Abstract
Arsenic and lead have been found in a number of traditional Ayurvedic medicines, and the practice of Rasa Shastra (combining herbs with metals, minerals and gems), or plant ingredients that contain these elements, may be possible sources. To obtain an estimate of arsenic and lead solubility in the human gastrointestinal tract, bioaccessibility of the two elements was measured in 42 medicines, using a physiologically-based extraction test. The test consisted of a gastric phase at pH 1.8 containing organic acids, pepsin and salt, followed by an intestinal phase, at pH 7 and containing bile and pancreatin. Arsenic speciation was measured in a subset of samples that had sufficiently high arsenic concentrations for the X-ray absorption near edge structure analysis used. Bioaccessible lead was found in 76% of samples, with a large range of bioaccessibility results, but only 29% of samples had bioaccessible arsenic. Lead bioaccessibility was high (close to 100%) in a medicine (Mahayograj Guggulu) that had been compounded with bhasmas (calcined minerals), including naga (lead) bhasma. For the samples in which arsenic speciation was measured, bioaccessible arsenic was correlated with the sum of As(V)–O and As(III)–O and negatively correlated with As–S. These results suggest that the bioaccessible species in the samples had been oxidized from assumed As–S raw medicinal ingredients (realgar, As4S4, added to naga (lead) bhasma and As(III)–S species in plants). Consumption at recommended doses of all medicines with bioaccessibile lead or arsenic would lead to the exceedance of at least one standard for acceptable daily intake of toxic elements.
Keywords: Arsenic, Lead, Bioaccessibility, Ayurvedic, Speciation, Risk
1. Introduction
Ayurveda, Siddha, and Unani are fields of traditional medicine practiced in India and are considered a form of complementary and alternative medicine in western society. Treatments include herbal medicines, dietary interventions, and massage. A special subset of herbal medicines is Rasa Shastra, where metals, minerals or gems are intentionally added to herbs. Although important micronutrients such as iron and zinc are commonly used, elements widely considered toxic in the west, such as lead, mercury or arsenic, are also used. Detailed protocols for the manipulation of metals, minerals, and gems are described in ancient Indian medical texts in order for them to be converted into bhasmas (Shastri, 1979; Satpute, 2003). Bhasmas are purported to contain non-toxic forms of lead, mercury and arsenic, but to our knowledge these claims have not been rigorously tested.
In previous studies we have shown that approximately one-fifth of traditional Indian medicines from a variety of different sources contain detectable lead (≥5 mg.kg−1), mercury (≥20 mg.kg−1) and arsenic (≥20 mg.kg−1). In most cases, the medicines that contained one or more of these elements exceeded one or more regulatory standards for their acceptable daily intake (Saper et al., 2004; Saper et al., 2008). In our previous work we identified that speciation of the elements, that is, the determination of their physicochemical form, in the medicines is a research area that has not yet been addressed. Non-toxic forms of lead may include species such as elemental lead, which is largely insoluble, whereas highly soluble species, such as lead acetate, are toxic (e.g., LD50 is 17 mg.kg−1 in mice) (Park et al., 2001). Likewise, mineral forms of arsenic like realgar (As4S4, LD50 3.2 g.kg−1 in mice) and orpiment (As2S3, LD50 not available) show a much lower toxicity than the more soluble forms such as arsenic(III) oxide (As2O3, LD50 33–39 mg.kg−1 in mice) (Liu et al., 2008) or sodium arsenate (Na2HAsO4, LD50 14–18 mg.kg−1 in rats) (Franke and Moxon, 1936). Clearly, the solubility of the different arsenic and lead species is an important determinant of toxicity, since the fraction that becomes soluble upon ingestion, also known as the bioaccessible fraction, is that which can be absorbed into the body and potentially cause toxic effects.
The bioaccessible fraction can be measured using a laboratory-based extraction test that mimics the gastro-intestinal environment in such details as the chemicals and enzymes present, temperature and exposure time, and pH. Bioaccessibility testing can be used as a surrogate for animal experiments commonly used for bioavailability testing. Standardized versions of bioaccessibility tests are used frequently in the pharmaceutical field to measure, for example, dissolution of formulations (e.g., US Pharmacopeia) (Younis et al., 2009). To measure inorganic elements present as contaminants, a field of bioaccessibility research has emerged that focuses on soil samples, with frequent interest in arsenic and lead, e.g., (Ruby et al., 1993; Ruby et al., 1996; Schroder et al., 2004; Van de Wiele et al., 2007; Juhasz et al., 2009). Fewer studies are available for bioaccessibility of these elements in other media, e.g., (Laparra et al., 2004; Amiard et al., 2008), especially in natural or traditional medicines (Koch et al., 2007; Jayawardene et al., 2010). A method for lead bioaccessibility in soil has gained regulatory acceptance based on agreement with results from animal studies for a series of U.S. soils (U.S.EPA and Office of Solid Waste and Emergency Response, 2007). Other methods have also shown agreement for lead and arsenic with animal studies of soils from other areas (Ruby et al., 1993; Ruby et al., 1996; Schroder et al., 2004; Basta et al., 2007; Juhasz et al., 2009). In the present study, we used a method originally developed to model rabbit absorption of lead from soil, where this model was assumed to represent the gastro-intestinal conditions of human children (Ruby et al., 1993; Ruby et al., 1996). Our previous studies using this model yielded results in reasonable agreement with clinical measurements of arsenic bioavailability measured by urinalysis (adult human) following ingestion of an arsenic-containing traditional Chinese medicine (Koch et al., 2007). We also found this method to be robust to changes in liquid to solid ratios for arsenic bioaccessibility measurements of soil and tailing materials (Meunier et al., 2010b), which is a desirable attribute for studies on new matrices in nonstandard liquid to solid ratios, as in the present study.
In the present study we aimed to refine the findings from previous studies reporting arsenic and lead concentrations that could pose risk following recommended ingestion regimes (Saper et al., 2008); in those studies only total concentrations were reported. Specifically, we focused on the following objectives: (1) determine the arsenic speciation in selected Indian traditional medicines, (2) measure the bioaccessibility of lead and arsenic in several medicines, and (3) recalculate the estimated daily intake of lead and arsenic from the medicines using the amount of bioaccessible metal rather than the total amount ingested.
2. Materials and methods
2.1. Traditional medicine samples
Forty-two traditional Indian medicine samples were studied. Of these, 40 were selected from a larger set of 193 products randomly purchased in 2005 via the Internet for one of our previous studies, and results were reported for 35 of them (Saper et al., 2008). These 40 were chosen because they contained detectable arsenic (>10 mg. kg−1) and/or lead (>5 mg.kg−1) by X-ray fluorescence (XRF) analysis. Two additional products containing lead and arsenic purchased via the Internet that were not included in the previous study were also analyzed. The samples used in the study are listed in Table 1, where information is also given about their status as Rasa Shastra medicines. Rasa Shastra medicines are elaborately prepared compounds combining herbs with metals, minerals, and gems. An Indian-trained Ayurvedic physician classified medicines as Rasa Shastra (Saper et al., 2008) if they contain metals, minerals, or gems traditionally used according to two classic Rasa Shastra texts (Shastri, 1979; Satpute, 2003). Details of the medicines’ contents, according to information on their labels, are given in the supporting information.
Table 1.
Products tested and arsenic and lead contents (mg.kg−1).
| ID | Product name | Rasa Shastraa | Total Asb (mg.kg−1) | Total Pbb (mg.kg−1) |
|---|---|---|---|---|
| 1 | Mahasudarshan specialty tablets | 1.4 | 8.5c | |
| 2 | Shilajit specialty tablets | Yes | 3.2 | 10d |
| 3 | Vital Lady | Yes | 2.1 | 5.5c |
| 4 | Worry Free | Yes | <1.0 | 7.0c |
| 5 | Yog-Raj Guggulu | 31d | 6d | |
| 6 | Commiphora mukul | <1.0 | 2.3 | |
| 7 | Bacopa monniera | <1.0 | 6c | |
| 8 | Yogaraj Guggulu | 2.2 | 2.0 | |
| 9 | Ezi Slim | 1.4 | 3.0c | |
| 10 | Shilajeet Tablets | Yes | 21d | 10d |
| 11 | Mahayograj Guggulu | Yes | 89 | 52,000 |
| 12 | Brahmi Capsules | <1.0 | 6.5c | |
| 13 | Amoebica | 3.8 | 11.0c | |
| 14 | Arogyavardhini Bati | Yes | 4.6 | 125c |
| 15 | Kanchanar Guggulu | <1.0 | 7.5c | |
| 16 | Shilajit | Yes | 1.6 | 10.5c |
| 17 | Lean Plus | <1.0 | 6.0c | |
| 18 | Neem Plus | <1.0 | 10.5c | |
| 19 | Prana: Breath of Life | 2.1 | 9.0c | |
| 20 | Bakuchi | <1.0 | 3.0c | |
| 21 | Brahmi | 1.1 | 6.5c | |
| 22 | Chairata | <1.0 | 6c | |
| 23 | Cold Aid | <1.0 | 5.5c | |
| 24 | Trifala Guggulu | 27c | 20.5c | |
| 25 | Heart Plus | 2.0 | 7.5c | |
| 26 | Jatamansi | 3.6 | 12.0c | |
| 27 | Kanta Kari | <1.0 | 20.5c | |
| 28 | Lavan Bhaskar | 3.4 | <1.0 | |
| 29 | Licorice — Glycyrrhiza Glabra | 1.0 | 5.5c | |
| 30 | Mahasudarshan | 140 d | 15d | |
| 31 | Praval Pisti | Yes | 27.5c | 7.5c |
| 32 | Prostate Rejuv | Yes | 1.2 | 11.5c |
| 33 | Shilajit | Yes | 12d | 1.6 |
| 34 | Sugar Fight | 6.4 | 7.5c | |
| 35 | Tagar | 2.3 | 12.0c | |
| 36 | Yograj Guggulu | 10.5c | 17.5c | |
| 37 | Ayu-Arthri-Tone | Yes | <1.0 | 63c |
| 38 | Ayu-Hemoridi-Tone | Yes | 7.5 | 3.9 |
| 39 | Ayu-Leuko-Tone | Yes | 2.6 | 33c |
| 40 | Ayu-Nephro-Tone | Yes | <1.0 | 340c |
| 41 | AyurRelief™ | <1.0 | 5.5c | |
| 42 | GlucoRite™ | 1.8 | 6.0c |
Products were classified as Rasa Shastra medicines (elaborately prepared compounds combining herbs with metals, minerals, and gems) by an Indian-trained Ayurvedic physician.
Unless otherwise noted, results were determined by aqua regia-ICP methods.
XRF results, previously published with significant figures as reported in Saper et al. (2008).
XRF results, reported for the first time in the present study.
2.2. Total arsenic and lead
All samples were analyzed by X-ray fluorescence (XRF) analysis and several of the results (35 of 42) were reported previously in Saper et al. (2008). For XRF results reported here for the first time, methods and quality control procedures, as well as quality control results, are described in detail in Saper et al. (2008). Samples that had arsenic and lead concentrations below the XRF limits of detection (10 mg.kg−1 for arsenic and 5 mg.kg−1 for lead) were reanalyzed by the methods described in the following paragraphs.
Samples 6 and 7 were powdered samples and did not require further sample preparation. The rest of the samples were first prepared by grinding/homogenizing pills using mortar and pestle, since an early trial run revealed that large differences in bioaccessible arsenic and lead content were apparent between individual pills and capsules. Since the pills and capsules were compounded from ingredients that had been previously ground, and were dissolved/suspended in the extraction solution by the end of the gastric phase, this additional homogenization step was not considered to substantially reduce the particle size or surface area of the samples. A minimum of three pills were used, and sufficient pills were used to obtain the mass required to complete the extraction and digestion analyses.
Total arsenic and lead contents in the ground pills and powders were obtained by digesting a 0.50 g portion of each in pyrex boiling tubes with 9 mL aqua regia (concentrated hydrochloric and nitric acids in a ratio of 3:1), followed by inductively coupled plasma-mass spectrometry (ICP-MS) analysis in collision cell mode (to reduce interferences from chloride) for all samples except 11. The m/z monitored were 75 (As), 206, 206 and 208 (all Pb and results were averaged); more instrumental details can be found in Smith et al. (2008).
Sample 11 was also subjected to the aqua regia digestion method because the lead concentration was very high (in weight percent levels) and exceeded the arsenic concentrations by more than two orders of magnitude. In this case the XRF values for arsenic were not accurate because of interference of lead on the arsenic analysis (United States Environmental Protection Agency (USEPA), 2007; Stark et al., 2008). The aqua regia digestion method was modified to ensure maximum digestion since the concentrations were very high; aqua regia to sample ratios greater than 250 were used (see supporting information for quality assurance/quality control details), and the digests were analyzed by ICP-optical emission spectrometry (OES) using a Perkin Elmer Optima 5300 V instrument, outfitted with a Perkin Elmer GemCone nebulizer and Perkin Elmer Baffied Cyclonic Spray Chamber. The emission lines monitored were as follows (where more than one emission line was monitored, the results were averaged): 188.979, 193.696, 197.197 (As); 220.353 (Pb) nm.
Quality control tests were carried out to accompany total arsenic and lead determinations. Specifically, a procedural blank, a standard reference material, and a duplicate sample were analyzed along with every batch of 10–12 samples. MESS-3 (NRC Canada), a sediment matrix, was used as the standard reference material to monitor dissolution of mineral components of the samples, which were thought to be the most challenging to dissolve, and also because this allowed us to ensure comparability to an accredited laboratory (who uses the same standard reference material). During the analysis of digested samples by ICP-MS, a five point calibration curve was used, and run before and after each batch of analysis and every 36 samples if the number of samples in a batch exceeded that number. Calibration check standards, prepared from a source separate from the calibration standard, were analyzed every 12 samples and the results were accepted only when the results were within 20% of the known value. Internal standards Sc, Y, Rh, In, Tb, Ho, Bi were included and interpolation (provided by the software) was used to correct for plasma drift and matrix effects. Internal standard elements appearing in a specific sample matrix were deleted from the calculation for the specific sample. For ICP-OES analysis, Y was used as an internal standard. The digested samples were diluted 10–10,000 times to ensure that the acid matrix was the same as the matrix of the calibration standards and concentrations were within the calibration curve.
2.3. Speciation analysis of arsenic
X-ray absorption near edge structure (XANES) spectra of the arsenic K-edge (11,868 eV) were recorded in fluorescence mode at the bending magnet beamline of the Pacific Northwest Consortium X-ray Sciences Division (PNC/XSD) facility, sector 20, Advanced Photon Source (APS) in Argonne, Illinois; and an arsenate–glycerol standard was analyzed at the hard X-ray microanalysis beamline, at the Canadian Light Source (CLS; Saskatoon, SK, Canada). Si(111) double-crystal monochromators used at both beamlines were calibrated using the first infection point of the gold LIII absorption edge (11919.7 eV) and a reference gold foil was measured simultaneously with samples. At the CLS, the monochromator was detuned to 50% of the maximum intensity for harmonic rejection. The arsenate–glycerol standard was maintained at 50 K using an Oxford liquid helium cryostat (CF 1208). Incident and transmitted intensities were measured using straight ion chamber detectors filled with 20% Ar and 80% He. As Kα data were collected in fluorescence mode using a 32-element solid state Ge detector (Canberra, 32 element Ultra LEGe Array). The configuration of APS is described elsewhere (Smith et al., 2005).
All samples were ground in a ball mill and mounted as a thin film on Kapton™ tape. Four layers of sample were affixed to a sample holder. Analyses were performed at room temperature. Three or six scans were collected and averaged for each sample.
XANES spectra were fit within −20 to +30 eV from E0 using ATHENA software (Ravel and Newville, 2005). Data were compared to linear combinations of solid As(III) (As2O3, Fluka reagent grade), solid As(V) (KH2AsO4, Fluka reagent grade), a synthesized arsenate–glycerol standard, and a synthesized amorphous orpiment standard. As(III) and As(V) samples were prepared and measured in the same way as the samples; the orpiment standard was measured as product on filter paper encased in Kapton™ tape and the arsenate–lycerol standard was measured as a liquid absorbed in a Kimwipe™. All fits were constrained to sum to 100% of measured arsenic. Error is reported both as the reduced χ2 and as three times the estimated standard deviation of the fit.
The amorphous orpiment standard was synthesized using the procedure described in detail elsewhere (Rochette et al., 2000). A 1.0 M acetate buffer solution, pH adjusted to 4.0, was deoxygenated by bubbling nitrogen gas through the solution for 10 min. Stock solutions of 0.133 M sodium sulfide and 0.0133 M arsenate were prepared. Sodium sulfide and arsenate were added to the acetate buffer solution in an amber jar at an As:S ratio of 1:2, and were shaken at 150 rpm for 72 h. Subsequently, the solution was filtered through a Whatman glass microfibre filter (45 μm) to collect the precipitated product.
A arsenate–glycerol standard solution was prepared by adding 0.7 mL of 1000 mg.L−1 arsenate aqueous solution (KAsH2PO4, > 98%, Fluka) to a large excess (8 mL) of glycerol (≥99%, Sigma), acidified by concentrated HCl (37%, OmniTrace, EMD), and adding 1.0 M NaOH (≥95% EMD) dropwise until pH 6 was reached, measured using pH paper.
2.4. Bioaccessibility of arsenic and lead
The bioaccessibility method was designed for a human receptor and consisted of a two phase (stomach and intestine) physiologically based extraction test (PBET). Two 0.1–0.2 g portions per sample were weighed into 50 mL plastic centrifuge tubes and 10–40 mL of simulated gastric solution, prewarmed to 37 °C, was added to each sample. The gastric solution was based on the physiologically based extraction test (PBET) first developed by Ruby et al. (1993), modified using variables proposed by Rodriguez et al. (1999) (pH and salt). It consisted of 1.25 g.L−1 pepsin (P7000 from porcine stomach mucosa, Sigma-Aldrich), 0.5 g.L−1 sodium citrate (Caledon), 0.5 g.L−1 malic acid (Sigma-Aldrich), 1 mL. L−1 glacial acetic acid (Fisher), 0.15 M NaCl (puriss p.a. Fluka), adjusted to pH 1.8±0.05 with HCl (Fisher). Exact masses and volumes for each sample are summarized in Table 2 and are based on a worst case scenario of ingesting the maximum recommended dose per day all at once in a fasted human stomach that was assumed to have a liquid volume of 417 mL over 5 h (2000 mL per day, adjusted for 5 h of extraction per 24 h). This scenario represents a higher liquid-to-solid ratio than would be calculated based on volumes obtained from either fasted stomach volumes (around 25 mL) (Scarr et al., 1989; Kong and Singh, 2008) or from a gastric basal secretion rate of 60 mL per h (300 mL over 5 h) (Deshpande et al., 1996; Kong and Singh, 2008), and is therefore more conservative (Richardson et al., 2006). Assuming fasted conditions with respect to pH (Kong and Singh, 2008) in the stomach compartment is also the most conservative assumption, and was used even when drug information recommended administration with meals (i.e., samples 12 and 19; sample 9 is with milk). Gastric extraction proceeded for 1 h, with a pH check and adjustment if necessary at 0.5 h, at 37 °C at 275 rpm on a temperature-controlled flatbed rotation incubator (New Brunswick Scientific Innova 4230). After 1 h, the gastric phase solutions were removed and centrifuged at 2970 g for 15 min, and the supernatant was filtered (0.45 μm, PVDF membrane, Millipore). To simulate intestinal conditions the remaining samples that had been subjected to the stomach extraction described above were neutralized to pH 7±0.2 with a saturated solution of Na2CO3 (Fluka), and 1.875 g.L−1 bile extract (B8631 porcine, Sigma) and 0.5 g.L−1 pancreatin (P1500 porcine, Sigma) were added. Intestinal extraction conditions were applied for 4 h at 37 °C with 275 rpm shaking, and samples were centrifuged and filtered in the same way as the gastric samples.
Table 2.
Liquid to solid ratios used in bioaccessibility extractions and recommended dosing information.
| Samples (IDs) | Ratio (mL:g)a | Minimum daily doseb (g) | Maximum daily doseb (g) |
|---|---|---|---|
| 4, 24, 26, 28, 35, 36 | 100:1 | 2.6 | 4.1 |
| 1, 2, 5, 8, 10, 14, 15, 16, 20, 21, 22, 25, 27, 29,30, 31, 32, 33, 34, 37, 38, 39, 40, 42 | 200:1 | 1.5 | 2.8 |
| 3, 11, 12, 13, 17, 18, 23, 41 | 400:1 | 1.0 | 1.4 |
| Powders 6, 7 | 100:1 | Standard ratioc | |
| 9 | 200:1 | Maximum dose of 6.1 g per day in 2000 mLd | |
| 19 | 400:1 | Maximum dose of 11 g per day in 2000 mLd |
Ratios are based on the daily gastric juice volume (2000 mL) corrected to the total extraction time (×5 h per 24 h=417 mL) to the maximum dose (g) of each medicine, except where noted.
Minimum and maximum doses are within the group of samples. All doses for individual samples were calculated as maximum, based on label information.
Dose could not be estimated so standard ratio was used.
Large daily masses resulted in ratios that were impractical for bioaccessibility extraction; 2000 mL used instead of 417 mL.
Each extract was kept refrigerated for a maximum of 1 week before analysis. Samples were first diluted with 2% v/v nitric acid (HNO3, Fisher) solution by 10–10,000× as needed and analyzed for total arsenic and lead concentrations by ICP-MS (Thermo Electron Corporation X-SeriesII) as described elsewhere (Smith et al., 2008), except that collision cell mode was used to reduce interference from chloride in the sample. Bioaccessibility results are expressed as a concentration, which is the quantity of a substance (mg.kg−1 dry weight) extracted during a bioaccessibility test, or as a percentage, which is the bioaccessible concentration divided by the total concentration in a given sample and multiplying by 100.
Quality control tests were carried out to accompany bioaccessibility tests. Specifically, a procedural blank, a standard reference material, and a duplicate sample were analyzed along with every batch of 9 samples. NIST 2710, a soil sample, was used to ensure quality control of the bioaccessibility tests because our laboratory has established control limits for this standard based on over 2 years of testing (mean±3 times the standard deviation). Instrumental quality control (ICP-MS and ICP-OES) was as described in Section 2.2.
3. Results
All results of quality control tests were within control limits, meaning they were acceptable according to established criteria, and quality control results are described in detail in the supporting information or, for XRF results, in Saper et al. (2008).
3.1. Total arsenic and lead concentrations
Total arsenic and lead concentrations are included in Table 1 and all samples had detectable values of lead and/or arsenic; previously published XRF values were used where available and are included for clarity in the present study. Samples that were reanalyzed using aqua regia digestion with ICP-MS analysis mostly had arsenic concentrations below the XRF detection limit of 10 mg.kg−1 and in a few samples lead values less than the XRF detection limit of 5 mg.kg−1 were obtained. As mentioned previously, sample 11 was reanalyzed (ICP-OES was used) because the XRF analysis was inaccurate for the high concentrations of lead and arsenic present. In these cases, and for sample 11, the results obtained by using the aqua regia-ICP method are used for further calculations in this study; otherwise XRF values were used.
3.2. Speciation analysis
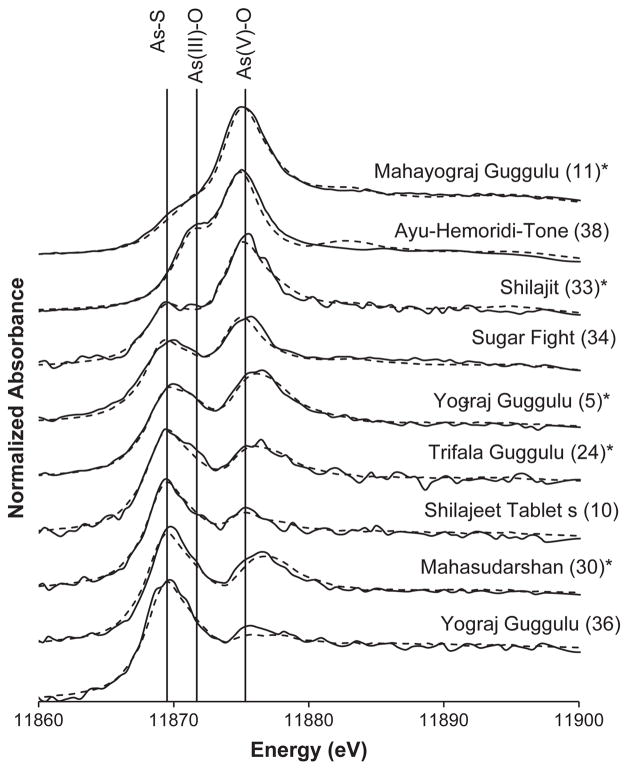
Arsenic species were determined by X-ray absorption spectroscopy (XAS) only in samples with arsenic contents greater than 10 mg.kg−1; the list of samples and results are summarized in Fig. 1 and Table 3, which also include the labeled ingredients of the samples (details for all samples can be found in the supporting information). The fluorescence signal from these traditional medicines was low, resulting in some fits that gave χ2 values higher than 0.003 (<0.003 is considered to be a good fit). However, the purpose of the XANES analysis for this paper was to investigate the oxidation state of arsenic within the pills with respect to the bioaccessibility results, rather than to unequivocally identify the specific arsenic compounds present. The speciation of arsenic in most samples is a mixture of arsenic-sulfur species (amorphous orpiment As2S3 was selected as the likeliest match and is from herein referred to as As–S), arsenic trioxide (As2O3, referred to as As(III)–O) and an arsenate (AsV) species (i.e., As(V)–O). In five samples (samples 5, 11, 24, 30, and 33), an arsenic species appeared that was shifted 1.0 eV higher than the arsenate compound used for fitting (KH2AsO4) and this species matched As(V)-glycerol.
Fig. 1.
Arsenic K-edge XAS spectra of traditional medicines containing arsenic around 10 mg.kg−1 or higher (product name is followed by ID in parentheses). Medicines are ordered from top to bottom from most % As(V)–O to least, and dotted lines indicate best fit. Solid vertical lines indicate locations of As–S, As(III)–O, and the usual position of As (V)–O white line. Samples marked with an * contained As(V)–O partially as As(V)–glycerol.
Table 3.
Arsenic concentration (mg.kg−1) and speciation (proportion of arsenic species, %, by X-ray absorption near-edge structure spectra) in a subset of samples; only the samples that contained arsenic at approximately 10 mg.kg−1 or greater could be analyzed.
| ID | As (mg.kg−1) | Product name | Ingredients | Amorphous orpiment% | As2O3 % | As(V)–O %
|
Red χ2a | |
|---|---|---|---|---|---|---|---|---|
| KH2AsO4 % | As(V)–glycerol % | |||||||
| 5 | 31 | Yog-Raj Guggulu | Resin and plants | 60(5) | 11(4) | 8(3) | 21(2) | 0.001 |
| 10 | 26 | Shilajeet Tablets | Bitumous substance collected from rocks | 81(3) | 19(3) | 0.003 | ||
| 11 | 89 | Mahayograj Guggulu | Resin, plants, seven bhasmasb | 11(6) | 19(4) | 59(4) | 11(2) | 0.002 |
| 24 | 27 | Trifala Guggulu | Resin and plants | 78(3) | 11(5) | 11(6) | 0.004 | |
| 30 | 140 | Mahasudarshan | Plants, including roots | 84(3) | 16(3) | 0.004 | ||
| 33 | 12 | Shilajit | Bitumous substance collected from rocks | 43(4) | 48(5) | 8(6) | 0.005 | |
| 34 | 6.4 | Sugar Fight | Plants, including roots | 60(2) | 40(2) | 0.002 | ||
| 36 | 11 | Yograj Guggulu | Resin and plants | 91(3) | 9(3) | 0.004 | ||
| 38 | 7.5 | Ayu-Hemoridi-Tone | Plants, sodium carbonate | 42(2) | 58(2) | 0.003 | ||
Numbers in parentheses indicate three times the standard deviation for the fit achieved. Arsenic species are named as those to which data were fitted.
Red χ2 indicates the goodness of the fitting to obtain the proportions of arsenic species (smaller values are better; <0.003 indicates a good fit).
The seven bhasmas used in Mahayograj Guggulu preparation are naga (lead), vanga (tin), rajata (silver), loha (iron), abhraka (mica), mandura (oxidized iron), and rasa sindura (red sulfide of mercury). Bhasma preparation often uses arsenic containing compounds. For example, in the classical preparation of naga (lead) bhasma, realgar (As4S4) is used. arsenate compound used for fitting (KH2AsO4) and this species matched As(V)–glycerol.
3.3. Bioaccessibility
The bioaccessibility results (Table 4) are included for both the gastric (G) phase and the gastric followed by intestinal (G+I) phase for arsenic, since the added extraction time and higher pH during the intestinal phase has been observed to increase arsenic bioaccessibility for certain kinds of samples (Meunier et al., 2010a; Meunier et al., 2010b). For lead, because the gastric phase results were always higher, only these results are included, which is consistent with standard risk assessment practice. Only detectable values are reported in Table 4. Excluded samples (those for which bioaccessible concentrations of both lead and arsenic were lower than the detection limit of 1 mg.kg−1) are samples 6, 7, 17, 18, 20, 21, 23, 28, and 29.
Table 4.
Bioaccessible (BA) arsenic (As) and lead (Pb) in concentration units (mg.kg−1) and percent of total (%).
| ID | BA As (mg.kg−1)
|
% BA As (%)
|
BA Pb (mg.kg−1)
|
% BA Pb (%)
|
||
|---|---|---|---|---|---|---|
| G | G+I | G | G+I | G | G | |
| 1 | <1.0 | <1.0 | <71 | <71 | 3.2 | 37 |
| 2 | <1.0 | <1.0 | <31 | <31 | 2.2 | 22 |
| 3 | 1.1 | <1.0 | 52 | <48 | 1.9 | 35 |
| 4 | 1.4 | 1.1 | 100 | 100 | 1.2 | 17 |
| 5 | 1.6 | 2.8 | 5.1 | 9.0 | 1.7 | 28 |
| 8 | <1.0 | <1.0 | <45 | <45 | 1.0 | 52 |
| 9 | <1.0 | <1.0 | <71 | <71 | 1.2 | 41 |
| 10 | 3.8 | 3.9 | 19 | 19 | 2.5 | 25 |
| 11 | 40 | 44 | 45 | 49 | 49,000 | 94 |
| 12 | <1.0 | <1.0 | N/a | N/a | 1.4 | 21 |
| 13 | <1.0 | <1.0 | <26 | <26 | 6.0 | 55 |
| 14 | <1.0 | <1.0 | <22 | <22 | 10 | 8 |
| 15 | <1.0 | <1.0 | N/a | N/a | 1.5 | 21 |
| 16 | <1.0 | <1.0 | <63 | <63 | 1.6 | 15 |
| 19 | <1.0 | <1.0 | <48 | <48 | 2.0 | 23 |
| 22 | <1.0 | <1.0 | N/a | N/a | 1.3 | 22 |
| 24 | 1.4 | 1.6 | 5.3 | 6.0 | 6.0 | 29 |
| 25 | 1.3 | <1.0 | 64 | <50 | 5.4 | 72 |
| 26 | <1.0 | <1.0 | <28 | <28 | 1.2 | 10 |
| 27 | <1.0 | <1.0 | N/a | N/a | 1.4 | 6.7 |
| 30 | 10 | 11 | 7.1 | 7.5 | 2.7 | 18 |
| 31 | <1.0 | <1.0 | <3.6 | <3.6 | 2.5 | 33 |
| 32 | <1.0 | <1.0 | <83 | <83 | 2.0 | 17 |
| 33 | 2.4 | 2.6 | 20 | 21 | <1.0 | <63 |
| 34 | 1.8 | 1.4 | 29 | 21 | 5.6 | 75 |
| 35 | <1.0 | <1.0 | <43 | <43 | 1.3 | 11 |
| 36 | <1.0 | <1.0 | <9.5 | <9.5 | 5.2 | 30 |
| 37 | <1.0 | <1.0 | N/a | N/a | 25 | 40 |
| 38 | 5.2 | 5.1 | 69 | 68 | 1.1 | 29 |
| 39 | <1.0 | <1.0 | <38 | <38 | 7.1 | 21 |
| 40 | <1.0 | <1.0 | N/a | N/a | 270 | 79 |
| 41 | <1.0 | <1.0 | N/a | N/a | 1.3 | 23 |
| 42 | 1.5 | <1.0 | 86 | <56 | 2.5 | 41 |
G = gastric phase and G+I = gastric followed by intestinal phase; both are shown for arsenic since both phases are relevant, but only the gastric phase results are shown for lead since these are the highest results. N/a = not applicable, because bioaccessible concentration detection limit is greater than total concentration, both bioaccessible concentration and total concentration are below detection, or total concentration is below detection.
The bioaccessibility results for both arsenic and lead were comparable to each other in terms of range and means, but almost all samples (32 out of 42, or 76%) had bioaccessible concentrations of lead, whereas the majority of samples were too low in arsenic to measure bioaccessibility (12 out of 42 samples, or 29% had detectable bioaccessible arsenic in the gastric phase and 9 out of 42, or 21% in the gastric+intestinal phase).
3.4. Comparison to acceptable daily intakes (ADIs)
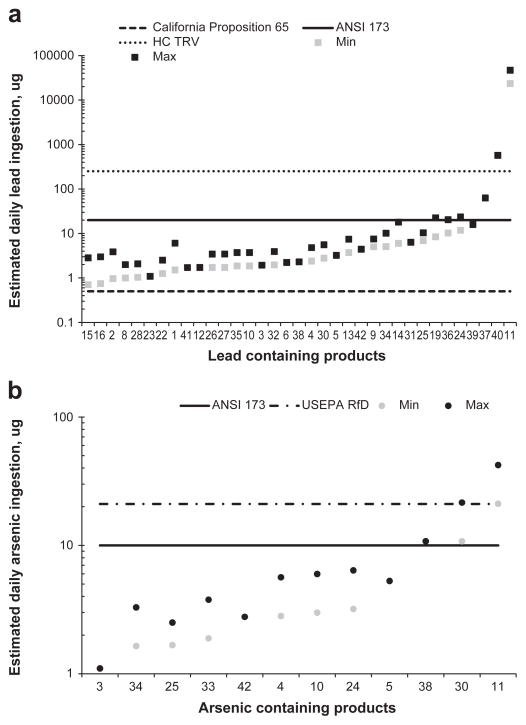
The daily amounts of lead and arsenic that would be ingested if medicines were taken according to manufacturers’ recommendations, based only on the amount that is bioaccessible (Fig. 2a and b), were calculated. The risk assessment approach used in the present study, that is, comparing estimated daily intakes with acceptable daily intakes (rather than the more conventional practice of calculating hazard quotients), was the same as that used in Saper et al. (2008) to facilitate comparisons with the results in that work. Standards for acceptable daily intakes were similar to those used in Saper et al. (2008), except that comparisons to Food and Agricultural Organization/World Health Organization Joint Expert committee on Food Additive (FAO/WHO JECFA) provisional tolerable daily intakes were not included, since these values for lead and arsenic have recently been withdrawn by FAO/WHO JECFA (Joint FAO/WHO Expert Committee on Food Additives, 2010a; Joint FAO/WHO Expert Committee on Food Additives, 2010b). Comparisons to Canadian standards (toxicological reference values, or TRVs) were added (Health Canada Contaminated Sites Division Safe Environments Programme, 2009).
Fig. 2.
Estimated daily ingestion amounts of (a) lead and (b) arsenic for Ayurvedic medicines containing bioaccessible lead and arsenic. Min = minimum amount based on minimum dose; max = maximum amount based on maximum dose. The California Proposition 65 maximum allowable dose level for lead is 0.5 μg.d−1; the American National Standard Institute/National Sanitation Foundation International Dietary Supplement Standard 173 (ANSI 173) states that dietary supplements should not contain undeclared metals that would cause intakes greater than 20 μg.d−1 for lead and 10 μg.d−1 for arsenic; the Health Canada Toxicological Reference Value (HC-TRV) for lead is 0.0036 mg.kg−1.d−1 corresponding to 252 μg.d−1 for a 70 kg person; the United States Environmental Protection Agency reference dose (USEPA RfD) for chronic oral intake of arsenic is 0.3 μg. kg−1.d−1, corresponding to 21 μg.d−1 for a 70 kg adult.
The daily amount of lead in all of the medicines that contained bioaccessible lead exceeded at least one regulatory standard (California Proposition 65), but the daily amounts of only two medicines (sample 40 Ayu-Nephro-Tone and 11 Mahayograj Guggulu) exceeded the Health Canada TRV. However, the California Proposition 65 daily intake standard (0.5 μg.d−1) is exceeded even at the detection limit of the bioaccessibility test, and thus lower detection limits are needed to assess the safety of the medicines that did not have detectable bioaccessible lead (samples 6, 7, 17, 18, 20, 21, 23, 28, 29), when this regulatory standard is considered in combination with the dosing regimes.
The arsenic daily amounts exceeded the lowest arsenic regulatory standard, American National Standard Institute/National Sanitation Foundation International Dietary Supplement Standard 173 (ANSI 173) for three medicines (samples 11 Mahayograj Guggulu, 30 Mahasudarshan, and 38 Ayu-Hemoridi-Tone) of the 13 that had bioaccessible arsenic. The maximum intakes of arsenic for a 70 kg adult ingesting Mahayograj Guggulu (sample 11) and Mahasudarshan (sample 30) exceeded the United States Environmental Protection Agency reference dose (USEPA RfD).
4. Discussion
In a previous study several Ayurvedic medicines with elevated arsenic and lead exceeded intake guidelines if ingested as recommended (Saper et al., 2008), but nothing was known about their speciation and associated solubility. Thus the present study addressed these issues in samples that were specifically selected to represent the products in the previous study with the highest concentrations of arsenic and lead, in addition to several others that had detectable amounts of these elements, including some that have not been studied previously. The process used to produce several of the products, known as Rasa Shastra, combines herbs with metals, minerals and gems, and is believed to produce medicines that are safe and therapeutic (Shastri, 1979; Satpute, 2003). Additionally, the processing of the minerals (shodhana or ‘purification’) is claimed to eliminate their harmful effects, rendering them non-toxic (Kumar et al., 2006). In the current study, sample 11 (Mahayograj Guggulu) contains a high concentration of lead, around 5% by weight (50,000 mg.kg−1), which was likely added as naga bhasma (lead ash) (Raza, 1975). Bhasmas are calcined or ashed minerals or gems; in chemical terms, the bhasma process is mainly oxidative roasting. The lead species in the sample could not be detected by X-ray diffraction analysis, likely because only amorphous forms were present, but the lead form was nearly 100% bioaccessible in the final product. This suggests that the bhasma process may actually increase the bioaccessibility of the lead starting material, most likely elemental lead, which is insoluble in water. Of note, Mahayograj Guggulu is the traditional Indian medicine most frequently associated with reported lead poisoning cases (Ernst, 2002; Centers for Disease Control and Prevention, 2004; Saper et al., 2008).
In the present study we report a reasonably high concentration of arsenic (around 90 mg.kg−1) in Mahayograj Guggulu (sample 11). This arsenic was likely introduced as part of a bhasma, since arsenic minerals like orpiment and realgar are often incorporated into the bhasma process (Thatte et al., 1993). These minerals may also be contaminants of other metal starting materials. The arsenic speciation in this sample was primarily As(V)–O along with a solid As(III)–O compound and an As–S compound (likely remaining from added arsenic-sulfide mineral such as orpiment or realgar) (Table 3). The high proportion of oxidized (and likely soluble) species As(III)–O and As(V)–O (89%) resulting from a likely starting material of insoluble orpiment or realgar gives additional evidence that the bhasma process changed the arsenic from a less soluble to a more soluble form. The speciation in starting materials and the end product naga bhasma should be studied directly to further examine this point.
Differences in total arsenic and lead, as well as percent bioaccessibility of these two elements, were noted between results in the present study and those reported recently for a sample of Mahayograj Guggulu (total lead and lead bioaccessibility were lower in the previous study, and arsenic was not detected) (Jayawardene et al., 2010). These differences probably reflect the heterogeneity of this sample from commercial sources, and the same heterogeneity probably exists for traditionally prepared preparations. Therefore, whether our findings with commercially prepared Mahayograj Guggulu are generalizable to such preparations requires further research.
Arsenic speciation was also determined in several other samples, chosen because their arsenic content is around or above 10 mg.kg−1 (Table 3). Most of these samples, including Yog-Raj (Yograj) Guggulu (samples 5, 36), Trifala Guggulu (sample 24), Mahasudarshan (sample 30), and Sugar Fight (sample 34) have not been classified as Rasa Shastra medicines, which means that they have no mineral ingredients. Consequently, the likeliest arsenic source is plant ingredients. The three other samples analyzed for arsenic speciation, Ayu-Hemoridi-Tone (sample 38), Shilajeet (sample 10) and Shilajit (sample 33), have been classified as Rasa Shastra medicines, indicating that they contain metals, minerals, or gems, although they are also derived from plant material (Shilajeet(jit) is made of “mineral pitch”, the dried exudate of the plant Asphaltum punjabinum from rock).
Plants have been observed to contain elevated arsenic concentrations when harvested from areas that have naturally or anthropogenically elevated soil arsenic concentrations, e.g., (Koch et al., 2000) and some plants used in Ayurvedic medicines have been shown to contain arsenic (Singh and Garg, 1997). Roots especially are susceptible to contamination, e.g., (Aldrich et al., 2007), and if these were included in the compounding of the medicines, they might have contributed to the arsenic content. The speciation of the arsenic in the samples indicates that an As–S compound is found in all samples except for Ayu-Hemoridi-Tone (sample 38) but the quality of the spectra was not sufficiently high to identify the As–S compound with certainty (Fig. 1, Table 3 Red χ2 values). As(III)–S compounds are commonly found in fresh and live plant samples using mass spectrometric (Bluemlein et al., 2009) and XAS methods (Pickering et al., 2000; Smith et al., 2008), although these compounds appear to be relatively unstable to processing steps like harvesting, drying and aging in air (Smith et al., 2008; Bluemlein et al., 2009). In some plants, however, As–S compounds have been observed to survive air-drying and grinding steps (Mir et al., 2007). Similar mild preparation techniques were likely used for all the medicines (e.g., cleaning, drying and grinding plants, aqueous extractions at 60–70 °C of guggulu), as described for Yogaraga Guggulu (Simha et al., 2008). In the absence of the knowledge of any mineral forms of arsenic added to the products that have not been classified as Rasa Shastra medicines, we propose that it is possible that the As–S species in these samples are derived from plants. This may be a possibility for Shilajeet (jit) (samples 10, 33) as well, but the possibility of the As–S compound as a mineral form, scraped from rocks that may have contained arsenic minerals, cannot be discounted.
In five samples, Yog-Raj Guggulu (sample 5), Mahayograj Guggulu (sample 11), Trifala Guggulu (sample 24), Mahasudarshan (sample 30), and Shilajit (sample 33), the AsV compound present had a white line position that was 1.0 eV higher than solid KH2AsO4 (Fig. 1). Arsenic white lines that are approximately 1.0 eV higher than arsenate have been seen previously in dried plant and insect residues (Mir et al., 2007; Andrahennadi et al., 2009) and the compound with this energy has been identified as an octahedrally coordinated As (V)–glycerol compound (Andrahennadi et al., 2009). When the As (V)–glycerol was included in the fittings, the higher energy compounds in the present study were tentatively identified as the glycerol compound. The presence of this compound may be attributable to the dried plant residues in the medicines.
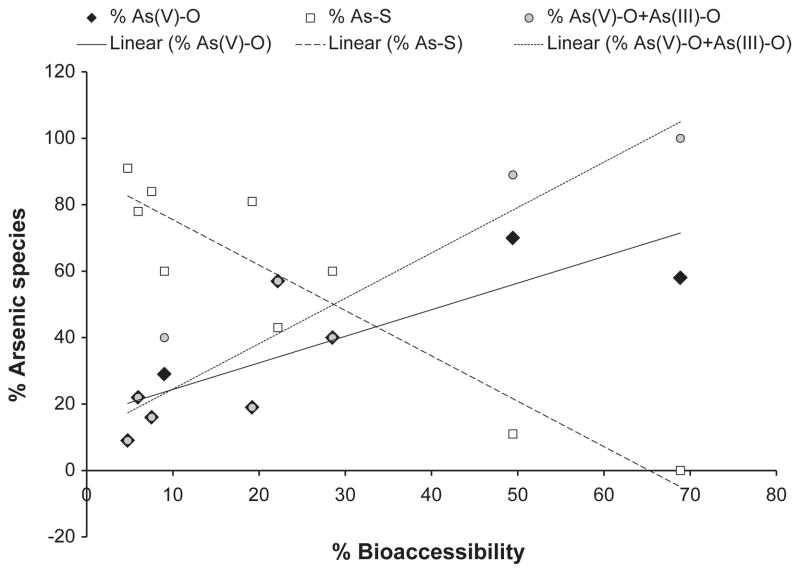
When the bioaccessibility results are compared with the speciation results, it appears that percent bioaccessibility increases with the proportion of pentavalent arsenic (Fig. 3) (n=9, r=0.82, p<0.05). The correlation is even better when As(III)–O is summed with As(V)–O (n=9, r=0.93, p<0.05). At the same time, a negative correlation is observed between the bioaccessibility and the proportion of the As–S species suggesting that these may be the arsenic species that are not bioaccessible (Fig. 3) (n=9, r=−0.93, p<0.05). Thus the appearance of oxidized As(III)–O and As(V)–O in these medicines, either introduced as an ingredient or resulting from processing, may make the arsenic more available for uptake into the human body, where toxic effects can be exerted. Since it is difficult with XANES to distinguish As(V)–O as a soluble species, such as the potassium arsenate (KH2AsO4) used as a standard in the present study, from insoluble species such as scorodite (FeAsO4 · 2H2O), we cannot state with certainty that the As(V)–O species found in these samples are indeed the forms dissolved in the bioaccessibility test; further research involving XANES testing of residues from the bioaccessibility test would be required. No organoarsenic species were detected in any samples, although they may be present at very low concentrations below the technique’s detection limit.
Fig. 3.
Percent arsenic species vs. percent bioaccessibility for ◆ As(V)–O species (sum of species matching KH2AsO4 +As(V)–glycerol),
 As(V)–O+As(III)–O, and □ As–S, based on samples (n=9) analyzed by XANES. The correlations of the data are significant (p<0.05, see text for r values), showing that the As(V)–O and As(III)–O species are likely the bioaccessible ones, whereas the As–S species are not.
As(V)–O+As(III)–O, and □ As–S, based on samples (n=9) analyzed by XANES. The correlations of the data are significant (p<0.05, see text for r values), showing that the As(V)–O and As(III)–O species are likely the bioaccessible ones, whereas the As–S species are not.
The implications of using the studied medicines were determined by comparing estimated daily intakes with acceptable daily intakes. Of the fourteen medicines included in the present study that previously exceeded the ANSI standard for lead in Saper et al. (2008), only five exceed this standard in Fig. 2a. Likewise, the three medicines that exceeded arsenic standards in Saper et al. (2008) no longer exceed any standards in the present work. However, one sample (sample 38, Ayu-Hemoridi-Tone) exceeds the ANSI arsenic standard in Fig. 2b, but was not included in the Saper et al. (2008) evaluation because its arsenic concentration was not detectable by the XRF method used. Incorporating bioaccessibility into the intake calculations therefore resulted in a decrease in the number of samples exceeding standards, compared with results using total concentrations.
5. Conclusions
The speciation for arsenic in a medicine that likely had a bhasma added to it indicates changes to more soluble forms, likely attributable to bhasma-type preparation procedures, and the high bioaccessibility of lead in the same material supports this theory. Arsenic appearing as As(V)–O and As(III)–O appears to be associated with bioaccessible arsenic and the remaining arsenic is as less-bioaccessible As–S compounds. These speciation trends are specific to the samples analyzed in the present study, and their interpretation in the context of other samples must be undertaken carefully. Nevertheless, the results suggest that increases in bioaccessibility, and likely toxicity (lead and arsenic are likely inorganic species in the bioaccessibility extracts), are associated with the processing steps used to compound these medicines (i.e., oxidation of arsenic minerals and elemental lead in naga bhasma, and oxidation of As–S in plants, (Smith et al., 2008)).
Daily intakes calculated with bioaccessible arsenic and lead concentrations are lower than previously estimated (Saper et al., 2008) but many are still above acceptable levels. Given our findings for Mahayograj Guggulu’s high level of bioaccessible lead and arsenic and case reports suggesting toxicity, its ongoing use should be carefully scrutinized. Mercury is an element of concern in the medicines that was not addressed in the present research, but future work is planned to measure the bioaccessibility of this element. Information about the elemental content should ideally be provided with medicines so practitioners and consumers can make informed decisions.
Supplementary Material
Acknowledgments
We would like to acknowledge funding from a Natural Sciences and Engineering Research Council (NSERC) Discovery grant and Department of National Defence Academic Research Program grant to KJR, WRC and IK. RBS was supported by a Career Development Award (K07 AT002915-03) from the National Center for Complementary and Alternative Medicine (NCCAM), National Institutes of Health. We would also like to thank Jun Zhang for his assistance with ICP-MS analysis; John Peters, Jessica Harris and Sara Dillabough for their assistance with bioaccessibility and total element measurements; Major Louise Meunier, Dr. Mark Button and beamline scientist Dr. Robert Gordon for their assistance with analysis at the Advanced Photon Source (APS), Argonne National Laboratories, Argonne, Illinois; Dr. Janet Paquin for conducting the XRF measurements at the U.S. Environmental Protection Agency New England Regional Laboratory, North Chelmsford, Massachusetts; and Anusha Sehgal MD (Ayurveda) for consultation regarding traditional Indian medicine. PNC/XSD facilities at the APS, and research at these facilities, are supported by the US Department of Energy — Basic Energy Sciences, a Major Resources Support grant from NSERC, University of Washington, Simon Fraser University and the APS. Use of the APS is also supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract DE-AC02-06CH11357. The Canadian Light Source is supported by NSERC, National Research Council (Canada), Canadian Institute of Health Research, and the University of Saskatchewan.
Appendix A. Supplementary data
Supplementary data to this article can be found online at doi:10.1016/j.scitotenv.2011.07.059.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Aldrich MV, Peralta-Videa JR, Parsons JG, Gardea-Torresdey JL. Examination of arsenic(III) and (V) uptake by the desert plant species mesquite (Prosopis spp) using X-ray absorption spectroscopy. Sci Total Environ. 2007;379:249–55. doi: 10.1016/j.scitotenv.2006.08.053. [DOI] [PubMed] [Google Scholar]
- Amiard JC, Amiard-Triquet C, Charbonnier L, Mesnil A, Rainbow PS, Wang WX. Bioaccessibility of essential and non-essential metals in commercial shellfish from Western Europe and Asia. Food Chem Toxicol. 2008;46:2010–22. doi: 10.1016/j.fct.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Andrahennadi R, Fu JX, Pushie MJ, Wiramanaden CIE, George GN, Pickering IJ. Insect excretes unusual six-coordinate pentavalent arsenic species. Environ Chem. 2009;6:298–304. [Google Scholar]
- Basta NT, Foster JN, Dayton EA, Rodriguez RR, Casteel SW. The effect of dosing vehicle on arsenic bioaccessibility in smelter-contaminated soils. J Environ Sci Health Part A-Tox/Haz Sub Environ Eng. 2007;42:1275–81. doi: 10.1080/10934520701434927. [DOI] [PubMed] [Google Scholar]
- Bluemlein K, Raab A, Feldmann J. Stability of arsenic peptides in plant extracts: off-line versus on-line parallel elemental and molecular mass spectrometric detection for liquid chromatographic separation. Anal Bioanal Chem. 2009;393:357–66. doi: 10.1007/s00216-008-2395-z. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Lead poisoning associated with use of Ayurvedic medications — five states, 2000–2003. MMWR Morb Mortal Wkly Rep. 2004;53:582–4. [PMC free article] [PubMed] [Google Scholar]
- Deshpande AA, Rhodes CT, Shah NH, Malick AW. Controlled-release drug delivery systems for prolonged gastric residence: an overview. Drug Dev Ind Pharm. 1996;22:531–9. [Google Scholar]
- Ernst E. Heavy metals in traditional Indian remedies. Eur J Clin Pharmacol. 2002;57:891–6. doi: 10.1007/s00228-001-0400-y. [DOI] [PubMed] [Google Scholar]
- Franke FW, Moxon AL. A comparsion of the minimun fatal doses of selenium, tellurium, arsenic and vanadium. J Pharmacol Exp Ther. 1936;58:454–9. [Google Scholar]
- Health Canada Contaminated Sites Division Safe Environments Programme. Federal contaminated site risk assessment in Canada: Part II: Health Canada Toxicological References Values (TRVs) and chemical-specific factors. Ottawa, ON: Government of Canada; 2009. [Google Scholar]
- Jayawardene I, Saper R, Lupoli N, Sehgal A, Wright RO, Amarasiriwardena C. Determination of in vitro bioaccessibility of Pb, As, Cd and Hg in selected traditional Indian medicines. J Anal At Spectrom. 2010;25:1275–82. doi: 10.1039/C003960H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Summary report of the seventy-second meeting of JECFA. Geneva, Switzerland: FAO/WHO; 2010a. pp. 1–16. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Summary report of the seventy-third meeting of JECFA. Geneva, Switzerland: FAO/WHO; 2010b. pp. 1–17. [Google Scholar]
- Juhasz AL, Weber J, Smith E, Naidu R, Rees M, Rofe A, et al. Assessment of four commonly employed in vitro arsenic bioaccessibility assays for predicting in vivo relative arsenic bioavailability in contaminated soils. Environ Sci Technol. 2009;43:9487–94. doi: 10.1021/es902427y. [DOI] [PubMed] [Google Scholar]
- Koch I, Steven S, Lai VWM, Owen A, Reimer KJ, Cullen WR. Bioaccessibility and excretion of arsenic in Niu Huang Jie Du Pian pills. Toxicol Appl Pharmacol. 2007;222:357–64. doi: 10.1016/j.taap.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Koch I, Wang LX, Ollson CA, Cullen WR, Reimer KJ. The predominance of inorganic arsenic species in plants from Yellowknife, Northwest Territories, Canada. Environ Sci Technol. 2000;34:22–6. [Google Scholar]
- Kong F, Singh RP. A model stomach system to investigate disintegration kinetics of solid foods during gastric digestion. J Food Sci. 2008;73:E202–10. doi: 10.1111/j.1750-3841.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Nair AGC, Reddy AVR, Garg AN. Unique ayurvedic metallic-herbal preparations, chemical characterization. Biol Trace Elem Res. 2006;109:231–54. doi: 10.1385/bter:109:3:231. [DOI] [PubMed] [Google Scholar]
- Laparra JM, Velez D, Montoro R, Barbera R, Farre R. Bioaccessibility of inorganic arsenic species in raw and cooked Hizikia fusiforme seaweed. Appl Organomet Chem. 2004;18:662–9. [Google Scholar]
- Liu J, Lu YF, Wu Q, Goyer RA, Waalkes MP. Mineral arsenicals in traditional medicines: orpiment, realgar, and arsenolite. J Pharmacol Exp Ther. 2008;326:363–8. doi: 10.1124/jpet.108.139543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier L, Walker SR, Wragg J, Parsons MB, Koch I, Jamieson HE, et al. Effects of soil composition and mineralogy on the bioaccessibility of arsenic from tailings and soil in gold mine districts of Nova Scotia. Environ Sci Technol. 2010a;44:2667–74. doi: 10.1021/es9035682. [DOI] [PubMed] [Google Scholar]
- Meunier L, Wragg J, Koch I, Reimer KJ. Method variables affecting the bioaccessibility of arsenic in soil. J Environ Sci Health Part A-Tox/Haz Sub Environ Eng. 2010b;45:517–26. doi: 10.1080/10934521003594863. [DOI] [PubMed] [Google Scholar]
- Mir KA, Rutter A, Koch I, Smith P, Reimer KJ, Poland JS. Extraction and speciation of arsenic in plants grown on arsenic contaminated soils. Talanta. 2007;72:1507–18. doi: 10.1016/j.talanta.2007.01.068. [DOI] [PubMed] [Google Scholar]
- Park JD, Liu YP, Klaassen CD. Protective effect of metallothionein against the toxicity of cadmium and other metals. Toxicology. 2001;163:93–100. doi: 10.1016/s0300-483x(01)00375-4. [DOI] [PubMed] [Google Scholar]
- Pickering IJ, Prince RC, George MJ, Smith RD, George GN, Salt DE. Reduction and coordination of arsenic in Indian mustard. Plant Physiol. 2000;122:1171–7. doi: 10.1104/pp.122.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel B, Newville M. Athena, Artemis, Hephaestus: data analysis for X-ray absorption spectroscopy using ifeffit. J Synchrotron Radiat. 2005;12:537–41. doi: 10.1107/S0909049505012719. [DOI] [PubMed] [Google Scholar]
- Raza ZB. Clinical trial in rheumatoid and osteoarthritis of an indigenous drug combination. Patna J Med. 1975;49:70–3. [Google Scholar]
- Richardson GM, Bright DA, Dodd M. Do current standards of practice in Canada measure what is relevant to human exposure at contaminated sites? II: oral bioaccessibility of contaminants in soil. Hum Ecol Risk Assess. 2006;12:606–16. [Google Scholar]
- Rochette EA, Bostick BC, Li GC, Fendorf S. Kinetics of arsenate reduction by dissolved sulfide. Environ Sci Technol. 2000;34:4714–20. [Google Scholar]
- Rodriguez RR, Basta NT, Casteel SW, Pace LW. An in vitro gastrointestinal method to estimate bioavailable arsenic in contaminated soils and solid media. Environ Sci Technol. 1999;33:642–9. [Google Scholar]
- Ruby MV, Davis A, Link TE, Schoof R, Chaney RL, Freeman GB, et al. Development of an in-vitro screening-test to evaluate the in-vivo bioaccessibility of ingested mine-waste lead. Environ Sci Technol. 1993;27:2870–7. [Google Scholar]
- Ruby MV, Davis A, Schoof R, Eberle S, Sellstone CM. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ Sci Technol. 1996;30:422–30. [Google Scholar]
- Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB, et al. Heavy metal content of Ayurvedic herbal medicine products. Jama-J Am Med Assoc. 2004;292:2868–73. doi: 10.1001/jama.292.23.2868. [DOI] [PubMed] [Google Scholar]
- Saper RB, Phillips RS, Sehgal A, Khouri N, Davis RB, Paquin J, et al. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the Internet. Jama-J Am Med Assoc. 2008;300:915–23. doi: 10.1001/jama.300.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute AD. Rasa Ratna Samuchaya of Vagbhatta, trans. Varanasi, India: Chaukhamba Sanskrit Pratishthan, Delhi; 2003. [Google Scholar]
- Scarr M, Maltby JR, Jani K, Sutherland LR. Volume and acidity of residual gastric fluid after oral fluid ingestion before elective ambulatory surgery. Can Med Assoc J. 1989;141:1151–4. [PMC free article] [PubMed] [Google Scholar]
- Schroder JL, Basta NT, Casteel SW, Evans TJ, Payton ME, Si J. Validation of the in vitro gastrointestinal (IVG) method to estimate relative bioavailable lead in contaminated soils. J Environ Qual. 2004;33:513–21. doi: 10.2134/jeq2004.5130. [DOI] [PubMed] [Google Scholar]
- Shastri K. Rasa Tarangini of Sadananda Sharma, trans. New Delhi, India: Motilal Banarsidass; 1979. [Google Scholar]
- Simha KRG, Laxminarayana V, Prasad SVLN, Khanum S. Standardisation of Yogaraja guggulu — an Ayurvedic polyherbal formulation. Indian J Tradit Knowl. 2008;7:389–96. [Google Scholar]
- Singh V, Garg AN. Availability of essential trace elements in Ayurvedic Indian medicinal herbs using instrumental neutron activation analysis. Appl Radiat Isot. 1997;48:97–101. doi: 10.1016/s0969-8043(96)00043-7. [DOI] [PubMed] [Google Scholar]
- Smith PG, Koch I, Gordon RA, Mandoli DF, Chapman BD, Reimer KJ. X-ray absorption near-edge structure analysis of arsenic species for application to biological environmental samples. Environ Sci Technol. 2005;39:248–54. doi: 10.1021/es049358b. [DOI] [PubMed] [Google Scholar]
- Smith PG, Koch I, Reimer KJ. Uptake, transport and transformation of arsenate in radishes (Raphanus sativus) Sci Total Environ. 2008;390:188–97. doi: 10.1016/j.scitotenv.2007.09.037. [DOI] [PubMed] [Google Scholar]
- Stark SC, Snape I, Graham NJ, Brennan JC, Gore DB. Assessment of metal contamination using X-ray fluorescence spectrometry and the toxicity characteristic reaching procedure (TCLP) during remediation of a waste disposal site in Antarctica. J Environ Monit. 2008;10:60–70. doi: 10.1039/b712631j. [DOI] [PubMed] [Google Scholar]
- Thatte UM, Rege NN, Phatak SD, Dahanukar SA. The flip side of Ayurveda. J Postgrad Med. 1993;39:179–82. [PubMed] [Google Scholar]
- U.S.EPA. Office of Solid Waste and Emergency Response. Estimation of relative bioavailability of lead in soil and soil-like materials using in vivo and in vitro methods. U.S. EPA; 2007. [Google Scholar]
- United States Environmental Protection Agency (USEPA) Method 6200: field portable X-ray florescence spectrometry for the determination of elemental concentrations in soil and sediment. Washington, DC: USEPA; 2007. p. SW-846. [Google Scholar]
- Van de Wiele TR, Oomen AG, Wragg J, Cave M, Minekus M, Hack A, et al. Comparison of five in vitro digestion models to in vivo experimental results: lead bioaccessibility in the human gastrointestinal tract. J Environ Sci Health Part A-Tox/Haz Sub Environ Eng. 2007;42:1203–11. doi: 10.1080/10934520701434919. [DOI] [PubMed] [Google Scholar]
- Younis IR, Stamatakis MK, Callery PS, Meyer-Stout PJ. Influence of pH on the dissolution of folic acid supplements. Int J Pharm. 2009;367:97–102. doi: 10.1016/j.ijpharm.2008.09.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.