Abstract
Background
Accurate quantification of mitral regurgitation (MR) is important for patient treatment and prognosis. Three-dimensional echocardiography allows for the direct measure of the regurgitant orifice area (ROA) by 3D-guided planimetry of the vena contracta area (VCA). We aimed to (1) establish 3D VCA ranges and cutoff values for MR grading, using the American Society of Echocardiography–recommended 2D integrative method as a reference, and (2) compare 2D and 3D methods of ROA to establish a common calibration for MR grading.
Methods and Results
Eighty-three patients with at least mild MR underwent 2D and 3D echocardiography. Direct planimetry of VCA was performed by 3D echocardiography. Two-dimensional quantification of MR included 2D ROA by proximal isovelocity surface area (PISA) method, vena contracta width, and ratio of jet area to left atrial area. There were significant differences in 3D VCA among patients with different MR grades. As assessed by receiver operating characteristic analysis, 3D VCA at a best cutoff value of 0.41 cm2 yielded 97% of sensitivity and 82% of specificity to differentiate moderate from severe MR. There was significant difference between 2D ROA and 3D VCA in patients with functional MR, resulting in an underestimation of ROA by 2D PISA method by 27% as compared with 3D VCA. Multivariable regression analysis showed functional MR as etiology was the only predictor of underestimation of ROA by the 2D PISA method.
Conclusions
Three-dimensional VCA provides a single, directly visualized, and reliable measurement of ROA, which classifies MR severity comparable to current clinical practice using the American Society of Echocardiography–recommended 2D integrative method. The 3D VCA method improves accuracy of MR grading compared with the 2D PISA method by eliminating geometric and flow assumptions, allowing for uniform clinical grading cutoffs and ranges that apply regardless of etiology and orifice shape.
Keywords: mitral regurgitation, diagnosis, 3D echocardiography, Doppler echocardiography
Accurate assessment of mitral regurgitation (MR) is important for clinical decision-making and outcome prediction. The calculation of an effective regurgitant orifice area (ROA) by the proximal isovelocity surface area method (PISA) is a main method for quantification of mitral regurgitation. However, it requires flow and geometric (hemisphere) assumptions, which has limited its clinical application. Recently, 3D echocardiography allowed for the direct measurement of effective regurgitant orifice area by 3D-guided planimetry of the vena contracta area (VCA). This single measure is not dependent on geometric and flow assumptions; therefore it can provide direct and more accurate quantification of MR than 2D measurements. In vitro validation and clinical studies showed that direct planimetry of the VCA was highly feasible, and this measurement correlated well with ROA derived by the volumetric Doppler approach.1,2 Currently, however, there are no data on the ranges and cutoff values of 3D VCA for MR grading, compared with standard reference grading criteria using the 2D integrative method proposed by the American Society of Echocardiography (ASE), which is the method of MR grading used by most clinical echocardiography laboratories. We aimed to (1) establish 3D VCA ranges and cutoff values for differentiation of MR grades, using the 2D integrative method as a reference, and (2) compare color Doppler 2D and 3D VCA for MR grading.
Methods
Patient Population
From September 2008 to August 2010, 102 patients with at least mild MR who underwent color Doppler 2D and 3D echocardiography were initially enrolled in this study.
Exclusion criteria included significant mitral stenosis (area <2.0 cm2), cleft mitral valve, mitral prosthesis or annuloplasty, infective endocarditis, irregular rhythm, poor 2D or 3D image quality, and undetermined etiology of MR.
The echocardiographic protocol and data were approved by the committee of the Massachusetts General Hospital Institutional Review Board.
Echocardiographic Examination
Two-dimensional and 3D color Doppler echocardiographic examinations were performed by using the iE33 ultrasound system (Philips Medical Systems, Andover, MA).
Three-Dimensional Color Doppler Acquisition and Data Analysis
A full-volume 3D color Doppler acquisition was obtained with the use of the X3-1 (1–3 MHz) matrix array transducer from the apical window over 7 to 14 consecutive cardiac cycles with ECG gating, the narrowest sector possible to maximize the frame rate, and Nyquist velocities of 50 to 80 cm/s. Patients were asked to hold respiration during acquisition if possible or to breathe quietly.
The 3D color MR jet dataset was analyzed at the Xcelera workstation using Philips Qlab 2.0 software. Two orthogonal image planes parallel to the regurgitant jet direction were manually cropped across the regurgitant jet; a third cropping plane, which was perpendicularly oriented to the jet direction, was then moved along the jet direction until the cross-sectional area at the level of the vena contracta was visualized. The frame with the largest VCA in systole was magnified, and VCA was measured by direct planimetry of the color Doppler flow signal3 (refer to the online-only Data Supplement Video). To analyze the circularity of the regurgitant orifice, the ratio of the long axis to the short axis of VCA (L/S ratio) was calculated.
Two-Dimensional Echocardiography and Measurements
Two-dimensional echocardiography was performed with the use of a S5-1 probe. LV end-diastolic and end-systolic diameters (LVEDD and LVESD) were measured by the 2D method from parasternal long-axis view. LV end-diastolic volume (EDV) and LV end-systolic volumes (ESV) were measured using the Simpson biplane method, and LVEF was calculated as (EDV–ESV)×100/EDV. Two-dimensional quantification of MR included the proximal isovelocity surface area (PISA) method, vena contacta width (VCW), and ratio of jet area to left atrial area. A narrow color flow sector width and the least depth were chosen to maximize image resolution.
PISA Method
Proximal flow convergence was acquired from magnified apical 4-chamber, 2-chamber, and long-axis views, with baseline shift of the Nyquist limit (30–40 cm/s) to optimize visualization of flow convergence. The ROA was calculated using the formula ROA=2 π×RPISA2×Valiasing/Vmax, where RPISA was the maximal PISA radius (cm), Valiasing was aliasing velocity of the proximal flow convergence (cm/s), and Vmax was maximal velocity of continuous-wave Doppler MR signal (cm/s). MR volume was calculated as (ROA×regurgitant time-velocity integral). The severity of MR was graded on the basis of current ASE recommendations as mild (<0.2 cm2), moderate (0.2 to 0.39 cm2), or severe (≥0.40 cm2).4
Vena Contracta Width
The vena contracta was acquired from a magnified parasternal long-axis view with the central beam through the leaflet tips. Vena contracta width (VCW) was defined as the narrowest width of the proximal jet measured at or in the immediate vicinity of the MR orifice at the leaflet tips. The severity of MR was graded on the basis of current recommendations as mild (<0.3 cm), moderate (0.3 to 0.69 cm), or severe (≥0.7 cm).4-6
Jet Area to Left Atrial Area Ratio
The color flow Doppler image of the MR jet was acquired from the apical 4- and 2-chamber views at a Nyquist limit of 50 to 60 cm/s. The ratio of MR jet area to left atrial area (JA/LAA) was calculated from the average of both views. The severity of MR was graded on the basis of current recommendations as mild (<20%), moderate (20% to 39%), or severe (≥40%).4
Two-Dimensional Integrative Method
The 2D integrative method recommended by the ASE as follows4 was used as the reference standard for MR grading because this method does not rely on only 1 color Doppler method and is used widely in clinical laboratories.7 To categorize MR within a certain grade, at least 2 of 3 color Doppler methods listed above were assessed within the same grade with at least 1 supportive data (pulmonary vein flow; mitral inflow; density of continuous wave Doppler MR jet; left atrial enlargement). The integrative grading and 3D VCA measurement were done independently, and the results were blinded to each other.
Statistical Analysis
Results are expressed as mean±SD for continuous variables and as percentages for categorical variables. Differences between groups were analyzed with the unpaired or paired t test. Differences among more than 2 groups were assessed by 1-way ANOVA test with the Bonferroni correction. The linear association between continuous variables was made with the Pearson correlation coefficient. The Spearman rank correlation method was used to assess the associations between grading of MR by the 2D integrative method and by each quantitative method. The diagnostic values of echocardiographic parameters for MR quantification were analyzed by receiver operating characteristic (ROC) curve. The optimal cutoff value was defined as the value for which the sum of sensitivity and specificity was maximized. Area under the curve (AUC), sensitivity, specificity, and positive and negative likelihood ratios were reported. Agreement between methods was tested by using Bland-Altman analysis and plotted with lines representing mean±2 SD. The degree of the underestimation of ROA by 2D PISA method was calculated as the ratio of 2D ROA to 3D VCA (2D ROA/3D VCA). Single predictor and multivariable linear regression with a stepwise approach were performed to identify the predictors associated with underestimation of ROA by 2D PISA method. A probability value of <0.01 was considered statistically significant for all analyses. SPSS 13.0 software was used for statistical analysis (SPSS Inc, Chicago, IL).
Results
Characteristics of the Study Population
One hundred two patients were initially enrolled in the study; 19 patients were excluded because of poor 3D image quality that was technically inadequate or suboptimal for 3D cropping or direct planimetry of 3D VCA. The majority (68%) of the excluded patients with poor image quality were patients with mild MR. The final study group consisted of a total 83 patients (mean age, 67±19 years; 41 men). The etiology of MR was functional in 39 patients (47%) and degenerative in 44 patients. Eccentric MR jet was present in 39 (47%) patients, whereas central MR jet was present in 44 patients. Mean LVEDD was 52.1±8.8 mm, mean LVESD was 38.8±11.8 mm, and mean LVEF was 50.3±19.2%. All patients were in sinus rhythm, with the mean heart rate of 71±17 bpm.
Assessment of VCA by 3D Color Doppler Echocardiography
MR severity was graded as mild, moderate, or severe, according to the 2D integrative method as a reference standard. The 2D and 3D echocardiographic quantitative variables in various grades are summarized in Table 1. There were significant differences in 3D VCA among mild, moderate, and severe MR groups as assessed by the 2D integrative criteria, as well as the 2D ROA, VCW, and JA/LAA (all P<0.001).
Table 1. Two-Dimensional and 3D Quantitative Variables in Mild, Moderate, and Severe MR as Assessed by the 2D Integrative Method.
| Mild MR (n=15) |
Moderate MR (n=34) |
Severe MR (n=34) |
|
|---|---|---|---|
| 3D VCA, cm2 | 0.15±0.06 | 0.34±0.09 | 0.66±0.21 |
| 2D ROA, cm2 | 0.13±0.05 | 0.25±0.08 | 0.57±0.25 |
| MR volume, mL | 25±11 | 42±12 | 83 ±37 |
| VCW, cm | 0.29±0.11 | 0.47±0.15 | 0.84±0.16 |
| JA/LAA | 0.17±0.06 | 0.29±0.09 | 0.40±0.12 |
MR indicates mitral regurgitation; VCA, vena contracta area; ROA, regurgitant orifice area; VCW, vena contracta width; and JA/LAA, ratio of jet area to left atrial area.
One-way ANOVA test was used to compare mean values for variables among different groups (all P<0.001).
Diagnostic Values of 3D VCA to Differentiate Between Moderate and Severe MR
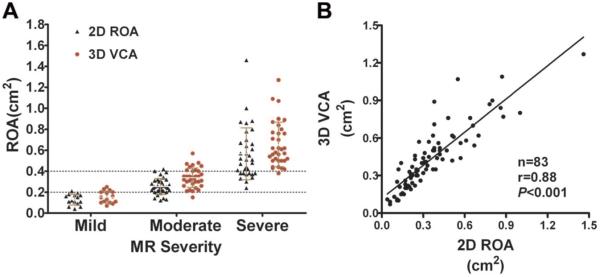
Spearman rank correlation showed that 3D VCA had the best correlation with the 2D integrative method (r=0.88). The correlation coefficient between 2D ROA, MR volume, VCW, JA/LAA, and the 2D integrative method was 0.86, 0.77, 0.83, and 0.65, respectively. As assessed by ROC analysis, the AUC was 0.96, 0.95, 0.94, and 0.78 for 3D VCA, 2D ROA, VCW, and JA/LAA, respectively, to differentiate moderate from severe MR. Three-dimensional VCA at an optimal cutoff value of 0.41 cm2 yielded a sensitivity of 97% and a specificity of 82% to differentiate moderate from severe MR (Figure 1). The best cutoff value for 2D ROA was 0.32 cm2, lower than 3D VCA. The diagnostic and cutoff values of these variables are demonstrated in Table 2.
Figure 1.
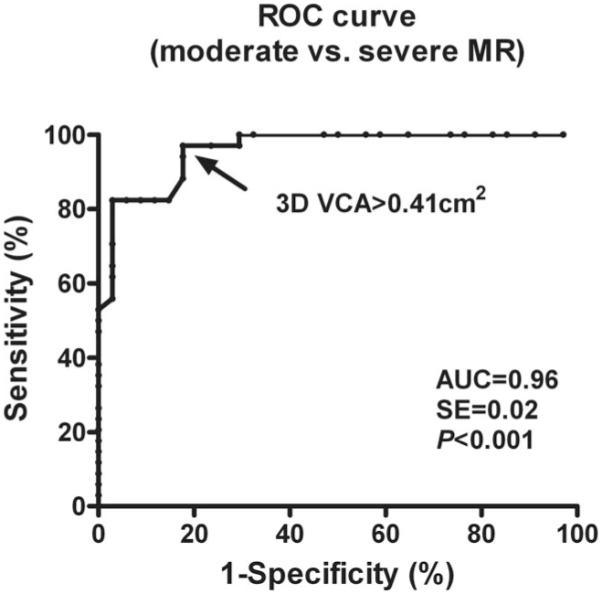
Receiver operating characteristic (ROC) analysis shows that 3D vena contracta area (VCA) with a cutoff value of 0.41 cm2 is a highly accurate parameter for the detection of severe mitral regurgitation (MR). AUC indicates area under the curve.
Table 2. ROC Analysis of Echocardiographic Parameters for Identification of Severe MR.
| Cutoff | AUC (95% CI) | Sensitivity, % | Specify, % | + LR | −LR | |
|---|---|---|---|---|---|---|
| 3D VCA, cm2 | 0.41 | 0.96 (0.88–0.99) | 97 | 82 | 5.50 | 0.04 |
| 2D ROA, cm2 | 0.32 | 0.95 (0.87–0.99) | 94 | 85 | 6.40 | 0.07 |
| VCW, cm | 0.62 | 0.94 (0.86–0.98) | 91 | 91 | 10.33 | 0.10 |
| JA/LAA | 0.39 | 0.78 (0.66–0.87) | 65 | 88 | 5.50 | 0.40 |
ROC indicates receiver operating characteristic; MR, mitral regurgitation; AUC, area under the curve; CI, confidence interval; +LR, positive likelihood ratio; −LR, negative likelihood ratio; VCA, vena contracta area; ROA, regurgitant orifice area; VCW, vena contracta width; and JA/LAA, ratio of jet area to left atrial area.
Correlations and Differences Between 2D and 3D Methods
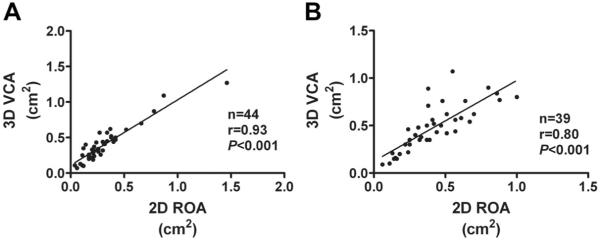
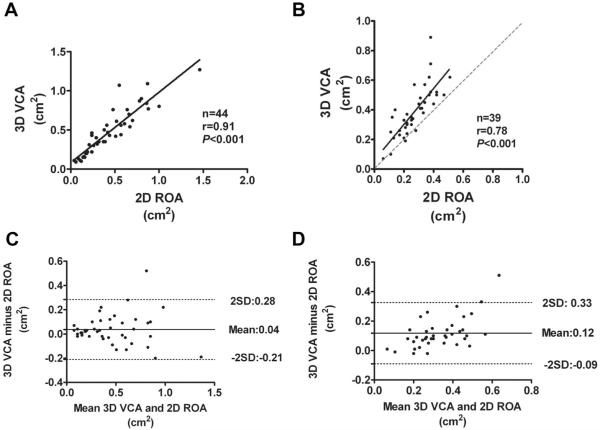
The ranges of 3D VCA and 2D ROA in different MR grades are demonstrated in Figure 2A. Three-dimensional VCA correlated well with 2D ROA(r=0.88, P<0.001, Figure 2B) in all patient sample. Correlations were strong for both central and eccentric MR jets, although patients with central MR jet showed a significantly better agreement than patients with eccentric MR jets (r=0.93 versus r=0.80, P<0.001, Figure 3A and 3B). The correlation between 3D VCA and 2D ROA demonstrated a significantly better agreement in patients with degenerative MR than functional MR (r=0.91 versus r=0.78, P<0.001, Figure 4A and 4B).
Figure 2.
A, Correlation between 3D vena contracta area (VCA) and 2D regurgitant orifice area (ROA) for the entire patient sample. B, Ranges of 3D VCA and 2D ROA in different mitral regurgitation (MR) grades as assessed by the 2D integrative method.
Figure 3.
Correlations between 3D vena contracta area (VCA) and 2D regurgitant orifice area (ROA) in patients with central mitral regurgitation (MR) jet (A) and patients with eccentric MR jet (B).
Figure 4.
Correlations between 3D vena contracta area (VCA) and 2D regurgitant orifice area (ROA) in patients with degenerative mitral regurgitation (MR) (A) and in patients with functional MR (B). Solid line represents linear regression fit through all the points; dashed line in B represents the line of identity. Bland-Altman plots depict ROA bias, using the 2D proximal isovelocity surface area method and 3D direct planimetry method in patients with degenerative MR (C) and patients with functional MR (D).
The paired t test showed that 3D VCA was significantly higher than 2D ROA in the entire patient sample (0.43±0.25 cm2 versus 0.36±0.24 cm2; mean difference, 0.08 cm2; P<0.001). Bland-Altman analysis showed that 3D VCA was slightly higher than 2D ROA in patients with degenerative MR (0.47±0.30 cm2 versus 0.44±0.30 cm2; mean difference, 0.04 cm2; P=0.05; Figure 4C); however, the difference was more statistically significant in patients with functional MR (0.39±0.17 cm2 versus 0.27±0.11 cm2; with a mean difference, 0.12 cm2; P<0.001; Figure 4D), resulting in an underestimation of ROA by 2D PISA method by a mean of 27% when compared with 3D VCA. This is demonstrated in Figure 4D, with an overall positive slope of overestimation by 3D VCA in patients with functional MR. If the currently recommended ranges and cutoff values of <0.2 cm2, 0.20–0.39 cm2, and ≥0.4 cm2 were applied for grading MR severity as mild, moderate, and severe, respectively, for both 2D ROA and 3D VCA, compared with 2D ROA, 31.3% of the patients would be upgraded to a more severe grade, based on the measurement of 3D VCA, among which 69% were the patients with functional MR. Two-dimensional ROA was in accordance with 3D VCA in MR grading in 67% of the patients. Similarly, when compared with 2D ROA, 19.3% of the patients would be upgraded 1 grade of MR severity by 2D integrative method, among which 75% were patients with functional MR.
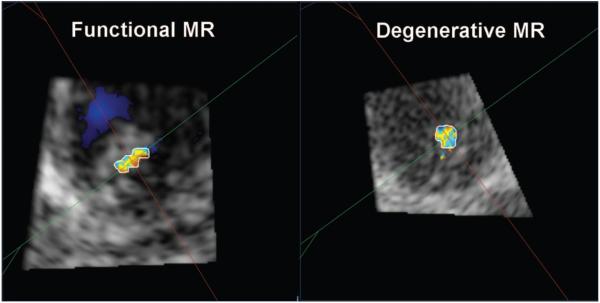
The geometry of VCA in the majority of patients was elliptical rather than circular, even in patients with degenerative MR. Patients with functional MR had a more elongated and elliptical shape of VCA (as assessed by L/S ratio of VCA) compared with patients with degenerative MR (2.25±0.85 versus 1.62±0.48 cm, P<0.01; Figure 5A and 5B).
Figure 5.
Geometry of vena contracta area is more elliptical in patients with functional mitral regurgitation (MR) compared with degenerative MR.
By single-predictor regression analysis, variables including LVESV, LVEF, eccentricity of MR jet, L/S ratio, and functional MR as etiology were statistically significant predictors for the underestimation of ROA by 2D PISA method (Table 3). Six variables with a probability value of <0.1 by single-predictor regression analysis were entered as covariates in the multivariable model, including LVESV, LVEF, eccentricity of MR jet, L/S ratio, functional MR as etiology, and heart rate. Multivariable analysis showed that functional MR as etiology was the only independent predictor of the underestimation of ROA by 2D PISA method (P<0.001).
Table 3. Single-Predictor and Multivariable Regression Analysis of Predictors of Underestimation of ROA by 2D PISA Method Compared With 3D VCA.
| Single-Predictor |
Multivariable |
|||
|---|---|---|---|---|
| β-Coefficient | P Value | β-Coefficient | P Value | |
| Age | −0.04 | 0.73 | … | … |
| LVEDV | −0.11 | 0.31 | … | … |
| LVESV | −0.24 | 0.03 | −0.006 | 0.96 |
| LVEF | 0.34 | 0.002 | 0.08 | 0.60 |
| MR severity | 0.08 | 0.48 | … | … |
| Eccentricity of MR jet | 0.27 | 0.01 | 0.18 | 0.09 |
| L/S ratio | −0.29 | 0.008 | −0.14 | 0.21 |
| Functional MR as etiology | −0.41 | <0.001 | −0.41 | <0.001 |
| Heart rate | −0.19 | 0.09 | −0.14 | 0.17 |
| Acquisition beats, 7 or 14 | −0.08 | 0.5 | … | … |
ROA indicates regurgitant orifice area; PISA, proximal isovelocity surface area; VCA, vena contracta area; LV, left ventricular; EDV, end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction; MR, mitral regurgitation; and L/S ratio, ratio of the long axis to the short axis of VCA.
Reproducibility of 3D VCA Measurement
To evaluate interobserver variability, 2 independent observers (X.Z. and L.H.) measured the 3D VCA in 15 patients, using the same 3D datasets. To test the intraobserver variability, measurement of 3D VCA was repeated by the same observer (X.Z.) 3 months later. Bland-Altman analysis and interclass correlation coefficient were used to assess the observer variability.
There was good interobserver variability for 3D VCA (interclass correlation coefficient, 0.92; 95% confidence interval, 0.79 to 0.97; mean difference, −0.03 cm2) as well as intraobserver variability (interclass correlation coefficient, 0.95; 95% confidence interval, 0.85 to 0.98; mean difference, −0.04 cm2). The Bland-Altman plot for observer variability is provided in the online-only Data Supplement Figure.
Discussion
The results of the present study showed that 3D VCA correlated well with the 2D integrative method of assessing MR severity. In addition, 3D VCA showed high diagnostic value for separating moderate from severe MR in all etiologies. The etiology of MR was the only factor independently affected the accuracy of ROA derived by the 2D PISA method. The 2D PISA method underestimated the ROA in patients with functional MR because of the hemispherical assumption when the PISA zone was more often hemielliptical. Three-dimensional VCA, which allows for direct planimetry of the regurgitant orifice area, circumvents this inherent limitation of the 2D PISA method and provides uniform clinical grading cutoffs and ranges that apply regardless of etiology and orifice shape.
Quantification of MR by Color Doppler 2D and 3D Echocardiography
Accurate assessment of MR is important but still challenging in daily clinical practice. Currently, various color Doppler 2D methods are used for MR quantification; however, each method has its limitations, based on technical issues or inaccurate geometric assumption. For example, the JA/LAA method is limited by instrument settings, hemodynamics, jet eccentricity, left atrial compliance, and so forth. The 2D PISA method provides an indirect measurement of the ROA, and the limitations arise from geometric assumption of a hemispherical regular shape, which appears to be less frequent than a hemi-elliptical one. Similarly, 1-dimensional measurement of VCW is used as a surrogate of an often irregular ROA, which limits its correlation. In individual cases, the results of different methods may be discordant. Even among experienced echocardiographers, intraobserver variability for these common parameters is high.8 Therefore, integrating different parameters would be more reliable for MR grading by minimizing the limitations of each method.
Besides the 2D methods, recent studies showed that planimetry of the VCA was highly feasible by using color Doppler 3D echocardiography, which provided a new approach for MR quantification by direct measurement of the ROA. Because there are no geometric and flow assumptions involved in the 3D method, direct planimetry of the VCA might be more accurate than conventional 2D calculations of effective ROA. A recent study by Marsan reported that 3D VCA was highly accurate compared with MR volume measured by velocity-encoded cardiac magnetic resonance in patients with functional MR.9 However, without the information of ranges and cutoff values for MR grading, it is difficult to apply 3D VCA in daily clinical practice. In the present study, our results showed that 3D VCA was a powerful method for accurate quantification of MR severity and had the best correlation with the 2D integrative method compared with other color Doppler methods. Moreover, the ability to accurately distinguish severe from nonsevere MR is of particular clinical importance, because severe MR is associated with significant LV remodeling, morbidity, and mortality10,11 and is often the grade at which clinical decision-making is based. Severity of MR has a significant influence on the decision toward timing of surgical intervention or watchful waiting as well; as a result, current American College of Cardiology/American Heart Association guidelines recommend surgery for asymptomatic severe MR.12 By using ROC curve analysis, we found that 3D VCA at a cutoff value of 0.41 cm2 had high sensitivity and specificity to identify severe MR for all etiologies. The current study establishes the ranges of 3D VCA in different MR grades and proposes a reliable cutoff value for differentiating severe from nonsevere MR, which would facilitate this new method in clinical use.
Comparisons of 2D and 3D Methods for MR Grading
The 2D PISA method is widely and frequently used for MR grading, which is based on the assumption of hemispheric symmetry of proximal flow convergence region.13 In our study, overall, the 2D PISA method correlated with both the 2D integrative method and 3D VCA. However, the correlation between the 2D PISA method and 3D VCA was significantly worse in patients with eccentric MR than central MR. It is well known that the PISA method has limitations in assessing eccentric jets due to the theoretical, technical, or measurement errors,8,14 whereas 3D VCA might be more accurate because it is derived by subsequent cropping, which is exactly perpendicular to the laminar vena contracta flow. Although no gold standard is available for comparison, this result supports the previous finding of the advantages of 3D VCA in assessing eccentric MR jets compared with 2D methods.1,2
The 2D PISA method can underestimate or overestimate the ROA when geometric assumptions do not fit. The geometry of PISA is often not hemispheric but hemi-elliptic, and the shape of the ROA may be noncircular.15 Earlier studies showed that the 2D PISA method, ignoring the horizontal length of PISA, could systematically underestimate the actual ROA in cases with a hemi-elliptical orifice.16-19 Furthermore, recent color Doppler 3D studies revealed that different geometry of PISA and shape of ROA might be associated with the etiology of MR. Matsumura et al20 demonstrated an elongated and slightly curved PISA geometry along the leaflet coaptation in functional MR, whereas the shape was rounder in mitral valve prolapse. Kahlert et al3 found significant asymmetry of the ROA in functional MR compared with organic MR. Our results are consistent with these previous findings. We observed elliptical-shaped VCA in most cases, even in degenerative MR. In addition, patients with functional MR had a significantly more elliptical shape of VCA than that in degenerative MR. By multivariable regression analysis, functional MR as etiology was the only predictor of underestimation of ROA by the 2D PISA method.
Our results support reevaluating the cutoff value for severe MR based on etiologies if the 2D PISA method is applied. The recommended cutoff value of the ROA according to ASE recommendation4 and the Dujardin et al study21 for severe MR is 0.4 cm2. However, Grigioni et al22,23 proposed a different cutoff value in patients with ischemic MR. ROA ≥0.2 cm2 by either 2D PISA or volumetric Doppler approach, which might otherwise be qualified as moderate MR, was independently associated with severe clinical consequences in post–myocardial infarction patients. Thus, such patients should be considered to have clinically severe MR. Our results are in agreement with these clinical findings. Because the 2D PISA method systematically underestimates the ROA in patients with functional MR, a lower cutoff value (<0.4 cm2) might be considered as severe in such patient samples. With the study population of all etiologies, our results showed that although the 2D PISA method had high accuracy in separating severe from nonsevere MR, the best cutoff value was 0.32 cm2, lower than the value recommended by ASE and the cutoff value of 3D VCA. Because the best cutoff point is subject to a specific study population, further studies with a larger population may be required to determine the cutoff value for severe functional MR by using the 2D PISA method. Current 2D ROA methods thus appear to require different grading scales for functional versus degenerative MR. Quantitative 3D VCA can be used to potentially establish unified normative ranges for MR grading that would apply to all MR etiologies.
Clinical Implications
This study proposes ranges for 3D VCA for MR grading, which is important for clinical reference and highlights the potential advantages of using 3D VCA over 2D ROA. Our study emphasizes the importance of etiology of MR in the underestimation of ROA by the 2D PISA method and supports redefining the ranges of MR degree based on etiologies if the 2D PISA method is applied. Three-dimensional VCA provides a reliable alternative method for MR grading with a unified range for all etiologies, which is likely to be more consistent in functional MR and probably when the ROA is elliptical, even in degenerative MR. Therefore, its clinical use can be applied in such patient populations or in cases of borderline severity.
Limitations
Some limitations should be acknowledged. First, 3D planimetry of the vena contracta has limited spatial resolution, particularly in the nonaxial planes (lateral resolution). In the present study, approximately 19% of patients were excluded because of technically inadequate image quality for planimetry of the VCA. To optimize spatial resolution, depth was adjusted for maximal resolution and 3D data acquisitions were performed by using a minimum of 7 beats and preferably 14 beats whenever possible. In addition, color gain settings were set in a standardized manner by increasing gain just below level where random noise develops, allowing for uniform color gain settings. The VCA was measured at aliased velocities, avoiding potential color bleeding that may occur at lower nonaliased velocities. Limited spatial resolution is more likely to affect mild MR ranges where the VCA is smallest and probably accounts for the varied results with mild MR.1,24 In the present study, planimetry of the VCA was inadequate or suboptimal in about 45% of patients with mild MR. However, clinical decision-making is based predominantly in the moderate to severe MR ranges, where spatial resolution limitations should have a lesser effect. Thus, our study primarily focused on the diagnostic value of 3D VCA for differentiating moderate from severe MR, which is also more clinically important. Second, variation in ROA during systole was not taken into account by this method. Although integrating instantaneous effective ROA throughout the cardiac cycle would take into account ROA variation, this is not practical in the clinical setting. No color Doppler technique avoids this limitation. Practice guidelines have supported the use of single ROA measurements for quantification of MR. The variation in ROA has less of an impact in degenerative MR such as flail leaflet, where the ROA is generally uniform throughout systole. In ischemic/functional MR, the temporal pattern of ROA during systole is bimodal, with peaks early and late in systole and minimum during midsystole. The bimodal pattern in ischemic MR is enhanced in lower ranges of MR (mild), where driving pressure can have a greater effect at midsystole. In larger degrees of MR (moderate or greater), where driving pressure has less of an impact due to the larger MR flows, the bimodal pattern is blunted. The impact of ROA should be minimized in the more clinically important situations involving moderate or greater MR ranges. Third, the accuracy of 3D VCA would be affected by the 3 cropping planes, especially the cross-sectional plane. In some cases with eccentric MR, the perfect plane of 3D VCA could be difficult to determine. Inappropriate cropping would result in overestimation of VCA. Fourth, stitch artifacts that might be caused by arrhythmias, respiration, patient motion, and movements of the probe by the operator also affect the accuracy of results. Last, because the sample was limited, ROC analysis was likely to be optimistic; further studies in larger patient populations are needed to confirm these results.
Conclusions
Three-dimensional VCA provides a single, directly visualized, and reliable measurement of ROA, independent of geometric and flow assumptions, which classifies MR severity comparable to current clinical practice using the ASE-recommended 2D integrative method. The 3D VCA method improves accuracy of MR grading compared with the 2D PISA ROA method by eliminating geometric assumptions that frequently fail to describe the true flow convergence shape. This is especially pertinent for functional MR, where the shape of the PISA region is mainly hemi-elliptical. The 3D VCA method provides uniform clinical grading cutoffs and ranges that apply regardless of etiology and orifice shape.
Supplementary Material
CLINICAL PERSPECTIVE.
The calculation of an effective regurgitant orifice area (ROA) by the proximal isovelocity surface area method (PISA) is the primary technique for quantification of mitral regurgitation (MR) by echocardiography. However, it requires flow and geometric (hemisphere) assumptions, which have limited its clinical application. The development of color Doppler 3D echocardiography offers the ability to directly measure the ROA by measuring the vena contracta area (VCA) as 3D-guided. However, there are no data on the ranges and cutoff values of VCA for MR grading. In this study, direct planimetry of the VCA by 3D echocardiography was compared against the American Society of Echocardiography–recommended 2D integrative method as a reference. This integrative method is widely used in clinical practice and is thus most relevant. There were significant differences in VCA among patients with different MR grades. VCA at a cutoff value of 0.41cm2 reliably differentiates severe from moderate MR. As compared with 3D VCA, the 2D PISA method underestimated MR in patients with functional MR. Multivariable regression analysis showed that functional MR as etiology was the predictor of underestimation of MR by the PISA method. Three-dimensional VCA provides a single, directly visualized, and reliable measurement of ROA, which classifies MR severity comparable to current clinical practice using the American Society of Echocardiography–recommended 2D integrative method. The 3D VCA method improves accuracy of MR grading compared with the 2D PISA method by eliminating geometric and flow assumptions, allowing for uniform clinical grading cutoffs and ranges that apply regardless of etiology and orifice shape.
Acknowledgments
Sources of Funding
This work was supported in part by grant R01 HL092101 from National Institutes of Health (NIH)/National Heart, Lung and Blood Institute (NHLBI) and by Echo-Investigator Award from the American Society of Echocardiography (Dr Hung); and grants R01 038176 and K24 HL67434 from NIH/NHLBI and grant 07CVD04 from the Leducq Foundation, Paris, France (Dr Levine).
Footnotes
Disclosures
None.
The online-only Data Supplement is available at http://circimaging.ahajournals.org/cgi/content/full/CIRCIMAGING.110.961649/DC1.
References
- 1.Little SH, Pirat B, Kumar R, Igo SR, McCulloch M, Hartley CJ, Xu J, Zoghbi WA. Three-dimensional color Doppler echocardiography for direct measurement of vena contracta area in mitral regurgitation: in vitro validation and clinical experience. J Am Coll Cardiol Cardiovasc Imaging. 2008;1:695–704. doi: 10.1016/j.jcmg.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yosefy C, Hung J, Chua S, Vaturi M, Ton-Nu TT, Handschumacher MD, Levine RA. Direct measurement of vena contracta area by real-time 3-dimensional echocardiography for assessing severity of mitral regurgitation. Am J Cardiol. 2009;104:978–983. doi: 10.1016/j.amjcard.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahlert P, Plicht B, Schenk IM, Janosi RA, Erbel R, Buck T. Direct assessment of size and shape of noncircular vena contracta area in functional versus organic mitral regurgitation using real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2008;21:912–921. doi: 10.1016/j.echo.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 5.Mele D, Vandervoort P, Palacios I, Rivera JM, Dinsmore RE, Schwammenthal E, Marshall JE, Weyman AE, Levine RA. Proximal jet size by Doppler color flow mapping predicts severity of mitral regurgitation: clinical studies. Circulation. 1995;91:746–754. doi: 10.1161/01.cir.91.3.746. [DOI] [PubMed] [Google Scholar]
- 6.Tribouilloy C, Shen WF, Quere JP, Rey JL, Choquet D, Dufosse H, Lesbre JP. Assessment of severity of mitral regurgitation by measuring regurgitant jet width at its origin with transesophageal Doppler color flow imaging. Circulation. 1992;85:1248–1253. doi: 10.1161/01.cir.85.4.1248. [DOI] [PubMed] [Google Scholar]
- 7.Grayburn PA, Bhella P. Grading severity of mitral regurgitation by echocardiography: science or art? J Am Coll Cardiol Cardiovasc Imaging. 2010;3:244–246. doi: 10.1016/j.jcmg.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Biner S, Rafique A, Rafii F, Tolstrup K, Noorani O, Shiota T, Gurudevan S, Siegel RJ. Reproducibility of proximal isovelocity surface area, vena contracta, and regurgitant jet area for assessment of mitral regurgitation severity. J Am Coll Cardiol Cardiovasc Imaging. 2010;3:235–243. doi: 10.1016/j.jcmg.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Marsan NA, Westenberg JJ, Ypenburg C, Delgado V, van Bommel RJ, Roes SD, Nucifora G, van der Geest RJ, de Roos A, Reiber JC, Schalij MJ, Bax JJ. Quantification of functional mitral regurgitation by real-time 3D echocardiography: comparison with 3D velocity-encoded cardiac magnetic resonance. J Am Coll Cardiol Cardiovasc Imaging. 2009;2:1245–1252. doi: 10.1016/j.jcmg.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, Scott C, Schaff HV, Tajik AJ. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–883. doi: 10.1056/NEJMoa041451. [DOI] [PubMed] [Google Scholar]
- 11.Bursi F, Enriquez-Sarano M, Nkomo VT, Jacobsen SJ, Weston SA, Meverden RA, Roger VL. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation. 2005;111:295–301. doi: 10.1161/01.CIR.0000151097.30779.04. [DOI] [PubMed] [Google Scholar]
- 12.Bonow RO, Carabello BA, Kanu C, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 13.Utsunomiya T, Doshi R, Patel D, Mehta K, Nguyen D, Henry WL, Gardin JM. Calculation of volume flow rate by the proximal isovelocity surface area method: simplified approach using color Doppler zero baseline shift. J Am Coll Cardiol. 1993;22:277–282. doi: 10.1016/0735-1097(93)90844-q. [DOI] [PubMed] [Google Scholar]
- 14.Simpson IA, Shiota T, Gharib M, Sahn DJ. Current status of flow convergence for clinical applications: is it a leaning tower of “PISA”? J Am Coll Cardiol. 1996;27:504–509. doi: 10.1016/0735-1097(95)00486-6. [DOI] [PubMed] [Google Scholar]
- 15.Yosefy C, Levine RA, Solis J, Vaturi M, Handschumacher MD, Hung J. Proximal flow convergence region as assessed by real-time 3-dimensional echocardiography: challenging the hemispheric assumption. J Am Soc Echocardiogr. 2007;20:389–396. doi: 10.1016/j.echo.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Coisne D, Erwan D, Christiaens L, Blouin P, Allal J, Barraine R. Quantitative assessment of regurgitant flow with total digital three-dimensional reconstruction of color Doppler flow in the convergent region: in vitro validation. J Am Soc Echocardiogr. 2002;15:233–240. doi: 10.1067/mje.2002.117901. [DOI] [PubMed] [Google Scholar]
- 17.Utsunomiya T, Ogawa T, Doshi R, Patel D, Quan M, Henry WL, Gardin JM. Doppler color flow “proximal isovelocity surface area” method for estimating volume flow rate: effects of orifice shape and machine factors. J Am Coll Cardiol. 1991;17:1103–1111. doi: 10.1016/0735-1097(91)90839-2. [DOI] [PubMed] [Google Scholar]
- 18.Iwakura K, Ito H, Kawano S, Okamura A, Kurotobi T, Date M, Inoue K, Fujii K. Comparison of orifice area by transthoracic three-dimensional Doppler echocardiography versus proximal isovelocity surface area (PISA) method for assessment of mitral regurgitation. Am J Cardiol. 2006;97:1630–1637. doi: 10.1016/j.amjcard.2005.12.065. [DOI] [PubMed] [Google Scholar]
- 19.Shiota T, Sinclair B, Ishii M, Zhou X, Ge S, Teien DE, Gharib M, Sahn DJ. Three-dimensional reconstruction of color Doppler flow convergence regions and regurgitant jets: an in vitro quantitative study. J Am Coll Cardiol. 1996;27:1511–1518. doi: 10.1016/0735-1097(96)00009-5. [DOI] [PubMed] [Google Scholar]
- 20.Matsumura Y, Fukuda S, Tran H, Greenberg NL, Agler DA, Wada N, Toyono M, Thomas JD, Shiota T. Geometry of the proximal isovelocity surface area in mitral regurgitation by 3-dimensional color Doppler echocardiography: difference between functional mitral regurgitation and prolapse regurgitation. Am Heart J. 2008;155:231–238. doi: 10.1016/j.ahj.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Dujardin KS, Enriquez-Sarano M, Bailey KR, Nishimura RA, Seward JB, Tajik AJ. Grading of mitral regurgitation by quantitative Doppler echocardiography: calibration by left ventricular angiography in routine clinical practice. Circulation. 1997;96:3409–3415. doi: 10.1161/01.cir.96.10.3409. [DOI] [PubMed] [Google Scholar]
- 22.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 23.Grigioni F, Detaint D, Avierinos JF, Scott C, Tajik J, Enriquez-Sarano M. Contribution of ischemic mitral regurgitation to congestive heart failure after myocardial infarction. J Am Coll Cardiol. 2005;45:260–267. doi: 10.1016/j.jacc.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 24.Skaug TR, Hergum T, Amundsen BH, Skjaerpe T, Torp H, Haugen BO. Quantification of mitral regurgitation using high pulse repetition frequency three-dimensional color Doppler. J Am Soc Echocardiogr. 2010;23:1–8. doi: 10.1016/j.echo.2009.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.