Abstract
Human brain imaging studies suggest that chronic neuropathic pain has a strong emotional component that is mediated by medial prefrontal cortex (mPFC) activity; in rodents, the mPFC is involved in emotional and cognitive aspects of behavior, including the extinction of Pavlovian fear conditioning. Together, these findings suggest that the cortex may modulate the memory trace of pain. As D-cycloserine (DCS), a partial agonist of the NMDA receptor, can enhance learning and potentiate the extinction of acquired fear, in the present study we tested its efficacy in neuropathic pain behavior. In rats with spared nerve injury (SNI), repeated daily oral administration of DCS reduced mechanical sensitivity of the injured limb in a dose-dependent manner; this effect continued for weeks after the cessation of DCS treatment. In addition, re-exposure to DCS further enhanced antinociceptive behavior. Repeated oral DCS administration also reduced cancer chemotherapy drug-induced neuropathic pain behavior. Infusions of DCS directly into the mPFC (especially within prelimbic cortex) or the amygdala (but not into thalamus, insula, or occipital cortex), acutely induced antinociception in SNI rats. The antinociceptive effect of intra-mPFC DCS infusions was, mimicked by NMDA and glycine, and blocked by HA −966. In the mPFC of SNI rats, NR2B expression was down regulated; however, this effect was reversed with repeated oral DCS. Lastly, infusions of DCS into mPFC reversed place avoidance behavior induced by mechanical stimulation of the injured paw in SNI rats. These findings indicate that limbic NMDA-mediated circuitry is involved in long-term reduction in neuropathic pain behavior.
Keywords: Fear extinction, NMDA receptor, NR2B, allodynia, aversion, prefrontal cortex, amygdala
Introduction
Neuropathic pain is a devastating consequence of nerve injury that is characterized by spontaneous pain and exaggerated responses to both painful (hyperalgesia) and non-painful stimuli (allodynia). The present study stems from the notion that manipulating prefrontal neuronal properties can diminish the suffering associated with the condition by controlling its memory traces.
Part of the survival value of pain is its intimate association with learning. Pain induces single event learning, the memory of which can persist for a lifetime. As such, Pavlovian paradigms of learning often utilize painful stimuli to study learning and memory processes, especially in fear conditioning where the more painful the unconditioned stimulus the fewer trials are required to establish an aversive negative emotional association with a conditioning stimulus that was originally affectively neutral (Schafe et al. 2001). The ability to extinguish aversive associations of fearful or painful events after repeated exposure to the conditioned stimulus is also important for normal behavior (Myers and Davis 2002; Sotres-Bayon et al. 2004). We hypothesize that chronic pain is a state of continuous learning, in which aversive emotional associations are made with incidental events simply as a consequence of unpredictable fluctuations in persistent pain (Foss et al. 2006). Moreover, persistent pain does not allow for extinction to take place, and instead may further reinforce aversive associations, as extinction requires repeated exposure to the conditioned stimulus in the absence of the unconditioned stimulus. Living with this conundrum, and the effort of disentangling such associations may contribute to the suffering in chronic pain, and is likely mediated via cortical circuitry that is involved in emotion and cognition and in associative learning and memory. This hypothesis is based on observations that chronic pain in humans activates medial prefrontal cortex (mPFC), is associated with atrophy in dorsolateral prefrontal cortex (DLPFC), and that such patients show deficits in emotional decision making (Grachev et al. 2000; Apkarian et al. 2004a; Apkarian et al. 2004b; Baliki et al. 2006).
D-cycloserine (DCS) given systemically or centrally enhances cognitive processes (Stromme and Myhrer 2002), improves attention and memory (Hughes 2004; Vertes 2006), and facilitates fear extinction (Walker et al. 2002; Richardson et al. 2004) through de novo memory trace formation involving n-methyl-D-aspartic acid (NMDA) plasticity (Falls et al. 1992; Milad and Quirk 2002; Santini et al. 2004). Therefore, in the present study we tested the effects of DCS on chronic neuropathic pain behavior. We hypothesized that DCS would enhance the extinction of pain-related memories and, thus, exhibit antinociceptive properties for neuropathic pain. In rodents, the ventral mPFC (prelimbic and infralimbic cortices) modulates emotional and cognitive aspects of goal directed behavior (Vertes 2006) and is critical in the extinction of fear (Morgan et al. 1993; Morgan and LeDoux 1995; Walker et al. 2002; Milad and Quirk 2002; Santini et al. 2004). Therefore, we also examined whether DCS effects on neuropathic pain are mediated by the mPFC via NMDA-mediated transmission.
Materials and Methods
Subjects
Sprague-Dawley rats (Harlan, Indianapolis, IN; 250–300 g) were housed in groups of three and kept on a 12-h light/dark cycle with free access to food and water. All experiments were performed during the light cycle. Experimental procedures were in accordance with the policies and recommendations of NIH guidelines (NIH publication No. 86–23, 1996), IASP guidelines for use of conscious animals in pain research (Zimmermann 1983), and all experiments were approved by the Northwestern University Institutional Animal Care and Use Committee. All behavioral measures were performed by a single experimenter, blinded as to treatment groups, and using the method of equal blocks to minimize environmental variation of response and bias due to expectation.
Drugs
DCS, glycine, NMDA, (±)-3-(2-carboxypiperazin-4-yL-propyl-1-phosphonic acid (CPP), and (±)1-hydroxy-3-aminopyrrolidine-2-one (HA 966); all purchased from Sigma-Aldrich, USA) were used in this experiment. DCS was administered orally (gavage, 2 ml/kg), intrathecally (i.t.) (10 μl/site while under brief gas anesthesia), or was directly infused into supraspinal structures (0.5 μl/3 min/site). Glycine (100 μg/site), NMDA (8 μg/site), CPP (0.5 μg/site), and HA 966 (5 μg/site) were directly infused into mPFC (1.0 μl/6 min/site) or the spinal cord (same doses as mPFC, 10 μl/rat), either alone or following administration of DCS.
Neuropathic pain
Two models of neuropathic pain were studied: spared nerve injury and cisplatin-induced neuropathy.
Spared nerve injury (SNI)
The method used to induce nerve injury has been previously described in detail (Decosterd and Woolf 2000). Rats were anesthetized with isofluorane 5%, and a mixture of 30% N2O and 70% O2. The left sciatic nerve was exposed at the level of its trifurcation into the sural, tibial, and common peroneal nerves. The tibial and common peroneal nerves were tightly ligated and then completely severed between ligations, leaving the sural nerve intact. Behavioral experiments began 2 wks after recovery from surgery.
Cisplatin-induced neuropathy
The method used to model chemical neuropathy has been previously described (Authier et al. 2003). Cisplatin (Sigma-Aldrich, USA) was administered intraperitoneally twice a week for 4 wks (2 mg/kg; cumulative dose, 16 mg/kg). Prior to each injection, 2 ml of sterile saline solution was subcutaneously administered to prevent renal damage due to hyperhydration. To avoid acute effects, injections were given after behavioral testing.
Behavioral tests
Tactile sensitivity
Hind paw withdrawal responses to von Frey filament stimulation were used to assess mechanical sensitivity of the hind paw. Animals were placed in a Plexiglas box with a wire grid floor and allowed to habituate to the environment for 15 min, at which point filaments of varying forces (Stoelting, USA) were applied to the plantar surface of the hind paw. Filaments were applied in either ascending or descending strengths as necessary to determine the filament strength closest to hind paw withdrawal threshold. Each filament was applied for a maximum of 10 seconds; paw withdrawal during the stimulation was considered a positive response. The minimum stimulus intensity was 0.008 g with a maximum cutoff of 45 g. Given the response pattern and the force of final filament, 50% response threshold (in grams) was calculated according to Chaplan et al. (1994).
Resting paw position
Paw position, in the absence of any stimulation, was evaluated before each tactile sensitivity measurement, as SNI animals modify their posture to protect the most sensitive part of their plantar surface (the lateral external edge of the paw). The categorical scale used in the present study was adapted from Attal et al. (1990): 0, normal position; 1, slightly protective position in which the external paw curvature is more marked, with all toes touching the floor; 2, marked protective position in which the external paw curvature is very marked, with some toes touching the floor; 3, prominent protective position with all toes raised, and only the heel touching the floor; 4, no paw contact with the floor. Half scores were assigned for positions intermediate to these five categories.
Paw immersion cold test
Behavioral responses to the immersion of the hind paws into a temperature-regulated bath were assessed. Rats were gently handled, and the right or left hind paw was immersed in a bath (10°C) until the animal struggled. The delay was used as cold sensitivity threshold. The test was repeated 3 times for each paw with a delay of 10 min between measurements. The average of the 3 assays was used as the individual threshold for each paw.
Motor activity
An open field apparatus (45 × 45 × 45 cm) was placed in a quiet room illuminated with dim white light. The floor of the apparatus was equally divided into nine squares (15 × 15 cm2). Rats were individually placed into the open field on the central square, and their spontaneous behavior was videotaped for 5 min. Subsequent analysis of the total number of squares visited was used to assess general motor activity. The number of rearings, entrees into the central square, and stretching in the direction of the central quadrant were also analyzed as indirect markers of stress caused by the novel environment.
Place Avoidance task
The place avoidance apparatus consisted of a Plexiglas chamber (60 × 30 × 50 cm) positioned on top of a mesh screen, which was placed in a quiet room illuminated with dim white light. The chamber was divided into 2 equal compartments (one black and one white) which were separated by a wall with a 10 × 10 cm opening. Animals were placed into the white chamber and allowed to freely move throughout the apparatus for the duration of a 30-min test period. A 476 mN (6 g) von Frey monofilament was applied to the plantar surface of the hind paws every 15 sec. The left paw (SNI) was stimulated in the black chamber, and the right paw in the white chamber. The percentage of time spent in the white compartment was calculated for every 5 min of testing (for details see Labuda and Fuchs, 2001) and was expressed as the animal’s preference. Shifts in preference were calculated as the difference in percentage of time spent in the white chamber between the first and last 5 min of testing.
Experimental design
The effects of varying doses of DCS on neuropathic pain behavior were assessed for the following conditions: single oral application (p.o.), single i.t. application, repeated oral application over multiple weeks, infusion in various brain sites, and for the combination of brain infusions before and after repeated oral applications. Acute i.t. injections of saline or DCS (10 or 50 μg/rat) were administered at L5-L6 level while under light gas anesthesia (isoflorane 5%).
Acute brain infusions of DCS in SNI animals
One week after SNI surgery, rats were anesthetized with deep gas anesthesia and implanted with one or two guide cannulas (26 gauge, Plastics One Inc., Canada). Cannulas were implanted into targeted brain structures using the following stereotaxic coordinates: right mPFC, (contralateral to SNI; anteroposterior (AP), +2.9 mm from bregma; mediolateral (ML), −1 mm from midline; dorsoventral (DV), +4.1 mm from skull surface, with ML angle at 11°); right and left basolateral amygdala (AP, −2.3 mm; ML, ±4.5 mm; DV, −7.8 mm); right thalamus (contralateral to SNI; AP, −2.6 mm; ML, −2.8 mm; DV, −6.5 mm); right insula (contralateral to SNI; AP, +2.2 mm; ML, −5 mm; DV, −5.8 mm); and right occipital cortex (contralateral to SNI; AP, −5.8 mm; ML, −3 mm; DV, −1.5 mm). After all behavioral procedures were completed, the animals were perfused with fixative under deep anesthesia, their brains were extracted, and the locations of the cannulas were verified histologically.
DCS infusion
Implanted animals were given infusions of either saline (0.5 μL/3 min) or DCS (50 μg/site).
Cortical and spinal cord infusions before and after repeated treatment with DCS
All animals were allowed to recover for at least 2 wks after SNI surgery and 1 wk after cortical cannulation of mPFC prior to cortical or i.t. infusions of various drugs. They then received twice-daily oral DCS for 14 consecutive days, and were tested again for cortical and i.t. infusion effects. At the end of the experiment, these animals were perfused with fixative under deep anesthesia, their brains were extracted, and the locations of cannulas were verified histologically.
DCS and HA 966 co-infusion
mPFC-implanted animals were infused with 1.0 μL/10 min saline or HA 966 (5 μg/site). After 10 min of rest, they received a second brain infusion of saline (0.5 μL/5 min) or DCS (50 μg/site). One week later the same animals received either saline alone or HA 966 alone.
Place avoidance task after acute mPFC infusions of DCS in SNI rats
Two groups of sham rats and 5 groups of SNI animals were tested (procedure described above). One sham group and 4 SNI groups were implanted with guide cannulas above the mPFC. The behavior of these animals was assessed 45 min or 24 h after an acute brain infusion of either saline or DCS (50 μg in 0.5 μL/3 min).
RNA extraction and RT-PCR for NMDA and AMPA receptors
Rats were anesthetized and decapitated, and their brains were quickly removed, iced, and blocked for slicing. Coronal (300 micron) slices were cut with a vibrating microtome (VT1000 slicer; Leica, Nussloch, Germany) in an ice-cold ACSF solution containing the following (in mM): 126 NaCl, 2.5 KCl, 1.2 NaHCO3’ 1 NaH2PO4, 2.5 KCl, pH 7.4 (300–305 mOsm/l). The mPFC was microdissected, placed in 0.5 ml RNase free Eppendorf tube and stored at −80°C. RNA was purified by RNAeasy kit (QIAGEN, Valencia, CA) and DNAse treated to avoid DNA contamination. Reverse transcription (RT) was done using SuperScriptIII kit (Invitrogen, Carlsbad, CA) according to the manufacture’s instructions. Real-time fluorescence-based PCR assay was performed with the LightCycler (Rosche Molecular Biochemicals, Indianapolis, IN) using SYBR green. Primers were developed from GenBank with the commercially available software OLIGO (National Biosciences, Plymouth, MN). Primers for NMDAR1 (GenBank accession U08261) were CACCACCCTCCCACGACATT (position 3806) and CAGCGTCTGAGGAAGCCTATT (position 3977). Primers for NMDAR2A (GenBank accession NM_012573) were GGAGGGCAACTTGTATGG (position 4049) and GACCTCAAGGATGACCGAAGAT (position 4288). Primers for NMDAR2B (GenBank accession NM_012574) were TCACGGCAGCAAATCCTACTTC (position 4538) and CTCCCGTACCCACCTTAACCT (position 4816). Primers for GluR1 (GenBank accession X17184) were TGATCCCACAGCAATCCATC (position 2728) and GGCATCCCTGAACTGTGACTCA (position 2878). Primers for GAPDH (GenBank accession X02231) were ATCGTGGAAGGGCTCATGACC (position 573) and GCCAGTGAGCTTCCCGTTCAG (position 735). For calibration curve serial dilutions of total cortical cDNA were used.
Statistical analyses
All statistical measures for tactile sensitivity thresholds were performed independently for each paw, as thresholds for injured and uninjured paws differ in the SNI model and the experimenter can visually identify the injured paw by its position. Mechanical response thresholds were measured for multiple doses at different time points. In all SNI experiments, drug effects were compared using a two-way analysis of variance (2 RM-ANOVA) for dose, with time as the repeated measure and tactile threshold as the dependent variable. Cold sensitivity is a cumulative behavioral score that measures discomfort over time, and drug effects were again compared with a 2 RM-ANOVA. As paw position rating is a categorical score, it requires formal testing for normality prior to the use of parametric statistics. This was determined with the Kolmogorov-Smirnov and Lilliefors one sample test of normality. Given the large number of observations per category, paw position data showed no evidence for deviation from normality. Therefore, paw position ratings were also analyzed with 2 RM-ANOVA. In each experiment, if ANOVA results were significant then a priori hypotheses were compared by planned comparisons between the means predicted by the statistical model tested, with the error terms computed from the sum of square residuals, and reported as t-values. Planned comparisons were performed to distinguish between doses and time courses of treatments. Degrees of freedom are indicated for all F and T values. The only condition where planned comparisons were not strictly applicable was the place avoidance task. However, the statistical outcomes for the latter were strong enough that specifics of secondary analyses were not important. The accepted level of statistical significance was p < 0.05.
Results
The effects of several doses of DCS were tested in two rat models of neuropathic pain. Various regimens of administration of DCS were tested (acute single doses; repeated application twice a day for multiple weeks; oral administration; and i.t. infusions or at specific cortical targets) on neuropathic pain behavior, including tactile thresholds, cold sensitivity, protective paw posture, and an operant pain behavior task. Two neuropathic animal models were studied: SNI (Decosterd and Woolf, 2000), and a cancer chemotherapy drug-induced neuropathy (Authier et al. 2003). SNI injury was always to the left sciatic nerve, resulting in increased tactile sensitivity for stimuli applied to the left paw, with smaller changes seen for tactile sensitivity in the right paw. Mechanical thresholds post-injury and prior to treatments were approximately 0.01 g for the left paw and 7.5 g for the right paw. After SNI injury, cold sensitivity also increased for the injured paw.
Antinociceptive effects of acute oral or i.t. D-Cycloserine
The effects of oral administration of saline or DCS (3, 10, or 30 mg/kg; n = 8 rats per group, Figure 1) was monitored for up to 300 min. Acute oral DCS resulted in decreased neuropathic pain behavior for specific times and doses (F3,26 = 1.1, p > 0.05 for dose; F5,135 = 2.4, p < 0.05 for time; and F15,135 = 2.3, p < 0.007 for the interaction). Paw withdrawal threshold was significant at 45 min for 10 mg/kg DCS (t14 = 2.3, p < 0.03). A similar transient acute analgesic effect of DCS has been observed in the past (Millan and Seguin 1994). Tactile sensitivity was not affected in the uninjured paw. Paw position scores were similarly effected by DCS treatment (F3,27 = 5.8, p < 0.005 for dose) with significant decreases in paw position scores observed for 3 mg/kg DCS at 180 min after treatment (t14 = −2.8, p < 0.01). There was no significant effect of DCS on cold sensitivity for the injured paw.
Figure 1.
Oral single dose of D-cycloserine (DCS) induced decreased neuropathic behavior, and was most effective at lower doses. Tactile threshold and paw position was tested before (time 0), and across 300 min after an acute treatment (n = 8 per group) with saline, or DCS (3, 10 or 30 mg/kg, p.o.). Asterisks indicate statistically significant differences when compared with the saline group. Error bars indicate S.E.M.
Effects of i.t. administration of saline and DCS (10 μg and 50 μg; n = 8 rats per group) were monitored for up to 60 min. No significant effects of i.t. DCS were found for tactile sensitivity of the injured or uninjured paw, or for paw position or cold sensitivity for the injured paw in SNI rats. Acute oral administration of DCS resulted in transient antinociception with a complex relationship to DCS dose. Importantly, the highest oral DCS dose tested (30 mg/kg) did not modify neuropathic pain behavior.
Repeated oral D-Cycloserine induced long-term antinociception
SNI rats were treated twice daily with saline or DCS (3, 10, or 30 mg/kg orally; n = 8 rats per group) for 14 consecutive days. Neuropathic pain behavior was tested twice per week during DCS treatment and for 21 days after the cessation of treatment. To minimize the contribution of acute DCS effects, pain behavior was assessed at least 2 h after the morning treatment.
DCS treatment resulted in a progressive reduction in tactile sensitivity and paw position ratings (Figure 2a): tactile sensitivity for the injured paw showed a dose (F3,20 = 4.3, p < 0.02) and time (F4,80 = 5.9, p < 0.0003) dependent decrease. Mean tactile thresholds across the 14 days of treatment were dose dependent: 0.09 ± 0.09 g for saline, and 0.26 ± 0.09 g, 0.42 ± 0.09 g, and 0.47 ± 0.09 g for DCS 3, 10, and 30 mg/kg, respectively. The effects of 10 and 30 mg/kg DCS on tactile thresholds were significantly different from saline (p < 0.05). Tactile thresholds for the uninjured paw showed no drug treatment effects. Similarly, paw position ratings for the injured paw diminished progressively with DCS treatment, showing a dose effect (F3,20 = 3.6, p < 0.03) but no time or interaction effects. Only paw position ratings for 30 mg/kg DCS was significantly different from saline treatment. Cold sensitivity of the injured paw did not change with DCS treatment.
Figure 2.
Repeated oral D-cycloserine (DCS) induced a long-term reduction in pain behavior in SNI rats. (A) Dose-response of pain behavior tested before (day 0), during a 2-wk treatment (gray background), and for 18 days after treatment. Rats were treated with saline or DCS (3, 10 or 30 mg/kg, p.o. twice-daily, n = 8 per group), and tested twice weekly. Tactile threshold and paw position rating of the SNI paw showed significant dose-dependent changes with treatment. (B) Re-exposure to DCS enhanced behavioral responses to the drug. Three of the four groups from (A) were re-exposed to DCS: The group that received saline in (A) was treated with DCS (30 mg/kg, naïve group), the group that received 3 mg/kg DCS was treated with saline (saline group), and the group that received 30 mg/kg DCS was re-exposed to the same treatment (redose group). Paw position and tactile threshold changes were larger for the redosed group than for the naïve group. (C) Uninjured paw tactile thresholds were not affected by treatment. Mobility, as measured in an open field, was not affected by the 2-wk DCS treatment (30 mg/kg). Asterisks indicate first and last statistically significant time points between the 30 mg/kg DCS and saline animals. Error bars indicate S.E.M.
After cessation of treatment, animals progressively returned to their baseline level of neuropathic pain behavior. Changes in tactile sensitivity for the injured paw, over the post-treatment period, show dose (F3,20 = 4.8, p < 0.01), and time effects (F5,120 = 7.4, p < 0.000005), and an interaction between both factors (F15,120 = 2.1, p < 0.02). Note that the dose effects, in this phase, are a reflection of the amount of DCS the animals received during treatment. Tactile thresholds returned to baseline around 18 days post-treatment. Injured paw position ratings also displayed dose (F3,20 = 3.1, p < 0.05) and time (F5,120 = 4.1, p < 0.002) effects across the 21 post-treatment days. There were no DCS treatment effects for the uninjured paw tactile thresholds or for cold sensitivity for the injured paw. Overall, these results indicate a dose dependent, primarily tactile, antinociceptive (or anti-allodynia) effect of repeated DCS treatment on SNI neuropathic behavior. The cumulative increase in antinociception with continued treatment and the long-lasting antinociception after cessation of treatment, as well as dose dependence, are important properties that distinguish the repeated treatment effects from acute DCS effects. We pursued this idea further in two related experiments.
Re-dosing potentiated D-cycloserine induced long-term antinociception
To evaluate the impact of pre-exposure to DCS on a second repeat-treatment with DCS, we used a sub-group of the animals from the above study in a re-dosing experiment (Figure 2b). These animals were treated again for 14 days and further monitored for another 18 days (3 groups; n = 8 rats per group): 1) Animals that initially received 3 mg/kg DCS were treated with saline (saline group); 2) Animals initially treated with saline received DCS 30 mg/kg (naïve group); and 3) Animals initially treated with DCS 30 mg/kg were re-treated with DCS 30 mg/kg again (redosed group).
Tactile sensitivity for the injured paw during re-treatment showed group (re-dosed vs. naïve; F2,21 = 6.1, p < 0.008), time (F4,84 = 11.9, p < 10−5), and group by time interaction (F8,84 = 5.4, p < 0.00002) effects. Importantly, the magnitude of response to DCS for the injured paw was larger for the re-dosed animals than for the naïve animals (mean tactile thresholds for the re-dose group = 1.0 ± 0.18 g, and for the naïve group = 0.41 ± 0.17 g; t14 = 2.5, p < 0.02). We observed a similar effect for injured paw position ratings: the magnitude of paw position change in response to DCS was larger for the re-dosed animals than for the naïve animals (t14 = −2.1, p < 0.05). Cold sensitivity for the injured paw and tactile sensitivity for uninjured paw showed no treatment effects.
The reversal of analgesic effects in the 18 days post-re-dosing was similar to that seen in the initial experiment, with injured paw tactile sensitivity displaying a time effect (F4,84 = 4.9, p < 0.001). It is noteworthy that the naïve animals showed reduced DCS responses in comparison with their counterparts in the first treatment (Figure 2a). This may be a consequence of the length of time from the initial injury rendering the condition more refractory, or due to repeated exposure to saline rendering reduced expectations (a nocebo-like effect). Overall, for both treatment and post-treatment periods, animals that were originally exposed to DCS exhibited a larger magnitude of antinociception.
Extended exposure to D-Cycloserine induced larger and longer-lasting antinociception
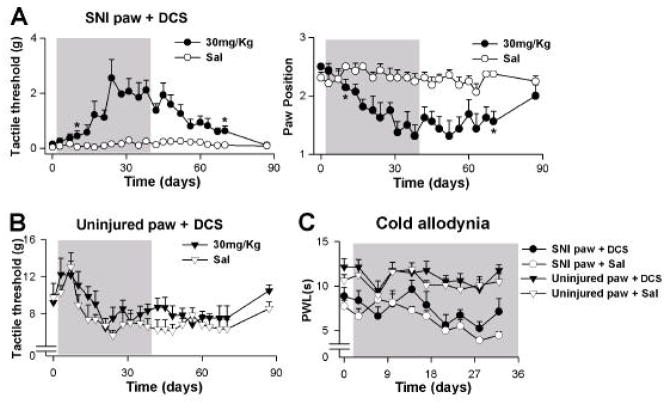
Both the initial treatment experiment, and the re-treatment of DCS, indicated that the antinociceptive effects of DCS are amplified over time, with post-treatment antinociception continuing for a time period corresponding to that of treatment. We further tested this finding in a new group of SNI animals, across a longer time scale: neuropathic behavior was monitored for 39 days of treatment (oral, twice daily, 30 mg/kg DCS or saline; 2 groups, n = 8 rats per group), and 49 days post-treatment.
In this experiment, tactile sensitivity for the injured paw continued to decrease for approximately 21 days, and reached a plateau at 21 to 28 days of DCS treatment (Figure 3A). There were significant effects of treatment (F1,14 = 40.1, p < 0.00002), time (F11,154 = 5.0, p < 0.000001), and their interaction (F11,154 = 4.2, p < 0.00002). Paw position ratings for the injured paw also showed strong treatment (F1,14 = 20.1, p < 0.0005), time (F11,154 = 3.2, p < 0.0007), and interaction (F11,154 = 4.5, p < 0.000007) effects. Cold sensitivity showed a strong laterality effect (injured vs. uninjured paw F1,28 = 91, p < 10−5), indicating that the injured paw was more sensitive to cold; in addition, there was a moderate treatment effect (F1,28 = 2.9, p < 0.1) and significant time (F7,196 = 4.9, p < 0.00004) and time by treatment interaction (F7,196 = 2.2, p < 0.03) effects (Figure 3C). Tactile sensitivity for the uninjured paw showed no treatment effects (Figure 3B). The effects of long-term DCS treatment on cold hyperalgesia were modest and manifested only after multiple weeks of treatment. It should be noted that in all cases examined, tactile sensitivity of the uninjured paw was unaffected by DCS treatment. However, it is possible that the effects of DCS on the uninjured paw were undetected due to the animal’s reluctance to shift weight off of the uninjured paw to the injured paw. Future studies should also include measures of tactile thresholds for the fore paws, to overcome the bias that the injured paw poses on the uninjured contralateral paw tactile sensitivity measures.
Figure 3.
(A) Extended treatment with D-cycloserine (DCS) resulted in larger decreases in pain behavior, which were sustained for a longer duration after cessation of treatment. Rats were treated with saline or DCS (30 mg/kg, twice daily, n = 8 each) for 39 days, and tested twice per week during treatment and for 49 days after treatment. (B) DCS treatment did not affect tactile sensitivity of the uninjured paw for the duration of treatment. (C) Cold sensitivity of the uninjured paw was not affected by DCS treatment. The SNI injured paw showed increased cold sensitivity and a moderate response to DCS treatment. Asterisks indicate first and last statistically significant time points between the 30 mg/kg DCS and saline animals. Error bars indicate S.E.M.
In the post-treatment period, injured paw tactile sensitivity and paw position ratings both show sustained antinociception for a duration proportional to the treatment time period. Both measures showed highly significant treatment dependence and remained significantly different from the saline treated animals for up to 32 days post-cessation of treatment (tactile threshold at 32 days, DCS group vs. saline group t14 = 2.9, p < 0.01; paw position ratings at 32 days, DCS group vs. saline group t14 = −3.8, p < 0.002), and only returned to baseline after 49 days post-treatment.
Repeated oral D-Cycloserine induced antinociception in chemical neuropathy
The effect of repeated treatment with DCS was evaluated on a chemically induced neuropathic pain model. Animals were given cisplatin (2 mg/kg, intraperitoneally, twice a week) for 28 days, and orally treated with DCS (30 mg/kg) or saline twice a day in the last 14 days (2 groups; n = 8–9 rats per group). Tactile sensitivity was tested before cisplatin, 14 days later, and again after drug treatment. Treatment with DCS significantly reduced the decreased tactile threshold induced by cisplatin (Figure 4). Tactile threshold after 14 days of cisplatin was 0.72 ± 0.09 g. Two weeks of DCS-treatment with continued cisplatin administration significantly increased these thresholds to 2.12 ± 0.35 g (paired t-test, t8 = 3.34, p < 0.01). Conversely, the group treated with saline continued to exhibit lower tactile thresholds, 1.1 ± 0.35 g, when compared to DCS-treated rats at day 28 (unpaired t-test, t15 = 2.0, p < 0.06). The mechanisms underlying cisplatin-induced neuropathy and SNI neuropathy are likely different; however, DCS seems to be similarly effective for both conditions.
Figure 4.
Repeated oral D-cycloserine (DCS) administration reduced chemical cisplatin-induced neuropathy pain behavior. Tactile threshold of the left paw was tested in rats prior any treatment (Before), after 14 days of twice-weekly 2 mg/kg cisplatin (14 days), and after 14 days of cisplatin together with twice-daily saline or DCS (30 mg/kg) treatment (28 days) (n = 8 each). Asterisks indicate statistically significant differences. Error bars indicate S.E.M.
Acute infusion of D-Cycloserine in the frontal cortex induced antinociception
In this experiment we tested the effects of DCS infusions in SNI rats into the mPFC, amygdala (bilaterally), thalamus, insula, and occipital cortex. The effects of DCS (50 μg in 0.5 μL) were tested over one hour and at 24 h post-infusion, for tactile sensitivity and paw position ratings. Over one hour, we observed brain site specific and DCS dependent changes in tactile sensitivity and paw position ratings, for the injured paw (Figure 5). In the first group of animals, the effect of DCS infusions into the mPFC, amygdala, and occipital cortex were assessed (n = 8–12 animals for each condition) (Figure 5A). In this experiment, tactile sensitivity of the SNI paw displayed brain area and treatment dependence (F4,45 = 11.9, p < 10−5), time of measurement dependence (0, 20, 40, 60 min after treatment, F3,135 = 4.1, p < 0.008), and their interaction (F12,135 = 9.92, p < 10−6). Amygdala infusion of DCS induced transient antinociception, significant only at 20 min for tactile threshold (t18 = 5.2, p < 10−5) and paw position (t18 = −2.9, p < 0.005). mPFC infusion of DCS induced progressively increasing antinociception over the 60 min of observation, with tactile thresholds significantly decreased from that of saline treatment at 40 and 60 min post-DCS (t20 = 5.1 and 5.6, p < 10−5 for both). Pain behavior was assessed 24 h after DCS or saline in a subset of animals. There were no differences in tactile thresholds or paw position ratings between pretreatment and 24 h post-treatment (p > 0.05). Saline infusions into the amygdala and mPFC, and DCS infusions into the occipital cortex did not have detectable effects on pain behavior. There were no DCS treatment effects on uninjured paw tactile thresholds.
Figure 5.
Acute infusions of D-cycloserine (DCS) into the mPFC and amygdala induced antinociception in SNI animals, while DCS infusions into the thalamus, insula, and occipital cortex were ineffective (n = 8–10 animals per site). Tactile thresholds for the SNI-injured paw were assessed after acute saline or DCS (50 μg/site) infusions at each brain sites. (A) In the first group of animals DCS was infused into the mPFC, bilateral amygdala, or occipital cortex. (B) In the second group of animals DCS was infused into the mPFC, thalamus, or insula. (C) Tips of infusion cannulas in the mPFC are shown for 30 animals (including animals used in Figures 5A, 5B, 7, 8, and 10) and for bilateral amygdala infusions in 5 animals (PrL = prelimbic; IL = infralimbic; ACC = anterior cingulate; Ce = Central nucleus; BLA = Basolateral nucleus). Coordinates are millimeters anterior (+) or posterior (−) from Bregma. Asterisks indicate statistically significant differences. Error bars indicate S.E.M.
In a second group of animals, we tested brain site specificity by comparing the effects of DCS infusions into the mPFC, thalamus, and insula at baseline, 20, 40, and 60 min post infusion (Figure 5B). Tactile sensitivity in the SNI paw again showed brain site and treatment dependence (6 conditions, n = 7–9 animals per condition). Each parameter (treatment, brain site, and time of measurement) was significant or moderately significant (p < 0.1); and the three-way interaction and all pairwise interactions were significant. Planned comparisons of mPFC, for DCS and saline, and across 20–60 min post injection, showed a significant difference (F1,46 = 13.5, p < 0.0006); while the same comparisons in the thalamus and insula showed no significant differences (p > 0.4). Paw position changes were not as robust as tactile sensitivity, but there was a significant difference between DCS and saline for mPFC (F1,46 = 4.3, p < 0.05). Overall, the findings indicate brain regional specificity of DCS effectiveness.
Localizing an optimal site of action for D-cycloserine effects within mPFC
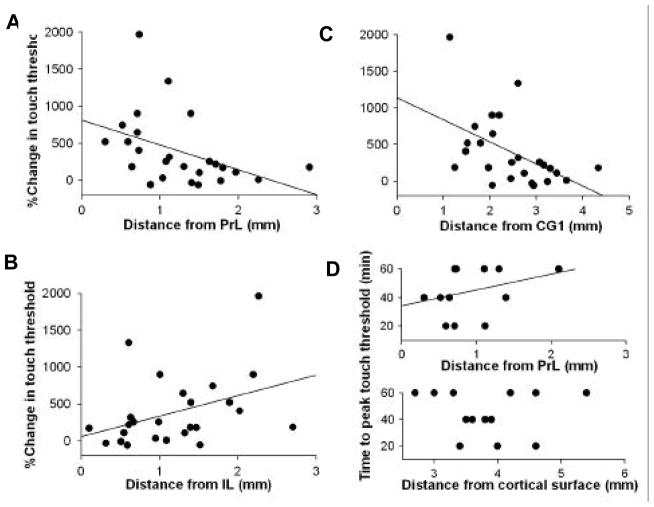
The mPFC is composed of multiple subdivisions: dorsally it is limited by the anterior cingulate (CG1), ventrally it is composed of prelimbic (PrL) and infralimbic (IL) cortical regions, and is ventrally limited by either medial orbital or dorsal peduncular cortex. Here we examined the effects of DCS infusions into these divisions of mPFC. The efficacy of DCS was examined by calculating the distance of cannula tips placed in mPFC from the center of different sub-regions, in relation to resultant antinociception, and to the time required to reach maximum antinociception by acute infusion of DCS. The distance-behavior relationship from the center of PrL (r = −0.48, p < 0.02) is opposite to that observed from the center of IL (r = 0.39, p < 0.06) (difference between these correlations was highly significant, p < 0.004) (Figure 6A and 6B). This indicated that DCS infusions closer to PrL and further away from IL were more antinociceptive. From the center of CG1, peak antinociception was found at 1–2 mm away from CG1 (corresponding to the distance of PrL from CG1) and decayed at further distances (r = −0.5, p < 0.01) (Figure 6C). In a subset of mPFC injection sites we measured the timecourse of DCS induced antinociception (tested at 20, 40, and 60 min after injection). This data showed a weak positive relationship with distance from PrL (non-significant) (Figure 6D, top panel). When the same data was displayed in relation to the distance from cortical surface, we found an inverted u-shaped dependence, with the fastest responses occurring at a depth of around 4 mm (distance of PrL from cortical surface), and slower responses occurring around 3 mm and 5–6 mm, which correspond to the coordinates of CG1 and IL respectively. Thus, the effects of DCS were greater and faster when administered into PrL, than when infused into IL or CG1.
Figure 6.
D-cycloserine infusions within mPFC are most effective when delivered into prelimbic (PrL) cortex. (A–C) Distances for infusion cannula tips within mPFC (from Figure 5) calculated from the center of PrL (A), center of infralimbic cortex (IL) (B), and center of anterior cingulate (CG 1) (C) are shown in relation to percent change in touch threshold induced by DCS for each infusion site. The greatest antinociceptive effect was seen when cannulas were in the proximity of PrL. (D) Times to peak increase in touch thresholds are shown relative to cannula tip distances from PrL (top panel), and relative to distances from the cortical surface (bottom panel). Both panels suggest that proximity to PrL induces faster antinociception. The linear regression fits in (A) and (C) are significant, only borderline significant in (B), and not significant in (D).
Site specificity within mPFC for DCS-induced antinociception diminishes the worry that DCS effects are due to its diffusion to remote unexplored sites. Moreover, PrL of mPFC in rodents is equivalent to DLPFC in primates (Vertes 2006). Thus, we speculate that the antinociceptive effects of DCS are due to modified cognitive abilities and only indirectly enhance extinction related circuitry.
Contrasting effects of D-cycloserine, NMDA, glycine, and CPP infusions into mPFC or spinal cord, before and after repeated oral treatment with D-cycloserine
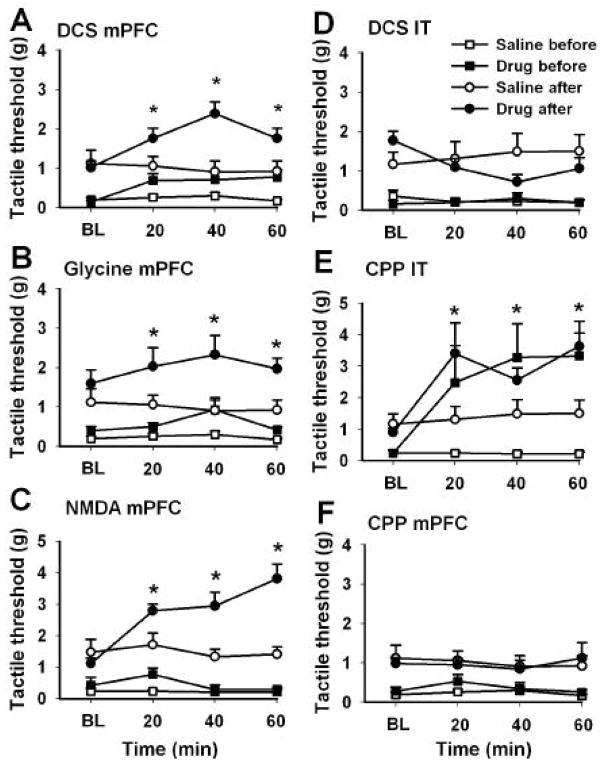
In this experiment, we studied the receptors involved in DCS antinociception by examining the effects of DCS, NMDA, glycine, and CPP infusions into the mPFC, as well as the effects of i.t. infusions of DCS and CPP given before and after a 2-wk treatment with repeated oral DCS (30 mg/kg twice a day). Glycine and NMDA infusion in mPFC should reveal the effects of activating their respective receptors, while CPP (a potent and selective NMDA receptor antagonist; Lehmann et al. 1987) identifies the effects of blocking NMDA mediated transmission. Similarities and differences between resultant responses show the receptor through which DCS induces antinociception within mPFC. Repeated oral DCS resulted in enhanced antinociception suggesting changes in central DCS efficacy. We test this directly by contrasting the effects of NMDA agonists and antagonists before and after repeated oral DCS treatment.
In comparison to the saline group, DCS infusions into mPFC (2 groups; n = 8–10 rats per group) increased tactile thresholds, both before and after the 2-wk oral DCS treatment, (replicating the results in Figure 5), increased baseline tactile sensitivity after the 2-wk oral DCS treatment (replicating the results in Figure 2), and enhanced the effect of subsequent DCS infusions into mPFC after the 2-wk oral DCS treatment (Figure 7A). There was a significant effect of treatment (DCS vs. saline infusions into mPFC, before and after oral DCS treatment, F3,32 = 21.4, p < 10−6), time (before, 20, 40, and 60 min after infusions; F3,96 = 4.9, p < 0.003), and a significant interaction between both factors (F9,96 = 3.0, p < 0.003). Oral DCS treatment significantly affected baseline tactile sensitivity (before vs. after 2-wk oral DCS, t17 = 3.5, p < 0.001). Effects of mPFC infusion of DCS was also significant independent of oral DCS treatment (prior to oral treatment, F1,16 = 5.7, p < 0.03; while post oral treatment, F1,16 = 8.7, p < 0.01). We also found that after the 2-wk oral DCS treatment, the efficacy of DCS infusions into the mPFC was potentiated for tactile thresholds (Figure 7A): There was a significant increase in magnitude of change in tactile thresholds between before and after oral DCS (threshold change relative to saline baseline before oral DCS was 0.48 +/− 0.57g, mean and S.D.; after oral DCS the change became 1.0 +/− 0.89g; the largest difference seen at 40 min t17 = 2.8, p < 0.01). Paw position ratings closely reflected the changes observed for tactile threshold (data not shown). It is noteworthy that oral DCS required multiple treatments to show antinociception while brain infusions showed acute effects. This difference may be due to central dose and duration of exposure to DCS, or perhaps due to distinct mechanisms. On the other hand, given that 2 wk oral DCS potentiated central DCS-evoked antinociception we presume a shared mechanism of action between both routes of treatment.
Figure 7.
Infusions of D-cycloserine (DCS), glycine, and NMDA into mPFC similarly reduced neuropathic behavior, both before (squares) and after (circles) repeated oral treatment with D-cycloserine. In contrast, D-cycloserine administered into the spinal cord (intrathecally, IT) showed minimal effects. (A) DCS (50 μg) infused into mPFC after a 2-wk oral treatment (30mg/kg twice a day) was more potent in reducing tactile allodynia than in the naïve condition (before). (B) Glycine (100 μg) and (C) NMDA (8 μg before, and 16 μg after 2 wks of oral DCS; given that the initial NMDA response was minimal, its dose was doubled after oral DCS) infusions exhibited the same profile as DCS, but not (F) CPP (0. 5μg). (D) Intrathecally injected, DCS (50 μg) did not reduce tactile allodynia in the naïve condition, and induced a borderline increased allodynia after 2 wks of oral treatment with DCS. (E) In contrast, intrathecal CPP (0.5 μg) exhibited a potent analgesic effect before and after repeated oral DCS for 2 wks. X-axis is time in min from central drug injections (BL, baseline). Asterisks indicate statistically significant differences; shown only between drug (filled) and saline (empty circles) after oral DCS. Error bars indicate S.E.M.
The effects of glycine and NMDA (note that the NMDA response after oral DCS is for a larger dose and does not indicate enhancement) infusions into mPFC before and after the 2-wk oral DCS treatment were similar to those of DCS infusions (Figure 7B and C). Therefore, we suppressed the statistical comparisons for these results. In contrast, CPP infusions into mPFC (Figure 7F), before and after the 2-wk oral DCS treatment, had no effect on touch thresholds or on paw position ratings. These results are consistent with the idea that the effects DCS in mPFC on neuropathic pain behavior are mediated through enhancement of glutamatergic transmission, with DCS acting as an agonist for glycine-recognition site of the NMDA receptor complex, and repeated oral DCS potentiating this agonistic activity.
We next studied the effects of DCS and CPP i.t. infusions, before and after a 2-wk oral DCS treatment (Figure 7D and E). Prior to oral DCS treatment, we observed no effect of i.t. DCS on tactile thresholds. However, after the 2-wk oral DCS treatment, tactile thresholds decreased significantly in response to i.t. DCS at 40 min (2 groups; n = 9–10 rats per group; baseline tactile threshold vs. 40 min after i.t. DCS, t17 =−2.2, p < 0.04). In contrast to CPP infusion into mPFC, i.t. CPP infusions (2 groups; n = 8 – 10 rats per group) resulted in increased tactile thresholds (and decreased paw position) both before and after oral DCS (treatment effect: i.t. CPP vs i.t. saline, before and after oral 2-wk DCS, F3,32 = 8.3, p < 0.0003; time effect: before, 20, 40, and 60 min post i.t. injections, F3,96 = 6.2, p < 0.0006, with a significant interaction F9,96 = 5.3, p < 0.05). The results highlight the differential effects of DCS in the cortex vs. spinal cord, and again suggest that its effects at both sites involve the NMDA receptor.
HA 966 blocked D-cycloserine-induced antinociception
The specificity of DCS infusions into the mPFC on pain behavior was studied by infusing HA 966 or saline into the mPFC 10 min prior to intra-mPFC DCS infusions in SNI rats (2 groups; n = 12 per group). Tactile thresholds for both paws were measured 1 h before and after the infusions. Infusions of HA 966 prior to DCS blocked the antinociceptive effect of intra-mPFC DCS infusions (t22 = 3.01, p < 0.006). Infusions of HA 966 or saline alone had no effect on tactile sensitivity (Figure 8).
Figure 8.
The strychnine-insensitive glycine-recognition site antagonist HA 966 blocked the effect of D-cycloserine (DCS) acutely infused into mPFC. DCS was infused into mPFC 10 min after infusing either saline (Sal) or HA966 into mPFC (n = 12 per group). Tactile thresholds were measured 1 h prior and 1 h after brain infusions, and are presented relative to the measurement prior to infusions. HA 966 infusions alone did not differ from saline alone and had minimal effects on tactile sensitivity in the uninjured paw. Asterisk indicates statistically significant difference. Error bars indicate S.E.M.
Upregulation of NR2B expression with long-term DCS treatment
We used RT-PCR analysis to evaluate the expression of AMPA and NMDA receptor components in the mPFC of three groups of rats: sham-injured, SNI-injured plus 2 wks of saline treatment, and SNI-injured plus 2 wks of DCS (30 mg/kg) treatment (treatments began 2 wks after injury, n = 5–7 per group; Figure 9). The level of NR2B expression in mPFC significantly decreased in SNI animals treated with saline (t10 = −2.4, p < 0.05). When SNI-injured rats were treated with DCS, NR2B expression was significantly upregulated (SNI-injured plus saline vs. SNI-injured plus DCS, t10 = 2.9, p < 0.02).
Figure 9.
Changes in the expression of NMDA NR2B with peripheral neuropathy and after treatment with D-cycloserine (DCS). Expression levels of NR2B were lower in the mPFC of SNI animals, in comparison with sham animals (n = 5–7 animals per group; arbitrary units normalized to GADPH). Moreover, treating SNI animals with DCS orally for 2 wks (30 mg/kg) reversed this pattern. Expression levels for NR2A, NR1, and gluR1 in mPFC were not affected by SNI injury or DCS treatment. Asterisks indicate statistically significant differences. Error bars indicate S.E.M.
We also performed RT-PCR analysis for other NMDA receptor subunits, NR1 and NR2A, and for AMPA receptor (gluR1), within the mPFC of sham-injured, SNI-injured and SNI-injured plus DCS treated rats. None of these receptor subunits showed any significant changes (p > 0.2).
D-cycloserine infusions into mPFC reversed place avoidance
The above measures of antinociception are based on reflexive responses. They localize the site of antinociception but do not necessarily reflect the impact of the antinociception on the organism as a whole. We studied the latter in a place avoidance task. The innate preference of a rat to remain in a dark environment was tested by mechanically stimulating the SNI-injured, hypersensitive, paw in the dark compartment and the uninjured, less sensitive, paw in the white compartment (Labuda and Fuchs 2001). The time spent in the white compartment was considered a measure of the aversiveness of mechanical stimulation of the injured paw relative to the aversiveness of the white compartment. We tested 7 conditions (n = 7–9 per group): sham animals with or without hind paw stimulation, SNI animals with or without hind paw stimulation, tested at 45 min and 24 h after an acute infusions of saline or DCS (50 μg) into the mPFC (Figure 10).
Figure 10.
Place avoidance was reversed by D-cycloserine (DCS) infusions into mPFC. (A) Timeline of preference of SNI animals at 5-minute time windows, across 30 min of monitoring. (B) Global preference (percent time in white chamber during testing) and (C) shifts in preference (time spent in white chamber in last 5 min – time spent in white chamber in first 5 min, in percent) in 7 groups of rats (n = 7 – 9 per group): sham or SNI animals (Sh or SNI), with or without paws stimulation (+, −), tested at 1 or 24 h after mPFC infusion, with saline or DCS. Asterisks indicate statistically significant differences; shown only in (B) Error bars indicate S.E.M.
Sham and non-stimulated SNI animals spent only about 25% of the time in the white chamber, and displayed a 10–20% shift in preference towards the black compartment. In contrast, saline-treated SNI animals that received hind paw stimulation spend the greatest amount of time in the white compartment (36 ± 5 %). Over time, they progressively shifted their preference to the white compartment (18 ± 10 %). Acute DCS infusions into mPFC reversed this behavior. The DCS-treated SNI animals that received hind paw stimulation spent most of their time in the dark chamber (time spent in white compartment was 7 ± 2 %) suggesting that recurrent stimulation of their SNI injured paw is not disturbing or painful enough to induce them to leave this compartment. Twenty-four hours after mPFC infusions, there were no differences between saline and DCS-treated animals in the place aversion task. Statistical analysis showed that preference was significantly dependent on group (F6,48 = 10.0, p < 10−5), time (F5,240 = 6.7, p < 10−5), and their interaction (F30,240 = 2.2, p < 0.0008). Specifically, saline-infused SNI animals that received stimulation were significantly different from DCS-infused SNI animals that received stimulation (t13 = 6.5, p < 10−5). Shift in preference (between first and last 5 min) also differed among groups (F6,48 = 5.3, p < 0.0003), with significant differences between saline- and DCS-infused animals that received stimulation (t13 = 4.3, p < 0.0001) as well as between SNI animals that either did or did not receive hind paw stimulation (t11 = −4.2, p < 0.0001) (Figure 10B).
DCS treatment and motor behavior
For all conditions tested (oral: acute, short-term chronic, and long-term chronic; and acute mPFC infusions; more than 60 rats tested), no modifications in motor behavior were detected in the open-field test (neither deficit nor hyperactivity). DCS-treated SNI animals moved as often to the central part of the enclosure as saline-treated SNI or sham rats. No anxiogenic or anxiolytic effects were detected. Therefore modifications in behavior after acute or repeated DCS-treatment were interpreted as specifically reflecting pain-related behavior.
Discussion
The main finding of the present study is that repeated treatment with DCS, a partial agonist at the strychnine-insensitive glycine-recognition site on the NMDA receptor complex (Furukawa and Gouaux 2003), robustly reduces tactile sensitivity and protective paw posturing in rat models of neuropathic pain. DCS appears to specifically affect hypersensitivity occurring in the injured paw (i.e. an anti-allodynia effect), as there were no behavioral changes observed for the contralateral uninjured paw. Moreover, as DCS treatment did not lead to changes in motor behavior, its effects cannot be attributed to neurotoxicity. The antinociception elicited by repeated DCS treatment was dose-dependent and increased in efficacy for up to 3 wks. Upon cessation of treatment, DCS effects on pain behavior persisted for a duration proportional to the length of treatment. When antinociception was assessed by measuring changes in mechanical sensitivity and in paw position, the effect of DCS treatment was relatively small (about 30%); however, a much larger effect of DCS treatment was revealed (complete reversal of preference) when antinociception was assessed by an operant stimulus avoidance task. In the present study we also showed that selective infusions of DCS into the PrL of mPFC and into the amygdala, (but not the spinal cord, thalamus, insula, or occipital cortices) are antinociceptive. This site specificity is consistent with the anatomy (mPFC and amygdala are reciprocally interconnected, Vertes 2006) and suggests that the effects of oral DCS treatment are mediated through these same brain regions, especially because the antinociceptive effect of intra-mPFC DCS infusions was enhanced in rats that received 2 wks of oral DCS treatment. The present finding that infusions of the glycine-recognition site antagonist HA 966 into the mPFC blocked DCS-induced antinociception strongly suggests that DCS effects are mediated by enhancing the NMDA receptor mediated neurotransmission. Importantly, infusions of HA 966 alone did not change neuropathic sensitivity. Furthermore, infusions of NMDA and glycine (but not CPP) into the mPFC mimicked DCS effects both acutely and after a 2-wk oral DCS treatment. Furthermore, the decrease observed in mPFC NR2B expression in SNI animals was reversed by oral DCS treatment.
The temporal profile of DCS-induced antinociception consisted of a slow onset, followed by a continued increase in efficacy for many weeks that was sustained after cessation of treatment. To our knowledge, this timeline is unique to DCS and has not been observed for other analgesics. This temporal profile reinforces the hypothesis that DCS’s site of action is outside of the areas known for classic analgesics (periphery, spinal cord, descending modulatory pathways). This temporal pattern, as well as the increased efficacy of DCS antinociception when the animals were re-exposed to the drug weeks after cessation of initial treatment, indicates that DCS continuously increases in efficacy and lacks tolerance, and has no observable side effects. This profile is different from that of opiate analgesics, in which repeated use results in decreased efficacy, hyperalgesia, and tolerance (Ossipov et al. 2004). The temporal property of DCS is also indicative of its molecular mechanisms. The half-life of DCS in the mouse CNS is 20 min (Conzelman, Jr. and Jones 1956), indicating that the drug is rapidly cleared. Thus, its increased efficacy with repeated treatment is not likely reflective of central concentration changes, but rather, of changes in receptor sensitivity as demonstrated by the enhancement of intra-mPFC DCS effects following a 2-wk oral treatment, perhaps as a direct consequence of increased NR2B expression in PrL of mPFC.
The present results of i.t. DCS and CPP infusions are consistent with extensive evidence that spinal cord NMDA receptors are up-regulated and contribute to central potentiation of nociceptive inputs in neuropathic pain (Davies and Lodge 1987; Woolf and Thompson 1991; Coderre and Melzack 1992; Wilson et al. 2005). Since specific subunits of the NMDA receptor are preferentially expressed in the spinal cord, many groups have used NMDA receptor antagonists as potential pharmacological means for controlling neuropathic pain (Garry and Fleetwood-Walker 2004). A recent study showed that rats with chronic constriction injury display increased NR2B, but not NR2A, and reduced NR1 in the superficial dorsal horn (Wilson et al. 2005). However, the increase in spinal cord NR2B may not be specific to neuropathic injury because increased NR2B phosphorylation is also observed in a rat model of inflammatory hyperalgesia (Guo et al. 2002). In the cortex, NR2B expression is increased in the anterior cingulate in inflammatory rat models, and this increase seems to directly contribute to the inflammatory pain (Wei et al. 2001; Wu et al. 2005). In contrast, we observe decreased NR2B in mPFC in neuropathic rats, implying that direction of change in cortical NR2B depends on the kind of persistent pain. Moreover, our results suggest that increased NR2B expression in the PrL of mPFC may be responsible for DCS treatment-induced antinociception, although we cannot exclude the possibility that NR2B expression may also be changing in CG1 and IL (mPFC tissue examined also included these regions). To our knowledge, this brain site specificity of DCS is also unique, because all other known classes of analgesics at least partially act on peripheral or spinal cord targets (Melzack and Wall PD 1999; De Vry et al. 2004; Lynch, III et al. 2004; Bayer et al. 2004).
The effect of repeat treatment with DCS for neuropathic pain appears to be very different from its effects on cognitive performance, and especially on fear extinction. DCS facilitation of conditioned fear extinction results in tolerance or desensitization if the animals are pre-exposed to DCS, and is reduced over time and requires weeks of abstinence to return to the efficacy seen in naïve animals (Parnas et al. 2005). In contrast, in the present study we observed dose-dependent and long-lasting antinociception after repeatedly treating animals with DCS. We interpret our finding to be consistent with the overall hypothesis that in chronic neuropathic states cognitive/emotional circuitry is actively depressed, as evidenced by decreased NR2B, and thus, DCS has a target that can be repeatedly used to overcome this depression. In addition, although neurons involved in fear extinction have been localized in IL of mPFC (Milad and Quirk 2002), in the present study the effects of DCS on neuropathic pain seem to be preferentially mediated by PrL rather than IL. Consistent with the idea that rodent PrL is equivalent to primate DLPFC (which is involved in cognition and decision making or planning) and that rodent IL is equivalent to primate mPFC (which is specific for emotional behavior) (Vertes 2006), PrL neurons increase in activity and IL neurons decrease during trace fear conditioning (Gilmartin and McEchron 2005). Similar reciprocal inhibitory fMRI responses in DLPFC and mPFC activity were recently found in chronic back pain (Baliki et al. 2006). Moreover, PrL in rodents seems to be specifically involved in the retrieval rather than the storage of memories (Simon et al. 2005), and in the selection of responses based on previously encoded associations (Corbit and Balleine 2003). Therefore, DCS-induced reinforcement of NMDA-receptor mediated transmission within PrL may facilitate the dissociation of relationships previously formed with spontaneous pain. Alternatively, it is also possible that DCS is mildly antihyperalgesic, and that repeated DCS exposure may uncouple incidental events from spontaneous pain as a result of sustained antihyperalgesia. These hypotheses remain to be explored in future studies.
Several recent studies have shown that cortical manipulations can modulate pain behavior (Baliki et al. 2003; Jasmin et al. 2003; Johansen and Fields 2004; Senapati et al. 2005; Han and Neugebauer 2005). These studies emphasize the role of the insula, anterior cingulate, mPFC, and amygdala in pain, all of which are limbic structures with strong interconnectivity. In particular, the study by Johansen and Fields (2004) is relevant because it suggests that anterior cingulate activity is both necessary and sufficient for noxious stimuli to produce an aversive memory. Anterior cingulate and PrL are in close proximity and tightly interconnected. It is possible that the two structures are involved in different components of pain-related memory traces.
Overall, our findings indicate that a cortical limbic pathway contributes to neuropathic pain via modulation of NMDA receptors. These results are pertinent to human clinical research on pain management. Brain imaging studies have shown that human mPFC is associated with fear extinction (Phelps et al. 2004), and is involved in emotion (Critchley et al. 2001; Dolan 2002) and in chronic pain (Baliki et al. 2006). Therefore, modulation of glutamatergic transmission between DLFPC and mPFC in humans could decrease the suffering associated with such conditions, which in the clinical setting is a primary complaint, and much harder to observe or test in rodents. Moreover, given that classical analgesics target different pathways, DCS-type treatments may be able to potentiate the effects of standard analgesics.
Acknowledgments
The authors thank Drs. D. R. Chialvo and J. Foss for valuable discussions throughout the course of this work. We also thank Drs. S. Khan, T. Schnitzer, M. Martina, J. Paice, and N Harden for reading earlier versions of this manuscript, and M. M. Baliki, and Drs. J. Katz and E. Mugniani for assistance in the completion of this study. Supported by National Institutes of Health NINDS NS 42660.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden R, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004a;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RE, Harden R, Parrish T, Gitelman D. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004b;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal N, Jazat F, Kayser V, Guilbaud G. Further evidence for ‘pain-related’ behaviours in a model of unilateral peripheral mononeuropathy. Pain. 1990;41:235–251. doi: 10.1016/0304-3959(90)90022-6. [DOI] [PubMed] [Google Scholar]
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp Neurol. 2003;182:12–20. doi: 10.1016/s0014-4886(03)00003-7. [DOI] [PubMed] [Google Scholar]
- Baliki M, Al Amin HA, Atweh SF, Jaber M, Hawwa N, Jabbur SJ, Apkarian AV, Saade NE. Attenuation of neuropathic manifestations by local block of the activities of the ventrolateral orbito-frontal area in the rat. Neuroscience. 2003;120:1093–1104. doi: 10.1016/s0306-4522(03)00408-1. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer K, Ahmadi S, Zeilhofer HU. Gabapentin may inhibit synaptic transmission in the mouse spinal cord dorsal horn through a preferential block of P/Q-type Ca2+ channels. Neuropharmacology. 2004;46:743–749. doi: 10.1016/j.neuropharm.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelman GM, Jr, Jones RK. On the physiologic disposition of cycloserine in experimental animals. Am Rev Tuberc. 1956;74:802–806. doi: 10.1164/artpd.1956.74.5.802. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146:145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Davies SN, Lodge D. Evidence for involvement of N-methylaspartate receptors in ‘wind-up’ of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987;424:402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- De Vry J, Kuhl E, Franken-Kunkel P, Eckel G. Pharmacological characterization of the chronic constriction injury model of neuropathic pain. Eur J Pharmacol. 2004;491:137–148. doi: 10.1016/j.ejphar.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss JM, Apkarian AV, Chialvo DR. Dynamics of pain: fractal dimension of temporal variability of spontaneous pain differentiates between pain States. J Neurophysiol. 2006;95:730–736. doi: 10.1152/jn.00768.2005. [DOI] [PubMed] [Google Scholar]
- Furukawa H, Gouaux E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J. 2003;22:2873–2885. doi: 10.1093/emboj/cdg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry EM, Fleetwood-Walker SM. Organizing pains. Trends Neurosci. 2004;27:292–294. doi: 10.1016/j.tins.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Grachev ID, Fredrickson BE, Apkarian AV. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain. 2000;89:7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Guo W, Zou S, Guan Y, Ikeda T, Tal M, Dubner R, Ren K. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J Neurosci. 2002;22:6208–6217. doi: 10.1523/JNEUROSCI.22-14-06208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Neugebauer V. mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain. 2005;113:211–222. doi: 10.1016/j.pain.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Hughes RN. Responsiveness to brightness change in male and female rats following treatment with the partial agonist of the N-methyl-D-aspartate receptor, D-cycloserine. Behav Brain Res. 2004;152:199–207. doi: 10.1016/j.bbr.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424:316–320. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- Labuda CJ, Fuchs PN. Low dose aspirin attenuates escape/avoidance behavior, but does not reduce mechanical hyperalgesia in a rodent model of inflammatory pain. Neurosci Lett. 2001;304:137–140. doi: 10.1016/s0304-3940(01)01787-6. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Schneider J, McPherson S, Murphy DE, Bernard P, Tsai C, Bennett DA, Pastor G, Steel DJ, Boehm C. CPP, a selective N-methyl-D-aspartate (NMDA)-type receptor antagonist: characterization in vitro and in vivo. J Pharmacol Exp Ther. 1987;240:737–746. [PubMed] [Google Scholar]
- Lynch JJ, III, Wade CL, Zhong CM, Mikusa JP, Honore P. Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy-induced neuropathic pain model. Pain. 2004;110:56–63. doi: 10.1016/j.pain.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Textbook of pain. London: Churchill livingston; 1999. pp. 1125–1250. [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Seguin L. Chemically-diverse ligands at the glycine B site coupled to N-methyl-D-aspartate (NMDA) receptors selectively block the late phase of formalin-induced pain in mice. Neurosci Lett. 1994;178:139–143. doi: 10.1016/0304-3940(94)90309-3. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, King T, Vanderah TW, Malan TP, Jr, Hruby VJ, Porreca F. Antinociceptive and nociceptive actions of opioids. J Neurobiol. 2004;61:126–148. doi: 10.1002/neu.20091. [DOI] [PubMed] [Google Scholar]
- Parnas AS, Weber M, Richardson R. Effects of multiple exposures to d-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83:224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena dO, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Senapati AK, Lagraize SC, Huntington PJ, Wilson HD, Fuchs PN, Peng YB. Electrical stimulation of the anterior cingulate cortex reduces responses of rat dorsal horn neurons to mechanical stimuli. J Neurophysiol. 2005;94:845–851. doi: 10.1152/jn.00040.2005. [DOI] [PubMed] [Google Scholar]
- Simon B, Knuckley B, Churchwell J, Powell DA. Post-training lesions of the medial prefrontal cortex interfere with subsequent performance of trace eyeblink conditioning. J Neurosci. 2005;25:10740–10746. doi: 10.1523/JNEUROSCI.3003-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Stromme JT, Myhrer T. Impaired visual memory in rats reared in isolation is reversed by D-cycloserine in the adult rat. Eur J Pharmacol. 2002;437:73–77. doi: 10.1016/s0014-2999(02)01282-7. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Wang GD, Kerchner GA, Kim SJ, Xu HM, Chen ZF, Zhuo M. Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nat Neurosci. 2001;4:164–169. doi: 10.1038/83993. [DOI] [PubMed] [Google Scholar]
- Wilson JA, Garry EM, Anderson HA, Rosie R, Colvin LA, Mitchell R, Fleetwood-Walker SM. NMDA receptor antagonist treatment at the time of nerve injury prevents injury-induced changes in spinal NR1 and NR2B subunit expression and increases the sensitivity of residual pain behaviours to subsequently administered NMDA receptor antagonists. Pain. 2005;117:421–432. doi: 10.1016/j.pain.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, Jia YH, Shum FW, Zerbinatti CV, Bu G, Wei F, Xu TL, Muglia LJ, Chen ZF, Auberson YP, Kaang BK, Zhuo M. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25:11107–11116. doi: 10.1523/JNEUROSCI.1678-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]