Abstract
Background
The effect on linear growth of daily long-term inhaled corticosteroid (ICS) therapy in preschool-aged children with recurrent wheezing is controversial.
Objective
To determine the effect of daily ICS given for 2 years on linear growth in preschool children with recurrent wheezing.
Methods
Children ages 2 and 3 years with recurrent wheezing and positive modified asthma predictive indices were randomized to a two-year treatment period of fluticasone propionate CFC (176 mcg/day) or masked-placebo delivered by valved chamber with mask and then followed 2 years off study medication. Height growth determined by stadiometry was compared between treatment groups.
Results
In the study cohort as a whole, the fluticasone group did not have significantly less linear growth than the placebo-group [change in height from baseline difference (ΔHt) of −0.2 cm (95% CI, −1.1, 0.6)] two years after discontinuation of study treatment. In post-hoc analyses, children 2 years old and who weighed < 15 kg at enrollment treated with fluticasone had less linear growth compared to placebo [ΔHt of −1.6 cm (95% CI, −2.8, −0.4), p=0.009].
Conclusion
Linear growth was not significantly different in high-risk, recurrent wheezing preschool age children treated with CFC fluticasone 176 mcg/day compared to placebo 2 years after fluticasone is discontinued. However, post-hoc subgroup analyses revealed that children who are younger in age and of lesser weight relative to the entire study cohort had significantly less linear growth, possibly due to a higher relative fluticasone exposure.
Keywords: Asthma predictive index, atopy, clinical trials, early childhood asthma, fluticasone, inhaled corticosteroids, intermittent wheezing, linear growth, research network
INTRODUCTION
Interpretation of study results evaluating linear growth in childhood asthma is difficult due to competing effects on growth related to the uncontrolled disease itself versus those related to its treatment. Children with persistent asthma of at least moderate severity eventually attain adult height usually in the predicted range(1). However, these children may demonstrate a delay in linear growth(2), associated with inhaled corticosteroid (ICS) therapy. In the Prevention of Early Asthma in Kids (PEAK) study, we previously reported that toddler-aged children with recurrent wheeze at high-risk for the development of asthma treated for two years with fluticasone (176 mcg/day) demonstrated a 1.1 cm reduction in height gained at the end of this treatment period caused by a delay in linear growth compared to those treated with placebo(3). However, following cessation of regularly scheduled fluticasone therapy in the next year, between group differences were no longer significant due to an increase in linear growth in the ICS-treated group. Based on these findings, we hypothesized that the cohort of ICS-treated children overall would have linear growth similar to the placebo group two years after treatment discontinuation. However, we also evaluated secondarily whether particular subgroups of children could be at higher risk for growth suppressing effects from ICS exposure.
METHODS
A detailed description of the screening, recruitment, design, algorithms for the addition and reduction/cessation of supplementary medications such as open-label ICS or montelukast and the criteria for assignment of treatment failure status and statistical analysis for the PEAK trial has been reported in detail elsewhere(4) but will be described briefly here.
Study design and treatments
PEAK is a multi-center, double-blind, randomized, placebo-controlled, parallel-group comparison trial of inhaled fluticasone to placebo in children 24–35 months (2 years) and 36–47 months (3 years) of age at high-risk for the development of asthma(4). These children were treated for two years using an AeroChamber® with mask (donated by Monaghan Medical, Plattsburgh, NY) with fluticasone propionate or Flovent®, 44 mcg/puff, 2 puffs twice daily, via metered-dose inhaler or matching placebo (both donated by GlaxoSmithKline, Research Triangle Park, NC) and then randomized treatment was stopped. Adherence was promoted by a standardized educational approach and measured using an electronic meter (Doser®) as detailed previously(4). The children were then followed for an additional year during which the primary outcome indicators were measured. Of the 285 children in the original study cohort, 204 enrolled in a 12 month extension and completed the entire four year study. The primary safety analysis in the PEAK study was linear growth and thus continued observation of this cohort was a high priority for the Childhood Asthma Research and Education (CARE) Network. The enrolled children had no clinically significant medical disorders apart from wheezing or allergy and were at high-risk for asthma-like symptoms to continue during the school years based on a positive, modified Asthma Predictive Index(3, 5).
Institutional Review Boards at all participating centers approved the protocol and consent forms; the trial was monitored by the CARE Network Data and Safety Monitoring Board. The role of commercial sponsors was limited to donating drug and matched placebo which they did after reviewing the drafted protocol. The text of the manuscript was made available to all the commercial sponsors 2 weeks prior to submission for finalization for comments.
Outcome Measures
Height was measured every 4 months during the 36 months of the study and at the 48 month extension study visit with an upright stadiometer (Harpenden, Holtain, UK) by established CARE procedures(4, 6). A medical history and symptom evaluation, family and environmental history, and an eosinophil count were obtained during the enrollment visit. An exacerbation was defined as the need for a prednisolone course to control asthma-like symptoms as directed by protocol. Skin-prick testing, with a core battery of 10 allergens in all clinical centers, was performed at enrollment(3, 4, 7).
Statistical Analyses
The primary analysis focused on the difference in linear growth between the ICS treated group and the placebo treated group in the 204 children that completed the 4 year study including the extension. Growth was characterized as change from baseline with two different metrics: absolute height measured in centimeters, and height z-score calculated with age and sex standardized growth charts (CDC 2000)(8). Linear mixed-effects regression using absolute height (cm), or height z-score, as the outcome was used to model the longitudinal effects of treatment at each study visit while adjusting for baseline covariates and for open-label ICS and oral corticosteroid use during both the treatment and follow-up periods. Linear contrasts were used to estimate the difference between treatment groups, with respect to change from baseline, at each study visit. We first examined models which incorporated age and weight as continuous variables. These analyses indicated a larger effect of ICS among the younger children of lesser weight (results fully reported below). Sensitivity analyses indicated that the results of the stratified analyses were very similar over a range of weight cut-points between 15–17 kg and age cut-points near 3 years. We further examined the effect of ICS incorporating an interaction between age, dichotomized at 3 years, and weight, dichotomized at 15 kg. The selected cut-points were chosen based on two considerations: first, that they were consistent with the effects seen in the continuous variable model, and second, that they were clinically useful. A complete description of the statistical methods is included in the electronic supplement.
Linear mixed-effects regression was performed using PROC MIXED in the SAS statistical software system version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Study Population
In the original cohort of 285 children, the two treatment groups were similar with respect to all baseline characteristics(3) except for a higher percentage of peripheral blood eosinophils in the ICS-group. There were no significant differences in the number of completed clinic visits, drop-outs, treatment failures or serious adverse events between groups(3). Less than 12% were lost to follow-up (drop-outs) in both groups one year after treatment discontinuation(3) and a total of 28% two years after treatment discontinuation leaving 204 children for analysis (e-Figure 1).
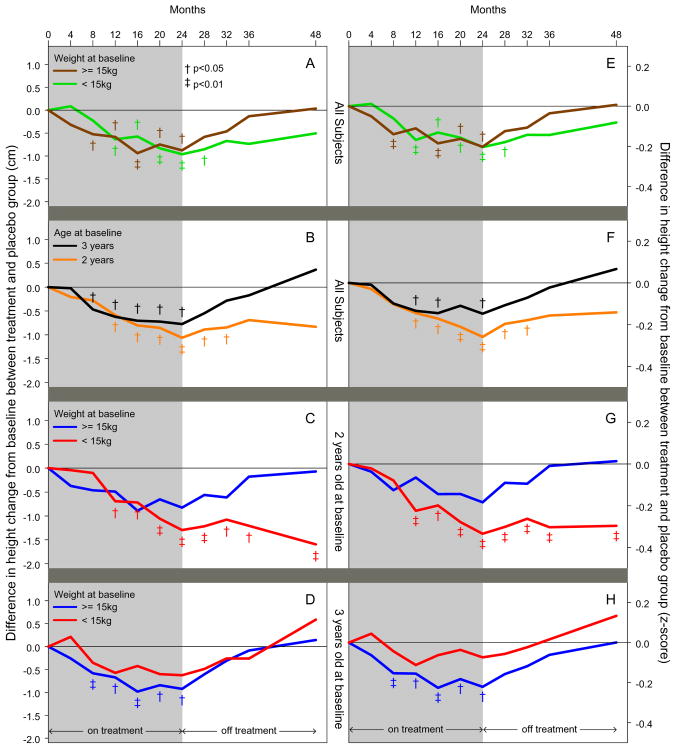
Figure 1.
Post-hoc subgroup analyses: Difference in height change from baseline between treatment and placebo group [height change (left panel) and z-score change (right panel)] from baseline to 48 months for fluticasone treated group as compared to placebo treated group. Children at particular risk for less linear growth during treatment were those children who were two years of age and <15 kg at enrollment. At the end of the two-year observation period, linear growth was only reduced in the children that were two years of age at enrollment of lesser weight (relative to the cohort) versus the younger children with a higher weight (Panels C & G) or the older children with any enrollment weight (Panel D & H). Results were obtained using linear mixed-effects regression including data from all study visits while adjusting for baseline covariates. Significant differences between treatment groups denoted by: † (p<0.05) and ‡(p<0.01).
The baseline characteristics of these 204 children who completed the extension study were similar to the original study cohort of 285 (p-values all > 0.5; electronic supplement-Table 1). It should be noted that the results did not change when the analyses also included partial follow-up data from children who did not complete the study or if the models did not include baseline covariates.
Overall Growth Analysis of the 204 Children Who Completed the Four Year Study (E-Figure 2; Tables 1 & 2)
Figure 2.
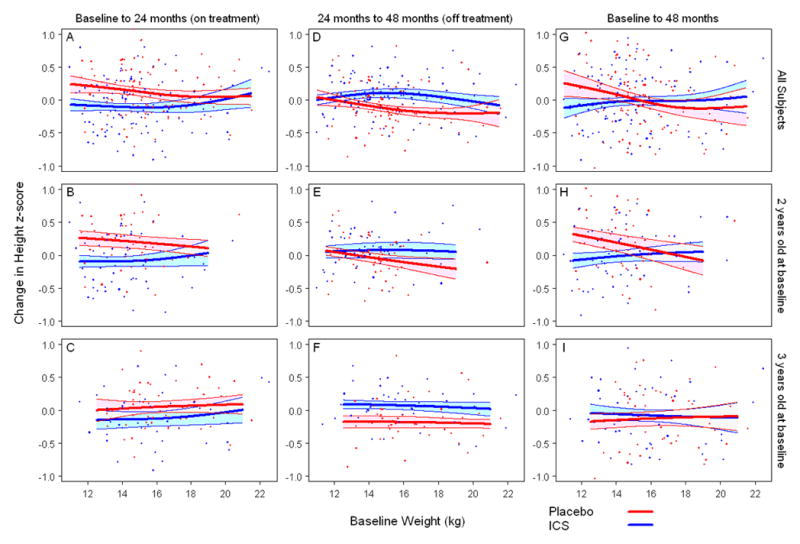
Scattergrams of growth from baseline (z-score) during treatment versus enrollment weight by treatment group. Top panels: overall cohort, middle panels: subjects 2 years old at enrollment, bottom panels: subjects 3 years old at enrollment. Children at particular risk for less linear growth during treatment were those that had an enrollment weight of <17 kg versus those that had a lesser exposure compared to their respective placebo counterparts (Panel A). This was more pronounced in the children who were two years of age at enrollment (Panel B). After the ICS treatment ended, these younger children of lesser weight (relative to the cohort) did not demonstrate the same catch-up in linear growth demonstrated by the younger, heavier children (Panel E) or older children of any weight (Panel F). Two years after treatment discontinuation, less linear growth was observed in only the younger children of lesser weight (Panel H). Solid lines (blue:ICS, red:Placebo) represent fitted values from non-parametric smoothing spline with 95% confidence bands (24). All data values were used for model fitting, but estimated regression lines are truncated to avoid extrapolation beyond the main part of the data.
Table 1.
Change in Height (cm) from Baseline to 24 and 48 months*
| Stratifying Variable | Strata | N | Change in Height from Baseline to 24 months | Change in Height from Baseline to 48 months | ||||
|---|---|---|---|---|---|---|---|---|
| ICS mean (95% CI) | Placebo mean (95% CI) | ICS vs Placebo (95% CI) | ICS mean (95% CI) | Placebo mean (95% CI) | ICS vs Placebo (95% CI) | |||
| Overall Cohort | 204 | 12.6 (12.2, 13.0) | 13.5 (13.2, 13.9) | −0.9 (−1.4, −0.4) ‡ | 25.7 (25.0, 26.3) | 25.9 (25.3, 26.5) | −0.2 (−1.1, 0.6) | |
| Age | 2 yrs | 105 | 13.4 (12.8, 13.9) | 14.4 (13.9, 15.0) | −1.1 (−1.8, −0.3) ‡ | 26.6 (25.8, 27.4) | 27.5 (26.7, 28.3) | −0.8 (−1.9, 0.3) |
| 3 yrs | 99 | 11.9 (11.3, 12.4) | 12.7 (12.2, 13.1) | −0.8 (−1.5, −0.1) + | 24.7 (23.8, 25.7) | 24.4 (23.5, 25.2) | 0.4 (−0.9, 1.6) | |
| Baseline Weight | < 15 kg | 104 | 12.3 (11.8, 12.8) | 13.2 (12.7, 13.7) | −1.0 (−1.7, −0.3) ‡ | 24.9 (24.2, 25.7) | 25.4 (24.6, 26.3) | −0.5 (−1.6, 0.6) |
| ≥ 15 kg | 100 | 13.0 (12.3, 13.6) | 13.8 (13.4, 14.3) | −0.9 (−1.6, −0.1) + | 26.4 (25.4, 27.4) | 26.4 (25.6, 27.2) | 0.0 (−1.1, 1.2) | |
| Gender | Female | 124 | 12.7 (12.1, 13.3) | 13.6 (13.1, 14.1) | −0.9 (−1.7, −0.1) + | 25.9 (24.9, 27.0) | 26.1 (25.1, 27.0) | −0.1 (−1.5, 1.2) |
| Male | 80 | 12.5 (12.0, 13.0) | 13.4 (13.0, 13.9) | −0.9 (−1.6, −0.2) ‡ | 25.4 (24.7, 26.1) | 25.8 (25.1, 26.4) | −0.3 (−1.3, 0.6) | |
| Race | Caucasian | 118 | 12.3 (11.7, 12.8) | 13.3 (12.9, 13.7) | −1.0 (−1.7, −0.4) ‡ | 25.7 (24.8, 26.5) | 26.0 (25.2, 26.7) | −0.3 (−1.3, 0.8) |
| non-Caucasian | 86 | 13.0 (12.4, 13.5) | 13.7 (13.2, 14.3) | −0.8 (−1.6, 0.0) | 25.7 (24.8, 26.6) | 25.9 (25.0, 26.8) | −0.2 (−1.4, 1.1) | |
| Age 2 yrs: Baseline Weight | < 15 kg | 70 | 12.9 (12.4, 13.5) | 14.2 (13.6, 14.8) | −1.3 (−2.1, −0.5) ‡ | 25.7 (25.0, 26.5) | 27.3 (26.4, 28.3) | −1.6 (−2.8, −0.4) ‡ |
| ≥ 15 kg | 35 | 13.8 (12.8, 14.8) | 14.6 (13.8, 15.4) | −0.8 (−2.1, 0.4) | 27.5 (26.2, 28.9) | 27.6 (26.4, 28.8) | −0.1 (−1.9, 1.7) | |
| Age 3 yrs: Baseline Weight | < 15 kg | 34 | 11.6 (10.8, 12.5) | 12.3 (11.5, 13.0) | −0.6 (−1.7, 0.5) | 24.1 (22.9, 25.4) | 23.5 (22.2, 24.9) | 0.6 (−1.3, 2.4) |
| ≥ 15 kg | 65 | 12.1 (11.3, 12.9) | 13.0 (12.6, 13.5) | −0.9 (−1.8, 0.0) + | 25.3 (24.0, 26.6) | 25.2 (24.2, 26.1) | 0.1 (−1.4, 1.7) | |
The linear mixed-effects regression model was adjusted for baseline covariates including clinical center, gender, race, history of eczema, during of percent blood eosinophils, aeroallergen skin test positivity, age, weight and height.
Difference between ICS and Placebo significant at p<0.05
Difference between ICS and Placebo significant at p<0.01
Table 2.
Change in Height z-score from Baseline to 24 and 48 months
| Stratifying Variable | Strata | N | Change in Height z-score from Baseline to 24 months | Change in Height z-score from Baseline to 48 months | ||||
|---|---|---|---|---|---|---|---|---|
| ICS mean (95% CI) | Placebo mean (95% CI) | ICS vs Placebo (95% CI) | ICS mean (95% CI) | Placebo mean (95% CI) | ICS vs Placebo (95% CI) | |||
| Overall Cohort | 204 | −0.10 (−0.19, −0.01) | 0.11 (0.03, 0.18) | −0.20 (−0.31, −0.09) ‡ | −0.08 (−0.20, 0.03) | −0.04 (−0.15, 0.06) | −0.04 (−0.18, 0.10) | |
| Age | 2 yrs | 105 | −0.07 (−0.20, 0.06) | 0.19 (0.07, 0.31) | −0.26 (−0.43, −0.09) ‡ | −0.05 (−0.20, 0.10) | 0.09 (−0.06, 0.24) | −0.14 (−0.34, 0.05) |
| 3 yrs | 99 | −0.13 (−0.24, −0.01) | 0.02 (−0.06, 0.11) | −0.15 (−0.28, −0.01) + | −0.11 (−0.26, 0.04) | −0.18 (−0.31, −0.05) | 0.07 (−0.12, 0.26) | |
| Baseline Weight | < 15 kg | 104 | −0.10 (−0.20, 0.00) | 0.11 (0.01, 0.21) | −0.20 (−0.34, −0.07) ‡ | −0.06 (−0.18, 0.07) | 0.03 (−0.10, 0.15) | −0.08 (−0.26, 0.09) |
| ≥ 15 kg | 100 | −0.10 (−0.24, 0.04) | 0.10 (0.00, 0.21) | −0.20 (−0.37, −0.03) + | −0.11 (−0.28, 0.06) | −0.11 (−0.26, 0.03) | 0.01 (−0.20, 0.21) | |
| Gender | Female | 124 | −0.10 (−0.23, 0.03) | 0.11 (0.01, 0.21) | −0.21 (−0.37, −0.05) + | −0.13 (−0.30, 0.05) | −0.13 (−0.27, 0.02) | 0.00 (−0.22, 0.21) |
| Male | 80 | −0.09 (−0.20, 0.02) | 0.10 (0.01, 0.20) | −0.20 (−0.34, −0.05) ‡ | −0.03 (−0.15, 0.09) | 0.04 (−0.08, 0.16) | −0.07 (−0.23, 0.09) | |
| Race | Caucasian | 118 | −0.17 (−0.28, −0.06) | 0.04 (−0.05, 0.13) | −0.21 (−0.34, −0.07) ‡ | −0.08 (−0.22, 0.06) | −0.07 (−0.19, 0.05) | −0.01 (−0.17, 0.15) |
| non-Caucasian | 86 | −0.03 (−0.16, 0.10) | 0.17 (0.06, 0.28) | −0.20 (−0.37, −0.03) + | −0.08 (−0.24, 0.07) | −0.02 (−0.18, 0.14) | −0.07 (−0.28, 0.15) | |
| Age 2 yrs: Baseline Weight | < 15 kg | 70 | −0.13 (−0.25, 0.00) | 0.21 (0.07, 0.34) | −0.33 (−0.51, −0.16) ‡ | −0.08 (−0.23, 0.06) | 0.21 (0.05, 0.37) | −0.30 (−0.50, −0.09) ‡ |
| ≥ 15 kg | 35 | −0.01 (−0.24, 0.21) | 0.17 (−0.02, 0.35) | −0.18 (−0.47, 0.10) | −0.02 (−0.27, 0.23) | −0.03 (−0.27, 0.20) | 0.01 (−0.31, 0.34) | |
| Age 3 yrs: Baseline Weight | < 15 kg | 34 | −0.07 (−0.22, 0.08) | 0.01 (−0.14, 0.15) | −0.07 (−0.28, 0.13) | −0.03 (−0.22, 0.17) | −0.16 (−0.35, 0.04) | 0.13 (−0.14, 0.41) |
| ≥ 15 kg | 65 | −0.18 (−0.34, −0.03) | 0.04 (−0.06, 0.13) | −0.22 (−0.40, −0.04) + | −0.19 (−0.39, 0.00) | −0.19 (−0.35, −0.03) | 0.00 (−0.24, 0.24) | |
The linear mixed-effects regression model was adjusted for baseline covariates including clinical center, gender, race, history of eczema, during of percent blood eosinophils, aeroallergen skin test positivity, age, weight and height.
Difference between ICS and Placebo significant at p<0.05
Difference between ICS and Placebo significant at p<0.01
The height and weight at enrollment were not significantly different between treatment groups at enrollment in the 204 children that completed the four year study. Similar to that previously reported, the fluticasone-group that completed the study had a significantly lower increase in height, adjusted for baseline covariates and for open-label ICS and oral corticosteroid use during both the treatment and follow-up periods, during treatment than the placebo group (ΔHt of 0.9 cm, p=0.0007)(3). Two years after study treatment discontinuation, the mean increase in height attained from baseline in the fluticasone group was not significantly different from the placebo-group (ΔHt of 0.2 cm, p=0.6). The fluticasone group grew significantly faster than the placebo group after treatment discontinuation (6.5 vs. 6.2 cm/year, p=0.02). The weight gain was not significantly different between treatment groups during treatment or after treatment discontinuation.
Subgroup Analyses
When examined in a post-hoc analysis, patterns of linear height growth, but not weight, were different in certain subgroups of children (detailed below).
Subgroup Analysis by Weight at Enrollment (Figure 1 Panels A and E)
Using a linear mixed-effects regression model using data from all study visits, children weighing less than 15 kg at enrollment demonstrated significantly less linear growth during fluticasone treatment compared to placebo (ΔHt of 1 cm, p=0.007), but linear growth was not significantly different two years after treatment discontinuation (ΔHt of 0.5 cm, p=0.38). A similar pattern was seen in those who weighed at least 15 kg.
Subgroup Analysis by Age at Enrollment (Figure 1 Panels B and F; Tables 1 & 2)
Using a linear mixed-effects regression model using data from all study visits, children aged two years at enrollment demonstrated significantly less linear growth during fluticasone treatment compared to placebo (ΔHt of 1.1 cm, p=0.006), but linear growth was not significantly different two years after treatment discontinuation (ΔHt of 0.8 cm, p=0.13). A similar effect was seen in those that were three years of age.
Subgroup Analyses by Age and Weight at Enrollment
Children at particular risk for less linear growth during treatment were those that were two years of age and <15–17 kg at enrollment (Figure 2, Panel B). After the ICS treatment ended, these younger children of lesser weight (relative to the cohort) did not demonstrate the same catch-up in linear growth demonstrated by the younger, heavier (relative to the cohort) children (Figure 2, Panel E) or older children of any weight (Figure 2, Panel F). Two years after treatment discontinuation ended, less linear growth was observed in only the younger children of lesser weight (Figure 2, Panel H).
When weight was dichotomized at 15 kg, children who were two years of age and <15 kg at enrollment demonstrated significantly less linear growth during fluticasone treatment compared to placebo ((ΔHt of 1.3 cm, p=0.002) (Figure 1, Panels C and G, Table 1 and 2). Two years after treatment discontinuation, these children still had significantly less linear growth from baseline ((ΔHt of 1.6 cm, p=0.008) (Figure 1, Panels C and G, Table 1 and 2). In contrast, linear growth was not significantly different after treatment discontinuation in the younger children of greater weight (relative to the cohort) (ΔHt of 0.1 cm, p=0.94) (Figure 1, Panels C and G, Table 1 and 2) or in the older children of any weight (<15 kg ΔHt of 0.6 cm, p=0.53; ≥15 kg ΔHt of 0.1 cm, p=0.85) (Figure 1, Panels D and H, Table 1 and 2). In the younger children of lesser weight (relative to the cohort), 31% received ICS, 51% received oral corticosteroids for respiratory symptoms, and 59% reported asthma symptoms or an asthma exacerbation in the past 12 months during the two-year period after treatment discontinuation. These are similar to the younger children of greater weight and the older children of any weight (Electronic Table 2).
Subgroup Analysis by Other Baseline Characteristics
No significant differences in linear growth were seen during treatment or two years after treatment discontinuation for the following baseline characteristics: gender, race, exacerbations in the past year on ICS treatment, allergic sensitization, eczema, or eosinophilia (Tables 1 & 2 with some data not shown). No significant interactions on linear growth were seen during fluticasone treatment or two years after treatment discontinuation by age and the aforementioned baseline characteristics (data not shown).
DISCUSSION
The present growth analyses in the PEAK preschool aged cohort with recurrent wheezing at high risk of developing asthma demonstrated that two years of treatment with fluticasone (176 mcg/day) is associated with less linear growth that dissipates over time after treatment discontinuation when the cohort as a whole is evaluated. The ICS treated group, as a whole, demonstrated catch-up linear growth during the two years after treatment discontinuation. However, post-hoc analysis identified a subgroup of younger children of lesser weight (two years of age and <17 kg at enrollment) that did not demonstrate catch-up linear growth. We confirm the findings of Murray et al.(9), who showed that ICS compared to placebo had a greater effect on linear growth in younger (age 2 years) versus older children (age 3 years). Similar to the PEAK study, school-aged children with persistent asthma treated with budesonide (400 mcg/day) for an average of 4.3 years in the Childhood Asthma Management Program (CAMP) Study had gained 1.1 cm less in height at the end of the study period compared to the placebo group(2). The difference in mean height in the budesonide group relative to the placebo group at the end of the trial diminished but remained statistically significant (0.9 cm; P = 0.01) after an additional 4.8 years (10) after the study medication was stopped. Participants in all CAMP treatment groups used inhaled corticosteroids during 30% of the post-trial period similar to the 40% observed in the PEAK study. In this study, the ICS treated groups had less linear growth than the placebo treated group; however, the effect diminished with time even while these children continued to receive ICS treatment and even more so once ICS treatment was discontinued. The PEAK study identified a group of younger children of lesser weight (relative to the cohort) for whom the growth suppressive effects of ICS were pronounced and this effect did not diminish with time or after the ICS treatment was discontinued. This is in contrast to the effects observed in the younger, heavier children (relative to the cohort) or the older children of any weight after adjusting for open-label ICS and oral corticosteroid use during both the treatment and follow-up periods.
The effect of ICS exposure on linear growth may be more profound in younger children of lesser weight (two years of age and <17 kg at enrollment) who may be predisposed to growth rates in the low-mid end of the normal range in early childhood(11). In addition, the weight gain was not significantly different between treatment groups at baseline, during treatment, or after treatment was discontinued. Indeed, these children with an enrollment weight of < 17 kg remained lighter relative to their peers at the end of the study. The younger children of lesser weight likely received a higher relative exposure (mcg/kg) of fluticasone than the older children. However, this does not appear to be solely a weight effect as the older children of lesser weight (three years of age and <17 kg at enrollment) did not show the same growth effect and demonstrated significant catch-up growth once the ICS treatment was discontinued. Thus, the inhaled corticosteroid exposure may have occurred during a key period of rapid growth in these children who were two years of age and <17 kg at enrollment and they were unable to accelerate their growth rate to the degree needed to catch up to their peers. Furthermore, no consistent, greater clinical benefit with respect to episode-free days or oral corticosteroid courses were seen in the younger children of lesser weight with a higher fluticasone exposure compared to those with a lower exposure (data not shown), and a previous analysis of this cohort demonstrated that two year old children did not have more favorable responses to fluticasone than placebo compared to three year olds(12). Thus, a higher relative ICS exposure resulted in an increased risk of less linear growth in the younger children of lesser weight without a correspondingly greater clinical benefit in symptom control or exacerbations.
In studies of this age group of children with recurrent wheezing(9, 13) and in other studies of older children with persistent wheezing(2, 14), the inhibitory effect of inhaled corticosteroids on growth appears “front-loaded”, or occurs during the first year of ICS treatment and the effect diminishes over time. However, in this study, the younger, lesser weight children (relative to the cohort) had a different pattern where they did not demonstrate catch-up growth over time. Unlike the CAMP study in older children with persistent asthma, we did not find that girls in the PEAK study were more likely to have less linear growth(10). In addition, these observations differ from the normal growth velocities reported in a study of fluticasone treatment for one year(15) in pre-school children with recurrent wheezing. A larger volume spacer(15, 16), which may have led to a different ICS deposition pattern, less adherence, or the higher dropout rate in that study(15), may explain these differences in findings.
Based on the observed effects of ICS on growth in the younger children of lesser weight (relative to the cohort),, an estimate for a cut-off for per-kg fluticasone dose that would be expected to have the largest growth effect was determined. In this analysis, we saw a growth effect in the children two years of age and <17 kg at enrollment (Figure 2 and E-Figure 3) that persisted two years after treatment was discontinued. Based on the fluticasone CFC dose of 176 mcg/day used in PEAK, we estimate that 10 mcg/kg/day is the upper limit of fluticasone dose to use to avoid the potential effects of long-term daily ICS use on growth in children 2–3 years of age. This dose range is speculative and would need to be studied prospectively.
Several limitations should be noted. The subgroup analyses described here are post-hoc as the original study was not designed or powered to examine the linear growth effects of ICS on smaller subgroups of children with particular baseline characteristics. The results presented herein can serve to generate hypotheses for future studies. The PEAK study was not initially designed to follow these children for an additional year of observation; thus, only 72% of the population had growth outcomes measured two years after the ICS treatment was stopped. However, these observations did not change when children who were lost to follow-up by the second observation period were added to the analysis. These results also did not change when the analyses were adjusted for parent-reported adherence. It should be noted that the sample size is smaller for the age by weight analyses. Height measurements in very young children can vary in their accuracy; however, we used a similar technique of repeated stadiometry measurements similar to that adopted in the CAMP trials and endocrinology literature(2, 4, 6). It is also possible that the height difference in the 2 year olds in this group may resolve with time and be attributable to a younger cohort effect.
The PEAK study used a small volume valved spacer [AeroChamber™ (Monaghan Medical Corporation, Plattsburgh, NY) with mask] and a CFC-based fluticasone inhaler and we do not know how other types of spacer devices, other types of ICS, or HFA-based or dry powder inhalers would alter these findings. Furthermore, Z-score analyses were used similar to other growth studies(17, 18) to standardize the study population to a larger, reference population(8). The average height percentile range of the younger children of lesser weight at enrollment using this reference population was 43.2%. Our placebo population did not perfectly mirror the growth of this reference population, but children with recurrent wheezing may not demonstrate the same growth as a healthier population nor have the same racial or ethnic composition, stressing the importance of a placebo group. An additional finding that may limit the generalizability of these results is that 33% of the 2–3 year old children enrolled in the PEAK study cohort were > 75th percentile for weight compared to a reference population [CDC], which may reflect recent observations of increased obesity rates in older children with asthma(19–23).
ICS continue to be recommended as the most effective therapy for children with persistent asthma(24) based on studies clearly demonstrating significantly decreased disease impairment and risk(2, 3, 25). In preschool children at high risk for subsequent asthma, we have also demonstrated that ICS treatment significantly reduces disease burden(3). Previous studies have shown that, on average, the growth suppressive effects of ICS are small(2) and may improve with time(1), but subgroups with variations in this response are potentially unrecognized when averaging the population. This study has identified a group of children in which ICS therapy was associated with an increased risk of adverse growth effects without apparent increase in benefit. Therefore, in this subgroup, these effects need to be balanced with prospective regular assessments of asthma control and possible appropriate ICS dose reductions, discontinuation of medication, use of alternative controller medications, or formulation adjustment to maximize the benefit risk ratios of inhaled corticosteroids.
Supplementary Material
Acknowledgments
Supported by: Grants # HL071742, HL004519-04, 5U10HL064287, 5U10HL064288, 5U10HL064295, 5U10HL064307, 5U10HL064305, 5U10HL064313 from the National Heart, Lung, and Blood Institute. This study was carried out in part in the General Clinical Research Centers at Washington University School of Medicine (M01 RR00036) and Colorado CTSA grant 1 UL1 RR025780 from the National Institutes of Health (NIH) and National Center for Research Resources (NCRR).
Abbreviations
- CARE
Childhood Asthma Research and Education Network
- CAMP
Childhood Asthma Management Program
- ICS
Inhaled corticosteroid
- IgE
Immunoglobulin E
- NHLBI
National Heart Lung and Blood Institute
- PEAK
Prevention of Early Asthma in Kids
Biographies
Theresa W. Guilbert, MD
Dr. Guilbert has been a consultant for the following pharmaceutical companies: AstraZeneca, GlaxoSmithKline, Genentech/Novartis, Merck/Schering Plough, MAP Pharmaceuticals, and Peerpoint Medical Education Institute. Her department has received research funding support from Altus Pharmaceuticals, Inspire Pharmaceuticals, and NHLBI.
David T. Mauger, PhD
Dr. Mauger has declared no relationships that are potential or real conflicts with pharmaceutical interests. His department has received research funding support from NHLBI.
David B. Allen, MD
Dr. Allen has declared no relationships that are potential or real conflicts with pharmaceutical interests.
Robert S. Zeiger, MD, PhD
Dr. Zeiger has been a consultant for the following pharmaceutical companies: Aerocrine, AstraZeneca, GlaxoSmithKline, Genentech, MedImmune, Schering Plough, and Novartis. His department has received research funding support from Aerocrine, AstraZeneca, GlaxoSmithKline, Genentech, NHLBI, and MERCK and Co.
Robert F. Lemanske, Jr., MD
Dr. Lemanske has been a consultant for the following pharmaceutical companies: AstraZeneca, MAP Pharmaceuticals, Merck Childhood Asthma Network and Novartis. He has been a speaker for AstraZeneca and Merck. His department has received research funding support from NHLBI.
Stanley J. Szefler, MD, PhD
Dr. Szefler has been a consultant for the following pharmaceutical companies: GlaxoSmithKline, Genentech, Merck, Boehringer-Ingelheim, Schering Plough, and Novartis. His department has received research funding support from GlaxoSmithKline, NIEHS/EPA, NIAID, and NHLBI.
Robert C. Strunk, MD
Dr. Strunk has declared no relationships that are potential or real conflicts with pharmaceutical interests. His department has received research funding support from NHLBI.
Leonard B. Bacharier, MD
Dr. Bacharier has been a consultant for the following pharmaceutical companies: Aerocrine, AstraZeneca, GlaxoSmithKline, Genentech, Merck, and Schering Plough. His department has received research funding support from NHLBI.
Ronina Covar, MD
Dr. Covar has declared no relationships that are potential or real conflicts with pharmaceutical interests. Her department has received research funding support from NHLBI.
Christine A. Sorkness, PharmD
Dr. Sorkness has been an Advisory Board member for GSK, Schering Plough, Astra Zeneca, Novartis (each less that $10,000 annually). Her departmental has received research funding from NHLBI, Schering Plough, and Compleware/Sandoz
Lynn M. Taussig, MD
Dr. Taussig has declared no relationships that are potential or real conflicts with pharmaceutical interests. His department has received research funding support from NHLBI.
Fernando D. Martinez, MD
Dr. Martinez has been a consultant for the following pharmaceutical companies: GlaxoSmithKline, MedImmune, and Merck. He has received lecture fees from Merck and Pfizer. His department has received research funding support from NHLBI.
Footnotes
Clinical Implications: The potential for reduced linear growth among children on inhaled corticosteroids who are two years of age and <17 kg should be balanced with level of asthma control to maximize the benefit-risk ratio of treatment.
Clinical Trials Registry: ClinicalTrials.gov number, NCT00272441.
This study was part of a large, multi-center clinical trial conducted through the NHLBI-sponsored Childhood Asthma Research and Education (CARE) Network. NHLBI funded this study. The role of commercial sponsors was limited to donating drug and matched placebo that they did after reviewing the drafted protocol. The text of the manuscript was made available to all the commercial sponsors 2 weeks prior to finalization for comments.
All the authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis. All authors have seen and approved the submission of this version of the manuscript and take full responsbility for the manuscript. The declaration of all sources of funding for each author are listed below. Potential conflicts of interest are also detailed on the COI forms which will be faxed to the JACI office at the time of manuscript submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med. 2000 Oct 12;343(15):1064–9. doi: 10.1056/NEJM200010123431502. [DOI] [PubMed] [Google Scholar]
- 2.The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000 Oct 12;343(15):1054–63. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 3.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006 May 11;354(19):1985–97. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 4.Guilbert TW, Morgan WJ, Krawiec M, Lemanske RF, Jr, Sorkness C, Szefler SJ, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials. 2004 Jun;25(3):286–310. doi: 10.1016/j.cct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000 Oct;162(4 Pt 1):1403–6. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 6.Childhood Asthma Management Program. Childhood Asthma Management Program Somatic Growth Measures Manual, Version 1.0. Springfield, VA: National Technical Information Service; 1994. [Google Scholar]
- 7.Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004 Dec;114(6):1282–7. doi: 10.1016/j.jaci.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000 Jun;8(314):1–27. [PubMed] [Google Scholar]
- 9.Murray CS, Woodcock A, Langley SJ, Morris J, Custovic A. Secondary prevention of asthma by the use of Inhaled Fluticasone propionate in Wheezy INfants (IFWIN): double-blind, randomised, controlled study. Lancet. 2006 Aug 26;368(9537):754–62. doi: 10.1016/S0140-6736(06)69285-4. [DOI] [PubMed] [Google Scholar]
- 10.Strunk RC, Sternberg AL, Szefler SJ, Zeiger RS, Bender B, Tonascia J. Long-term budesonide or nedocromil treatment, once discontinued, does not alter the course of mild to moderate asthma in children and adolescents. J Pediatr. 2009 May;154(5):682–7. doi: 10.1016/j.jpeds.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen DB. Inhaled corticosteroid therapy for asthma in preschool children: growth issues. Pediatrics. 2002 Feb;109(2 Suppl):373–80. [PubMed] [Google Scholar]
- 12.Bacharier LB, Guilbert TW, Zeiger RS, Strunk RC, Morgan WJ, Lemanske RF, Jr, et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. J Allergy Clin Immunol. 2009 May;123(5):1077–82. 82, e1–5. doi: 10.1016/j.jaci.2008.12.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skoner DP, Szefler SJ, Welch M, Walton-Bowen K, Cruz-Rivera M, Smith JA. Longitudinal growth in infants and young children treated with budesonide inhalation suspension for persistent asthma. J Allergy Clin Immunol. 2000 Feb;105(2 Pt 1):259–68. doi: 10.1016/s0091-6749(00)90074-5. [DOI] [PubMed] [Google Scholar]
- 14.Simons FE. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study Group. N Engl J Med. 1997 Dec 4;337(23):1659–65. doi: 10.1056/NEJM199712043372304. [DOI] [PubMed] [Google Scholar]
- 15.Bisgaard H, Allen D, Milanowski J, Kalev I, Willits L, Davies P. Twelvemonth safety and efficacy of inhaled fluticasone propionate in children aged 1 to 3 years with recurrent wheezing. Pediatrics. 2004 Feb;113(2):e87–94. doi: 10.1542/peds.113.2.e87. [DOI] [PubMed] [Google Scholar]
- 16.Dolovich M. Aerosols. In: Barnes P, Grunstein MM, Leff AR, Woolcock AJ, editors. Asthma. Philadelphia, PA: Lippincott-Raven; 1997. pp. 1359–61. [Google Scholar]
- 17.van Dijk CE, Innis SM. Growth-curve standards and the assessment of early excess weight gain in infancy. Pediatrics. 2009 Jan;123(1):102–8. doi: 10.1542/peds.2007-3382. [DOI] [PubMed] [Google Scholar]
- 18.de Onis M, Garza C, Onyango AW, Borghi E. Comparison of the WHO child growth standards and the CDC 2000 growth charts. J Nutr. 2007 Jan;137(1):144–8. doi: 10.1093/jn/137.1.144. [DOI] [PubMed] [Google Scholar]
- 19.Ross KR, Hart MA, Storfer-Isser A, Kibler AM, Johnson NL, Rosen CL, et al. Obesity and obesity related co-morbidities in a referral population of children with asthma. Pediatric pulmonology. 2009 Sep;44(9):877–84. doi: 10.1002/ppul.21065. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G. Metabolic abnormalities in children with asthma. American journal of respiratory and critical care medicine. 2011 Feb 15;183(4):441–8. doi: 10.1164/rccm.201004-0603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu YT, Chen WY, Wang TN, Tseng HI, Wu JR, Ko YC. Extreme BMI predicts higher asthma prevalence and is associated with lung function impairment in school-aged children. Pediatric pulmonology. 2009 May;44(5):472–9. doi: 10.1002/ppul.21023. [DOI] [PubMed] [Google Scholar]
- 22.Scholtens S, Wijga AH, Seidell JC, Brunekreef B, de Jongste JC, Gehring U, et al. Overweight and changes in weight status during childhood in relation to asthma symptoms at 8 years of age. The Journal of allergy and clinical immunology. [Research Support, Non-U.S. Gov’t] 2009 Jun;123(6):1312–8. e2. doi: 10.1016/j.jaci.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 23.Jeong Y, Jung-Choi K, Lee JH, Lee HY, Park EA, Kim YJ, et al. Body weight at birth and at age three and respiratory illness in preschool children. Journal of preventive medicine and public health = Yebang Uihakhoe chi. [Comparative Study] 2010 Sep;43(5):369–76. doi: 10.3961/jpmph.2010.43.5.369. [DOI] [PubMed] [Google Scholar]
- 24.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007 Nov;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 25.Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007 Jan;119(1):64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.