Abstract
High doses of niacin (nicotinic acid) used to treat dyslipidemias cause flushing, due to high levels of prostaglandin D2 (PGD2). GPR109A, a G-protein coupled receptor, triggers the flushing in the skin. In addition to boosting PGD2, niacin binding to GPR109A activates the entire prostanoid cascade. We found that GPR109A occurs throughout the gastrointestinal tract. Mice that alternated between a 1% niacin diet and a control diet had higher urinary prostaglandin E2 (PGE2) metabolite levels when on niacin (2.8-fold increase; 95% confidence interval, 1.8–3.9). PGE2 promotes tumors in the intestines, whereas PGD2 may have an opposite effect, on the basis of our report showing that transgenic hematopoietic prostaglandin D synthase suppresses intestinal adenomas in ApcMin/+ mice. To determine if either tumor growth or tumor suppression prevails, we fed ApcMin/+ mice a 1% niacin diet and assessed tumor development. A 1% niacin diet did not affect the number of tumors scored histologically in ApcMin/+ mice at 14 weeks (33 mice on niacin, 33 controls). Although niacin stimulates production of various prostaglandins, our results support an interpretation that very high intakes of niacin are safe in relation to intestinal tumors in this model.
Keywords: niacin, colorectal cancer, animal models, eicosanoids/prostaglandins
INTRODUCTION
Niacin (the nicotinic acid form of vitamin B3) has been a treatment for hyperlipidemia for some 50 years (1). In doses of up to 3 grams per day, niacin raises high-density lipoprotein cholesterol and lowers triglycerides and low-density lipoprotein cholesterol (2). Currently in the United States, only 6% of individuals being treated for lipid disorders use niacin, mainly due to the side effect of vasodilatation in the skin -- known as flushing. Compounds that can prevent flushing are being developed, which may lead to wider niacin usage (3).
In 1989, Morrow et al. (4–6) found that niacin-induced vasodilatation is caused by a marked rise of PGD2 in the skin. Lorenzen et al. (7) hypothesized that skin macrophages might be responsible and showed nicotinic acid binding to membranes of RAW 264.7 macrophage cells. Binding was inhibited by guanine nucleotides, supporting an interpretation that nicotinic acid binds to a G protein-coupled receptor. Tunaru et al. (8) identified the nicotinic acid receptor (protein upregulated in macrophages by interferon-gamma, or PUMA-G) -- now called GPR109A. Niacin-induced lipid changes and flushing disappeared in mice with knockouts of Gpr109a (9). Antigen-presenting Langerhans cells are the PGD2-producing cells in the skin (10, 11).
The ketone body, 3-hydroxy-butyrate, is a natural GPR109A ligand. By recognizing 3-hydroxy-butyrate, GPR109A coupled to Gi-type G proteins is thought to be a metabolic sensor. Specifically, during increased fatty acid oxidation and formation of ketone bodies, binding to GPR109A inhibits adipocyte adenylate cyclase and produces an anti-lipolytic effect (12).
GPR109A has also been hypothesized to be a colon tumor suppressor, through binding to butyrate released at high levels (~20 mM) by intestinal flora. GPR109A occurs throughout the mouse and human gastrointestinal tract, on the luminal face of epithelial cells (13). Human colon cancer samples have lower GPR109A expression, compared to normal colon. Findings were similar in ApcMin/+ mice. Methylation appeared to be a mechanism for GPR109A silencing, because colon cancer specimens had higher expression of DNA (cytosine-5-)-methyltransferases DNMT1 and DNMT3b.
With transgenic mice, we showed that overexpression of hematopoietic prostaglandin D synthase (HPGDS) suppresses tumors in ApcMin/+ mice. Conversely, knockout of Hpgds had the opposite effect -- causing more tumors (14). The results support a concept that PGD2 inhibits intestinal tumors. Thus, tumor inhibition by PGD2 is a possible mechanism for tumor suppression by GPR109A.
However, niacin binding to GPR109A stimulates the entire prostanoid cascade, so that PGE2 is also produced. PGE2 is pro-tumorigenic in the intestines (15, 16). In mouse skin, 2 phases of prostaglandin production occur in response to niacin. Antigen presenting Langerhans cells promptly produce both PGD2 and PGE2 in the first phase, whereas keratinocytes produce PGE2 in a second phase (17). Antigen presenting dendritic cells (18) and PGE2-producing cells (16) are also present in the intestines.
To assess the impact of high niacin intakes on occurrence of intestinal tumors, we fed ApcMin/+ mice a high niacin diet (1%) or a control diet (0.003%) and scored adenomas in the small bowel and colon. Neither tumor suppression nor promotion prevailed with the high niacin diet, supporting a conclusion that high doses of niacin seem to be safe with respect to risk for intestinal tumors in this model.
MATERIALS AND METHODS
Mice
ApcMin/+ mice on a C57BL/6 background came from Jackson (Bar Harbor, ME; stock number 002020; 19) and were bred with wild-type C57BL/6 mice to produce animals for the experiments below (unless otherwise specified). HPGDS transgenic mice are described in Park et al. (14). All procedures were approved by the Institutional Animal Care and Use Committee.
1% Niacin (10 g per kg) and control diets
Diets were from Dyets, Inc. (Bethlehem, PA). For urine prostaglandin studies with wild-type mice, 1% niacin and control diets were prepared from the same lots of Purina lab rodent diet (LabDiet 5001; PMI Nutrition International; Richmond, IN), re-pelleted with and without niacin. For tumor studies with ApcMin/+ mice, 1% niacin and control diets (both in powder form) were prepared from the AIN-93G diet (modified to meet 1995 NRC values, which contains 0.003% niacin) with and without added niacin. Diets were started at 3 weeks of age (weaning).
Urine prostaglandins in wild-type mice
We housed mice individually in metabolic cages to collect urine. Specimens were obtained over 24 hours, centrifuged to remove debris, and stored at −80 °C. 11β-PGF2α and PGE-M (9,15-dioxo-11α-hydroxy-2,3,4,5-tetranor-prostan-1,20-dioic acid) were measured by enzyme immunoassay (Cayman Chemical; Ann Arbor, MI). Creatinine was measured by Antech Diagnostics (Irvine, CA). Prostaglandin excretion values (in pg/mg of creatinine) were transformed to logarithms (base 10) for statistical comparisons.
Most comparisons were with mice on a continuous 1% niacin diet, versus mice on a control diet or HPGDS transgenic mice (also on a control diet). However, additional analyses were performed for 4 mice whose diets alternated between the control diet (Thursdays through Mondays) and the 1% niacin diet. These 4 mice received 1% niacin on Tuesdays and Wednesdays, and 24-hour urines were collected on Mondays for control levels and on Wednesdays for levels on 1% niacin.
Statistical calculations for prostaglandin data were performed with linear mixed model analysis (which is a generalization of ordinary ANOVA; 20), in order to account for varying numbers of urine specimens per mouse and for alternating periods on the 1% niacin diet and on the control diet for 4 of the mice.
mRNA quantitation
Tissue specimens (~100 mg) were placed in RNAlater (Ambion; Austin, TX) and frozen at the time of sacrifice. RNA was extracted, purified (RNeasy Lipid Tissue Mini Kit, Qiagen; Germantown, MD), reverse transcribed to cDNA, and quantitated via RT-PCR. Oligonucleotide primers for PCR were: mouse Pla2ga (cytosolic phospholipase A2), 5’-CAT GAC CCT GAG TAG TTT GAA GGA-3’ (forward) and 5’-GAC TAA ATT CCA CCC AAT CGG-3’ (reverse); mouse Gpr109a, 5’-GTC GCA CGA TGC TAT GTT CCT-3’ (forward) and 5’-TGC CTG AGC AGA ACA AGA TGA-3’ (reverse); mouse Hpgds, 5’-CAG AGC CTC GCA ATA GCA AGA-3’ (forward) and 5’-CGT GTC CAC CAC TGC ATC AG-3’ (reverse). Fluorescent oligonucleotide probes were: mouse Pla2ga, 6FAM AGG TCA ATG CCG CCC GGT TAMRA; mouse Gpr109a, 6FAM TTG GAA TTC TTC TTG CCC CTG GCC TAMRA; mouse Hpgds, 6FAM CTG GGA AGA CAG CGT TGG AGC AAT G TAMRA. Assays for Myc, Ccnd1 (cyclin D1), Gapdh (glyceraldehyde-3-phosphate dehydrogenase), and Vegfa (vascular endothelial growth factor A) were performed with kits (Applied Biosystems; Foster City, CA; Mm00487803_m1, Mm00432359_m1, Mm99999915_g1, Mm00437304_m1, respectively). P-values for comparisons of mice on the 1% niacin diet to controls were calculated by the use of the Mann-Whitney method.
Intestinal histopathology
Mice were sacrificed at 14 weeks. Intestines were immediately removed in one piece, flushed with ice-cold Ringer solution, and opened on blotter paper (Whatman 3MM; Florham Park, NJ). Six cm of the proximal small bowel were removed, cut into small pieces, and then stored at −80 °C for possible future analyses. The remaining intestines were fixed in buffered formalin for 3–4 hours, coiled as “Swiss rolls” (14, 21), transferred to 70% ethanol (at 4 °C), embedded in paraffin, and sectioned (4 µm). Sections spaced 250 µm apart were mounted on slides, stained (hematoxylin and eosin), and cover slipped. We examined 8 sections per mouse, by use of a Leica MZ6 stereo microscope (with up to 40× magnification) to identify and count adenomas. Higher magnification (100–400×) was used to confirm very early neoplasms that were contained within single villi. Slides were examined without knowledge of the diet. Color coding was used to prevent over-counting (14). Sizes of adenomas were gauged by the number of sections spanned. Numbers of adenomas were statistically analyzed by non-parametric methods (Mann-Whitney).
RESULTS
Urine prostaglandins
We measured urinary excretion of PGD2 and PGE2 metabolites in wild-type mice (with no ApcMin alleles), to obtain information on effects of niacin on prostaglandin production. PGD2 degrades quickly in vivo and is removed from the bloodstream. 11β-PGF2α is the first metabolite that appears in the urine and is used as an indicator of PGD2 production. Similarly, PGE2 is rapidly catabolized in the lungs and excreted in the urine, where PGE-M reflects PGE2 production in the body.
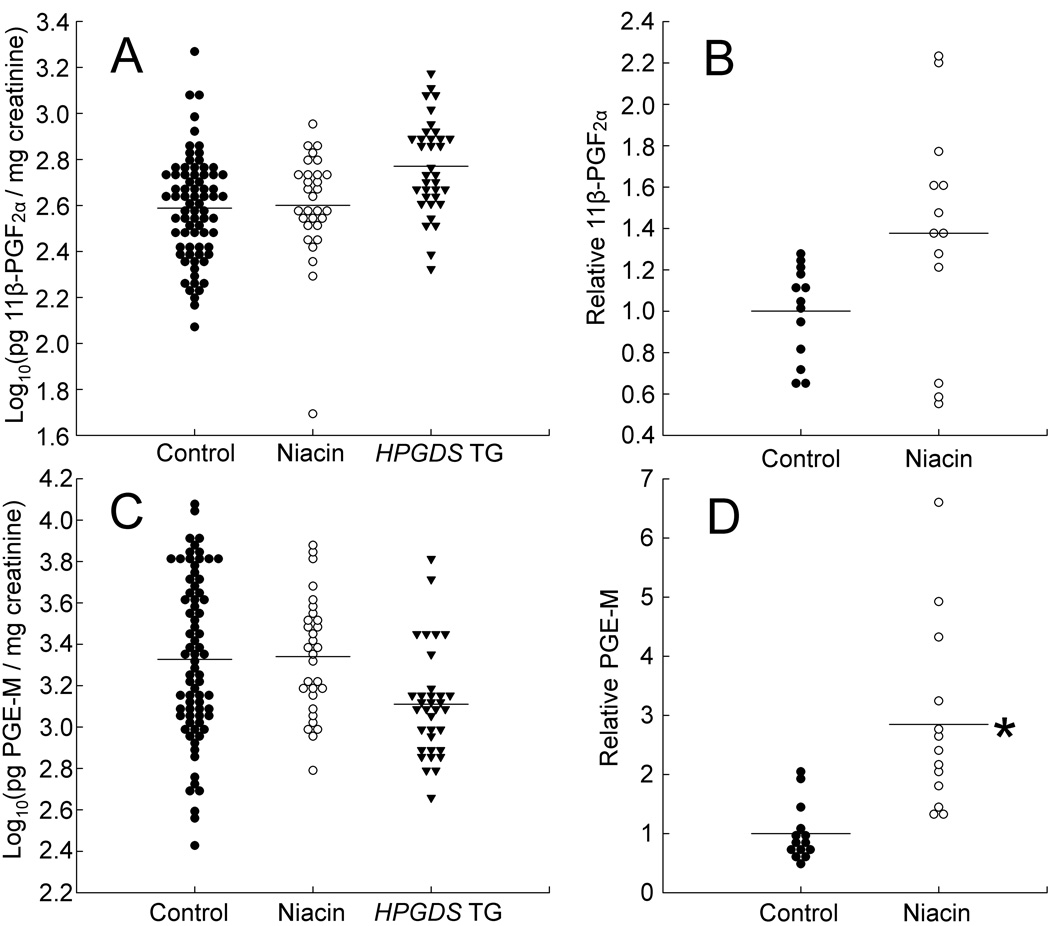
Urine 11β-PGF2α and PGE-M levels were highly variable from specimen to specimen (Table 1; Figs. 1A and 1C). Estimated means for 11β-PGF2α excretion were 403 and 385 pg per mg of creatinine, for controls and for mice on the 1% niacin diet, respectively (P = 0.94). For PGE-M excretion, the corresponding means were 1,995 (for controls) and 2,196 (for mice on 1% niacin; P = 0.92). By these comparisons, there was no statistical difference in urine prostaglandin levels between mice fed the control diet and a different set of mice on 1% niacin.
TABLE 1.
Urinary 11β-PGF2α and PGE-M in relation to 1% niacin and HPGDS transgenesa
| A. Estimated mean prostaglandin excretion (and 95% confidence intervals; in units of pg/mg of creatinine) among mice on the control diet, mice on 1% niacin, and HPGDS transgenic mice | |||
|---|---|---|---|
| Controlb | 1% Niacin | HPGDS transgenicc | |
| 11β-PGF2α | 403 (285–571) | 385 (230–646) | 633 (326–1,228) |
| No. of urine samples | 75 | 30 | 35 |
| No. of mice | 32 | 15 | 9 |
| P-value | -- | 0.94 | 0.19 |
| PGE-M | 1,995 (1,224–3,251) | 2,196 (1,364–3,532) | 1,622 (542–4,854) |
| No. of urine samples | 74 | 30 | 34 |
| No. of mice | 30 | 15 | 8 |
| P-value | -- | 0.92 | 0.72 |
| B. Relative prostaglandin excretion (and 95% confidence intervals), as compared to control values, in 4 mice that were assessed on both the control diet and the 1% niacin diet | |||
|---|---|---|---|
| Control | 1% Niacin | P-value | |
| 11β-PGF2α | 1 (0.43–1.47) | 1.34 (0.83–1.86) | 0.07 |
| No. of urine samples | 13 | 13 | |
| No. of mice | 4 | 4 | |
| PGE-M | 1 (0–2.04) | 2.85 (1.80–3.89) | 0.02 |
| No. of urine samples | 14 | 12 | |
| No. of mice | 4 | 4 | |
Data are plotted in Fig. 1. None of the mice represented here had ApcMin alleles.
Twenty-seven of the control 11β-PGF2α measurements (from 19 mice) were previously shown in Park et al. (14), as were 17 of the control PGE-M measurements (from 9 mice).
A subset of the data from HPGDS transgenic mice was published in Park et al. (14). However, the results shown here include data from 13 new urine specimens (including 3 specimens from 2 new mice) for 11β-PGF2α and 5 new urine specimens (including 2 specimens from one new mouse) for PGE-M.
FIG. 1.
Urinary excretion of 11β-PGF2α (a major PGD2 metabolite) and PGE-M (the major PGE2 metabolite). Numbers of urine specimens, numbers of mice, and results are summarized in Table 1. A: Urinary 11β-PGF2α in relation to 1% niacin and HPGDS transgenes. 11β-PGF2α levels (in pg per mg of creatinine) were transformed to logarithms (base 10). Estimated mean levels were 403, 385 (P = 0.94), and 633 (P = 0.19), for controls, mice on 1% niacin, and HPGDS transgenic mice, respectively. The graph includes data on mixed background mice (C57BL/6 × FVB/N) as well as C57BL/6 mice, but no ApcMin/+ mice. B: Relative urinary 11β-PGF2α excretion levels in 4 mice that alternated between the control diet and the 1% niacin diet. For each of these 4 mice, urine prostaglandins were measured on the control diet and on the 1% niacin diet. All prostaglandin levels for each mouse were divided by the mean value of the control measurements for that mouse. Data for all 4 mice were then pooled and analyzed. The mean increase in 11β-PGF2α excretion on the 1% niacin diet was 1.34-fold (P = 0.07). Numbers of urine specimens and of mice are shown in Table 1. C: Urinary PGE-M in relation to 1% niacin and HPGDS transgenes. PGE-M levels (in pg per mg of creatinine) were transformed to logarithms (base 10). Estimated mean levels were 1,995, 2,196 (P = 0.92), and 1,622 (P = 0.72), for controls, mice on 1% niacin, and HPGDS transgenic mice, respectively. The mice were the same as used for panel A, except there were a few missing data, due to inadequate amounts of urine for a few of the mice. D: Relative urinary PGE-M excretion levels in 4 mice that alternated between the control diet and the 1% niacin diet. Data were collected and analyzed as for panel B. The mean increase in PGE-M excretion on the 1% niacin diet was 2.85-fold (P = 0.02).
However, niacin effects may have been difficult to detect in these experiments, because prostaglandin levels varied from mouse-to-mouse and day-to-day. Adaptation or tolerance to chronic, frequent niacin doses also occurs (see Discussion). To limit the impact of mouse-to-mouse variation and tolerance to chronic niacin, we measured prostaglandin excretion on and off niacin in 4 wild-type mice that received the 1% niacin diet (for 2 days) alternating with the control diet (for 5 days). We collected urine both before and during the intervals in which the diet contained 1% niacin, over a period of several weeks. For each mouse, we then normalized prostaglandin levels to the mean value obtained on the control diet for that same mouse and pooled data from all 4 mice for statistical analyses (Figs. 1B and 1D). Mean 11β-PGF2α excretion increased 1.34-fold, and PGE-M excretion increased 2.85-fold on the 1% niacin diet, relative to levels on the control diet (P-values were 0.07 and 0.02, respectively). Thus, there is evidence that 1% niacin given intermittently (2 consecutive days per week) increased tissue production of PGE2, in addition to its putative effects on PGD2.
For comparison with mice presumed to have very high PGD2 production due to over-expression of HPGDS, we obtained new data on urine 11β-PGF2α and PGE-M excretion in HPGDS transgenic mice and combined them with data from Park et al. (14; Table 1 and Figs. 1A and 1C). HPGDS transgenic mice had 1.6-fold higher excretion of 11β-PGF2α and 0.8-fold lower excretion of PGE-M compared to wild-type mice on the control diet, but the differences were not statistically significant (P-values were 0.19 and 0.72, respectively).
Expression of selected genes
GPR109A
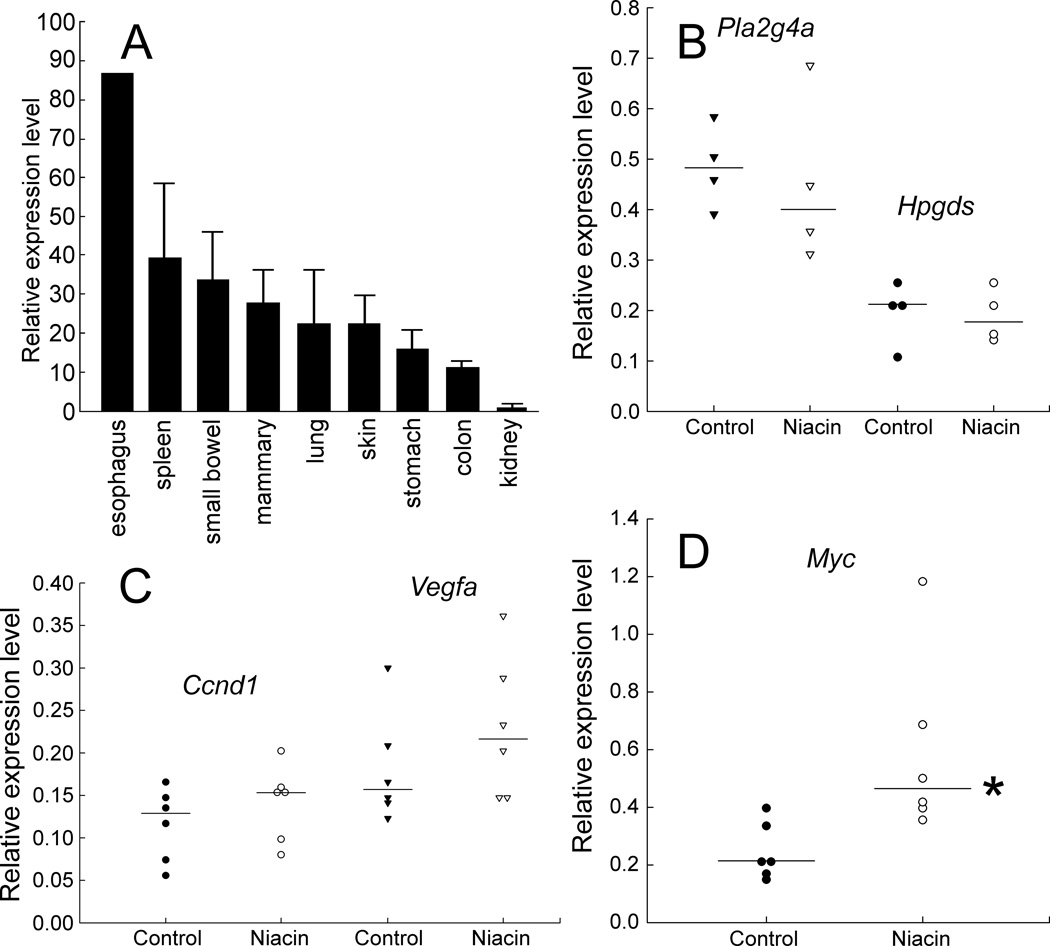
We measured Gpr109a mRNA transcript levels in 9 tissues from wild-type mice on a control diet (Fig. 2A). Levels were reported relative to endogenous glyceraldehyde-3-phosphate dehydrogenase (Gapdh) transcription levels. Among tissues tested, Gpr109a expression was lowest in the kidney and highest in the esophagus. Levels were fairly similar in the spleen, small intestine, mammary gland, lung, and skin. The data show that there are Gpr109a-producing cells throughout the gastrointestinal tract, which would respond to niacin by synthesizing prostaglandins.
FIG. 2.
Expression of Gpr109a, selected prostaglandin biosynthetic enzymes, and growth factors in the experiment. A: Gpr109a mRNA in 9 tissues in wild-type, mixed background mice (C57BL/6 × FVB/N). All mice were on a diet with no supplemental niacin. Levels were expressed relative to mRNA levels for endogenous mouse glyceraldehyde-3-phosphate dehydrogenase (Gapdh). The Gpr109a level in the kidney was set to 1.0 (lowest value). Thick bars represent the average expression among 3 mice. Lines indicate standard deviations. mRNA levels for all tissues were measured in triplicate for each mouse. No standard deviation is shown for the esophagus, because tissue specimens from the 3 mice were combined (specimens were too small to assay individually). The esophagus had the highest Gpr109a expression among the tissues tested, followed by the spleen. Gpr109a expression levels were fairly comparable among the small intestine, mammary gland, lung, and skin. B: Cytosolic phospholipase A2 (Pla2g4a) and hematopoietic prostaglandin D synthase (Hpgds) expression in the colon. mRNA was measured in colon tissue of ApcMin/+ mice on a control diet or daily 1% niacin. Levels were expressed relative to mRNA levels for endogenous mouse Gapdh. The plotted points represent triplicate measurements in different mice. Horizontal bars indicate medians. Median values for control and niacin-fed mice (respectively) were: for Pla2g4a, 0.48 and 0.40 (P = 0.71); for Hpgds, 0.21 and 0.18 (P = 0.83). C and D: Ccnd1, Vegfa, and Myc oncogene expression. mRNA was measured in colon tissue of ApcMin/+ mice on a control diet or daily 1% niacin, as described for panel B above. Median values for control and niacin-fed mice (respectively) were: for Ccnd1, 0.13 and 0.15 (P = 0.30); for Vegfa, 0.16 and 0.22 (P = 0.30); for Myc, 0.21 and 0.46 (P = 0.005). * indicates P < 0.05.
Cytosolic phospholipase A2 (PLA2G4A) and HPGDS
In the skin, Langerhans cells respond to nicotinic acid with a transient increase in the cytoplasmic Ca2+ concentration, triggering the activation of PLA2G4A and release of arachidonic acid from membrane phospholipids. Arachidonic acid is then further metabolized by cyclooxygenases and by PGD2 and PGE2 synthases (11). To examine possible effects of daily dietary niacin on the expression of genes involved in PGD2 production in the gastrointestinal tract, we measured colon mRNA levels of Pla2g4a and Hpgds. There was no statistical difference in expression of these genes between mice on 1% niacin and controls (Fig. 2B). Niacin thus does not appear to influence expression of these genes involved in prostaglandin metabolism.
Cyclin D1 (CCND1), vascular endothelial growth factor A (VEGFA), and MYC
As shown in Fig. 1 and by others (2), niacin binding to GPR109A can increase production of PGE2, which can trigger signaling cascades that activate genes involved in cell proliferation, invasion, and angiogenesis in colorectal neoplasia. Therefore, we measured expression of Ccnd1, Vegfa, and Myc, as markers of tumor promoting responses (Figs. 2C and 2D). Expression of Ccnd1 and Vegfa were not statistically different for mice on 1% niacin versus controls. However, median levels of Myc mRNA increased roughly 2-fold (P = 0.005).
Intestinal tumors in ApcMin/+ mice
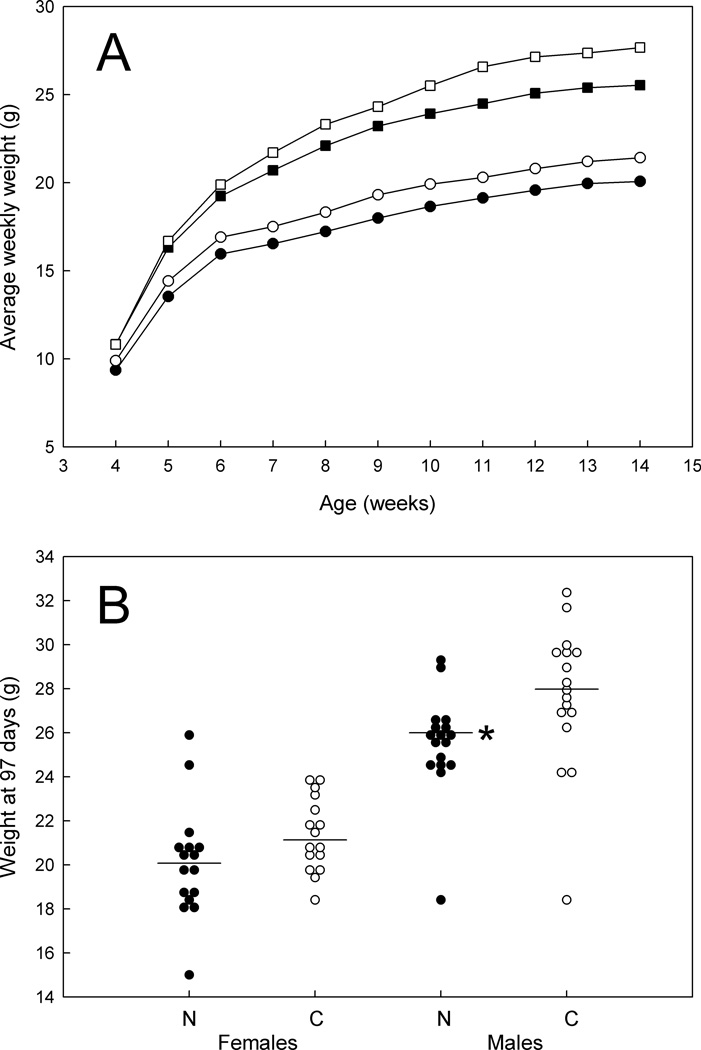
We fed ApcMin/+ mice a 1% niacin diet and assessed tumor development histologically at 14 weeks in 34 males and 32 females (17 and 16 on niacin, respectively). After weaning at 3 weeks, food consumption was estimated 3 times per week until the time of sacrifice at 14 weeks. Amounts of food consumed were fairly comparable for the 1% niacin and the control diets (not shown). However, median weights at 14 weeks on the 1% niacin diet were somewhat less than weights on the control diet for males (25.5 g versus 27.6 g; P = 0.001; Fig. 3).
FIG. 3.
Growth of ApcMin/+ mice on 1% niacin versus control diets. A: Combined average weights of the mice, by week. Open squares, males on the control diet. Filled squares, males on the 1% niacin diet. Open circles, females on the control diet. Filled circles, females on the 1% niacin diet. B: Weights at 97 days (14 weeks). N, mice on the 1% niacin diet. C, mice on the control diet. * indicates P < 0.05.
Histological sections of “Swiss rolls” allowed us to sample nearly the entire intestine and identify tumors at various stages. Tumors in individual mice were fairly uniformly distributed across the 8 sections examined for each mouse. More than 95% of the lesions occurred in the small bowel.
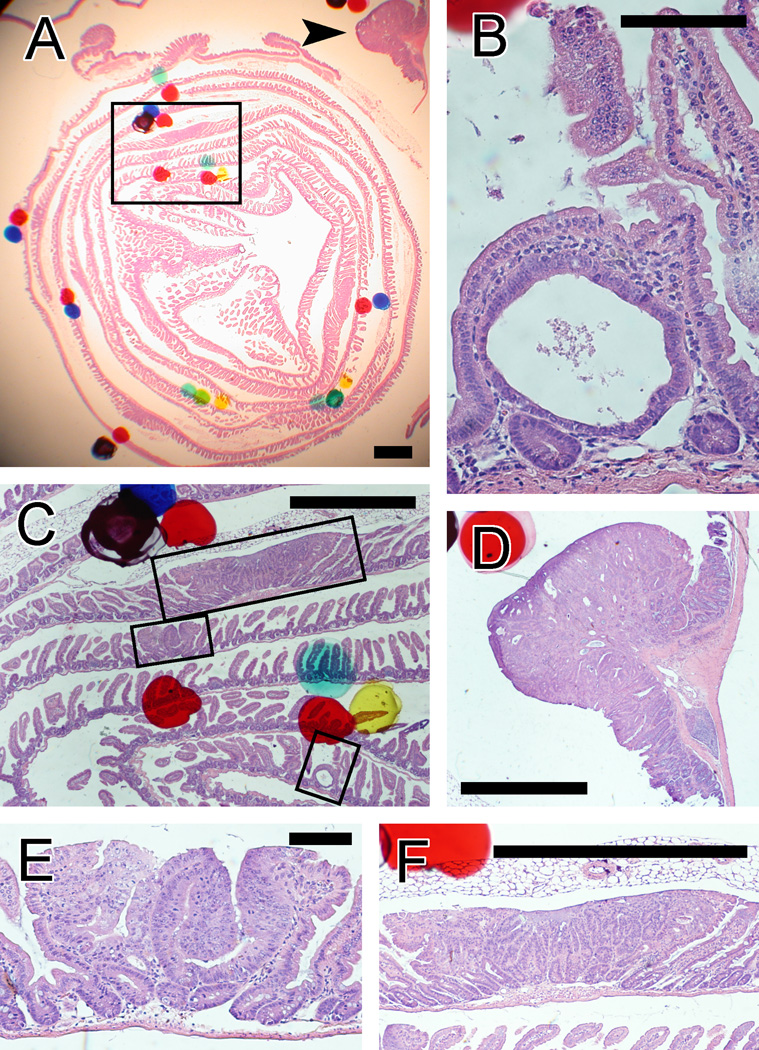
We examined tumors by use of a stereo microscope with up to 40× magnification (Fig. 4A) and at higher magnifications (Figs. 4B to 4E). We classified tumors into 3 size categories, from minute early lesions to the largest tumors identified. The earliest detectable tumors were uniglandular, intravillar neoplasms with a simple cystic configuration, designated here as intravillar neoplasms (Fig. 4B). Sizes of more advanced tumors were gauged by the number of profiles seen on the slides. Thus, small tumors were those seen in only one section (i.e., tumors up to 250–500 µm in diameter; Figs. 4C and 4E), excluding the intravillar lesions mentioned above. Large tumors were those seen in multiple sections (i.e., larger than 250–500 µm in diameter; Figs. 4C and 4F). Colon tumors (e.g., Fig. 4D) were included in these categories and were also considered as a separate group. Some of the small and large tumors represent gastrointestinal intraepithelial neoplasia (GIN), as defined in Boivin et al. (22).
FIG. 4.
Examples of intestinal tumors in a Swiss roll section from an ApcMin/+ mouse, seen through a stereomicroscope (A) and at higher magnification (B–F). Hematoxylin and eosin staining. A: Tumors are visible with very low magnification (<40×), although screening of slides was also done with higher magnification, to detect early tumors. The box indicates the area shown in panel C. The arrow indicates the tumor seen in panel D. Scale bar = 1 mm. B: An early, intravillar adenoma with a single neoplastic gland. Scale bar = 100 µm. C: Adenomas of all sizes, from intravillar tumors to large adenomas. The upper, middle, and lower boxes indicate the areas shown in panels F, E, and B, respectively. Scale bar = 1 mm. D: A large colon tumor. Scale bar = 1 mm. E: A small adenoma, defined as seen on only one section. Scale bar = 100 µm. F: A large adenoma, which was seen in 5 sections. Scale bar = 1 mm.
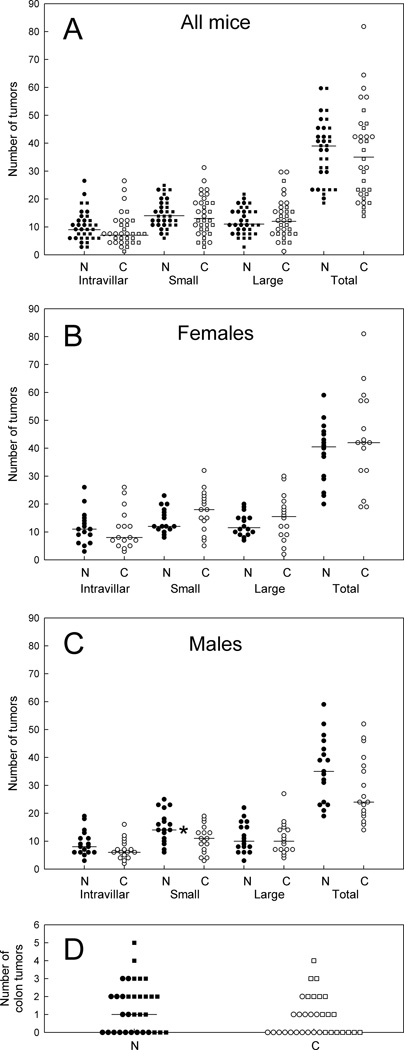
Overall, there was no difference in the number of tumors scored for mice on the 1% niacin diet versus controls, for males or females (Figs. 5A to 5C) or for colon tumors (Fig. 5D). There was a statistically significant increase in the median number of small tumors for males on the 1% niacin diet, but the ranges in numbers of these small tumors were comparable for the 1% niacin and the control diets.
FIG. 5.
Numbers of adenomas in ApcMin/+ mice on a 1% niacin diet and controls. A: Data for female and male mice combined. B: Female mice. C: Male mice. D: Colon tumors for male and female mice combined. Horizontal bars indicate medians. * indicates P < 0.05.
DISCUSSION
Niacin and tumors in animals
Historically, there have been only a few reports on tumors in animals exposed to niacin or related compounds. Results have been somewhat inconsistent. In one of the first reports, nicotinamide increased the frequency of streptozotocin-induced pancreatic islet cell tumors in rats (64%, compared to 4% for streptozotocin alone; 23). On the other hand, nicotinamide in drinking water (0.25–0.4%) or food (2%) reduced the number of urethane-induced lung adenomas in mice (24). Lifelong administration of 1% nicotinamide in mice did not affect numbers of tumors found by complete necropsies (25). However, nicotinamide raised numbers of renal tubular tumors in diethylnitrosamine-exposed mice (59% versus 5% for diethylnitrosamine alone; 26).
The early experiments did not address the role of GPR109A, because the studies used nicotinamide instead of nicotinic acid. Nicotinamide does not bind to GPR109A. More recently, Bartleman et al. (27) fed rats a niacin-deficient, control, or high nicotinic acid diet and assessed for tumors after exposure to ethylnitrosourea, an alkylating agent. A high nicotinic acid diet (0.4%) reduced incidence of leukemias. As a possible mechanism, niacin deficiency causes chromosome aberrations in bone marrow cells in rats exposed to ethylnitrosourea (28). However, niacin had no effect on occurrence of solid tumors, such as lung cancer, in these animals.
Focusing on the nicotinic acid receptor, Thangaraju et al. (13) found that GPR109A acts like a tumor suppressor in the intestines -- with lower expression in human colon cancers, in colon cancer cell lines, and in tumors from ApcMin/+ mice. Methylation was part of the silencing mechanism. Transfection of GPR109A in cultured KM12L4 colon cancer cells followed by activation with nicotinic acid or butyrate caused apoptosis. Butyrate from gut flora is thought to be a tumor suppressing GPR109A ligand in the colon.
Niacin, GPR109A, and the intestines
A diet containing 1% niacin amounts to ingestion of roughly 2,000 mg/kg of niacin per day, based on a daily intake of 5 grams of chow for a 25 g mouse. Mice feed roughly 16 times a day (29), corresponding to 125 mg/kg of niacin at a time. Cheng et al. (30) showed that a 100-mg/kg oral niacin dose raised plasma PGD2 levels in mice at least 1.5-fold. Levels return to normal by 30 minutes. Thus, 1% niacin in the diet is expected to transiently activate GPR109A many times a day.
Moreover, Yeganeh et al. (31) showed that dietary niacin (0.5%) combined with a phytosterol mixture raised plasma high-density lipoprotein cholesterol levels in apolipoprotein E-knockout mice. These results further support an interpretation that 0.5–1% niacin in the daily diet activates the mouse GPR109A receptor.
We found that the nicotinic acid receptor is readily expressed in the gut, from the esophagus to the colon (Fig. 2A). Expression in the small bowel was comparable to expression in skin. Thus, the mouse gastrointestinal tract has the potential to produce prostaglandins in response to niacin, as in the skin. Our GPR109A expression results are consistent with findings of Maciejewski-Lenoir et al. (10), who analyzed >120 different human tissues or cell lines. GPR109A expression was high in neutrophils, adipocytes, and Langerhans cells.
Safety of high doses of niacin in relation to intestinal tumors
Our experiments were designed to assess effects of high nicotinic acid intake on intestinal tumors in ApcMin/+ mice, which are prone to adenomas. On a mg per body weight basis, mice on a 1% niacin diet consume far more nicotinic acid (2,000 mg/kg/day) than do humans who take niacin for lipid disorders (3 g per 70 kg of body weight, or 43 mg/kg/day). However, humans eat roughly 300–400 g of dry matter per day (32) -- far less than what mice eat, on a g per body weight basis. Thus, a 1% niacin diet in mice simulates the concentrations of niacin in food consumed by humans who are on standard doses of niacin for lipid disorders (3 g of niacin per 300 g of dry matter). Moreover, niacin concentrations in consumed food may be important in determining niacin effects within the gut.
Because activation of GPR109A has several metabolic consequences, one may hypothesize either pro- or anti-tumorigenic effects. Niacin binding to GPR109A stimulates the entire prostanoid cascade. Thus, we observed a 2.85-fold higher excretion of PGE-M, a PGE2 metabolite, in mice on 1% niacin for 2 consecutive days per week (Fig. 1D). Mice on 1% niacin had higher colon expression of Myc (Fig. 2D), compatible with pro-tumorigenic effects of PGE2 (16, 33). MYC is required for tumor development in ApcMin/+ mice (34). On the other hand, PGD2 appears to suppress intestinal tumors (14).
Neither tumor promotion nor tumor suppression prevailed in our experiments. Pertaining to safety, there was no increase in numbers of tumors in ApcMin/+ mice on a continuous 1% niacin diet. The result supports an interpretation that high intakes of niacin do not promote tumors in this model, despite evidence for higher levels of molecules that can be associated with tumor growth. In humans, tolerance to flushing develops with repeated niacin doses, and prostaglandin production declines (35). Therefore, a decline in prostaglandin production with continuing, frequent niacin doses may explain why niacin had no effect on tumors in our experiments. Opposing effects of PGD2 versus PGE2 may also be possible.
Studies on niacin and tumors are useful, because drug companies are developing products to encourage wider use of niacin for long-term treatment of lipid disorders. For example, in Europe, nicotinic acid is combined with laropiprant, an antagonist of PTGDR (a PGD2 receptor, also known as DP1), with the goal of reducing flushing (3, 36). Moreover, new GPR109A agonists are being developed with different tissue distributions, to reduce vasodilation in the skin (37). In view of the effects of both PGE2 and PGD2 on intestinal tumors in ApcMin/+ mice, these animals may represent a useful model for obtaining data on GPR109A agonists and tumor occurrence (38).
TABLE 2.
Intestinal tumors in ApcMin/+ mice on a 1% niacin diet and controlsa
| All mice | Females | Males | ||||
|---|---|---|---|---|---|---|
| A. Total numbers of adenomas in the entire intestine: | ||||||
| Niacin | Control | Niacin | Control | Niacin | Control | |
| Median | 38 | 35 | 40.5 | 42 | 35 | 24 |
| Range | 19–59 | 14–81 | 20–59 | 19–81 | 19–59 | 14–52 |
| No. of mice | 33 | 33 | 16 | 16 | 17 | 17 |
| P-valueb | 0.49 | 0.46 | 0.10 | |||
| Ratio (95% CI)c | 1.09 (0.84–1.40) | 0.92 (0.70–1.20) | 1.27 (0.98–1.65) | |||
| B. Numbers of intravillar neoplasms in the entire intestine: | ||||||
|---|---|---|---|---|---|---|
| Niacin | Control | Niacin | Control | Niacin | Control | |
| Median | 9 | 7 | 11 | 8 | 8 | 6 |
| Range | 3–26 | 2–26 | 3–26 | 3–26 | 3–19 | 2–16 |
| No. of mice | 33 | 33 | 16 | 16 | 17 | 17 |
| P-valueb | 0.13 | 0.53 | 0.13 | |||
| Ratio (95% CI) c | 1.23 (0.86–1.75) | 1.13 (0.73–1.73) | 1.33 (0.94–1.89) | |||
| C. Numbers of smalld tumors in the entire intestine: | ||||||
|---|---|---|---|---|---|---|
| Niacin | Control | Niacin | Control | Niacin | Control | |
| Median | 14 | 13 | 12 | 18 | 14 | 11 |
| Range | 6–25 | 3–32 | 8–23 | 5–32 | 6–25 | 3–19 |
| No. of mice | 33 | 33 | 16 | 16 | 17 | 17 |
| P-valueb | 0.55 | 0.17 | 0.033 | |||
| Ratio (95% CI)c | 1.14 (0.83–1.58) | 0.86 (0.63–1.44) | 1.49 (1.06–2.10) | |||
| D. Numbers of larged tumors in the entire intestine: | ||||||
|---|---|---|---|---|---|---|
| Niacin | Control | Niacin | Control | Niacin | Control | |
| Median | 11 | 12 | 11.5 | 15.5 | 10 | 10 |
| Range | 3–22 | 2–30 | 7–20 | 2–30 | 3–22 | 4–27 |
| No. of mice | 33 | 33 | 16 | 16 | 17 | 17 |
| P-valueb | 0.82 | 0.36 | 0.74 | |||
| Ratio (95% CI) c | 0.99 (0.72–1.38) | 0.96 (0.64–1.44) | 1.02 (0.71–1.46) | |||
| E. Numbers of tumors in the colon: | ||||||
|---|---|---|---|---|---|---|
| Niacin | Control | Niacin | Control | Niacin | Control | |
| Median | 1 | 0 | 0 | 0 | 2 | 1 |
| Range | 0–5 | 0–4 | 0–3 | 0–2 | 0–5 | 0–4 |
| No. of mice | 33 | 33 | 16 | 16 | 17 | 17 |
| P-valueb | 0.10 | 0.45 | 0.15 | |||
| Ratio (95% CI) c | 1.41 (0.83–2.41) | 1.27 (0.75–2.14) | 1.56 (0.89–2.73) | |||
We counted adenomas histologically at 14 weeks of age in 8 Swiss roll sections spaced 250 µm apart. Data are plotted in Fig. 5.
The P-values refer to comparison between mice on the 1% niacin diet and controls by use of the Mann-Whitney test.
Ratio of the geometric mean number of adenomas in mice on the 1% niacin diet to the geometric mean number of adenomas in controls, and 95% confidence intervals.
Small tumors were defined as those seen in only one section (excluding intravillar neoplasms), whereas large tumors were designated as those with profiles seen in multiple sections (i.e., size >250–500 µm in diameter).
ACKNOWLEDGMENTS
Work was done at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. General Clinical Research Center grant M01 RR00425 at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center provided statistical (Dr. Peter D. Christenson) and Core Laboratory (Ms. Stephanie Griffiths) support. Grant support was from NIH grants CA73403, CA 91179, and CA132184 (H. J. Lin) and AA08116 (S. W. French).
REFERENCES
- 1.Vosper H. Niacin: a re-emerging pharmaceutical for the treatment of dyslipidaemia. Br J Pharmacol. 2009;158:429–441. doi: 10.1111/j.1476-5381.2009.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Offermanns S. The nicotinic acid receptor GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends Pharmacol Sci. 2006;27:384–390. doi: 10.1016/j.tips.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Shah S, Ceska R, Gil-Extremera B, Paolini JF, Giezek H, et al. Efficacy and safety of extended-release niacin/laropiprant plus statin vs. doubling the dose of statin in patients with primary hypercholesterolaemia or mixed dyslipidaemia. Int J Clin Pract. 2010;64:727–738. doi: 10.1111/j.1742-1241.2010.02370.x. [DOI] [PubMed] [Google Scholar]
- 4.Morrow JD, Parsons WG, III, Roberts LJ., II Release of markedly increased quantities of prostaglandin D2 in vivo in humans following the administration of nicotinic acid. Prostaglandins. 1989;38:263–274. doi: 10.1016/0090-6980(89)90088-9. [DOI] [PubMed] [Google Scholar]
- 5.Morrow JD, Awad JA, Oates JA, Roberts LJ., II Identification of skin as a major site of prostaglandin D2 release following oral administration of niacin in humans. J Invest Dermatol. 1992;98:812–815. doi: 10.1111/1523-1747.ep12499963. [DOI] [PubMed] [Google Scholar]
- 6.Morrow JD, Roberts LD., II . Lipid-derived autacoids. Eicosanoids and platelet-activating factor. In: Hardman JG, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill; 2001. pp. 669–685. [Google Scholar]
- 7.Lorenzen A, Stannek C, Burmeister A, Kalvinsh I, Schwabe U. G protein-coupled receptor for nicotinic acid in mouse macrophages. Biochem Pharmacol. 2002;64:645–648. doi: 10.1016/s0006-2952(02)01220-0. [DOI] [PubMed] [Google Scholar]
- 8.Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9:352–355. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 9.Benyó Z, Gille A, Kero J, Csiky M, Suchánková MC, et al. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest. 2005;115:3634–3640. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maciejewski-Lenoir D, Richman JG, Hakak Y, Gaidarov I, Behan DP, et al. Langerhans cells release prostaglandin D2 in response to nicotinic acid. J Invest Dermatol. 2006;126:2637–2646. doi: 10.1038/sj.jid.5700586. [DOI] [PubMed] [Google Scholar]
- 11.Benyó Z, Gille A, Bennett CL, Clausen BE, Offermanns S. Nicotinic acid-induced flushing is mediated by activation of epidermal Langerhans cells. Mol Pharmacol. 2006;70:1844–1896. doi: 10.1124/mol.106.030833. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed K, Tunaru S, Offermanns S. GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol Sci. 2009;30:557–562. doi: 10.1016/j.tips.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JM, Kanaoka Y, Eguchi N, Aritake K, Grujic S, et al. Hematopoietic prostaglandin D synthase suppresses intestinal adenomas in ApcMin/+ mice. Cancer Res. 2007;67:881–889. doi: 10.1158/0008-5472.CAN-05-3767. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005;65:1822–1829. doi: 10.1158/0008-5472.CAN-04-3671. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson J, Gille A, Zwykiel S, Lukasova M, Clausen BE, et al. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J Clin Invest. 2010;120:2910–2919. doi: 10.1172/JCI42273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 19.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 20.Brown H, Prescott R. Applied mixed models in medicine. New York, NY: Wiley and Sons; 1999. pp. 199–259. [Google Scholar]
- 21.Moolenbeek C, Ruitenberg EJ. The ‘Swiss roll’: a simple technique for histological studies of the rodent intestine. Lab Anim. 1981;15:57–59. doi: 10.1258/002367781780958577. [DOI] [PubMed] [Google Scholar]
- 22.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 23.Rakieten N, Gordon BS, Beaty A, Cooney DA, Schein PS, et al. Modification of renal tumorigenic effect of streptozotocin by nicotinamide: spontaneous reversibility of streptozotocin diabetes. Proc Soc Exp Biol Med. 1976;151:356–361. doi: 10.3181/00379727-151-39209. [DOI] [PubMed] [Google Scholar]
- 24.French FA. The influence of nutritional factors on pulmonary adenomas in mice. Adv Exp Med Biol. 1977;91:281–292. doi: 10.1007/978-1-4684-0796-9_19. [DOI] [PubMed] [Google Scholar]
- 25.Toth B. Lack of carcinogenicity of nicotinamide and isonicotinamide following lifelong administration to mice. Oncology. 1983;40:72–75. doi: 10.1159/000225695. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg MR, Novicki DL, Jirtle RL, Novotny A, Michalopoulos G. Promoting effect of nicotinamide on the development of renal tubular cell tumors in rats initiated with diethylnitrosamine. Cancer Res. 1985;45:809–814. [PubMed] [Google Scholar]
- 27.Bartleman AP, Jacobs R, Kirkland JB. Niacin supplementation decreases the incidence of alkylation-induced nonlymphocytic leukemia in Long-Evans rats. Nutr Cancer. 2008;60:251–258. doi: 10.1080/01635580701649628. [DOI] [PubMed] [Google Scholar]
- 28.Kostecki LM, Thomas M, Linford G, Lizotte M, Toxopeus L, et al. Niacin deficiency delays DNA excision repair and increases spontaneous and nitrosourea-induced chromosomal instability in rat bone marrow. Mutat Res. 2007;625:50–61. doi: 10.1016/j.mrfmmm.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Ritskes-Hoitinga M. Nutrition of laboratory mice. In: Hedrich H, editor. The Laboratory Mouse. New York, NY: Elsevier Academic Press; 2004. pp. 463–479. [Google Scholar]
- 30.Cheng K, Wu T-J, Wu KK, Sturino C, Metters K, et al. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc Natl Acad Sci USA. 2006;103:6682–6687. doi: 10.1073/pnas.0601574103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeganeh B, Moshtaghi-Kashanian G-R, DeClercq V, Moghadasian MH. Combination of dietary phytosterols plus niacin or fenofibrate: effects on lipid profile and atherosclerosis in apo E-KO mice. J Nutr Biochem. 2005;16:222–228. doi: 10.1016/j.jnutbio.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Holzinger S, Anke M, Röhrig B, Gonzalez D. Molybdenum intake of adults in Germany and Mexico. Analyst. 1998;123:447–450. doi: 10.1039/a706882d. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi M, Montrose DC, Clark P, Nambiar PR, Belinsky GS, et al. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 2008;68:3251–3259. doi: 10.1158/0008-5472.CAN-07-6100. [DOI] [PubMed] [Google Scholar]
- 34.Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 35.Stern RH, Spence JD, Freeman DJ, Parbtani A. Tolerance to nicotinic acid flushing. Clin Pharmacol Ther. 1991;50:66–70. doi: 10.1038/clpt.1991.104. [DOI] [PubMed] [Google Scholar]
- 36.Sturino CF, O'Neill G, Lachance N, Boyd M, Berthelette C, et al. Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]-acetic acid (MK-0524) J Med Chem. 2007;50:794–806. doi: 10.1021/jm0603668. [DOI] [PubMed] [Google Scholar]
- 37.Shen HC, Ding FX, Raghavan S, Deng Q, Luell S, et al. Discovery of a biaryl cyclohexene carboxylic acid (MK-6892): a potent and selective high affinity niacin receptor full agonist with reduced flushing profiles in animals as a preclinical candidate. J Med Chem. 2010;53:2666–2670. doi: 10.1021/jm100022r. [DOI] [PubMed] [Google Scholar]
- 38.Tammariello AE, Milner JA. Mouse models for unraveling the importance of diet in colon cancer prevention. J Nutr Biochem. 2010;21:77–88. doi: 10.1016/j.jnutbio.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]