Abstract
Power spectral analysis of heart rate variability (HRV) has been used frequently to assess cardiac autonomic function; however, the relationship of low frequency (LF) power of HRV to cardiac sympathetic tone has been unclear. With or without adjustment for high frequency (HF) power, total power, or respiration, LF power seems to provide an index not of cardiac sympathetic tone but of baroreflex function. Manipulations and drugs that change LF power or LF:HF may do so not by affecting cardiac autonomic outflows directly but by affecting modulation of those outflows by baroreflexes.
The autonomic nervous system plays major roles in maintaining cardiovascular homeostasis and in the pathophysiology of a wide variety of disease states. The system includes vagal cholinergic and sympathetic noradrenergic nerves that supply the heart and sympathetic noradrenergic nerves that enmesh arterioles—a major determinant of total peripheral resistance to blood flow in the body and therefore of the blood pressure. Clinicians and researchers have long sought valid, non-invasive, quantitative means to identify pathophysiologically relevant abnormalities of these systems.
As will be emphasized later, one must distinguish autonomic “tone” from modulation of that tone. If one considers a home heating system, there is a difference between measuring how much the furnace is working and measuring how much one can regulate the furnace by adjusting the thermostat. We will be proposing that power spectral analysis of heart rate variability (HRV) provides means to evaluate the ability to modulate autonomic outflows via baroreflexes rather than means to evaluate autonomic tone per se.
LF Power is Unrelated to Cardiac Sympathetic Tone during Supine Rest
About a century ago, the great Dutch cardiologist, Karel Frederik Wenckebach—the same Wenckebach for whom a type of second degree heart block still bears his name—wrote that a variable pulse rate is a sign of a healthy heart (Wenckebach, 1914). Since then many studies have shown that both tachycardia and decreased HRV are adverse prognostic signs in a variety of common conditions such as ischemic heart disease, congestive heart failure, myocardial infarction, and stroke.
Heart rate variability can be assessed in the time and frequency domains. Measures in the time domain include the standard deviation of heart rate and the standard deviation of heart rate normalized for absolute heart rate. It is generally accepted that under resting conditions HRV in the time domain mainly reflects respiratory sinus arrhythmia, which is mediated by parasympathetic cardiovagal outflow. Respiratory sinus arrhythmia corresponds to Wenckebach's sign of a healthy heart. In heart failure, myocardial infarction, and stroke, respiratory sinus arrhythmia usually is either blunted or absent. In the frequency domain, the most commonly used approach is based on power spectral analysis of HRV and in particular quantification of low frequency (LF) and high frequency (HF) power. The latter corresponds to the frequency of breathing, and most investigators agree that just as for HRV in the time domain, HF power in the frequency domain mainly reflects respiratory sinus arrhythmia.
The origins and clinical significance of LF power have aroused intense interest and persistent controversy (Akselrod et al., 1981). The source and meaning of LF power are the main subject matter of this review. Many studies have presumed that LF power, especially if adjusted for HF power, total power, or respiration, provides an index of cardiac sympathetic “tone” and that the ratio of LF:HF power indicates “sympathovagal balance.” Thus, a PubMed search on low frequency power of heart rate variability and cardiac sympathetic tone yielded 227 citations.
Several lines of evidence, however, argue against the validity of LF power, with or without adjustment for HF or total power, as an index of sympathetic outflow especially to the heart.
The rate of entry of the sympathetic neurotransmitter, norepinephrine, into the cardiac venous drainage (cardiac norepinephrine spillover) provides a “gold standard” index of cardiac sympathetic outflow. Individual values for LF power and LF:HF are not correlated with cardiac norepinephrine spillover (Kingwell et al., 1994; Alvarenga et al., 2006; Moak et al., 2007; Baumert et al., 2009).
Cardiac sympathetic neuroimaging by 18F-fluorodopamine or 11C-hydroxyephedrine positron emission tomographic scanning or 123I-metaiodobenzylguanidine single photon emission computed tomographic scanning enables quantitative assessment of noradrenergic innervation of the left ventricular myocardium. The imaging agents are taken up by sympathetic nerves and then translocated from the cytosol to intraneuronal vesicles. In other words, the scanning depicts the radioactivity in the vesicles in sympathetic nerves. As shown in Figure 1, the log of LF power is not correlated with left ventricular myocardial concentrations of 18F-fluorodopamine. Neither is LF:HF (Moak et al., 2007). LF:HF is also unrelated to cardiac sympathetic innervation assessed by 123I-metaiodobenzylguanidine scanning in patients with Parkinson disease (Haensch et al., 2009). Analogously, LF power is not associated with myocardial sympathetic innervation by 11C-hydroxyephedrine scanning (Vesalainen et al., 1999).
Drugs that increase release of norepinephrine from cardiac sympathetic nerves (e.g., tyramine, yohimbine) increase LF power even in patients with neuroimaging evidence of cardiac sympathetic denervation (Moak et al., 2007).
In patients with congestive heart failure, cardiac sympathetic outflow is known to be markedly increased (Eisenhofer et al., 1996), yet such patients have very low LF power (Adamopoulos et al., 1992; Creager, 1992; Guzzetti et al., 1995; Vesalainen et al., 1999). LF power in this setting and in the setting of pulmonary hypertension can actually be negatively related to skeletal muscle sympathetic outflow (Notarius et al., 1999; McGowan et al., 2009).
Blockade of preganglionic cardiac sympathetic outflow by segmental spinal anesthesia does not affect LF power or LF:HF, although segmental spinal anesthesia does attenuate the increase in LF:HF during head-up tilt (Hopf et al., 1995).
With aging, cardiac and total body norepinephrine spillover and skeletal muscle sympathetic outflow increase. That is, delivery of norepinephrine to its receptors increases with aging. In contrast, LF power decreases and LF:HF remains unchanged (Lipsitz et al., 1990; Ryan et al., 1992; Piccirillo et al., 1995; Karas et al., 2008).
Bilateral thoracic sympathectomies or sympathotomies, which produce partial cardiac sympathetic denervation (Moak et al., 2005), do not decrease LF power of LF:HF (Noppen et al., 1996; Tedoriya et al., 1999). We recently confirmed this finding in a cohort of patients with bilateral thoracic sympathectomies (Figure 1). In the study of Noppen et al., sympathectomized patients had decreased LF power during orthostasis, but in the study of Tedoriya et al. they did not.
Cardiac beta-adrenergic stimulation with isoproterenol, which increases heart rate and plasma norepinephrine levels (Goldstein et al., 1986), decreases LF power (Ahmed et al., 1994).
Considering that LF power is influenced by respiration-related changes in cardiovagal outflow, it has been suggested that accounting for respiratory influences on LF power improves the accuracy of power spectral analysis of HRV in assessment of cardiac sympathetic tone (Aysin et al., 2007; Colombo et al., 2008). The ANSAR ANX 3.0 system (ANSAR Medical Technologies Inc., Philadelphia, PA) is the only commercially available device that makes this adjustment. The ANX 3.0 uses a proprietary algorithm that yields a variable termed LFa. In conjunction with a testing protocol for beat-to-beat heart rate, respiration, and blood pressure during baseline sitting, the Valsalva maneuver, and standing, the ANX 3.0 calculates values for LFa and HF and interprets those values in terms of sympathetic and parasympathetic modulation and sympathovagal balance. Our recent findings that patients with low baroreflex-cardiovagal slopes have decreased values for LFa compared to subjects with normal baroreflex slopes and that these decreases are independent of cardiac sympathetic innervation cast doubt on the notion that even with respiratory adjustment LF power provides a measure of cardiac sympathetic tone (Rahman et al., 2011).
LF power is unrelated to several measures of extra-cardiac sympathetic outflow, such as peroneal skeletal muscle sympathetic traffic and plasma norepinephrine levels (Saul et al., 1990).
In dogs, heart failure increases sympathetic outflow as measured by direct recording of stellate ganglionic nervous activity, whereas in this setting LF power decreases (Piccirillo et al., 2009).
Perhaps most convincingly, in sheep with pacing-induced heart failure, in which directly measured cardiac sympathetic outflow is increased markedly, LF power in not increased, with or without normalization for total or mid-frequency power (Watson et al., 2007).
Figure 1.
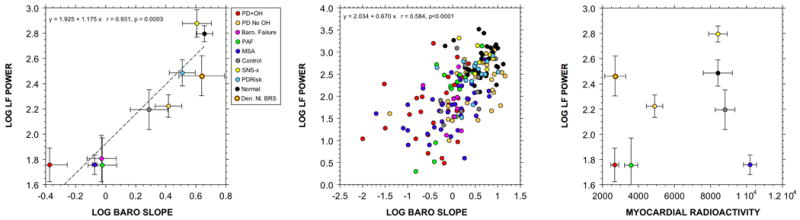
(Left) Group mean (± SEM) values for the log of low frequency (LF) power of heart rate variability (in ms2) as a function of mean values for the log of baroreflex-cardiovagal gain, calculated from the slope of the linear relationship between cardiac interbeat interval and systolic blood pressure during the descent of pressure in Phase II of the Valsalva maneuver (in ms/mm Hg). (Middle) Individual values for the log of LF power as a function of baroreflex-cardiovagal gain. (Right) Group mean (± SEM) values for the log of LF power of heart rate variability as a function of mean values for septal myocardial 18F-fluorodopamine-derived radioactivity in the 5-minute interval beginning about 5 minutes after initiation of 3-minute infusion of the tracer. Abbreviations: Den. Nl. BRS=denervated, normal baroreflex sensitivity; PD+OH=Parkinson disease with orthostatic hypotension; PD No OH=Parkinson disease without orthostatic hypotension; Baro. Failure=baroreflex failure from head/neck cancer and neck irradiation; PAF=pure autonomic failure; MSA=multiple system atrophy; SNS-x=bilateral; thoracic sympathectomies; PDRisk=individuals with multiple statistical risk factors for Parkinson disease (at least 3 of the following: family history of Parkinson disease; symptoms of REM behavior disorder; decreased olfaction; orthostatic intolerance from orthostatic hypotension. Dashed line shows linear line of best fit. Note positive correlation between the log of LF power and baroreflex-cardiovagal gain and no correlation between the log of LF power and myocardial radioactivity. Data adapted and updated from Rahman et al. (Rahman et al., 2011)
LF Power does not Relate to Sympathetic Nervous Responses to Acute Manipulations
One might propose that acute changes in LF power reflect phasic changes in sympathetic outflow, even if there were no relationship at baseline. We considered four manipulations used in clinical laboratory testing—head-up tilt, exercise, mental arithmetic, and meal ingestion.
Many studies have noted increases in LF:HF ratios and normalized LF power during orthostasis. In this setting HF power usually decreases, and so the ratio of LF:HF and LF normalized for total HRV would be expected to decrease, even if LF power remained unchanged. LF power considered alone does not consistently increase with orthostasis (Lipsitz et al., 1990; Hopf et al., 1995; Peles et al., 1995b; Piccirillo et al., 1995; Vicek et al., 2008), despite approximately a doubling of plasma norepinephrine levels, although there are exceptions (Peles et al., 1995a).
LF power also does not increase during exercise, with or without normalization for total power (Warren et al., 1997), whereas there are clear-cut increases in cardiac and extra-cardiac sympathetic outflows (Eisenhofer et al., 1992).
During laboratory psychological challenges such as mental arithmetic, video games, or the Stroop color word conflict test, total body and cardiac spillovers of norepinephrine increase (Eisenhofer et al., 1992; Esler et al., 1995), indicating increased sympathoneural outflows. Concurrently, normalized LF power may (Moriguchi et al., 1992) or may not (Sloan et al., 1996) increase. Without normalization or adjustment for HF power, LF power does not increase during laboratory psychological challenges (Madden & Savard, 1995; Sloan et al., 1996; Hoshikawa & Yamamoto, 1997; Bernardi et al., 2000).
Meal ingestion represents a situation in which both parasympathetic and sympathetic outflows might be expected to increase, the former as part of the cephalic phase of digestion and the latter as a response to a tendency toward decreased total peripheral resistance because of post-prandial shunting of blood toward the gut. Studies have disagreed about LF and HF power responses to meal ingestion. Ryan et al. reported that LF power increases in young but not old subjects, and HF power does not change regardless of subject age (Ryan et al., 1992); Miyajima et al. noted no change in LF power and an increase in HF power (Miyajima et al., 2001); Kamath et al. found a tendency to increase LF power and no change in HF power after sham feeding (“chew and spit”); Friesen et al., studying responses of children to meal ingestion, described increased LF and decreased HF power (Friesen et al., 2007); and Vaz et al. reported no changes in LF or HF power, despite significant increases in total body norepinephrine spillover (Vaz et al., 1995).
LF Power is Related to Baroreflex Function
A different perspective on the physiological meaning of LF power is based on a distinction between tone and modulation of autonomic outflows.
In the 1920s, Hering described reflexive falls in heart rate and blood pressure upon stimulation of a branch of the glossopharyngeal nerve located near the bifurcation of the carotid arteries or upon intravascular stretching of this carotid sinus area (Hering, 1927). Subsequent studies of reflexive responses to increases or decreases in blood pressure in the carotid sinus (the baroreceptor reflex or baroreflex) and of reflexive responses to hypoxia and hypercarbia in the nearby carotid bodies (chemoreflexes) led to the Nobel Prize for Corneille Heymans in 1938 (Heymans & Neil, 1958). Relevant to the current discussion, Heymans emphasized effects of baroreflex stimulation on respiration. Carotid sinus stretching decreases the frequency of breathing.
When blood pressure increases acutely, heart rate decreases because of baroreflex stimulation. In the late 1960s, Smyth, Sleight, and Pickering described a clinical method to measure baroreflex-cardiovagal gain (often called baroreflex sensitivity), based on the slope of the linear relationship between cardiac interbeat interval and systolic blood pressure after bolus i.v. injection of a pressor agent (Smyth et al., 1969), originally angiotensin but replaced soon after by phenylephrine (Bristow et al., 1969). Responses also to i.v. injection of a vasodilator enabled construction of baroreflex function curves. It soon became clear that baroreflex-cardiovagal failure is associated with the conditions noted above that are associated with decreased HRV, such as congestive heart failure, hypertension, and myocardial infarction.
Several lines of evidence fit with the concept that LF power is of central origin (Cooley et al., 1998) and in particular support an association between LF power and baroreflex modulation of autonomic outflows (Saul et al., 1990), as summarized below.
Sleight et al. (Sleight et al., 1995) demonstrated that carotid sinus stimulation produced by neck suction increases LF power only in individuals with normal baroreflex function and not in those with impaired baroreflex sensitivity.
Patients with baroreflex failure, whether from carotid endarterectomy (Timmers et al., 2004), head and neck irradiation (Timmers et al., 1999; Sharabi et al., 2003), mixed cranial nerve neuroma (Guasti et al., 2010), neurosarcoidosis (Jardine et al., 2000), or brainstem stroke (Phillips et al., 2000) have very low values for LF power. Patients who have undergone neck irradiation also have attenuated responses of LF power to drugs that increase norepinephrine release from sympathetic nerves (yohimbine, which increases exocytotic release, or tyramine, which increases non-exocytotic release, independently of cardiac sympathetic innervation (Moak et al., 2007).
Whereas individual values for the log of LF power do not correlate with cardiac sympathetic outflow, as indicated by cardiac norepinephrine spillover, they do correlate positively with the log of baroreflex-cardiovagal gain (Moak et al., 2007; Rahman et al., 2011).
Patients who are status post bilateral thoracic sympathectomies have normal baroreflex function and normal LF power, even though they have evidence for partial cardiac sympathetic denervation (Moak et al., 2005) (Figure 1).
-
Evaluation of patient groups with chronic autonomic failure provides a powerful means to determine if LF is related to cardiac sympathetic innervation, baroreflex function, or both, because chronic autonomic failure syndromes vary greatly in terms of cardiac sympathetic innervation. Three well-studied forms are pure autonomic failure, multiple system atrophy, and Parkinson disease with orthostatic hypotension. Patients with pure autonomic failure and Parkinson disease with orthostatic hypotension have neuroimaging, neurochemical, and post-mortem neuropathologic evidence of cardiac sympathetic denervation (Goldstein et al., 2000; Goldstein & Orimo, 2009), whereas most patients with multiple system atrophy have intact cardiac sympathetic innervation (Orimo et al., 2002) and normal cardiac norepinephrine spillover (Goldstein et al., 2000). All three diseases are associated with baroreflex-cardiovagal failure (Goldstein et al., 2003), and all three are associated with low LF power (Rahman et al., 2011).
In our series, across all subjects the log of LF power has been strongly positively correlated with the log of HF power (r=0.74, p<0.0001). Because of this, we cannot separate baroreflex modulation of sympathetic outflow from baroreflex modulation of parasympathetic outflow.
Although most patients with cardiac sympathetic denervation also have baroreflex failure, there are rare exceptions. Among subjects in our series with cardiac sympathetic denervation (less than 5000 nCi-kg/cc-mCi of septal 18F-fluorodopamine-derived radioactivity) and normal baroreflex-cardiovagal gain (more than 2 msec/mm Hg), LF power is approximately normal (Moak et al., 2007). Mean values for this important group (N=5) are depicted by the yellow circle with brown rim in Figure 1).
A recent study showed that in conscious mice, carotid sinus, aortic, and combined sino-aortic baroreceptor denervation decreases both LF and HF power (Rodrigues et al., 2011).
Conclusion
With or without adjustment for HF power, total power, or respiration, LF power seems to provide an index not of cardiac sympathetic tone but of baroreflex function. Manipulations and drugs that change LF power or LF:HF may do so not by affecting cardiac autonomic outflows directly but by affecting modulation of those outflows by baroreflexes.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Neurological Disorders and Stroke.
Ms. Tereza Jenkins coordinated patient travel.
References
- Adamopoulos S, Piepoli M, McCance A, Bernardi L, Rocadaelli A, Ormerod O, Forfar C, Sleight P, Coats AJ. Comparison of different methods for assessing sympathovagal balance in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1992;70:1576–1582. doi: 10.1016/0002-9149(92)90460-g. [DOI] [PubMed] [Google Scholar]
- Ahmed MW, Kadish AH, Parker MA, Goldberger JJ. Effect of physiologic and pharmacologic adrenergic stimulation on heart rate variability. J Am Coll Cardiol. 1994;24:1082–1090. doi: 10.1016/0735-1097(94)90874-5. [DOI] [PubMed] [Google Scholar]
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Alvarenga ME, Richards JC, Lambert G, Esler MD. Psychophysiological mechanisms in panic disorder: a correlative analysis of noradrenaline spillover, neuronal noradrenaline reuptake, power spectral analysis of heart rate variability, and psychological variables. Psychosom Med. 2006;68:8–16. doi: 10.1097/01.psy.0000195872.00987.db. [DOI] [PubMed] [Google Scholar]
- Aysin B, Colombo J, Aysin E. Comparison of HRV analysis methods during orthostatic challenge: HRV with respiration or without? Conf Proc IEEE Eng Med Biol Soc. 2007;2007:5047–5050. doi: 10.1109/IEMBS.2007.4353474. [DOI] [PubMed] [Google Scholar]
- Baumert M, Lambert GW, Dawood T, Lambert EA, Esler MD, McGrane M, Barton D, Sanders P, Nalivaiko E. Short-term heart rate variability and cardiac norepinephrine spillover in patients with depression and panic disorder. Am J Physiol Heart Circ Physiol. 2009;297:H674–679. doi: 10.1152/ajpheart.00236.2009. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Wdowczyk-Szulc J, Valenti C, Castoldi S, Passino C, Spadacini G, Sleight P. Effects of controlled breathing, mental activity and mental stress with or without verbalization on heart rate variability. J Am Coll Cardiol. 2000;35:1462–1469. doi: 10.1016/s0735-1097(00)00595-7. [DOI] [PubMed] [Google Scholar]
- Bristow JD, Honour J, Pickering GW, Sleight P, Smyth HS. Diminished baroreflex sensitivity in high blood pressure. Circulation. 1969;39:48–54. doi: 10.1161/01.cir.39.1.48. [DOI] [PubMed] [Google Scholar]
- Colombo J, Shoemaker WC, Belzberg H, Hatzakis G, Fathizadeh P, Demetriades D. Noninvasive monitoring of the autonomic nervous system and hemodynamics of patients with blunt and penetrating trauma. J Trauma. 2008;65:1364–1373. doi: 10.1097/TA.0b013e31818cc307. [DOI] [PubMed] [Google Scholar]
- Cooley RL, Montano N, Cogliati C, van de Borne P, Richenbacher W, Oren R, Somers VK. Evidence for a central origin of the low-frequency oscillation in RR-interval variability. Circulation. 1998;98:556–561. doi: 10.1161/01.cir.98.6.556. [DOI] [PubMed] [Google Scholar]
- Creager MA. Baroreceptor reflex function in congestive heart failure. Am J Cardiol. 1992;69:10G–15G. doi: 10.1016/0002-9149(92)91250-8. discussion 15G-16G. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Esler MD, Meredith IT, Dart A, Cannon RO, 3rd, Quyyumi AA, Lambert G, Chin J, Jennings GL, Goldstein DS. Sympathetic nervous function in human heart as assessed by cardiac spillovers of dihydroxyphenylglycol and norepinephrine. Circulation. 1992;85:1775–1785. doi: 10.1161/01.cir.85.5.1775. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Friberg P, Rundqvist B, Quyyumi AA, Lambert G, Kaye DM, Kopin IJ, Goldstein DS, Esler MD. Cardiac sympathetic nerve function in congestive heart failure. Circulation. 1996;93:1667–1676. doi: 10.1161/01.cir.93.9.1667. [DOI] [PubMed] [Google Scholar]
- Esler MD, Thompson JM, Kaye DM, Turner AG, Jennings GL, Cox HS, Lambert GW, Seals DR. Effects of aging on the responsiveness of the human cardiac sympathetic nerves to stressors. Circulation. 1995;91:351–358. doi: 10.1161/01.cir.91.2.351. [DOI] [PubMed] [Google Scholar]
- Friesen CA, Lin Z, Schurman JV, Andre L, Mc Callum RW. Autonomic nervous system response to a solid meal and water loading in healthy children: its relation to gastric myoelectrical activity. Neurogastroenterol Motil. 2007;19:376–382. doi: 10.1111/j.1365-2982.2007.00906.x. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO., 3rd Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Orimo S. Cardiac sympathetic neuroimaging: summary of the First International Symposium. Clin Auton Res. 2009;19:133–136. doi: 10.1007/s10286-009-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Pechnik S, Holmes C, Eldadah B, Sharabi Y. Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension. 2003;42:136–142. doi: 10.1161/01.HYP.0000081216.11623.C3. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Zimlichman R, Stull R, Keiser HR. Plasma catecholamine and hemodynamic responses during isoproterenol infusions in humans. Clin Pharmacol Ther. 1986;40:233–238. doi: 10.1038/clpt.1986.168. [DOI] [PubMed] [Google Scholar]
- Guasti L, Mainardi LT, Baselli G, Simoni C, Cimpanelli M, Braga SS, Pedretti R, Castiglioni L, Maroni L, Codari R, Gaudio G, Grandi AM, Marino F, Cosentino M, Venco A. Components of arterial systolic pressure and RR-interval oscillation spectra in a case of baroreflex failure, a human open-loop model of vascular control. J Hum Hypertens. 2010;24:417–426. doi: 10.1038/jhh.2009.79. [DOI] [PubMed] [Google Scholar]
- Guzzetti S, Cogliati C, Turiel M, Crema C, Lombardi F, Malliani A. Sympathetic predominance followed by functional denervation in the progression of chronic heart failure. Eur Heart J. 1995;16:1100–1107. doi: 10.1093/oxfordjournals.eurheartj.a061053. [DOI] [PubMed] [Google Scholar]
- Haensch CA, Lerch H, Jorg J, Isenmann S. Cardiac denervation occurs independent of orthostatic hypotension and impaired heart rate variability in Parkinson's disease. Parkinsonism Relat Disord. 2009;15:134–137. doi: 10.1016/j.parkreldis.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Hering HE. Die Karotissinusreflexe auf Herz und Gefasse. Steinkopff; Dresden: 1927. [Google Scholar]
- Heymans C, Neil E. Reflexogenic Areas of the Cardiovascular System. Little, Brown & Co.; Boston: 1958. [DOI] [PubMed] [Google Scholar]
- Hopf HB, Skyschally A, Heusch G, Peters J. Low-frequency spectral power of heart rate variability is not a specific marker of cardiac sympathetic modulation. Anesthesiology. 1995;82:609–619. doi: 10.1097/00000542-199503000-00002. [DOI] [PubMed] [Google Scholar]
- Hoshikawa Y, Yamamoto Y. Effects of Stroop color-word conflict test on the autonomic nervous system responses. Am J Physiol. 1997;272:H1113–1121. doi: 10.1152/ajpheart.1997.272.3.H1113. [DOI] [PubMed] [Google Scholar]
- Jardine DL, Melton IC, Bennett SI, Crozier IG, Donaldson IM, Ikram H. Baroreceptor denervation presenting as part of a vagal mononeuropathy. Clin Auton Res. 2000;10:69–75. doi: 10.1007/BF02279894. [DOI] [PubMed] [Google Scholar]
- Karas M, Larochelle P, LeBlanc RA, Dube B, Nadeau R, Champlain J. Attenuation of autonomic nervous system functions in hypertensive patients at rest and during orthostatic stimulation. J Clin Hypertens (Greenwich) 2008;10:97–104. doi: 10.1111/j.1751-7176.2008.07324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation. 1994;90:234–240. doi: 10.1161/01.cir.90.1.234. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Mietus J, Moody GB, Goldberger AL. Spectral characteristics of heart rate variability before and during postural tilt. Relations to aging and risk of syncope. Circulation. 1990;81:1803–1810. doi: 10.1161/01.cir.81.6.1803. [DOI] [PubMed] [Google Scholar]
- Madden K, Savard GK. Effects of mental state on heart rate and blood pressure variability in men and women. Clin Physiol. 1995;15:557–569. doi: 10.1111/j.1475-097x.1995.tb00544.x. [DOI] [PubMed] [Google Scholar]
- McGowan CL, Swiston JS, Notarius CF, Mak S, Morris BL, Picton PE, Granton JT, Floras JS. Discordance between microneurographic and heart-rate spectral indices of sympathetic activity in pulmonary arterial hypertension. Heart. 2009;95:754–758. doi: 10.1136/hrt.2008.157115. [DOI] [PubMed] [Google Scholar]
- Miyajima H, Nomura M, Muguruma N, Okahisa T, Shibata H, Okamura S, Honda H, Shimizu I, Harada M, Saito K, Nakaya Y, Ito S. Relationship among gastric motility, autonomic activity, and portal hemodynamics in patients with liver cirrhosis. J Gastroenterol Hepatol. 2001;16:647–659. doi: 10.1046/j.1440-1746.2001.02493.x. [DOI] [PubMed] [Google Scholar]
- Moak JP, Eldadah B, Holmes C, Pechnik S, Goldstein DS. Partial cardiac sympathetic denervation after bilateral thoracic sympathectomy in humans. Heart Rhythm. 2005;2:602–609. doi: 10.1016/j.hrthm.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Moak JP, Goldstein DS, Eldadah BA, Saleem A, Holmes C, Pechnik S, Sharabi Y. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm. 2007;4:1523–1529. doi: 10.1016/j.hrthm.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi A, Otsuka A, Kohara K, Mikami H, Katahira K, Tsunetoshi T, Higashimori K, Ohishi M, Yo Y, Ogihara T. Spectral change in heart rate variability in response to mental arithmetic before and after the beta-adrenoceptor blocker, carteolol. Clin Auton Res. 1992;2:267–270. doi: 10.1007/BF01819547. [DOI] [PubMed] [Google Scholar]
- Noppen M, Dendale P, Hagers Y, Herregodts P, Vincken W, D'Haens J. Changes in cardiocirculatory autonomic function after thoracoscopic upper dorsal sympathicolysis for essential hyperhidrosis. J Auton Nerv Syst. 1996;60:115–120. doi: 10.1016/0165-1838(96)00034-3. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Butler GC, Ando S, Pollard MJ, Senn BL, Floras JS. Dissociation between microneurographic and heart rate variability estimates of sympathetic tone in normal subjects and patients with heart failure. Clin Sci (Lond) 1999;96:557–565. [PubMed] [Google Scholar]
- Orimo S, Oka T, Miura H, Tsuchiya K, Mori F, Wakabayashi K, Nagao T, Yokochi M. Sympathetic cardiac denervation in Parkinson's disease and pure autonomic failure but not in multiple system atrophy. J Neurol Neurosurg Psychiatry. 2002;73:776–777. doi: 10.1136/jnnp.73.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Goldstein DS, Akselrod S, Nitzan H, Azaria M, Almog S, Dolphin D, Halkin H, Modan M. Interrelationships among measures of autonomic activity and cardiovascular risk factors during orthostasis and the oral glucose tolerance test. Clin Auton Res. 1995a;5:271–278. doi: 10.1007/BF01818892. [DOI] [PubMed] [Google Scholar]
- Peles E, Goldstein DS, Akselrod S, Nitzan H, Azaria M, Almog S, Dolphin D, Halkin H, Modan M. Interrelationships among measures of autonomic activity and cardiovascular risk factors during orthostasis and the oral glucose tolerance test. Clin Auton Res. 1995b;5:271–278. doi: 10.1007/BF01818892. [DOI] [PubMed] [Google Scholar]
- Phillips AM, Jardine DL, Parkin PJ, Hughes T, Ikram H. Brain stem stroke causing baroreflex failure and paroxysmal hypertension. Stroke. 2000;31:1997–2001. doi: 10.1161/01.str.31.8.1997. [DOI] [PubMed] [Google Scholar]
- Piccirillo G, Fimognari FL, Viola E, Marigliano V. Age-adjusted normal confidence intervals for heart rate variability in healthy subjects during head-up tilt. Int J Cardiol. 1995;50:117–124. doi: 10.1016/0167-5273(95)93680-q. [DOI] [PubMed] [Google Scholar]
- Piccirillo G, Ogawa M, Song J, Chong VJ, Joung B, Han S, Magri D, Chen LS, Lin SF, Chen PS. Power spectral analysis of heart rate variability and autonomic nervous system activity measured directly in healthy dogs and dogs with tachycardia-induced heart failure. Heart Rhythm. 2009;6:546–552. doi: 10.1016/j.hrthm.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman F, Pechnik S, Gross D, Sewell L, Goldstein DS. Low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Clin Auton Res. 2011;21:133–141. doi: 10.1007/s10286-010-0098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues FL, Oliveira M, Salgado HC, Fazanjr R. Effect of baroreceptor denervation on the autonomic control of arterial pressure in conscious mice. Exp Physiol. 2011 doi: 10.1113/expphysiol.2011.057067. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Goldberger AL, Ruthazer R, Mietus J, Lipsitz LA. Spectral analysis of heart rate dynamics in elderly persons with postprandial hypotension. Am J Cardiol. 1992;69:201–205. doi: 10.1016/0002-9149(92)91305-n. [DOI] [PubMed] [Google Scholar]
- Saul JP, Rea RF, Eckberg DL, Berger RD, Cohen RJ. Heart rate and muscle sympathetic nerve variability during reflex changes of autonomic activity. Am J Physiol. 1990;258:H713–721. doi: 10.1152/ajpheart.1990.258.3.H713. [DOI] [PubMed] [Google Scholar]
- Sharabi Y, Dendi R, Holmes C, Goldstein DS. Baroreflex failure as a late sequela of neck irradiation. Hypertension. 2003;42:110–116. doi: 10.1161/01.HYP.0000077441.45309.08. [DOI] [PubMed] [Google Scholar]
- Sleight P, La Rovere MT, Mortara A, Pinna G, Maestri R, Leuzzi S, Bianchini B, Tavazzi L, Bernardi L. Physiology and pathophysiology of heart rate and blood pressure variability in humans: is power spectral analysis largely an index of baroreflex gain? Clin Sci (Lond) 1995;88:103–109. doi: 10.1042/cs0880103. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Bagiella E, Bigger JT, Jr, Lo ES, Gorman JM. Relationships between circulating catecholamines and low frequency heart period variability as indices of cardiac sympathetic activity during mental stress. Psychosom Med. 1996;58:25–31. doi: 10.1097/00006842-199601000-00005. [DOI] [PubMed] [Google Scholar]
- Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man: quantitative method of assessing baroreflex sensitivity. Circ Res. 1969;24:109–121. doi: 10.1161/01.res.24.1.109. [DOI] [PubMed] [Google Scholar]
- Tedoriya T, Sakagami S, Ueyama T, Thompson L, Hetzer R. Influences of bilateral endoscopic transthoracic sympathicotomy on cardiac autonomic nervous activity. Eur J Cardiothorac Surg. 1999;15:194–198. doi: 10.1016/s1010-7940(98)00309-1. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Buskens FG, Wieling W, Karemaker JM, Lenders JW. Long-term effects of unilateral carotid endarterectomy on arterial baroreflex function. Clin Auton Res. 2004;14:72–79. doi: 10.1007/s10286-004-0165-3. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Karemaker JM, Lenders JW, Wieling W. Baroreflex failure following radiation therapy for nasopharyngeal carcinoma. Clin Auton Res. 1999;9:317–324. doi: 10.1007/BF02318378. [DOI] [PubMed] [Google Scholar]
- Vaz M, Turner A, Kingwell B, Chin J, Koff E, Cox H, Jennings G, Esler M. Postprandial sympatho-adrenal activity: its relation to metabolic and cardiovascular events and to changes in meal frequency. Clin Sci. 1995;89:349–357. doi: 10.1042/cs0890349. [DOI] [PubMed] [Google Scholar]
- Vesalainen RK, Pietila M, Tahvanainen KU, Jartti T, Teras M, Nagren K, Lehikoinen P, Huupponen R, Ukkonen H, Saraste M, Knuuti J, Voipio-Pulkki LM. Cardiac positron emission tomography imaging with [11C]hydroxyephedrine, a specific tracer for sympathetic nerve endings, and its functional correlates in congestive heart failure. Am J Cardiol. 1999;84:568–574. doi: 10.1016/s0002-9149(99)00379-3. [DOI] [PubMed] [Google Scholar]
- Vicek M, Radikova Z, Penesova A, Kvetnansky R, Imrich R. Heart rate variability and catecholamines during hypoglycemia and orthostasis. Auton Neurosci: Bas Clin. 2008;143:53–57. doi: 10.1016/j.autneu.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Warren JH, Jaffe RS, Wraa CE, Stebbins CL. Effect of autonomic blockade on power spectrum of heart rate variability during exercise. Am J Physiol. 1997;273:R495–R502. doi: 10.1152/ajpregu.1997.273.2.R495. [DOI] [PubMed] [Google Scholar]
- Watson AM, Hood SG, Ramchandra R, McAllen RM, May CN. Increased cardiac sympathetic nerve activity in heart failure is not due to desensitization of the arterial baroreflex. Am J Physiol Heart Circ Physiol. 2007;293:H798–804. doi: 10.1152/ajpheart.00147.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenckebach KF. Die Unregelmassige Hertztatigkeit und Ihre Klinische Bedeutung. Verlag von Wilhelm Engelmann; Berlin: 1914. [Google Scholar]


