 did not have fetuin-A measurements available at the preceding visit, yielding a study sample of 382 individuals for the CAC analysis.
Peripheral Arterial Disease
PAD was defined on the basis of ankle brachial index (ABI) measurements made concurrent with collection of samples for fetuin-A measurements (1992–96 study visit). With the participant in the supine position after 5 minutes of rest, a specially trained and certified nurse used a mercury sphygmomanometer to measure the blood pressure in each arm and leg. Systolic blood pressure of the brachial artery was used for the upper extremities, whereas duplicate systolic blood pressures of the posterior tibial artery were used for the 2 lower extremities. The ABI was calculated separately for each leg by taking the higher systolic blood pressure in each lower extremity and dividing by the highest upper-extremity systolic blood pressure. The lowest of these two leg-specific ABIs was used to classify the ABI score for each participant.(22) PAD was defined as an ABI < 0.90. Among 1,375 individuals, 1,047 had ABI measurements available; 2 were excluded for ABI values > 1.30, as such individuals frequently have medial arterial calcification and artifactually high ABI measurements.(23,24) This provided a final analytic sample of 1,063 for PAD.
Carotid Intima Media Thickness
cIMT measurements were made at the follow-up visit subsequent to when blood samples for fetuin-A measurements were obtained (1998–2002). B-mode ultrasonography of the left and right common and internal carotid arteries was performed by a specially trained radiology technician. Briefly, 4 standardized images were obtained on each participant: 1 at the lateral angle of the common carotid artery, defined as the segment 1 cm proximal to the dilation of the carotid bulb, and 3 for the internal carotid artery at the site of maximal thickness in 3 distinct angles (anterior, posterior, and lateral). Ultrasound measurements were recorded on super-VHS tapes and sent to a central reading facility, where data were processed blinded to all clinical data except for the cIMT ultrasound images. The common cIMT thickness was calculated as the mean of the left and right measurements. Similarly, the internal cIMT thickness was determined as the mean of the 6 internal cIMT measurements.(25) All results are reported in millimeters. Of the 1,375 individuals included in this study, 597 returned and participated in the cIMT testing at the next follow-up visit. All had available common cIMT measurements while 14 had internal cIMT measurements that were deemed not interpretable, providing a sample size of 583 individuals for cIMT analysis
Other Measurements
Information on medical history, medication use, physical exercise (≥3 times per week, yes or no), alcohol consumption (≥3 per week vs. less), and current smoking (yes/no) was obtained using standardized questionnaires. Current medication use was validated by examination of pills and prescriptions brought to the clinic for that purpose. Participants were asked to rate their overall health on a 5-point scale (excellent, very good, good, fair, or poor) and to report the number of hospitalizations in the prior year.
Diabetes mellitus was defined as a fasting glucose ≥ 126 mg/dL, reported physician’s diagnosis, or use of hypoglycemic medications. Systolic (SBP) and diastolic (DBP) blood pressure were measured twice in the seated position after 5 minutes of rest, using the Hypertension Detection and Follow-up Program protocol.(26) Hypertension was defined as SBP > 140mmHg, DBP > 90mmHg, or use of antihypertensive medications. Height and weight were measured with participants wearing light clothes and no shoes, and body mass index (BMI) was calculated (weight [kg]/height [meters]2). Participants with BMI > 30 kg/m2 were classified as obese. Blood samples were obtained by venapuncture between 07:30 and 11:00 AM after a requested 12 hour fast; serum and plasma were separated and frozen at −70° Celsius. Plasma total and HDL cholesterol and triglycerides were measured in a Centers for Disease Control-certified Lipid Research Clinic laboratory. LDL cholesterol was calculated using the Friedewald equation.(27) Serum creatinine was measured using the rate Jaffe reaction, and combined with age, sex, and race in the Modification of Diet in Renal Disease equation to estimate glomerular filtration rate (eGFR).(28) Participants with eGFR < 60 ml/min/1.73m2 were classified as having moderate chronic kidney disease (CKD), according to the National Kidney Foundation criteria.(29)
Statistical Analysis
We categorized participants into quartiles of fetuin-A defined by the distribution within our study population. Differences in demographics and baseline variables across the fetuin-A quartiles were evaluated using ANOVA or the Chi Square test for categorical variables. We visually inspected histograms of the distribution of each continuous variables in table 1. When skewed variables were observed, we elected used the Kruskal Wallis test in place of ANOVA. In these instances, we report the median and interquartile range within each quartile of fetuin-A. To maximize statistical power, we used multiple linear regression to evaluate the association of fetuin-A with each subclinical CVD measure on a continuous scale. CAC scores were right skewed, and 25% had CAC scores of 0. Thus, we added 1 to each individual’s CAC score, natural log-transformed the resulting variable, and evaluated ln(CAC+1) as the primary outcome. The resulting transformed variable more closely approximated a normal distribution. In companion analyses, we evaluated categories of severity of CAC using cut-points that have been associated with increased risk of incident CVD in prior studies (CAC categories: 0, 1–100, 101–300, >300)(30) and used ordinal logistic regression models in analysis. The proportional odds assumption was evaluated with the Score test, and no violations were observed. We also evaluated PAD (ABI < 0.90 vs. greater) and common and internal IMT (>75 percentile of each) on the basis of dichotomous cut-points used in the Cardiovascular Health Study and the Multi-Ethnic Study of Atherosclerosis.(31–33) Logistic regression was used to evaluate these outcomes.
Table 1.
Baseline Characteristics of Community-Living Individuals by Quartiles of Plasma Fetuin-A: The Rancho Bernardo Study
| Fetuin-A Quartiles | ||||||
|---|---|---|---|---|---|---|
| Fetuin-A Range (g/L) N |
All 1375 |
I < 0.45 342 |
II 0.45–0.51 343 |
III 0.52–0.58 345 |
IV ≥ 0.59 345 |
P-value† |
| Demographics | ||||||
| Age (years) ± SD | 70 ± 11 | 73 ± 12 | 70 ± 11 | 69 ± 11 | 68 ± 11 | <0.001 |
| Male n (%) | 492 (36%) | 138 (40%) | 132 (38%) | 133 (39%) | 89 (26%) | <0.001 |
| Lifestyle Factors | ||||||
| Current smoking n (%) | 107 (8%) | 28 (8%) | 30 (9%) | 21 (6%) | 28 (8%) | 0.58 |
| Regular physical exercise n (%) | 979 (71%) | 243 (71%) | 261 (76%) | 243 (70%) | 232 (67%) | 0.08 |
| Alcohol ≥ 3/week n (%) | 626 (46%) | 178 (52%) | 165 (48%) | 155 (45%) | 128 (37%) | 0.001 |
| Health Status | ||||||
| Oral Estrogen Use n (%)** | 350 (25%) | 53 (26%) | 75 (36%) | 81 (38%) | 141 (55%) | <0.001 |
| Fair or Poor Health Status n (%) | 83 (6%) | 27 (8%) | 21 (6%) | 18 (5%) | 17 (5%) | 0.34 |
| Any hospitalizations in past year n (%) | 106 (8%) | 25 (8%) | 27 (8%) | 28 (8%) | 26 (8%) | 0.98 |
| Co-morbidities* | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 0.29 |
| Medications* | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 0.91 |
| Co-morbidities | ||||||
| Obese n (%) | 161 (12%) | 23 (7%) | 41 (12%) | 50 (14%) | 47 (14%) | 0.01 |
| Diabetes n (%) | 170 (12%) | 35 (10%) | 37 (11%) | 40 (12%) | 58 (17%) | 0.03 |
| Hypertension n (%) | 674 (49%) | 171 (50%) | 155 (45%) | 171 (50%) | 177 (51%) | 0.41 |
| Cardiovascular Risk Factors | ||||||
| Body mass index (kg/m2) ± SD | 25 ± 4 | 25 ± 4 | 26 ± 4 | 26 ± 4 | 26 ± 4 | <0.001 |
| Systolic blood pressure (mmHg) ± SD | 135 ± 22 | 134 ± 21 | 134 ± 22 | 135 ± 24 | 135 ± 21 | 0.94 |
| Diastolic blood pressure (mmHg) ± SD | 76 ± 9 | 75 ± 10 | 76 ± 9 | 77 ± 9 | 77 ± 9 | 0.03 |
| Total cholesterol (mg/dL) ± SD | 211 ± 35 | 205 ± 34 | 209 ± 36 | 212 ± 35 | 217 ± 36 | <0.001 |
| LDL cholesterol (mg/dL) ± SD | 128 ± 32 | 122 ± 32 | 128 ± 32 | 130 ± 32 | 130 ± 31 | 0.05 |
| HDL cholesterol (mg/dL) ± SD | 59 ± 17 | 61 ± 17 | 59 ± 16 | 58 ± 17 | 59 ± 18 | 0.18 |
| Triglycerides (mg/dL)* | 103 (73, 146) | 90 (67, 121) | 96 (67, 139) | 110 (76, 156) | 123 (85, 187) | <0.001 |
| eGFR (ml/min/1.73m2) ± SD | 68 ± 16 | 68 ± 16 | 68 ± 14 | 69 ± 18 | 68 ± 14 | 0.48 |
| eGFR < 60 ml/min/1.73m2, n (%) | 413 (30%) | 104 (30%) | 105 (31%) | 104 (30%) | 1060 (29%) | 0.97 |
Median (interquartile range), p-value obtained by Kruskal Wallis test.
Women only
P-value across quartiles by ANOVA, Chi Square unless otherwise indicated.
For each outcome, identical models were used to facilitate comparisons. An initial model was adjusted for age and sex. A second model additionally adjusted for lifestyle factors (current smoking, alcohol use, regular physical exercise, and oral estrogen use), and a final model additionally adjusted for traditional CVD risk factors (diabetes, BMI, total cholesterol, triglycerides) and eGFR. On the basis of our prior work, we evaluated for effect modification in the associations of fetuin-A with subclinical CVD by diabetes by evaluating multiplicative interaction terms within the final adjusted models.(15,34) P-values < 0.05 were considered statistically significant for all analyses including interaction terms and all analyses were performed using STATA version 11.0 SE (StataCorp LP, College Station, TX).
RESULTS
Among the 1,375 person study sample, the mean age was 70 ± 11 years, and 64% (n=883) were female, among whom 40% (n=350) were using oral estrogen therapy (ET). Twelve percent had diabetes, and 30% had moderate CKD. The median CAC score was 71 (interquartile range [IQR] 0, 319), and the mean ankle brachial index (ABI), common, and internal IMT were 1.06 ± 0.15, 0.95±0.21 mm, and 1.47 ± 0.74 mm, respectively.
Table 1 shows the distribution of demographics, life-style factors, and CVD risk factors by fetuin-A quartiles. Compared to the lowest quartile, individuals with higher fetuin-A levels were younger, more frequently female, and consumed less alcohol. Among women, use of oral estrogen was associated with higher fetuin-A levels. Individuals with higher fetuin-A levels and were also more likely to have obesity, diabetes, and an atherogenic lipid profile.
Table 2 shows the association of fetuin-A, both by quartiles and as a continuous predictor variable, with each marker of subclinical CVD evaluated as a continuous outcome measure. Fetuin-A was inversely associated with CAC when evaluated as a continuous variable. This finding remained consistent in models that adjusted for age and sex, life-style factors, traditional CVD risk factors and kidney function. We observed no association of fetuin-A with ABI in age and sex or life-style adjusted models, but a statistically significant direct association emerged in the final adjusted model. This association was of modest strength (each SD higher fetuin-A associated with 0.01 higher ABI units). We observed no association of fetuin-A with common or internal IMT across the sequence of models.
Table 2.
Association of Fetuin-A with Continuous Measures of Subclinical CVD: The Rancho Bernardo Study
| Fetuin-A Quartiles | Fetuin-A Continuous | ||||
|---|---|---|---|---|---|
| Fetuin-A range (g/L) | I < 0.45 |
II 0.45–0.51 |
III 0.52–0.58 |
IV ≥ 0.59 |
Per SD (0.11 g/L) greater |
| Coronary Artery Calcification (Ln[CAC + 1]) | |||||
| Number | 86 | 97 | 95 | 104 | 382 |
| Age and sex adjusted; β (95% CI) P-value | 1.00 (Ref) | −0.33 (−0.95, 0.30); 0.31 | −0.32 (−0.95, 0.31); 0.32 | −0.63 (−1.25, −0.02); 0.04 | −0.24 (−0.45, −0.02); 0.03 |
| Lifestyle Adjusted; β (95% CI) P-value* | 1.00 (Ref) | −0.32 (−0.95,0.31); 0.32 | −0.31 (−0.95, 0.32); 0.33 | −0.58 (−1.21, 0.04); 0.07 | −0.21 (−0.44, 0.01); 0.06 |
| Fully Adjusted; β (95% CI) P-value** | 1.00 (Ref) | −0.40 (−1.03, 0.22); 0.21 | −0.44 (−1.07, 0.20); 0.18 | −0.80 (−1.44, −0.16); 0.01 | −0.31 (−0.54, −0.08); 0.008 |
| Ankle Brachial Index | |||||
| Number | 280 | 270 | 250 | 245 | 1045 |
| Age and sex adjusted; β (95% CI) P-value | 1.00 (Ref) | 0.01 (−0.01, 0.04); 0.36 | 0.03 (0.01, 0.04); 0.02 | 0.01 (−0.01, 0.04); 0.34 | 0.01 (−0.002, 0.02); 0.15 |
| Lifestyle Adjusted; β (95% CI) P-value* | 1.00 (Ref) | 0.01 (−0.01, 0.03); 0.46 | 0.03 (0.002, 0.05); 0.04 | 0.01 (−0.01, 0.04); 0.39 | 0.01 (−0.003, 0.02); 0.16 |
| Fully Adjusted; β (95% CI) P-value** | 1.00 (Ref) | 0.01 (−0.01, 0.04); 0.44 | 0.03 (0.002, 0.05); 0.03 | 0.02 (−0.01, 0.04); 0.16 | 0.01 (0.000, 0.02); 0.04 |
| Common Carotid Intima Media Thickness (ln[common cIMT]) | |||||
| Number | 131 | 150 | 148 | 168 | 597 |
| Age and sex adjusted; β (95% CI) P-value | 1.00 (Ref) | −0.02 (−0.06, 0.02); 0.30 | −0.01 (−0.05, 0.04); 0.79 | 0.03 (−0.01, 0.07); 0.20 | 0.01 (−0.002, 0.03); 0.10 |
| Lifestyle Adjusted; β (95% CI) P-value* | 1.00 (Ref) | −0.02 (−0.06, 0.02); 0.37 | −0.01 (−0.05, 0.04); 0.82 | 0.03 (−0.01, 0.07); 0.20 | 0.01 (−0.003, 0.03); 0.11 |
| Fully Adjusted; β (95% CI) P-value** | 1.00 (Ref) | −0.03 (−0.07, 0.02); 0.25 | −0.01 (−0.06, 0.03); 0.51 | 0.01 (−0.03, 0.06); 0.53 | 0.01 (−0.01, 0.02); 0.36 |
| Internal Carotid Intima Media Thickness (ln[internal cIMT)]) | |||||
| Number | 125 | 149 | 143 | 166 | 583 |
| Age and sex adjusted; β (95% CI) P-value | 1.00 (Ref) | −0.004 (−0.11, 0.10); 0.94 | 0.04 (−0.07, 0.14); 0.47 | 0.06 (−0.04, 0.17); 0.23 | 0.02 (−0.02, 0.06); 0.26 |
| Lifestyle Adjusted; β (95% CI) P-value* | 1.00 (Ref) | 0.01 (−0.10, 0.11); 0.89 | 0.04 (−0.06, 0.15); 0.41 | 0.07 (−0.03, 0.18); 0.19 | 0.02 (−0.01, 0.06); 0.21 |
| Fully Adjusted; β (95% CI) P-value** | 1.00 (Ref) | −0.004 (−0.11, 0.10); 0.95 | 0.02 (−0.09, 0.13); 0.71 | 0.02 (−0.09, 0.12); 0.75 | 0.001 (−0.04, 0.04); 0.95 |
Adjusted for age, sex, current smoking, alcohol use, regular physical exercise, and oral estrogen use.
Adjusted for lifestyle model (*) plus diabetes, bmi, total chol., triglycerides, and eGFR.
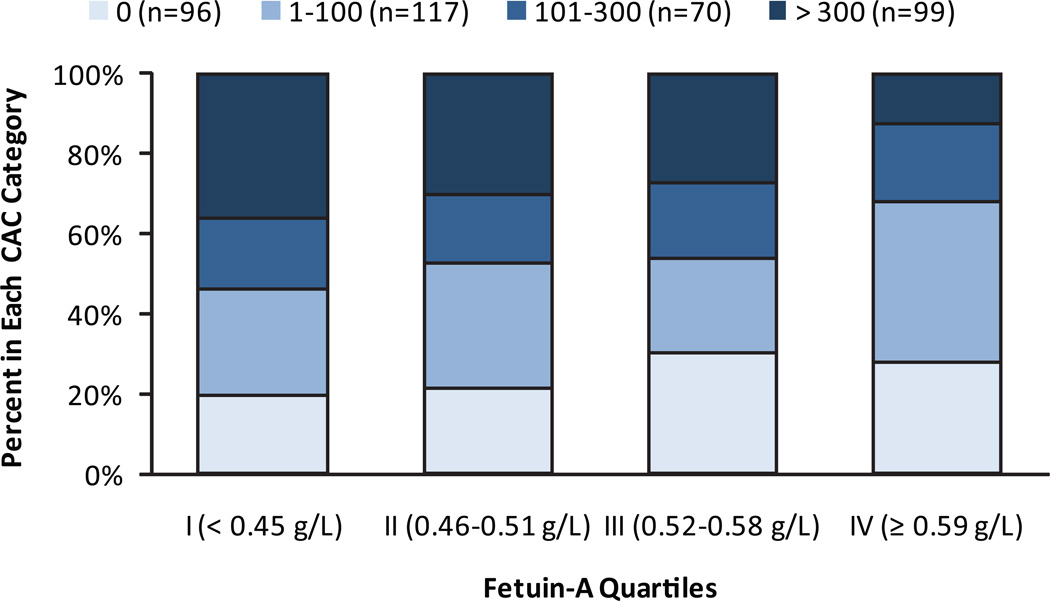
Table 3 shows the association of fetuin-A with each outcome evaluated using clinically defined cut-points. In this analysis, higher fetuin-A was also associated with less CAC severity. Quartile analysis demonstrated a dose-response relationship between fetuin-A and CAC categories (Figure 1; chi square p=0.02). Among persons without any CAC, 20% were in the lowest fetuin-A quartile whereas 28% were in the highest quartile. Conversely, among those with CAC scores > 300, 36% were in the lowest fetuin-A quartile, whereas only 13% were in the highest. In contrast, there was no association of fetuin-A with PAD or high internal cIMT defined as categorical variables across the sequence of models (Table 3). Each standard deviation higher fetuin-A level was associated with 27% greater odds of high common cIMT in a model adjusted for lifestyle factors, but this association was attenuated and rendered no longer statistically significant when adjusted for traditional CVD risk factors and kidney function.
Table 3.
Association of Fetuin-A with Categorical Definitions of Subclinical Cardiovascular Disease: The Rancho Bernardo Study
| Fetuin-A Quartiles | Fetuin-A Continuous | ||||
|---|---|---|---|---|---|
| Fetuin-A range (g/L) | I ≤ 0.45 |
II 0.45–0.51 |
III 0.52–0.58 |
IV ≥ 0.59 |
Per SD (0.11 g/L) greater |
| CAC severity (0, 1–100, 101–300, >300) | |||||
| N with CAC>0/Total (%) | 69/86 | 76/97 | 66/95 | 75/104 | 286/382 |
| Age and sex adjusted; OR (95% CI) P-value | 1.00 (Ref) | 0.74 (0.43, 1.27); 0.27 | 0.72 (0.42, 1.25); 0.24 | 0.58 (0.34, 0.99); 0.04 | 0.81 (0.67, 0.98); 0.03 |
| Lifestyle Adjusted; OR (95% CI) P-value* | 1.00 (Ref) | 0.71 (0.41, 1.24); 0.23 | 0.71 (0.41, 1.23); 0.22 | 0.56 (0.33, 0.98); 0.04 | 0.81 (0.66, 0.98); 0.03 |
| Fully Adjusted; OR (95% CI) P-value** | 1.00 (Ref) | 0.65 (0.37, 1.14); 0.13 | 0.61 (0.35, 1.08); 0.09 | 0.45 (0.25, 0.79); 0.006 | 0.72 (0.59, 0.89); 0.002 |
| PAD (ABI < 0.90) | |||||
| N with PAD/Total (%) | 40/280 | 36/270 | 25/250 | 28/245 | 129/1045 |
| Age and sex adjusted; OR (95% CI) P-value | 1.00 (Ref) | 0.99 (0.61, 1.62); 0.98 | 0.73 (0.43, 1.23); 0.24 | 0.88 (0.52, 1.48); 0.62 | 0.93 (0.76, 1.12); 0.43 |
| Lifestyle Adjusted; OR (95% CI) P-value* | 1.00 (Ref) | 1.02 (0.62, 1.67); 0.94 | 0.72 (0.42, 1.24); 0.24 | 0.87 (0.51, 1.48); 0.60 | 0.92 (0.75, 1.12); 0.40 |
| Fully Adjusted; OR (95% CI) P-value** | 1.00 (Ref) | 1.05 (0.63, 1.73); 0.86 | 0.73 (0.42, 1.26); 0.26 | 0.78 (0.45, 1.37); 0.39 | 0.88 (0.71, 1.08); 0.22 |
| Common cIMT (> 75th Percentile vs. Lower) | |||||
| N with high common cIMT/Total (%) | 36/131 | 29/150 | 32/148 | 52/168 | 149/597 |
| Age and sex adjusted; OR (95% CI) P-value | 1.00 (Ref) | 0.73 (0.40, 1.32); 0.30 | 0.77 (0.43, 1.38); 0.38 | 1.79 (1.03, 3.11); 0.04 | 1.27 (1.03, 1.57); 0.02 |
| Lifestyle Adjusted; OR (95% CI) P-value* | 1.00 (Ref) | 0.72 (0.39, 1.31); 0.28 | 0.76 (0.42, 1.37); 0.35 | 1.76 (1.00, 3.10); 0.05 | 1.27 (1.03, 1.58); 0.03 |
| Fully Adjusted; OR (95% CI) P-value** | 1.00 (Ref) | 0.65 (0.35, 1.20); 0.17 | 0.65 (0.35, 1.20); 0.17 | 1.50 (0.84, 2.69); 0.17 | 1.20 (0.96, 1.50); 0.11 |
| Internal cIMT (> 75th Percentile vs. Lower) | |||||
| N with high internal cIMT/Total (%) | 27/125 | 33/149 | 38/143 | 47/166 | 145/583 |
| Age and sex adjusted; OR (95% CI) P-value | 1.00 (Ref) | 1.06 (0.60, 1.90); 0.83 | 1.32 (0.75, 2.34); 0.34 | 1.66 (0.95, 2.88); 0.07 | 1.19 (0.98, 1.46); 0.08 |
| Lifestyle Adjusted; OR (95% CI) P-value* | 1.00 (Ref) | 1.09 (0.60, 1.95); 0.78 | 1.34 (0.75, 2.39); 0.32 | 1.66 (0.94, 2.92); 0.08 | 1.20 (0.97, 1.47); 0.09 |
| Fully Adjusted; OR (95% CI) P-value** | 1.00 (Ref) | 1.05 (0.57, 1.93); 0.87 | 1.26 (0.69, 2.30); 0.45 | 1.35 (0.74, 2.47); 0.32 | 1.10 (0.88, 1.36); 0.40 |
Adjusted for age, sex, current smoking, alcohol use, regular physical exercise, and oral estrogen use.
Adjusted for lifestyle model (*) plus diabetes, bmi, total chol., triglycerides, and eGFR.
Figure 1. Coronary Artery Calcification Severity by Fetuin-A Quartiles. The Rancho Bernardo Study.
Distribution of coronary artery calcification scores by fetuin-A quartiles among 382 community-living individuals without clinically apparent cardiovascular disease: The Rancho Bernardo Study
Last, we tested for effect modification in the association of fetuin-A with each continuous measure of subclinical CVD by diabetes. In all cases, results were similar (all P values for interaction > 0.24).
DISCUSSION
This study evaluated for the first time the associations of plasma fetuin-A levels with subclinical CVD in a population sample of community-dwelling men and women who are free of clinical CVD. We observed that fetuin-A levels were inversely associated with CAC severity, independent of traditional CVD risk factors and kidney function. In contrast, fetuin-A levels were not associated with PAD or carotid IMT. In vitro studies, knock-out studies in rodents, and clinical studies in ESRD populations, all suggest that fetuin-A inhibits arterial calcification.(2,3,5,35) Here, we report that higher fetuin-A levels are also associated with lower CAC prevalence in community-living individuals, suggesting that fetuin-A may be serving a similar biological function in this setting.
We had hypothesized that lower fetuin-A levels would be associated with greater prevalence of PAD and higher cIMT and so the inverse association of fetuin-A with CAC but not with PAD and cIMT, was not expected. However, if confirmed, these findings may provide new insights to vascular disease. Fetuin-A inhibits arterial calcium deposition, and any individual atherosclerotic lesion may be characterized by more or less calcium content. Thus, low fetuin-A may predispose to greater calcium deposition, but not necessarily to the burden or progression of atherosclerosis per se. Above its inverse association with CAC, we also found that higher fetuin-A levels were statistically significantly associated with higher ABI measurements in the final adjusted model. Individuals with high ABI measurements are known to have marked peripheral arterial stiffness and high prevalence of medial arterial calcification;(24,36) a pattern of calcification observed frequently in persons with end-stage renal disease.(37,38) If these findings are confirmed, low fetuin-A may be marking arterial calcium burden in the deeper tunica media, but not with the burden of atherosclerosis per se.
As low fetuin-A levels were associated with CAC but not with PAD, common, or internal IMT, an alternative possibility is that fetuin-A is associated with subclinical coronary atherosclerosis, but not with atherosclerosis in other vascular beds. Future studies that evaluate whether low fetuin-A levels are more strongly associated with incident MI as compared to stroke or PAD may provide additional insights to this competing hypothesis.
Individuals with fetuin-A levels in the lowest quartile were most likely to have severe CAC, yet less likely to have traditional CVD risk factors including diabetes, hypertension, and hyperlipidemia. These findings raise the intriguing hypothesis that measurement of fetuin-A may prove useful as a risk marker for arterial calcification or CVD events in individuals who would otherwise be considered lower risk by traditional CVD risk factors. However, the biological significance of calcium deposition in atherosclerotic lesions remains a matter of debate. While it is well established that CAC score is a strong risk marker for CVD events,(30) this may be because CAC is marking the total burden of atherosclerosis rather than causally related to disease. Within individual lesions, prior investigators have suggested that calcium nodules may stimulate plaque rupture,(39) while others have suggested that calcium has a stabilizing effect on individual plaques.(40,41) Thus, the association of fetuin-A with CVD events will be an important topic for future research. Moreover, while low fetuin-A was associated with a greater burden of CAC, higher fetuin-A is also known to induce peripheral insulin resistance in vitro,(42–45) and in humans, higher fetuin-A levels are associated with future risk of diabetes.(46,47) Diabetes is a well established independent CVD risk factor. Thus, there are plausible mechanisms through which either low or high fetuin-A levels could be linked with incident CVD, and evaluation of this association should be a high priority moving forward. Few studies have evaluated this association, and the existing studies are conflicting. The largest to date, and the only study to evaluate community-dwelling individuals, observed that higher fetuin-A levels were associated with incident myocardial infarction (MI) and ischemic stroke.(48) However, another study evaluated individuals presented in the emergency room with ST segment elevation MI, and reported that lower levels were associated with mortality.(49) We previously evaluated a large sample of individuals with known prevalent CVD at baseline who experienced 182 recurrent CVD events and 220 deaths over 6 years. In this sample, we observed no statistically significant association of fetuin-A with recurrent events or death.(50) Future large-scale studies are required to confirm the nature, direction, and strength of the association of fetuin-A with incident CVD events in the general community, and to determine if such associations may differ by diabetes status.
Strengths of this study include well-characterized community-dwelling study sample without prevalent CVD and availability of three measures of subclinical CVD. The study also has important limitations. CAC and IMT measurements were made at a follow-up visit 4 years after measurement of fetuin-A, and not all participants had these measurements available. Since fetuin-A and each subclinical CVD measure were made only once, this study cannot determine the temporal directions of associations. Associations of fetuin-A with PAD and IMT were null, and it is possible that a true association with these outcomes was missed. We investigated each measure as a continuous variable to decrease the chance of type 2 errors. Nonetheless, results should be interpreted within the context of the 95% confidence intervals, which suggest that any missed association would likely be modest, at best. Measurement of other novel proteins that may inhibit arterial calcification were not available concurrently with fetuin-A. Participants were predominantly older and Caucasian. Results may not generalize to other settings.
In summary, in community-living individuals free of clinically apparent CVD, low fetuin-A levels are independently associated with CAC severity, but not with PAD or cIMT. Future studies are required to determine if blood measurement of fetuin-A is useful as a method to assess systemic burden of arterial calcification, and to determine whether fetuin-A levels may identify risk of incident CVD events in community-dwelling individuals.
Acknowledgments
This study was sponsored by a grant from the National Heart Lung and Blood Institute (R01HL096851) supporting Drs. Ix, Barrett-Connor, Wassel, and Laughlin. The Rancho Bernardo Study was funded by research grants AG028507 and AG018339 from the National Institute on Aging and grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases. Drs. Laughlin and Daniels were also supported by grants from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Price PA, Lim JE. The inhibition of calcium phosphate precipitation by fetuin is accompanied by the formation of a fetuin-mineral complex. J Biol Chem. 2003;278:22144–22152. doi: 10.1074/jbc.M300744200. [DOI] [PubMed] [Google Scholar]
- 2.Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westenfeld R, Jahnen-Dechent W, Ketteler M. Vascular calcification and fetuin-A deficiency in chronic kidney disease. Trends Cardiovasc Med. 2007;17:124–128. doi: 10.1016/j.tcm.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Hermans MM, Brandenburg V, Ketteler M, et al. Association of serum fetuin-A levels with mortality in dialysis patients. Kidney Int. 2007;72:202–207. doi: 10.1038/sj.ki.5002178. [DOI] [PubMed] [Google Scholar]
- 5.Ketteler M, Bongartz P, Westenfeld R, et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361:827–833. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 6.Stenvinkel P, Wang K, Qureshi AR, et al. Low fetuin-A levels are associated with cardiovascular death: Impact of variations in the gene encoding fetuin. Kidney Int. 2005;67:2383–2392. doi: 10.1111/j.1523-1755.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang AY, Woo J, Lam CW, et al. Associations of serum fetuin-A with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol Dial Transplant. 2005;20:1676–1685. doi: 10.1093/ndt/gfh891. [DOI] [PubMed] [Google Scholar]
- 8.Zheng S, de Las Fuentes L, Bierhals A, et al. Relation of serum fetuin-A levels to coronary artery calcium in African-American patients on chronic hemodialysis. Am J Cardiol. 2009;103:46–49. doi: 10.1016/j.amjcard.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shroff RC, Shah V, Hiorns MP, et al. The circulating calcification inhibitors, fetuin-A and osteoprotegerin, but not Matrix Gla protein, are associated with vascular stiffness and calcification in children on dialysis. Nephrol Dial Transplant. 2008 doi: 10.1093/ndt/gfn226. [DOI] [PubMed] [Google Scholar]
- 10.Kirkpantur A, Altun B, Hazirolan T, et al. Association among serum fetuin-A level, coronary artery calcification, and bone mineral densitometry in maintenance hemodialysis patients. Artif Organs. 2009;33:844–854. doi: 10.1111/j.1525-1594.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Gan LY, Li SJ, Hong N, Zhang M. Vascular calcification in maintenance hemodialysis patients. Blood Purif. 2009;28:15–20. doi: 10.1159/000210033. [DOI] [PubMed] [Google Scholar]
- 12.Schlieper G, Brandenburg V, Djuric Z, et al. Risk factors for cardiovascular calcifications in non-diabetic Caucasian haemodialysis patients. Kidney Blood Press Res. 2009;32:161–168. doi: 10.1159/000221064. [DOI] [PubMed] [Google Scholar]
- 13.Cianciolo G, La Manna G, Donati G, et al. Coronary calcifications in end-stage renal disease patients: a new link between osteoprotegerin, diabetes and body mass index? Blood Purif. 29:13–22. doi: 10.1159/000245042. [DOI] [PubMed] [Google Scholar]
- 14.Mori K, Ikari Y, Jono S, et al. Fetuin-A is associated with calcified coronary artery disease. Coron Artery Dis. 21:281–285. doi: 10.1097/MCA.0b013e32832fe5d5. [DOI] [PubMed] [Google Scholar]
- 15.Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley MA. Association of fetuin-A with mitral annular calcification and aortic stenosis among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:2533–2539. doi: 10.1161/CIRCULATIONAHA.106.682450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eraso LH, Ginwala N, Qasim AN, et al. Association of lower plasma fetuin-a levels with peripheral arterial disease in type 2 diabetes. Diabetes Care. 33:408–410. doi: 10.2337/dc09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rittig K, Thamer C, Haupt A, et al. High plasma fetuin-A is associated with increased carotid intima-media thickness in a middle-aged population. Atherosclerosis. 2009;207:341–342. doi: 10.1016/j.atherosclerosis.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Fiore CE, Celotta G, Politi GG, et al. Association of high alpha2-Heremans-Schmid glycoprotein/fetuin concentration in serum and intima-media thickness in patients with atherosclerotic vascular disease and low bone mass. Atherosclerosis. 2007;195:110–115. doi: 10.1016/j.atherosclerosis.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 19.Uz O, Kardesoglu E, Yiginer O, et al. The relationship between coronary calcification and the metabolic markers of osteopontin, fetuin-A, and visfatin. Turk Kardiyol Dern Ars. 2009;37:397–402. [PubMed] [Google Scholar]
- 20.Roos M, Lutz J, Salmhofer H, et al. Relation between plasma fibroblast growth factor-23, serum fetuin-A levels and coronary artery calcification evaluated by multislice computed tomography in patients with normal kidney function. Clin Endocrinol (Oxf) 2008;68:660–665. doi: 10.1111/j.1365-2265.2007.03074.x. [DOI] [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 22.Criqui MH, Fronek A, Klauber MR, Barrett-Connor E, Gabriel S. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: results from noninvasive testing in a defined population. Circulation. 1985;71:516–522. doi: 10.1161/01.cir.71.3.516. [DOI] [PubMed] [Google Scholar]
- 23.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg. 2008;48:1197–1203. doi: 10.1016/j.jvs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Young MJ, Adams JE, Anderson GF, Boulton AJ, Cavanagh PR. Medial arterial calcification in the feet of diabetic patients and matched non-diabetic control subjects. Diabetologia. 1993;36:615–621. doi: 10.1007/BF00404070. [DOI] [PubMed] [Google Scholar]
- 25.O'Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 26.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med. 1976;5:207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 27.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 28.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract] J Am Soc Nephrol. 2001;11:A0828. [Google Scholar]
- 29.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49:S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 31.Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukamal KJ, Kronmal RA, Mittleman MA, et al. Alcohol consumption and carotid atherosclerosis in older adults: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2003;23:2252–2259. doi: 10.1161/01.ATV.0000101183.58453.39. [DOI] [PubMed] [Google Scholar]
- 33.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 34.Barrett-Connor E, Laughlin GA. Hormone therapy and coronary artery calcification in asymptomatic postmenopausal women: the Rancho Bernardo Study. Menopause. 2005;12:40–48. doi: 10.1097/00042192-200512010-00009. [DOI] [PubMed] [Google Scholar]
- 35.Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, Jahnen-Dechent W. Theserum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J Biol Chem. 1996;271:20789–20796. doi: 10.1074/jbc.271.34.20789. [DOI] [PubMed] [Google Scholar]
- 36.Orchard TJ, Strandness DE., Jr Assessment of peripheral vascular disease in diabetes. Report and recommendations of an international workshop sponsored by the American Diabetes Association and the American Heart Association September 18–20, 1992 New Orleans, Louisiana. Circulation. 1993;88:819–828. doi: 10.1161/01.cir.88.2.819. [DOI] [PubMed] [Google Scholar]
- 37.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 38.Monckeberg J. Uber die reine mediaverkalkung der extremitatenarterien und ihr verhalten zur arteriosklerose. Virchows Arch Pathol Anat. 1902;171:141–167. [Google Scholar]
- 39.Virmani R, Burke AP, Farb A. Plaque morphology in sudden coronary death. Cardiologia. 1998;43:267–271. [PubMed] [Google Scholar]
- 40.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 41.Ge J, Chirillo F, Schwedtmann J, et al. Screening of ruptured plaques in patients with coronary artery disease by intravascular ultrasound. Heart. 1999;81:621–627. doi: 10.1136/hrt.81.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Auberger P, Falquerho L, Contreres JO, et al. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell. 1989;58:631–640. doi: 10.1016/0092-8674(89)90098-6. [DOI] [PubMed] [Google Scholar]
- 43.Mathews ST, Singh GP, Ranalletta M, et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51:2450–2458. doi: 10.2337/diabetes.51.8.2450. [DOI] [PubMed] [Google Scholar]
- 44.Mathews ST, Srinivas PR, Leon MA, Grunberger G. Bovine fetuin is an inhibitor of insulin receptor tyrosine kinase. Life Sci. 1997;61:1583–1592. doi: 10.1016/s0024-3205(97)00737-6. [DOI] [PubMed] [Google Scholar]
- 45.Rauth G, Poschke O, Fink E, et al. The nucleotide and partial amino acid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. Eur J Biochem. 1992;204:523–529. doi: 10.1111/j.1432-1033.1992.tb16663.x. [DOI] [PubMed] [Google Scholar]
- 46.Ix JH, Wassel CL, Kanaya AM, et al. Fetuin-A and incident diabetes mellitus in older persons. Jama. 2008;300:182–188. doi: 10.1001/jama.300.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stefan N, Fritsche A, Weikert C, et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes. 2008;57:2762–2767. doi: 10.2337/db08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weikert C, Stefan N, Schulze MB, et al. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118:2555–2562. doi: 10.1161/CIRCULATIONAHA.108.814418. [DOI] [PubMed] [Google Scholar]
- 49.Lim P, Collet JP, Moutereau S, et al. Fetuin-A is an independent predictor of death after st-elevation myocardial infarction. Clinical Chemistry. 2007;53:1835–1840. doi: 10.1373/clinchem.2006.084947. [DOI] [PubMed] [Google Scholar]
- 50.Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 152:640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]