Abstract
Texaphyrins, a class of tumor selective expanded porphyrins capable of coordinating large metals, have been found to act as redox mediators within biological systems. This review summarizes studies involving their experimentaluse in cancer chemotherapy. Mechanistic insights involving their presumed mode of action are also described, as well as certain structure activity relationships. Finally, newer texaphyrin-based applications associated with targeted drug delivery are presented.
Keywords: Texaphyrin, reactive oxygen species, anticancer activity, mechanism of action, zinc, platinum delivery, alpha and beta core emitters
INTRODUCTION
The regulation of reactive oxygen species (ROS) within biological systems plays a vital role in the health and longevity of the organism. For example, the serendipitous discovery by McCord and Fridovich [1] of superoxide dismutase (SOD), a major enzyme responsible for the regulation of ROS, helped pave the way for developing the disciplineof free radical biology [2]. Herein, we discuss our approach to cancer chemotherapy through alteration of the redox environment within cancer cells. Our research has focused on a class of expanded porphyrins called texaphyrins. The foremost of these, motexafin gadolinium (MGd) and motexafin lutetium (MLu) (Fig. 1), were selected for clinical development in the context of radiation therapy and photodynamic therapy, respectively, by Pharmacyclics, Inc [3, 4]. This work has provided us with insights into the cellular antioxidant system and inspired the discovery and investigation of new molecules capable of ROS generation within cancer [5, 6].
Fig. 1.
Expanded pentaaza porphyrins, MGd (1) and MLu (2). The two acetate counter anions present in the neutral complex are not shown.
REDOX ACTIVE DRUGS
The regulation and control of ROS is crucially important in many biological processes. While such ROS generation can be beneficial when effected in a cancer-selective manner, it is interesting to note that the over-production of ROS is implicated in a variety of inflammatory responses and diseases, including amyotrophic lateral sclerosis (ALS), Parkinson's Disease (PD), and Alzheimer’s Disease (AD) [7–9]. The majority of redox active drug candidates are designed to decompose ROS and related species, including superoxide, hydrogen peroxide, and peroxynitrite. These systems generally employ a redox active transition metal catalyst and are often biologically inspired by metalloenzymes such as SOD and catalase.
Groves and co-workers have studied the use of both manganese and iron metalloporphyrins for the decomposition of peroxynitrite under physiological conditions [10–13]. These systems rely on oxidized (FeIII or MnIII) species that react with ONOO− to form initially high-valent (FeIV or MnIV) oxo-intermediates. As shown in a collaboration between Sessler and Groves, Mn(II)-texaphyrins also proved to be effective peroxynitrite decomposition catalysts in the presence of ascorbate [14]. More recently, Crow and co-workers have demonstrated that a Mn(II)-texaphyrin complex that is analogous to MGd, except for the central metal and the overall complex charge, extends the lifespan of transgenic mice which over-express the G93A human Cu, Zn superoxide dismutase (SOD1) protein (a murine model for ALS) [15]. Gratifyingly, this texaphyrin complex proved to be as efficacious as the very best of the many porphyrin systems tested to date by Crow [15–18].
There is great interest in using manganese complexes for decomposition of ROS. For instance, Doctrow and colleagues have developed Mn-salen complexes that act as mimics of SOD, cata-lase, and peroxidase; these chemical entities show promising activity in vivo as supplemental antioxidants [8, 19–25] and are currently being developed by Eukarion, Inc. as potential treatments of neu-rodegenerative events such as stroke, AD, and PD. Many other small molecule SOD mimics have been developed [26]. These include Mn(III) and Fe(III) metalloporphyrins, [27–29] Cu(II) complexes, [30–32] nitroxides, [33–35] and other Fe (II/III) and Mn(II) macrocycles [36]. Riley and co-workers have developed this latter class of Fe/Mn macrocyclic complexes to the point of ongoing clinical trials [7, 9, 36]. Fridovich, Batinic-Haberle and co-workers have recently developed Mn-porphyrins as SOD-mimics [37–44] and decomposition catalysts for hydrogen peroxide, [45, 46] per-oxynitrite, [47] and carbonate radical anion [47].
Several classes of anticancer drugs, including quinone-based agents, [48] are believed to promote the generation of ROS at tumor sites via a redox cycling mechanism. Bioreductive drugs may also rely on in vivo reduction, by biological reductants such as NADPH, to produce reactive species that damage DNA. For instance, bioreductive activation of mitomycin C by one-electron reduction, can lead to the generation of ROS as well as alkylation of DNA (through opening of the aziridine ring) [49]. Other quinone antibiotics are known to form semiquinones or hydroquinones as the result of bioreduction [50]. Depending on the position and degree of cationic functionality, these bind to, and are hence capable of reacting directly with, DNA.
Natural products have been used in combination chemotherapy to treat cancers as diverse as lymphomas, Kaposi's sarcoma, cervical cancer, and squamous cell carcinoma of the head and neck. Among these, bleomycins represent a class of anticancer agents whose mode of action is believed to involve redox chemistry. This class of natural products is thought to derive its cytotoxicty from hydroxyl radical mediated cleavage of nucleic acids. However, the clinical usage of bleomycins has been limited, most notably due to pulmonary toxicity [51].
Biological systems have a host of natural defenses to ROS, including enzymes such as SOD, glutathione peroxidase, and cata-lase. Additional cofactors, such as glutathione (GSH), thioredoxin (TRX)/thioredoxin reductase (TRXR), ascorbate (vitamin C), and α-tocopherol (vitamin E), are able to serve as ROS scavengers. Many strategies to enhance the efficacy of radiation therapy involve “shutting off these defense mechanisms. For instance, L-buthionine-(S,R)-sulfoximine (BSO) acts as a selective inhibitor of GSH synthesis [52, 53] and has been found to potentiate the effects of MGd, [54] thus providing important support for its proposed mode of action. TRX is further believed to be responsible for mediating cellular response to environmental stress, including radiation therapy [55]. Inhibition of TRX/TRXR is thus an attractive strate-gyfor patients undergoing radiation therapy. Early work to target TRX involved the use of alkyl 2-imidazolyl disulfides, which thioalkylate critical cysteine residues on TRX, thereby rendering it biologically inactive [56]. More recently, rationally designed compounds, such as the cyclodextrin-derived diorganyl tellurides, have been developed as inhibitors of thioredoxin reductase and have shown inhibition of cancer cell growth [57].
STRUCTURE ACTIVITY RELATIONSHIPS OF TEXAPHYRINS: EFFECT OF REDOX POTENTIAL
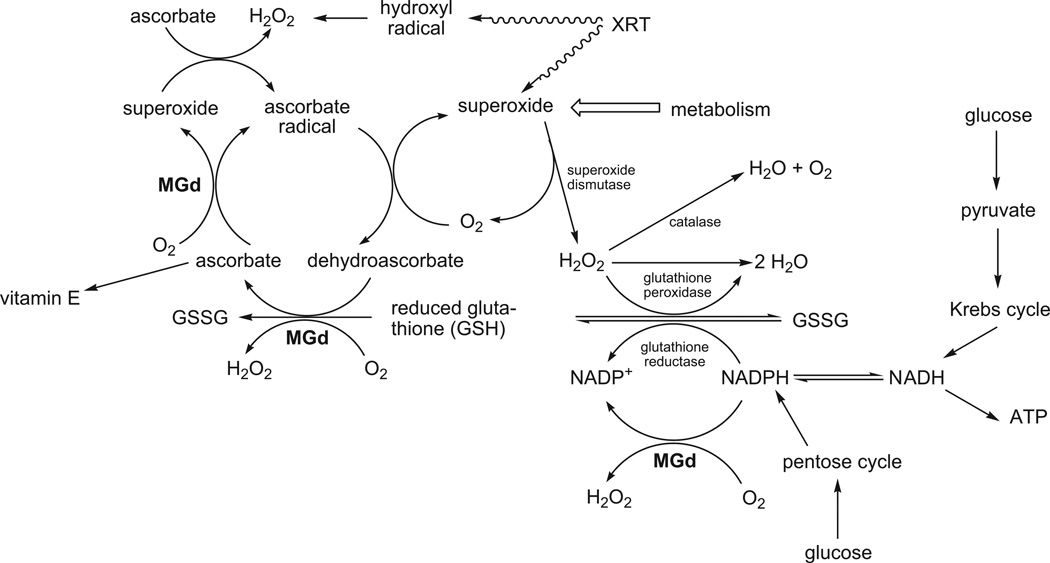
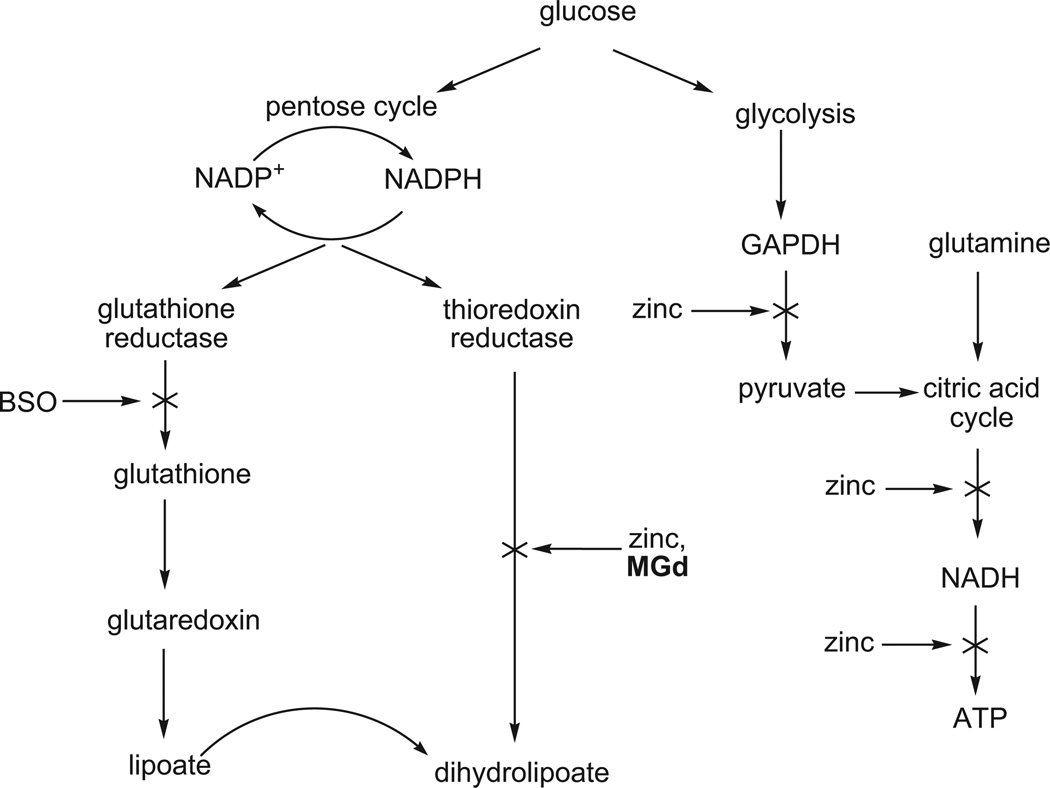
One of the most important “lessons” learned is that MGd mediates its observed radiation sensitizing function, at least in part, by acting as a redox mediator [54]. This mechanistic rationale, summarized in Fig. (2), relies on the fact that MGd readily accepts an electron from an endogenous electron rich species (also referred to as reducing metabolites), such as ascorbate, NAD(P)H, or glutathione (GSH). This produces a reduced texaphyrin radical that reacts with oxygen to produce superoxide in a rapid equilibrium process which in turn regenerates MGd. In vitro, and presumably in vivo, this superoxide is converted quickly into hydrogen peroxide, a species that is a known to be a potent apoptosis trigger. (The rate constant for non-enzymatic disproportionation of superoxide is estimated to be 1 × 105 M−1 s−1 at pH 7.4 [58]).
Fig. 2.
Metabolic processes affected by MGd. The essence of this mechanistic proposal is that MGd deactivates the cellular antioxidant system while producing ROS in a catalytic manner. This figure is a modification of one that first appeared in ref. 54.
Texaphyrins have also been shown to localize specifically to tumors [6]. In vitro, MGd uptake was found to be dependent on medium supplementation with serum protein and temperature [59, 60]. Tumor uptake and enhancement of MGd has been well documented with MRI signal enhancement, [61] fluorescence bioimaging, [62] and radiolabeling [63] in vivo with persistent uptake in the tumor relative to blood and neighboring tissue.
Because MGd, in contrast to other redox active agents, localizes in tumors, the net effect of this catalytic (in MGd) process is (1) the site selective production of ROS and enhancement of cancer cell death and (2) a corresponding decrease in the concentration of reducing metabolites, such as ascorbate and glutathione, that under normal circumstances serve to mitigate the effects of oxidative stress and ionizing radiation. Consistent with this rationale was the observation that the addition of BSO, antimycins A, or diamide, compounds involved in inhibiting glutathione metabolism or forming reactive oxygen species, enhanced the effects of MGd.
We see the mechanism presented in Fig. (2) as the beginning, rather than the end, of our structure-function based understanding of MGd. Indeed, as described below much of the work carried out over the last few years has been designed to extend and refine this working hypothesis. For instance, it provided a rationale for why MGd could prove active under certain in vitro conditions but not others (a realistic concentration of reducing metabolites is required for activity, as independently established by Rockwell [64]) [65]. This mechanism also led to the inference that MGd should (1) show activity in the absence of ionizing radiation and (2) prove particularly useful in treating cancers characterized by a high level of oxidative stress. Such considerations, supported by follow-up preclinical work, led to further clinical testing of MGd, both in conjunction with a range of other cancer drugs or, in the case of chronic lymphocytic leukemia (CLL) [66] (a disease particularly susceptible to oxidative stress) and non-small cell lung cancer (NSCLC), as a single agent.
As implied above, much of our effort has been devoted to extending and refining our mechanistic understanding of MGd. For instance, we now know that replacing the central Gd(III) by a heavier (and smaller) lanthanide(III) cation facilitates reduction of the texaphyrin (ligand, not metal) and makes the electron transfer process more favorable in a thermodynamic sense (for instance, E1/2 = −239 vs. −294 mV for the first one-electron reduction of MLu and MGd, respectively, vs. Ag/AgCl in DMSO) [67]. However, depending on the nature of the biological reductant in question, the kinetics of the reaction can be slowed. In the case of ascorbate, where this process was studied in some detail, this disparity between kinetics and thermodynamics was rationalized in structural terms [68]. In particular, it was proposed that the larger lanthanide(III) cations, including Gd(III), which resides further above of the texaphyrin plane than, e.g., Lu(III) as in 2 (MLu), allow for better binding interactions between the anionic reductant and the positively charged metal center, thus facilitating inner sphere electron transfer. Unfortunately, MLu, in contrast to MGd, is aggregated in buffer and likely bound with greater affinity to serum proteins in vitro and in vivo, thus complicating interpretations based solely on structural characteristics.
A second effect of cation-anion binding interactions was also noted, namely that the products of the reaction between MGd, ascorbate, and air form an oxalate bridged coordination polymer in saline buffer [69]. This polymer, characterized by Gd-oxalate-Gd bridging interactions, was found to be taken up into A549 human lung cancer cells more rapidly than MGd and led to greater oxidative stress. This raises the intriguing possibility, as yet not pursued, that these or related polymers could provide the basis for new kinds of formulations that might improve further the efficacy of the compound.
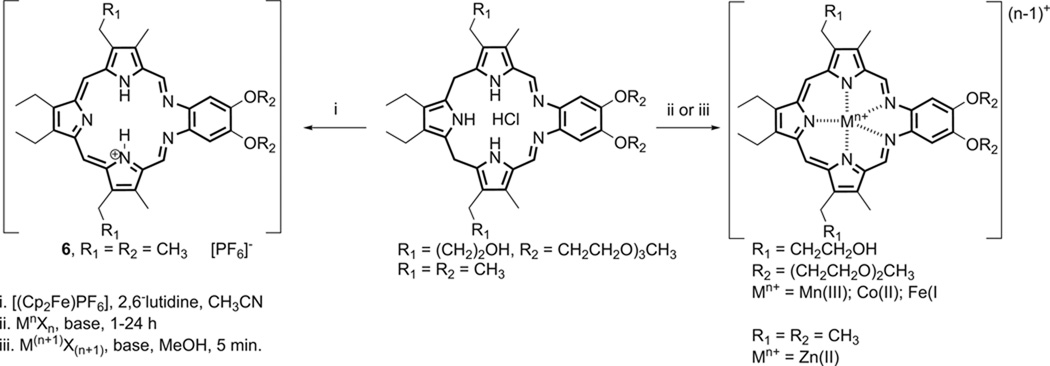
The potential utility of the reactions with ascorbate as a possible indicator of biological function provided an incentive for us to study whether other metallotexaphyrins besides those containing lanthanide(III) cations could function as oxidation catalysts for this key reducing metabolite. Accordingly, several transition metal complexes of texaphyrin were prepared (cf. Scheme 1) and characterized including the water-soluble Mn(II) (3), Co(II) (4), and μ-oxo Fe(III) dimer (5) analogues of MGd (referred to as Mn-Tex, Co-Tex, and Fe-Tex, respectively) [70]. Again, the effect of the centrally coordinated metal center was found to be substantial. While the Mn(II) complex (3) displayed an initial rate that was ca. 3 times slower than MGd under identical experimental conditions (Vo = 3.0 (µM/min vs. 8.7 (µM/min, respectively), the Co(II) (4) and μ-oxo Fe(III) dimer (5) complexes gave initial rate values (Vo = 23.8 and 30.6 µM, respectively) that were substantially larger [70]. This proved true in spite of the fact that these species are harder to reduce than MGd (E1/2 = −571 for Co-Tex vs. −294 for MGd; vs. Ag/AgCl in DMSO) [67]. In this instance, it is thought that the redox active metal centers participate in ascorbate decomposition. Unfortunately, the Co(II) complex (4) and the Fe(III) complex (5) are too lipophilic to be attractive in terms of further drug development, at least for the indications for which MGd is being tested. (Note: Fe-Tex (5) exists as a monomeric species and in equilibrium with a μ-oxo dimeric species at neutral pH.) On the other hand, the Mn(II) texaphyrin complex (3) has taken on a “life of its own” as a potential ALS treatment and has been studied carefully in this regard by Crow, as noted above.
Scheme 1.
Synthesis of transition metal texaphyrin complexes.
The zinc complex of texaphyrin was prepared and found to contain a highly unusual η3-texaphyrin-metal center coordination mode [70]. Unfortunately, preliminary biological studies revealed that this latter complex is unstable in cell culture medium. On the other hand, the lability of this metal center led us to suggest that this complex could serve as a precursor for the generation of other more stable metal systems, an idea that was subsequently developed by researchers at Pharmacyclics using the corresponding calcium texaphyrin derivative [71]. Meanwhile, in an effort to achieve this same goal, and because it represented an important “missing link”, we worked to prepare the oxidized, metal-free form of texaphyrin (6); we succeeded in doing so by subjecting the starting “sp3 form” of an organic soluble texaphyrin to oxidation using ferrocenium as the oxidant, as shown in Scheme 1 [72]. While relatively unstable in its unprotonated form, this product did allow for the formation of certain metal complexes, including a Mn(II) derivative.
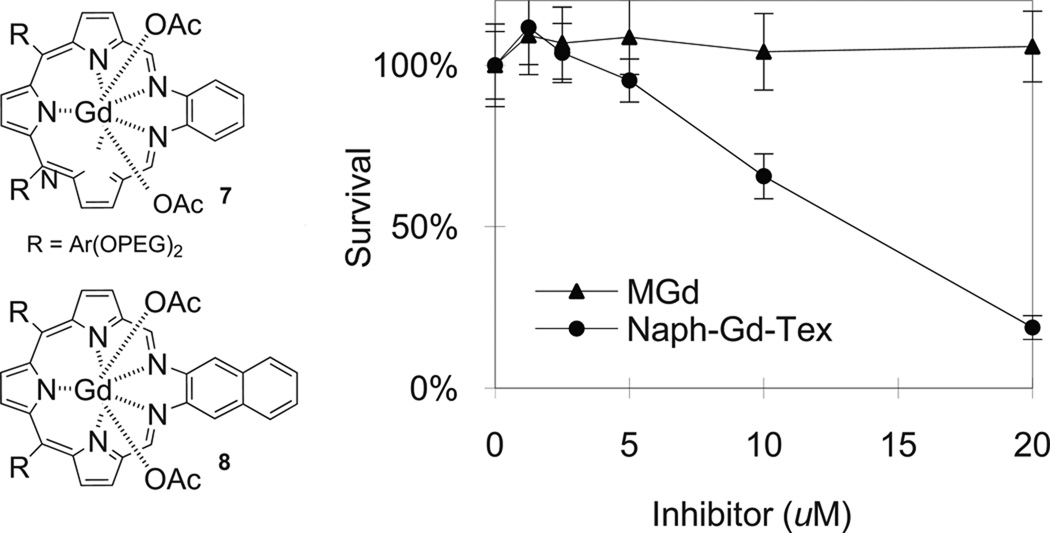
Another way of adjusting the redox potential of the texaphyrin ligand involves modifying the π-system. While there are a number of ways this could be done, one that looked particularly promising involves replacing the o-phenylenediamine moiety by the corresponding diaminonaphthalene (cf. Fig. 3). Doing so leads to a substantial enhancement of in vitro activity relative to MGd [73]. This result is rationalized in terms of a change in the redox properties of the system. Specifically, the “exchange” of naphthyl for phenyl leads to a ca. 70 mV shift in the reduction potential in favor of initial electron transfer to the texaphyrin (−122 mV, −130, and −329 mV vs. SCE in DMF in the case of 7, 8, and MGd, respectively).Assuming this latter rationale is correct, it serves to justify the synthesis of other electronically modified texaphyrins. In a more general sense, these findings are important because they show that by modifying the texaphyrin skeleton appropriately it is indeed possible to prepare new systems that are more active than MGd, at least as judged from initial tests involving well-validated in vitro model systems (A549 human lung cancer; Fig. 3).
Fig. 3.
Results of a cell proliferation assay showing the enhanced activity of the naphthalene texaphyrin (8) under conditions (RPMI media; A549 cells) where MGd is not active (ref. 73).
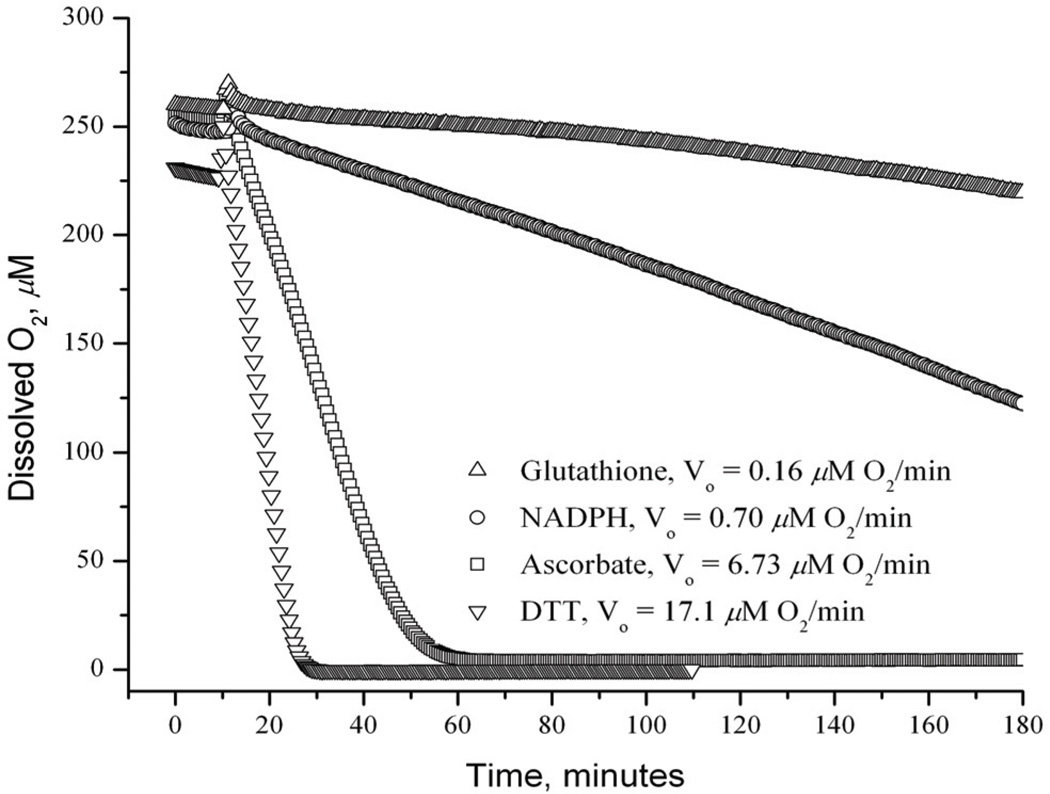
In the case of MGd, substantial effort has been devoted to exploring its reactions with reducing metabolites other than ascorbate. Although such analyses are far from complete, we have found that dithiothreitol (DTT), a model substrate containing two thiols in close proximity (a so-called “vicinal thiol”), reacts particularly quickly with MGd (cf. Fig. 4) [68, 74]. Presumably, this reflects the fact that the ultimate product of thiol oxidation is (generally) a disulfide and that eliminating the need for a bimolecular process is beneficial. In accord with such thinking, it was found that thiore-doxin reductase provides a particularly favorable substrate for MGd [68, 72]. This enzyme is characterized by a thiol-selenothiol “active site” and serves as a general purpose cellular reductant [75]. Its deactivation in vivo is thus expected to play a key role in mediating MGd-induced oxidative stress [76]. We have also found that other enzymes, including cytochrome c reductase and cytochrome P450 reductase, may be effectively substituted for thioredoxin reductase, again leading to the concurrent formation of reactive oxygen species and oxidation of the enzyme cofactor, NADPH. These and similar studies led us to propose a probable role for MGd in inhibiting the thioredoxin reductase-derived antioxidant system, as distinguished from the glutathione-dependent system (see Fig. 5 for a schematic overview).
Fig. 4.
Combined data for MGd-catalyzed, air-based oxidation of reducing metabolites in pH 7.5 NaCl/HEPES buffered aqueous media. The concentration of dissolved O2 in 1 mm sealed cuvettes was monitored as a function of time for each sample. The initial reductant concentration was 1.25 mM and the substrate:MGd ratio was 20:1 in all cases. Taken from ref. 68.
Fig. 5.
Generalized effect of MGd and zinc on metabolism. Both agents have an effect on the thioredoxin reductase based antioxidant pathway and are thus expected to complement the activity predicted on the basis of Fig. (2).
Another class of thiol rich proteins whose oxidation may play a critical role in mediating the effects of MGd, are the metallothione-ins [77]. Metallothioneins (strictly speaking thioneins) possess multiple cysteine-rich sites that can bind metal cations. They are believed to be a major site of intracellular zinc storage and transport [78, 79] and, as such, play a key role in maintaining zinc homeosta-sis. Metallothionein oxidation, in the context of MGd reduction and catalytic ROS production, would thus liberate free (or, more precisely, chelatable) zinc in a similar fashion to the effect of MGd on thioredoxin reductase described above. The presence of this free zinc, in turn, would trigger biochemical responses designed to mitigate the effects of excess zinc, including the expression of thionein and an increased production of zinc transporter 1 (ZnT1), a plasma membrane-bound protein that transports zinc to the outside of the cell [80–86]. However, the catalytic nature of the MGd-mediated oxidation process is expected to overwhelm these natural “defenses” leading to toxicity effects associated with excess free zinc (cf. Fig. 5). Moreover, it was appreciated that the disulfide bond in metal-free thionein must be reduced by thioredoxin reductase, a species that is also deactivated by MGd (vide supra), in order to (re)coordinate zinc. Thus, it was expected that MGd would act as a potent agent for the disruption of zinc homeostasis.
Considerable evidence has already been obtained in support of the above hypothesis [87]. For instance, MGd was found to lead to the release of free zinc in A549 human lung cancer, PC3 prostate cancer, and Ramos B-cell lymphoma cells [87]. This was inferred from Northern blotting and lipoate reduction inhibition measurements, as well as from direct measurements made using a zinc-specific fluorescent probe (FluoZin-3™) in combination with flow cytometry [88, 89]. Lipoate reduction and FluoZin-3 measurements also show that increased intracellular zinc levels can be observed in the absence of extracellular zinc (i.e., in RPMI 1640 medium without serum or exogenous zinc), and that inhibition of cellular transcription or translation by addition of actinomycin D or cyclohexi-mide increases zinc levels and potentiates the effect of MGd treatment. Conversely, the addition of a zinc ionophore, zinc 1 hydroxypyridine-2-thione (ZnHPT), increases the inhibition of lipoate reduction following treatment of cells with MGd. It also decreases cell viability. Finally, gene expression profiling experiments, carried out in collaboration with Hacia and coworkers (Univ. of Southern California), revealed increased levels of metal-lothionein isoforms and ZnT1 transcripts in plateau phase A549 cell cultures treated with MGd [87]. More recent studies demonstrated that MGd or zinc each elicited transcriptional responses in Ramos B-cell lymphoma cultures that were remarkably similar and consistent with those seen in the plateau phase A549 cultures. Interestingly, treatment with either MGd or zinc also led to the induction of hypoxia inducible transcription factor 1 (HIF-1) regulated genes. Stabilization of HIF-1 after treatment with MGd or zinc was confirmed on the protein level using an ELISA for HIF-1. Cultures co-treated with both MGd and zinc displayed further increases in the levels of MTF-1 and HIF-1 regulated transcripts as well as additional transcripts regulated by NF-E2-related transcription factor 2 (NRF-2), a sign of oxidative stress [90]. Taken together, these findings lead us to suggest that an important component of the anticancer activity of MGd derives from its ability to alter the cellular availability of zinc.
The upregulation of zinc by MGd is of interest because the role intracellular free (non–protein bound) zinc plays in regulating cellular functions is of considerable relevance to cancer. For example, increased free zinc has been proposed to stabilize hypoxia-inducible factor-1 (HIF-1) and thus influence processes such as glycolysis, apoptosis, and angiogenesis [90–93]. Moreover, free zinc inhibits thioredoxin reductase, [79] a key mediator in the cellular response to oxidative stress that is frequently overexpressed in cancer [76, 94, 95]. The importance of these targets in tumor development led to the hypothesis that small molecules capable of modulating free zinc levels can serve as anticancer agents. As previously mentioned, MGd increases intracellular free zinc levels, enhances the cellular toxicity of zinc, and inhibits cellular bioreductive activity in several human cancer cell lines [79, 89]. In fact, the ability of MGd to disrupt cellular zinc homeostasis provides a possible mechanistic explanation for its anticancer activity noted in clinical trials [96]. Such considerations led to the proposal that synergistic interactions between MGd and exogenous zinc acetate are important for activity and that these interactions would occur regardless of the means of zinc delivery.
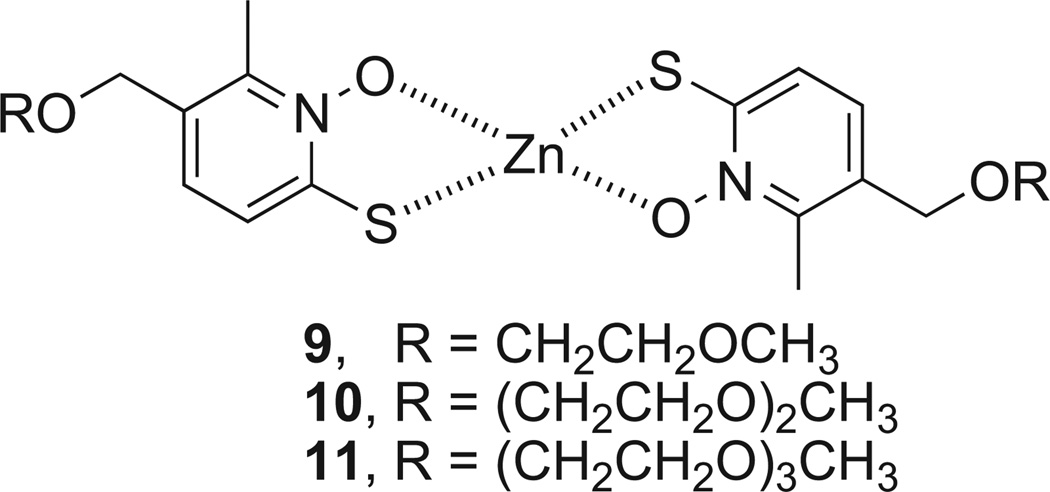
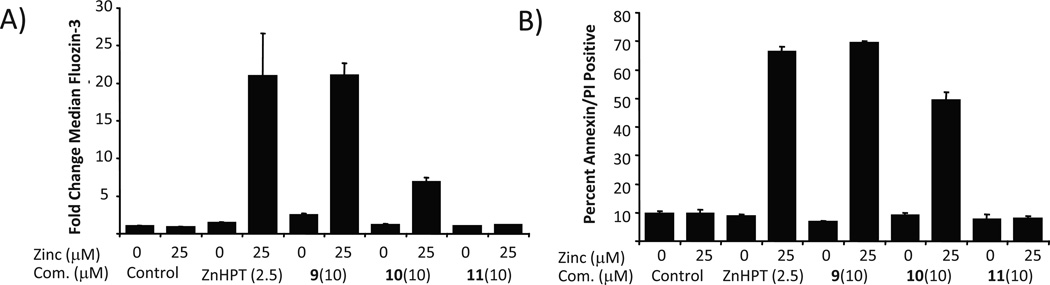
Indeed, MGd displayed synergy with the zinc ionophore ZnHPT in an in vitro assay measuring bioreductive activity as noted above [87]. ZnHPT is commonly used to increase intracellular zinc in vitro and has been given p.o. to laboratory animals for toxicity testing and to probe zinc homeostasis in vivo [97–100]. ZnHPT is extensively used as a bacteriocide and fungicide [101]. However, the complex is insoluble in aqueous media and is poorly bioavail-able [100]. Functionalizing the ZnHPT cores with short, water solubilizing polyethylene glycol (PEG) chains (i.e. complexes 9, 10, and 11, Fig. 6) increased solubility and biological utility [102]. No benefit was seen above a certain length PEG group, seemingly due to an unfavorable decrease in logP. It was also observed that co-incubation with additional Zn (Zn(OAc)2 supplemented media) potentiated increases in intracellular free Zn and this trend directly correlated to rates of cell death (cf. Fig. 7).
Fig. 6.
Water-soluble zinc ionophores.
Fig. 7.
A) Fluozin-3 fluorescence assay of A549 cells incubated with various ionophores and supplemental zinc. Results indicate increased levels of intracellular zinc in the presence of the representative zinc ionophore. B) Annexin A5 affinity assay of A549 cells with various zinc ionophores and supplemental zinc indicating that the presence of the representative zinc ionophore induces apoptosis and is correlated to increased intracellular zinc from data in A).
Even more promising, these modified Zn ionophores also proved to suppress tumor growth in nude mice bearing either A549 or PC3 tumors. 10 and 11 were sufficiently soluble in aqueous media to permit i.v. administration and testing in these A549 and PC3 xenograft models. A549 tumor growth was inhibited transiently by both agents. Since we had shown that MGd would potentiate the effects of zinc in vitro, [87] the effect of i.p. treatment with MGd was also examined, followed after 1 hour by 11. MGd treatment alone does not significantly inhibit A549 tumor growth using this protocol. However, in combination with 11, significant tumor growth inhibition was observed. In the PC3 model, tumor growth was inhibited significantly by treatment using 11 after four doses, but not after two doses. This leads us to suggest that more effective tumor control might be observed after more prolonged treatment or in combination with MGd.
CURRENT INVESTIGATIONS GENERATED FROM BIOMECHANISTIC STUDIES OF TEXAPHYRIN
Selective Platinum Delivery
Building on the characterization of texaphyrins as tumor selective redox active agents, our group has been inspired to develop the texaphyrin core as an active delivery vehicle for other known cancer therapeutics. We rationalized this approach versus other methods (i.e. pegylation, liposomal formulation) in that the carrier (i.e. texaphyrin) is itself an anticancer agent thus delivering two different agents in one.
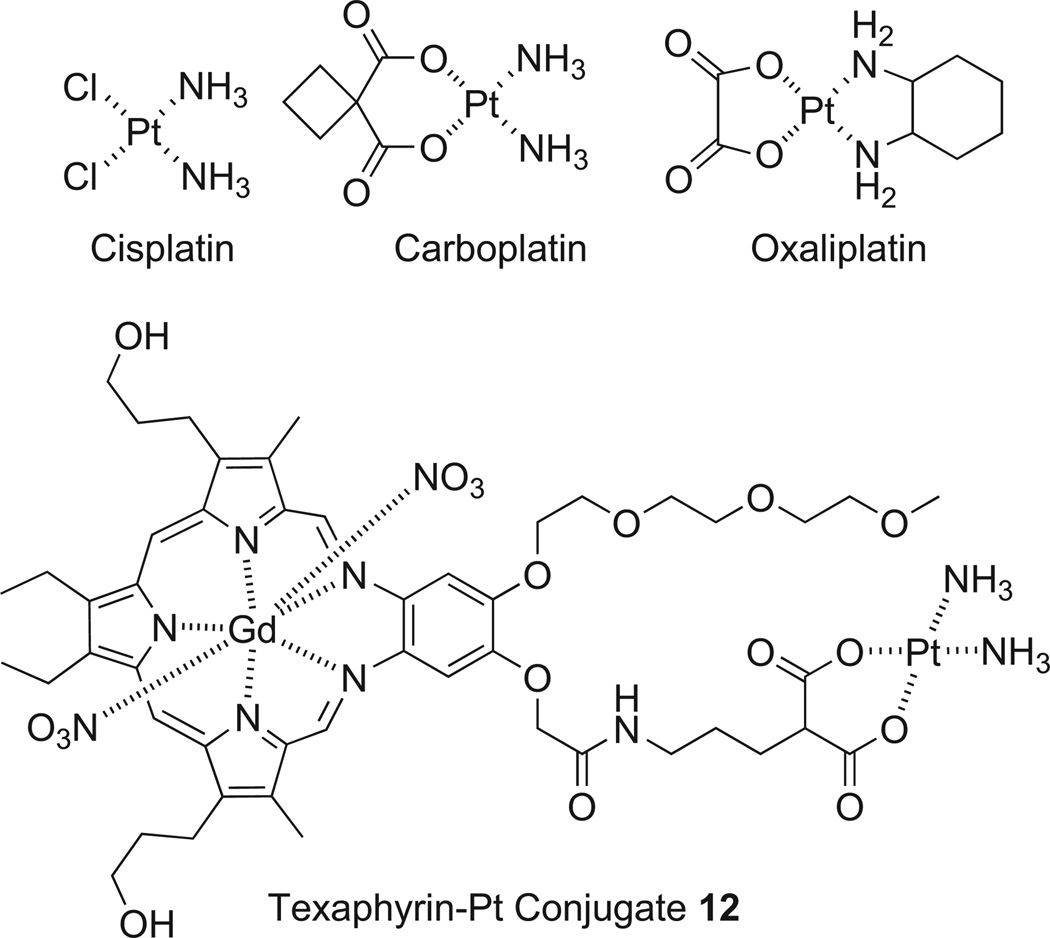
A major thrust involves the use of texaphyrin as a “carrier” with the goal of overcoming Pt-drug resistance, particularly as applied to ovarian cancer. The FDA-approved Pt drugs, cisplatin, carboplatin, and oxaliplatin (Fig. 8) are widely used cancer therapeutic agents [103–106]. Cisplatin and carboplatin, however, are the main agents used in ovarian cancer [107]. The mode of action in platinum based agents is the formation of Pt-DNA adducts, which in turn initiate several transduction pathways eventually leading to apoptosis. In several cell lines, Pt resistance has become a major factor, recapitulating a key limitation in terms of the clinical use of Pt-based drugs. This observed resistance has been attributed to a reduction in intra-cellular drug accumulation, drug inactivation by thiol-containing molecules, increased Pt-DNA adduct repair, and inactivation of apoptotic pathways (i.e., p53), to just name a few. In the clinic, resistance serves to compound the inherent limitations of the Pt drugs, including systemic (and often dose limiting) toxicity that reflects, at least in part, a lack of tumor-specific tissue distribution. Many strategies have been put forth in an effort to increase the tumor specificity and accumulation of platinum drugs, including conjugation to potentially site-directing molecules, such as folate, poly-(ethyleneglycol) (PEG), porphyrins, peptides, and nanoparti-cles among others [108–115]. While increased delivery of a Pt drug can augment tumor cell killing effects, we believe that the Pt species delivered to resistant tumor cells should also have intrinsic properties that overcome diverse mechanisms of resistance.
Fig. 8.
FDA approved Pt agents and texaphyrin-Pt conjugate 12.
Over the past two years, much of our effort has been focused on the development of texaphyrin-platinum conjugates with the ultimate goal of overcoming Pt resistance in ovarian cancer. Working in collaboration with Zahid Siddik at the M. D. Anderson Cancer Center, we began exploring the hypothesis that conjugation of platinum to a tumor localizing texaphyrin would serve to overcome some platinum resistance pathways such as reduced accumulation, reduced Pt-DNA lesions, and inactivation of p53 regulated apopto-sis.Towards this end, we designed and synthesized a novel texaphy-rin Pt conjugate (complex 12; Fig. 8). An ovarian cancer model, consisting of a platinum sensitive A2780 cell line and its isogenic platinum resistant 2780CP cell line, was chosen to determine whether this conjugate was effective in overcoming resistance [116].
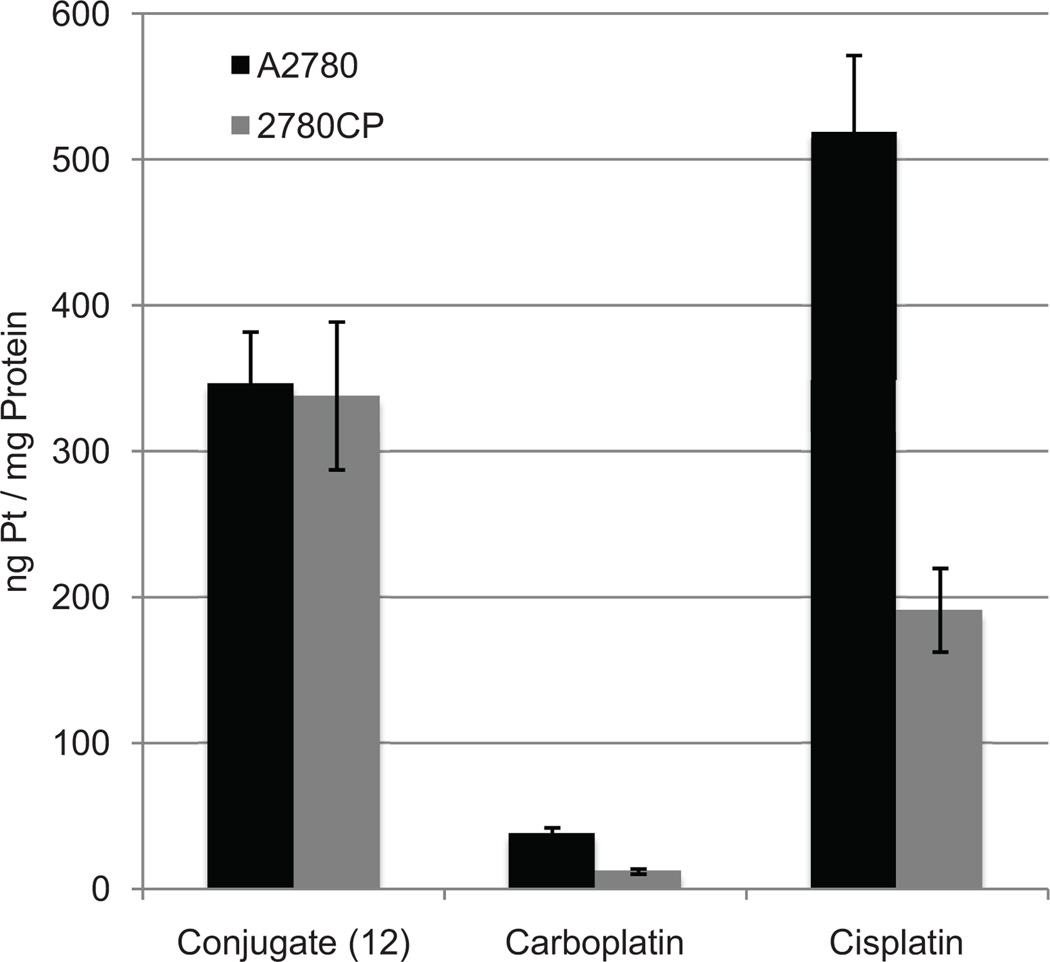
Cell proliferation assays were used initially to detect cytotoxic-ity and probe anti-resistance benefits (Table 1). Conjugate 12 provided cytotoxicity profiles similar to that of carboplatin and other controls in ovarian A2780. In addition, complex 12 provided higher cytotoxicity than MGd. Conjugate 12 provided greater cytotoxicity (i.e. lower IC50) than carboplatin in platinum sensitive 2780CP. In terms of resistance factor, conjugate 12 provided the lowest resistance factor and about 32–55% lower relative to other platinum complexes. This is indicative of partial circumvention of cisplatin resistance. It was later discovered that the decrease in resistance factor of conjugate 12 is due to increased intracellular platinum provided by conjugation to texaphyrin (cf. Fig. 9) [117]. In fact, a 12-fold increase in intracellular platinum from conjugate 12 was detected relative to carboplatin. Additionally, no reduction was seen in the uptake of platinum between A2780 and 2780CP with conjugate 12 whereas a 50% reduction was observed in platinum based controls including cisplatin. This significant increase in intracellular platinum resulted in increased production of Pt-DNA adducts in both A2780 and 2780CP, presumably accounting for the reduced resistance as compared to control complexes.
Table 1.
IC50 Values of Platinum Complexes with Cisplatin Sensitive A2780 Ovarian and Its Isogenic Cisplatin Resistant Cell Line (2780CP)
| Complex | IC50 (µM) | IC50 (µM) | Resistance |
|---|---|---|---|
| A2780 | 2780CP | Factor | |
| 12(MGd-Pt) | 1.4 | 14.4 | 10.3 |
| Carboplatin | 1.6 | 26.3 | 16.4 |
| Cisplatin | 0.31 | 7.1 | 22.9 |
| MGd | 6.3 | 13.7 | 2.2 |
Fig. 9.
Intracellular platinum concentration of cells after a 4-hour incubation with 200 µM of each complex.
Bismuth and Lead Coordinated Texaphyrins
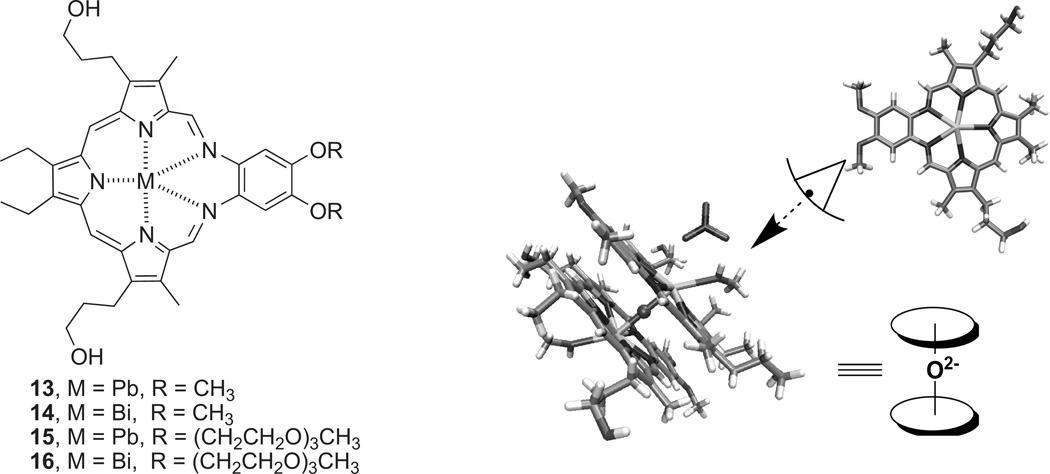
In addition to exploring biomechanistic studies of texaphyrins, much of our ongoing research effort is focused on exploiting their highly versatile complexation ability. In porphyrin chemistry, complexes with post-transition elements such as Ga, In, Tl, Pb, and Bi are rare as compared to thatof the transition elements [118]. Yet the chemistry of bismuth hasbecome of increasing interest as its Bi and123 Bi isotopeshave some potential as α-emitters in radiotherapy [119] due to effective cancer cell killing even under hypoxic conditions owing to high ionization density radiation of 100 keV/um. This ultimately leads to double-strand DNA breaks with little chance of cell repair and survival. However, the shorthalf-life of the isotopes (60.55 min and 45.65 min for212 Bi and213 Bi, respectively) gave birth to the concept of an in situ generator based on the initial complexation of lead [120]. Lead-212 has a half-life of 10.64 hours and produces 212Bi as its primary decay product along with a β-particle.
Finding suitable ligands for bismuth or lead has proved challenging. An ideal ligand would be one that is able to form rapidly stable complexes with both bismuth and lead and do so under mild conditions. Complexes of bismuth and lead that possess inherent tumor selectivity would be further advantageous since they would allow the radioactive species in question, namely 212Bi, 213Bi, or 212Pb, to be delivered selectively to cancerous tissues. This led us to suggest that texaphyrin would be an ideal ligand for these metals. Texaphyrins have been shown to localize to, or be retained selectively in, rapidly growing tissues, including cancerous lesions and could therefore act as carriers for these radioisotopes in question (cf. Fig. 10) [121]. Moreover, we demonstrated recently that texaphyrin is indeed able to complex these two cations rapidly. Specifically, spectroscopic and mass spectrometric evidence was put forward to support the formation of the first lead(II) texaphyrin complex. Similar methods were used to confirm the formation of the first discrete binuclear μ-oxo bismuth(III) macrocyclic complex (14) to be described in the literature, a system that was then characterized in a definitive manner via a single crystal X-ray diffraction analysis [122]. These newly prepared Pb(II) and Bi(III) texaphyrin complexes proved chemically stable despite the μ-oxo bond being present in the latter complex. This allowed the water soluble derivatives to be studied in vitro using the A2780 ovarian cancer cell line. On this basis, it was concluded that the Pb(II) texaphyrin 15 and the Bi(III) texaphyrin 16 gave IC50 values of 2.9 and 2.2 µM, respectively. This represents a 2–3 fold increase in cytotoxicity relative to MGd (6.3 µM) [116]. Based on these findings and considering the tumor selectivity properties of texaphyrins, we suggest that the texaphyrins could emerge as useful complexants for 212Bi, 213Bi, or 212Pb and, as such, warrant further study as candidates for radiotherapy.
Fig. 10.
Lead and bismuth texaphyrins 13–16, and views of the single crystal X-ray diffraction-derived structure of complex 14.
CONCLUSIONS
The results obtained to date provide support for the notion that texaphyrins could have a role to play in a variety of biomedical application areas, including as anticancer agents, site-localizing carriers, isotope delivery vehicles, and treatments for neurodegenerative diseases. Their unique mode of action, involving in some instances the manipulation of reactive oxygen species, and their inherent versatility in terms of sites for chemical modification and functionalization certainly make them attractive scaffolds for further study. It is hoped that this overview will inspire additional efforts to develop fully the potential of this unique class of compounds, as well as investigations of findings, such as the potential therapeutic utility of zinc ionophores, that have emerged from the work carried out to date.
ACKNOWLEDGMENTS
We thank Dr. Zahid Siddik for his collaborative efforts to develop Pt-texaphyrin conjugates. The work described herein was supported in part by the NIH (grant CA-68682 to J.L.S.) and the Robert A. Welch Foundation (Grant F-1018 to J.L.S.). Support under the Korean WCU program (grant R32-2008-000-10217-0) is acknowledged. Collaborative grant support for JFA from UT Austin TI-3D (Robert A. Welch Foundation Grant H-F-0032) and UT MD Anderson Cancer Center CCD (Grant 1003020-2100) is also acknowledged. JFA is also supported by a postdoctoral fellowship (Grant PF-11-015-01-CDD) by the American Cancer Society.
REFERENCES
- 1.McCord JM, Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J. Biol. Chem. 1968;243:5753. [PubMed] [Google Scholar]
- 2.McCord JM, Fridovich I. Superoxide dismutase: the first twenty years (1968–1988) Free Rad. Biol. Med. 1988;5:363. doi: 10.1016/0891-5849(88)90109-8. [DOI] [PubMed] [Google Scholar]
- 3.Mehta MP, Shapiro WR, Phan SC, Gervais R, Carrie C, Chabot P, Patchell RA, Glantz MJ, Recht L, Langer C, Sur RK, Roa WH, Mahe MA, Fortin A, Nieder C, Meyers CA, Smith JA, Miller RA, Renschler MF. Motexafin gadolinium combined with prompt whole brain radiotherapy prolongs time to neurologic progression in non-small-cell lung cancer patients with brain metastases: results of a phase III trial. Int. J. Radiat. Oncol. Biol. Phys. 2009;73:1069. doi: 10.1016/j.ijrobp.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 4.Patel H, Mick R, Finlay J, Zhu TC, Rickter E, Cengel KA, Malkowicz SB, Hahn SM, Busch TM. Motexafin lute-tium-photodynamic therapy of prostate cancer: short- and long-term effects on prostate-specific antigen. Clin. Cancer Res. 2008;14:4869. doi: 10.1158/1078-0432.CCR-08-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sessler JL, Hemmi G, Mody TD, Murai T, Burrell A, Young SW. Texaphyrins: Synthesis and applications. Acc. Chem. Res. 1994;27:43. [Google Scholar]
- 6.Magda D, Miller RA. Motexafin gadolinium: A novel redox active drug for cancer therapy. Seminars in Cancer Biol. 2006;16:446. doi: 10.1016/j.semcancer.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Riley DP. Functional mimics of superoxide dismutase enzymes as therapeutic agents. Chem. Rev. 1999;99:2573. doi: 10.1021/cr980432g. [DOI] [PubMed] [Google Scholar]
- 8.Doctrow SR, Adinolfi CA, Baudry M, Huffman K, Malfroy B, Marcus CB, Melov S, Pong K, Rong Y, Smart JL, Tocco G. Salen Manganese complexes, combined with superoxide dismutase/catalase mimetics, demonstrate potential for treating neurodegenerative and other age-associated diseases. Crit. Rev. Oxid. Stress Aging. 2003;2:1324. [Google Scholar]
- 9.Salvemini D, Muscoli C, Riley DP, Cuzzocrea S. Superoxide dismutase mimetics. Pulmonary Pharmacol. and Therap. 2002;15:439. doi: 10.1006/pupt.2002.0374. [DOI] [PubMed] [Google Scholar]
- 10.Groves JT. Peroxynitrite: Reactive Invasive, and Enigmatic. Peroxynitrite: Reactive, Invasive, and Enigmatic. Curr. Op. Chem. Biol. 1999;3:226. doi: 10.1016/S1367-5931(99)80036-2. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Hunt JA, Groves JT. Mechanisms of Iron Porphyrin Reactions with Peroxynitrite. J. Am. Chem. Soc. 1998;120:7493. [Google Scholar]
- 12.Lee J, Hunt JA, Groves JT. Manganese porphyrins as redox-coupled peroxynitrite reductases. J. Am. Chem. Soc. 1998;120:6053. [Google Scholar]
- 13.Shimanovich R, Groves JT. Mechanisms of peroxynitrite decomposition catalyzed by FeTMPS, a bioactive sulfonated iron porphyrin. Arch. Biochem. Biophys. 2001;387:307. doi: 10.1006/abbi.2000.2247. [DOI] [PubMed] [Google Scholar]
- 14.Shimanovich R, Hannah S, Lynch V, Gerasimchuk N, Mody TD, Magda D, Sessler JL, Groves JT. Mn(II)-Texaphyrin as a catalyst for the decomposition of peroxynitrite. J. Am. Chem. Soc. 2001;123:3613. doi: 10.1021/ja005856i. [DOI] [PubMed] [Google Scholar]
- 15.Crow JP. Administration of Mn porphyrin and Mn Texaphyrin at symptom onset extends survival of ALS Mice. In: Sessler JL, Doctrow SR, McMurry T, Lippard SJ, editors. ACS Symposium Series No. 903. Washington, DC: American Chemical Society; 2005. [Google Scholar]
- 16.Wu AS, Kiaei M, Aguirre N, Crow JP, Calingasan NY, Browne SE, Beal MF. Iron porphyrin treatment extends survival in a transgenic animal model of amyotrophic lateral sclerosis. J. Neurochem. 2003;85:142. doi: 10.1046/j.1471-4159.2003.01639.x. [DOI] [PubMed] [Google Scholar]
- 17.Crow JP. Peroxynitrite scavenging by metalloporphyrins and thiolates. Free Rad. Biol. Med. 2000;28:1487. doi: 10.1016/s0891-5849(00)00249-5. [DOI] [PubMed] [Google Scholar]
- 18.Crow JP. Manganese and iron porphyrins catalyze peroxynitrite decomposition and simultaneously increase nitration and oxidant yield: Implications for their use as peroxynitrite scavengers in vivo. Arch. Biochem. Biophys. 1999;371:41. doi: 10.1006/abbi.1999.1414. [DOI] [PubMed] [Google Scholar]
- 19.Browne SE, Roberts II LJ, Dennery PA, Doctrow SR, Beal MF, Barlow C, Levine RL. Treatment with a catalytic antioxidant corrects the neurobehavioral defect in ataxia-telangiectasia mice. Free Rad. Biol. Med. 2004;36:938. doi: 10.1016/j.freeradbiomed.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Doctrow SR, Huffman K, Marcus CB, Tocco G, Malfroy E, Adinolfi CA, Kruk H, Baker K, Lazarowych N, Mas-carenhas J, Malfroy B. Salen-manganese complexes as catalytic scavengers of hyrogen peroxide and cytoprotective agents: Structure-activity relationships Studies. J. Med. Chem. 2002;45:4549. doi: 10.1021/jm020207y. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc. Natl. Acad. Sci., USA. 2003;100:8526. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melov S, Ravenscroft J, Sarwatt M, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/ catalase mimetics. Science. 2000;289:1567. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- 23.Melov S, Wolf N, Strozyk D, Doctrow SR, Bush AI. Mice transgenic for alzheimer disease β-Amyloid develop lens cataracts that are rescued by antioxidant treatment. Free Rad. Biol. Med. 2005;38:258. doi: 10.1016/j.freeradbiomed.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Zhang HJ, Doctrow SR, Xu L, Oberley LW, Beecher B, Morrison J, Oberley TD, Kregel KC. Redox Modulation of the liver with chronic antioxidant enzyme mimetic treatment prevents age-related oxidative damage associated with environmental stress. FASEB J. 2004;18:1547. doi: 10.1096/fj.04-1629fje. [DOI] [PubMed] [Google Scholar]
- 25.Doctrow SR, Baudry M, Huffman K, Malfroy B, Melov S. Salen manganese complexes. multifunctional catalytic antioxidants protective models for neurodegenerative diseases of aging. In: Sessler JL, Doctrow SR, McMurry T, Lippard SJ, editors. ACS Symposium Series No. 903. Washington, DC: American Chemical Society; 2005. [Google Scholar]
- 26.Cabelli D, Riley D, Rodriguez JA, Valentine JS, Zhu H. Models of superoxide dismutases. In: Meunier B, editor. Biomimetic Oxidations Catalyzed by Transition Metal Complexes. London, UK: Imperial College Press; 2000. [Google Scholar]
- 27.Szabo C, Day BJ, Salzman AL. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and peroxynitrite scavenger. FEBS Lett. 1996;381:82. doi: 10.1016/0014-5793(96)00087-7. [DOI] [PubMed] [Google Scholar]
- 28.Day BJ, Batinic-Haberle I, Crapo JD. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Rad. Biol. Med. 1999;26:730. doi: 10.1016/s0891-5849(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 29.Kasugi N, Murase T, Ohse T, Nagaoka S, Kawakami H, Kubota S. Selective cell death by water-soluble Fe-porphyrins with superoxide dismutase (SOD) activity. J. Inorg. Biochem. 2002;91:349. doi: 10.1016/s0162-0134(02)00455-5. [DOI] [PubMed] [Google Scholar]
- 30.Casolaro M, Chelli M, Ginanneschi M, Laschi F, Messori L, Muniz-Miranda M, Papini AM, Kowalik-Jankowska T, Ko-zlowski H. Spectroscopic and potentiometric study of the SOD mimic system copper(II)/Acetyl-L-Histidylglycyl-L-Histidylglycine. J. Inorg. Biochem. 2002;89:181. doi: 10.1016/s0162-0134(02)00365-3. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Alvarez; Alzuet G, Borras J, Agudo LC, Montejo-Bernardo JM, Garcia-Granda S. Development of novel copper(II) complexes of Benzothiazole-N-sulfonamides as protective agents against superoxide anion. J. Biol. Inorg. Chem. 2003;8:112. doi: 10.1007/s00775-002-0394-7. [DOI] [PubMed] [Google Scholar]
- 32.Bailey MA, Ingram MJ, Naughton DP. A novel anti-oxidant and anti-cancer strategy: A peptoid anti-inflammatory drug conjugate with SOD mimic activity. Biochem. Biophys. Res. Comm. 2004;317:1155. doi: 10.1016/j.bbrc.2004.03.162. [DOI] [PubMed] [Google Scholar]
- 33.Haj-Yehia A, Nassar T, Lotan C, Munzel T, Benet L, Anggard EE. Development of 3-Nitratomethyl-PROXYL (NMP): A novel, bifunctional superoxide dismutase-mimic-nitric oxide-donor. Drug Development Res. 2000;50:528. [Google Scholar]
- 34.Goldstein S, Merenyi G, Russo A, Samun A. The Role of Oxoammonium Cation in the SOD-Mimic Activity of Cyclic Nitroxides. J. Am. Chem. Soc. 2003;125:789. doi: 10.1021/ja028190w. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman A, Goldstein S, Samuni A, Borman JB, Schwalb H. Effect of nitric oxide and nitroxide SOD-mimic on the recovery of isolated rat heart following ischemia and repersfusion. Biochem. Pharmacol. 2003;66:1279. doi: 10.1016/s0006-2952(03)00441-6. [DOI] [PubMed] [Google Scholar]
- 36.Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemer-mann C, Wang Z-Q, Salvemini D. On the selectivity of super-oxide dismutase mimetics and its importance in pharmacological studies. Brit. J. Pharmacol. 2003;140:445. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batinic-Haberle I, Reboucas JS, Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Sig. 2010;13:877. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batinic-Haberle I, Spasojevic I, Fridovich I. Tetrahydrobiop-terin rapidly reduces the SOD Mimic Mn(III) ortho-Tetrakis(N-Ethylpyridinium-2-yl)Porphyrin. Free Rad. Biol. Med. 2004;37:367. doi: 10.1016/j.freeradbiomed.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 39.Reboucas JS, Kos I, Vujaskovic Z, Batinic-Haberle I. Determination of residual manganese in Mn porphyrin-based superoxide dismutase (SOD) and peroxynitrite reductase mimics. J. Pharm. Biomed. Anal. 2009;50:1088. doi: 10.1016/j.jpba.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batinic-Haberle I, Spasojevic I, Stevens RD, Hambright P, Neta P, Okado-Matsumoto A, Fridovich I. New class of potent catalysts of O2 dismutation.Mn(III) ortho-methoxyethylpyridyl-and di-ortho-methoxyethyl-imidazolylporphyrins. Dalton Trans. 2004:1696. doi: 10.1039/b400818a. [DOI] [PubMed] [Google Scholar]
- 41.Okado-Matsumoto A, Batinic-Haberle I, Fridovich I. Complementation of SOD-deficient escherichia coli by manganese porphy-rin mimics of superoxide dismutase activity. Free Rad. Biol. Med. 2004;37:401. doi: 10.1016/j.freeradbiomed.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 42.Spasojevic I, Batinic-Haberle I, Reboucas JS, Idemori YM, Fridovich I. Electrostatic contribution in the catalysis of O2*-dismutation by superoxide dismutase mimics. MnIIITE-2-PyP5+ versus MnIIIBr8T-2-PyP+ J. Biol. Chem. 2003;278:6831. doi: 10.1074/jbc.M211346200. [DOI] [PubMed] [Google Scholar]
- 43.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng Q-F, Kang SK, Spasojevic I, Samulski TV, Fridovich I, De-whirst MW, Anscher MS. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Rad. Biol. Med. 2002;33:857. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 44.Spasojevic I, Batinic-Haberle I, Stevens RD, Hambright P, Thorpe AN, Grodkowski J, Neta P, Fridovich I. Manga-nese(III) biliverdin IX dimethyl ester: a powerful catalytic scavenger of superoxide employing the Mn(III)/Mn(IV) redox couple. Inorg. Chem. 2001;40:726. doi: 10.1021/ic0004986. [DOI] [PubMed] [Google Scholar]
- 45.Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch. Biochem. Biophys. 1997;347:256. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- 46.Liochev SI, Fridovich I. Carbon dioxide mediates Mn(II)-catalyzed decomposition of hydrogen peroxide and peroxidation reactions. Proc. Natl. Acad. Sci. U.S.A. 2004;101:12485. doi: 10.1073/pnas.0404911101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrer-Sueta G, Vitturi D, Batinic-Haberle I, Fridovich I, Goldstein S, Czapski G, Radi R. Reactions of manganese por-phyrins with peroxynitrite and carbonate radical anion. J. Biol. Chem. 2003;278:27432. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- 48.Galm U, Hager MH, Van Lanen SG, Ju J, Thorson JS, Shen B. Antitumor antibiotics: bleomycin, enediynes, and mito-mycin. Chem. Rev. 2005;105:739. doi: 10.1021/cr030117g. [DOI] [PubMed] [Google Scholar]
- 49.Tomasz M. Mitomycin C: small, fast and deadly (but very selective) Chem. Biol. 1995;2:575. doi: 10.1016/1074-5521(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 50.de Groot FMH, Damen EWP, Scheeren HW. Anticancer prodrugs for application in monotherapy: targeting hypoxia, tumor-associated enzymes, and receptors. Curr. Med. Chem. 2001;8:1093. doi: 10.2174/0929867013372634. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Stubbe JA. Bleomycins: new methods will allow rein-vestigation of old issues. Curr. Opinion Chem. Biol. 2004;8:175. doi: 10.1016/j.cbpa.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Tuttle S, Horan AM, Koch CJ, Held K, Manevich Y, Biaglow J. Radiation-sensitive tyrosine phosphorylation of cellular proteins: sensitive to changes in GSH content induced by pretreat-ment with N-acetyl-L-cysteine or L-buthionine-S,R-sulfoximine. Int. J. Radiat. Oncol. Biol. Phys. 1998;42:833. doi: 10.1016/s0360-3016(98)00331-9. [DOI] [PubMed] [Google Scholar]
- 53.Obrador E, Navarro J, Mompo J, Asensi M, Pellicer JA, Estrela JM. Regulation of tumour cell sensitivity to TNF-induced oxidative stress and cytotoxicity: role of glutathione. BioFactors. 1998;8:23. doi: 10.1002/biof.5520080105. [DOI] [PubMed] [Google Scholar]
- 54.Magda D, Lepp C, Gerasimchuk N, Lee I, Sessler JL, Lin A, Biaglow J, Miller RA. Redox cycling by motexafin gadolinium enhances cellular response to ionizing radiation by forming reactive oxygen species. Int. J. Radiat. Biol. Oncol. Phys. 2001;51:1025. doi: 10.1016/s0360-3016(01)01810-7. [DOI] [PubMed] [Google Scholar]
- 55.Wei SJ, Botero A, Hirota K, Bradbury CM, Markovina S, Laszlo A, Spitz DR, Goswami PC, Yodoi J, Gius D. Thi-oredoxin nuclear translocation and interaction with redox factor-1 activates the activator protein-1 transcription factor in response to ionizing radiation. Cancer. Res. 2000;60:6688. [PubMed] [Google Scholar]
- 56.Kirkpatrick DL, Ehrmantraut G, Stettner S, Kunkel M, Powis G. Redox active disulfides: the thioredoxin system as a drug target. Oncol. Res. 1997;9:351. [PubMed] [Google Scholar]
- 57.McNaughton M, Engman L, Birmingham A, Powis G, Cot-greave IA. Cyclodextrin-derived Diorganyl Tellurides as Glu-tathione Peroxidase Mimics and Inhibitors of Thioredoxin Reduc-tase and Cancer Cell Growth. J. Med. Chem. 2004;47:233. doi: 10.1021/jm030916r. [DOI] [PubMed] [Google Scholar]
- 58.Buettner GR. The Pecking Order of Free Radicals and Antioxi-dants: Lipid Peroxidation. Arch. Biochem. Biophys. 1993;300:535. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 59.Byrne AT, Magda D, Nguyen H, Miles D, Boswell G, Miller RA. Selective tumor localization with motexafin gadolinium (MGd) and motexafin lutetium (MLu) occurs by active transport. Toronto, Ontario, Canada: AACR; American Association for Cancer Research (AACR); 2003. p. 393. [Google Scholar]
- 60.Woodburn KW, Fan Q, Miles DR, Kessel D, Luo Y, Young SW. Localization and efficacy analysis of the photothera-peutic lutetium texaphyrin (PCI-0123) in the murine EMT6 sarcoma model. Photochem. Photobiol. Photochem. Photobiol. 1997;65:410. doi: 10.1111/j.1751-1097.1997.tb08579.x. [DOI] [PubMed] [Google Scholar]
- 61.Miles DR, Mesfin M, Mody TD, Stiles M, Lee J, Fiene J, Denis B, Boswell GW. Validation and use of three complementary analytical methods (LC-FLS, LC-MS/MS and ICP-MS) to evaluate the pharmacokinetics, biodistribution and stability of motexafin gadolinium in plasma and tissues. Anal. Bioanal. Chem. 2006;385:345. doi: 10.1007/s00216-006-0414-5. [DOI] [PubMed] [Google Scholar]
- 62.Donnelly ET, Liu Y, Fatunmbi YO, Lee I, Magda D, Rockwell S. Effects of texaphyrins on the oxygenation of EMT6 mouse mammary tumors. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:1570. doi: 10.1016/j.ijrobp.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 63.Bohmer RM, Morstyn G. Uptake of hematoporphyrin derivative by normal and malignant cells: effect of serum, pH, temperature, and cell size. Cancer Res. 1985;45:5328. [PubMed] [Google Scholar]
- 64.Rockwell S, Donnelly ET, Liu Y, Tang L-Q. Preliminary studies of the effects of gadolinium texaphyrin on the growth and radiosensitivity of EMT6 cells in vitro. Int. J. Radiat. Oncol. Biol. Phys. 2002;54:536. doi: 10.1016/s0360-3016(02)02962-0. [DOI] [PubMed] [Google Scholar]
- 65.Bernhard EJ, Mitchell JB, Deen D, Cardell M, Rosenthal DI, Brown JM. Re-evaluating gadolinium(III) texaphyrin as a radiosensitizing agent. Canc. Res. 2000;60:86. [PubMed] [Google Scholar]
- 66.Lin TS, Naumovski L, Lecane PS, Lucas MS, Moran ME, Cheney C, Lucas DM, Phan SC, Miller RA, Byrd JC. Effects of motexafin gadolinium in a phase II trial in refractory chronic lymphocytic leukemia. Leuk. Lymphoma. 2009;50:1977. doi: 10.3109/10428190903288464. [DOI] [PubMed] [Google Scholar]
- 67.Guldi DM, Mody TD, Gerasimchuk NN, Magda D, Sessler JL. Influence of Large Metal Cations on the Photophysical Properties of Texaphyrin, a Rigid Aromatic Chromophore. J. Am. Chem. Soc. 2000;122:8289. [Google Scholar]
- 68.Magda DJ, Sessler JL, Gerasimchuk N, Miller RA. Gadolium(III) Texaphyrin (Xcytrin®): A New Class of Redox Active Drug Leads. In: Sessler JL, Doctrow SR, McMurry T, Lippard SJ, editors. Medicinal Inorganic Chemistry -- ACS Symposium Series No. 903. Oxford University Press; 2005. [Google Scholar]
- 69.Magda D, Lepp C, Gerasimchuk N, Lecane P, Miller RA, Biaglow JE, Sessler JL. Motexafin gadolinium reacts with ascorbate to produce reactive oxygen species. Chem. Commun. 2002:2730. doi: 10.1039/b208760j. [DOI] [PubMed] [Google Scholar]
- 70.Hannah S, Lynch V, Guldi DM, Gerasimchuk N, MacDonald CLB, Magda D, Sessler JL. Late first-row transition-metal complexes of texaphyrin. J. Am. Chem. Soc. 2002;124:8416. doi: 10.1021/ja012747a. [DOI] [PubMed] [Google Scholar]
- 71.Fu L, Carey D, Mody T. Preparation of Metallotexaphyrins. WO2004050716. U.S. Patent. 2004
- 72.Biaglow JE, Miller RA, Magda D, Lee I, Tuttle S. Prog. Abst. Radiat. Res. Soc. 2002:107. [Google Scholar]
- 73.Wei W-h, Wang Z, Magda D, Sessler JL. New polyethyle-neglycol-functionalized texaphyrins: synthesis and in vitro biological studies. Dalton Trans. 2006:1934. doi: 10.1039/b515636j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hashemy SI, Ungerstedt JS, Zahedi Avval F, Holmgren A. Motexafin gadolinium, a tumor-selective drug targeting thiore-doxin reductase and ribonucleotide reductase. J. Biol. Chem. 2006;281:10691. doi: 10.1074/jbc.M511373200. [DOI] [PubMed] [Google Scholar]
- 75.Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267:6102. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 76.Smart DK, Ortiz KL, Mattson D, Bradbury CM, Bisht KS, Sieck LK, Brechbiel MW, Gius D. Thioredoxin reductase as a potential molecular target for anticancer agents that induce oxidative stress. Cancer Res. 2004;64:6716. doi: 10.1158/0008-5472.CAN-03-3990. [DOI] [PubMed] [Google Scholar]
- 77.Kagi JHR, Schaffer A. Bichemistry of metallothionein. Biochemistry. 1988;27:8509. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- 78.Vallee BL. Introduction to metallothionein. Methods Enzymology. 1991;205:3–7. doi: 10.1016/0076-6879(91)05077-9. [DOI] [PubMed] [Google Scholar]
- 79.Cherian MG, Jayasurya A, Bay BH. Metallothioneins in human tumors and potential roles in carcionogenesis. Mutat. Res. 2003;533:201. doi: 10.1016/j.mrfmmm.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 80.Giedroc DP, Chen X, Apuy JL. Metal response element (MRE)-binding transcription factor-2 (MTF-1): structure, function, and regulation. Antioxid. Redox Signal. 2001;3:577. doi: 10.1089/15230860152542943. [DOI] [PubMed] [Google Scholar]
- 81.Hasumi M, Suzuki K, Matsui H, Koike H, Ito K, Yamanaka H. Regulation of metallothionein and zinc transporter expression in human prostate cancer cells and tissues. Cancer Lett. 2003;200:187. doi: 10.1016/s0304-3835(03)00441-5. [DOI] [PubMed] [Google Scholar]
- 82.Cousins RJ, Blanchard RK, Popp MP, Liu L, Cao J, Moore JB, Green CL. A global view of the selectivity of zinc deprivation and excess on genes expressed in human THP-1 mono-nuclear cells. Proc. Natl. Acad. Sci., USA. 2003;100:6952. doi: 10.1073/pnas.0732111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J. Biol. Chem. 2000;275:34803. doi: 10.1074/jbc.M007339200. [DOI] [PubMed] [Google Scholar]
- 84.Lichtlen P, Schaffner W. Putting its fingers on stressful situations: the heavy metal-regulatory transcription factor MTF-1. Bioessays. 2001;23:1010. doi: 10.1002/bies.1146. [DOI] [PubMed] [Google Scholar]
- 85.Lichtlen P, Wang Y, Belser T, Georgiev O, Certa U, Sack R, Schaffner W. Target gene search for the metal-responsive transcription factor MTF-1. Nucleic Acids Res. 2001;29:1514. doi: 10.1093/nar/29.7.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andrews GK. Cellular zinc sensors: MTF-1 regulation of gene expression. Biometals. 2001;14:223. doi: 10.1023/a:1012932712483. [DOI] [PubMed] [Google Scholar]
- 87.Magda D, Lecane P, Miller RA, Lepp C, Miles D, Mesfin M, Biaglow JE, Ho VV, Chawannakul D, Nagpal S, Karaman MW, Hacia JG. Motexafin Gadolinium and Zinc Induce Oxidative Stress Responses and Apoptosis in B-Cell Lym-phoma Lines. Canc. Res. 2005;65:3837. doi: 10.1158/0008-5472.CAN-04-4099. [DOI] [PubMed] [Google Scholar]
- 88.Biaglow JE, Donahue J, Tuttle S, Held K, Chrestensen C, Mieyal JA. method for measuring disulfide reduction by cultured ammalian cells: relative contributions of gluthione-dependent and glutathione-independent mechanisms. Anal. Biochem. 2000;281:77. doi: 10.1006/abio.2000.4533. [DOI] [PubMed] [Google Scholar]
- 89.Gee KR, Zhou ZL, Ton-That D, Sensi SL, Weiss JH. Measuring zinc in living cells. A new generation of sensitive and selective fluorescent probes. Cell Calcium. 2002;31:245. doi: 10.1016/S0143-4160(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 90.Lecane P, Karaman MW, Sirisawad M, Naumovski L, Miller RA, Hacia JG, Magda D. Motexafin Gadolinium and Zinc Induce Oxidative Stress Responses and Apoptosis in B-Cell Lymphoma Lines. Cancer Res. 2005;65:11676. doi: 10.1158/0008-5472.CAN-05-2754. [DOI] [PubMed] [Google Scholar]
- 91.Hirsila M, Koivunen P, Xu L, Seeley T, Kivirikko KI, Myllyharju J. Effect of desferrioxamine and metals on the hydroxylases in the oxygen sensing pathway. FASEBJ. 2005;19:1308. doi: 10.1096/fj.04-3399fje. [DOI] [PubMed] [Google Scholar]
- 92.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxy-lases. Nat. Rev. Mol. Cell Biol. 2004;5:343. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 93.Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 94.Choi JH, Kim TN, Kim S, Baek S, Kim J, Lee S, Kim JR. Overexpression of mitochondrial thioredoxin reductase and peroxiredoxin III in hepatocellular carcinomas. Anticancer Res. 2002;22:3331. [PubMed] [Google Scholar]
- 95.Kirkpatrick DL, Kuperus M, Dowdeswell M, Potier N, Donald L, Kunkel M, Berggren M, Angulo M, Powis G. Mechanisms of inhibition of the thioredoxin growth factor system by antitumor 2-imidazolyl disulfides. Biochem. Pharmacol. 1998;55:987–94. doi: 10.1016/s0006-2952(97)00597-2. [DOI] [PubMed] [Google Scholar]
- 96.Mehta MP, Rodrigus P, Terhaard CH, Rao A, Suh J, Roa W, Souhami L, Bezjak A, Leibenhaut M, Komaki R, Schultz C, Timmerman R, Curran W, Smith J, Phan S, Miller RA, Renschler M. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J. Clin. Oncol. 2003;21:2529. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 97.Gibson WB, Jeffcoat AR, Turan TS, Wendt RH, Hughes PF, Twine ME. Zinc pyridinethione: serum metabolites of zinc pyridinethione in rabbits, rats, monkeys, and dogs after oral dosing. Toxicol. Appl. Pharmacol. 1982;62:237. doi: 10.1016/0041-008x(82)90122-3. [DOI] [PubMed] [Google Scholar]
- 98.Jeffcoat AR, Gibson WB, Rodriguez PA, Turan TS, Hughes PF, Twine ME. Zinc pyridinethione: urinary metabolites of zinc pyridinethione in rabbits, rats, monkeys, and dogs after oral dosing. Toxicol. Appl. Pharmacol. 1980;56:141. doi: 10.1016/0041-008x(80)90139-8. [DOI] [PubMed] [Google Scholar]
- 99.Jasim S, Tjalve H. Effect of zinc pyridinethione on the tissue disposition of nickel and cadmium in mice. Acta. Pharmacol. Toxicol. (Copenh) 1986;59:204. doi: 10.1111/j.1600-0773.1986.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 100.Jasim S, Tjalve H. Effect of sodium pyridinethione on the uptake and distribution of nickel, cadmium and zinc in pregnant and non-pregnant mice. Toxicology. 1986;38:327. doi: 10.1016/0300-483x(86)90148-4. [DOI] [PubMed] [Google Scholar]
- 101.Doose CA, Ranke J, Stock F, Bottin-Weber U, Jastorff B. Structure-activity relationships of pyrithiones-IPC-81 toxicity tests with the antifouling biocide zinc pyrithione and structural analogs. Green Chem. 2004;6:259. [Google Scholar]
- 102.Magda D, Lecane P, Wang Z, Hu W, Thiemann P, Ma X, Dranchak P, Wang X, Lynch V, Wei W, Csokai V, Hacia J, Sessler J. Synthesis and Anticancer Properties of Water-Soluble Zinc Ionophores. Cancer Res. 2008;68:13. doi: 10.1158/0008-5472.CAN-08-0601. 5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bosl GJ, Bajorin DF, Sheinfeld J. In: Cancer of the Testis. DeVita VTJ, Hellman S, Rosenberg SA, editors. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 104.Jamieson ER, Lippard SJ. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem. Rev. 1999;99:2467. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 105.Kelland LR, Sharp SY, O'Neill CF, Raynaud FI, Beale PJ, Judson IR. Mini-review: discovery and development of platinum complexes designed to circumvent cisplatin resistance. J. Inorg. Biochem. 1999;77:111. [PubMed] [Google Scholar]
- 106.Fuertes MA, Alonso C, Pérez J-M. Biochemical modulation of Cisplatin mechanisms of action:enhancement of antitumor activity circumvention of drug resistance. Chem. Rev. 2003;103:645. doi: 10.1021/cr020010d. [DOI] [PubMed] [Google Scholar]
- 107.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 108.Aronov O, Horowitz AT, Gabizon A, Fuertes MA, Perez JM, Gibson D. Nuclear Localization signal-targeted poly(ethylene glycol) conjugates as potential carriers and nuclear localizing agents for carboplatin analogues. Bioconjugate Chem. 2004;15:814. doi: 10.1021/bc0499331. [DOI] [PubMed] [Google Scholar]
- 109.Aronov O, Horowitz AT, Gabizon A, Gibson DFolate-Targeted PEG as a Potential Carrier for Carboplatin Analogs. Synthesis and in Vitro Studies. Bioconjugate Chem. 2003;14:563. doi: 10.1021/bc025642l. [DOI] [PubMed] [Google Scholar]
- 110.Lottner C, Knuechel R, Bernhardt G, Brunner H. Distribution and cellular localization of a water-soluble hematoporphyrin-platinum(II) complex in human bladder cancer cells. Cancer Lett. 2004;215:167. doi: 10.1016/j.canlet.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 111.Barnes KR, Kutikov A, Lippard SJ. Synthesis, characterization and cytotoxicity of a series of estrogen-tethered platinum(IV) complexes. Chem. Biol. 2004;11:557. doi: 10.1016/j.chembiol.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 112.Galanski M, Keppler BK. Searching for the magic bullet: anticancer platinum drugs which can be accumulated or activated in the tumor tissue. Anti-Cancer Agents in Med. Chem. 2007;7:55. doi: 10.2174/187152007779314017. [DOI] [PubMed] [Google Scholar]
- 113.van Zutphen S, Reedijk J. Targeting platinum anti-tumour drugs: Overview of strategies employed to reduce systemic toxicity. Coord. Chem. Rev. 2005;249:2845. [Google Scholar]
- 114.Mukhopadhyay S, Barnes CM, Haskel A, Short SM, Barnes KR, Lippard SJ. Conjugated Platinum(IV)-Peptide Complexes for Targeting Angiogenic Tumor Vasculature. Biocon-jugate Chem. 2008;19:39. doi: 10.1021/bc070031k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dhar S, Daniel WL, Giljohann DA, Mirkin CA, Lippard SJ. Polyvalent Oligonucleotide Gold Nanoparticle Conjugates as Delivery Vehicles for Platinum(IV) Warheads. J. Am. Chem. Soc. 2009;131:14652. doi: 10.1021/ja9071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arambula JF, Sessler JL, Fountain M, Wei W-h, Magda D, Siddik ZH. Gadolinium texaphyrin (Gd-Tex)-malonato-platinum conjugates: Synthesis and comparison with carboplatin in normal and Pt-resistent cells. Dalton Trans. 2009;48:10834. doi: 10.1039/b912089k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arambula JF, Sessler JL, Siddik ZH. Overcoming biochemical pharmacologic mechanisms of platinum resistance with a texaphyrin-platinum conjugate. Bioorg. Med. Chem. Lett. 2011 doi: 10.1016/j.bmcl.2011.01.092. doi:10.1016/ j.bmcl.2011.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. 3rd ed. New York: Wiley; 1972. [Google Scholar]
- 119.(a) Kozak RW, Atcher RW, Gansow OA, Friedman AM, Hines JJ, Waldmann TA. Bismuth-212-labeled anti-TAC monoclinal antibody: a-particle emitting radionucleotides as modalities for radioimmunotherapy. Proc. Natl. Acad. Sci. USA. 1991;83:474. doi: 10.1073/pnas.83.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brechbiel MW, Pippin CG, McMurry TJ, Milenic D, Roselli M, Colcher D, Gansow OA. An effective chelating agent for abeling of monoclonal antibody with bismuth-212 for a-particle mediated radiomimmunotherapy. J. Chem. Soc. Chem. Commun. 1991:1169. [Google Scholar]
- 120.Kumar K, Magerstaedt M, Gansow OA. Lead(II) and bismuth(III) complexes of the polyazacycloalkane-N-acetic acids 1,4,7-triazacyclononane-N,N',N"-triacetic acid, 1,4,7,10-tetraazacyclododecane-N,N',N",N"'-tetraacetic acid, and 1,4,8,11-tetraazacyclotetradecanetetraacetic acid. J. Chem. Soc. Chem. Commun. 1989:145. [Google Scholar]
- 121.Sessler JL, Miller RATexaphyrins. New Drugs with diverse clinical applications in radiation and photodynamic therapy. Bio-chem. Pharmacol. 2000;59:733. doi: 10.1016/s0006-2952(99)00314-7. [DOI] [PubMed] [Google Scholar]
- 122.Preihs C, Arambula JF, Lynch VM, Siddik ZH, Sessler JL. Bismuth- and lead-texaphyrin complexes: Towards potential α-core emitters for radiotherapy. Chem. Commun. 2010;46:7900. doi: 10.1039/c0cc03528a. [DOI] [PMC free article] [PubMed] [Google Scholar]