Non-technical summary
Administration of cannabinoids can impair several cognitive functions, including memory by altering synchronous activities in cortical networks. We show that the gamma frequency (40 Hz) oscillations in hippocampal slices, that are prominent oscillations in electroencephalogram during awake states in vivo, are reduced by cannabinoids. This effect can be explained by the suppression of the excitatory synaptic transmission onto fast spiking basket cells, GABAergic cells that are key players in oscillogenesis. The reduced excitatory drive onto these interneurons leads to a reduction in neuronal firing frequency and precision, and thus to smaller field potentials. Our data further our understanding of the synaptic mechanisms of how cannabinoids alter neuronal operation.
Abstract
Abstract
CB1 cannabinoid receptor (CB1R) activation by exogenous ligands can impair memory processes, which critically depend on synchronous neuronal activities that are temporarily structured by oscillations. In this study, we aimed to reveal the mechanisms underlying the cannabinoid-induced decrease in gamma oscillations. We first verified that cannabinoids (CP55,940 and WIN55,212-2) readily suppressed carbachol-induced gamma oscillations in the CA3 region of hippocampal slices via activation of CB1Rs. The cannabinoid-induced decrease in the peak power of oscillations was accompanied by reduced and less precise firing activity in CA3 pyramidal cells and fast spiking basket cells. By examining the cannabinoid sensitivity of synaptic inputs we found that the amplitude of evoked excitatory postsynaptic currents was significantly suppressed upon CB1R activation in both CA3 pyramidal cells and fast spiking basket cells. In contrast, evoked inhibitory postsynaptic currents in CA3 pyramidal cells were unaltered. Furthermore, we observed that a CB1R agonist-induced decrease in the oscillation power at the beginning of the drug application was accompanied primarily by the reduced discharge of fast spiking basket cells, while pyramidal cell firing was unaltered. This result implies that the dampening of cholinergically induced gamma oscillations in the hippocampus by cannabinoids can be explained by a reduced excitatory input predominantly onto fast spiking basket cells, which leads to a reduction in neuronal firing frequency and precision, and thus to smaller field potentials. In addition, we uncovered that the spontaneously occurring sharp wave-ripple activities in hippocampal slices could also be suppressed by CB1R activation suggesting that cannabinoids profoundly reduce the intrinsically generated oscillatory activities at distinct frequencies in CA3 networks by reducing synaptic neurotransmission.
Introduction
The emergence of gamma frequency (30–80 Hz) oscillations in cortical neuronal networks is often associated with higher cognitive functions, including attention, sensory encoding and memory storage or retrieval (Singer, 1993; Tiitinen et al. 1993; Sederberg et al. 2003; Montgomery & Buzsaki, 2007). Recent evidence suggests that these oscillations can support neuronal communication and synaptic plasticity (Fell & Axmacher, 2011). To understand the cellular and network mechanisms underlying the generation of gamma oscillations in cortical structures, a number of in vitro models of gamma oscillations have been introduced (Whittington et al. 1995; Fisahn et al. 1998; Hajos et al. 2000).
In one of these models, cholinergic receptor activation induces gamma oscillations in the CA3 region of hippocampal slices, a brain region that is able to intrinsically generate synchronous network activities both in behaving animals and in vitro (Fisahn et al. 1998; Csicsvari et al. 2003). Importantly, this model has been shown to capture several features of gamma oscillations recorded in vivo (Hajos & Paulsen, 2009). Recent studies uncovered that the cholinergically induced gamma oscillations in the hippocampus are generated by a recurrent synaptic feedback loop comprised of CA3 pyramidal cells and fast spiking basket cells (Mann et al. 2005; Gulyas et al. 2010). During such oscillatory activity, the discharge of principal cells is governed by perisomatic inhibition, whereas the firing of GABAergic interneurons is driven by excitatory input (Oren et al. 2006). The frequency and the magnitude of these oscillations are primarily determined by the decaying phase and the amplitude of perisomatic inhibitory currents, respectively (Fisahn et al. 1998; Oren et al. 2010).
Previous in vitro and in vivo studies (Hajos et al. 2000; Robbe et al. 2006; Robbe & Buzsaki, 2009) indicated that cannabinoids, which affect several cognitive processes including short term memory (Lichtman et al. 1995; Hampson & Deadwyler, 1998), can effectively suppress oscillatory activities in, among others, the gamma frequency range. This effect of cannabinoids is probably accomplished via activation of CB1 cannabinoid receptors (CB1Rs) (Robbe et al. 2006). The G-protein-coupled CB1Rs in cortical networks are predominantly, if not exclusively, located at axon terminals (Katona et al. 1999, 2006; Kawamura et al. 2006), thereby efficiently controlling neurotransmitter release (Kano et al. 2009). In the hippocampus, these receptors are present at axon terminals of pyramidal cells and one type of GABAergic interneuron that express cholecystokinin (CCK) (Katona et al. 1999; Hajos et al. 2000). Thus, cannabinoids can regulate both excitatory transmission and a subset of inhibitory synapses in hippocampal circuits (Katona & Freund, 2008; Kano et al. 2009).
As in vitro studies revealed, application of cholinergic receptor agonists can block GABA release from the axon terminals of CCK-containing basket cells, an effect that was shown to be accomplished via CB1R activation (Fukudome et al. 2004; Neu et al. 2007; Szabo et al. 2010). We recently verified that these interneurons do not contribute to oscillations induced by the cholinergic receptor agonist carbachol (Gulyas et al. 2010). Hence, we hypothesize that cannabinoid receptor agonists reduce the extent of excitatory synaptic transmission, resulting in the suppression of gamma oscillations. Whereas the transmitter release from recurrent collaterals in CA3 pyramidal cells has been shown to be regulated by CB1R activation (Hofmann et al. 2008), excitatory input onto hippocampal interneurons seems to be insensitive to cannabinoids (Hoffman et al. 2003). Therefore, we tested the hypothesis of whether the suppression of recurrent excitation between CA3 pyramidal cells by cannabinoid agonists can account for the cellular mechanisms underlying the dampening of cholinergically induced gamma oscillations in the hippocampus. In addition, we examined the cannabinoid sensitivity of sharp wave-ripple activities (SWRs) occurring spontaneously in the CA3 region of hippocampal slices. In intact animals, these synchronous events in local electroencephalogram are present predominantly during consummatory activity, immobility and slow wave sleep (Buzsáki, 1989; Buzsáki et al. 1992).
Methods
Slice preparation
All experiments were performed in accordance with the Hungarian Act of Animal Care and Experimentation (1998, XXVIII, section 243/1998), and with the guidelines of the institutional ethical code. The experiments comply with the policies and regulations as required by The Journal of Physiology (Drummond, 2009). A total of 59 mice were used in this study. CD1 mice of both sexes (Charles River, Budapest, Hungary) or CB1R knockout mice and their wild type littermates (Zimmer et al. 1999) (postnatal day 14 (P14)–P26) were deeply anaesthetized with isoflurane. After decapitation, the brain was quickly removed and placed into ice-cold cutting solution containing (in mm): sucrose, 252; KCl, 2.5; NaHCO3, 26; CaCl2, 0.5; MgCl2, 5; NaH2PO4, 1.25; glucose, 10; and bubbled with 95% O2 and 5% CO2. Horizontal slices 350–400 μm thick for studying oscillations and 150–200 μm thick for investigating postsynaptic currents, respectively, were prepared using a Leica (Nussloch, Germany) VT1000S or VT1200S vibratome. Slices containing the hippocampal formation were trimmed from other brain regions and kept in an interface-type holding chamber at room temperature for at least 60 min before recording in standard aCSF containing (in mm): NaCl, 126; KCl, 2.5; NaHCO3, 26; CaCl2, 2; MgCl2, 2; NaH2PO4, 1.25; glucose, 10; and bubbled with 95% O2 and 5% CO2 (pH 7.2–7.4).
Electrophysiological recordings
To examine the cannabinoid effects on oscillations, experiments were performed in the CA3 region of the hippocampus using a dual-superfusion recording chamber in aCSF at 30–32°C at a flow rate of 3.5–5 ml min−1 (Hajos & Mody, 2009). Oscillatory activities emerged either spontaneously in the case of SWRs or were induced by bath application of 10 μm carbachol, which was present throughout the experiments. The power and the frequency of oscillations stabilized within 10–15 min after carbachol application (Hajos & Mody, 2009). Local field potentials and the spiking activity of cells were simultaneously recorded with two patch pipettes (pulled from borosilicate glass capillaries, resistance 3–6 MΩ) filled with aCSF. One pipette was placed within the pyramidal cell layer of the CA3b region at a depth of 100–200 μm to monitor local field oscillations. The other pipette was used under visual guidance to detect action potentials extracellularly from neurons. Pyramidal cells were selected based on the shape of their cell bodies, whereas interneurons with somata at the border of strata pyramidale and oriens were targeted if they fired almost in every oscillation cycle. After the extracellular recording, the recorded cell was labelled with a different pipette using the whole-cell patch-clamp technique. For labelling, the intracellular solution contained (in mm): potassium gluconate, 110; NaCl, 4; creatine phosphate, 10; Hepes, 10; ATP, 2; GTP, 0.4; and 0.3–0.5% biocytin (pH 7.3; 290–300 mosmol l−1). Only interneurons that could be identified as fast spiking basket cells based on both electrophysiological and anatomical criteria were included in this study (n = 7). All such interneurons showed high spiking rates and phase-coupling strengths, and often fired doublets of action potentials, characteristic features that are only observed in fast spiking basket cells during carbachol-induced oscillations (Gulyas et al. 2010). The axon arbor of each interneuron was restricted to stratum pyramidale, and using double fluorescent staining we determined that their labelled boutons avoided axon initial segments of pyramidal cells, indicating that they were not axo-axonic cells (see below).
For stimulation experiments, a theta glass electrode was placed in the stratum radiatum at a distance of 200–400 μm from the recorded cells. To detect evoked excitatory postsynaptic currents (EPSCs), the pipette solution was the same as above, but 600–800 μm picrotoxin was added to intracellularly block GABAA receptor-mediated currents. For evoked inhibitory postsynaptic currents (IPSCs), 2–3 mm kynurenic acid was added to the bath solution, while the pipette solution contained (in mm): CsCl, 80; caesium gluconate, 60; NaCl, 3; creatine phosphate, 10; MgCl2, 1; Hepes, 10; ATP, 2; and QX314, 5 (pH 7.3, 290–300 mosmol l−1). The flow rate was ∼2 ml min−1. Recordings were performed at a holding potential of −65 mV. Access resistance (between 5–15 MΩ, compensated 70–80%) was frequently monitored and cells were excluded from analysis if the series resistance changed more than 25%. All recorded neurons were post hoc anatomically identified. Only CA3 pyramidal cells (n = 29) and anatomically identified basket cells with a fast spiking phenotype (n = 18) were included in the study. The fast spiking character of interneurons was tested and distinguished from a regular spiking discharge pattern by injection of depolarizing current steps immediately after obtaining the whole-cell recording configuration, whereas the morphological identification was performed post hoc (Gulyas et al. 2010).
All data were recorded with a Multiclamp 700B amplifier (Axon Instruments, Foster City, CA, USA). Signals were low-pass filtered at 2 kHz. Data acquisition was done with a PCI-6024E board (National Instruments, Austin, TX, USA) using EVAN 1.3 (courtesy of Professor I. Mody, Departments of Neurology and Physiology, UCLA, CA, USA) and analysed offline using Origin 8.0 (OriginLab Corp. Northampton, MA, USA) and Matlab 6.0 (MathWorks Natick, MA, USA) softwares.
Data analysis
Power spectra density analysis was performed on 120–180 s epochs. Time windows of 1 s with 50% overlap were multiplied by a Hanning window before a fast Fourier transform was performed. Peak power and peak frequency at that power value were used for comparison. A custom-written firing phase detection algorithm was used as described in detail previously (Gulyas et al. 2010). Spikes recorded in a loose-patch mode for 120–180 s were detected by manually setting the threshold on the unfiltered trace. The negative peak of the trough of the oscillation was considered as phase zero for field potentials band-pass filtered with an RC filter between 5 and 500 Hz. The phase of individual spikes was specified by calculating the position of the unit spikes in relation to two subsequent negative phase time points. The amplitude and the instantaneous frequency of the oscillation varied, and often the detection algorithm skipped one or more oscillation cycles. Therefore, our spike phase detection algorithm checked for the actual detected cycle length and assigned a phase to a spike only if the actual cycle length did not differ from the mean of the average cycle length by more than a chosen fraction of 0.3 standard deviations of the cycle length. Phase values of individual cells were analysed by circular statistical methods using Oriana 2.0 software (Kovach Computing Services, Anglesey, UK). Significant deviation from uniform (random) phase distribution along the circle indicated directionality. This was tested with Rao's spacing test and Rayleigh's uniformity test. To characterize a non-uniform distribution, two parameters of its mean vector (calculated from individual observations) were used, the mean angle and the length of the mean vector, i.e. the phase-coupling strength.
To evaluate the changes in the peak amplitude of evoked postsynaptic currents upon CB1R activation, control amplitudes in a 2–3 min time window were compared to those measured after 20 min drug application for the same period of time. Only those experiments were included that had stable amplitudes at least for 10 min before drug application. After each experiment, the Teflon tubing of the setup was washed with ethanol for 10 min and with aCSF for 15 min.
The peak amplitudes of SWRs and their incidence before and after the drug application were analysed by EVAN software.
Data are presented as mean ± SEM unless otherwise indicated. Statistical comparisons were performed in OriginPro 8.0 using the Student's paired t test for normally distributed linear data and the Wilcoxon's signed rank test for linear data that were not normally distributed.
Anatomical identification of cells
After recording, slices were fixed in 4% paraformaldehyde in 0.1 m phosphate buffer (PB; pH 7.4) for at least 60 min, followed by washout with PB several times, cryoprotected in 20% sucrose and then repeatedly freeze-thawed (for details see Hajos et al. 2004). Biocytin was visualized using an avidin–biotinylated horseradish peroxidase complex reaction (ABC; Vector Laboratories, Burlingame, CA, USA) with nickel-intensified 3,3-diaminobenzidine as chromogen.
Identification of fast spiking basket cells using double immunofluorescent labelling
The details of this procedure have been published previously (Gulyas et al. 2010). Briefly, after recording, the slices were fixed as above, washed, cryoprotected, embedded in 1% agar and re-sectioned at 60 μm thickness. The sections were treated with 0.2 mg ml−1 pepsin (Cat. No. S3002; Dako, Glostrup, Denmark) in 0.2 m HCl at 37°C for 5 min and were washed in 0.1 m PB. Sections were blocked in normal goat serum (NGS, 10%) made up in Tris-buffered saline (TBS, pH 7.4) followed by incubation in mouse anti-AnkyrinG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:100 in TBS containing 2% NGS and 0.3% Triton X-100. Following several washes in TBS, Cy3-conjugated goat anti-mouse (1:500, Jackson Laboratories, Bar Harbor, ME, USA) was used to visualize the immunoreactions, and Alexa488-conjugated strepavidin (1:500; Invitrogen, Carlsbad, CA, USA) to visualize the biocytin. Sections were then mounted on slides in Vectashield (Vector Laboratories, Burlingame, CA, USA). Images were taken using an AxioImager.Z1 (Carl Zeiss GmbH, Vienna, Austria).
Drugs
WIN55,212-2, CP55,940 and NBQX were purchased from Tocris (Tocris Bioscience Ltd, Bristol, UK). WIN55,212-2 was dissolved in 0.1n HCl giving a 20 mm stock solution stored at 4°C prior to dilution to working concentration. CP55,940 and NBQX were dissolved in DMSO and in water, respectively (both at a concentration of 100 mm), and stored at –20°C. SR141716A (dissolved as 10 mm stock in DMSO and stored at 4°C) was provided by the National Institute on Drug Abuse (NIDA) drug supply service. In control solutions, the vehicle was diluted in the same concentration as in the solutions containing drugs. All other drugs were obtained from Sigma-Aldrich (St Louis, MO, USA).
Results
Effect of CB1R activation on cholinergically induced oscillations in the hippocampus
Bath application of the acetylcholine receptor agonist carbachol at a concentration of 10 μm readily induced synchronous network activities in mouse hippocampal slices at 30–32°C, which could be detected as oscillations in local field potentials recorded in the pyramidal cell layer of the hippocampal CA3 region (Fig. 1A). The average peak frequency (30.1 ± 1.5 Hz, n = 13) and mean peak power (87.9 ± 20.3 μV2, n = 13) of these oscillations calculated from the power spectral density function were comparable to results reported previously in studies using slices prepared from the rat hippocampus (Fisahn et al. 1998; Hajos et al. 2004; Oren et al. 2006). First, we asked whether cholinergically induced oscillations could be controlled by CB1R activation, similarly to that observed for kainate-induced network oscillations (Hajos et al. 2000). Indeed, addition of CB1R agonists CP55,940 or WIN55,212-2 to the bath solution at a concentration of 1 μm significantly decreased the power of the oscillations (Fig. 1A and B, left panels). After 20 min of bath application of these agonists, the peak power of oscillatory activities was significantly reduced to 62.1 ± 6.3% of control and 54.7 ± 15.2% of control for CP55,940 and WIN55,212-2, respectively (Fig. 1C and D, left panels, Table 1) without a substantial change in the frequency (105.9 ± 2.8% of control for CP55,940 and 103.3 ± 5.1% of control for WIN55,212-2; Fig. 1C and D, right panels, Table 1). The reduction in the peak power caused by CP55,940 (control: 98.1 ± 16.2 μV2; in CP55,940: 61.8 ± 11.8 μV2; n = 3; P = 0.024) could be fully reversed by subsequent co-application of the CB1R antagonist SR141716A at a concentration of 1 μm (in CP55,940 + SR141716A: 90.1 ± 12.9 μV2; n = 3; P = 0.019). Moreover, the pre-treatment of slices with SR141716A for 1 h prevented the CP55,940-induced drop in oscillation power (in SR141716A: 67.4 ± 9.3 μV2; in SR141716A + CP55,940: 64.9 ± 8.8 μV2; P = 0.23; n = 3). Importantly, SR141716A alone did not change the parameters of the oscillation (control: 69.8 ± 10.4 μV2 and 34.3 ± 2.2 Hz; in SR141716A: 67.4 ± 9.3 μV2 and 31.8 ± 2.4 Hz; P = 0.15 and P = 0.19, respectively, n = 3), suggesting the lack of tonic CB1R activation in our slice preparations that could interfere with oscillogenesis. To further confirm that cannabinoids acted exclusively via CB1Rs, we tested the specificity of these agonists using CB1R knockout mice (Fig. 1A and B right panels). The properties of carbachol-induced oscillations in slices prepared from CB1R knockout mice were not different from those observed in their wild type littermates (peak frequency: 31.1 ± 1.1 Hz, n = 10, P = 0.64; peak gamma power: 103.6 ± 21.5 μV2, n = 10, P = 0.6). Bath application of CB1R agonists for 20 min did not change the peak power (101.4 ± 6.4% of control for CP55,940 and 102.4 ± 12.3% for WIN55,212-2; Fig. 1C and D, left panel, Table 1) or frequency (96.3 ± 2.8% of control for CP55,940 and 95.4 ± 2.3% of control for WIN55,212-2; Fig. 1C and D, right panels, Table 1) of the oscillations. These results indicated that both synthetic cannabinoids could significantly reduce the peak power of cholinergically induced oscillations in the CA3 subfield via activation of CB1Rs.
Figure 1. CB1R activation reduces the power of the cholinergically induced gamma oscillations in the CA3 region of the hippocampus.
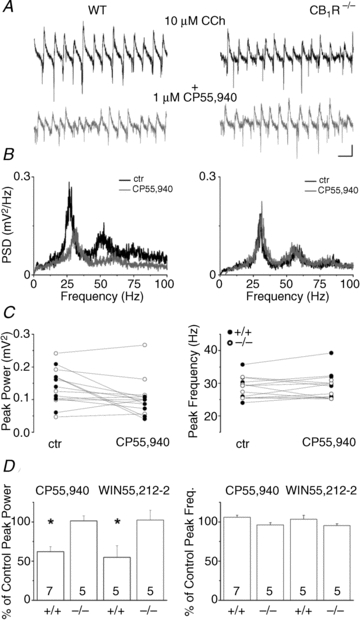
A, example traces of extracellular field recordings of cholinergically induced oscillations (10 μm carbachol) in the pyramidal cell layer of CA3 in a wild type animal (WT, left traces) and in a CB1R knockout mouse (CB1R KO (CB1R−/−), right traces) before (upper black traces) and after bath application of the cannabinoid receptor agonist CP55,940 (1 μm) (lower grey traces). B, power spectra calculated from the power spectral density function (PSD) of the traces in A showing peaks between 25–30 Hz (black), with harmonics both in the WT and in the CB1R KO animals. CB1R agonist application reduced the peak power and is accompanied by a modest shift in the peak frequency (grey). No change was detected in the CB1R KO animals (right). C, comparison of the peak power (left) and peak frequency (right) change in wild type (filled circles) and CB1R KO (open circles) animals. D, summary graphs showing the reduction in peak power (left) due to the bath application of either CP55,940 or WIN55,212-2 (1 μm, asterisks indicates P < 0.05) without substantial changes in the peak frequency (right). Scale bars, 0.1 mV and 50 ms.
Table 1.
Basic properties of cholinergically induced network oscillations in the CA3 region of the hippocampus under control conditions and 20 min after drug application
| Genotype | Property | Control | CP55,940 | P value | Number |
|---|---|---|---|---|---|
| WT | Peak frequency (Hz) | 28.9 ± 1.5 | 30.6 ± 1.7 | 0.097 | 7 |
| Peak power (μV2) | 136 ± 18.5 | 80.4 ± 10.4 | 0.0098 | 7 | |
| CB1R KO | Peak frequency (Hz) | 28.7 ± 1.1 | 27.8 ± 1.0 | 0.32 | 5 |
| Peak power (μV2) | 140.8 ± 34.3 | 153.3 ± 85.9 | 0.11 | 5 |
| Genotype | Property | Control | WIN55,212-2 | P value | Number |
|---|---|---|---|---|---|
| WT | Peak frequency (Hz) | 30.1 ± 2.2 | 30.7 ± 1.2 | 0.69 | 5 |
| Peak power (μV2) | 70.3 ± 17.5 | 45.2 ± 13.7 | 0.027 | 5 | |
| CB1R KO | Peak frequency (Hz) | 33.4 ± 1.1 | 31.8 ± 0.7 | 0.12 | 5 |
| Peak power (μV2) | 66.5 ± 14.8 | 67.5 ± 12.9 | 0.91 | 5 |
Data were compared using the Student's paired t test: significant differences are indicated in italics. Data are presented as mean ± SEM.
CB1R activation suppresses the firing rate of CA3 pyramidal cells and fast spiking interneurons during gamma oscillations
What might be the mechanism underlying the reduction of the oscillation power as a consequence of the cannabinoid agonist treatment? In the in vitro model of gamma oscillations induced by carbachol, the rhythmic inhibitory currents originating from the periodic discharge of parvalbumin-containing fast spiking basket cells (FSBCs) generate the majority of field potential oscillations (Mann et al. 2005; Atallah & Scanziani, 2009; Sohal et al. 2009; Gulyas et al. 2010; Oren et al. 2010). Hence, we hypothesized that perisomatic inhibition should be suppressed by cannabinoids in order to reduce the power of gamma oscillations. Since FSBCs do not express CB1Rs at their axon terminals (Katona et al. 1999; Hajos et al. 2000), cannabinoids cannot directly control their synaptic output (Hajos et al. 2000; Ohno-Shosaku et al. 2002). Thus, the reduction of synchronized GABA release during gamma oscillations can only be achieved either by a decrease in the firing frequency of FSBCs or by the de-synchronization of their spiking activity without a change in the mean frequency (Andersson et al. 2010; Gulyas et al. 2010). To reveal which scenario might be responsible for the CB1R-mediated reduction in oscillations, we simultaneously recorded local field potentials and the spiking activity of individual neurons, which were post hoc anatomically identified. In this set of experiments, we first monitored the discharge of CA3 pyramidal cells using a loose-patch mode in parallel with the field oscillation. As Fig. 2 shows, bath application of CP55,940 (1 μm) resulted in a significant decrease in the firing rate of pyramidal cells to 55.2 ± 9.2% of control (Fig. 2A, left panel, and B, Table 2) along with a change in the power of the oscillation (50.2 ± 5.3% of control, control: 146.5 ± 16.7 μV2, in CP55,940: 72.2 ± 8.1 μV2, n = 6, P = 0.002). In addition to the spiking activity, the phase-coupling strength of spiking was also found to be reduced after drug treatment without any change in the mean phase (Table 2). Under identical conditions, we next investigated the firing behaviour of FSBCs, which discharged in almost every oscillation cycle and often fired doublets of action potentials (Fig. 2A, right panel, and C). In addition to the substantial dampening of the field oscillation upon CB1R activation (53.3 ± 7.1% of control, control: 117.3 ± 25.6 μV2, CP55,940: 58.4 ± 13.2 μV2, n = 7, P = 0.028), the spiking activity of FSBCs was also suppressed to 62.2 ± 7.1% of control (Fig. 2, Table 2). The reduced firing rate was complemented by a decrease in the phase-coupling strength, but not in the mean phase (Table 2). When comparing the change in the peak power of the oscillation and the change in the firing rate of the recorded cells, we found a linear relationship both in the case of pyramidal cells (r = 0.58, n = 6, P = 0.04; Fig. 2B, right panel) and of FSBCs (r = 0.48, n = 7, P = 0.04, Fig. 2C, right panel). Thus, larger reductions in the peak power of gamma oscillations caused by CB1R activation were accompanied by a more suppressed and less precise firing activity in CA3 neurons.
Figure 2. Effect of CB1R activation on the firing frequency of CA3 pyramidal cells and fast spiking basket cells during carbachol-induced gamma oscillations.
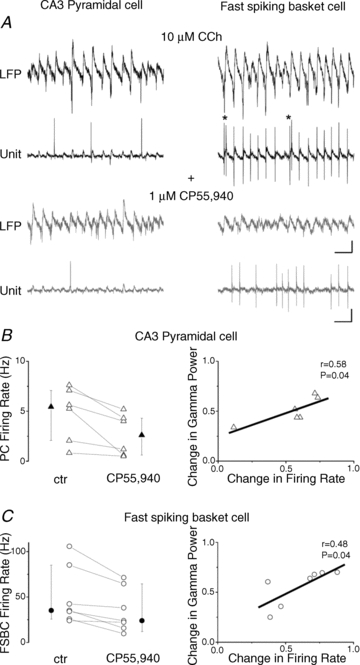
A, simultaneous extracellular recordings of field potentials in the stratum pyramidale of CA3 (top black traces) during carbachol-induced oscillations and spike trains recorded in a loose-patch mode (bottom black traces) from a post hoc anatomically identified CA3 pyramidal cell (left) and a fast spiking basket cell (right). The CB1R agonist CP55,940 (1 μm) reduces the firing frequency of both cell types (lower grey traces) along with the power of the oscillation (upper grey traces). CCh, carbachol. Scale bars, 0.1 mV (field potentials) or 0.2 mV (spike recordings) and 50 ms. B and C, left, summary of the effect of the CP55,940 application on the firing rate of each measured CA3 pyramidal cell (triangles) and FSBC (circles) showing a clear reduction in all cases. The vertical bars adjacent to the pairwise comparisons indicate medians and interquartile ranges of the corresponding data (filled symbols). B and C, right, a linear relationship was found between the change in the peak power of the oscillation and the change in the firing rate of the recorded cells, both normalized to control.
Table 2.
Firing properties of CA3 pyramidal cells and fast spiking basket cells during carbachol-induced oscillations under control conditions and 20 min after drug application
| CA3 pyramidal cells (n = 6) | |||
|---|---|---|---|
| Control | CP55,940 | P value | |
| Firing frequency (Hz) | 5.4 (1.7–7.2) | 2.6 (0.6–4.5) | 0.031 |
| Spikes/oscillation cycle | 0.19 (0.07–0.26) | 0.08 (0.02–0.17) | 0.031 |
| Phase (deg) | 7.7 ± 6.3 | 10 ± 7.8 | 0.62 |
| Phase-coupling strength | 0.69 (0.67–0.72) | 0.58 (0.44–0.69) | 0.031 |
| CA3 fast spiking basket cells (n = 7) | |||
|---|---|---|---|
| Control | CP55,940 | P value | |
| Firing frequency (Hz) | 35.1 (25.7–85.1) | 23.8 (12.1–64.6) | 0.015 |
| Spikes/oscillation cycle | 1.07 (0.75–2.46) | 0.74 (0.43–1.82) | 0.015 |
| Phase (deg) | 23 ± 13.3 | 33.1 ± 22.6 | 0.358 |
| Phase-coupling strength | 0.83 (0.8–0.85) | 0.78 (0.73–0.79) | 0.015 |
Data are presented as the median with interquartile range in parentheses, except for phase values, which are given as the mean phase ± circular SD. Significant changes are indicated in italics.
CB1R activation suppresses monosynaptically evoked EPSCs in CA3 pyramidal cells and fast spiking basket cells in the presence of carbachol
The microcircuits comprising pyramidal cells and FSBCs generate the cholinergically induced gamma oscillation in hippocampal slices, but at which synapses do cannabinoids control the neurotransmission? A recent study elucidated that synaptic communication between CA3 pyramidal cells is regulated by CB1R activation (Hofmann et al. 2008), similar to that observed in other hippocampal subfields (Misner & Sullivan, 1999; Chiu & Castillo, 2008; Xu et al. 2010). To test whether excitatory synaptic transmission between CA3 pyramidal cells would be sensitive to cannabinoids under the conditions used to induce oscillations in this study, we performed the following experiments in the presence of 10 μm carbachol. Excitatory postsynaptic currents (EPSCs) were evoked by stimulation of fibres in the stratum radiatum. In CA3 pyramidal cells, both CP55,940 and WIN55,212-2 (bath applied at 1 μm) significantly reduced the peak amplitude of the evoked currents (68.9 ± 5.1% and 76.4 ± 9.1% of control, n = 6 and 5, respectively; Table 3; Fig. 3A and D). Next, we examined the sensitivity of EPSCs in FSBCs measured under identical conditions. Similar to the findings obtained in CA3 pyramidal cells, but in contrast to previous data (Hoffman et al. 2003), application of both cannabinoid agonists (CP55,940 and WIN55,212-2 at a concentration of 1 μm) substantially suppressed the amplitude of evoked events in FSBCs (63.9 ± 3.8% and 68.3 ± 7.2%, n = 9 and n = 5, respectively; Table 3, Fig. 3B and D). After 20 min of cannabinoid treatment, 1 μl of 10 mm NBQX applied directly to the recording chamber (volume of 1 ml) readily eliminated all evoked currents recorded in both cell types, confirming that primarily AMPA/kainate-mediated synaptic currents were evoked (Fig. 3A and B). In addition, the specificity of the effects of cannabinoids was also tested in CB1R knockout animals. No significant effect was found in the case of CP55,940 application on evoked EPSCs in CA3 pyramidal cells (97.4 ± 8.9% of control n = 6; Table 3, Fig. 3D) or in FSBCs (93.6 ± 4.3% of control n = 4; Table 3, Fig. 3D), verifying the selective activation of CB1Rs by CP55,940. These observations suggest that excitatory synaptic transmission received by both CA3 pyramidal cells and FSBCs could be substantially reduced by cannabinoids in a CB1R-dependent manner during cholinergic receptor activation.
Table 3.
Effect of cannabinoid receptor agonists on the peak amplitude of electrically evoked postsynaptic currents (PSC) recorded in CA3 neurons
| Genotype | Cell type | PSC type | Control (pA) | CP55,940 (pA) | P value | Number |
|---|---|---|---|---|---|---|
| WT | PC | EPSC | 114.8 ± 40.4 | 79.2 ± 37.7 | 0.041 | 6 |
| FSBC | EPSC | 343.9 ± 59.2 | 220.4 ± 42.8 | 0.0007 | 9 | |
| PC | IPSC | 141.1 ± 11.9 | 148.6 ± 21.1 | 0.29 | 5 | |
| CB1R KO | PC | EPSC | 106.1 ± 57.1 | 98.2 ± 53.8 | 0.16 | 6 |
| FSBC | EPSC | 109.3 ± 21.7 | 101.3 ± 20.1 | 0.53 | 4 |
| Genotype | Cell type | PSC type | Control (pA) | WIN55,212-2 (pA) | P value | Number |
|---|---|---|---|---|---|---|
| WT | PC | EPSC | 110.5 ± 61.2 | 84.5 ± 62.3 | 0.047 | 5 |
| FSBC | EPSC | 242.5 ± 48.5 | 164 ± 39. 9 | 0.025 | 5 | |
| PC | IPSC | 100.7 ± 61.5 | 101.5 ± 76.2 | 0.56 | 4 |
Data were compared using the Student's paired t test: significant differences are indicated in italics. Data are presented as mean ± SEM. PC, pyramidal cell; FSBC, fast spiking basket cell.
Figure 3. Cannabinoid agonists reduce the monosynaptically evoked EPSCs (eEPSCs) recorded in CA3 pyramidal cells and fast spiking basket cells, but have no effect on IPSCs measured in CA3 pyramidal cells in the presence of carbachol.
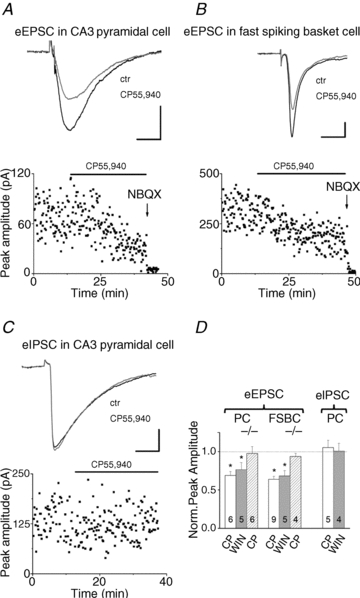
A and B, upper traces show typical mean EPSCs evoked in the stratum radiatum of CA3 recorded in a CA3 pyramidal cell (A) or in a FSBC (B) in control (black) and after CP55,940 (1 μm, grey) application. Plots (lower panels) of the peak amplitude of eEPSCs show a reduction upon CP55,940 application (black lines indicate the wash-in period of the drug). NBQX, an antagonist of AMPA/kainate-type glutamate receptors, was applied in each case at the end of the recording to verify that EPSCs were recorded. C, typical mean IPSC (upper panel) recorded in a CA3 pyramidal cell before (black trace) and after (grey trace) application of CP55,940 (1 μm). Lower panel shows the amplitude plot of eIPSCs. No change in the peak amplitude can be detected after the application of the CB1R agonist (black line). D, graph summarizing the effect of CP55,940 (CP, open columns) and WIN55,212-2 (WIN, grey columns) application on the evoked excitatory and inhibitory PSCs. The normalized peak amplitudes after drug application compared to control (dashed line) are shown. Hashed columns represent the results obtained in CB1R knockout animals. Scale bars, 30 pA and 10 ms.
CB1R activation has no effect on monosynaptically evoked IPSCs recorded in CA3 pyramidal cells in the presence of carbachol
Next, we checked the cannabinoid sensitivity of synaptic inhibitory transmission under the present recording conditions. Pharmacologically isolated IPSCs evoked by stimulation of fibres in the stratum pyramidale were recorded in CA3 pyramidal cells in the presence of carbachol (10 μm). Bath application of CB1R agonists (CP55,940 and WIN55,212-2 at 1 μm) did not produce any effect on the IPSC amplitude (101.59 ± 9.32% and 100.87 ± 9.85% of control, n = 5 and 4, respectively; Table 3, Fig. 3C and D). These data are in line with earlier findings showing that perisomatic inhibition, which is critical for gamma oscillations, predominantly originates from cannabinoid-insensitive GABAergic terminals (Fukudome et al. 2004; Neu et al. 2007; Szabo et al. 2010), since GABA release from the axon endings of CB1R-expressing interneurons is almost completely muted in the presence of carbachol at a concentration higher than 5 μm (Gulyas et al. 2010). Moreover, our results are also consistent with the hypothesis that cannabinoids do not suppress gamma oscillations through the direct reduction of phasic inhibitory input onto CA3 pyramidal cells, but rather act by decreasing the excitatory drive in the hippocampal network.
The reduced spiking frequency of fast spiking basket cells alone may account for a significant drop in the oscillation power upon CB1R activation
Finally, we attempted to differentiate the relative roles of CA3 pyramidal cells and FSBCs in mediating the cannabinoid effect on gamma oscillations. To address this question, we took advantage of the distinct temporal efficacy of CP55,940 on the suppression of EPSC amplitudes observed in the two cell types. As Fig. 3A and B indicated, this cannabinoid receptor ligand seemed to reduce the EPSC amplitude recorded in FSBCs faster than in pyramidal neurons. To quantify this difference, we calculated the changes in the peak amplitude every 5 min from the beginning of the drug superfusion. EPSC amplitudes evoked in FSBCs showed a significant decrease at 5 and 10 min (P < 0.001) compared to control, but no change in the EPSC amplitudes recorded in CA3 pyramidal cells could be observed (Fig. 4A, Table 4). This significant difference in the changes of the peak amplitudes between the two cell types upon CP55,940 treatment could not be observed at the second half of the 20 min period (15 min: P = 0.52; 20 min: P = 0.48), as the peak amplitude of EPSCs recorded in CA3 pyramidal cells started to decrease gradually from 15 min onwards (Fig. 4A). Examination of the effect of CP55,940 on the spiking activity of individual neurons as well as on the oscillation power at the same time points showed that at 5 min pyramidal cell spiking was not significantly different from the control (90.8 ± 2.5% of control, n = 6, P = 0.09), whereas the firing frequency of FSBCs was already significantly suppressed (73.9 ± 6.7% of control, n = 7, P = 0.01). The peak power of gamma oscillations decreased in parallel with the FSBC firing (Fig. 4B and C; peak power in slices where pyramidal cells were recorded: 75.9 ± 3.4% of control, n = 6, P = 0.001; peak power in slices where FSBCs were recorded: 69.1 ± 7.9% of control, n = 7, P = 0.047). At the 10, 15 and 20 min time points no significant difference could be detected in the changes of spiking activity and oscillation power (Fig. 4B and C, P > 0.1). Figure 4C demonstrates that there was no significant difference in the kinetics of the power decrease between the two slice populations, where the CA3 pyramidal cells and the FSBCs were measured. Similarly, the peak power of the oscillations in control conditions did not differ either (peak power in slices where pyramidal cells were recorded: 146.5 ± 16.7 μV2, n = 6; peak power in slices where FSBCs were recorded: 117.3 ± 25.6 μV2, n = 7, P = 0.45). The coincidence of the drop in oscillation power and the decrease in the firing activity of FSBCs, but not of pyramidal cells, at the beginning of CP55,940 treatment suggests that cannabinoid effects on gamma oscillations could primarily be mediated by the suppression of FSBC function, producing less perisomatic inhibitory current and thus smaller oscillations (Gulyas et al. 2010; Oren et al. 2010). However, upon longer cannabinoid application the reduced discharge rate of pyramidal cells, causing even less excitatory drive onto FSBCs, can also contribute to the full reduction in the oscillation power.
Figure 4. The reduced spiking frequency of fast spiking basket cells is already accompanied by a significant drop in the oscillation power, while pyramidal cell firing is still unaltered.
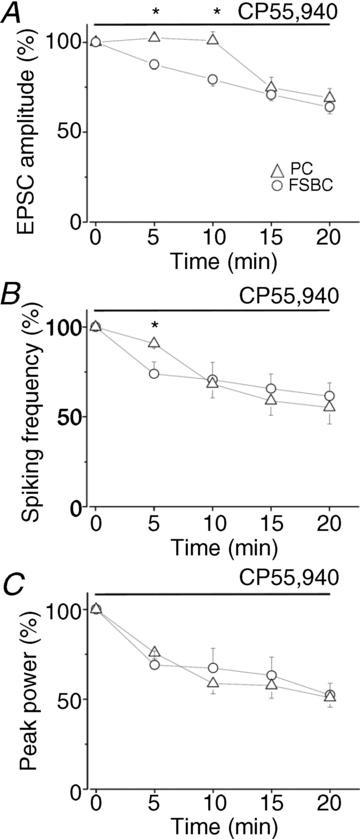
A, time course of the decrease of EPSC amplitudes recorded in CA3 pyramidal cells (PC) and in fast spiking basket cells (FSBC) during CP55,940 wash-in. Note that in the first 10 min no change in the EPSC amplitude measured in pyramidal cells could be detected, whereas EPSCs in fast spiking basket cells were already depressed even after 5 min of drug application. Asterisks indicate the significant difference between the two cell types. B, superfusion of CP55,940 caused a gradual decrease in spiking frequency of pyramidal cells and fast spiking basket cells during ongoing gamma oscillations. At 5 min, however, there was no substantial change in the spiking frequency of pyramidal cells, but the discharge rate of fast spiking basket cells was significantly suppressed (asterisk). At later time points no significant difference in the magnitude of the reduction in firing rate could be noticed between the two cell types. C, in the same experiments as in B, the oscillation power was significantly reduced already 5 min after drug application, a reduction that further developed as CP55,940 was perfused. Circles and triangles represent data obtained in two populations of slices, where CA3 pyramidal cells and FSBCs, respectively, were measured. Data are presented as mean ± SEM.
Table 4.
Change in the peak amplitude of electrically evoked EPSCs recorded in CA3 neurons at different time points from the beginning of CP55,940 application
| Time points | % of control amplitude in PC (n = 6) | P value | % of control amplitude in FSBC (n = 9) | P value |
|---|---|---|---|---|
| 5 min | 102.4 ± 2.1 | 0.27 | 87.6 ± 2.7 | 0.031 |
| 10 min | 100.9 ± 4.9 | 0.85 | 79.3 ± 3.6 | 0.009 |
| 15 min | 74.6 ± 5.9 | 0.03 | 70.7 ± 3.3 | 0.008 |
| 20 min | 68.9 ± 5.1 | 0.041 | 63.9 ± 3.8 | 0.0007 |
Data were compared using the Student's paired t test: significant differences are indicated in italics. Data are presented as mean ± SEM. PC, pyramidal cell; FSBC, fast spiking basket cell.
Effect of CB1R activation on sharp wave-ripple activities in the hippocampus
In addition to gamma oscillations, the CA3 region of the hippocampus can intrinsically generate SWRs that propagate to CA1 driven by synaptic excitation originated from Schaffer collaterals (Buzsáki et al. 1992). A previous in vivo study showed that SWRs in CA1 could be reduced by cannabinoids, an effect that can be explained by the depressed glutamate release from Schaffer collaterals in CA1 upon CB1Rs activation (Misner & Sullivan, 1999; Kawamura et al. 2006; Robbe et al. 2006). However, it is not clear whether cannabinoids could suppress SWRs in CA3 or if only their propagation to CA1 is impaired by CB1R activation. Therefore, we tested the cannabinoid sensitivity of SWRs in the CA3 region of the hippocampal slices. A patch pipette filled with aCSF was placed to the stratum pyramidale to monitor the local field potentials. After obtaining a control period, CP55,940 at a concentration of 1 μm was bath applied. We observed that both the peak amplitude of SWRs (control: 254.9 μV, 125.2 μV (median and interquartile range, respectively); in CP55,940: 215.4 μV, 116.1 μV, n = 8, P = 0.007) and their incidence (control: 0.69 Hz, 0.36 Hz (median and interquartile range, respectively); in CP55,940: 0.38 Hz, 0.46 Hz, n = 8, P = 0.01, Wilcoxon Signed Rank test) was significantly reduced (Fig. 5). These data propose that not only gamma oscillations, but SWRs in the hippocampal CA3 networks can be readily suppressed by cannabinoids.
Figure 5. CB1R activation by CP55,940 significantly suppresses sharp wave-ripple activities in the CA3 region of hippocampal slices.
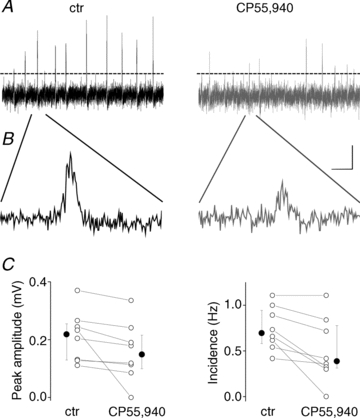
A, 10-s-long field recordings before and after drug application indicate marked decrease in the peak amplitude of spontaneously occurring population events. Dashed lines show the detection threshold. B, example events are enlarged. Scale bars, 0.1 mV and 25 ms. C, the mean values of individual experiments (open circles) and the corresponding medians and the interquartile ranges (filled circles) obtained in two conditions are shown.
Discussion
The main findings of the present study are as follows: (1) Cholinergically induced hippocampal gamma oscillations can be dampened by exogenous cannabinoids in a CB1R-dependent manner; (2) Decreased peak oscillatory power upon CB1R activation is accompanied by reduced and less precise firing activity in CA3 pyramidal cells and FSBCs, neuron types that are critically involved in the generation of field oscillations in this model; (3) In the presence of carbachol, CB1R activation can significantly suppress excitatory synaptic transmission recorded in CA3 pyramidal cells and FSBCs, whereas pyramidal cell perisomatic inhibitory currents are unaffected; (4) At the beginning of CP55,940 superfusion in slices, a significant drop in oscillation power coincides with the reduced firing of FSBCs, while pyramidal cell spiking is still unchanged; (5) Cannabinoids can also suppress SWRs in the CA3 region of hippocampal slices. Since CB1Rs are predominantly, if not exclusively, located at axon terminals in the hippocampus, our data propose that the decrease in excitatory synaptic input upon activation of these receptors can result in fewer action potential discharges with lower fidelity during synchronous network activities, which may account for the impairment of hippocampal oscillations observed after cannabinoid treatment.
Our results clearly show that the excitatory input not only onto CA3 pyramidal cells but also onto FSBCs could be reduced by cannabinoids activating CB1Rs. This finding seems to be in contrast to that published previously (Hoffman et al. 2003), where evoked EPSCs recorded in CA1 interneurons located outside of the pyramidal cell layer were found to be insensitive to cannabinoid ligands. Since FSBCs can be predominantly found within the stratum pyramidale (Katsumaru et al. 1988), it is likely that this GABAergic cell type was not among the interneurons tested by Hoffman et al. (2003). Alternatively, we cannot rule out the possibility that recurrent collaterals in CA3 targeting local interneurons express CB1Rs, but that Schaffer collaterals in CA1 synapsing on GABAergic cells do not. Further studies might clarify the apparent contradiction between these observations.
In a previous report, we found that the μ-opioid receptor agonist DAMGO could effectively diminish cholinergically induced network oscillations in hippocampal slices (Gulyas et al. 2010). Although both DAMGO and cannabinoids reduced the oscillation power, the cellular mechanisms underlying their effects were quite different. The activation of μ-opioid receptors located specifically at axon terminals of parvalbumin-containing interneurons suppressed GABA release (Drake & Milner, 2002; Glickfeld et al. 2008; Gulyas et al. 2010) and led to de-synchronization of neuronal firing without changing the mean discharge rate. Similarly, it was recently proposed that the agonists of H3 histamine receptors could dampen gamma oscillations predominantly via the de-synchronization of firing of CA3 pyramidal cells with no effects on their spiking activity (Andersson et al. 2010). In contrast, we find here that the cannabinoid-induced decrease in the excitatory synaptic transmission predominantly onto FSBCs can already cause a significant reduction in their mean firing frequency (Fig. 4). As a result, the magnitude of synchronous GABA release from FSBC axon terminals generating the field oscillation in CA3 is substantially depressed, producing smaller fluctuations in the field potentials (Oren et al. 2010). However, the reduced discharge rate of pyramidal cells (Figs 2 and 4), producing even smaller excitatory drive onto FSBCs, may contribute to the full suppression of the oscillation power upon cannabinoid treatment. These results are consistent with the notion that the reduction of field oscillations, e.g. by anaesthetics, may indicate the suppression of synaptic transmission (Destexhe et al. 2003).
Excitatory synaptic transmission is critical for carbachol-induced oscillations, since blocking AMPA receptors diminishes rhythmic activities (Fisahn et al. 1998; Mann et al. 2005). Furthermore, application of a selective AMPA receptor antagonist resulted in the hyperpolarization of the resting membrane potential of CA3 pyramidal cells, and abolished the rhythmic firing of FSBCs (Mann et al. 2005), which has been shown to be driven by phasic excitatory input (Traub et al. 2000; Oren et al. 2006). The tonic depolarizing effect of AMPA receptor activation probably promotes pyramidal cell discharge, which is consistent with results indicating that slow, non-rhythmic excitatory input onto pyramidal cells during gamma oscillations may be enough to support the spiking of principal neurons synchronized by perisomatic inhibition (Fisahn et al. 1998; Traub et al. 2000; Mann et al. 2005; Morita et al. 2008). These observations together suggest that a partial reduction in excitatory synaptic transmission, e.g. after cannabinoid treatment (Fig. 3), can reduce the excitability of CA3 pyramidal cells in a recurrent network. The prediction that this will reduce the number of neurons firing synchronously during a gamma cycle is supported by our recordings (Fig. 2) and by modelling studies (Traub et al. 2000; Morita et al. 2008). The resulting smaller neuronal assembly will produce a lower excitatory drive onto FSBCs. This reduced phasic excitation from a smaller neuronal population together with the direct reduction of glutamate release onto FSBCs upon CB1R activation is likely to be responsible for the full cannabinoid effect on gamma oscillations. Our observation that the significant suppression of the FSBC discharge rate was already accompanied by a drop in the oscillation power, while the pyramidal cell spiking was still unaltered, is in full agreement with a recent study proposing that the reduction of excitatory input solely onto FSBCs might be sufficient to suppress in vitro gamma oscillations (Fuchs et al. 2007).
In vivo studies uncovered that cannabinoids could effectively depress oscillatory activities in several frequency bands in the CA1 region of the hippocampus (Robbe et al. 2006; Hajos et al. 2008). At low doses, CP55,940 caused only de-synchronization of the firing of CA1 neurons without a significant change in their frequency, an effect that was already accomplished by the dampening of the rhythmic activities detected in local field potentials. At high doses, however, the firing rate was significantly suppressed (Robbe et al. 2006). During exploratory behaviour, when the theta rhythm dominates hippocampal field potentials, input from the entorhinal cortex might prevalently influence the firing of CA1 neurons (Buzsáki, 1989; Brun et al. 2002). In contrast, their spiking during SWRs, which characterize local field potentials in CA1 during consummatory behaviour and slow wave sleep, is mainly controlled by excitation from CA3 (Buzsáki, 1989; Buzsáki et al. 1992; Nakashiba et al. 2009). Importantly, a recent in vitro study revealed that excitatory synaptic input from the entorhinal cortex onto CA1 pyramidal cells could be depressed only to a small extent upon CB1R activation, in contrast to excitatory synaptic transmission originating from CA3, which was significantly reduced (Xu et al. 2010). Taking into account these results, we hypothesize that CP55,940 at low doses may not significantly attenuate the excitatory drive onto CA1 neurons from entorhinal cortex during theta rhythms, and thus the frequency of spiking would not be substantially changed, whereas the impairment in the temporal structure of the firing may be explained by the reduced excitation from CA3 (Jarsky et al. 2005; Katz et al. 2007). This proposal is supported by in vivo findings, showing that SWRs in CA1 that are generated by the synchronous population discharge of CA3 pyramidal cells via Schaffer collaterals (Buzsáki et al. 1992) are significantly suppressed after CP55,940 treatment (Fig. 5) (Robbe et al. 2006). These observations are in line with our present results indicating that the reduction in synaptic excitation of CA3 pyramidal cells may underlie the cellular mechanisms of the CB1R-dependent dampening of synchronous activities in hippocampal networks. Thus, cannabinoids could mainly alter intrahippocampal excitatory synaptic transmission, leading to impairment of oscillatory activities and memory formation (Hampson & Deadwyler, 1998; Robbe & Buzsaki, 2009).
Acknowledgments
This work was supported by the Wellcome Trust International Senior Research Fellowship, the National Office for Research and Technology (OMFB-01678/2009), and the Hungarian Scientific Research Fund (T49517, NNF 85659). N.Ho. is a recipient of Bolyai Research Fellowship. We acknowledge Prof. Andreas Zimmer (University of Bonn, Germany) for generously providing the CB1 knockout mice. We thank Dr Attila I. Gulyás (Institute of Experimental Medicine, Hungarian Academy of Sciences, Budapest, Hungary) for the analysis programs and Erzsébet Gregori for her excellent technical assistance. The participation of Gergely Szabó and Zsolt Kohus at the initial stages of these experiments is also acknowledged.
Glossary
Abbreviations
- CA
cornu ammonis
- CB1R
cannabinoid receptor type 1
- CCh
carbachol
- CCK
cholecystokinin
- CP55,940
(-)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- FSBC
fast spiking basket cell
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione
- PC
pyramidal cell
- SR141716A
5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)- 1H-pyrazole-3-carboxamide
- SWR
sharp wave-ripple activitiy
- WIN55,212-2
(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate
Author contributions
N.Ho. and N.Há. contributed to the conception and design of the experiments. N.Ho., B.N., O.I.P., G.A.N. and J.M.V. conducted the electrophysiological experiments. N.Ho., O.I.P. and N.Há. analysed the data. N.Ho. and N.Há. drafted the manuscript. All authors participated in the interpretation of the data, revised the article and approved the final version for publication. Experiments were conducted in the Department of Cellular and Network Neurobiology, Institute of Experimental Medicine, Hungarian Academy of Sciences, Budapest, Hungary.
References
- Andersson R, Lindskog M, Fisahn A. Histamine H3 receptor activation decreases kainate-induced hippocampal gamma oscillations in vitro by action potential desynchronization in pyramidal neurons. J Physiol. 2010;588:1241–1249. doi: 10.1113/jphysiol.2009.180984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah BV, Scanziani M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron. 2009;62:566–577. doi: 10.1016/j.neuron.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience. 1989;310:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Chiu CQ, Castillo PE. Input-specific plasticity at excitatory synapses mediated by endocannabinoids in the dentate gyrus. Neuropharmacology. 2008;54:68–78. doi: 10.1016/j.neuropharm.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4:739–751. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. μ-Opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12:119–136. doi: 10.1002/hipo.1107. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12:105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl E, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, Monyer H. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci. 2004;19:2682–2692. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Atallah BV, Scanziani M. Complementary modulation of somatic inhibition by opioids and cannabinoids. J Neurosci. 2008;28:1824–1832. doi: 10.1523/JNEUROSCI.4700-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Szabo GG, Ulbert I, Holderith N, Monyer H, Erdelyi F, Szabo G, Freund TF, Hajos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos M, Hoffmann WE, Kocsis B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: relevance to schizophrenia. Biol Psychiatry. 2008;63:1075–1083. doi: 10.1016/j.biopsych.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hajos N, Mody I. Establishing a physiological environment for visualized in vitro brain slice recordings by increasing oxygen supply and modifying aCSF content. J Neurosci Methods. 2009;183:107–113. doi: 10.1016/j.jneumeth.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Palhalmi J, Mann EO, Nemeth B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Paulsen O. Network mechanisms of gamma oscillations in the CA3 region of the hippocampus. Neural Netw. 2009;22:1113–1119. doi: 10.1016/j.neunet.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Role of cannabinoid receptors in memory storage. Neurobiol Dis. 1998;5:474–482. doi: 10.1006/nbdi.1998.0223. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Riegel AC, Lupica CR. Functional localization of cannabinoid receptors and endogenous cannabinoid production in distinct neuron populations of the hippocampus. Eur J Neurosci. 2003;18:524–534. doi: 10.1046/j.1460-9568.2003.02773.x. [DOI] [PubMed] [Google Scholar]
- Hofmann ME, Nahir B, Frazier CJ. Excitatory afferents to CA3 pyramidal cells display differential sensitivity to CB1 dependent inhibition of synaptic transmission. Neuropharmacology. 2008;55:1140–1146. doi: 10.1016/j.neuropharm.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Roxin A, Kath WL, Spruston N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat Neurosci. 2005;8:1667–1676. doi: 10.1038/nn1599. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumaru H, Kosaka T, Heizmann CW, Hama K. Immunocytochemical study of GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus. Exp Brain Res. 1988;72:347–362. doi: 10.1007/BF00250256. [DOI] [PubMed] [Google Scholar]
- Katz Y, Kath WL, Spruston N, Hasselmo ME. Coincidence detection of place and temporal context in a network model of spiking hippocampal neurons. PLoS Comput Biol. 2007;3:e234. doi: 10.1371/journal.pcbi.0030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 1995;119:282–290. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Misner DL, Sullivan JM. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J Neurosci. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci U S A. 2007;104:14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Kalra R, Aihara K, Robinson HP. Recurrent synaptic input and the timing of gamma-frequency-modulated firing of pyramidal cells during neocortical ‘UP’ states. J Neurosci. 2008;28:1871–1881. doi: 10.1523/JNEUROSCI.3948-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Buhl DL, McHugh TJ, Tonegawa S. Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron. 2009;62:781–787. doi: 10.1016/j.neuron.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren I, Hajos N, Paulsen O. Identification of the current generator underlying cholinergically induced gamma frequency field potential oscillations in the hippocampal CA3 region. J Physiol. 2010;588:785–797. doi: 10.1113/jphysiol.2009.180851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren I, Mann EO, Paulsen O, Hajos N. Synaptic currents in anatomically identified CA3 neurons during hippocampal gamma oscillations in vitro. J Neurosci. 2006;26:9923–9934. doi: 10.1523/JNEUROSCI.1580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Buzsaki G. Alteration of theta timescale dynamics of hippocampal place cells by a cannabinoid is associated with memory impairment. J Neurosci. 2009;29:12597–12605. doi: 10.1523/JNEUROSCI.2407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W( Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo GG, Holderith N, Gulyas AI, Freund TF, Hajos N. Distinct synaptic properties of perisomatic inhibitory cell types and their different modulation by cholinergic receptor activation in the CA3 region of the mouse hippocampus. Eur J Neurosci. 2010;31:2234–2246. doi: 10.1111/j.1460-9568.2010.07292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Naatanen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- Traub RD, Bibbig A, Fisahn A, LeBeau FE, Whittington MA, Buhl EH. A model of gamma-frequency network oscillations induced in the rat CA3 region by carbachol in vitro. Eur J Neurosci. 2000;12:4093–4106. doi: 10.1046/j.1460-9568.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jeffreys JGR. Synchronized oscillations in interneurons driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Xu JY, Chen R, Zhang J, Chen C. Endocannabinoids differentially modulate synaptic plasticity in rat hippocampal CA1 pyramidal neurons. PloS One. 2010;5:e10306. doi: 10.1371/journal.pone.0010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]


