Non-technical summary
The serotonin 3 receptor is a ligand-gated ion channel which is activated by the neurotransmitter serotonin. This receptor is present in many parts of the brain, yet there is hardly any information on its function in the cerebellum. In this study, we use transgenic mice to show that the serotonin 3 receptor is localised in the cerebellum during the first three weeks after birth. We found that during this period, the serotonin 3 receptor regulates communication between two major types of neurons in the cerebellum, the granule cells and the Purkinje cells. In the absence of this receptor during early postnatal development, synaptic transmission between those neurons is disturbed. Taken together, these findings can help us understand the way serotonin affects the function of the cerebellum, and may provide insight into pathophysiological conditions in which the serotonergic system is compromised.
Abstract
Abstract
The serotonin 5-HT3 receptor is the only ligand-gated ion channel activated by serotonin and is expressed by GABAergic interneurons in many brain regions, including the cortex, amygdala and hippocampus. Furthermore, 5-HT3 receptors are expressed by glutamatergic Cajal–Retzius cells in the cerebral cortex. We used 5-HT3A/enhanced green fluorescent protein (EGFP) transgenic mice to show that 5-HT3 receptors are also ubiquitously expressed by glutamatergic granule cells in the cerebellum during the first three postnatal weeks. Using whole-cell patch clamp recordings, we show that local application of either serotonin or the selective 5-HT3 receptor agonist SR57227A to granule cells results in a small inward current, demonstrating a post- and/or extrasynaptic localisation of the 5-HT3 receptors. Functional 5-HT3 receptors were also observed presynaptically at the parallel fibre–Purkinje cell synapse. Pharmacological block using the selective 5-HT3 receptor antagonist tropisetron induced a reduction in the frequency of miniature synaptic events recorded from Purkinje cells. Paired-pulse stimulation of parallel fibres on whole-cell voltage clamped Purkinje cells from 1-week-old mice did not yet show synaptic plasticity. In the presence of tropisetron, the parallel fibre–Purkinje cell synapse showed paired-pulse depression. Taken together, these results show that functional 5-HT3 receptors are present during early postnatal development in the cerebellum, where they modulate synaptic plasticity.
Introduction
The serotonin 3 (5-HT3) receptor is the only ligand-gated ion channel for serotonin (Derkach et al. 1989; Maricq et al. 1991). Functional receptors are either hetero- or homopentamers with at least two obligatory 5-HT3A subunits (Davies et al. 1999; Barrera et al. 2005), and are permeable to cations such as potassium, calcium and sodium (Yakel et al. 1990). The 5-HT3 receptor is expressed throughout the CNS, in particular by GABAergic interneurons, with high levels of expression in the cortex, hippocampus, amygdala, several brainstem nuclei and the spinal cord (reviewed by Chameau & van Hooft, 2006). In the cortex, selective subpopulations of interneurons characterized by expression of vasoactive intestinal peptide or neuropeptide Y express the 5-HT3 receptor from early postmitotic to adult stages (Vucurovic et al. 2010). In addition, 5-HT3 receptors are expressed postsynaptically by Cajal–Retzius cells, located in layer I of the cortex during the first two postnatal weeks (Chameau et al. 2009).
In the cortex, it has been shown that electrical stimulation of a single serotonergic fibre originating from the raphe nuclei and contacting the recorded cells via multiple synaptic contacts results in a large and fast 5-HT3 receptor-mediated synaptic current in both GABAergic interneurons and Cajal–Retzius cells (Férézou et al. 2002; Chameau et al. 2009). In both hippocampal and cortical neurons, 5-HT3 receptor-mediated ion currents show a characteristic voltage-dependent block in their I–V curve, caused by external Ca2+ ions and modulated by intracellular phosphates such as ATP (Kawa, 1994; McMahon & Kauer, 1997; Roerig et al. 1997; van Hooft & Wadman, 2003; Noam et al. 2008).
Literature on the presence of 5-HT3 receptors in the cerebellum is still inconsistent. Most studies have shown that there are no or few 5-HT3 receptors in the cerebellum in different species such as mice (Kilpatrick et al. 1989), rats (Kilpatrick et al. 1987, 1989; Barnes et al. 1990; Hewlett et al. 1998; Miquel et al. 2002), ferrets (Kilpatrick et al. 1989), rabbits (Kilpatrick et al. 1989), macaque monkeys (Thorell et al. 1997) and humans (Kilpatrick et al. 1989; Parker et al. 1996; Marazziti et al. 2001). However, one study reported moderate levels of 5-HT3 receptor expression in rats (Ge et al. 1997). Others found that local application of the 5-HT3 receptor agonist 1-(m-chlorophenyl)-biguanide (mCPBG) induced changes in relative intracellular Ca2+ concentration in 32% of synaptosomes isolated from the cerebellum (Nayak et al. 1999). This indicates a presynaptic localisation of 5-HT3 receptors in the cerebellum. Furthermore, Geurts et al. (2002) found fine varicose 5-HT3 receptor-positive fibres in low density in both the molecular and the granular layer in the cerebellum from adult rats. More recently, Arpin-Bott et al. (2006) found 5-HT3 receptor immunoreactivity on Bergmann glial cells and their radial fibres, and less intense and scattered immunoreactivity was observed throughout the granular layer. However, all of these studies investigated adult animals, and no information is available on the expression of 5-HT3 receptors in the cerebellum of younger animals. It is especially during the early period of cerebellar development that fundamental neuronal connections are laid for motor coordination and cognitive functioning. Because 5-HT3 receptors are previously associated with early postnatal development in the cortex (Chameau et al. 2009) and this period is of high importance to the cerebellum, we investigated the expression and function of 5-HT3 receptors in the developing postnatal cerebellum using the transgenic 5-HT3A/EGFP mouse line. This mouse line has been found to be a reliable and useful tool to study 5-HT3 receptor expression throughout the CNS (Inta et al. 2008; Chameau et al. 2009; Vucurovic et al. 2010; Lee et al. 2010).
In this study we provide evidence that 5-HT3 receptors are abundantly expressed by glutamatergic granule cells in the cerebellum during the first three postnatal weeks. Cerebellar 5-HT3 receptors induce a fast inward current upon local activation by either serotonin or the selective agonist SR57227A ((4-amino)-(6-chloro-2-pyridyl)l-piperidine hydrochloride), indicating a post- and/or extrasynaptic localization. In addition, presynaptic 5-HT3 receptors were observed at the parallel fibre–Purkinje cell synapse. Together, these data indicate for the first time functional 5-HT3 receptors, located both pre- and postsynaptically, on glutamatergic granule cells in the cerebellum during early postnatal development.
Methods
Ethical approval
Wild-type and 5-HT3A/enhanced green fluorescent protein (EGFP) transgenic Swiss mice (GENSAT, http://www.gensat.org) between the age of postnatal day 0 and postnatal day 48, both males and females, were used for this study. In total 54 animals were used for all experiments in this study. All experiments were performed in accordance with the committee on animal bioethics of the University of Amsterdam.
Immunohistochemistry
Sagittal slices from transcardially perfused 5-HT3A/EGFP transgenic mice between P0 and P48 were cut using a vibrating blade microtome (Leica VT1000S) at a thickness of 50–100 μm. Slices were washed in phosphate-buffered saline (PBS), then permeabilized for 30 min in PBS–0.25% Triton X-100 (PBST), and kept for 1 h in PBST + 10% normal goat serum (NGS), after which they were kept overnight at 4°C in the primary antibody in PBS + 5% NGS. The next day, slices were washed in PBS and incubated for 2 h at room temperature in the secondary antibody in PBS + 5% NGS. After washing in PBS, slices were mounted using Vectashield Hard Set Mounting Medium (Vector Laboratories, Peterborough, UK). Coexpression of the EGFP signal from 5-HT3A/EGFP transgenic mice with glutamate decarboxylase (GAD) at P7 was tested by staining slices with rabbit polyclonal GAD65 + GAD67 antibody (1:500, Abcam) and goat anti-rabbit IgG Alexa 594 (1:200, Invitrogen) as secondary antibody. Coexpression of the EGFP signal with glial fibrillary acidic protein (GFAP) at P7 was visualised by staining slices with polyclonal rabbit anti-GFAP antibody (1:1000, DAKO) and goat anti-rabbit IgG Alexa 594 (1:200, Invitrogen). Images from all stainings were made using a confocal microscope (Zeiss LSM 510) equipped with a 20× objective and using the 488 and 543 nm lines of an argon–krypton laser.
Electrophysiological recordings
Animals were killed by decapitation between P6 and P21 for electrophysiological recordings. Sagittal brain slices were cut using a vibrating blade microtome (Leica VT1200S) at a thickness of 300 μm for whole-cell patch clamp recordings. During slicing the brains were kept in cooled (4°C) oxygenated ACSF which was composed of the following (in mm): NaCl (120), KCl (3.5), CaCl2 (2.5), MgSO4 (1.3), NaH2PO4 (1.25), NaHCO3 (25), glucose (25), continuously bubbled with 95% O2 and 5% CO2 (pH 7.4). During the experiments slices were kept submerged at room temperature and continuously superfused with ACSF. Patch pipettes were pulled from borosilicate glass with a resistance of 2–3 MΩ for Purkinje cells and 8–10 MΩ for granule cells and were filled with internal solution consisting of (in mm): potassium gluconate (105), KCl (30), EGTA (5), CaCl2 (0.5), Hepes (10), and Mg-ATP (5), pH 7.3 with KOH. EGFP-positive neurons in the cerebellum were visualised using differential interference contrast videomicroscopy on a Zeiss Axioskop 2 FS Plus microscope equipped with standard epifluorescence. Whole-cell recordings were made using an EPC9 patch-clamp amplifier and PULSE software (HEKA Electronik, Lambrecht, Germany) or an in-house software package running under MATLAB (The MathWorks, Natick, MA, USA). Off-line analysis was performed using Igor 4.07 (Wavemetrics, Lake Oswego, OR, USA) or MATLAB. Signals were filtered at 1–5 kHz and sampled at 10 kHz. Series resistance ranged from 2 to 12 MΩ for Purkinje cells and 5 to 40 MΩ for granule cells and was compensated for at least 60%. Cells were voltage clamped at –70 mV, corrected for liquid junction potential.
Granule cells were patched at P6–P10, and a second pipette connected to a picospritzer II (General Valve, Fairfield, NJ, USA), and containing 100 μm 5-HT (Sigma) or 100 μm SR57227A (Sigma, Bachy et al. 1993) in ACSF was positioned in the vicinity of the cell soma and the drug was applied for 500 ms at 35–100 kPa. Purkinje cells were patched at P7–P21 and glutamatergic synaptic currents in Purkinje cells were evoked by stimulation of the parallel fibres with a glass electrode filled with ACSF. Paired stimuli (100–400 μA, 200 μs duration, interstimulus interval 50 ms) were delivered to the parallel fibres using a custom-made isolated bipolar current stimulator. Paired-pulse stimulations were delivered with a 20 s interval, and only recordings which were stable for at least 15 min were used to analyse the paired-pulse ratio. Blockade of 5-HT3 receptors was achieved by pre-incubation of the slices with 30 nm of the specific antagonist tropisetron (ICS 205–930; Sigma) for at least 20 min. During the recordings tropisetron was also applied via the superfusion system. Activation of 5-HT3 receptors was achieved by local application of 10 μm of the specific 5-HT3 receptor agonist mCPBG (Sigma, Kilpatrick et al. 1990) to the soma of the recorded cell, using a picospritzer.
Miniature synaptic events from Purkinje cells were recorded at P8–P10 in the presence of 0.5 μm TTX (Latoxan, Valence, France). Five-minute control recordings were followed by wash-in of 30 nm tropisetron via the superfusion system for 20 min, after which another five-minute recording in the presence of tropisetron was used for analysis. Activation of 5-HT3 receptors was achieved by local application of 10 μm mCPBG to the soma of the recorded cell. Traces of 5 min were used for analysis during which all miniature synaptic events were pooled per group, each containing over 1400 miniature synaptic events. The amplitude threshold for detection of spontaneous miniature synaptic currents was set above the noise level and events were subsequently verified visually. Average cumulative distributions were calculated using 200 bins per distribution. Both inter-event interval times and amplitudes of each event were analysed.
Morphology
In order to reveal granule cell and Purkinje cell morphology, cells were filled with biocytin (Sigma, 2–3 mg ml−1 dissolved in internal solution as described above) during the whole-cell patch clamp recordings. Slices were fixed overnight in paraformaldehyde (PFA, 4% in PBS, pH 7.4) at 4°C and visualised using immunohistochemical methods. Slices were washed in PBS for 5 × 8 min, and endogenous peroxidase was inhibited by a 30 min incubation in H2O2 (3% in PBS). After 60 min permeabilization in 2% Triton X-100 (2% in PBS), slices were incubated for 2 h in avidin–biotin–peroxidase complex (Vectastain ABC Elite kit, Vector Laboratories). Biocytin was visualised as a dark brown substrate using a DAB (3,3′-diaminobenzidine-4 HCl, Sigma) reaction. Slices were washed 3 × 8 min before being mounted on a glass slide with mowiol 4–88 (Sigma; dissolved in 0.2 m Tris-HCl and glycerol, pH 8.5). Images were made using the same confocal microscope as described above.
Statistical analysis
Values are expressed as means ± standard error of the mean. Comparisons were made using Student's t test. Frequency and amplitude distributions of the miniature synaptic events were compared with a two-sample Kolmogorov–Smirnov test. P < 0.05 was assumed to indicate a significant difference.
Results
The 5-HT3 receptor is transiently expressed by granule cells in early postnatal cerebellum
We used the 5-HT3A/EGFP transgenic mouse line to study the expression of 5-HT3 receptors in the cerebellum. EGFP expression is apparent during early postnatal development, with relatively high expression in lobules I–VI and to a lesser extent in lobules VII–X (Fig. 1A). At birth, EGFP expression is found only in the external granule cell layer (Fig. 1Ba), and subsequently parallels the pattern of radial migration of granule cells during early postnatal development. By P7, the expression is found ubiquitously in the cerebellum in both the external and the internal granule cell layer, with a clear lack of expression in the Purkinje cell layer (Fig. 1Bb). At P14, EGFP expression is only found in the internal granule cell layer and is starting to diminish (Fig. 1Bc). The EGFP expression is almost absent in the internal granule cell layer at P21 (Fig. 1Bd), and is completely absent from the cerebellum at P28 and P48 (not shown). EGFP expression neither colocalises with GAD65 + 67, a marker of Purkinje cells and interneurons (Fig. 1C), nor with GFAP, a marker of glial cells (Fig. 1D).
Figure 1. The 5-HT3 receptor is transiently expressed by glutamatergic granule cells in the cerebellum.
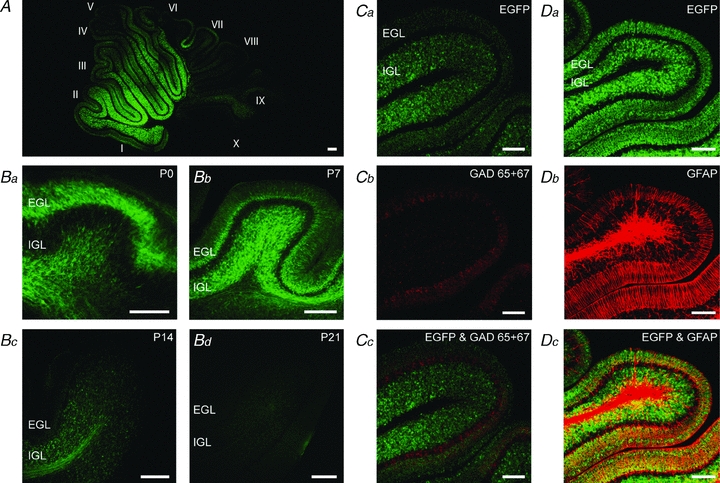
A, EGFP expression pattern in a P8 5-HT3A/EGFP transgenic mouse. Relatively high expression can be found in lobules I–VI. Roman numerals indicate cerebellar lobules. B, EGFP expression pattern in 5-HT3A/EGFP transgenic mice at P0 (Ba), P7 (Bb), P14 (Bc), and P21 (Bd). The expression pattern follows the migration pathway of the cerebellar granule cells from the external (EGL) to the internal (IGL) granule cell layer, with diminished expression from P14 onwards and no expression after P21. C, immunostaining of GAD65 + 67 in EGFP-expressing cerebellum (Ca–Cc) from a P7 5-HT3A/EGFP transgenic mouse shows no coexpression of 5-HT3 receptors with GABAergic neurons. D, immunostaining of GFAP in EGFP-expressing cerebellum (Da–Dc) from a P7 5-HT3A/EGFP transgenic mouse shows no coexpression of 5-HT3 receptors with glial cells, further confirming the expression of 5-HT3 receptors by glutamatergic granule cells in the cerebellum. Scale bars indicate 100 μm.
Activation of post- and/or extrasynaptic 5-HT3 receptors on granule cells induces a fast inward current
We performed whole-cell patch clamp recordings from granule cells located in the internal granule cell layer from 5-HT3A/EGFP transgenic mice at P6–P10. All recorded granule cells showed a mature morphology, with a small soma and several multipolar short dendrites (Fig. 2A). Granule cells at this stage of development showed only occasionally action potentials (Fig. 2Ba) in response to depolarizing current injections (Fig. 2Bb) due to a small amplitude of voltage-gated Na+ currents. These cells were furthermore characterized by a high input resistance (1.10 ± 0.16 GΩ, n = 9), a low capacitance (4.13 ± 0.35 pF, n = 15) and a resting membrane potential of –66.6 ± 3.8 mV (n = 9). None of the cells (n = 10) in the external granule cell layer responded to the application of 5-HT. Also not all cells in the internal granule cell layer responded to the application of either 5-HT or the selective 5-HT3 receptor agonist SR57227A: 34.7% (n = 72) of these cells displayed a 5-HT3 receptor-mediated current. Activation of 5-HT3 receptors on whole-cell voltage clamped granule cells by local application of 100 μm 5-HT resulted in a small inward current of 23.6 ± 6.3 pA (n = 15, Fig. 2Ca). The responses could be mimicked by application of 100 μm SR57227A, which resulted in an inward current of 22.1 ± 3.5 pA (n = 10, Fig. 2Cb), confirming that the currents were mediated by 5-HT3 receptors. The I–V curve of the 5-HT3 receptor-mediated inward current showed a region of negative-slope conductance (Fig. 2D). Taken together, the results show that a subset of cerebellar granule cells located in the inner granule cell layer expresses functional 5-HT3 receptors.
Figure 2. Cerebellar granule cells show a fast inward current upon 5-HT3 receptor activation with a characteristic voltage-dependent Ca2+ block.
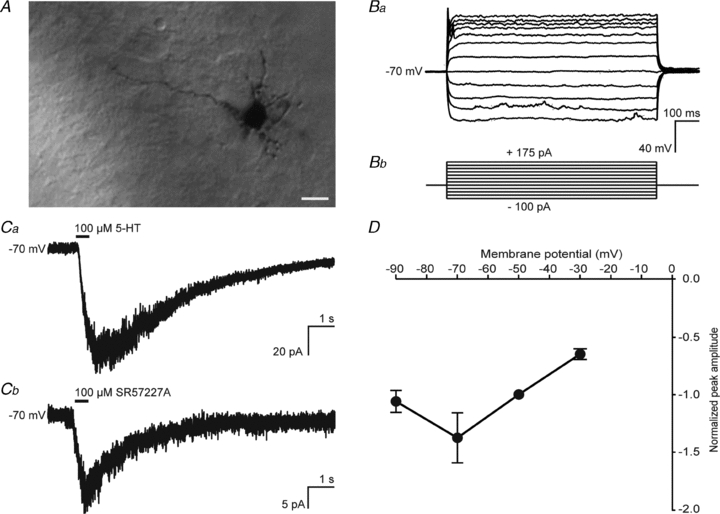
A, P7 granule cell used for electrophysiological recordings, showing a mature morphology. Ba, physiological characteristics of the recorded granule cells were less mature, with occasionally one spike at the beginning of depolarization steps. Bb, protocol of injected current steps used for Ba. C, examples of a 5-HT3 receptor-mediated response to local application of 100 μm 5-HT (Ca) and 100 μm SR57227A (Cb) for 500 ms. D, I–V curve of the 5-HT3 receptor-mediated ion current shows the characteristic voltage-dependent Ca2+ block.
Functional presynaptic 5-HT3 receptors modulate synaptic plasticity at the parallel fibre–Purkinje cell synapse
5-HT3 receptors are located on a subset of cerebellar synaptosomes, where they mediate presynaptic Ca2+ influx (Nichols & Mollard, 1996; Nayak et al. 1999), which can modulate the release of neurotransmitters. We therefore investigated whether 5-HT3 receptors can modulate spontaneous miniature synaptic events recorded from Purkinje cells. Miniature postsynaptic currents (mPSCs) from whole-cell voltage clamped Purkinje cells were recorded before and after the application of either the specific 5-HT3 receptor antagonist tropisetron (30 nm) or the specific 5-HT3 receptor agonist mCPBG (10 μm, Fig. 3A). Pharmacological block of 5-HT3 receptors by using 30 nm of the specific 5-HT3 receptor antagonist tropisetron resulted in an increase in inter-event interval distribution (n = 4; P < 0.001; Fig. 3Ba), showing a reduction in frequency, without a change in amplitude distribution (n.s.; Fig. 3Bb). Conversely, activation of 5-HT3 receptors by local application of 10 μm of the specific 5-HT3 receptor agonist mCPBG reduced the inter-event interval distribution (n = 11 versus n = 8 control recordings; P < 0.001; Fig. 3Ca), showing an increase in frequency, with no change in the amplitude distribution (n.s.; Fig. 3Cb).
Figure 3. Presynaptic 5-HT3 receptors on granule cells modulate the inter-event interval distribution of spontaneous miniature synaptic events recorded from Purkinje cells.
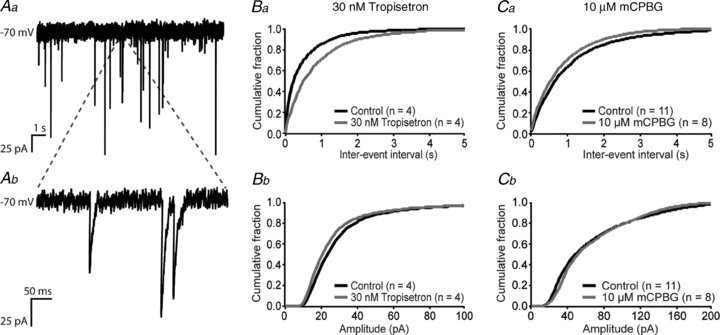
A, example trace (Aa, with magnification in Ab) of miniature synaptic currents recorded from Purkinje cells at –70 mV. B, inter-event interval distribution (Ba) and amplitude distribution (Bb) of mPSCs in Purkinje cells show an increase in inter-event intervals (a reduction in frequency) but no change in amplitude after application of 30 nm of the specific 5-HT3 receptor antagonist tropisetron. C, inter-event interval distribution (Ca) and amplitude distribution (Cb) of Purkinje cell mPSCs show a reduction in inter-event intervals (an increase in frequency) but no change in amplitude after application of 10 μm of the 5-HT3 receptor-specific agonist mCPBG.
Given that granule cells express functional 5-HT3 receptors, we tested whether 5-HT3 receptor activity can modulate the glutamatergic parallel fibre–Purkinje cell synapse. Whole-cell patch clamp recordings of Purkinje cells from 7-, 8- and 9-day-old mice were performed and parallel fibres were stimulated with a paired-pulse protocol. At 1 week after birth, Purkinje cells can be in two distinct developmental stages: immature with multiple primary dendrites (Fig. 4Aa) or in the more mature stage in which they have only one primary dendrite left (Fig. 4Ab). Only cells which displayed a mature morphology were included in the analysis. The paired-pulse ratio (PPR), defined as the amplitude of the second EPSC (EPSC2) divided by the amplitude of the first EPSC (EPSC1), had a value at P7, P8 and P9 of respectively 0.98 ± 0.06 (n = 4), 0.99 ± 0.11 (n = 6) and 1.07 ± 0.07 (n = 8; Fig. 4Ba and C). In the presence of 30 nm of the selective 5-HT3 receptor antagonist tropisetron the synapse showed clear paired-pulse depression: the PPR was changed to 0.51 ± 0.14 (n = 5, P < 0.05) at P7, 0.54 ± 0.13 (n = 5, P < 0.05) at P8 and 0.64 ± 0.07 (n = 10, P < 0.001) at P9 (Fig. 4Bb and C). Time constants for both EPSCs were not different when compared to control experiments (Table 1), suggesting that tropisetron does not affect postsynaptic receptor properties. At P21, when the expression of 5-HT3 receptors has faded, the parallel fibre–Purkinje cell synapse showed paired-pulse facilitation (PPR = 1.53 ± 0.20; n = 7; Fig. 4Bc and C). Application of 30 nm tropisetron did not affect the PPR at this age (1.35 ± 0.08, n = 7, n.s.; Fig. 4Bd and C). Mean time constants of both EPSCs at P21 remained unaffected by the application of tropisetron (Table 1). Taken together, these results show that functional 5-HT3 receptors are also located presynaptically, regulating short-term synaptic plasticity at the parallel fibre–Purkinje cell synapse.
Figure 4. Presynaptic 5-HT3 receptors on granule cells modulate plasticity at the parallel fibre–Purkinje cell synapse.
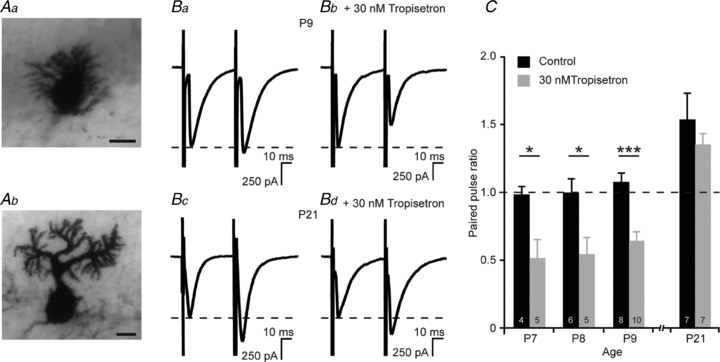
A, Purkinje cells at P9 can be in different developmental stages: immature with multiple short primary dendrites (Aa), or in a mature stage with only one primary dendrite (Ab). Only Purkinje cells which showed a mature morphology were included in the analysis. Scale bars indicate 10 μm. B, paired-pulse recordings from Purkinje cells at P9 at a holding potential of –70 mV, stimulated at the parallel fibres. Control recordings show no paired-pulse plasticity (Ba), while pre-incubation in 30 nm tropisetron leads to paired-pulse depression (Bb). Paired-pulse recordings at –70 mV at the parallel fibre–Purkinje cell synapse at P21 show paired-pulse facilitation in both control recordings (Bc) and after pre-incubation in 30 nm tropisetron (Bd). C, synaptic plasticity at the parallel fibre–Purkinje cell synapse is regulated by 5-HT3 receptors at P7–P9, but not at P21. Blocking 5-HT3 receptors by 30 nm tropisetron turns the parallel fibre–Purkinje cell synapse into a depressing one at P7–P9.
Table 1.
Time constant (τ) of EPSCs from paired-pulse recordings at different ages
| Control | 30 nm tropisetron | |||
|---|---|---|---|---|
| τEPSC1 (ms) | τEPSC2 (ms) | τEPSC1 (ms) | τEPSC2 (ms) | |
| P7 | 9.2 ± 1.9 (n = 4) | 10.1 ± 1.7 (n = 4) | 7.9 ± 1.6 (n = 5) | 8.6 ± 0.6 (n = 5) |
| P8 | 10.5 ± 1.1 (n = 6) | 11.3 ± 1.2 (n = 6) | 8.4 ± 2.1 (n = 5) | 12.9 ± 4.5 (n = 5) |
| P9 | 14.2 ± 4.7 (n = 8) | 16.0 ± 3.1 (n = 8) | 12.4 ± 1.6 (n = 10) | 13.9 ± 1.5 (n = 10) |
| P21 | 35.1 ± 10.3 (n = 7) | 28.4 ± 7.2 (n = 7) | 20.5 ± 4.9 (n = 7) | 21.8 ± 6.8 (n = 7) |
Discussion
The results in this study show functional 5-HT3 receptors and their physiological properties in the cerebellum during early postnatal development. Expression of 5-HT3 receptors was found on granule cells, which could be identified by their migration pattern from the external granule cell layer towards the internal granule cell layer during the first three postnatal weeks. Using immunohistochemistry, we also show that 5-HT3 receptors are not expressed by interneurons or glial cells, specifying its presence on the glutamatergic granule cells. We furthermore confirm the presence of the 5-HT3 receptors on granule cells by electrophysiological methods. Local application of either 5-HT or the 5-HT3 receptor-specific agonist SR57227A to EGFP-expressing cells resulted in a fast inward current. All recorded cells had a low cell capacitance, a high input resistance and a resting membrane potential with values characteristic of cerebellar granule cells in the last stage of maturation (Okazawa et al. 2009). Thus, as opposed to 5-HT3 receptor expression on GABAergic interneurons, and in concordance with recent findings of 5-HT3 receptors on glutamatergic Cajal–Retzius cells (Chameau et al. 2009), we show here expression of 5-HT3 receptors on another glutamatergic cell type.
Functional 5-HT3 receptors were identified at the post- and/or extrasynaptic site of granule cells by local application of either 5-HT or SR57227A. In addition, the ion currents evoked by both 5-HT and SR57227A showed a region of negative-slope conductance, similar to the voltage-dependent Ca2+ block of 5-HT3 receptor-mediated currents in the cortex (Roerig et al. 1997), the hippocampus (Kawa, 1994; McMahon & Kauer, 1997; van Hooft & Wadman, 2003) and those mediated by cloned 5-HT3 receptors expressed in HEK293 cells (Noam et al. 2008). Interestingly, we could only evoke responses to the agonists in cells which had several multipolar short dendrites around the cell body, which is indicative of a mature morphology (Powell et al. 1997). These cells did, however, display some physiologically immature features: they only had small voltage-gated Na+ currents and they rarely showed a full spike train in current clamp mode, indicating that those cells which did give a response could still maturate further. We could not record currents from immature granule cells in the external granule cell layer. We hypothesize that this is because they express 5-HT3 receptors which are not yet fully functional, or the density of 5-HT3 receptor expression is too low to detect a response, or a combination of both.
The frequency of spontaneous miniature postsynaptic currents recorded from Purkinje cells was modulated by 5-HT3 receptors, without an effect on the amplitude distributions (Fig. 3B and C). This is in concordance with the previous notion that 5-HT3 receptors are located on presynaptic nerve terminals in the cerebellum (Nichols & Mollard, 1996; Nayak et al. 1999). During early postnatal development, the parallel fibre–Purkinje cell synapse does not yet show short-term synaptic plasticity in response to paired-pulse stimulation. However, stimulation of parallel fibres of the granule cells resulted in paired-pulse depression after pharmacological blockade of 5-HT3 receptors. It is known that neurotransmitter release is modulated by 5-HT3 receptors by increasing the intracellular Ca2+ concentration via Ca2+ entry, either directly through the 5-HT3 receptor-operated ion channel or indirectly via activation of voltage-gated Ca2+ channels on presynaptic sites (Nichols & Mollard, 1996; Nayak et al. 1999). It therefore seems likely that 5-HT3 receptors regulate Ca2+ influx at the presynaptic site of the parallel fibre–Purkinje cell synapse, thereby increasing neurotransmitter release probability at the presynaptic terminal. The release probability is simultaneously reduced by inhibitory mechanisms, either through inhibitory receptors at the presynaptic site or via feedback inhibition of parallel fibre–Purkinje cell transmission. In young mice, the 5-HT3 receptor-mediated Ca2+ influx is presumably involved in maintaining a paired-pulse ratio around 1. Preventing the presynaptic Ca2+ influx by blocking 5-HT3 receptors could give rise to inhibitory signals at the presynaptic terminal, as reflected by paired-pulse depression. In summary, these findings indicate a role for the 5-HT3 receptor at an immature stage at the parallel fibre–Purkinje cell synapse and the adjacent cerebellar neuronal network. This happens at a time when the parallel fibre–Purkinje cell synapse is still maturating, reflected by a paired-pulse ratio around 1 in contrast to a facilitating paired-pulse ratio in the mature synapse. In this way serotonergic input via 5-HT3 receptors could play a role in the formation of the cerebellar neuronal network by enhancing the probability that an otherwise non-facilitating synapse develops into the fully mature state with paired-pulse facilitation.
Taken together, the findings presented above provide evidence for functional 5-HT3 receptors in the cerebellum. We show the presence of 5-HT3 receptors on glutamatergic granule cells during the first three postnatal weeks, at both the pre- and postsynaptic site. We speculate that proper functioning of 5-HT3 receptors in the cerebellum is part of the underlying mechanism regulating development of the cerebellar network and parallel fibre–Purkinje cell synapses in particular. This novel aspect of postnatal cerebellar maturation could have implications for the development of motor coordination and motor learning.
Acknowledgments
We thank Pascal Chameau and Wytse Wadman for support and reading of the manuscript, and Erik Manders and Ronald Breedijk from the Centre of Advanced Microscopy (University of Amsterdam, the Netherlands) for their assistance and use of their confocal microscope. This research was in part supported by the Research Council for Earth and Life Sciences (ALW) with financial aid from the Netherlands Organization for Scientific Research (NWO).
Glossary
Abbreviations
- EGL
external granule cell layer
- GFAP
glial fibrillary acidic protein
- IGL
internal granule cell layer
- mPSC
miniature postsynaptic current
- P
postnatal day
- PPR
paired-pulse ratio
Author contributions
M.O. and J.S. performed the experiments and analysed the data with help of J.A.v.H.; M.O. and J.A.v.H. wrote the manuscript. All authors approved the final version of the manuscript.
References
- Arpin-Bott M-P, Dietrich J-B, Dirrig-Grosch S, Aunis D, Zwiller J. Induction by cocaine of the serotonergic 5-HT3 receptor in rat cerebellum. Ann NY Acad Sci. 2006;1074:382–389. doi: 10.1196/annals.1369.038. [DOI] [PubMed] [Google Scholar]
- Bachy A, Héaulme M, Giudice A, Michaud J-C, Lefevre IA, Souilhac J, Manara L, Emerit MB, Gozlan H, Hamon M, Keane PE, Soubrié P, Le Fur G. SR 57227A: a potent and selective agonist at central and peripheral 5-HT3 receptors in vitro and in vivo. Eu J Pharmacol. 1993;237:299–309. doi: 10.1016/0014-2999(93)90282-m. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Barnes NM, Champaneria S, Costall B, Naylor RJ. Characterisation and autoradiographic localisation of 5-HT3 receptor recognition sites identified with [3H]-(S)-zacopride in the forebrain of the rat. Neuropharmacology. 1990;29:1037–1045. doi: 10.1016/0028-3908(90)90110-d. [DOI] [PubMed] [Google Scholar]
- Barrera NP, Herbert P, Henderson RM, Martin IL, Edwardson JM. Atomic force microscopy reveals the stoichiometry and subunit arrangement of 5-HT3 receptors. Proc Natl Acad Sci U S A. 2005;102:12595–12600. doi: 10.1073/pnas.0503253102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chameau P, Inta D, Vitalis T, Monyer H, Wadman WJ, van Hooft JA. The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc Natl Acad Sci U S A. 2009;106:7227–7232. doi: 10.1073/pnas.0810764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chameau P, van Hooft JA. Serotonin 5-HT3 receptors in the central nervous system. Cell Tissue Res. 2006;326:573–581. doi: 10.1007/s00441-006-0255-8. [DOI] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature. 1999;397:359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- Derkach V, Surprenant A, North RA. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- Férézou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Barnes JM, Towers P, Barnes NM. Distribution of S(–)-zacopride-insensitive [125I]R(+)-zacopride binding sites in the rat brain and peripheral tissues. Eur J Pharmacol. 1997;332:307–312. doi: 10.1016/s0014-2999(97)01091-1. [DOI] [PubMed] [Google Scholar]
- Geurts FJ, De Schutter E, Timmermans J-P. Localization of 5-HT2A, 5-HT3, 5-HT5A and 5-HT7 receptor-like immunoreactivity in the rat cerebellum. J Chem Neuroanat. 2002;24:65–74. doi: 10.1016/s0891-0618(02)00020-0. [DOI] [PubMed] [Google Scholar]
- Hewlett WA, Fridman S, Trivedi BL, Schmidt DE, De Paulis T, Ebert MH. Characterization of desamino-5-[125I]iodo-3-methoxy-zacopride ([125I]MIZAC) binding to 5-HT3 receptors in the rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:397–410. doi: 10.1016/s0278-5846(98)00012-8. [DOI] [PubMed] [Google Scholar]
- Inta D, Alfonso J, von Engelhardt J, Kreuzberg MM, Meyer AH, van Hooft JA, Monyer H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci U S A. 2008;105:20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa K. Distribution and functional properties of 5-HT3 receptors in the rat hippocampal dentate gyrus: a patch-clamp study. J Neurophysiol. 1994;71:1935–1947. doi: 10.1152/jn.1994.71.5.1935. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Jones BJ, Tyers MB. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987;330:746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Jones BJ, Tyers MB. Binding of the 5-HT3 ligand, [3H]GR65630, to rat area postrema, vagus nerve and the brains of several species. Eu J Pharmacol. 1989;159:157–164. doi: 10.1016/0014-2999(89)90700-0. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Butler A, Burridge J, Oxford AW. 1-(m-Chlorophenyl)-biguanide, a potent high affinity 5-HT3 receptor agonist. Eu J Pharmacol. 1990;182:193–197. doi: 10.1016/0014-2999(90)90513-6. [DOI] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Betti L, Giannaccini G, Rossi A, Masala I, Baroni S, Cassano GB, Lucacchini A. Distribution of [3H]GR65630 binding in human brain postmortem. Neurochemic Res. 2001;26:187–190. doi: 10.1023/a:1010939530412. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. Hippocampal interneurons are excited via serotonin-gated ion channels. J Neurophysiol. 1997;78:2493–2502. doi: 10.1152/jn.1997.78.5.2493. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Emerit MB, Nosjean A, Simon A, Rumajogee P, Brisorgueil MJ, Doucet E, Hamon M, Vergé D. Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. Eur J Neurosci. 2002;15:449–457. doi: 10.1046/j.0953-816x.2001.01872.x. [DOI] [PubMed] [Google Scholar]
- Nayak SV, Rondé P, Spier AD, Lummis SCR, Nichols RA. Calcium changes induced by presynaptic 5-hydroxytryptamine-3 serotonin receptors on isolated terminals from various regions of the rat brain. Neuroscience. 1999;91:107–117. doi: 10.1016/s0306-4522(98)00520-x. [DOI] [PubMed] [Google Scholar]
- Nichols RA, Mollard P. Direct observation of serotonin 5-HT3 receptor-induced increases in calcium levels in individual brain nerve terminals. J Neurochem. 1996;67:581–592. doi: 10.1046/j.1471-4159.1996.67020581.x. [DOI] [PubMed] [Google Scholar]
- Noam Y, Wadman WJ, van Hooft JA. On the voltage-dependent Ca2+ block of serotonin 5-HT3 receptors: a critical role of intracellular phosphates. J Physiol. 2008;586:3629–3638. doi: 10.1113/jphysiol.2008.153486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa M, Abe H, Katsukawa M, Iijima K, Kiwada T, Nakanishi S. Role of calcineurin signaling in membrane potential-regulated maturation of cerebellar granule cells. J Neurosci. 2009;29:2938–2947. doi: 10.1523/JNEUROSCI.5932-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RMC, Barnes JM, Ge J, Barber PC, Barnes NM. Autoradiographic distribution of [3H]-(S)-zacopride-labelled 5-HT3 receptors in human brain. J Neurol Sci. 1996;144:119–127. doi: 10.1016/s0022-510x(96)00211-0. [DOI] [PubMed] [Google Scholar]
- Powell SK, Rivas RJ, Rodriguez-Boulan E, Hatten ME. Development of polarity in cerebellar granule neurons. J Neurobiol. 1997;32:223–236. doi: 10.1002/(sici)1097-4695(199702)32:2<223::aid-neu7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Roerig B, Nelson DA, Katz LC. Fast synaptic signaling by nicotinic acetylcholine and serotonin 5-HT3 receptors in developing visual cortex. J Neurosci. 1997;17:8353–8362. doi: 10.1523/JNEUROSCI.17-21-08353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell J-O, Stone-Elander S, Eriksson L, Ingvar M. N-methylquipazine: Carbon-11 labelling of the 5-HT3 agonist and in vivo evaluation of its biodistribution using PET. Nucl Med Biol. 1997;24:405–412. doi: 10.1016/s0969-8051(97)00003-6. [DOI] [PubMed] [Google Scholar]
- van Hooft JA, Wadman WJ. Ca2+ ions block and permeate serotonin 5-HT3 receptor channels in rat hippocampal interneurons. J Neurophysiol. 2003;89:1864–1869. doi: 10.1152/jn.00948.2002. [DOI] [PubMed] [Google Scholar]
- Vucurovic K, Gallopin T, Ferezou I, Rancillac A, Chameau P, van Hooft JA, Geoffroy H, Monyer H, Rossier J, Vitalis T. Serotonin 3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cereb Cortex. 2010;20:2333–2347. doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel JL, Shao XM, Jackson MB. The selectivity of the channel coupled to the 5-HT3 receptor. Brain Res. 1990;533:46–52. doi: 10.1016/0006-8993(90)91793-g. [DOI] [PubMed] [Google Scholar]


