Abstract
Cell death under physiologic conditions does not lead to the induction of immunity. However recognition of stressed or opsonized cells can trigger immune responses. Recent studies have begun to illustrate the critical role of molecular chaperones such as inducible heat shock proteins in mediating immunogenicity of stressed cells. Immunity to opsonized cells depends in part on the engagement and the balance of activating and inhibitory FcγRs on antigen presenting dendritic cells. Understanding both these pathways of immunogenic cell death may yield novel approaches to regulate immunity.
Two forms of immunogenic cell death
Billions of cells die each day and are replaced by newly differentiated progeny. Corpses of such dying cells are rapidly removed by phagocytes; do not elicit immunity and may induce tolerance. However under some settings, recognition of dying cells leads to inflammatory responses and immunity[1]•. Two aspects of such “immunogenic death” are the focus of this review. A common response to cellular stress is the transcriptional activation of molecular chaperones that belong to the class of inducible heat shock proteins (HSPs). Another setting wherein dying cells might induce immunity is when they are opsonized by antibodies due to antibody mediated recognition of determinants on dying cells. Such opsonized cells can be recognized by FcγR mediated pathways on antigen presenting cells (APCs). Below, we will discuss recent insights into how both of these pathways play an important role in regulating immunogenicity of dying cells and can be dysregulated in autoimmunity.
Heat shock proteins (HSPs) and immunogenic cell death
HSPs are referred to as stress proteins or molecular chaperones, although most HSPs are constitutively expressed. Known best for their roles in regulating intracellular protein homeostasis, HSPs were immunologically implicated because of the observations that tumor-derived HSPs, although having no tumor-specific mutations, can immunize for tumor-specific T cell immunity[2]•. Efforts to explain the phenomenon have led to two hypotheses[2]: HSPs chaperone peptides for presentation to MHC molecules, and HSPs activate professional APCs. Studies have shown that most of HSPs are indeed able to chaperone peptides and HSP-peptide complexes are critical for cross-priming of peptide-specific CD8+ T cells[3]••. The interaction between HSPs and APCs is thought to depend on receptors on APCs. Several candidate receptors have been suggested, including CD91, SR-A (scavenger receptor A), TLR2/4, CD40 and Lox1[4]. However the nature of specific HSP receptors remains to be completely resolved.
gp96 is a prototype HSP, whose immunological properties have received the most attention. In addition to the biochemical evidence, roles of gp96 in immune response are also supported genetically. Tumor cells engineered to express gp96 on cell surfaces [5] or secrete gp96[6] are able to activate dendritic cells (DCs) and prime CD8+ T cells. Transgenic mice with ubiquitous expression of membrane-bound gp96 suffered from lupus-like autoimmune diseases spontaneously, which was dependent on MyD88[7]. This line of research suggests that extracellular gp96 might be able to engage innate receptors to cause inflammation. An important recent insight into the biology of gp96 is its ability to serve as a master chaperone for toll like receptors, and regulate their function[8••,9]. Surface translocation of HSPs might therefore result in the alteration of cellular responsiveness to environmental stimuli. For example, surface translocation of gp96 leads to hyperresponsiveness to endotoxin, due to the TLR4 chaperoning function of gp96 [8,10]. Pro-inflammatory properties of extracellular HSPs have also been observed with HSP60, HSP70, GRP170, HSP90 and calreticulin [11]. Collectively, the intrinsic immunological properties of extracellular HSPs indicate that exposure of HSPs, as a result of cell death or stress, is likely to have immunological consequences.
Regulation of HSP export
The presence of HSP receptors on the surface of DCs strongly suggests that HSPs, being exclusively intracellular molecules under normal conditions, may gain access to the extracellular milieu to elicit their proinflammatory properties. gp96 possesses an endoplasmic reticulum (ER) retention signal lys-asp-glu-leu (KDEL) at the carboxyl terminus [12] and resides mostly in the lumen of the ER. Cell surface expression of gp96 in several studies has been shown to depend on the active ER to Golgi transport. The cell biological basis for the plasticity of the cellular transport of gp96 remains unknown. It was recently shown that gp96 associates tightly with p43, a versatile protein known for its ability to associate with multiprotein complexes (such as tRNA synthetase) [13]. The interaction between gp96 and p43 leads to retention of gp96 inside cells, as overexpression of p43 significantly reduced the cell surface gp96. Cell surface gp96 is also significantly increased in many cell types in p43-/- mice. Therefore, one of the functions of p43 may be to “hold” gp96 intracellularly to prevent unnecessary DC activation and detrimental immune responses, due to gp96-DC interactions. The mechanism of the export of other HSPs is less clear. There is a membrane-bound variant of HSP90 whose regulation is yet to be worked out [14]. A recent study implicated sphingolipid Gb3 in membrane localization of HSP70 in tumor cells[15]. Understanding the pathways or molecules that regulate the cellular localization of HSPs may have major implications for immune activation.
Exposure of HSPs during cell death
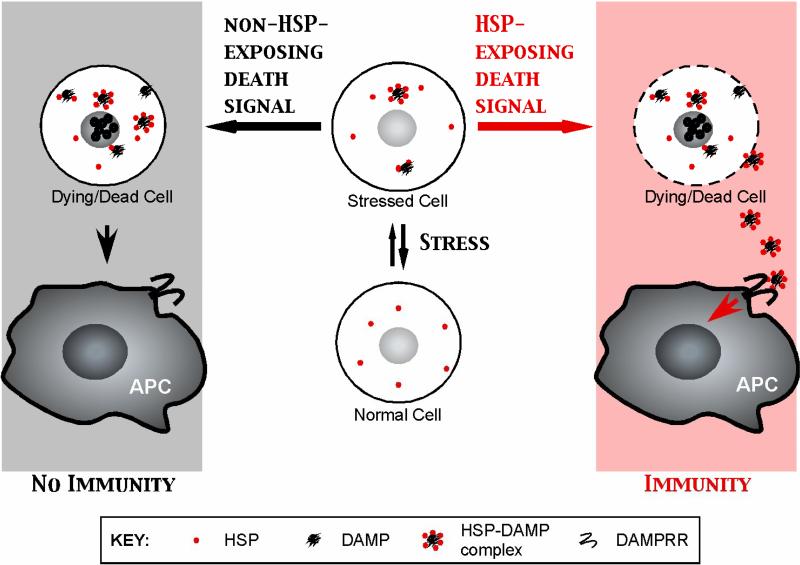
Initial studies focused on the differences in HSP content between cells undergoing apoptotic or necrotic cell death, and subsequent activation of phagocytic DCs [2]. However the distinction between apoptosis and necrosis as immunogenic or nonimmunogenic forms of cell death respectively, may be too simplistic. Recent studies have shown that apoptotic death of tumor cells by certain agents (such as anthracyclines, proteasome inhibitors, or viral infection) can be immunogenic[16-19], while necrotic cell death may impair tumor immunity in vivo[20]. Moreover destroying the anthracycline induced apoptotic bodies by freeze thaw abolished their immunogenicity[18]. Recently, Obeid et al. identified the exposure of calreticulin (CRT) as a critical marker of immunogenicity of tumor apoptosis induced by anthracyclines and γ-irradiation [18••,21]. In these experiments, the immunogenicity of CRT appears to be linked to its capacity as an “eat me” signal, rather than as a chaperone for tumor derived peptides. The mechanism behind CRT exposure is not known but may involve phosphorylation of eukaryotic initiation factor 2α (eIF2α), as a result of unfolded protein response[18]. Another example of immunogenicity of HSP exposure came from in vitro studies of human myeloma. Bortezomib (a proteasome inhibitor) induces the exposure of HSP90 on the surface of dying human myeloma tumor cells [19]••. Recognition of such tumor cells by human monocyte derived DCs leads to efficient generation of anti-tumor T cells. Collectively, we propose that immunity and tolerance from cell death are dictated mechanistically not by necrosis and apoptosis, but rather by whether HSPs are exposed or not (Figure 1). Further studies are needed to better understand the mechanism of HSP exposure on dying cells and which additional signals are needed to mediate immunity to dying cells by phagocytic DCs[22]. In addition to activation of APCs, extracellular HSPs have also been implicated in activation of NK cells[23].
Figure 1. Exposure of HSPs dictates the immunological fate of dying cells.
Intracellular HSPs patrol cells for misfolded proteins that contain DAMPs and are responsible for their refolding. After encountering HSP-exposing death signals, HSP-DAMP complexes engage DAMP recognition receptors (DAMPRR) on APCs to elicit immune response. Cells dying under conditions of no HSP exposure are rapidly cleared by APCs to achieve tissue homeostasis and do not lead to immunity, unless dying cells are presented in other proinflammatory contexts such as opsonization or viral infection (not depicted).
HSPs as carriers for damage-associate molecular patterns: a hypothesis
It remains unclear if extracellular HSPs possess unique structural moieties for recognition by innate receptors. The presence of HSPs predates the emergence of immune system in evolution. As stress proteins whose functions are primarily in accelerating protein folding and preventing protein misfolding, HSPs in the unicellular organisms can be considered as a primitive form of defense. As discussed earlier, in the steady state, HSPs in multicellular organisms are primarily confined intracellularly. In the situation of stress, they are induced and exposed to external milieu. Thus if there is a molecular definition of cell stress or danger, HSPs may fulfill such a role due to their inducibility by stress, their ability to interact with and to alarm immune cells, and their ubiquity to signal stress of all cells and all tissues. Just like microbes whose presence can be sensed by the pattern recognition receptors (PRR) on host cells, it has been argued that the pattern of stress or damage can be found in the extracellular HSPs. Since HSPs prefer to interact with hydrophobic stretches of proteins and have been found to be in complex with misfolded proteins, we argue that HSPs are the carriers of damage-associated molecular patterns (DAMP) [24] ••. We suggest that the fundamental stimuli in the immunogenic cell death are not HSPs or DAMPs, but the HSP-DAMP complex (Figure 1). We term the receptors for such a complex as DAMPRR, or DAMP recognition receptor. This hypothesis can potentially explain many conflicting data in the field and current opposing views of HSPs in innate immunity. For example, tissue-derived HSPs (containing stress peptides) are clearly immunostimulatory [2], whereas recombinant HSPs are generally not [25]. It is notable that HSPs may in addition, provide additional signals such as “eat me” signals that promote immunogenicity[18]. Overall, the “context” in which HSPs are released or prepared may have profound impact on the immunogenicity of HSPs. HSPs from stressed cells are more immunogenic than these isolated from un-stressed cells, which may explain why inducible HSPs are more immunogenic than HSPs expressed constitutively [26,27]. Improved understanding of interactions between HSPs and DAMPs may provide novel insights into autoimmunity and improving vaccines.
FcR-mediated recognition of dying cells
FcγR as a balance of activating and inhibitory receptors
Recognition of cells opsonized with antibodies is mediated in part by Fcγ receptors (FcγRs). The FcγR system represents a balance of activating and inhibitory receptors that determines the outcome of immune complex-mediated inflammation and immunity. In human cells, the major activating receptors are FcγRI, IIa and III, while the sole inhibitory receptor is FcγRIIB. Activating receptors are characterized by an immune tyrosine activating motif (ITAM) in their cytoplasmic domain. Signaling via the activating receptors generally leads to the recruitment of signaling adaptor molecules (e.g. syk) to these receptors and downstream activation of PI3 kinase and other pathways[28] •. The net result is generally cellular activation, although it is now apparent that in some instances, some ITAM containing receptors can also mediate inhibitory signals[29]. Inhibitory Fcγ receptors contain an immune tyrosine inhibitory motif (ITIM) and inhibit signaling through the activating receptors by recruitment of phosphatases including inositol phosphatase SHIP and the tyrosine phosphatase SHP-1[28]. Most FcγR bearing cells express both activating and inhibitory receptors. Therefore, the relative engagement of activating versus inhibitory FcγRs represents a balance that determines the net cellular effects of these signals.
Role of FcγR balance in adaptive immunity
Dendritic cells (DCs) play a critical role in the induction of immunity and tolerance. Recognition, uptake and presentation of dying cells by DCs plays a critical role in immunologic consequences of cell death[30]. Uptake of opsonized cells and immune complexes depends on both Fc receptors and complement system. Several aspects of the interaction between dying cells and DCs or macrophages is altered by FcR mediated uptake. Opsonized cells taken up via FcγRs undergo reduced processing in the phagosomes, a process termed as phagosome maturation[31]. Altered degradation of antigens in DCs may facilitate both the induction of antibody responses[32] as well as T cell immunity[33]. Antigen uptake in the form of immune complexes or opsonized cells is associated with enhanced antigen presentation by DCs and the generation of antigen specific T cells[34-37]. This pathway may also be important for immunity to opsonized pathogens[38]. FcγR mediated internalization enhances not only MHC II restricted antigen presentation, but also presentation on MHC I molecules (termed cross presentation), thereby activating both CD4 and CD8+ T cell responses[35].
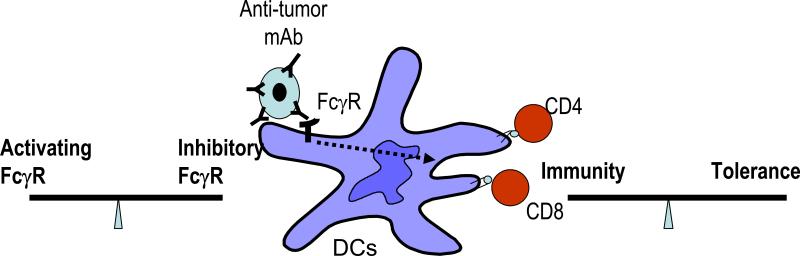
DCs express both activating and inhibitory Fcγ receptors, and the balance of these receptors may have a major effect on DC function[39-41••] (Figure 2). Selective blockade of the inhibitory Fcγ receptor, FcγRIIB leads to maturation of human DCs and presentation of antigens derived from phagocytosed tumor cells and immune complexes[40,42,43]. The balance of activating versus inhibitory FcγRs may also be altered by cytokines, thereby regulating FcγR mediated cross presentation[44]. In mice genetically lacking inhibitory FcγRIIB, targeting immune complexes to DCs can lead to enhanced generation of antigen specific CD8+ T cell immunity in vitro and in vivo[45,46]. Likewise, genetic deletion of FcγRIIB leads to spontaneous autoimmunity in genetically prone mice[47]. The importance of FcγRs on DCs has been further supported by recent studies showing that targeting antigen to activating FcγRs on DCs helps break tolerance in mice, while the ligation of inhibitory FcγR promotes tolerance[48,49] ••. The ability of IC to enhance antigen presentation depends on the recruitment of syk to the activating FcRs, as well as activation of PI3 kinase pathway[50,51]. FcγRs mediated activation leads to a distinct form of DC activation, including the induction of a type I interferon response program and proinflammatory cytokines[39,52]. Polymorphisms that impact the balance of activating versus inhibitory FcγRs may therefore have implications for both autoimmunity and resistance to pathogens[53]. In addition to FcγRs, the complement proteins also interact with immune complexes and opsonized cells. A recent study described a novel role for complement factor C1q in augmenting presentation of antigens from immune complexes[54]. In addition to ICs, targeting of pathogens to activating FcγRs via C-reactive protein also leads to enhanced immunity[55].
Figure 2. Balance of activating versus inhibitory FcγRs regulates immunity versus tolerance.
Uptake of opsonized dying cells is mediated in part by FcγRs expressed on DCs. The balance of activating versus inhibitory FcγRs determines the activation status of DCs and resultant T cell activation. Alteration of this balance may lead to inadvertent immune activation.
Summary
Herein, we have discussed two distinct pathways (HSPs and FcγR) that regulate the immunogenicity of dying cells. Dysregulation of these pathways, for example, by exposure of HSPs on dying cells and altered balance of activating versus inhibitory FcγRs may have implications for the development of autoimmunity. These pathways may also be useful targets for enhancing immunity to tumors and pathogens in the clinic. Targeting HSPs may in principle be accomplished both by injection of purified HSPs, as well as with drugs (such as bortezomib, anthracyclines) that induce these genes[18,19]. Similarly, FcγR signaling may impact the efficacy of anti-tumor antibodies in cancer and provide novel approaches to improve their efficacy by antibody engineering[56].
Acknowledgement
This work is supported in part by funds from the National Institutes of Health (CA106802, CA109465 to MD; AI054375 to KD; and AI070603, CA100191 to ZL); Dana Foundation (to MD and KD); and Sinsheimer Fund (to KD). ZL is a clinical scholar of the Leukemia and Lymphoma Society.
Abbreviations
- HSP
heat shock proteins
- APC
Antigen presenting cells
- MHC
Major histocompatibility complex
- TLR
Toll like receptors
- ER
Endoplasmic reticulum
- CRT
calreticulin
- DAMP
damage associated molecular pattern
- DAMPRR
DAMP recognition receptor
- ITAM
Immune tyrosine activation motif
- DC
Dendritic cell
- FcγR
Fcγ receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1•.Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15:3–12. doi: 10.1038/sj.cdd.4402269. [An excellent summary of emerging evidence for properties of immunogenic cell death.] [DOI] [PubMed] [Google Scholar]
- 2•.Srivastava PK. Roles of heat shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [A summary of supporting evidence for HSP to chaperone antigenic peptides, activate APC and prime T cells.] [DOI] [PubMed] [Google Scholar]
- 3••.Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat Immunol. 2005;6:593–599. doi: 10.1038/ni1201. [By immunodepleting various HSPs from total cell lysates and examining the impact on subsequent T cell priming of remaining components, the critical roles of HSP in T cell priming in vivo were demonstrated.] [DOI] [PubMed] [Google Scholar]
- 4.Binder RJ, Vatner R, Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64:442–451. doi: 10.1111/j.1399-0039.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 5.Dai J, Liu B, Caudill MM, Zheng H, Qiao Y, Podack ER, Li Z. Cell surface expression of heat shock protein gp96 enhances cross-presentation of cellular antigens and the generation of tumor-specific T cell memory. Cancer Immun. 2003;3:1. [PubMed] [Google Scholar]
- 6.Oizumi S, Strbo N, Pahwa S, Deyev V, Podack ER. Molecular and cellular requirements for enhanced antigen cross-presentation to CD8 cytotoxic T lymphocytes. J Immunol. 2007;179:2310–2317. doi: 10.4049/jimmunol.179.4.2310. [DOI] [PubMed] [Google Scholar]
- 7.Liu B, Dai J, Zheng H, Stoilova D, Sun S, Li Z. Cell surface expression of an endoplasmic reticulum resident heat shock protein gp96 triggers MyD88-dependent systemic autoimmune diseases. Proc Natl Acad Sci U S A. 2003;100:15824–15829. doi: 10.1073/pnas.2635458100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, Li Z. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–226. doi: 10.1016/j.immuni.2006.12.005. [By conditionally deleting gp96 gene from murine macrophages, this study demonstrated the critical roles of ER HSP gp96 in chaperoning multiple TLRs and highlighted the importance of HSP in regulating immunity by post-translational regulation of TLR expression.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol. 2001;3:891–896. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Yang Y, Dai J, Medzhitov R, Freudenberg MA, Zhang PL, Li Z. TLR4 up-regulation at protein or gene level is pathogenic for lupus-like autoimmune disease. J Immunol. 2006;177:6880–6888. doi: 10.4049/jimmunol.177.10.6880. [DOI] [PubMed] [Google Scholar]
- 11.Calderwood SK, Mambula SS, Gray PJ., Jr. Extracellular heat shock proteins in cell signaling and immunity. Ann N Y Acad Sci. 2007;1113:28–39. doi: 10.1196/annals.1391.019. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Dai J, Zheng H, Liu B, Caudill M. An integrated view of the roles and mechanisms of heat shock protein gp96-peptide complex in eliciting immune response. Front Biosci. 2002;7:d731–751. doi: 10.2741/a808. [DOI] [PubMed] [Google Scholar]
- 13.Han JM, Park SG, Liu B, Park BJ, Kim JY, Jin CH, Song YW, Li Z, Kim S. Aminoacyl-tRNA synthetase-interacting multifunctional protein 1/p43 controls endoplasmic reticulum retention of heat shock protein gp96: its pathological implications in lupus-like autoimmune diseases. Am J Pathol. 2007;170:2042–2054. doi: 10.2353/ajpath.2007.061266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, Zehetmeier C, Lain B, Torella C, Henning SW, Beste G, et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- 15.Gehrmann M, Liebisch G, Schmitz G, Anderson R, Steinem C, De Maio A, Pockley G, Multhoff G. Tumor-specific Hsp70 plasma membrane localization is enabled by the glycosphingolipid Gb3. PLoS ONE. 2008;3:e1925. doi: 10.1371/journal.pone.0001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blachere NE, Darnell RB, Albert ML. Apoptotic cells deliver processed antigen to dendritic cells for cross-presentation. PLoS Biol. 2005;3:e185. doi: 10.1371/journal.pbio.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [This pioneering work demonstrated that anthracyclines induce preapoptotic translocation of CRT to the cell surface, and subsequent DC activation and tumor immunity via CRT.] [DOI] [PubMed] [Google Scholar]
- 19••.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC) mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109:4839–4845. doi: 10.1182/blood-2006-10-054221. [The study demonstrates that bortezomib, a proteasome inhibitor, induces the expression of cell surface HSP90 on dying myeloma cells, with increased immunogenicity in HSP90-dependent manner. References 18, 19 and 21 together suggest a role for exposure of HSPs on dying cells as a marker for immunogenicity of tumors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamrekelashvili J, Kruger C, von Wasielewski R, Hoffmann M, Huster KM, Busch DH, Manns MP, Korangy F, Greten TF. Necrotic tumor cell death in vivo impairs tumor-specific immune responses. J Immunol. 2007;178:1573–1580. doi: 10.4049/jimmunol.178.3.1573. [DOI] [PubMed] [Google Scholar]
- 21.Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, Zitvogel L, Kroemer G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 22.Spisek R, Dhodapkar MV. Towards a better way to die with chemotherapy: role of heat shock protein exposure on dying tumor cells. Cell Cycle. 2007;6:1962–1965. doi: 10.4161/cc.6.16.4601. [DOI] [PubMed] [Google Scholar]
- 23.Elsner L, Muppala V, Gehrmann M, Lozano J, Malzahn D, Bickeboller H, Brunner E, Zientkowska M, Herrmann T, Walter L, et al. The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J Immunol. 2007;179:5523–5533. doi: 10.4049/jimmunol.179.8.5523. [DOI] [PubMed] [Google Scholar]
- 24••.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [This essay argues compellingly that PAMPs and tissue-derived danger signals are not mutually exclusive, but rather share similar biochemical features in the form of exposed hydrophobicity or DAMPs which initiate immunity.] [DOI] [PubMed] [Google Scholar]
- 25.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 26.Menoret A, Patry Y, Burg C, Le Pendu J. Co-segregation of tumor immunogenicity with expression of inducible but not constitutive hsp70 in rat colon carcinomas. J Immunol. 1995;155:740–747. [PubMed] [Google Scholar]
- 27.Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat.Med. 1998;4:581–587. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 28•.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [An excellent review of the role of Fcγ receptors as regulators of immune responses.] [DOI] [PubMed] [Google Scholar]
- 29.Hamerman JA, Lanier LL. Inhibition of immune responses by ITAM-bearing receptors. Sci STKE. 2006;2006:re1. doi: 10.1126/stke.3202006re1. [DOI] [PubMed] [Google Scholar]
- 30.Dhodapkar MV, Dhodapkar KM, Palucka AK. Interactions of tumor cells with dendritic cells: balancing immunity and tolerance. Cell Death Differ. 2008;15:39–50. doi: 10.1038/sj.cdd.4402247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erwig LP, McPhilips KA, Wynes MW, Ivetic A, Ridley AJ, Henson PM. Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc Natl Acad Sci U S A. 2006;103:12825–12830. doi: 10.1073/pnas.0605331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Delamarre L, Couture R, Mellman I, Trombetta ES. Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J Exp Med. 2006;203:2049–2055. doi: 10.1084/jem.20052442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhodapkar KM, Krasovsky J, Williamson B, Dhodapkar MV. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J Exp Med. 2002;195:125–133. doi: 10.1084/jem.20011097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groh V, Li YQ, Cioca D, Hunder NN, Wang W, Riddell SR, Yee C, Spies T. Efficient cross-priming of tumor antigen specific T cells by dendritic cells sensitized with diverse anti-MICA opsonized tumor cells. Proc Natl Acad Sci U S A. 2005;102:6461–6466. doi: 10.1073/pnas.0501953102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhodapkar KM, Dhodapkar MV. Recruiting dendritic cells to improve antibody therapy of cancer. Proc Natl Acad Sci U S A. 2005;102:6243–6244. doi: 10.1073/pnas.0502547102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woelbing F, Kostka SL, Moelle K, Belkaid Y, Sunderkoetter C, Verbeek S, Waisman A, Nigg AP, Knop J, Udey MC, et al. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J Exp Med. 2006;203:177–188. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhodapkar KM, Banerjee D, Connolly J, Kukreja A, Matayeva E, Veri MC, Ravetch JV, Steinman RM. Dhodapkar MV: Selective blockade of the inhibitory Fc{gamma} receptor (Fc{gamma}RIIB) in human dendritic cells and monocytes induces a type I interferon response program. J Exp Med. 2007;204:1359–1369. doi: 10.1084/jem.20062545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhodapkar KM, Kaufman JL, Ehlers M, Banerjee DK, Bonvini E, Koenig S, Steinman RM, Ravetch JV, Dhodapkar MV. Selective blockade of inhibitory Fc gamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc Natl Acad Sci U S A. 2005;102:2910–2915. doi: 10.1073/pnas.0500014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest. 2005;115:2914–2923. doi: 10.1172/JCI24772. [References 40 and 41 demonstrate the importance of the balance between activating and inhibitory Fc receptors in regulating dendritic cell activation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuurhuis DH, van Montfoort N, Ioan-Facsinay A, Jiawan R, Camps M, Nouta J, Melief CJ, Verbeek JS, Ossendorp F. Immune complex-loaded dendritic cells are superior to soluble immune complexes as antitumor vaccine. J Immunol. 2006;176:4573–4580. doi: 10.4049/jimmunol.176.8.4573. [DOI] [PubMed] [Google Scholar]
- 43.Banerjee D, Matthews P, Matayeva E, Kaufman JL, Steinman R, Dhodapkar K. Enhanced T cell responses to glioma cells coated with anti-EGF receptor antibody and targeted to activating FcgRs on human dendritic cells. J Immunotherapy. 2008 doi: 10.1097/CJI.0b013e31815a5892. in press. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Gao X, Masuda E, Redecha PB, Blank MC, Pricop L. Regulated expression of FcgammaR in human dendritic cells controls cross-presentation of antigen-antibody complexes. J Immunol. 2006;177:8440–8447. doi: 10.4049/jimmunol.177.12.8440. [DOI] [PubMed] [Google Scholar]
- 45.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fc receptors on dendritic cells. J Exp Med. 2002;195:1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. J Clin Invest. 2002;110:71–79. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 48.Desai DD, Harbers SO, Flores M, Colonna L, Downie MP, Bergtold A, Jung S, Clynes R. Fc{gamma} Receptor IIB on Dendritic Cells Enforces Peripheral Tolerance by Inhibiting Effector T Cell Responses. J Immunol. 2007;178:6217–6226. doi: 10.4049/jimmunol.178.10.6217. [DOI] [PubMed] [Google Scholar]
- 49••.Harbers SO, Crocker A, Catalano G, D'Agati V, Jung S, Desai DD, Clynes R. Antibody-enhanced cross-presentation of self antigen breaks T cell tolerance. J Clin Invest. 2007;117:1361–1369. doi: 10.1172/JCI29470. [References 48 and 49 demonstrate the critical role for inhibitory FcRs in maintaining peripheral tolerance to self antigens.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sedlik C, Orbach D, Veron P, Schweighoffer E, Colucci F, Gamberale R, Ioan-Facsinay A, Verbeek S, Ricciardi-Castagnoli P, Bonnerot C, et al. A critical role for Syk protein tyrosine kinase in Fc receptor-mediated antigen presentation and induction of dendritic cell maturation. J Immunol. 2003;170:846–852. doi: 10.4049/jimmunol.170.2.846. [DOI] [PubMed] [Google Scholar]
- 51.Herrada AA, Contreras FJ, Tobar JA, Pacheco R, Kalergis AM. Immune complex-induced enhancement of bacterial antigen presentation requires Fcgamma receptor III expression on dendritic cells. Proc Natl Acad Sci U S A. 2007;104:13402–13407. doi: 10.1073/pnas.0700999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laborde EA, Vanzulli S, Beigier-Bompadre M, Isturiz MA, Ruggiero RA, Fourcade MG, Catalan Pellet AC, Sozzani S, Vulcano M. Immune complexes inhibit differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2007;179:673–681. doi: 10.4049/jimmunol.179.1.673. [DOI] [PubMed] [Google Scholar]
- 53.Clatworthy MR, Willcocks L, Urban B, Langhorne J, Williams TN, Peshu N, Watkins NA, Floto RA, Smith KG. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proc Natl Acad Sci U S A. 2007;104:7169–7174. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Montfoort N, de Jong JM, Schuurhuis DH, van der Voort EI, Camps MG, Huizinga TW, van Kooten C, Daha MR, Verbeek JS, Ossendorp F, et al. A novel role of complement factor C1q in augmenting the presentation of antigen captured in immune complexes to CD8+ T lymphocytes. J Immunol. 2007;178:7581–7586. doi: 10.4049/jimmunol.178.12.7581. [DOI] [PubMed] [Google Scholar]
- 55.Thomas-Rudolph D, Du Clos TW, Snapper CM, Mold C. C-reactive protein enhances immunity to Streptococcus pneumoniae by targeting uptake to Fc gamma R on dendritic cells. J Immunol. 2007;178:7283–7291. doi: 10.4049/jimmunol.178.11.7283. [DOI] [PubMed] [Google Scholar]
- 56.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]