Abstract
Since their identification in 2005, T helper (Th)17 cells have been proposed to play important roles in several human diseases, including various autoimmune conditions, allergy, the development and progression of tumors, and the acceptance or rejection of transplanted organs and bone marrow. Focusing on human studies, here we review recent developments regarding Th17 biology and function in each of these fields. Th17 cells actively participate in the pathogenesis of autoimmune disease, allergy and transplantation rejection. Th17 cells contribute to protective anti-tumor immunity in human epithelial malignancy, while Th17-associated cytokines may also be associated with tumor initiation and growth in the context of chronic inflammation and infection. Also discussed is how the in vivo plasticity of Th17 cells may be an important feature of Th17 cell biology in human disease.
Keywords: Th17, cancer, allergy, autoimmune disease, transplantation
Human Th17 cells in the pathological microenvironment
The expression of IL-17 characterizes a subset of CD4+ helper T cells (Th17 cells). In healthy individuals, approximately one percent of CD4+ T cells in peripheral blood are Th17 cells. Only marginal increases in the number of these cells are detected in the peripheral blood of patients with cancer or autoimmune disease. However, together with IL-17+CD8+ (Tc17) cells, Th17 cell numbers can dramatically increase in the pathological microenvironment, where Th17 and Tc17 cells can secrete high amounts of IL-17 [1–7]. Thus Th17 cells might be actively recruited to or/and expanded in the pathological site, and their tissue localization could be important for Th17 cell-associated pathology. Supporting the idea of active recruitment, the chemokine receptor CCR6 and the integrin CD49 molecules implicated in Th17 tissue trafficking are highly expressed on Th17 cells in human blood and tissues [4, 5]. The integrin CD161 is also highly expressed on Th17 cells but its function is poorly understood.
The development of Th17 cells is distinct from the development of Th1, Th2 and regulatory T (Treg) cells, and is characterized by unique transcriptional factors and cytokine requirements [8–12]. However, although Th17 cells form a distinct Th lineage under specific conditions in vitro, it is emerging that Th17 cells exhibit plasticity in some in vivo settings [13, 14] and the cytokine profile of Th17 cells may be altered in tissues. For example, human Th17 cells may express IL-4 [15], IFNγ [4, 5] and Foxp3 [6] in different pathological environments. Although the precise role and underlying mechanisms of Th17 cells in tissues in human disease, relative to other effector T cell subsets, are unclear, it is thought that the plasticity could be important for Th17 cell-associated pathology and -mediated immunity [13, 14]. Furthermore, Th17 cells might mediate effects on tissue homeostasis and local immune responses via mechanisms in addition to production of Th17-associated cytokines. In this article we explore the literature on Th17 cells and human disease, and briefly discuss several clinical trials that target the Th17 pathway to treat patients with autoimmune disease. When we consider the function of Th17 cells, the specific research context, including research model, disease stage, cellular target, and Th17 versus Th17-associated cytokines should be taken into account. In particular, as we discuss below, Th17 cell phenotypic plasticity may be an important factor in determining the role of Th17 cells in vivo in different pathological scenarios.
Th17 cells may promote human cancer-associated immunity
In the tumor microenvironment, suppressive macrophages[16], Treg-inducing plasmacytoid dendritic cells [17, 18], myeloid-derived suppressor cells, inhibitory B7-H1 and B7-H4-expressing antigen presenting cells (APCs) [19], and Treg cells [20], together form suppressive networks that can mediate tumor immune escape and temper the efficacy of vaccination and other immune therapies [21–23]. Th17 cells are also found in several human tumors. Although, studies into the role of Th17 cells in tumor initiation, development, and metastasis are complicated by variables, such as infection or inflammatory status and tumor type, recent evidence suggests that Th17 may be beneficial to cancer patients, especially those with advanced stages of disease [4, 24, 25]. In the context of epithelial cancer, Th17 cells usually constitute only a small fraction of the effector T cell population in the tumor microenvironment [4, 25]. This is despite the high concentration of TGFβ, IL-6 and IL-1 factors that promote mouse Th17 cell development [21–23] suggesting that Th17 cell development may be suppressed in the microenvironment of these tumors. In support of this, Th17 cells are tightly regulated by the local cytokine environment [26] and Treg cells inhibit Th17 cell expansion in the tumor microenvironment[4, 25].
Human solid epithelial cancer-associated Th17 cells express HLA-DR, CD25, and granzyme B, albeit in low amounts, and are thus not “conventional” effector cells. They do not, however, express programmed cell death 1 (PD-1) or FoxP3, and are unlikely to be immunosuppressive. These Th17 cells express granulocyte-macrophage colony stimulating factor (GM-CSF), tumor necrosis factor-alpha (TNFα), interleukin (IL)-2, and interferon-gamma (IFNγ) but not IL-10 [4]. This polyfunctional cytokine profile is also observed in patients with certain viral infections and might contribute to the effects of tumor-associated Th17 cells to immune responses in the tumor microenvironment. Supporting this, within an ovarian tumor microenvironment, Th17 cell-derived IFNγ and IL-17 synergize to increase production of the chemokines CXCL9 and CXCL10. CXCL9 and CXCL10 attract Th1 cells, natural killer (NK) cells, and cytotoxic T lymphocytes (CTLs) to the tumor microenvironment, cells that play an active role in antitumor immunity[4]. This might explain why in patients with advanced ovarian cancer, both intratumoral Th17 numbers and IL-17 concentrations within patient ascites correlate with improved survival [4]. The prevalence of Th17 cells in prostate cancer inversely correlates with the stage of tumor progression, which also supports a beneficial role for Th17 cells in cancer [27]. Malignant pleural fluid from patients with lung adenocarcinoma or squamous cell carcinoma is chemotactic for Th17 cells, which is partially abrogated by CCL20 and/or CCL22 blockade. In lung cancer patients, increased accumulation of Th17 cells in malignant pleural effusion predicted longer survival [28]. Similar results were observed in patients with gastric cancer [29].
Not all studies examining Th17 in cancer patients are as conclusive. For example, in a study of tumor-infiltrating lymphocytes (TIL) in nasopharyngeal carcinoma (NPC), no correlation was found between Th17 cell numbers and patient clinicopathological characteristics or survival [30]. Furthermore, in some situations, Th17 cells may hasten tumor development and serve as a detriment to the host. In patients with hormone-resistant prostate cancer, an inverse correlation is reported between pre-treatment circulating levels of Th17 cells and time to disease progression [31]. Increased Th17 cell numbers in blood and/or tissues might indicate an underlying infection or active inflammatory state, a scenario that can accelerate speed of tumor initiation and development [32]. However, it is arguable that it is the inflammatory status that promotes tumor development rather than direct effects of increased Th17 cell numbers. Supporting this, high Th17 cell numbers and IL-17+ Treg cells are detected in the microenvironment of ulcerative colitis and associated colon cancer. These IL-17-expressing cells induce the production of inflammatory cytokines, including IL-1, IL-8, and TNFα, and promote neutrophil trafficking [6]. It is tempting to reason that Th17 cells in patients with existing chronic inflammation may further promote inflammation, and be associated with early tumor initiation through accelerated DNA damage, enhanced tumor angiogenesis and impaired protective immunity in the local environment. Clinical examples of this phenomenon include patients with colitis-associated colon cancer, and hepatitis-associated hepatocellular carcinoma.
In conclusion, the key feature of human tumor-associated Th17 cells identified thus far is their polyfunctional cytokine profile [4, 12]. This supports the notion of Th17 cell plasticity in vivo, and a fraction of Th17 cells may be shifted to Th1-type cells in the inflammatory environments [13, 33–36]. This could partially explain why Th17 cells are associated with protective tumor immunity in advanced epithelial cancer [4]. However, it remains unknown why Th17 cells are functionally better than Th1 and CD8+ effector T cells in mediating anti-tumor immunity [12], and why a small population of Th17 cells could positively predict patient outcome [4, 27, 29, 37] [28]. Nonetheless, the functional relevance of Th17 cells may not be black and white. The role of Th17 cells might be associated with their quantity and quality (prevalence, phenotype and cytokine profile), the stage of the disease, as well as the specific characteristics of the tumor microenvironment (Figure 1).
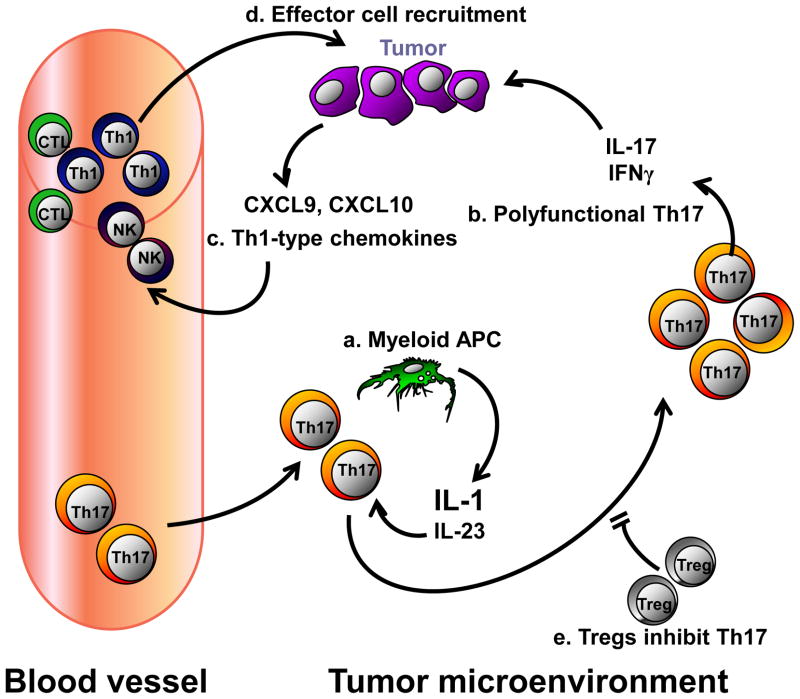
Figure 1. IL-17+ cells and cancer.
(a) Th17 cells from blood and peripheral tissues are recruited into the tumor environment. Tumor-associated myeloid antigen-presenting cells (APCs) secrete IL-1 and IL-23, which results in Th17 cell expansion. (b) Th17 cells express polyfunctional cytokines including IL-17 and IFNγ. (c) Th17 cell-derived IL-17, IFNγ, along with Th1-derived IFNγ, stimulates expression of CXCL9 and CXCL10 in the tumor environment. (d) These chemokines recruit T cells and NK cells into the local environment, where they execute antitumor responses. (e) Treg cells are enriched in the tumor environment, which suppress Th17 cell expansion through an adenosinergic pathway. This model is based on published information.
Th17 cells play a pathogenic role in autoimmune disease
Th17 cells have been linked to multiple human autoimmune conditions, including as psoriasis, multiple sclerosis (MS), rheumatoid arthritis (RA) and inflammatory bowel disease (IBD). Here we provide a disease-specific account of these findings.
Th17 cells and psoriasis
Psoriasis is a chronic inflammatory disease of the skin involving epidermal infiltration of T cells, dendritic cells (DC), and monocytes. The condition is characterized by epidermal hyperplasia and angiogenesis in the dermis. Both Th1-type and Th17-type cytokines are overexpressed in lesional skin and serum of patients. Expression of retinoic acid receptor-related orphan receptor C (RORC) and the Th17-associated cytokines, IL-1β, IL-6 and IL-23 are increased in psoriatic skin. Both Tc17 and Th17 cells are expanded in psoriatic lesions, and myeloid APCs isolated from psoriasis samples induce expansion of these T cells [5, 38]. In addition, IFNγ, which is increased in psoriatic blood and skin, programs myeloid APCs to induce human IL-17+ T cells via IL-1 and IL-23. IFNγ also stimulates APC production of CCL20, a chemokine which supports IL-17+ T cell migration. The synergistic interaction between IL-17 and IFNγ induces the production of IL-1, IL-23, CCL20, and β-defensin 2 by APC and keratinocytes, and results in increased keratinocyte proliferation and accelerated local inflammation. Therefore, IL-17 and IFNγ promote Th17 cell expansion, via IL-1 and IL-23, enhance Th17 cell recruitment via CCL20/CCR6, and further mediate psoriasis pathogenesis through β-defensin 2 [5]. Thus, Th1 and IL-17+ T cells, including dual IL-17+IFNγ+ cells, collaboratively contribute to the pathogenesis of human psoriasis(Figure 2) [5].
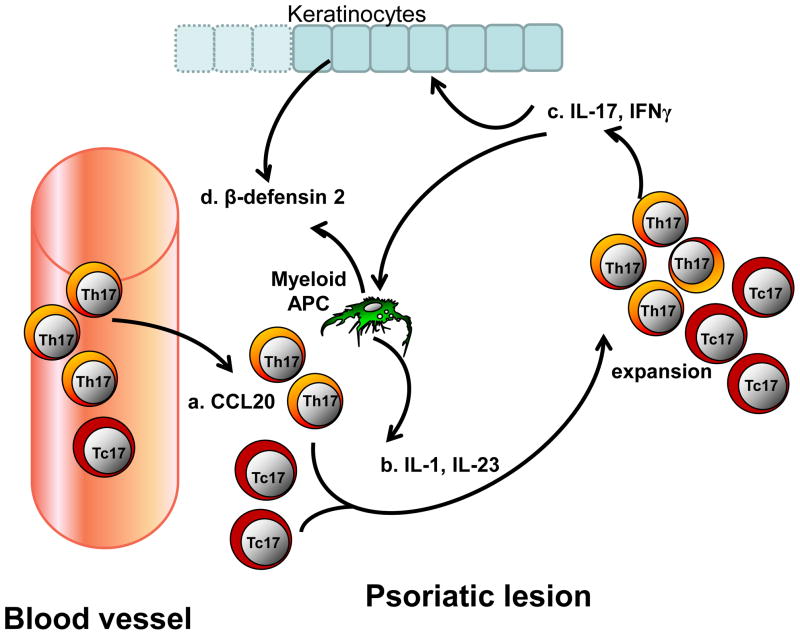
Figure 2. IL-17+ cells and psoriasis.
(a) CCL20 mediates the recruitment of Th17 and Tc17 into a psoriatic lesion. (b) IL-1 and IL-23 derived from psoriatic myeloid APCs and possibly BCDA− dendritic cells (DCs) expand both local Th17 and Tc17 populations. Psoriatic Th17 and Tc17 cells express IL-17 and IFNγ. (c) Psoriatic Th17 and Tc17 cells express polyfunctional cytokines including IL-17 and IFNγ. (d) Th17 and Th1 derived cells induce keratinocytes to secrete β-defensin 2 and CCL20, which further increase the recruitment of Th17 and Tc17 cells into the lesion and promote keratinocyte proliferation. (e) IFNγ derived from Th1 and Th17 cells stimulates myeloid APCs to produce higher amounts of the Th17-polarizing cytokines IL-1 and IL-23, and further enhances Th17 and Tc17 development. The interactions between Th1 and IL-17+ cells positively impact IL-17+ cell development, recruitment and function in the psoriatic environment. The information summarizes established concepts in literature.
Th17 cells and RA
RA patients suffer chronic inflammation in multiple joints, which is associated with bone and cartilage destruction. IL-17 is increased in the sera and synovial fluid of RA patients [39–42]. It is established that the development of a cytokine environment favoring Th17 cell generation is an early event in RA pathogenesis[43]. In patients experiencing active RA, an increase in peripheral Th17 cell numbers and Th1 and Th17-associated cytokines is observed. Although RA was at one time considered a Th1-mediated disease, Th17 cells from RA patients are more efficient than Th1 cells at inducing matrix metalloprotease (MMP) and proinflammatory cytokine production from synovial fibroblasts [39–42, 44]. Although not as well studied as IL-17A, IL-17F might also participate in the pathogenesis of RA; in synergy with TNFα, IL-17A and IL-17F induce a similar cytokine and chemokine expression patterns in synoviocytes [45].
Higher amounts of IL-17 and TNFα in the synovium are linked to more severe joint damage over time[46]. A polymorphism in the IL-4 receptor (IL4R) that affects signaling strength downstream of IL-4 binding is also associated with more rapid joint destruction. This might result from a diminished capacity of IL-4 to downregulate IL-17 secretion by Th17 cells [47]. IL-17 can induce production of proinflammatory mediators from myeloid cells and synovial fibroblasts, therefore perpetuating the existence of an inflammatory environment and positively feeding back into Th17 development and maintenance [44, 48, 49]. Th17 cells can also upregulate receptor activator of nuclear factor κβ (RANK) ligand to effect downstream bone destruction [50, 51]. Not surprisingly, then, the presence of Th17 cells in the joint of arthritis patients correlates positively with other synovial and systemic markers of inflammation [52]. Interestingly, recent studies have revealed that in the synovial fluid of joints of children affected from Juvenile Idiopathic Arthritis (JIA), a shifting from the Th17 to the Th1 phenotype occurs, that appears to be associated with the degree of inflammation[36, 53]. This further supports the plasticity of Th17 cells in vivo and the notion that Th17 and Th1 cells collaboratively impact human autoimmune disease and tumor [12]. Thus, IL-17 and Th17 cells contribute to maintenance of the chronically inflamed environment observed in joints affected by RA [54].
Th17 cells and MS
MS is characterized by damage to the myelin sheaths surrounding the axons of nerves in the brain and spinal cord. A pathological role for Th17 cells in the mouse model of MS, experimental autoimmune encephalomyelitis (EAE) is established [8–11]. There is also data supporting a role for Th17 cells in MS in humans. In 1999 it was reported that IL17 mRNA is increased in the blood and cerebrospinal fluid (CSF) of MS patients [55]. Higher concentrations of IL-17 protein were reported in the CSF of Asian patients with the more severe opticospinal form of disease when compared to patients with conventional MS [56, 57]. However, it is not understood why Th17 cells or IL-17 are increased in MS patients. microRNA326 in peripheral blood mononuclear cells has recently been reported to promote Th17 cell development, and its expression in these cells correlates positively with disease severity in MS patients and mice with EAE [58]. Increased Th17 cell trafficking to the CNS may be also important in MS. In vitro-polarized Th17 cells were found to more easily migrate through a layer of blood brain barrier endothelial cells (BBB-EC) than Th1-polarized cells. Furthermore, treatment of BBB-EC with IL-17 or IL-22 made it easier for human PBMC CD4+ T cells to travel through the monolayer[59]. It is possible that Th17 cells in MS serve to weaken the BBB, facilitating the influx of other immune cells into the CNS. Notably, although Th17 cells play a pathogenic role in MS, it appears that Th17 cells polarized by IL-23, but not TGFβ and IL-6, induce MS-like pathology in mouse EAE. This is due to a high amount of IL-10 expression in TGFβ- and IL-6-polarized Th17 cells, but not by IL-23[60]. This suggests that the phenotype of Th17 cells is crucial for pathogenicity in MS. Notably, in addition to the Th17-differentiation cytokine cocktail, other cells and factors including glycogen synthase kinase-3 [61], peroxisome proliferator-activated receptor [62], CNS-resident NK cells [63], IL-9 [29], gut flora [64], and αvβ8 integrin expression on colonic DCs [65] are reported to impact Th17 development, trafficking, and disease severity in mouse EAE model. Although it is clear that mouse EAE model does not fully recapitulate human MS, we hope that these data may inform future human studies.
Th17 cells and IBD
Recent studies demonstrate that Th17 cells play a role in human IBD, including ulcerative colitis (UC) and Crohn’s disease (CD). Multiple studies of murine intestinal inflammation have established that IL-23 is requisite in both spontaneous and infection-induced disease [66–68]. In support of this, an IL-23R coding variant is associated with reduced risk of IBD in humans [69]. Examination of tissues from IBD patients revealed that IL-17 was expressed in the inflamed colon, and that Th17 cells were clustered in the lamina propria [1, 3, 5, 70–73]. Human lamina propria myeloid APCs efficiently induce Th17 cells through cytokines IL-1β, IL-6 and IL-23 [5, 74, 75] . In addition to Th17 cells, IL-17+IFNγ+ and IL-17+Foxp3+ T cells are found in the colon mucosa of IBD patients [5]. This supports the idea that Th17 cells are phenotypically plastic in vivo in the inflammatory environment. TGFβ and IL-2 induce IL-17+Foxp3+ T cells [5]. In patients with ulcerative colitis and associated colon cancer, IL-17+Foxp3+ T cells are able to suppress T cell activation and stimulate inflammation in colon mucosa. Therefore, IL-17+Foxp3+ T cells are inflammatory Treg cells. These cells have dual biological activities which may favor tumor initiation and development via uncontrolled inflammation and suppressed adaptive T cell immunity (Figure 3) [5].
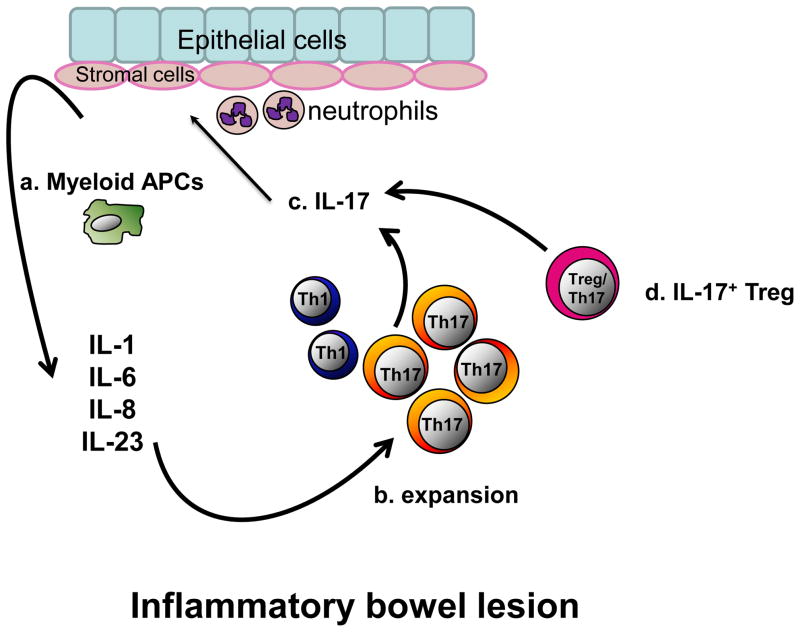
Figure 3. IL-17+ cells and inflammatory bowel disease (IBD).
(a) High levels of inflammatory cytokines are found in IBD environments. These cytokines are produced by a variety of cells including myeloid APCs, stromal cells, epithelial cells and neutrophils. (b) Myeloid APCs along with IL-1, IL-6 and IL-23 lead to Th17 cell expansion. (c) Th17 cells further induce epithelial and stromal cells to express more proinflammatory cytokines, promote neutrophil recruitment and accelerates local inflammation. (d) Treg/Th17 (IL-17+Foxp3+) cells are also found in IBD environments. Treg/Th17 cells suppress adaptive T cell immunity but also secrete inflammatory cytokines. So these cells are termed as inflammatory Treg cells. The information summarizes established concepts in literature.
Altogether, it is clear that Th17 cell-derived IL-17, and the inflammatory cytokines IL-1, IL-6 and IL-23 [74, 75] that induce Th17 cells, synergistically or/and collaboratively act to mediate potent local inflammation, and result in tissue damage in IBD. Moreover, IL-17 may inhibit the proliferation of intestinal epithelial cells, a phenomenon that may contribute to the maintenance of the chronic inflammatory environment in IBD by preventing damaged tissue from healing [76]. Thus, these studies on Th17 cells have helped unravel the pathogenesis of IBD.
Targeting the Th17 cell pathway as a treatment for autoimmune disease
Targeting the Th17 cell pathway has been preliminarily tested as a treatment for patients with autoimmune disease. The strategies include the suppression of Th17 cell generation and the blockade of Th17-associated cytokines.
Given the defined role of IL-23 in Th17 cell development, a monoclonal antibody (mAb) against the IL-12/IL23 p40 subunit has been tested in use to treat patients with psoriasis[77] and CD [78, 79]. Clinical improvement was observed and associated with a decrease in multiple proinflammatory cytokines and chemokines [77, 80]. IFNβ is often used to treat patient with relapsing-remitting MS. Although its therapeutic mechanisms remain poorly understood, Th17 cells may be a target. IFNβ treatment leads to decreased expression of Th17-polarizing cytokines from B cells and increases the production of IL-12 and IL-27, both of which inhibit RORC and several other Th17-associated genes [81, 82]. However, there is also a report that IFNβ, while effective in reducing EAE symptoms induced by Th1 cells, exacerbates Th17 cells-mediated disease [83]. This may explain why IFNβ treatment is not universally effective in MS patients. Furthermore patient-oriented studies will clarify the working mode of IFNβ treatment in different pathological types or stages of MS. In this context, the predominant immune profile (Th1, Th17, monocytes and B cells) could be determined in MS patients and used to guide treatment.
TNFα signaling pathway is activated and involved in multiple autoimmune diseases. In patient with psoriasis, Etanercept (a soluble p75 TNF receptor) treatment ameliorates epidermal hyperplasia. Etanercept reduces the expression of dendritic cell-derived Th17-driving cytokines, which may be associated with improved clinical efficacy in patients [84]. Recently, blockade of IL-17 using a humanized anti-IL-17 mAb has been investigated in patients with psoriasis, rheumatoid arthritis, and uveitis. This treatment improved symptoms of disease, with no strong adverse safety signal [85, 86] supporting neutralization of IL-17 as a potential treatment for autoimmune diseases.
Th17 cells in organ and bone marrow transplantation
Organ transplantation
A role is emerging for Th17 cells in the human transplant setting. IL-17 is upregulated in bronchoalveolar lavage (BAL) during acute rejection of human lung allografts [87]. In human lung transplants the presence of collagen type 5-reactive Th17 cells correlates with the development of bronchiolitis obliterans [88]. Mouse studies also support a role for Th17 cells, Tc17 cells and IL-17 in transplanted tissue rejection [89].
Serum levels of IL-17 (and IL-23) rise dramatically in patients undergoing acute liver rejection [90]. In studies of chronically rejected kidneys, grafts containing higher numbers of IL-17- and IL-21-producing CD4+ T cells were shorter-lived in the host [91]. In B cells, AICDA mRNA, which encodes the B cell enzyme activation-induced cytidine deaminase (AID) and controls germinal center formation [92], correlates positively with both intragraft RORC2 and IL-21 expression [91]. Thus Th17 might contribute to graft rejection via IL-21 production, which could induce the formation of new lymphoid structures to support development of a local humoral immune response. Further experiments are required to confirm this. Dramatic increases in IL-17+ cells and T-bet+ cells are detected in the blood of kidney transplant patients who were experiencing delayed graft function (DGF). The majority of T-bet+ cells were CD4+, while fewer than 5% of the IL-17+ cells were CD4+ [93]. This suggests that the presence of Th1 cells, and not Th17, in the graft may be associated with DGF. In cardiac transplantation patients with stable grafts or acute cellular rejection, TBX21, IFNG, RORC, IL17A, IL23 and FOXP3 transcripts were all elevated in endomyocardial biopsies from patients experiencing acute graft rejection. Th1, Th17 and Treg cells increased in the blood of these patients, suggesting that all mediate acute graft rejection [94] (Figure 4). The balance of Th1, Th17 and Treg cells may be one of the factors determining the fate of transplanted organs.
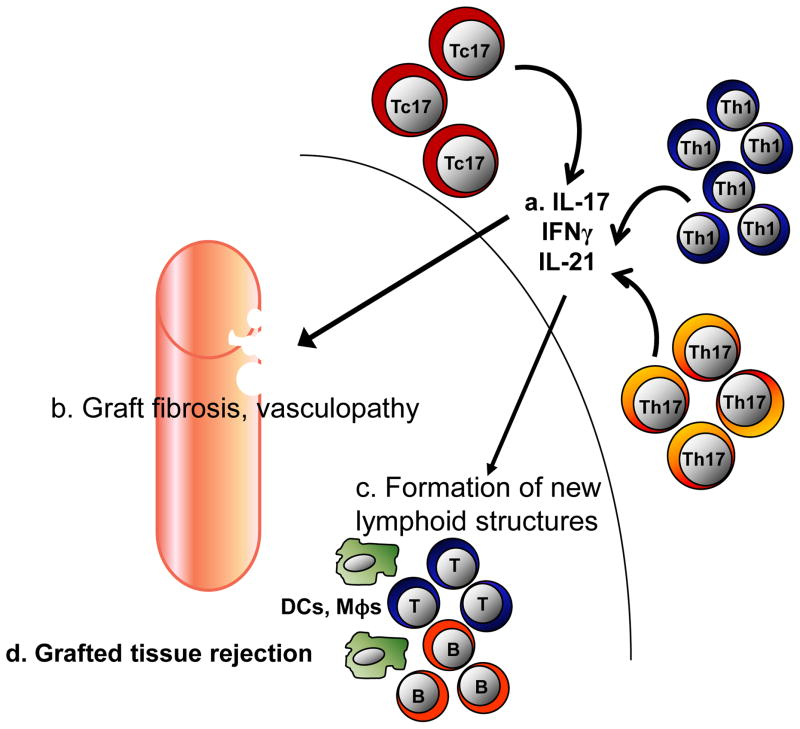
Figure 4. IL-17+ cells and transplant.
(a) Th17, Tc17 and Th1 cells are found in grafted tissues. These cells release IL-21, IL-17 and IFNγ. (b) IL-21 serves to initiate the formation of new lymphoid structures. (c) Tc17 and Th17-derived IL-17 contributes to graft fibrosis, rejection and local vasculopathy. (d) Th17 cells, along with Th1-derived IFNγ also impedes graft acceptance. Multiple immune cells work together and result in grafted tissue rejection. The model presented here is partially hypothetical and partially based on published data.
Bone marrow transplantation
In bone marrow transplantation, the role of Th17 cells appears complex. In acute and chronic active graft-versus-host disease (GVHD) patient Th17 cell numbers are increased in the peripheral blood, whereas in patients with inactive chronic GVHD circulating Th17 cell numbers are reduced. These Th17 cells included both IFNγ− and IFNγ+ subpopulations and the IFNγ+Th17 cells could migrate into GVHD lesions in the skin and liver. Th17 cell numbers were inversely correlated with Treg cell numbers in the blood and other GVHD-affected tissues. More Th17 cells in the blood of GVHD patients correlates with a flare in disease activity [95]. It was recently shown that patients who develop acute GVHD have Th17 cells in their peripheral blood, but in lower numbers than in the days preceding disease onset [96]. Intriguingly, a sequence variation in IL17A in stem cell transplant recipients is associated with a higher risk of acute GVHD development [97]. IL-17 production was recently examined in patients undergoing granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood progenitor cell (PBPC) and G-CSF-primed bone marrow (G-BM) transplantation. Those who received a higher dose of Th17 cells in the G-BM or a higher dose of Tc17 in PBPC displayed a higher incidence of acute GVHD [29]. The Th17 cell population was highest at the onset of acute disease and the percent of Th17 cells decreased drastically in GVHD patients following administration of various treatments to induce remission, while both Th17 and Tc17 numbers were reduced after in vivo G-CSF application. These data suggest that Th17 cells contribute to GVHD. A recent mouse study has suggested that Th17 cells are sufficient but not necessary to induce GVHD [98]. Future studies should examine how and exactly when Th17 cells participate in GVHD exacerbation in settings of bone marrow transplantation.
Th17 cells in allergy
Allergy was classically thought to be a Th2-mediated condition. However, clinical trials of Th2-targeted therapies in asthma have not achieved satisfactory outcomes [72, 99, 100]; as a result, several recent studies have begun to look beyond the Th2 paradigm. Because IL-17A and IL-17F are instrumental in mobilizing and attracting neutrophils [101, 102], key cellular players in the inflammation associated with allergic disease, many laboratories have recently investigated Th17 cells in the context of allergy and asthma.
In asthmatic patients, peripheral Th17 cell numbers, CCR6 expression, ex vivo CD4+ T cell IL-23 production and IL-22 production by stimulated PBMCs were all elevated compared with control subjects [103]. Increased IL-23 production may exacerbate asthma by promoting Th17 development; these Th17 cells may then release IL-22, which can increase inflammation. Interestingly, IL-17A can directly induce IgE production by human B cells. Several groups have noted that allergic patients have higher numbers of IL-17A+ cells than do healthy donors. Removing IL-17A+ cells from PBMCs of allergic donors (with allergic rhinitis, asthma, or atopic dermatitis) reduces IgE levels, while addition of recombinant IL-17A to PBMCs restores it. IL-17A could promote the differentiation of IgE-secreting cells and, in combination with anti-CD40 and IL-4 stimulation, could induce IgE production [104]. Mechanistically, IL-17A promoted IκBα degradation and nuclear translocation of NF-κB, and transcription of epsilon germ-line (εGLT), which is necessary to initiate IgE class switch recombination. εGLT marks the sites for AID to create DNA breaks [105]. Given the central role of IgE in allergy, this is persuasive evidence that Th17 cells can contribute to disease.
IL-17A is also increased in bronchial tissue from mildly asthmatic patients [106]. In primary epithelial cells from asthmatics, glucocorticoid receptor beta (GR-beta; the transcriptionally inactive GR) [107] was upregulated to a greater extent in response to IL-17A and IL-17F treatment than in cells from healthy controls. Patients with asthma can become insensitive to corticosteroid treatment, which is associated with increased GR-beta expression in many cell types. It is possible that IL-17A and/or IL-17F participate in the development of steroid hypo-responsiveness in asthmatics. Perhaps not surprisingly, in PBMCs from adults with allergic asthma, Th2 and Th17 populations and their related cytokines were higher than in healthy controls, even after some patients had been treated with glucocorticoids [108–110]. Th17 cell numbers and plasma concentrations of IL-17 and IL-22 tended to increase with disease severity. RORC mRNA in activated PBMCs was also significantly higher in asthmatic patients. A higher concentration of IL-17 in the sputum of patients positively correlates with airway hyperresponsiveness, and is associated with more severe disease [108–110]. Higher amounts of IL-17A and IL-17F in the lung, and increased airway expression of IL-17F, are also associated with more pronounced disease. A recent study established the existence of IL-4+ Th17 cells, Th17 and Th2 double-feature cells in the blood of asthmatic patients [15]; this population is extremely small in healthy donors. Because Th17 cells express the IL-4R and were reactive to IL-4, it is possible that a cytokine milieu rich in IL-4 may in fact polarize Th17 cells towards a double-feature phenotype. If this is true, this insight into the pathogenesis of asthma may help to guide the development of new, more efficacious therapies. Again, this supports the concept that Th17 cells are phenotypically plastic and can be differentiated into IL-4+ Th17 cells in vivo, and perform dual biological function in patients with asthma. Therefore, it is becoming clear that abnormal Th17 immunity may be significantly involved, alongside Th2 responses, in the pathogenesis of allergy.
Concluding remarks
Data are emerging that associate human Th17 cells with disease. In cancer, Th17 cells seem to be protective, at least in the context of advanced epithelial disease. In contrast, patients with expanded numbers of Th17 cells in blood or tissues, probably resulting from inflammation or infection, may experience accelerated tumor initiation, although this may be related to the inflammatory state rather than direct effects of Th17 cells. In autoimmune disease, Th17 cells are detrimental: they contribute to tissue destruction and to the induction of other proinflammatory mediators that feedback into existing chronic inflammation. In transplant and allergy, Th17 cells are not only mediators of tissue rejection and the inflammation that contributes to disease pathogenesis. They may also induce the development of new lymphoid structures, recruit or affect other cellular mediators of disease, or assimilate functionalities of other disease-perpetuating populations. Current human research clearly demonstrates that Th17 cells are phenotypically and functionally plastic. In this regard, IFNγ+, IL-4+, and Foxp3+ Th17 cells are found in the human microenvironments of chronic asthma, inflammation and cancer. However, the in vivo dynamics and evolution, and the pathological contribution of these different T cell subsets to human diseases remain largely unknown. Further patient-oriented studies are required in all of the disease areas discussed herein. Perhaps the next five years will allow for a more comprehensive understanding of the roles of Th17 cells in tumor, autoimmunity, transplant and allergy, and lead to more efficacious clinical therapies based upon such knowledge.
Acknowledgments
This work was supported in part by research grants from the NIH/NCI R01 grants, and the Ovarian Cancer Research Foundation (WZ) and the NIH through the University of Michigan’s Cancer Center Support Grant (P30CA46592).
Footnotes
Competing interests: The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosmi L, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleinschek MA, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kryczek I, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kryczek I, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kryczek I, et al. IL-17+ Regulatory T Cells in the Microenvironments of Chronic Inflammation and Cancer. J Immunol. 2011;186:4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 7.Maggi L, et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol. 2010;40:2174–2181. doi: 10.1002/eji.200940257. [DOI] [PubMed] [Google Scholar]
- 8.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 9.Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli E, et al. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 12.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosmi L, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125:222–230. e221–224. doi: 10.1016/j.jaci.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Kryczek I, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou W, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 18.Wei S, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 19.Curiel TJ, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 20.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 21.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 22.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 23.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 24.Kryczek I, et al. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kryczek I, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 26.Kryczek I, et al. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol. 2007;179:1423–1426. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 27.Sfanos KS, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye ZJ, et al. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185:6348–6354. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 29.Chen JG, et al. Intratumoral expression of IL-17 and its prognostic role in gastric adenocarcinoma patients. Int J Biol Sci. 2011;7:53–60. doi: 10.7150/ijbs.7.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang YL, et al. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol Cancer. 2010;9:4. doi: 10.1186/1476-4598-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derhovanessian E, et al. Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int J Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]
- 32.Wu S, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annunziato F, Romagnani S. The transient nature of the Th17 phenotype. Eur J Immunol. 2010;40:3312–3316. doi: 10.1002/eji.201041145. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Orozco N, et al. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bending D, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nistala K, et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci U S A. 2010;107:14751–14756. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acharya M, et al. alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120:4445–4452. doi: 10.1172/JCI43796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaba LC, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chabaud M, et al. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 40.Ziolkowska M, et al. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164:2832–2838. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- 41.Hwang SY, Kim HY. Expression of IL-17 homologs and their receptors in the synovial cells of rheumatoid arthritis patients. Mol Cells. 2005;19:180–184. [PubMed] [Google Scholar]
- 42.Leipe J, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–2885. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 43.Cascao R, et al. Identification of a cytokine network sustaining neutrophil and Th17 activation in untreated early rheumatoid arthritis. Arthritis Res Ther. 2010;12:R196. doi: 10.1186/ar3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Hamburg JP, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83. doi: 10.1002/art.30093. [DOI] [PubMed] [Google Scholar]
- 45.Hot A, Miossec P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann Rheum Dis. 2011;70:727–732. doi: 10.1136/ard.2010.143768. [DOI] [PubMed] [Google Scholar]
- 46.Kirkham BW, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54:1122–1131. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 47.Wallis SK, et al. A polymorphism in the interleukin-4 receptor affects the ability of interleukin-4 to regulate Th17 cells: a possible immunoregulatory mechanism for genetic control of the severity of rheumatoid arthritis. Arthritis Res Ther. 2011;13:R15. doi: 10.1186/ar3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lundy SK, et al. Cells of the synovium in rheumatoid arthritis. T lymphocytes. Arthritis Res Ther. 2007;9:202. doi: 10.1186/ar2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tran CN, et al. Molecular interactions between T cells and fibroblast-like synoviocytes: role of membrane tumor necrosis factor-alpha on cytokine-activated T cells. Am J Pathol. 2007;171:1588–1598. doi: 10.2353/ajpath.2007.070004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollinger B, et al. Th17 Cells, Not IL-17+ {gamma}{delta} T Cells, Drive Arthritic Bone Destruction in Mice and Humans. J Immunol. 2011;186:2602–2612. doi: 10.4049/jimmunol.1003370. [DOI] [PubMed] [Google Scholar]
- 52.Zizzo G, et al. Synovial fluid-derived T helper 17 cells correlate with inflammatory activity in arthritis, irrespectively of diagnosis. Clin Immunol. 2011;138:107–116. doi: 10.1016/j.clim.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Cosmi L, et al. Evidence of the transient nature of the Th17 phenotype of CD4+CD161+ T cells in the synovial fluid of patients with juvenile idiopathic arthritis. Arthritis Rheum. 2011;63:2504–2515. doi: 10.1002/art.30332. [DOI] [PubMed] [Google Scholar]
- 54.Cooney LA, et al. Sensitivity and resistance to regulation by IL-4 during Th17 maturation. 2011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matusevicius D, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 56.Ishizu T, et al. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain. 2005;128:988–1002. doi: 10.1093/brain/awh453. [DOI] [PubMed] [Google Scholar]
- 57.Tesmer LA, et al. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Du C, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10:1252–1259. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- 59.Kebir H, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 61.Beurel E, et al. Glycogen synthase kinase-3 is an early determinant in the differentiation of pathogenic Th17 cells. J Immunol. 2011;186:1391–1398. doi: 10.4049/jimmunol.1003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanakasabai S, et al. PPARdelta deficient mice develop elevated Th1/Th17 responses and prolonged experimental autoimmune encephalomyelitis. Brain Res. 2011;1376:101–112. doi: 10.1016/j.brainres.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 63.Hao J, et al. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med. 2010;207:1907–1921. doi: 10.1084/jem.20092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ochoa-Reparaz J, et al. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes. 2010;1:103–108. doi: 10.4161/gmic.1.2.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melton AC, et al. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120:4436–4444. doi: 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hue S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kullberg MC, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duerr RH, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nielsen OH, et al. Upregulation of interleukin-12 and -17 in active inflammatory bowel disease. Scand J Gastroenterol. 2003;38:180–185. doi: 10.1080/00365520310000672. [DOI] [PubMed] [Google Scholar]
- 71.Rovedatti L, et al. Differential regulation of interleukin 17 and interferon gamma production in inflammatory bowel disease. Gut. 2009;58:1629–1636. doi: 10.1136/gut.2009.182170. [DOI] [PubMed] [Google Scholar]
- 72.Bogaert S, et al. Differential mucosal expression of Th17-related genes between the inflamed colon and ileum of patients with inflammatory bowel disease. BMC Immunol. 2010;11:61. doi: 10.1186/1471-2172-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saruta M, et al. Phenotype and effector function of CC chemokine receptor 9-expressing lymphocytes in small intestinal Crohn's disease. J Immunol. 2007;178:3293–3300. doi: 10.4049/jimmunol.178.5.3293. [DOI] [PubMed] [Google Scholar]
- 74.Stevens C, et al. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992;37:818–826. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- 75.Reinecker HC, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwartz S, et al. Interleukin-17 is a potent immuno-modulator and regulator of normal human intestinal epithelial cell growth. Biochem Biophys Res Commun. 2005;337:505–509. doi: 10.1016/j.bbrc.2005.09.075. [DOI] [PubMed] [Google Scholar]
- 77.Krueger GG, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 78.Mannon PJ, et al. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 79.Burakoff R, et al. A phase 1/2A trial of STA 5326, an oral interleukin-12/23 inhibitor, in patients with active moderate to severe Crohn's disease. Inflamm Bowel Dis. 2006;12:558–565. doi: 10.1097/01.ibd.0000225337.14356.31. [DOI] [PubMed] [Google Scholar]
- 80.Toichi E, et al. An anti-IL-12p40 antibody down-regulates type 1 cytokines, chemokines, and IL-12/IL-23 in psoriasis. J Immunol. 2006;177:4917–4926. doi: 10.4049/jimmunol.177.7.4917. [DOI] [PubMed] [Google Scholar]
- 81.Ramgolam VS, et al. B Cells as a Therapeutic Target for IFN-{beta} in Relapsing-Remitting Multiple Sclerosis. J Immunol. 2011;186:4518–4526. doi: 10.4049/jimmunol.1000271. [DOI] [PubMed] [Google Scholar]
- 82.Ramgolam VS, Markovic-Plese S. Interferon-beta inhibits Th17 cell differentiation in patients with multiple sclerosis. Endocr Metab Immune Disord Drug Targets. 2010;10:161–167. doi: 10.2174/187153010791213029. [DOI] [PubMed] [Google Scholar]
- 83.Axtell RC, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zaba LC, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hueber W, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 86.Genovese MC, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 87.Vanaudenaerde BM, et al. The role of interleukin-17 during acute rejection after lung transplantation. Eur Respir J. 2006;27:779–787. doi: 10.1183/09031936.06.00019405. [DOI] [PubMed] [Google Scholar]
- 88.Burlingham WJ, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Faust SM, et al. Role of T cell TGFbeta signaling and IL-17 in allograft acceptance and fibrosis associated with chronic rejection. J Immunol. 2009;183:7297–7306. doi: 10.4049/jimmunol.0902446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fabrega E, et al. Changes in the serum levels of interleukin-17/interleukin-23 during acute rejection in liver transplantation. Liver Transpl. 2009;15:629–633. doi: 10.1002/lt.21724. [DOI] [PubMed] [Google Scholar]
- 91.Deteix C, et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol. 2010;184:5344–5351. doi: 10.4049/jimmunol.0902999. [DOI] [PubMed] [Google Scholar]
- 92.Durandy A. Activation-induced cytidine deaminase: a dual role in class-switch recombination and somatic hypermutation. Eur J Immunol. 2003;33:2069–2073. doi: 10.1002/eji.200324133. [DOI] [PubMed] [Google Scholar]
- 93.Loverre A, et al. T helper 1, 2 and 17 cell subsets in renal transplant patients with delayed graft function. Transpl Int. 2011;24:233–242. doi: 10.1111/j.1432-2277.2010.01157.x. [DOI] [PubMed] [Google Scholar]
- 94.Wang S, et al. Dynamic changes in Th1, Th17, and FoxP3(+) T cells in patients with acute cellular rejection after cardiac transplantation. Clin Transplant. 2010;25:E177–E186. doi: 10.1111/j.1399-0012.2010.01362.x. [DOI] [PubMed] [Google Scholar]
- 95.Dander E, et al. Interleukin-17-producing T-helper cells as new potential player mediating graft-versus-host disease in patients undergoing allogeneic stem-cell transplantation. Transplantation. 2009;88:1261–1272. doi: 10.1097/TP.0b013e3181bc267e. [DOI] [PubMed] [Google Scholar]
- 96.Dlubek D, et al. Interleukin-17-producing cells increase among CD4+ lymphocytes before overt manifestation of acute graft-versus-host disease. Transplant Proc. 2010;42:3277–3279. doi: 10.1016/j.transproceed.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 97.Carvalho A, et al. Prognostic significance of genetic variants in the IL-23/Th17 pathway for the outcome of T cell-depleted allogeneic stem cell transplantation. Bone Marrow Transplant. 2010;45:1645–1652. doi: 10.1038/bmt.2010.28. [DOI] [PubMed] [Google Scholar]
- 98.Iclozan C, et al. T helper17 cells are sufficient but not necessary to induce acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:170–178. doi: 10.1016/j.bbmt.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 100.Cosmi L, et al. Th17 cells: new players in asthma pathogenesis. Allergy. 2011;66:989–998. doi: 10.1111/j.1398-9995.2011.02576.x. [DOI] [PubMed] [Google Scholar]
- 101.Laan M, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 102.Hellings PW, et al. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28:42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 103.Wong CK, et al. Interleukin-17A activation on bronchial epithelium and basophils: a novel inflammatory mechanism. Eur Respir J. 2010;35:883–893. doi: 10.1183/09031936.00088309. [DOI] [PubMed] [Google Scholar]
- 104.Milovanovic M, et al. Interleukin-17A promotes IgE production in human B cells. J Invest Dermatol. 2010;130:2621–2628. doi: 10.1038/jid.2010.175. [DOI] [PubMed] [Google Scholar]
- 105.Geha RS. Allergy and hypersensitivity. Nature versus nurture in allergy and hypersensitivity. Curr Opin Immunol. 2003;15:603–608. doi: 10.1016/j.coi.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 106.Vazquez-Tello A, et al. Induction of glucocorticoid receptor-beta expression in epithelial cells of asthmatic airways by T-helper type 17 cytokines. Clin Exp Allergy. 2010;40:1312–1322. doi: 10.1111/j.1365-2222.2010.03544.x. [DOI] [PubMed] [Google Scholar]
- 107.Bamberger CM, et al. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest. 1995;95:2435–2441. doi: 10.1172/JCI117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao Y, et al. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol. 2010;151:297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- 109.Barczyk A, et al. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97:726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 110.Al-Ramli W, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]