Abstract
Human T cell leukemia virus type 1 (HTLV-1) is associated with two immunologically distinct diseases: HTLV-1–associated myelopathy/tropical spastic paraparesis and adult T cell leukemia. We observed previously that depletion of dendritic cells (DCs) in CD11c-diphtheria toxin receptor transgenic mice followed by infection with cell-free virus led to greater proviral and Tax mRNA loads and diminished cellular immune response compared with mice infected with cell-associated virus. To understand the significance of these in vivo results and explore the host–pathogen interaction between DCs and cell-free HTLV-1, we used FLT3 ligand-cultured mouse bone marrow-derived DCs (FL-DCs) and chimeric HTLV-1. Phenotypically, the FL-DCs upregulated expression of surface markers (CD80, CD86, and MHC class II) on infection; however, the level of MHC class I remained unchanged. We performed kinetic studies to understand viral entry, proviral integration, and expression of the viral protein Tax. Multiplex cytokine profiling revealed production of an array of proinflammatory cytokines and type 1 IFN (IFN-α) by FL-DCs treated with virus. Virus-matured FL-DCs stimulated proliferation of autologous CD3+ T cells as shown by intracellular nuclear Ki67 staining and produced IFN-γ when cultured with infected FL-DCs. Gene expression studies using type 1 IFN-specific and DC-specific arrays revealed upregulation of IFN-stimulated genes, most cytokines, and transcription factors, but a distinct downregulation of many chemokines. Overall, these results highlight the critical early responses generated by FL-DCs on challenge with cell-free chimeric HTLV-1.
Human T cell leukemia virus type 1 (HTLV-1) has infected 10–20 million people worldwide (1). It is endemic in Japan, the Caribbean, parts of South America, and Central Africa. A majority of infected individuals remain asymptomatic carriers; only a small percentage (<5%) experience development of the disease (1–3). Why the disease develops in some infected individuals whereas others remain healthy carriers remains unknown. Causative studies have linked HTLV-1 with two predominant, immunologically distinct diseases: oncogenic adult T cell leukemia (ATL) (4) and the neuroinflammatory HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP) (5, 6), as well as with many different clinical syndromes (7). It is believed that age and route of primary infection play a role in determining the clinical outcome after infection with HTLV-1. Studies have suggested that the risk for development of HAM/TSP is greater if HTLV-1 infection is acquired during adulthood, especially through sexual transmission (8), whereas individuals infected early in life through breast-feeding are believed to be at greater risk for ATL because the infected immature thymocytes have more chances to develop into malignant cells (9). The i.v. route of viral transmission has a predisposition to lead to the neurologic disease associated with the hyperimmune response, as was shown with a cohort of HAM patients, most of whom received blood transfusions (10). On the contrary, rats orally inoculated with HTLV-1 developed persistent infection, immune unresponsiveness, and T cell lymphomas (11, 12), suggesting that mucosal exposure may lead to ATL.
Viruses, like other pathogens, exhibit a variety of pathogen-associated molecular patterns such as RNA replication intermediates with genomic modifications, high repetition of capsomers on the surfaces of virions, and others that are recognized by pattern recognition receptors on various APCs such as dendritic cells (DCs). The ability of DCs to respond to the viral threat through an array of defense mechanisms makes their role critical in thwarting viral attacks. DCs are not only potent activators of CD8+ and CD4+ T cells, but they release a plethora of cytokines that serve an important role in determining the phenotype of Th cells and the eventual immunologic response. In addition, DCs are the key producers of the type 1 IFNs that are one of the early key antiviral cytokines released that can create an overall antiviral state through the activation of IFN-stimulated genes (ISGs).
DCs behave and respond in a contrastingly different manner in the two immunologically distinct and diverse HTLV-1–associated diseases, ATL and HAM/TSP. In the immunosuppressive ATL, the DCs fail to mature (13–15); however, during hyperinflammatory HAM/TSP, the DCs mature rapidly (16). Thus, the DCs play an important role in two disparate disease states. HTLV-1 infection of DCs has been demonstrated in patients with HAM/TSP (17), as well as in vitro (16, 18, 19). In addition, autologously infected DCs, as well as those pulsed with inactivated HTLV-1 virions, can lead to a strong proliferative response of both CD4+ and CD8+ T cells (20). The route of viral exposure (mucosal versus peripheral blood) is believed to have an important bearing on the eventual outcome of the disease and warrants attention, considering the fact that different subsets of DCs are known to reside in these exclusively separate compartments. The mucosal route may be linked to the development of ATL (12), whereas peripheral blood exposure has been associated with HAM/TSP (10). Although the mucosa is enriched with unique DC subsets that sample pathogens in the gut and local area (21–23), the peripheral blood is enriched predominantly with the viral-sensing, type I IFN-producing plasmacytoid DCs (pDCs) (24). Another level of complexity is the mode of viral delivery: cell free versus cell associated. Viruses may enter the cell through an array of different mechanisms, such as receptor-dependent and -independent endocytosis, pinocytosis, phagocytosis, as well as lipid raft-mediated endocytosis (25). Therefore, depending on the route of entry, different pattern recognition receptors maybe triggered, thereby eliciting different responses. In this respect, it was recently shown that cell-free virus can induce the production of IFN-α from pDCs in a TLR7-dependent manner (26). Thus, we believe that the two distinct compartmental subsets of DCs may respond to the virus differently and may play a role in the generation of immunologically separate diseases.
We showed previously that DCs exhibit higher binding affinity to cell-free HTLV-1 than do other susceptible cell types including CD4+ T cells (27), the primary target cell population in patients. In addition, several studies have demonstrated that cell-free HTLV-1 productively infects DCs, which can, in turn, transfer the virus to CD4+ T cells (18, 19, 28), suggesting that DCs play a critical role in primary infection and subsequent transmission. A unique feature of HTLV-1–associated neuroinflammatory disease is the intense proliferation of CD8+ T cells, the majority of which are targeted against the viral transcription transactivating protein, Tax (29). Our previous studies with the murine DC cell line JAWS II and with human monocyte-derived DCs demonstrated that, after treatment of DCs with Tax, the DCs undergo activation and maturation-exhibiting changes in mRNA levels of activation markers, surface phenotype, and the secretion of cytokines and chemokines leading to allogenic and Ag (Tax)-specific immune responses (30–33). We later showed that DCs can successfully prime a functional Tax-specific cytotoxic T cell response in naive human PBLs in vitro and in the line HHD II HLA-A*0201 transgenic mice in vivo (19). Finally, to understand the role of DCs in determining the degree of infection engendered by cell-free and cell-associated virus, we showed that infection with cell-free virus in CD11c-diphtheria toxin receptor transgenic mice depleted of DCs led to significantly increased proviral and Tax mRNA loads, as well as a diminished cellular immune response (34).
Having demonstrated the absolute necessity of DCs in controlling cell-free virus that may represent an early stage of viral infection (34), we wanted to further understand how the DCs interacted with cell-free HTLV-1 and responded to the viral threat. Unfortunately, the low frequency of DCs in mice, coupled with the tedious isolation strategies, rendered functional studies difficult. To obtain large numbers of DCs for our studies, we cultured mouse bone marrow cells in the presence of FLT3 ligand (FLT3L) to generate bone marrow-derived DCs (35). When the FLT3 ligand-cultured bone marrow-derived DCs (FL-DCs) were analyzed for the surface expression of various molecules after maturation with virus, they were found to upregulate CD80, CD86, and MHC class II. The kinetics of viral entry, proviral integration, and viral replication through the active production of the viral transcriptional trans-activating protein Tax demonstrated that the virus was able to enter the FL-DCs as early as 2 h postinfection, and that the provirus, as well as Tax, could be detected around 12 h postinfection. Multiplex cytokine profiling demonstrated the production of a large number of proinflammatory cytokines, such as IL-6, IL-12, IL-23, IFN-γ, and TNF-α. In addition, robust amounts of type 1 IFN (IFN-α) were produced by the FL-DCs postinfection. The maturation of such activated DCs was also functionally demonstrated in an MLR, wherein the DCs, after culturing with virus, were found to stimulate the proliferation of autologous CD3+ T cells, which were able to produce IFN-γ in response to the infected DCs. Gene expression studies demonstrated the upregulation of genes related to type 1 IFNs and their receptors, ISGs, most cytokines, and transcription factors, as well as the intriguing downregulation of many chemo-kines, notably CCR5, the receptor for CCL5 (RANTES). These results clearly delineate and identify the critical role that DCs play during early HTLV-1 infection through the release of proinflammatory cytokines, the potent antiviral IFN-α, surface expression of costimulatory molecules, as well as the upregulation of IFN-specific genes, thereby allowing them to control viral spread and infection. These observations may help us to explain the increased infection results seen in mice depleted of DCs (34).
Materials and Methods
Generation and isolation of bone marrow-derived DCs
FL-DCs were generated as described previously (36). In brief, 30 × 106 bone marrow cells were obtained from 6- to 10-wk-old C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) and cultured in 100-mm dishes containing 15 ml complete RPMI media 1640 (Mediatech, Manassas, VA) supplemented with 10% FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, 100 U penicillin-streptomycin, 0.05 mM 2-ME, and 20% of FLT3L-containing supernatant, produced from an SP2/0 transfected cell line that secretes murine recombinant FLT3L (37). On day 9, the cells were collected by gentle trypsinization; CD11c+ murine DCs (FL-DCs) were isolated using the EasySep mouse CD11c+ selection kit (StemCell Technologies, Vancouver, BC, Canada), and their purity was confirmed by flow cytometry on the FACSCalibur (BD Biosciences, San Jose, CA) and the data analyzed using FlowJo software (Tree Star, Ashland, OR).
CD3+ T cell separation from splenocytes
The autologous spleens (purchased from The Jackson Laboratory, Bar Harbor, ME) were harvested in RPMI media 1640 (Mediatech), macerated, and passed through a 0.45-µm sterile nylon filter (BD Biosciences) to obtain a single-cell suspension. The splenocytes were then subjected to CD3+ separation using the EasySep mouse T cell enrichment kit (StemCell Technologies). The isolated autologous T cells were used with FL-DCs for the MLR.
Production of cell-free chimeric HTLV-1
The chimeric HTLV-1 plasmid (pCS-HTLV-chimΔR) encoding the HTLV-1 with Moloney murine leukemia virus envelope and the Tax-expressing plasmid (pCMV-envΔPvuII) were obtained from Dr. Frederic Tangy (Pasteur Institute, Paris, France) (38, 39). The human embryonic kidney 293 T cells were cotransfected with 3 µg pCS-HTLV-chimΔR and 0.5 µg pCMV-envΔPvuII per million cells by the calcium phosphate (Invitrogen, Carlsbad, CA) method, as described previously (34). Five hours posttransfection, the medium was washed off and replaced with fresh medium. The successful production of active virus was confirmed 36 h posttransfection both in the supernatant and cell lysate of transfected cells by the HTLV-1 Ag (p19) ELISA kit (ZeptoMetrix, Buffalo, NY). For isolating cell-free virus (34), the collected supernatant was first passed through a 0.45-µm filter (Millipore, Billerica, MA) and then overlaid on a 20% (w/v) sucrose cushion in polyallomer tubes (Beckman Coulter, Fullerton, CA). The virus particles were concentrated from the filtered supernatant by ultracentrifugation for 2 h at 25,000 rpm on the Optima CE-80K (Beckman Coulter). The virus pellet was resuspended in 1/100th volume of PBS and quantified before use by ELISA (ZeptoMetrix).
A portion of the virus stock was irradiated with UV light to inactivate the chimeric HTLV-1, and thus incapacitate its ability to replicate (40). About 5 ml of the concentrated viral stock was placed in a 15-ml tube, kept on ice to eliminate thermal effects, and gently agitated to ensure uniform irradiation. The UV irradiation was performed in a Stratalinker UV crosslinker (Stratagene, La Jolla, CA) at 200 Jm−2. The incident UV dose rate was determined using a Spectroline DRC-100× dosimeter (Spectronics, West-bury, NY).
Phenotyping of FL-DCs in response to chimeric HTLV-1
The cells were treated with media (control), 10 µg/ml LPS (Sigma-Aldrich, St. Louis, MO), and cell-free virus to evaluate for the maturation of FL-DCs and expression of costimulatory molecules. After 24 h, the cells were collected and stained for the mouse surface markers CD80, CD86, and MHC class I and II (eBioscience, San Diego, CA).
Infection of FL-DCs with cell-free chimeric HTLV-1
Two million FL-DCs were mixed with 100 ng cell-free virus in a six-well plate. For viral entry, proviral load, and replication (Tax production) kinetic studies, the infected cells were isolated at 0, 2, 4, 8, 12, and 24 h postinfection. The collected cells were gently trypsinized to remove any unbound virus. For the viral entry and Tax production studies, cells were stained intracellularly with anti-HTLV-1 Gag p19 and anti-Tax Ab. The unconjugated anti-p19 (ZeptoMetrix) and anti-Tax (generously provided by Dr. Yuetsu Tanaka, Kitasato University, Kanagawa, Japan) Abs were fluorochrome-conjugated using an allophycocyanin labeling kit (Dojindo Molecular Technologies, Rockville, MD). Stained cells were analyzed by flow cytometry on the FACSCalibur (BD Biosciences), and the data were analyzed using FlowJo software (Tree Star).
Quantitative HTLV-1 proviral detection by real-time PCR
To determine the proviral load at various time points, we isolated DNA from the infected cells using the Wizard SV Genomic Purification kit (Promega, Madison, WI) and subjected them to real-time PCR analysis to detect the HTLV-1 Gag gene. Real-time PCR was performed on an ABI Prism 7300 Sequence Detector (Applied Biosystems, Foster City, CA). The standard real-time PCR reaction using SYBR Green I consisted of 15 µl SYBR Green PCR Master Mix (Applied Biosystems), 10 µM each of forward and reverse primers (Integrated DNA Technologies, Coralville, IA), and 10 µl DNA in a total volume of 30 µl. The thermal cycling conditions comprised an initial activation step at 95˚C for 10 min followed by 40 cycles including denaturation at 95˚C for 15 s and annealing and extension at 60˚C for 1 min. The following primers were used for HTLV-1 Gag (F: 5′-CTTACCACGCCTTCGTAGA-3′ and R: 5′-ACA AGCCCGCAACATATCTC-3′) and mouse GAPDH (F: 5′-CCTTAAACAGGCCCACTTGA-3′ and R: 5′-CCTTCCACAATGCCAAAGTT-3′) yielding products of 180 and 201 bp, respectively. By using the 2−ΔΔCT method (41), we quantified the levels of HTLV-1 Gag gene relative to the control group and normalized them to the endogenous housekeeping GAPDH. Experiments were performed in duplicate for each data point. Dissociation curve analysis was performed to ensure the presence of a single peak at the correct melting temperature.
Cytokine profiling and kinetics
For multiplex cytokine profiling, the FL-DCs were cultured with media (control), 10 µg/ml LPS (Sigma-Aldrich), and increasing concentrations of cell-free virus (25, 50, and 100 ng) for various times (0, 2, 4, 8, 12, and 24 h) to determine the dose-dependent response and kinetics of cytokine production. The supernatant was collected and multiplex cytokine profiling was performed using the Multi-Analyte ELISArray kit (SABiosciences, Frederick, MD), which allowed us to screen and quantitate 12 independent cytokines. For analysis of IFN-α, the FL-DCs were cultured with media (control), 3 µg/ml TLR7 agonist imiquimod (InvivoGen, San Diego, CA), and 100 ng UV-irradiated and competent cell-free virus for various time points (0, 2, 4, 8, 12, 24, and 48 h) to determine the kinetics of cytokine production using the VeriKine Mouse IFN-α ELISA kit (PBL InterferonSource, Piscataway, NJ).
To study the cytokine profile during an ongoing infection strategy, the cells were treated with media (control), LPS (Sigma), imiquimod (InvivoGen), and 100 ng UV-irradiated and competent virus for 6 h. The cells were then washed, and multiplex cytokine profiling (SABiosciences) was performed on the supernatant on day 3. The FL-DCs were cultured with media (control), imiquimod (InvivoGen), and 100 ng UV-irradiated virus and competent virus for 6 h to analyze the IFN-α response. Thereafter, the cells were gently trypsinized to remove any unbound virus or TLR agonist and placed in fresh medium. The supernatant was collected on days 3 and 5, and analyzed for IFN-α (PBL InterferonSource), as well as HTLV-1 p19 (ZeptoMetrix), as explained earlier. All ELISAs were performed as per the manufacturer’s instructions and minimum with the duplicate set of samples.
MLR
To determine the stimulating and activating capacity of DCs to proliferate T cells, we performed an MLR. The FL-DCs were treated for 24 h with one of the following: media (control), LPS, UV-irradiated virus, or competent cell-free virus. Thereafter, the cells were gently trypsinized to wash off any unbound virus and mixed with autologous CD3+ T cells (isolated as explained earlier) in increasing ratios (1:1, 1:20, 1:40, 1:80, and 1:160). The mixed cells were left undisturbed for 5 d. The cells were surface stained for CD3-FITC (eBioscience) followed by treatment with intracellular fixation buffer (eBioscience) and washed with permeabilization buffer (eBioscience) before staining intracellularly for nuclear proliferation marker Ki67 (BD Biosciences) and IFN-γ (eBisocience). The stained cells were analyzed by flow cytometry on the FACSCalibur (BD Biosciences), and the data were analyzed using FlowJo software (Tree Star).
Gene expression profiling
To determine the effect of viral infection on the expression of type 1 IFN-specific (at 4 h) and DC-specific genes (at 12 h) of the FL-DCs, we performed quantitative real-time PCR using the mouse RT2-IFN-α,β response, as well as the dendritic and APC PCR array kits (SABiosciences), respectively. RNA was collected from the treated cells at 4 and 12 h postinfection using the RNeasy Mini Kit (Qiagen, Valencia, CA) and then reverse transcribed into cDNA using the RT2-First Strand kit (SABiosciences). PCR arrays were performed as per the manufacturer’s instructions. The quantitative real-time PCR data are accessible at the National Center for Biotechnology Information in the Gene Expression Omnibus database with the accession number GSE24962 (http://www.ncbi.nlm.nih.gov/geo/).
Results
Evaluating FL-DCs activation in response to chimeric HTLV-1
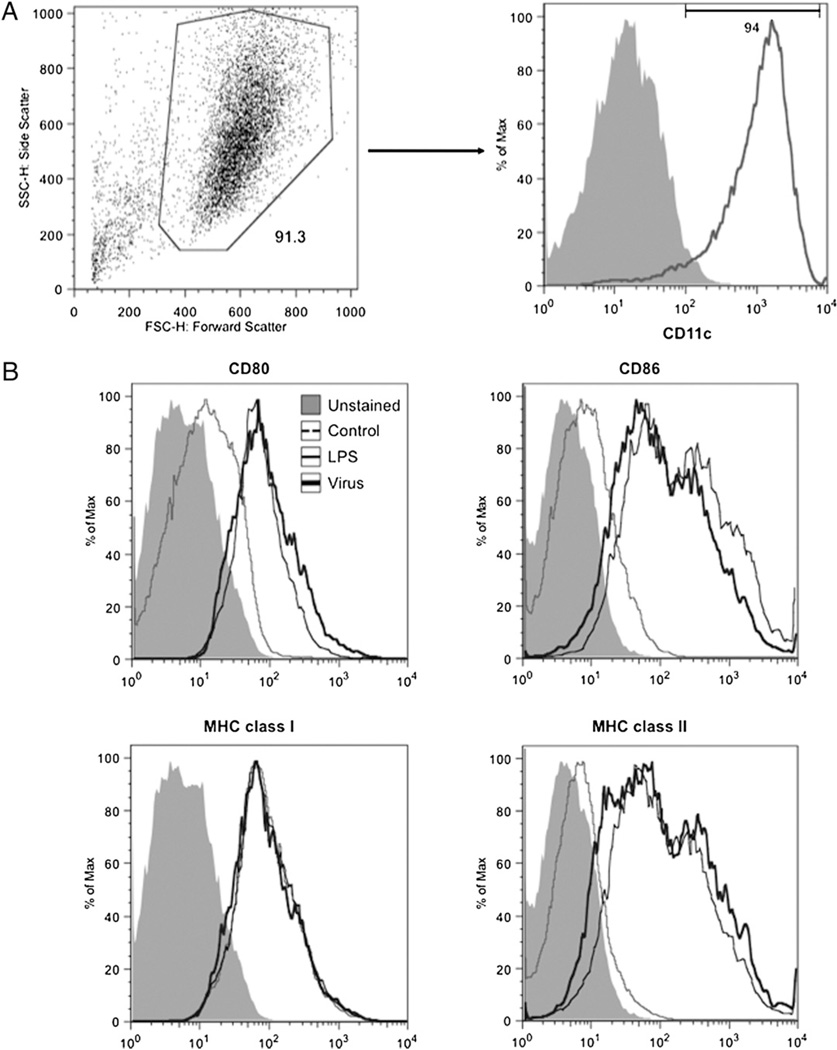
To generate FL-DCs, we cultured extracted mouse bone marrow in complete RPMI media 1640 supplemented with mouse FLT3L. After 9 d, the culture was processed for the positive isolation of CD11c+ murine DCs and was found to be a distinctly pure population (>90%) of CD11c+ cells (Fig. 1A). To demonstrate the ability of FL-DCs to mature and express costimulatory and other surface markers after activation and maturation, we mixed the cells with media (control), LPS (positive control), and cell-free virus. After 24 h, the cells were stained for the various markers—CD80, CD86, and MHC class I and II—and analyzed by flow cytometry. As expected, the FL-DCs responded to the positive control LPS and virus (Fig. 1B). The chimeric virus greatly upregulated the expression of CD80 and the costimulatory molecules CD86 and MHC class II. However, the levels of MHC class I remained unchanged (Fig. 1B) by both LPS and the virus.
FIGURE 1.
Demonstration of purity and phenotypic characterization of CD11c+ murine DCs. A, The bone marrow cells were cultured in FLT3L media for 9 d, and thereafter isolated and analyzed for purity by flow cytometry. B, Phenotypic characterization of the ability of FL-DCs to upregulate surface markers (CD80, CD86, and MHC classes I and II) in response to LPS (positive control) or virus. Shaded histogram represents unstained sample; faint gray line represents unstimulated FL-DCs; dark gray line represents LPS; solid black line represents challenge with virus.
Examining the kinetics of viral entry, integration, and expression of the viral protein Tax
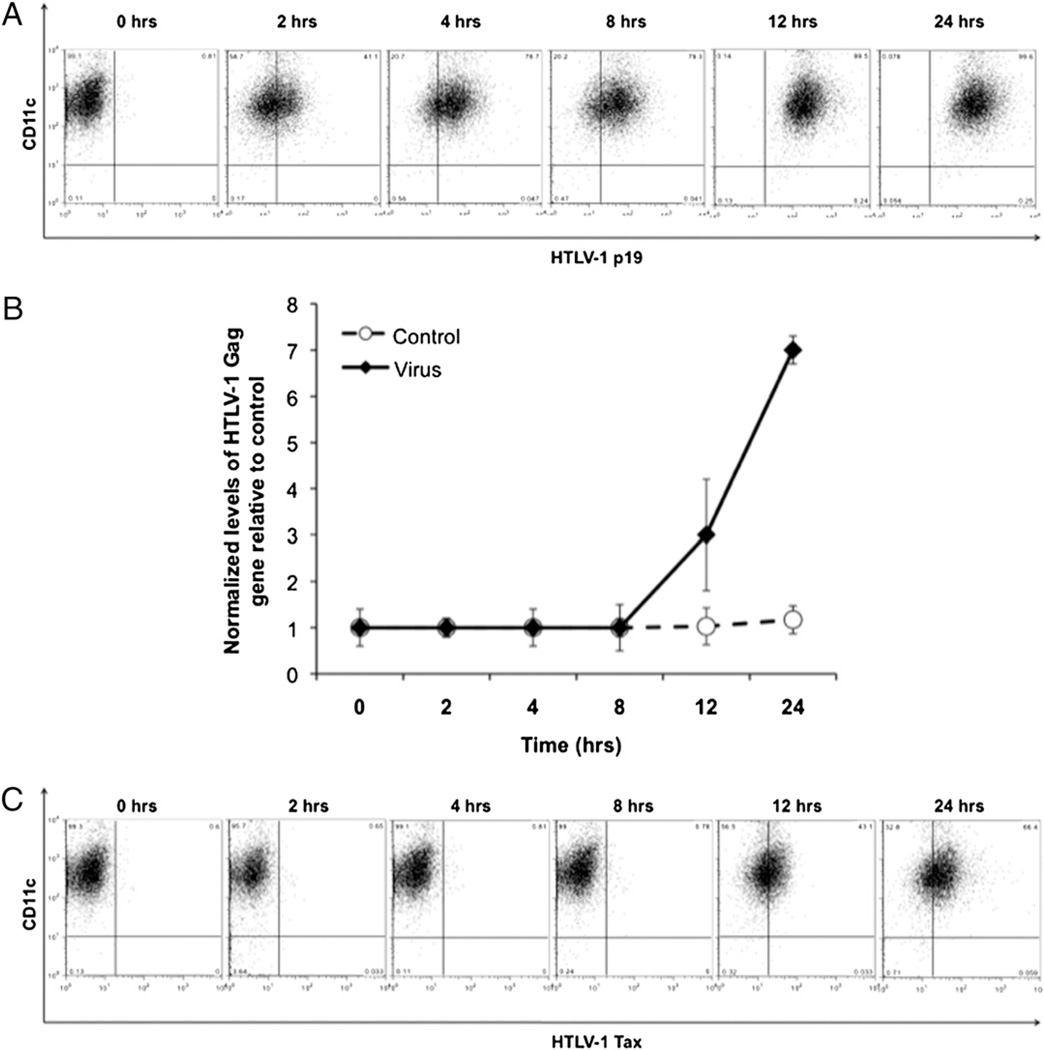
To examine the close interaction between the chimeric virus and the FL-DCs, we cultured the two together and examined them at regular intervals (0, 2, 4, 8, 12, and 24 h) to understand the kinetics of viral uptake by the FL-DCs. The cells were also examined concurrently for proviral integration and the subsequent expression of Tax protein, indicative of freely replicating virus. As observed by anti-p19 intracellular staining, the FL-DCs started taking in the cell-free virus as early as 2 h, and by 24 h, almost all cells showed staining for viral protein (Fig. 2A). When we examined for the proviral load by real-time PCR, we found that the proviral DNA could be detected by 12 h (Fig. 2B). Finally, in concurrence with the presence of the integrated provirus, we detected the expression of Tax around the same time (Fig. 2C).
FIGURE 2.
Kinetics of viral entry, integration, and replication in CD11c+ FL-DCs. A, The FL-DCs were mixed with virus and analyzed at various intervals (0, 2, 4, 8, 12, and 24 h) for viral entry by intracellular staining for HTLV-1 p19 Gag protein. B, The DNA of the FL-DCs was isolated and analyzed for HTLV-1 Gag to determine proviral integration. C, The cells were analyzed for viral replication by intracellular staining for HTLV-1 Tax protein to determine the ability of the integrated virus to undergo replication.
Strong proinflammatory cytokine and type 1 IFN responses against chimeric HTLV-1
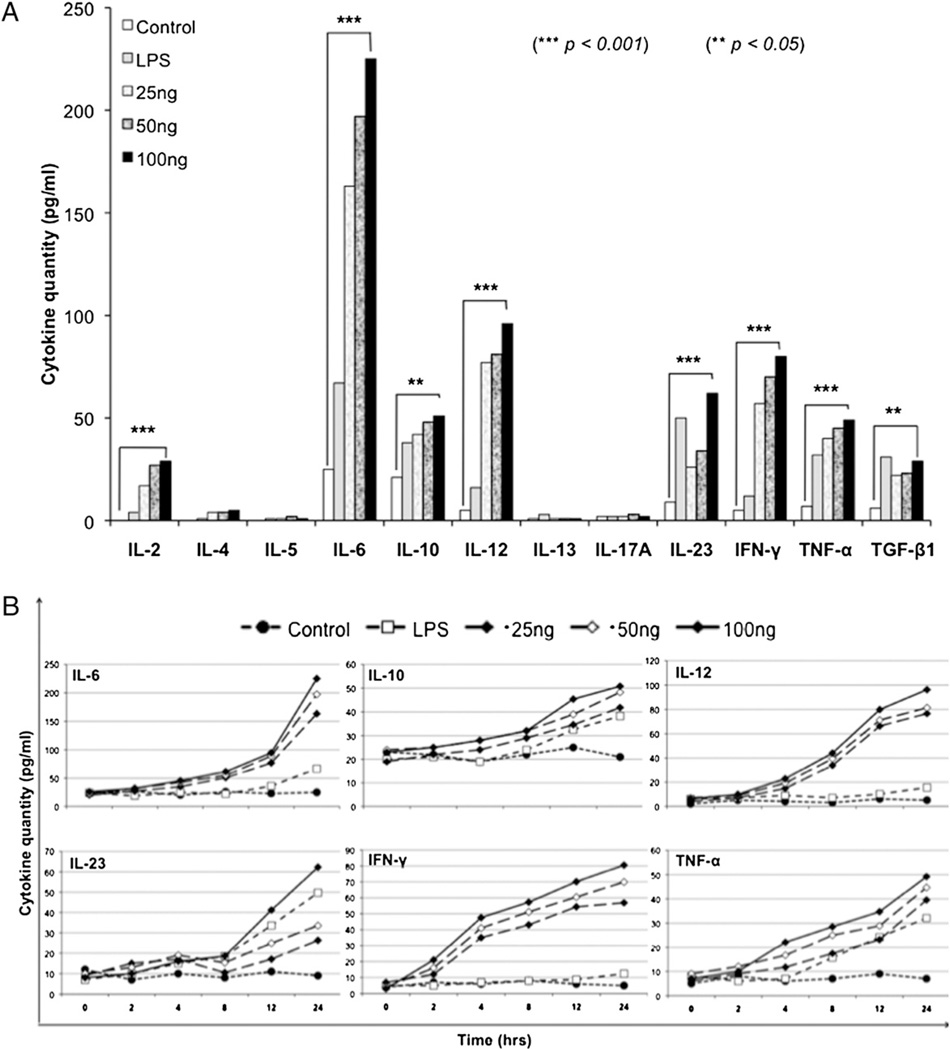
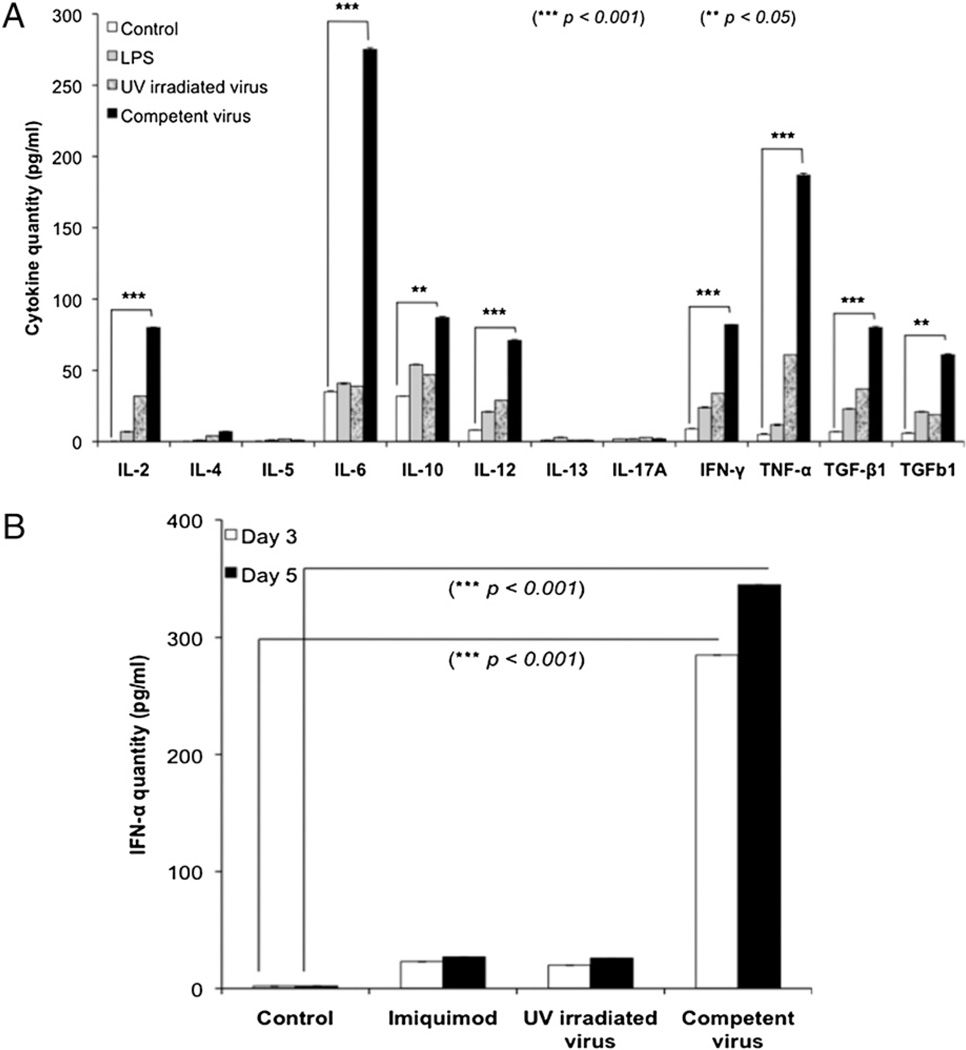
To evaluate the cytokines that could be generated by the FL-DCs in response to the virus, we performed a multiplex cytokine profiling that allowed us to examine 12 different cytokines at one time. When the FL-DCs were treated with increasing concentrations of the virus (25, 50, and 100 ng) and evaluated at various intervals, the cytokines had a characteristic Th1 cell profile, as seen by the production of the proinflammatory cytokines IL-2, IL-6, IL-12, IL-23, IFN-γ, and TNF-α (Fig. 3A). We analyzed the kinetics of these select cytokine responders and found that they responded in both a dose-and time-dependent manner (Fig. 3B).
FIGURE 3.
Multiplex cytokine profiling of FL-DCs. A, The FL-DCs were cultured with various doses of virus (25, 50, and 100 ng) and analyzed for their ability to produce different cytokines. LPS was used as positive control. B, Kinetics of select cytokines. The cytokines (IL-6, IL-10, IL-12, IL-23, IFN-γ, and TNF-α) observed in A were further analyzed for their kinetics and were found to respond in a dose- (25, 50, and 100 ng virus) and time-dependent (0, 2, 4, 8, 12, and 24 h) manner. Statistical significance was determined by the Student t test. **p < 0.05; ***p < 0.001.
One of the first early antiviral cytokines to be produced by FL-DCs was the type 1 IFN (IFN-α and IFN-β). To evaluate the ability of FL-DCs to produce IFN-α after challenge with virus, we did a 24-h kinetic study. The cells were treated with media (control), a TLR7 agonist, imiquimod (because HTLV-1 is a single-stranded RNA virus and TLR7 recognizes single-stranded RNA), and virus. The supernatant was collected at 0, 2, 4, 8, 12, 24, and 48 h to analyze the kinetics (Fig. 4). Robust IFN-α production was observed within 24 h in response to the virus. However, the cytokine level seemed to plateau at 48 h (Fig. 4). Thus, the FL-DCs responded to the viral challenge by the massive production of an array of proinflammatory cytokines, as well as IFN-α, to activate other cells and eventually contain the threat.
FIGURE 4.
Kinetics of type 1 IFN (IFN-α). The FL-DCs were cultured with either imiquimod (TLR7 agonist) or virus (100 ng) and analyzed for their ability to produce IFN-α at various points (0, 2, 4, 8, 12, 24, and 48 h). Statistical significance between virus treatment at 0 and 24 h was determined by the Student t test. ***p < 0.001.
Examining the kinetics of viral entry and expression using UV-irradiated chimeric HTLV-1
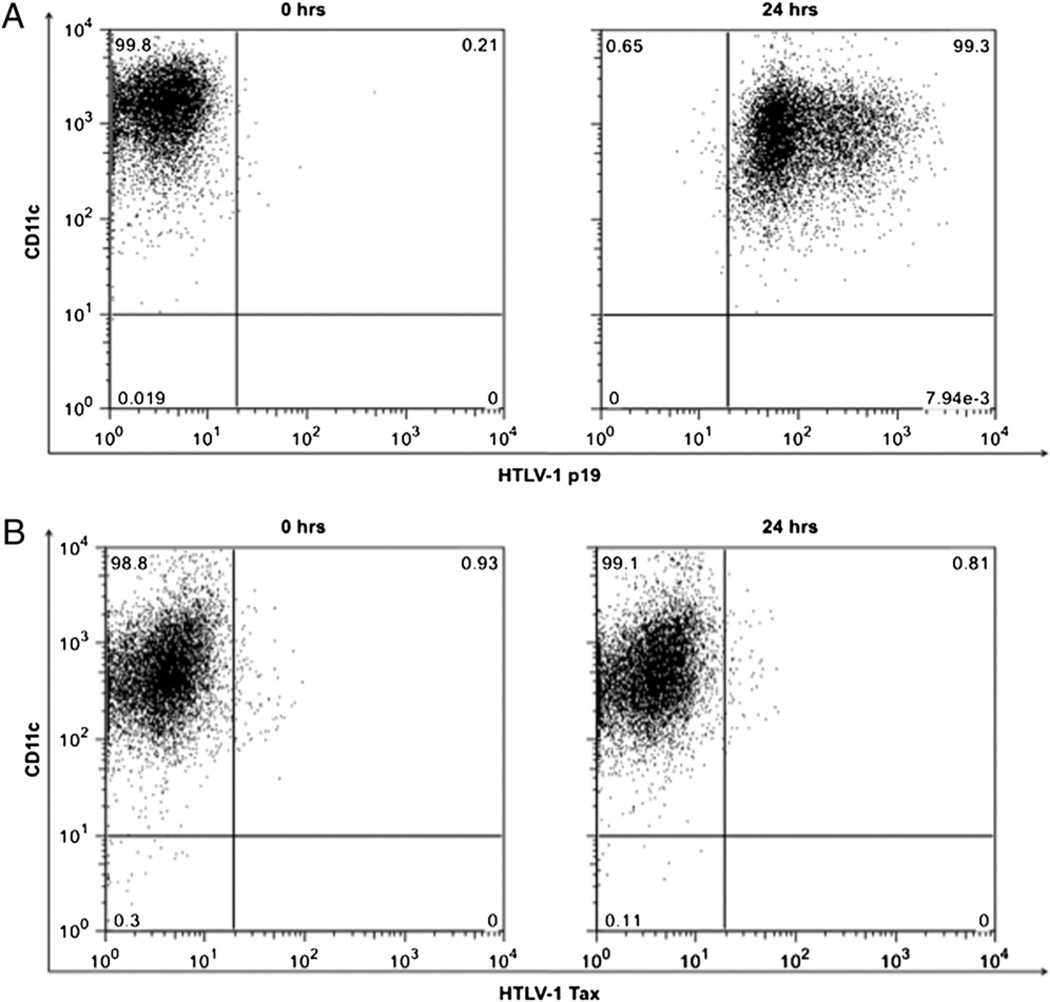
To rule out the possibility of the generation of a DC-mediated response to any contamination in the concentrated viral stock and as a control for subsequent experiments, we wanted to use an inactivated virus. The aim was to prevent the replicative capacity of the virus without causing any damage to the overall structure of the virus, which occurs when it is inactivated by heat. To avoid the loss of structure but still effectively dent the virus’s replicating potential, UV irradiation was performed as demonstrated earlier (40). When the inactivated virus was tested for its entry at 24 h, similar to the competent virus, it was effectively taken in by the FL-DCs (Fig. 5A). However, unlike the competent virus, the incapacitated virus was unable to integrate as provirus (data not shown) or express Tax even at 24 h (Fig. 5B).
FIGURE 5.
Kinetics of viral entry, integration, and replication in UV-irradiated CD11c+ FL-DCs. A, The FL-DCs were mixed with virus and analyzed at various intervals (0 and 24 h) for viral entry by intracellular staining for HTLV-1 p19 Gag protein. B, Demonstration of the inability of the virus to replicate because of lack of production of HTLV-1 Tax protein.
Cytokine response generated only by competent virus during ongoing productive infection
The FL-DCs generated a strong proinflammatory cytokine profile and type 1 IFN response when infected with chimeric HTLV-1. However, the FL-DCs mediated a similar response against the TLR7 agonist imiquimod. To mimic a physiologically relevant in vivo infection model, the cells were first treated with the TLR7 agonist, UV-irradiated virus, or competent virus for 6 h and then washed to remove any TLR agonist or unbound virus. In such a scenario, we would expect only the competent virus to keep replicating within the cells and, therefore, keep stimulating the cells to produce the cytokines; imiquimod and UV-irradiated virus would not be able to do so.
New virions could be detected in the supernatant of the FL-DCs infected with competent virus but not in that infected with the UV-irradiated virus, demonstrating that the former were releasing new virus and thus productively infected (data not shown). To determine the cytokine profile during productive infection of the FL-DCs, we treated the cells as described earlier and analyzed the supernatant on day 3. The cytokine profile showed that the continued presence of replicative virus, indicative of productive infection, induced the cells to continuously produce the proinflammatory cytokines; cells treated with imiquimod or UV-irradiated virus were able to generate a response but were unable to maintain it (Fig. 6A). To analyze for IFN-α during productive infection, we treated the cells as explained earlier and analyzed the supernatant on days 3 and 5. A limited amount of IFN-α was produced when the cells treated with agonist or UV-irradiated virus were washed off (Fig. 6B). However, in the case of productive infection with the competent virus, the cells continued to produce IFN-α in response to the replicating virus within (Fig. 6B).
FIGURE 6.
Cytokine profiling during productive infection of FL-DCs. A, Multiplex cytokine profiling demonstrates the significantly increased production of cytokines after infection with competent virus. B, Analysis of IFN-α demonstrates significantly robust production of this cytokine only by competent virus. Error bars represent SD. Statistical significance was determined by the Student t test. **p < 0.05; ***p < 0.001.
MLR
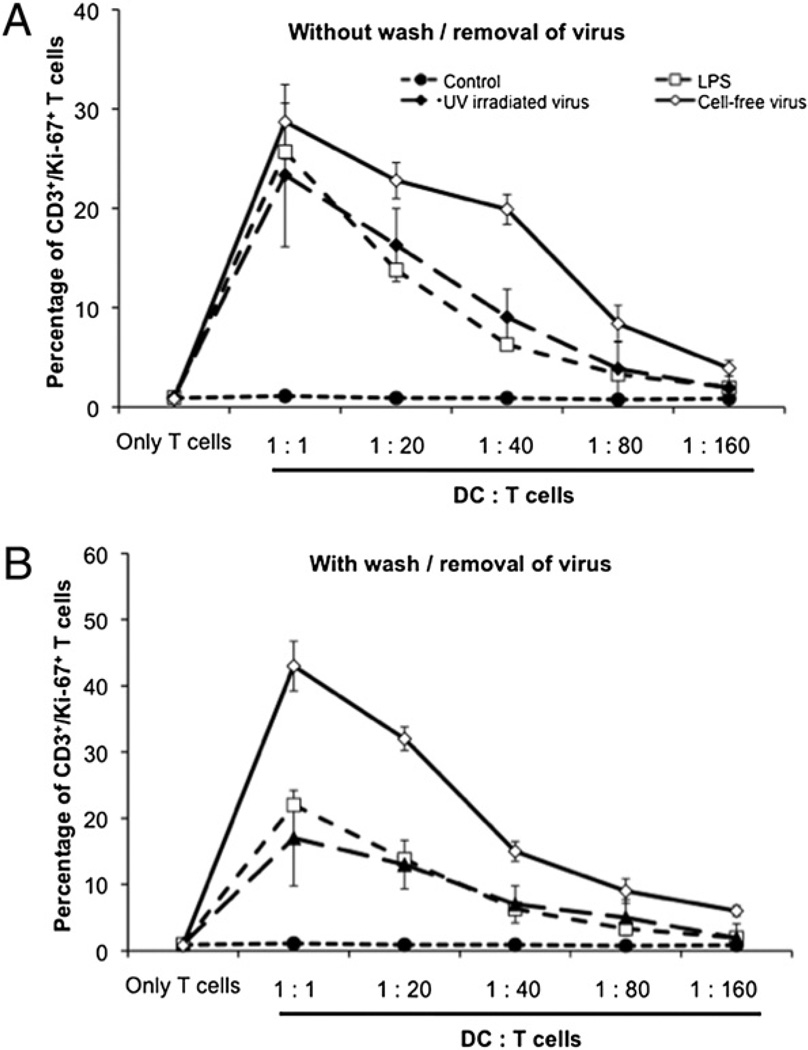
Treating FL-DCs with the virus caused them to mature and upregulate the surface expression of various costimulatory molecules (Fig. 1B). To determine their ability to proliferate T cells, as well as the ability of T cells to produce IFN-γ in response to the infected FL-DCs, we performed an MLR. The FL-DCs were treated for 6 h with media, LPS, UV-irradiated, or competent virus. Thereafter, the cells were either left untouched or gently trypsinized to wash off any unbound virus and then mixed with autologous CD3+ T cells in increasing ratios (1:1, 1:20, 1:40, 1:80, and 1:160). We observed that the activated and matured FL-DCs caused the proliferation of T cells as measured through an increase in the amount of the T cell nuclear proliferation marker Ki67 (Fig. 7A, 7B). However, the levels of proliferation were much greater with competent virus than with UV-irradiated virus when the FL-DCs were washed (Fig. 7B) compared with the cells that had not been washed (Fig. 7A). Similarly, when analyzed for the ability of the T cells to produce IFN-γ on coculture with the productively infected FL-DCs, we noted robust production of IFN-γ by FL-DCs that had been infected with competent virus compared with those infected with UV-irradiated virus (Fig. 8A, 8B). As observed earlier, unlike the undisturbed setup (Fig. 8A), when the cells were washed after treatment with virus, the competent virus maintained the ability of CD8+ T cells to produce greater amounts of IFN-γ compared with the UV-irradiated virus (Fig. 8B). Overall, the MLR results clearly demonstrated that the continued presence of a replicative competent virus was critical to allow the FL-DCs to keep stimulating the proliferation of the autologous T cells and also in prompting the CD8+ T cells to produce IFN-γ to keep the viral threat in check.
FIGURE 7.
Proliferative capacity of FL-DCs. To determine the ability of FL-DCs to proliferate autologous CD3 T cells, the FL-DCs were allowed to mature after activation with either UV-irradiated or competent virus and then later (A) left undisturbed or (B) washed after 6 h to remove any unbound virus. Proliferation of the CD3 T cells was analyzed on day 5 by intracellular staining for the nuclear proliferation marker Ki67.
FIGURE 8.
Proliferative capacity of FL-DCs. To determine the ability of the CD3 T cells to respond to the infected FL-DCs by producing IFN-γ, the FL-DCs were allowed to mature after activation with either UV-irradiated or competent virus and then later (A) left undisturbed or (B) washed after 6 h to remove any unbound virus. Production of IFN-γ by the CD3 T cells was analyzed on day 5 by staining intracellularly for IFN-γ.
Gene expression profiling studies
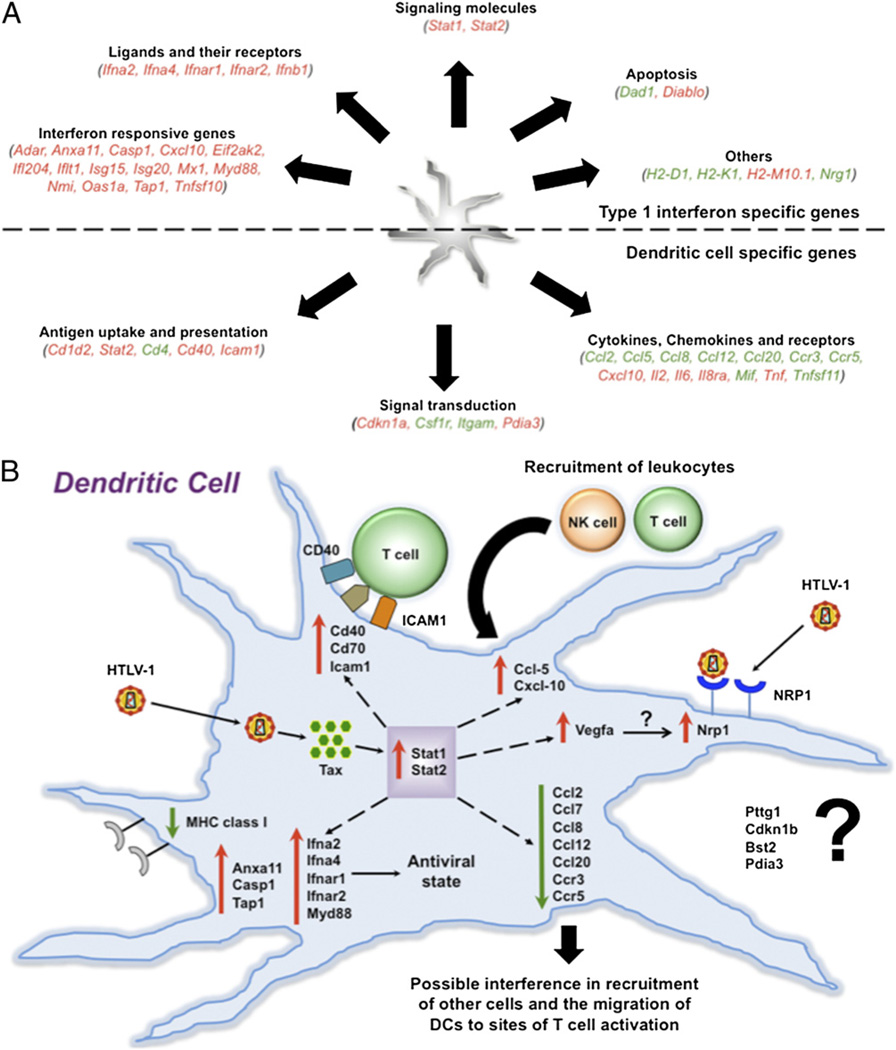
To identify an early molecular signature, we performed gene expression profiling to determine the effect of viral infection on the expression of FL-DC genes involved in type 1 IFN pathways and DC functions (Fig. 9A). One of the early responses against viruses is mediated through the action of type 1 IFNs (IFN-α and IFN-β). To evaluate the downstream targets and effects of type 1 IFNs after HTLV-1 infection of DCs, we performed an array evaluating the expression of genes involved in the IFN-α and IFN-β immune responses (Table 1). The genes upregulated were for IFN and its receptors (Ifna2, Ifna4, IfnaR1, IfnaR2, and Ifnb1), as well as for various signaling molecules (stat1 and stat2). Strikingly, the majority of genes upregulated were ISGs such as Adar, Anxa11, Casp1, Ccxl10, Eif2ak2, Ifl204, Irf2, Isg15, Isg20, Mx2, MyD88, Nmi, stat1, Tap1, and Tnfsf10 (Table I). These results suggest the importance of the type 1 IFN response during viral threat. Interestingly, we observed the genes for MHC class I (H2-D and H2-K) to be downregulated. In addition, the levels of CD70 Ag and vascular endothelial growth factor A (Vegfa) were highly upregulated (59- and 170-fold, respectively).
FIGURE 9.
Gene expression profiling of FL-DCs. A, The FL-DCs were infected with competent virus and analyzed to determine their ability to upregulate or downregulate various cellular genes after infection with HTLV-1. Two independent sets of arrays were performed. The first was designed to identify genes involved in type 1 IFN pathway; the second was to identify those involved in DC functions. B, Model to explain the role of various genes in DCs after infection with HTLV-1.
Table I.
Type 1 IFN response gene profiling of FL-DCs after HTLV-1 infection compared with control (noninfected) cells
| Symbol | Description | Fold Change |
|---|---|---|
| Type 1 IFN response genes | ||
| Ligands and their receptors | ||
| Ifna2 | IFN-α2 | 12.4 |
| Ifna4 | IFN-α4 | 18.3 |
| Ifnar1 | IFN (α and β) receptor 1 | 10.0 |
| Ifnar2 | IFN (α and β) receptor 2 | 31.8 |
| Ifnb1 | IFN-β1, fibroblast | 19.3 |
| Signaling molecules | ||
| stat1 | Signal transducer and activator of transcription 1 | 8.1 |
| stat2 | Signal transducer and activator of transcription 2 | 11.0 |
| IFN-responsive genes | ||
| Adar | Adenosine deaminase, RNA-specific | 7.7 |
| Anxa11 | Annexin A11 | 8.6 |
| Casp1 | Caspase 1 | 9.8 |
| Cxcl10 | Chemokine (CXC motif) ligand 10 | 15.5 |
| Eif2ak2 | Eukaryotic translation initiation factor 2α kinase 2 | 9.9 |
| Ifi204 | IFN-activated gene 204 | 8.4 |
| Ifit1 | IFN-induced protein with tetratricopeptide repeats 1 | 2.5 |
| Isg15 | ISG15 ubiquitin-like modifier | 12.6 |
| Isg20 | IFN-stimulated protein | 14.6 |
| Mx1 | Myxovirus (influenza virus) resistance 1 | 4.5 |
| MyD88 | Myeloid differentiation primary response gene 88 | 7.3 |
| Nmi | N-myc (and stat) interactor | 2.6 |
| Oas1a | 2′-5′ oligoadenylate synthetase 1A | 13.6 |
| Tap1 | Transporter 1, ATP-binding cassette, subfamily B (MDR/TAP) |
13.4 |
| Tnfsf10 | TNF (ligand) superfamily, member 10 | 26.2 |
| Genes associated with intrinsic IFN resistance | ||
| Signal transduction | ||
| Prkcz | Protein kinase C, ζ | −2.2 |
| Apoptosis | ||
| Dad1 | Defender against cell death 1 | −2.2 |
| Diablo | Diablo homolog (Drosophila) | 9.7 |
| Transcription regulation | ||
| Atf5 | Activating transcription factor 5 | 2.1 |
| Irf2 | IFN regulatory factor 2 | 3.1 |
| Irf3 | IFN regulatory factor 3 | −3.1 |
| Pttg1 | Pituitary tumor-transforming gene 1 | 5.5 |
| Cell growth | ||
| Cd70 | CD70 Ag | 59.3 |
| Cdkn1b | Cyclin-dependent kinase inhibitor 1B | −7.5 |
| Hoxb2 | Homeo box B2 | 2.0 |
| Ifi204 | IFN-activated gene 204 | 8.4 |
| Vegfa | Vascular endothelial growth factor A | 170.8 |
| Others | ||
| Bst2 | Bone marrow stromal cell Ag 2 | −5.2 |
| Cnp | 2′,3′-cyclic nucleotide 3′ phosphodiesterase | −3.5 |
| H2-D1 | Histocompatibility 2, D region locus 1 | −3.8 |
| H2-K1 | Histocompatibility 2, K1, K region | −2.3 |
| H2-M10.1 | Histocompatibility 2, M region locus 10.1 | 3.7 |
| Nrg1 | Neuregulin 1 | −4.2 |
The array was repeated three times to validate the findings.
−, Downregulation.
When we analyzed for the genes involved in DC functions (Table II), we found that the genes for Il2, Il6, and Tnf-α were upregulated in correlation with the observed ELISA results (Fig. 3). In addition, the genes for Ag uptake (Icam1) and presentation (Cd40) were also upregulated. However, a majority of the chemokines and their receptors (Ccl2, Ccl7, Ccl8, Ccl12, Ccl20, Ccr3, and Ccr5), as well as a few key signaling molecules (Csfir and Itgam), were downregulated (Table II). These results suggest the plausible role of viral interference in cell recruitment, as well as DC migration through the downregulation of key chemokines and their receptors.
Table II.
DC functional gene profiling of FL-DCs after HTLV-1 infection compared with control (noninfected) cells
| Symbol | Description | Fold Change |
|---|---|---|
| Cytokines, chemokines, and their receptors | ||
| Ccl2 | Chemokine (CC motif) ligand 2 | −5.2 |
| Ccl5 | Chemokine (CC motif) ligand 5 | 49.0 |
| Ccl7 | Chemokine (CC motif) ligand 7 | −7.5 |
| Ccl8 | Chemokine (CC motif) ligand 8 | −3.5 |
| Ccl12 | Chemokine (CC motif) ligand 12 | −14.3 |
| Ccl20 | Chemokine (CC motif) ligand 20 | −2.0 |
| Ccr3 | Chemokine (CC motif) receptor 3 | −2.2 |
| Ccr5 | Chemokine (CC motif) receptor 5 | −2.1 |
| Cxcl10 | Chemokine (CXC motif) ligand 10 | 2.5 |
| Il2 | IL-2 | 4.5 |
| Il6 | IL-6 | 2.1 |
| Il8ra | IL-8Rα | 2.6 |
| Mif | Macrophage migration inhibitory factor | −2.2 |
| Tnf | TNF | 2.0 |
| Ag uptake and presentation | ||
| Cd1d2 | CD1d2 Ag | 2.4 |
| Stat2 | Signal transducer and activator of transcription 2 | 11.0 |
| Cd4 | CD4 Ag | −2.3 |
| Cd40 | CD40 Ag | 3.7 |
| Icam1 | ICAM-1 | 2.5 |
| Cell surface receptors | ||
| Cd36 | CD36 Ag | −3.8 |
| Fcgr1 | FcR, IgG, high affinity I | −3.1 |
| Fcer2a | FcR, IgE, low affinity II, α polypeptide | 3.1 |
| Lrp1 | Low-density lipoprotein receptor-related protein 1 | −4.4 |
| Signal transduction | ||
| Cdkn1a | Cyclin-dependent kinase inhibitor 1A (P21) | 2.0 |
| Csf1r | CSF 1 receptor | −2.2 |
| Fcamr | FcR, IgA, IgM, high affinity | 2.6 |
| Itgam | Integrin α M | −4.2 |
| Pdia3 | Protein disulfide isomerase-associated 3 | 5.5 |
The array was repeated three times to validate the findings.
−, Downregulation.
Discussion
Our previous in vivo studies with the transgenic CD11c-diphtheria toxin receptor mice and chimeric HTLV-1 showed that, in the absence of DCs, infection with cell-free virus led to significantly increased proviral and Tax mRNA loads leading to an enhanced susceptibility of mice to infection (34). These results suggest that DCs are crucial for controlling cell-free HTLV-1, which may represent an early stage of infection. Therefore, we proceeded to examine the interaction of cell-free virus and DCs in isolation. However, large amounts of murine DCs would be required for extensive subsequent studies, which is not economically viable or feasible to isolate. To overcome this shortcoming, we isolated the mouse bone marrow cells and cultured them in vitro in the presence of murine FLT3L to generate large numbers of FL-DCs. Using the purified FL-DCs (Fig. 1A), we first examined the phenotypic changes in these cells in response to incubation with virus. Chimeric HTLV-1 matured the cells through the increased expression of surface markers CD80, CD86, and MHC class II (Fig. 1B). However, the levels of MHC class I remained unchanged (Fig. 1B), possibly because of the effect of the HTLV-1 protein p12 that is known to downregulate the expression of MHC class I and thus escape immune surveillance (42).
We then attempted to evaluate closely the interaction kinetics between cell-free chimeric HTLV-1 and FL-DCs in terms of viral uptake, integration, and replication. After infection of FL-DCs, the virus was taken in as early as 2 h, and the provirus could be detected around 12 h (Fig. 2A, 2B). In correlation with the observed proviral load, the Tax protein could also be detected around the same time (Fig. 2C). In an earlier study, Tax was detectable around 16 h after infection of pDCs (18). Based on these results, we believe that the earlier observed increase in surface expression of the maturation markers (seen after 24-h incubation with virus), rather than being an effect of the Mo-MLV envelope, could be possibly caused by HTLV-1 replication and more so Tax expression (seen after 12 h) that has been previously demonstrated by us to activate both the murine JAWS II cells and human monocyte-derived DCs (30, 33). To rule out any bias in the HTLV-1-specific response and also as a specificity control for subsequent experiments, we used a UV-irradiated chimeric viral stock that was replicatively incapacitated by being unable to integrate as provirus or to produce the Tax protein (Fig. 5). Overall, these results suggest that cell-free HTLV-1 can enter the FL-DCs as early as 2 h, soon thereafter integrate, and begin producing Tax protein by around 12 h. However, in contrast with competent virus, the virus incapacitated after UV irradiation lost its replication potential and thus could neither integrate nor replicate.
To analyze the spectrum of cytokines produced, we performed a viral dose and time kinetics study after infection of FL-DCs. The cytokines had a characteristic Th1 cell profile, with the production of the proinflammatory cytokines IL-2, IL-6, IL-12, IL-23, IFN-γ, and TNF-α (Fig. 3A). We then analyzed the time kinetics of these select cytokine responders (Fig. 3B). One of the first lines of defensive mediators produced by DCs during early viral infection is the type 1 IFNs. We examined the kinetics of IFN-α and observed that the cells started producing significant amounts of this cytokine as early as 2 h and platitude around 24 h (Fig. 4). In the previous study by Delebecque et al. (39), only a modest increase in infection was observed in the 129Sv IFNAR−/− mice compared with the parental strain, suggesting that the innate immune response does not play a major role in controlling this virus. However, in this study, the mode of HTLV-1 infection was cell associated as opposed to cell-free virus used here. The reduced benefit of type 1 IFN observed previously could be because cell-associated virus represents a late stage of infection wherein it has escaped the early immune responses, whereas cell-free virus represents an early stage of infection; therefore, the role of type 1 IFNs during this critical stage of infection may be vital.
Under an ideal in vivo ongoing productive infection scenario, the cells should keep responding only to an integrated, replicating virus. We thus used a UV-irradiated virus in addition to a competent virus to determine the ability of FL-DCs to respond to a one-time triggering of DCs compared with an ongoing replicative infection. As a mark of productive infection, we detected new virions being released only from the infected cells into the supernatant starting on day 3 (data not shown). When we examined the cytokine profile after ongoing productive infection on day 3, we found that only the cells that were productively infected with competent virus kept producing the cytokines because these were constantly challenged by the virus (Fig. 6A). However, the cells that were infected with UV-irradiated virus or treated with imiquimod and then washed showed reduced production of the cytokines (Fig. 6A). Similarly, with type 1 IFN, only the cells that were infected with competent virus continued to produce significant amounts of IFN-α on days 3 and 5; the cells that were treated with imiquimod or with UV-irradiated virus stopped producing the cytokine after being washed (Fig. 6B). The results so far demonstrate that the productively infected FL-DCs responded to the viral challenge by a massive production of proinflammatory cytokines characteristic of a Th1 cell profile and of type 1 IFN, IFN-α, to activate other immune cells and eventually to contain the threat.
By the MLR, we addressed whether the FL-DCs, once matured and activated, could, in turn, stimulate proliferation of the CD3+ T cells. We noted that the activated FL-DCs could indeed stimulate proliferation of the autologous T cells as measured by an increase in the nuclear proliferation marker Ki67 (Fig. 7). In addition, when the same T cells were analyzed for their ability to produce IFN-γ in response to the productively infected FL-DCs, many cells were found to produce IFN-γ (Fig. 8). Thus, the activated FL-DCs stimulated proliferation of the autologous T cells, thereby demonstrating their functional maturation. Finally, the T cells produced IFN-γ in response to the productively infected FL-DCs.
To determine the genes involved in type 1 IFN and DC functions and pathways that were differentially expressed during and after infection of FL-DCs with HTLV-1, we performed an exhaustive gene expression profile. We analyzed the effects of the type 1 IFNs (IFN-α and IFN-β) produced by the FL-DCs and found that many genes were upregulated (Fig. 9A, Table I). Most of the upregulated genes were those for IFN and its receptor (Ifna2, Ifna4, Ifnar1, Ifnar2, and Ifnb1) and/or IFN-responsive genes. Interestingly, the genes for mouse MHC class I (H2-D and H2-K) were downregulated, whereas no observable difference was seen by flow cytometry when challenged with virus (Fig. 1B). This is probably because changes in gene expression are not always accompanied by changes in protein expression (30, 43). Notably, the levels of two genes, CD70 Ag and Vegfa, were highly upregulated. It was recently shown that HTLV-1–infected cell lines and primary cells upregulated the expression of CD70 Ag (44), which is a costimulatory ligand expressed on DC maturation. Of particular interest was the distinctly high upregulation of Vegfa, which is known to selectively upregulate the expression of the neuronal receptor neuropilin-1 (45). The neuropilin-1 protein plays an important role in mediating the interaction between DCs and T cells, which leads to generation of a primary immune response, as well as the ability of HTLV-1 to enter cells using this critical receptor (46, 47). Overall, the selective upregulation of Vegfa may represent a viral-mediated strategy to increase the expression of neuropilin-1 protein and thereby aid in the further entry of new virions.
Similarly, when analyzed for the genes involved in DC functions (Fig. 9A, Table II), most of the upregulated genes were those for cytokines (Il2, Il6, and Tnf-α) and for those involved in Ag uptake (Icam1) and presentation (Cd40). The cytokine gene result corroborates observed ELISA results (Fig. 3). Most strikingly, a number of chemokines and their receptors were downregulated (Ccl2, Ccl7, Ccl8, Ccl12, Ccl20, Ccr3, and Ccr5), except for Ccl5 (RANTES) and Cxcl10, which were upregulated. Previous studies have clearly shown increased levels of Ccl5 in both HAM/TSP and ATL patients (48, 49). In addition, it was recently shown that Tax-dependent TGF-β–activated kinase 1 activation induces the activation and the expression of several IFN-inducible genes, including Ccl5 and Cxcl10 (50). The ability of HTLV-1 to down-regulate the expression of a number of chemokines and, in particular, the RANTES receptor (Ccr5) is particularly intriguing. These data suggest one of the many ways by which the virus can interfere in the generation of a successful cell-mediated immune response. Overall and as outlined in our model (Fig. 9B), the gene expression studies clearly demonstrate the important role that DCs play during the early phase of infection with cell-free HTLV-1 because of their ability to upregulate the expression of various ISGs and to produce an array of antiviral cytokines that, in turn, can help to control the viral challenge, as well as the ability of the virus to counter the threat by downregulating the critical chemokines. We do understand that some of these results might be specific to the murine model of HTLV-1 and may not fully translate to the human system. Nevertheless, these results highlight the importance of DCs during early HTLV-1 infection and their ability to control the infection through an array of innate immune responses in our murine model of HTLV-1.
Acknowledgments
We thank Dr. Robert Rottapel (Ontario Cancer Institute, Toronto Medical Discovery Tower, Toronto, Ontario, Canada) for the kind gift of SP2/0 transfectants secreting mouse recombinant FLT3L.
This work was supported by the U.S. Public Health Service/National Institutes of Health Grants R01 AI077414 (to P.J.) and R01 CA054559 (to B.W.).
Abbreviations used in this paper
- ATL
adult T cell leukemia
- DC
dendritic cell
- FL-DC
FLT3 ligand-cultured bone marrow-derived dendritic cell
- FLT3L
FLT3 ligand
- HAM/TSP
human T cell leukemia virus type 1-associated myelopathy/tropical spastic paraparesis
- HTLV-1
human T cell leukemia virus type 1
- ISG
IFN-stimulated gene
- pDC
plasmacytoid dendritic cell
- Vegfa
vascular endothelial growth factor A
Footnotes
The sequences presented in this article have been submitted to Gene Expression Omnibus under accession number GSE24962.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan JE, Osame M, Kubota H, Igata A, Nishitani H, Maeda Y, Khabbaz RF, Janssen RS. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J. Acquir. Immune Defic. Syndr. 1990;3:1096–1101. [PubMed] [Google Scholar]
- 3.Murphy EL, Hanchard B, Figueroa JP, Gibbs WN, Lofters WS, Campbell M, Goedert JJ, Blattner WA. Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type. I. Int. J. Cancer. 1989;43:250–253. doi: 10.1002/ijc.2910430214. [DOI] [PubMed] [Google Scholar]
- 4.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. [PubMed] [Google Scholar]
- 5.Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de The´ G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 6.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 7.Ohshima K. Pathological features of diseases associated with human T-cell leukemia virus type I. Cancer Sci. 2007;98:772–778. doi: 10.1111/j.1349-7006.2007.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maloney EM, Cleghorn FR, Morgan OS, Rodgers-Johnson P, Cranston B, Jack N, Blattner WA, Bartholomew C, Manns A. Incidence of HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Jamaica and Trinidad. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1998;17:167–170. doi: 10.1097/00042560-199802010-00011. [DOI] [PubMed] [Google Scholar]
- 9.Maguer-Satta V, Gazzolo L, Dodon MD. Human immature thymocytes as target cells of the leukemogenic activity of human T-cell leukemia virus type. I. Blood. 1995;86:1444–1452. [PubMed] [Google Scholar]
- 10.Osame M, Janssen R, Kubota H, Nishitani H, Igata A, Nagataki S, Mori M, Goto I, Shimabukuro H, Khabbaz R, et al. Nationwide survey of HTLV-I-associated myelopathy in Japan: association with blood transfusion. Ann. Neurol. 1990;28:50–56. doi: 10.1002/ana.410280110. [DOI] [PubMed] [Google Scholar]
- 11.Ohashi T, Hanabuchi S, Kato H, Koya Y, Takemura F, Hirokawa K, Yoshiki T, Tanaka Y, Fujii M, Kannagi M. Induction of adult T-cell leukemia-like lymphoproliferative disease and its inhibition by adoptive immunotherapy in T-cell-deficient nude rats inoculated with syngeneic human T-cell leukemia virus type 1-immortalized cells. J. Virol. 1999;73:6031–6040. doi: 10.1128/jvi.73.7.6031-6040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannagi M, Ohashi T, Hanabuchi S, Kato H, Koya Y, Hasegawa A, Masuda T, Yoshiki T. Immunological aspects of rat models of HTLV type 1-infected T lymphoproliferative disease. AIDS Res. Hum. Retroviruses. 2000;16:1737–1740. doi: 10.1089/08892220050193236. [DOI] [PubMed] [Google Scholar]
- 13.Makino M, Wakamatsu S, Shimokubo S, Arima N, Baba M. Production of functionally deficient dendritic cells from HTLV-I-infected monocytes: implications for the dendritic cell defect in adult T cell leukemia. Virology. 2000;274:140–148. doi: 10.1006/viro.2000.0445. [DOI] [PubMed] [Google Scholar]
- 14.Makino M, Utsunomiya A, Maeda Y, Shimokubo S, Izumo S, Baba M. Association of CD40 ligand expression on HTLV-I-infected T cells and maturation of dendritic cells. Scand. J. Immunol. 2001;54:574–581. doi: 10.1046/j.1365-3083.2001.01003.x. [DOI] [PubMed] [Google Scholar]
- 15.Hishizawa M, Imada K, Kitawaki T, Ueda M, Kadowaki N, Uchiyama T. Depletion and impaired interferon-alpha-producing capacity of blood plasmacytoid dendritic cells in human T-cell leukaemia virus type I-infected individuals. Br. J. Haematol. 2004;125:568–575. doi: 10.1111/j.1365-2141.2004.04956.x. [DOI] [PubMed] [Google Scholar]
- 16.Ali A, Patterson S, Cruickshank K, Rudge P, Dalgleish AG, Knight SC. Dendritic cells infected in vitro with human T cell leukaemia/lymphoma virus type-1 (HTLV-1); enhanced lymphocytic proliferation and tropical spastic paraparesis. Clin. Exp. Immunol. 1993;94:32–37. doi: 10.1111/j.1365-2249.1993.tb05973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macatonia SE, Cruickshank JK, Rudge P, Knight SC. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res. Hum. Retroviruses. 1992;8:1699–1706. doi: 10.1089/aid.1992.8.1699. [DOI] [PubMed] [Google Scholar]
- 18.Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat. Med. 2008;14:429–436. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- 19.Manuel SL, Schell TD, Acheampong E, Rahman S, Khan ZK, Jain P. Presentation of human T cell leukemia virus type 1 (HTLV-1) Tax protein by dendritic cells: the underlying mechanism of HTLV-1-associated neuro-inflammatory disease. J. Leukoc. Biol. 2009;86:1205–1216. doi: 10.1189/jlb.0309172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makino M, Shimokubo S, Wakamatsu SI, Izumo S, Baba M. The role of human T-lymphotropic virus type 1 (HTLV-1)-infected dendritic cells in the development of HTLV-1-associated myelopathy/tropical spastic paraparesis. J. Virol. 1999;73:4575–4581. doi: 10.1128/jvi.73.6.4575-4581.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelsall BL, Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer’s patch. J. Exp. Med. 1996;183:237–247. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 23.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 24.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 25.Schelhaas M. Come in and take your coat off - how host cells provide endocytosis for virus entry. Cell Microbiol. 2010;12:1378–1388. doi: 10.1111/j.1462-5822.2010.01510.x. [DOI] [PubMed] [Google Scholar]
- 26.Colisson R, Barblu L, Gras C, Raynaud F, Hadj-Slimane R, Pique C, Hermine O, Lepelletier Y, Herbeuval JP. Free HTLV-1 induces TLR7-dependent innate immune response and TRAIL relocalization in killer plasmacytoid dendritic cells. Blood. 2010;115:2177–2185. doi: 10.1182/blood-2009-06-224741. [DOI] [PubMed] [Google Scholar]
- 27.Kampani K, Quann K, Ahuja J, Wigdahl B, Khan ZK, Jain P. A novel high throughput quantum dot-based fluorescence assay for quantitation of virus binding and attachment. J. Virol. Methods. 2007;141:125–132. doi: 10.1016/j.jviromet.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain P, Manuel SL, Khan ZK, Ahuja J, Quann K, Wigdahl B. DC-SIGN mediates cell-free infection and transmission of human T-cell lym-photropic virus type 1 by dendritic cells. J. Virol. 2009;83:10908–10921. doi: 10.1128/JVI.01054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 30.Mostoller K, Norbury CC, Jain P, Wigdahl B. Human T-cell leukemia virus type I Tax induces the expression of dendritic cell markers associated with maturation and activation. J. Neurovirol. 2004;10:358–371. doi: 10.1080/13550280490521104. [DOI] [PubMed] [Google Scholar]
- 31.Ahuja J, Kampani K, Datta S, Wigdahl B, Flaig KE, Jain P. Use of human antigen presenting cell gene array profiling to examine the effect of human T-cell leukemia virus type 1 Tax on primary human dendritic cells. J. Neurovirol. 2006;12:47–59. doi: 10.1080/13550280600614981. [DOI] [PubMed] [Google Scholar]
- 32.Ahuja J, Lepoutre V, Wigdahl B, Khan ZK, Jain P. Induction of pro-inflammatory cytokines by human T-cell leukemia virus type-1 Tax protein as determined by multiplexed cytokine protein array analyses of human dendritic cells. Biomed Pharmacother. 2007;61:201–208. doi: 10.1016/j.biopha.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain P, Ahuja J, Khan ZK, Shimizu S, Meucci O, Jennings SR, Wigdahl B. Modulation of dendritic cell maturation and function by the Tax protein of human T cell leukemia virus type 1. J. Leukoc. Biol. 2007;82:44–56. doi: 10.1189/jlb.1006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman S, Manuel SL, Khan ZK, Wigdahl B, Acheampong E, Tangy F, Jain P. Depletion of dendritic cells enhances susceptibility to cell-free infection of human T cell leukemia virus type 1 in CD11c-diphtheria toxin receptor transgenic mice. J. Immunol. 2010;184:5553–5561. doi: 10.4049/jimmunol.0903226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–3039. [PubMed] [Google Scholar]
- 36.Jain P, Coisne C, Enzmann G, Rottapel R, Engelhardt B. Alpha4beta1 integrin mediates the recruitment of immature dendritic cells across the blood-brain barrier during experimental autoimmune encephalomyelitis. J. Immunol. 2010;184:7196–7206. doi: 10.4049/jimmunol.0901404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dehlin M, Bokarewa M, Rottapel R, Foster SJ, Magnusson M, Dahlberg LE, Tarkowski A. Intra-articular fms-like tyrosine kinase 3 ligand expression is a driving force in induction and progression of arthritis. PLoS ONE. 2008;3:e3633. doi: 10.1371/journal.pone.0003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delebecque F, Pramberger K, Pre´vost MC, Brahic M, Tangy F. A chimeric human T-cell lymphotropic virus type 1 with the envelope glycoprotein of Moloney murine leukemia virus is infectious for murine cells. J. Virol. 2002;76:7883–7889. doi: 10.1128/JVI.76.15.7883-7889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delebecque F, Combredet C, Gabet AS, Wattel E, Brahic M, Tangy F. A chimeric human T cell leukemia virus type I bearing a deltaR Moloney-murine leukemia virus envelope infects mice persistently and induces humoral and cellular immune responses. J. Infect. Dis. 2005;191:255–263. doi: 10.1086/426825. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu A, Shimizu N, Tanaka A, Jinno-Oue A, Roy BB, Shinagawa M, Ishikawa O, Hoshino H. Human T-cell leukaemia virus type I is highly sensitive to UV-C light. J. Gen. Virol. 2004;85:2397–2406. doi: 10.1099/vir.0.19578-0. [DOI] [PubMed] [Google Scholar]
- 41.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 42.Johnson JM, Nicot C, Fullen J, Ciminale V, Casareto L, Mulloy JC, Jacobson S, Franchini G. Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12(I) protein. J. Virol. 2001;75:6086–6094. doi: 10.1128/JVI.75.13.6086-6094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jørgensen TN, Haase C, Michelsen BK. Treatment of an immortalized APC cell line with both cytokines and LPS ensures effective T-cell activation in vitro. Scand. J. Immunol. 2002;56:492–503. doi: 10.1046/j.1365-3083.2002.01166.x. [DOI] [PubMed] [Google Scholar]
- 44.Baba M, Okamoto M, Hamasaki T, Horai S, Wang X, Ito Y, Suda Y, Arima N. Highly enhanced expression of CD70 on human T-lymphotropic virus type 1-carrying T-cell lines and adult T-cell leukemia cells. J. Virol. 2008;82:3843–3852. doi: 10.1128/JVI.02013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh H, Takagi H, Otani A, Koyama S, Kemmochi S, Uemura A, Honda Y. Selective induction of neuropilin-1 by vascular endothelial growth factor (VEGF): a mechanism contributing to VEGF-induced angiogenesis. Proc. Natl. Acad. Sci. USA. 2002;99:383–388. doi: 10.1073/pnas.012074399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tordjman R, Lepelletier Y, Lemarchandel V, Cambot M, Gaulard P, Hermine O, Rome´o PH. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 2002;3:477–482. doi: 10.1038/ni789. [DOI] [PubMed] [Google Scholar]
- 47.Ghez D, Lepelletier Y, Lambert S, Fourneau JM, Blot V, Janvier S, Arnulf B, van Endert PM, Heveker N, Pique C, Hermine O. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J. Virol. 2006;80:6844–6854. doi: 10.1128/JVI.02719-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montanheiro P, Vergara MP, Smid J, da Silva Duarte AJ, de Oliveira AC, Casseb J. High production of RANTES and MIP-1alpha in the tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) J. Neuroimmunol. 2007;188:138–142. doi: 10.1016/j.jneuroim.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 49.Mori N, Krensky AM, Ohshima K, Tomita M, Matsuda T, Ohta T, Yamada Y, Tomonaga M, Ikeda S, Yamamoto N. Elevated expression of CCL5/RANTES in adult T-cell leukemia cells: possible trans-activation of the CCL5 gene by human T-cell leukemia virus type I tax. Int. J. Cancer. 2004;111:548–557. doi: 10.1002/ijc.20266. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki S, Zhou Y, Refaat A, Takasaki I, Koizumi K, Yamaoka S, Tabuchi Y, Saiki I, Sakurai H. Human T cell lymphotropic virus 1 manipulates interferon regulatory signals by controlling the TAK1-IRF3 and IRF4 pathways. J. Biol. Chem. 2010;285:4441–4446. doi: 10.1074/jbc.M109.031476. [DOI] [PMC free article] [PubMed] [Google Scholar]