Abstract
Systemic and microvascular hemodynamic responses to hemorrhagic shock resuscitation with hypertonic saline (HTS, 7.5% NaCl) followed with a small volume of plasma expander were studied in the hamster window chamber model to determine the role of plasma expander viscosity in the acute resuscitation outcome. Moderate hemorrhagic shock was induced by arterial controlled bleeding of 50% of blood volume (BV) and the hypovolemic state was maintained for one hour. Volume restitution was performed by infusion of HTS, 3.5% of BV followed by 10% of BV plasma expanders. Resuscitation was followed for 90 min. The experimental groups were named based on the plasma expanders infused after the HTS, namely: [Hextend], Hextend® (6% Hetastarch 670 kDa in lactated electrolyte solution, 4 cp), [Hextend+V], Hextend® with viscosity enhanced by the addition of 0.4% alginate, 8 cp, and [NVR] no volume resuscitation as control group. Measurement of systemic parameters, microvascular hemodynamics and capillary perfusion were performed during hemorrhage, shock and resuscitation. Restitution with Hextend yielded the higher mean arterial pressure (MAP), followed by Hextend+V and NVR. Increasing plasma viscosity did not increase peripheral vascular resistance. Functional capillary density (FCD) was higher for Hextend+V than Hextend and NVR. The level of restoration of acid-base balance correlated with microvascular perfusion and was significantly improved with Hextend+V when compared to Hextend and NVR. These results suggest the importance of restoration of blood rheological properties through enhancing plasma viscosity, influencing the re-establishment of microvascular perfusion during small volume resuscitation from hemorrhagic shock.
Keywords: Microcirculation, hemorrhage, hemodilution, plasma expander, intravascular oxygen, plasma viscosity, hypertonic saline, functional capillary density
INTRODUCTION
Hemorrhage after penetrating trauma is a major combat hazard managed, when possible, according to the Advanced Trauma Life Support guidelines, which rely on aggressive fluid resuscitation until definitive control of the hemorrhage has been achieved.1,2 Initial resuscitation strategies are based on recovering intravascular volume to restore blood pressure and metabolic imbalance under the assumption that early and rapid fluid resuscitation will prolong survival. In this context, fluid bolus administration is assumed to rapidly expand plasma volume, resulting in the restoration of circulation and facilitating the correction of metabolic acidosis associated with hypoperfusion and shock.3,4 This strategy can resolve the hypovolemic syndrome but may lead to fluid overloading disorders that may influence length of recovery, days required for mechanical ventilation and mortality. Current studies suggest that overload morbidity can be significant and are exploring alternative fluid resuscitation methods based on permissive hypotensive resuscitation using relatively small volumes of hypertonic fluids.5–7
Current experimental studies focused on the microvascular component of shock resuscitation define restoration of perfusion to be more effective than the recovery of oxygen carrying capacity in determining tissue metabolic conditions, leading to better short and long term outcome.8 This outcome, however, is critically dependant on the biophysical properties of the resuscitation fluid (plasma expander). To control damage and prevent further injury during resuscitation, the fluid needs to insure recovery of microvascular perfusion, i.e., functional capillary density (FCD), allowing the remaining red blood cells (RBCs) to sustain tissue oxygen delivery. Microvascular strategy does not rely on the restoration of systemic blood pressure as an end point; it emphasizes the restoration of capillary perfusion using low volume resuscitation and limited reoxygenation as a way to prevent and control multiorgan failure from becoming established.8–10
To attain the goal of hypotensive recovery of microvascular functional during resuscitation from hemorrhagic shock, the plasma expander is require to restore and sustain FCD and to produce the required volume expansion to sustain cardiac function and a moderate level of central blood pressure. An appropriate plasma expander would also be required to not cause RBCs aggregation, as well as being effective at low concentration.5,11 How to achieve these properties has been a subject of controversy, particularly regarding the viscosity, colloid osmotic pressure (COP) and the type of colloid which ensure adequate organ perfusion.12–14
The objective of the study was to determine whether a currently available and relatively high viscosity plasma expander could reinstate systemic/microvascular conditions from a severe experimental hemorrhagic shock model when used in conjunction with HTS in a moderate volume resuscitation strategy. To achieve this objective, our experimental hamster model was subjected to a hemorrhage of 50% of blood volume (BV) followed by one hour hypovolemic shock. The resuscitation was implemented in two steps: the initial phase was hypertonic saline (HTS, 7.5% NaCl) 3.5% of BV, and five minutes after HTS, 10% of BV of volume resuscitation was provided. The solutions used for were Hextend® (Hospira; Lake Forest, IL, 6% Hetastarch 670 kDa in Lactated Electrolyte Injection) or Hextend® with enhanced viscosity. The viscosity of Hextend® was increased by the addition of 0.4 g/dl of alginates (FMC Biopolymer, Brakrøya, Norway). Findings were also compared to resuscitation with only HTS. Alginates are produced by brown seaweed (Phaeophyceae, mainly Laminaria) resulting in a viscogenic additive. At a comparatively low concentration (0.7 g/dl), alginate diluted in normal saline has a viscosity of 7.6 cp and low COP. It can be mix or diluted in conventional plasma expander, allowing the design of a plasma expander.
METHODS
Animal Preparation
Investigations were performed in 55 – 65 g male Golden Syrian Hamsters (Charles River Laboratories, Boston, MA) fitted with a dorsal window chamber. Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the local animal care committee. The hamster window chamber model is widely used for microvascular studies in the unanesthetized state, and the complete surgical technique is described in detail elsewhere.15,16 Catheters were tunneled under the skin, exteriorized at the dorsal side of the neck, and securely attached to the window frame.
Inclusion Criteria
Animals were suitable for the experiments if: 1) systemic parameters were within normal range, namely, heart rate (HR) > 340 beat/min, mean arterial blood pressure (MAP) > 80 mmHg, systemic Hct > 45%, and arterial oxygen partial pressure (PaO2) > 50 mmHg; and 2) microscopic examination of the tissue in the chamber observed under a ×650 magnification did not reveal signs of edema or bleeding.
Systemic Parameters
MAP and heart rate (HR) were recorded continuously (MP 150, Biopac System; Santa Barbara, CA). Hct was measured from centrifuged arterial blood samples taken in heparinized capillary tubes. Hb content was determined spectrophotometrically (B-Hemoglobin, Hemocue, Stockholm, Sweden).
Blood Chemistry and Biophysical Properties
Arterial blood was collected in heparinized glass capillaries (0.05 ml) and immediately analyzed for PaO2, PaCO2, base excess (BE) and pH (Blood Chemistry Analyzer 248, Bayer, Norwood, MA). Viscosity was measured at a shear rate of 160/sec (Brookfield Engineering Laboratories, Middleboro, MA). Colloid osmotic pressure was measured using a 4420 Colloid Osmometer (Wescor, Logan, UT).
Acute hemorrhage and volume replacement protocol
Acute hemorrhage was induced by withdrawing 50% of estimated BV via the carotid artery catheter within 5 min. Total BV was estimated as 7% of body weight. One hour after hemorrhage induction, animals received a single bolus infusion of HTS 3.5% of BV, and five minutes after HTS, 10% of BV of volume resuscitation fluids (see experimental groups) were infused within 10 min via the jugular vein catheter. Restoration of 13.5% of the blood volume does not cause reinstating normovolemia, because autotransfusion from the extravascular space is not able to restore the rest of the shed volume during the shock period. Parameters were analyzed before hemorrhage (baseline), after hemorrhage (shock), and up to 90 min after volume replacement (resuscitation).
Experimental groups
Animals were randomly divided into the following three experimental groups before the experiment: 1) Hextend, HTS infusion followed by volume resuscitation performed with Hextend® (degree of substitution 0.75, COP 36 mmHg and viscosity of 4 cp); 2) Hextend+V, HTS infusion followed by volume resuscitation Hextend® with enhanced viscosity (Hextend + 0.4% alginate; COP 36 mmHg and viscosity of 8 cp); 3) NVR, HTS treatment only, no volume resuscitation was provided.
Microvascular Experimental Setup
The unanesthetized animal was placed in a restraining tube with a longitudinal slit from which the window chamber protruded, then fixed to the microscopic stage of a transillumination intravital microscope (BX51WI, Olympus, New Hyde Park, NY). The animals were given 20 min to adjust to the change in the tube environment before measurements. Measurements were carried out using a 40X (LUMPFL-WIR, numerical aperture 0.8, Olympus) water immersion objective.
Functional Capillary Density (FCD)
Functional capillaries, defined as those capillary segments that have RBC transit of at least one RBC in a 60s period in 10 successive microscopic fields were assessed, totaling a region of 0.46 mm2. Each field had between two and five capillary segments with RBC flow. FCD (cm−1), i.e., total length of RBC perfused capillaries divided by the area of the microscopic field of view, was evaluated by measuring and adding the length of capillaries that had RBC transit in the field of view. The relative change in FCD from baseline levels after each intervention is indicative of the extent of capillary perfusion 14,17.
Microhemodynamics
Arteriolar and venular blood flow velocities were measured online by using the photodiode cross-correlation method 18 (Photo-Diode/Velocity, Vista Electronics, San Diego, CA). The measured centerline velocity (V) was corrected according to vessel size to obtain the mean RBC velocity 19. A video image-shearing method was used to measure vessel diameter (D) 20. Blood flow (Q) was calculated from the measured values as Q = π × V (D/2)2. Wall shear stress (WSS) was defined by WSS = WSR × η, where WSR is the wall shear rate given by 8VD−1, and η is the microvascular blood viscosity or plasma viscosity.
Data analysis
Results are presented as mean ± standard deviation. Data within each group were analyzed using analysis of variance for repeated measurements (ANOVA, Kruskal-Wallis test). When appropriate, post hoc analyses were performed with the Dunns multiple comparison test. Microhemodynamic data are presented as absolute values and ratios relative to baseline values. A ratio of 1.0 signifies no change from baseline while lower and higher ratios are indicative of changes proportionally lower and higher than baseline (i.e., 1.5 would mean a 50% increase from the baseline level). The same vessels and capillary fields were followed so that direct comparisons to their baseline levels could be performed, allowing for more robust statistics for small sample populations. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, Inc., San Diego, CA). Changes were considered statistically significant if P<0.05.
RESULTS
Eighteen animals were entered into the study; all tolerated the entire protocol without visible signs of discomfort. Animals were randomly assigned to the following experimental groups: Hextend (n=6); Hextend+V (n=6); and NVR (n=6). Systemic data for baseline and shock were obtained by combining all experimental groups, data for baseline and shock was not statistically different among groups.
Systemic parameters
Systemic hemodynamic and blood parameters are presented in detail on Table 1. Hemorrhage and shock reduced Hct and Hb from baseline (P<0.05). Resuscitation by means of HTS followed with Hextend and Hextend+V decreased Hct and Hb further. Resuscitation with only HTS (NVR) also lowered Hct and Hb, although to a lesser extent than Hextend and Hextend+V. Prolonged volume expansion of the colloid was observed between 60 and 90 min after of resuscitation. Volume expansion of HTS only (NVR) was lower and without long term effects, no changes in Hct or Hb were measured between 60 and 90 min after resuscitation.
Table 1.
Laboratory Parameter
|
Resuscitation 60 min |
Resuscitation 90 min |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Shock | Hex | Hex+V | NVR | Hex | Hex+V | NVR | ||
| Hct, % | 48 ± 1 | 29 ± 1 | 22 ± 1† | 23 ± 1† | 25 ± 1 | 20 ± 1 | 21 ± 1 | 24 ± 1 | |
| Hb, g/dl | 14.7 ± 0.6 | 9.6 ± 0.4 | 7.1 +0.4† | 7.4 ± 0.3† | 8.0 ± 0.4 | 6.4 ± 0.5† | 6.6 ± 0.6† | 7.8 ± 0.5 | |
| MAP, mmHg | 108 ± 7 | 41 ± 4 | 74 ± 5† | 70 ± 6† | 52 ± 5 | 79 ± 6† | 70 ± 8† | 54 ± 4 | |
| Heart Rate, bpm | 438 ± 28 | 449 ± 37 | 421 ± 29 | 411 ± 26 | 397 ± 40 | 402 ± 34 | 417 ± 31 | 377 ± 42 | |
| PaO2, mmHg | 55.6 ± 4.1 | 89.9 ± 8.2 | 73.4 ± 6.1 | 75.3 ± 5.7 | 77.5 ± 7.8 | 70.5 ± 5.6† | 69.4 ± 5.2† | 79.2 ± 5.7 | |
| PaCO2, mmHg | 53.9 ± 4.8 | 38.6 ± 5.7 | 42.5 ± 5.2 | 43.3 ± 5.4 | 38.4 ± 5.0 | 41.7 ± 6.0 | 43.7 ± 5.4 | 37.7 ± 5.2 | |
| Arterial pH | 7.335 ± 0.014 | 7.289 ± 0.027 | 7.314 ± 0.020 | 7.321 ± 0.022 | 7.319 ± 0.022 | 7.324 ± 0.021 | 7.332 ± 0.018 | 7.322 ± 0.023 | |
| BE, mmol/l | 3.2 ± 1.6 | −6.1 ± 2.0 | −1.3 ± 1.6 | −0.4 ± 1.4 | −2.4 ± 1.4 | −1.8 ± 2.0 | 0.6 ± 1.7 | −2.6 ± 1.9 | |
| Viscosity, cP | |||||||||
| Blood | 4.2 ± 0.4 | 3.0 ± 0.3 | 3.7 ± 0.4† | 2.9 ± 0.3 | |||||
| Plasma | 1.2 ± 0.1 | 1.4 ± 0.1† | 2.0 ± 0.3† | 1.1 ± 0.2 | |||||
| COP, mmHg | 17.8 ± 1.6 | 15.4 ± 1.1 | 15.6 ± 0.9 | 15.3 ± 0.8 | |||||
HEX, volume resuscitation with Hextend; HEX+V, volume resuscitation with Hextend with added alginate; NVR, no volume resuscitation. Baseline and shock included all the animals. Hct, systemic hematocrit; Hb, hemoglobin content of blood; MAP, mean arterial blood pressure; PaO2, arterial partial O2 pressure; PaCO2, arterial partial pressure of CO2; BE, base excess. Shear rate of 160 s-1 at 37°C; COP, colloid osmotic pressure at 27°C. Values are means ± SD.
P<0.05 compared NVR;
P<0.05 compared HES.
Hemorrhage and shock decreased MAP (P<0.05). Changes in MAP during the hemorrhagic shock resuscitation protocol for all experimental groups are presented in Figure 1. Resuscitation with HST followed by Hextend partially restored MAP from the shock (P<0.05); however, it was still lower than baseline (P<0.05). A similar result was observed with HST followed by Hextend+V, where MAP was restored from shock state without reaching baseline MAP. MAP after resuscitation did not differ between Hextend and Hextend+V at any time point. Resuscitation with only HTS (NVR) recovered MAP from the shock (P<0.05) without reaching baseline MAP levels and was statistically lower than Hextend and Hextend+V (P<0.05). Heart rate after resuscitation with HTS only (NVR) was statistically lower than baseline (P<0.05), but no different from the other groups.
Figure 1.
Mean arterial pressure during the hemorrhage, shock and resuscitation protocol. †, P<0.05 compared NVR.
Gas laboratory parameters and calculated acid base balance are presented in Table 1. Hemorrhagic shock decreased arterial pH and pCO2, significantly compromising acid based balance. Resuscitation with HTS followed by Hextend partially recovered blood gas parameters, although acid base balance remained negative. Resuscitation with HTS and Hextend+V significantly recovered blood gas parameters, restoring positive acid base balance 90 min after resuscitation. HTS only (NVR) infusion did not recover shock induced negative acid base balance. All resuscitation strategies mitigated arterial acidosis produced during shock conditions.
Resuscitation with HTS followed with Hextend or Hextend+V increased plasma viscosity compared to baseline (P<0.05). Resuscitation with only HTS (NVR) did not affect plasma viscosity. Blood viscosity and plasma COP were reduced after resuscitation for all groups. Table 1 shows the blood rheological properties and COP for normal blood and after resuscitation. Baseline rheological properties and COP were obtained from animals that did not undergo the shock protocol.
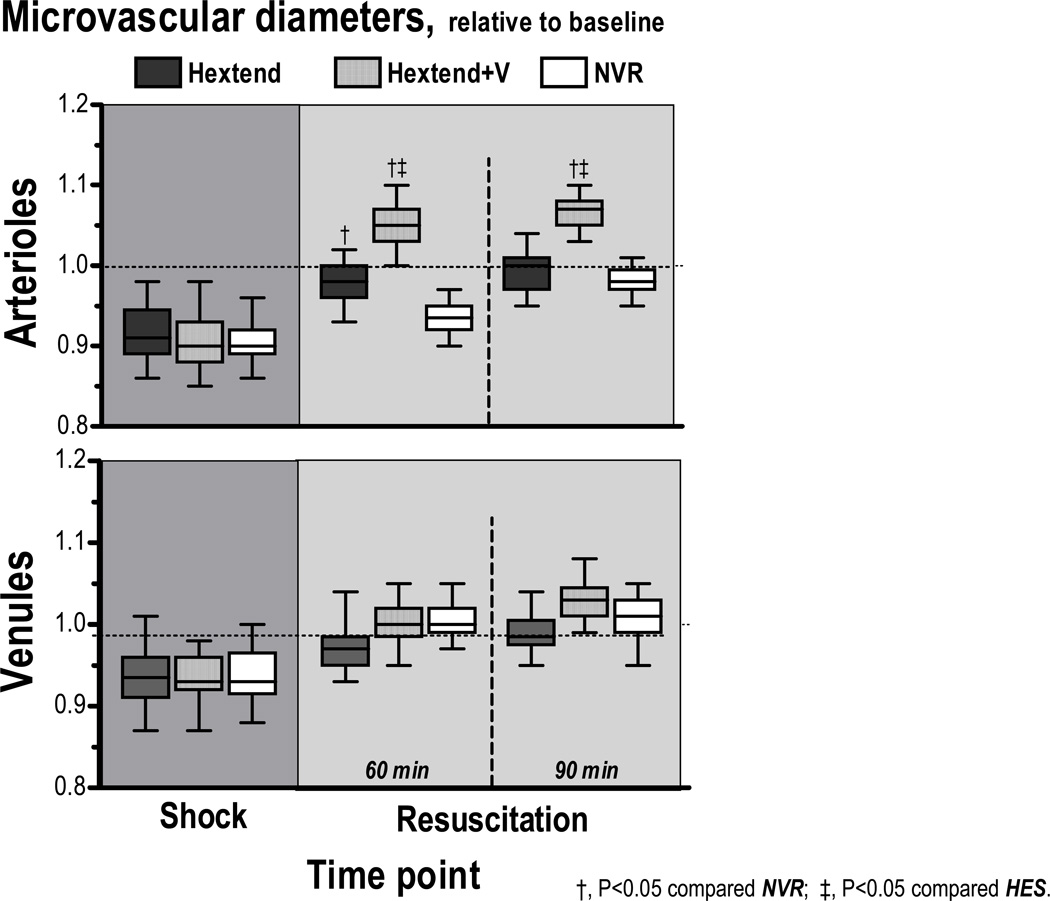
Microvascular diameter and blood flow are presented in Figures 2 & 3. Arteriolar and venular diameters during shock were significantly constricted from baseline (P<0.05). Resuscitation with HTS followed by Hextend reversed vasoconstriction. Infusion of Hextend+V after HTS overcame vasoconstriction and even had arteriolar dilation when compared to baseline (P<0.05). Resuscitation with only HTS (NVR) reversed only venular constriction, but not arteriolar constriction.
Figure 2.
Relative changes to baseline in arteriolar and venular diameter. Broken line represents baseline level. †, P<0.05 compared NVR; ‡, P<0.05 compared Hextend. Baseline diameters (µm, mean ± SD) for each animal group were as follows: Hextend (arterioles (A): 63.0 ± 9.2, n = 26; venules (V): 64.4 ± 8.2, n = 34); Hextend+V (A: 62.4 ± 8.8, n = 30; V: 61.9 ± 9.0, n = 32); NVR (A: 61.9 ± 8.9, n = 29, V: 61.3 ± 9.2, n = 34). n = number of vessels studied.
Figure 3.
Relative changes to baseline in arteriolar and venular blood flow. Broken line represents baseline level. †, P<0.05 compared NVR; ‡, P<0.05 compared Hextend. Calculated baseline blood flows (nl/s, mean ± SD) for each animal group were as follows: Hextend (arterioles (A): 12.1 ± 11.0, n = 26; venules (V): 7.2 ± 8.3, n = 34); Hextend+V (A: 12.3 ± 10.8, n = 30; V: 7.2 ± 9.1, n = 32); NVR (A: 11.9 ± 8.2, n = 29, V: 7.2 ± 8.7, n = 34). n = number of vessels studied.
Arteriolar and venular flows during shock were statistically significantly lower than baseline (P<0.05). Resuscitation increased microvascular flows in all groups. Infusion of Hextend+V presented statistically higher arteriolar and venular blood flows than Hextend or NVR (P<0.05). Infusion of small colloid volume (Hextend or Hextend+V) after HTS produced higher and more stable microvascular blood flows than HTS alone (NVR). Counterintuitively, at lower MAP and higher plasma and blood viscosity, resuscitation with HTS and Hextend+V caused higher microvascular perfusion.
Calculated hemodynamic parameters are presented in Table 2. Peripheral vascular resistance was statically lower for Hextend+V when compared to Hextend and NVR (P<0.05). Furthermore, peripheral vascular hindrance, which reflects the contribution of vascular geometry, decreased maximally for Hextend+V when compared to Hextend or NVR (P<0.05). Parallel with the decrease in vascular resistance, animals resuscitated with HTS and Hextend+V presented higher levels of microvascular wall shear rate and wall shear stress.
Table 2.
Calculated Hemodynamic Parameters, 90 min after resuscitation
| Arterioles |
Venules |
|||||
|---|---|---|---|---|---|---|
| PVR | PVH | WSR | WSS | WSR | WSS | |
|
values reported here are relative to baseline for all calcualted parameters (mean ±std) |
||||||
| Hexted | 1.73 ± 0.25§ | 2.20 ± 0.32§ | 0.47 ± 0.12§† | 0.34 ± 0.09§ | 0.39 ± 0.12§† | 0.34 ± 0.09§ |
| Hexted +V | 1.19 ± 0.28†‡ | 1.37 ± 0.31†‡ | 0.45 ± 0.16§† | 0.42 ± 0.12†§ | 0.49 ± 0.14§† | 0.44 ± 0.12§† |
| NVR | 1.84 ± 0.26§ | 2.65 ± 0.34§ | 0.29 ± 0.14§ | 0.22 ± 0.08§ | 0.27 ± 0.12§ | 0.19 ± 0.08§ |
HEX, volume resuscitation with Hextend; HEX+V, volume resuscitation with Hextend with added alginate; NVR, no volume resuscitation. Values are means ± SD. PVR, peripheral vascular resistance; PVH, peripheral vascular hindrance; WSR, wall shear rate; WSS, wall shear stress.
P<0.05 compared Baseline;
P<0.05 compared NVR;
P<0.05 compared HES.
Changes in capillary perfusion during the protocol are presented in Figure 4. FCD was significantly reduced after hemorrhage and shock (P<0.05). Resuscitation partially restored FCD in all groups. Differences in FCD between groups were observed at 60 min: Hextend+V presented statistically higher FCD compared to Hextend or NVR (P<0.05). At 90 min, differences among groups were maintained. Infusion of only HTS (NVR) produced a minimal recovery in FCD, statistically lower than Hextend or Hextend+V.
Figure 4.
Effects of resuscitation on capillary perfusion during hemodilution. Functional capillary density (FCD) was drastically reduced after hemorrhage. FCD was lower after resuscitation with HES compared to volume restitution with RBCs. FCD (cm−1) at baseline was as follows: Hextend (104 ± 12); Hextend+V (110 ± 8); NVR (A: 106 ± 9).
DISCUSSION
The principal finding of the study is that small volume resuscitation from hemorrhagic shock by infusion of HTS followed by low volume infusion of high viscosity colloid provides superior restoration of systemic and microhemodynamic parameters when compared to hypertonic infusion followed with a conventional colloid or the HTS alone. The importance of increasing plasma viscosity during anemic and hypovolemic conditions is shown by the sustained recovery of microhemodynamic conditions post resuscitation using Hextend with enhanced viscosity (Hextend+V) when compared with Hextend alone (Hextend). Colloidal osmotic properties were not affected by alginate as shown by the lack of differences in Hct and Hb between the two colloidal formulations used. Therefore, since there were no differences in oxygen transport capacity, the differences found between groups were due to the change in blood rheological properties.
Optimal resuscitation was attained when blood viscosity was restored to 3.7 cp, a value not significantly different from normal blood viscosity (4.2 cp). However, this effective viscosity during resuscitation has a significantly greater component of plasma viscosity, a condition previously associated with increased capillary pressure, a critical determinant of FCD.14 Therefore, our findings suggest that both the elevation of blood and plasma viscosity are beneficial in resuscitation.
The recovery observed after infusion of HTS is due to rapid intercompartment fluid shifts and restoration of blood volume. Our results suggest that small volume resuscitation accentuates and stabilizes the fluids shifted during hypovolemic shock state and ensures an adequate hemodynamic restoration. Furthermore, this restoration can be sustained and improved if blood rheological properties, especially plasma viscosity, are manipulated to reinstate endothelium mechanical signals at the hypovolemic and anemic states.21
The role of plasma viscosity and consequently blood viscosity in maintaining systemic blood pressure and blood gases are highlighted by the results obtained here, since increasing plasma viscosity by the means of alginate provided consistent restoration of homeostasis compared to volume resuscitation with Hextend alone. Although Hextend’s high molecular weight results in a higher viscosity than other hydroxyethyl starch solutions, the addition of a viscogenic agent improved recovery of microhemodynamic conditions. This result suggests that Hextend, as with other similar plasma expanders, could be formulated with higher viscosities in order to produce a maximal benefit during volume restitution when used during the appropriate conditions (moderate and extreme anemia). Acid/base balance, post volume restitution with Hextend alone, clearly showed an initial recovery when compared to HTS; however, increasing the viscosity of Hextend sustained recovery for a longer period. The beneficial effects of maintaining blood viscosity after resuscitation from hemorrhage were similar to the results found using high viscosity plasma expanders during extreme hemodilution (11% Hct) and resuscitation during continuous bleeding.14,22
The use of alginate as an additive to Hextend increased plasma viscosity by 50% and blood viscosity by 23% relative to volume resuscitation with Hextend alone; however, peripheral vascular resistance and blood pressure were not affected. This effect is due to arteriolar vasodilatation which is probably due to the restoration of shear stress. Shear stress exerted by the cell free plasma layer moving near the endothelial surface influences vessel diameter and modulates the release of dilatory autocoids (prostacyclin, nitric oxide, etc).23 Shear stress induced by the flowing column of blood occurs within the plasma layer and is mostly affected by changes in plasma viscosity, supporting the concept that a small amount of viscogenic agent can exert shear dependent mechanisms without increasing the blood flow resistance. A previous study by Tsai et al. showed that increased shear stress due to moderate augmentation of plasma viscosity was associated with increased concentration of perivascular microvascular nitric oxide and vasodilation.21 Current studies show that the resuscitation limit for hemorrhagic shock is reached when blood viscosity is severely decreased by blood dilution, causing the impairment of microvascular function, local blood flow mal-distribution and insufficient oxygen delivery.24–26
Deliberate hypotensive resuscitation by a limited volume infusion is suitable for conditions where bleeding has stopped but definitive control of bleeding has not been attained, a condition typical of pre-hospital settings. When resuscitation is performed without repairing the cause of bleeding, the risk of re-bleeding increases as MAP increases, especially when large volumes of resuscitation solutions are infused, due to disruption of the coagulation cascade, dilution of coagulating factors and hypervolemia per se.27
In the present study, hemorrhage was set to 50% of the blood volume, and at this point autoregulatory mechanisms are still capable of maintaining life for up to 4 hours if microvascular perfusion and FCD are maintained.28 The infusion of HTS minimally restored volume and briefly restored microvascular and systemic conditions. A more sustained recovery of FCD and microvascular flow was attained when HTS was followed with colloids, a result mostly related to volume stabilization of intercompartment sifted fluids by HTS and the increase in plasma viscosity. Notably, the increase of plasma viscosity has been erroneously related to the increase of blood flow resistance, due to increased loss of viscosity (resistance). This rationalization, however, is only applicable to a rigid circulation since peripheral resistance is determined by blood viscosity, and more importantly, by the varying diameter of the resistance vessels (arterioles and precapillary arterioles).
In conclusion, this study shows that restoration of vascular homeostasis during hemorrhagic shock requires the compensation of microvascular malfunction. Minimal restoration of volume using a fluid with adequate rheological properties appears to restore blood/blood vessel endothelium interaction and redistributing pressures along the circulatory tree. Hextend is a comparably high viscosity plasma expander, relative to the conventional viscosity value for these fluids; however, the full benefit of plasma expansion appears to still be limited by low viscosity. It also indicates that the upper limit to which plasma viscosity should be increased during hypovolemic and anemic conditions is not yet clearly defined. Furthermore, the appropriate value could be a moving target, since optimal systemic and microvascular resuscitation should ultimately be determined by the combination of volume, oxygen carrying capacity and viscosity, while limiting blood pressure recovery. Therefore, the subject’s condition may be the ultimate determinant of the combination of parameters which provides optimal recovery.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Program project P01 HL071064 and grants R01-HL62354, R01-HL62318 and R01-HL76182. The authors thank Froilan P. Barra and Cynthia Walser for the surgical preparation of the animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement,
All authors do not have any financial or personal relationships with other people or organizations that could inappropriately influence (bias) in their work.
REFERENCES
- 1.Bellamy RF. The causes of death in conventional land warfare: implications for combat casualty care research. Mil Med. 1984;149:55–62. [PubMed] [Google Scholar]
- 2.Advanced Trauma Life Support Course for Physicians Committee on Trauma. Chicago: American College of Surgeons; 1997. pp. 87–107. [Google Scholar]
- 3.Kentner R, Safar P, Prueckner S, et al. Titrated hypertonic/hyperoncotic solution for hypotensive fluid resuscitation during uncontrolled hemorrhagic shock in rats. Resuscitation. 2005;65:87–95. doi: 10.1016/j.resuscitation.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Krausz MM, Hirsh M. Bolus versus continuous fluid resuscitation and splenectomy for treatment of uncontrolled hemorrhagic shock after massive splenic injury. J Trauma. 2003;55:62–68. doi: 10.1097/01.TA.0000074110.77122.46. [DOI] [PubMed] [Google Scholar]
- 5.Dubick MA, Atkins JL. Small-volume fluid resuscitation for the far-forward combat environment: current concepts. J Trauma. 2003;54:S43–S45. doi: 10.1097/01.TA.0000064514.42470.3B. [DOI] [PubMed] [Google Scholar]
- 6.Stern SA. Low-volume fluid resuscitation for presumed hemorrhagic shock: helpful or harmful? Curr Opin Crit Care. 2001;7:422–430. doi: 10.1097/00075198-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds PS, Barbee RW, Skaflen MD, Ward KR. Low-Volume Resuscitation Cocktail Extends Survival after Severe Hemorrhagic Shock. Shock. 2007;28:45–52. doi: 10.1097/shk.0b013e31802eb779. [DOI] [PubMed] [Google Scholar]
- 8.Cabrales P, Tsai AG, Intaglietta M. Is resuscitation from hemorrhagic shock limited by blood oxygen-carrying capacity or blood viscosity? Shock. 2007;27:380–389. doi: 10.1097/01.shk.0000239782.71516.ba. [DOI] [PubMed] [Google Scholar]
- 9.Cabrales P, Tsai AG, Intaglietta M. Hemorrhagic shock resuscitation with carbon monoxide saturated blood. Resuscitation. 2007;72:306–318. doi: 10.1016/j.resuscitation.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Cabrales P, Intaglietta M, Tsai AG. Transfusion restores blood viscosity and reinstates microvascular conditions from hemorrhagic shock independent of oxygen carrying capacity. Resuscitation. 2007 doi: 10.1016/j.resuscitation.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welch HG, Meehan KR, Goodnough LT. Prudent strategies for elective red blood cell transfusion. Ann Intern Med. 1992;116:393–402. doi: 10.7326/0003-4819-116-5-393. [DOI] [PubMed] [Google Scholar]
- 12.Moore FA, McKinley BA, Moore EE. The next generation in shock resuscitation. Lancet. 2004;363:1988–1996. doi: 10.1016/S0140-6736(04)16415-5. [DOI] [PubMed] [Google Scholar]
- 13.Driessen G, Scheidt H, Inhoffen W, et al. A comparative study: perfusion of the micro- and macrocirculation as a function of the hematocrit value. Microvasc Res. 1988;35:73–85. doi: 10.1016/0026-2862(88)90051-9. [DOI] [PubMed] [Google Scholar]
- 14.Cabrales P, Tsai AG, Intaglietta M. Microvascular pressure and functional capillary density in extreme hemodilution with low- and high-viscosity dextran and a low-viscosity Hb-based O2 carrier. Am J Physiol Heart Circ Physiol. 2004;287:H363–H373. doi: 10.1152/ajpheart.01039.2003. [DOI] [PubMed] [Google Scholar]
- 15.Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol. 1984;246:H508–H517. doi: 10.1152/ajpheart.1984.246.4.H508. [DOI] [PubMed] [Google Scholar]
- 16.Endrich B, Asaishi K, Götz A, Messmer K. Technical report: A new chamber technique for microvascular studies in unanaesthetized hamsters. Res Exp Med. 1980;177:125–134. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- 17.Tsai AG, Cabrales P, Winslow RM, Intaglietta M. Microvascular oxygen distribution in awake hamster window chamber model during hyperoxia. Am J Physiol Heart Circ Physiol. 2003;285:H1537–H1545. doi: 10.1152/ajpheart.00176.2003. [DOI] [PubMed] [Google Scholar]
- 18.Intaglietta M, Silverman NR, Tompkins WR. Capillary flow velocity measurements in vivo and in situ by television methods. Microvasc Res. 1975;10:165–179. doi: 10.1016/0026-2862(75)90004-7. [DOI] [PubMed] [Google Scholar]
- 19.Chien S, Lipowsky HH. Correlation of hemodynamics in macrocirculation and microcirculation. Int J Microcirc Clin Exp. 1982;1:351–365. [PubMed] [Google Scholar]
- 20.Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res. 1973;5:309–312. doi: 10.1016/0026-2862(73)90042-3. [DOI] [PubMed] [Google Scholar]
- 21.Tsai AG, Acero C, Nance PR, et al. Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am J Physiol Heart Circ Physiol. 2005;288:H1730–H1739. doi: 10.1152/ajpheart.00998.2004. [DOI] [PubMed] [Google Scholar]
- 22.Cabrales P, Intaglietta M, Tsai AG. Increase plasma viscosity sustains microcirculation after resuscitation from hemorrhagic shock and continuous bleeding. Shock. 2005;23:549–555. [PubMed] [Google Scholar]
- 23.Ballermann BJ, Dardik A, Eng E, Liu A. Shear stress and the endothelium. Kidney Int Suppl. 1998;67:S100–S108. doi: 10.1046/j.1523-1755.1998.06720.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsai AG, Friesenecker B, McCarthy M, et al. Plasma viscosity regulates capillary perfusion during extreme hemodilution in hamster skin fold model. Am J Physiol. 1998;275:H2170–H2180. doi: 10.1152/ajpheart.1998.275.6.H2170. [DOI] [PubMed] [Google Scholar]
- 25.Cabrales P, Tsai AG. Plasma viscosity regulates systemic and microvascular perfusion during acute extreme anemic conditions. Am J Physiol Heart Circ Physiol. 2006;291:H2445–H2452. doi: 10.1152/ajpheart.00394.2006. [DOI] [PubMed] [Google Scholar]
- 26.Cabrales P, Nacharaju P, Manjula BN, et al. Early difference in tissue pH and microvascular hemodynamics in hemorrhagic shock resuscitation using polyethylene glycol-albumin- and hydroxyethyl starch-based plasma expanders. Shock. 2005;24:66–73. doi: 10.1097/01.shk.0000167111.80753.ef. [DOI] [PubMed] [Google Scholar]
- 27.Cabrales P, Tsai AG, Intaglietta M. Resuscitation from hemorrhagic shock with hydroxyethyl starch and coagulation changes. Shock. 2007;28:461–467. doi: 10.1097/shk.0b013e31804880a1. [DOI] [PubMed] [Google Scholar]
- 28.Kerger H, Saltzman DJ, Menger MD, et al. Systemic and subcutaneous microvascular pO2 dissociation during 4-h hemorrhagic shock in conscious hamsters. Am J Physiol. 1996;270:H827–H836. doi: 10.1152/ajpheart.1996.270.3.H827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.