Non-technical summary
Non-invasive neuromodulation of the human brain – with pulsed magnetic fields or small direct currents – is becoming increasingly popular for treating a variety of neurological and neuropsychiatric disorders. In the present work we investigated in healthy humans the possibility of a non-invasive modulation of motor cortex excitability by the application of static magnetic fields through the scalp. We found that transcranial static magnetic field stimulation (tSMS) can reduce the excitability of the motor cortex for a period that outlasts the time of the application of the magnetic field. Moreover, we demonstrated that these excitability changes take origin at the cortical level. These results suggest that tSMS using small static magnets may be a promising tool to modulate cerebral excitability in a non-invasive, painless and reversible way.
Abstract
Abstract
The aim of the present study was to investigate in healthy humans the possibility of a non-invasive modulation of motor cortex excitability by the application of static magnetic fields through the scalp. Static magnetic fields were obtained by using cylindrical NdFeB magnets. We performed four sets of experiments. In Experiment 1, we recorded motor potentials evoked by single-pulse transcranial magnetic stimulation (TMS) of the motor cortex before and after 10 min of transcranial static magnetic field stimulation (tSMS) in conscious subjects. We observed an average reduction of motor cortex excitability of up to 25%, as revealed by TMS, which lasted for several minutes after the end of tSMS, and was dose dependent (intensity of the magnetic field) but not polarity dependent. In Experiment 2, we confirmed the reduction of motor cortex excitability induced by tSMS using a double-blind sham-controlled design. In Experiment 3, we investigated the duration of tSMS that was necessary to modulate motor cortex excitability. We found that 10 min of tSMS (compared to 1 min and 5 min) were necessary to induce significant effects. In Experiment 4, we used transcranial electric stimulation (TES) to establish that the tSMS-induced reduction of motor cortex excitability was not due to corticospinal axon and/or spinal excitability, but specifically involved intracortical networks. These results suggest that tSMS using small static magnets may be a promising tool to modulate cerebral excitability in a non-invasive, painless, and reversible way.
Introduction
In animal experiments, it has long been possible to probe and manipulate the efficacy of synaptic transmission by repetitive electrical stimulation of central nervous system pathways (Cooke & Bliss, 2006). This led to the discovery of the well-studied phenomena of long-term potentiation (LTP) and long-term depression (LTD) of synaptic connections (Cooke & Bliss, 2006). Repetitive transcranial magnetic stimulation (rTMS), which is a non-invasive method of stimulating the brain of conscious human subjects through the intact scalp, has obvious potential for mimicking the effects that have been observed in animal models (Siebner & Rothwell, 2003). Also other stimulation methods have been used to try to induce plastic changes in human cortex, for example paired associative stimulation (PAS) (Stefan et al. 2000), theta-burst stimulation (TBS) (Huang et al. 2005), or transcranial direct current stimulation (tDCS) (Nitsche et al. 2008). Most of these protocols typically require periods of conditioning lasting from a few minutes or less for the theta burst stimulation protocols, up to tens of minutes for some of the others. All of these stimulation techniques are potentially uncomfortable for some subjects, require more or less expensive devices, need highly qualified personnel and lack an absolute convincing sham stimulation to be easily used in clinical trials.
Static magnetic fields, unlike time-varying magnetic fields, are not associated with induced electric currents and have been shown to influence a variety of biological systems (Rosen, 2003). In a number of studies, static magnetic fields have been suggested to act primarily at the synapse and it has been proposed that these fields alter the function of membrane ion channels (Rosen, 2003; Coots et al. 2004). Moreover, the effects of application of static magnetic fields to different animal preparations seem to have an effect that outlasts the time of stimulation (Rosen & Lubowski, 1987).
In the present experiments, we tested the hypothesis that it is possible to produce after-effects in the human motor cortex by applying transcranial static magnetic field stimulation (tSMS) focally through the scalp. We made use of transcranial magnetic stimulation (TMS) for evaluating cortical excitability changes, because it allows the quantification of motor cortex output in a painless and non-invasive manner (Hallett, 2000). The amplitude of the resulting motor evoked potential (MEP) represents the excitability of the motor system. We show that tSMS of sufficiently high intensity decreases the excitability of the motor cortex independently of the polarity of the magnetic field (Experiment 1). We confirm the tSMS-induced reduction of cortical excitability with a double-blind sham-controlled design (Experiment 2). We characterize the dependence of this reduction of cortical excitability on the duration of tSMS (Experiment 3). Finally, we use transcranial electrical stimulation (TES) to establish an intracortical origin for the action of tSMS (Experiment 4).
Methods
Subjects
We performed a total of 107 experimental sessions in 19 healthy subjects. Experiment 1 was performed on 11 subjects (8 males and 3 females, mean age 33.8 ± 6.8 (SD); range 22–46). Experiments 2 and 3 were performed on a new set of 11 subjects (3 males and 8 females, mean age 33.1 ± 5.8; range 27–41), three of which also participated in Experiment 1. Experiment 4 was performed on a subset of four subjects (2 males and 2 females, mean age 36.2 ± 6.3; range 29–42), who had participated in all previous experiments. The participants were screened for history of hormonal, metabolic, circulatory, psychiatric and neurological disorders, and were medication-free at the time of the study. The participants were seated comfortably in a semi-darkened room, and were instructed to refrain from speaking and to remain awake while in a calm, relaxed state. All participants gave their informed consent; the procedures had the approval of the hospital ethics committee and were conducted in accordance with the Declaration of Helsinki. No more than one experiment was conducted per subject per week.
Transcranial static magnetic field stimulation (tSMS) of the motor cortex
We used two different sizes of magnets, which will be referred to as the Big Magnet and Small Magnet. The Big Magnet was a cylindrical nickel-plated (Ni–Cu–Ni) NdFeB magnet of 45 mm diameter and 30 mm of thickness, with a weight of 360 g (Model S-45-30-N; Supermagnete, Gottmadingen, Germany). The maximum amount of magnetic energy stored in this magnet was 45 MGOe (megagauss-oersteds), with a nominal strength of 765 N (78 kg). The Small Magnet was a cylindrical nickel-plated (Ni–Cu–Ni) NdFeB magnet of 30 mm diameter and 15 mm of thickness, with a weight of 81 g (Model S-30-15-N; Supermagnete). The maximum amount of magnetic energy stored in this magnet was 42 MGOe, with a nominal strength of 225 N (23 kg). The distance between the scalp and the motor cortex was about 20 mm. At this distance, the magnetic energy (in vacuum) is about 5.57 MGOe (range 18–22 mm, 6.32–4.92 MGOe) for the Big Magnet and about 2.50 MGOe (range 18–22 mm, 2.98–2.11 MGOe) for the Small Magnet. Two different magnetic field polarities were used: north and south. The magnet was held over the representational field of the left first dorsal interosseous (FDI) muscle identified by TMS. A non-magnetic metal cylinder was used for sham stimulation. This had the same size, weight and appearance of the Big Magnet.
For Experiments 2 and 3 we only used the Big Magnet (or the sham) and one polarity (south). Subjects were not able to feel any sensation apart from the physical contact with the magnet and they were not able to identify any difference between the magnet and the non-magnetic metal cylinder we used for sham stimulation. In Experiment 2, the sensation was formally tested by asking the subjects if they thought it was the real intervention or the sham.
Measurement of motor cortex excitability by TMS
MEPs were recorded from the FDI by single pulse TMS (duration 300 μs). These pulses were induced using a Magstim 200 magnetic stimulator (Magstim Company, Whiteland, Dyfed, UK) and a figure-of-eight magnetic coil. The coil was held tangentially to the skull, with the handle pointing backwards and laterally at 45 deg from the midline. The optimal position was defined as the site where TMS resulted consistently in the largest MEP. Surface electromyography (EMG) was recorded from the FDI by use of adhesive electrodes in a belly tendon montage. MEPs were amplified and filtered (bandwidth 3 Hz to 3 kHz) by D360 amplifiers (Digitimer, Welwyn Garden City, UK). Data were sampled at 10 kHz, collected on a computer and stored for later analysis using a CED 1401 A/D converter (Cambridge Electronic Design, Cambridge, UK).
Experimental procedures
Each experiment was conducted according to a repeated measurement design. The subject was seated in a reclining chair. First, the ‘hot spot’ of the left FDI was identified by TMS (coil position which led to the largest MEPs of FDI). The ‘hot spot’ was marked over the scalp with a marker. After the identification of the hot spot, a baseline of 20 MEPs was recorded at 0.15 Hz. TMS intensity was set to obtain MEPs of an amplitude of ∼1 mV. Afterwards the magnet was fixed over the hot spot. In Experiments 2 and 3 (see below) the effects of tSMS on resting motor threshold (RMT) were also evaluated. RMT was defined as the minimum stimulus intensity that produced a liminal motor evoked response (about 50 μV in 50% of 10 trials) at rest. This definition of RMT was used in baseline recordings and 20 min after the intervention. Five minutes after the intervention a quicker way to test the RMT was needed due to the short duration of the tSMS after-effects. For this reason, we tested the RMT starting with the value of the baseline and RMT was defined as the minimum stimulus intensity that produced a motor evoked response of about 50 μV in 50% of four trials at rest.
Experiment 1
The aim of Experiment 1 was to test whether and how tSMS alters cortical excitability. tSMS duration was always 10 min, with magnetic field intensity (Big Magnet or Small Magnet) and polarity (north or south) varying between the experiments (see below). After removing the magnet, MEPs were recorded for 20 min at 0.15 Hz using the same intensity as in baseline condition. Each subject underwent four experimental sessions using real stimulation (1: Big Magnet with north polarity, 2: Big Magnet with south polarity, 3: Small Magnet with north polarity, 4: Small Magnet with south polarity), and also an experimental session using sham stimulation. For sham stimulation a non-magnetic metal cylinder was used. The order of the experimental sessions was randomly assigned among subjects. Only for this experiment, the MEPs were recorded using 50 Hz notch filter, which was used in all conditions and all subjects. Figs. 1 and 2A summarize the experimental set-up.
Figure 1. The magnet and its location.
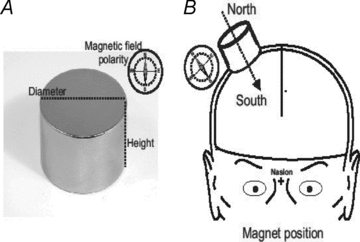
A, the magnet. B, schematic illustration of the magnet located over the cortical representation of the hand (hot spot).
Figure 2. The set-up for Experiments 1–3.
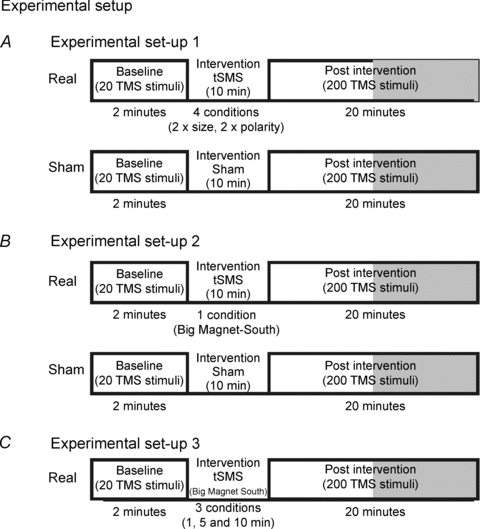
A, experimental set-up for real and sham stimulation (Experiment 1). B, experimental set-up for real and sham stimulation (Experiment 2, double blind). C, experimental set-up for real stimulation using different duration of the tSMS (Experiment 3).
Experiment 2
The aim of Experiment 2 was to replicate the main results obtained in Experiment 1 using a rigorous double-blind sham-controlled design. The experimenter performing TMS and evaluating MEPs off-line did not know whether real or sham tSMS was applied. The subjects were not aware if they received real or sham tSMS. Moreover, at the end of each experimental session the subjects were given a forced choice question about which magnet/sham they received. The intervention duration was always 10 min. We used only one magnetic field intensity (Big Magnet) and one polarity (south). After removing the magnet, MEPs were recorded for 20 min at 0.15 Hz using the same intensity as in baseline condition. RMT was also evaluated at baseline and 5 and 20 min after removing the magnet. Each subject underwent two experimental sessions (Big Magnet with south polarity, and sham stimulation). The order of the experimental sessions was randomly assigned among subjects. Figure 2B summarizes the experimental set-up.
Experiment 3
The aim of Experiment 3 was to study the dependence of tSMS-induced effects on the duration of tSMS. Using only one magnetic field intensity (Big Magnet) and polarity (south), we tested the effects of 1 min, 5 min and 10 min of tSMS. After removing the magnet, MEPs were recorded for 20 min at 0.15 Hz using the same intensity as in baseline condition. RMT was also evaluated at baseline and 5 and 20 min after removing the magnet. The order of the experimental sessions was randomly assigned among subjects. Figure 2C summarizes the experimental set-up.
Experiment 4
The aim of Experiment 4 was to understand whether tSMS-induced after-effects involved only intracortical networks, or also corticospinal axon and spinal excitability. To this end, we tested cortical excitability using transcranial electric stimulation (TES) at near-threshold intensity at rest and during slight tonic voluntary contraction, before and after tSMS (Big Magnet, south polarity). These experimental conditions guarantee that the component of the descending volley along the corticospinal pathway due to direct axonal activation (D-wave) maximally contributes to the evoked response in the target muscle (Di Lazzaro et al. 1998). The D-wave arises from the direct activation of the corticospinal axons in the white matter, below the cerebral cortex (Patton & Amassian, 1954; Di Lazzaro et al. 2004). TES was delivered by a Digitimer D180 stimulator connected to a pair of Ag–AgCl electrodes over the scalp: the anode placed above the motor cortex and the cathode above the vertex. We measured four different parameters: the active and resting motor threshold (AMTE and RMTE) and the MEP amplitude at rest and during voluntary contraction. The AMTE was defined as the minimum TES intensity eliciting a peak-to-peak MEP amplitude of 200 μV or more in slightly active muscle, in at least 5 out of 10 measurements. The RMTE was defined as the minimum TES intensity eliciting a peak-to-peak MEP amplitude of 50 μV or more at rest, in at least 5 out of 10 measurements. For MEP amplitude measurements, TES intensity was set to obtain MEPs of an amplitude of ∼1 mV. Using TES, we only evaluated two time points (baseline and 1–3 min after tSMS). A total of 20 TES stimuli were delivered at 0.1 Hz for each time point. MEPs were recorded and the peak-to-peak amplitude was measured. The measurements at rest and during voluntary contraction were performed in two separate experimental sessions.
Statistical analyses
Experiment 1
We analysed the effects of tSMS on MEP amplitudes evoked by TMS. To reduce the number of multiple comparisons, statistical analyses were limited to the first 10 min after the end of stimulation (baseline plus 5 time points of 2 min each). Amplitude data were divided by the mean of the baseline value in order to normalize their spread. Using this normalization, the baseline mean is always equal to 1 but the variance is the same as in the raw data. MEP amplitudes were entered into a three-way repeated-measures analysis of variance (ANOVA) incorporating, where necessary, a Greenhouse–Geisser correction for non-sphericity. Within-subject factors were SIZE (Big or Small), POLARITY (north or south) and TIME (six time points: baseline and five time points after tSMS). In case of significant effects, post hoc analyses using Tukey's honestly significant difference test were applied. Note that in Experiment 1 the sham stimulation was delivered as a control condition that was not intended to be included in the main ANOVA design, and was thus separately analysed with a one-way repeated-measures ANOVA with TIME as main factor.
Experiment 2
We analysed the effects of the tSMS or sham stimulation on MEP amplitudes evoked by TMS and RMT. MEP amplitude data were divided by the mean of the baseline value in order to normalize their spread. MEP amplitudes and RMT values were separately entered into a two-way repeated-measures ANOVA incorporating, where necessary, a Greenhouse–Geisser correction for non-sphericity. Within-subject factors were STIMULATION (Real or Sham) and TIME (six time points: baseline and five time points after tSMS). The sensation of the patient (Real or Sham) was evaluated using the Z test for two proportions.
Experiment 3
We analysed the effects of the duration of tSMS on MEP amplitudes evoked by TMS. To reduce the number of multiple comparisons, statistical analyses were limited to the time points after the tSMS that were significant in experiments 1 and 2 (3 time points of 2 min each). Amplitude data were divided by the mean of the baseline value in order to normalize their spread. MEP amplitudes were entered into a repeated-measures ANOVA incorporating, where necessary, a Greenhouse–Geisser correction for non-sphericity. Within-subject factors were DURATION (1 min, 5 min, 10 min), and TIME (four time points: baseline and three time points after tSMS).
Experiment 4
We analysed the effects of tSMS on active and resting motor thresholds and MEP amplitudes evoked by TES. All measures were compared using Student's paired t test.
In all experiments, post hoc comparisons were performed using Tukey's honestly significant difference test. All results were considered significant at P < 0.05.
Results
Experiment 1
Experiment 1 showed how tSMS affects the amplitude of MEPs evoked by TMS over motor cortex. Examples of the averaged MEPs obtained from a representative subject before and 2–4 min after both sham stimulation and tSMS are shown in Fig. 3. Figure 4 shows an overview of the total population with the time courses of the effects of tSMS and sham stimulation on the amplitudes of the MEPs. Repeated-measures ANOVA on normalized data showed significant effect of TIME (F5,50 = 2.668, P = 0.033) and SIZE (F1,10 = 9.572, P = 0.011), while no effect was observed for POLARITY (F1,10 = 0.030, P = 0.867). Interaction SIZE × TIME was also significant (F5,50 = 5.051, P = 0.001). Post hoc analysis confirmed that there was a significant amplitude decrease – when the Big Magnet was used – in the first 6 min compared to the baseline (0–2 min, P = 0.0060; 2–4 min, P = 0.0013; 4–6 min, P = 0.0466). The average MEP amplitude reduction was about 25%.
Figure 3. Experiment 1. Effects of transcranial static magnetic field stimulation (tSMS) on motor evoked potential (MEP) amplitude evoked by transcranial magnetic stimulation (TMS) pulses in a representative subject.
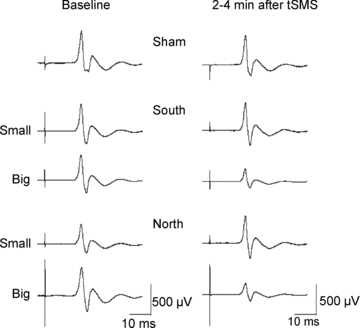
The left column shows the baseline MEPs and the right column shows the MEPs 2–4 min after the application of the tSMS at different intensities (Big Magnet and Small Magnet) and different polarities (south and north). Sham stimulation is reported in the first line.
Figure 4. Experiment 1. Time course of the effects of the different kinds of tSMS and of sham stimulation on normalized MEPs.
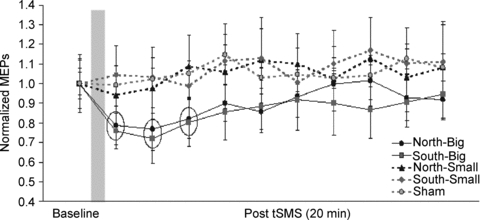
The grey column represents schematically the time of stimulation. Each time point is the average of 2 min. To reduce the number of multiple comparisons, statistical analyses were limited to the first 10 min after the end of stimulation (baseline plus 5 time points of 2 min each). The dotted circles indicate those data that are statistically significant. Error bars are standard errors of the mean.
As far as the sham stimulation is concerned, repeated-measures ANOVA on normalized data showed no effect of TIME (F5,50 = 0.926, P = 0.472).
To summarize, no changes of the MEP amplitude were observed after tSMS with either polarity of the Small Magnet, nor after sham stimulation. Using the Big Magnet, mean MEP amplitudes were decreased relative to baseline for about 6 min after the end of tSMS. Similar effects were obtained using both north and south stimulation.
Experiment 2
Experiment 2 replicated the main results obtained in Experiment 1 using a rigorous double-blind sham-controlled design. Figure 5 shows an overview of the total population with the time course of the effects of tSMS and sham stimulation on the mean amplitudes of the MEPs evoked by TMS. Figure 6 shows the effects of tSMS and of sham stimulation on the individual subjects. Repeated-measures ANOVA on normalized data showed significant interaction STIMULATION × TIME (F5,50 = 3.223, P = 0.013). Post hoc analysis confirmed that there was a significant amplitude decrease in the first 6 min after real tSMS – but not after sham – compared to the baseline (0–2 min, P = 0.0086; 2–4 min, P = 0.0071; 4–6 min, P = 0.0425). The average MEP amplitude reduction was about 25%.
Figure 5. Experiment 2. Time course of the effects of tSMS and sham stimulation on the mean amplitudes of the MEPs evoked by TMS.
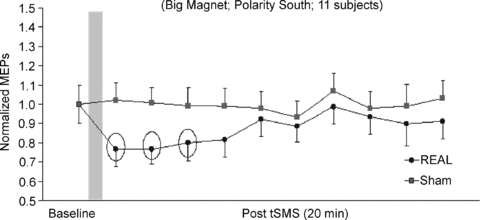
This experiment was conducted using a double blind protocol. Time course of the effects of 10 min of tSMS using a big magnet and south polarity and of sham stimulation on normalized MEPs. The grey column represents schematically the time of stimulation. Each time point is the average of 2 min. To reduce the number of multiple comparisons, statistical analyses were limited to the first 10 min after the end of stimulation (baseline plus 5 time points of 2 min each). The dotted circles indicate those data that are statistically significant. Error bars are standard errors of the mean.
Figure 6. Experiment 2. MEP amplitudes in individual subjects.
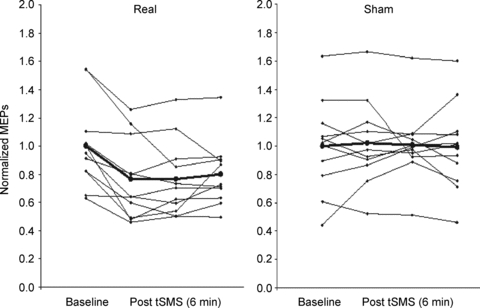
Time course of the effects of 10 min of tSMS using the Big Magnet and south polarity and of sham stimulation on normalized MEPs. Each time point is the average of 2 min. Here we show the baseline plus 3 time points of 2 min each (6 min). The bold line and circles indicate the mean.
RMT was unchanged after both the real and sham tSMS. At the end of the experimental session with real tSMS, 55% of the subjects (6 of 11) answered correctly (forced choice = real). At the end of the experimental session with sham tSMS, 55% of the subjects (6 of 11) answered correctly (forced choice = sham). The Z test for two proportions demonstrated that the subjects were not able to distinguish the magnet and the metal cylinder (P = 0.67).
To summarize, using a double-blind sham-controlled design, mean MEP amplitudes were decreased relative to baseline for about 6 min after the end of real tSMS but not after sham. No difference was observed in RMT. The subjects were not able to recognize whether the metallic cylinder used was the real magnet or the sham.
Experiment 3
Experiment 3 showed the effects of tSMS duration on MEP amplitudes evoked by TMS. Figure 7 shows an overview of the total population with the time courses of the effects of 1, 5 and 10 min tSMS on the amplitudes of the MEPs. Repeated-measures ANOVA on normalized data showed significant effect of TIME (F3,30 = 3.391, P = 0.030). The interaction DURATION × TIME was also significant (F6,60 = 2.345, P = 0.042). Post hoc analysis confirmed that there was a significant amplitude decrease in the first 6 min compared to the baseline only when the larger duration (10 min) was used (0–2 min, P = 0.0013; 2–4 min, P = 0.0010; 4–6 min, P = 0.0098). RMT was unchanged after 1, 5 and 10 min of tSMS.
Figure 7. Experiment 3. Time course of the effects of the different duration of tSMS on normalized MEPs.
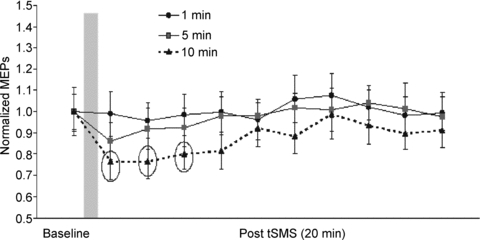
The grey column represents schematically the time of stimulation. Each time point is the average of two minutes. To reduce the number of multiple comparisons, statistical analyses were limited to the first 6 min after the end of stimulation (baseline plus 3 time points of 2 min each). The dotted circles indicate those data that are statistically significant. Error bars are standard errors.
To summarize, mean MEP amplitudes were decreased relative to baseline for about 6 min after the end of tSMS only when the tSMS had a duration of 10 min.
Experiment 4
Experiment 4 investigated the possible effects of tSMS on active and resting motor thresholds and MEP amplitudes evoked by TES (Fig. 8). The mean threshold for responses in FDI using anodal TES at rest was 363.3 ± 73 V in baseline and 380 ± 60 V after tSMS (paired t test, P = 0.3700). The mean MEP amplitude using anodal TES at rest was 0.64 ± 0.16 mV at baseline and 0.68 ± 0.23 mV after tSMS (paired t test, P = 0.5025). The mean threshold for responses in FDI using anodal TES during voluntary contraction was 213.3 ± 25 V in baseline and 215 ± 22 V after tSMS (paired t test, P = 0.7418). The mean MEP amplitude using anodal TES during voluntary contraction was 0.77 ± 0.11 mV at baseline and 0.78 ± 0.14 mV after tSMS (paired t test, P = 0.5783).
Figure 8. Experiment 4. Effects of tSMS on MEP amplitude evoked by transcranial electrical stimulation (TES).
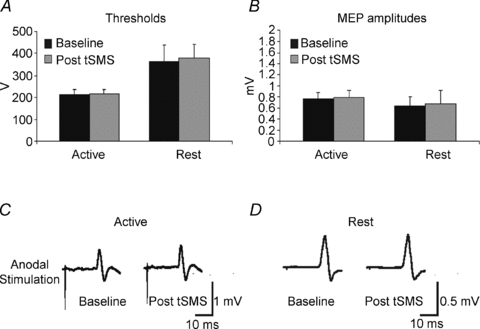
Upper part of the figure shows active and resting motor thresholds before and 2–4 min after the application of the tSMS. Lower part of the figure shows effects of tSMS on MEP amplitude evoked by TES at rest and during voluntary contraction. Error bars are standard deviations.
To summarize, using TES no difference was observed in threshold and MEP amplitude after tSMS both at rest and during voluntary contraction.
Discussion
Our main finding is that the application of tSMS in humans reduces the output of motor cortex – tested using TMS – for a few minutes after the end of stimulation. On the contrary, TES parameters were unchanged after tSMS. TES activates the axons of pyramidal neurons directly, whereas TMS activates the same neurons trans-synaptically (Di Lazzaro et al. 1998). Therefore, our first conclusion is that reduced motor output after tSMS can be explained by reduced motor cortex excitability. Moreover, the decrease of cortical excitability is unlikely to be due to a general alteration in arousal of subjects as neither sham stimulation nor the weaker static magnetic field of the smaller magnet produce any cortical excitability change.
TMS-based techniques (rTMS, TBS, PAS, etc.) and tDCS deliver electric currents to the cortex to obtain short or long term effects on cortical excitability. To the best of our knowledge, the tSMS introduced here is the only neuromodulation technique that is able to produce a lasting change in cortical excitability that is not associated directly with induced electric currents.
Static magnetic fields have been suggested to act primarily at the synapse and it has been proposed that these fields alter the function of membrane ion channels (Rosen & Lubowsky, 1987; Rosen, 2003; Coots et al. 2004). Moreover, the application of static magnetic fields to different animal preparations seems to have an effect that lasts longer than the time of stimulation (Rosen & Lubowsky, 1987). Future studies will clarify if this is a form of plasticity or just a perturbation of the normal functioning of the cortex that takes time to recover.
We also found that the polarity of the magnetic field is not an important factor for this kind of neuromodulation. In fact, when the static magnetic field is strong enough (the Big Magnet in our experiments) it does not matter if the north or south pole is pointing towards the motor cortex. This is important for our understanding of the underlying mechanisms of the after-effects induced by tSMS. If tSMS modulates the cortical excitability by acting on magnetic polar structures or molecules, then we would expect to see different effects as a function of the polarity of the magnetic field (i.e. north–south); however, this is not the case. As both polarities produced equivalent effects, the structures or molecules modulated by tSMS are likely to be influenced in the same way by the opposite directions of the magnetic field. Ferromagnetic (attracted by the two poles) or diamagnetic (repulsed by the two poles) structures or molecules can be the target. Nonetheless, our data suggest that the duration of tSMS is an important factor to obtain an aftereffect.
Na+, K+, Ca2+ and Mg2+ are abundant in the extracellular and intracellular spaces and they are all diamagnetic. All these ions will be displaced by tSMS, but when the stimulation is removed they are likely to return immediately to the previous state. So it is improbable that the after-effects of tSMS can be explained only by ion movements. Within the cortex, however, different Na+, K+ and Ca2+ channels and Mg2+ regulated channels are implicated in the regulation of neuron excitability and synaptic plasticity. The interference with the conductance characteristics of these ions might produce short and long-term effects. For example, modulation of the Na+ and K+ persistent channels at the dendrite level of neurons could explain the change in cortical excitability that we observed in the present experiments. Indeed, it has been previously shown that Ca2+ and Na+ channels are transiently slowed during exposure to static magnetic fields (Rosen, 2003). The mechanism proposed to explain this phenomenon is based on the diamagnetic anisotropic properties of membrane phospholipids (Rosen, 2003). Reorientation of these molecules during static magnetic field exposure results in deformation of embedded ion channels, thereby altering their activation kinetics (Rosen, 2003). Interestingly, a similar mechanism of action has been proposed for tDCS: the constant gradient of voltage – produced by tDCS – may induce ionic shifts and transmembrane protein changes that finally result in changes of cortical excitability (Zaghi et al. 2010). Interestingly, application of cathodal tDCS over the motor cortex has similar effects to tSMS (reduced MEP amplitudes with unchanged motor thresholds) (Nietsche & Paulus, 2000; Lang et al. 2011). One might speculate that these two neuromodulation techniques share some common mechanism of action that is based on the alteration of ion channel kinetics (due to the change of membrane properties) induced by an artificially induced gradient of some ions.
Certainly, tSMS (and tDCS) may affect the intrinsic properties of neurons, but – as a speculative hypothesis – it could also affect the intrinsic properties of astrocytes. Astrocytes greatly outnumber neurons, and occupy 25–50% of brain volume, so it is conceivable that they can be modulated by tSMS (and tDCS). Recently, it has been proposed that astrocytes may play a role in long-term changes in synaptic efficacy and that calcium elevation in astrocytes enhances the probability of neurotransmitter (glutamate) release (Perea & Araque, 2007). Astrocytes are strongly coupled to one another by gap junctions. Such communication is believed to mediate the coordinated action of adjacent individual cells, and equalize their intracellular ion concentrations. In the case of tDCS, application of direct current could polarize the strongly interconnected astrocyte network. If so, positive charges (calcium) will be displaced (intracellularly) towards the anode (Islam et al. 1995; Stagg & Nitsche, 2011) while negative charges will be moved towards the cathode. Similarly to cathodal tDCS, application of tSMS could ‘push away’ the diamagnetic Ca2+ ions throughout the strongly interconnected astrocyte network. The gradient of the static magnetic field will direct the flow of Ca2+ ions far away from the site of tSMS application. Reduction of positive ions, including calcium, in astrocytes may reduce the probability of glutamate release, ultimately reducing cortical excitability.
A final possible candidate to understand the mechanisms of tSMS is the ferromagnetic mineral magnetite, which is known to be present in the human brain (Kirschvink et al. 1992). Nonetheless, to understand the exact mechanism by which tSMS could modulate the activity of motor cortex in our experiments will require further investigation.
Magnetic resonance imaging (MRI) is widely used worldwide for clinical and research purpose. Surprisingly, little is known about the possible effects of MRI static magnetic fields on cortical excitability. In a very recent study, Schlamann et al. (2010) did not find any effect of MRI static magnetic fields on cortical excitability, as measured by resting motor threshold to TMS stimuli. However, the resting motor threshold has been proposed to represent a marker of membrane excitability in the pyramidal output cells, because it is relatively insensitive to pharmacological manipulations involving neurotransmission (Ziemann et al. 1996). For this reason, if the main effects of static magnetic fields are at the synaptic level, the study of resting motor thresholds may be inadequate.
In fact, in our experiments the resting motor thresholds were also not affected by tSMS. Our study shows that tSMS oriented in one direction perpendicular to the motor strip alters cortical excitability, as measured by MEP amplitude. This suggests that the changes in cortical excitability induced by tSMS may not depend on the membrane excitability of pyramidal neurons but on alterations at the synaptic level (Rosen, 2003). Furthermore, one main difference between the tSMS and the MRI magnetic field is that the magnet used in the present study induces a strong magnetic gradient over a relatively small volume of brain tissue underlying the magnet. By contrast, in MRI the brain is immersed in a static uniform magnetic field. If diamagnetic ion movements are an important factor for the tSMS effects, these gradient difference might explain why our procedure was relatively effective in inducing excitability changes. It is worth pointing out, however, that few studies have systematically assessed changes in cortical or corticospinal excitability during exposure to homogeneous static magnetic fields experienced during MR imaging (Schlamann et al. 2010) in a similar well-controlled way as done in the present study. However, the clear differences in the geometry of the magnetic fields in MRI and in the current study prevent direct comparison of the two procedures. Finally, we note that our effects largely occurred immediately after the application of the magnetic field. This makes direct comparison with exposure to MRI technically difficult, because measures of cortical excitability as done in our present study are technically difficult to conduct (Bestmann et al. 2006). Whether or not the homogeneous static magnetic fields over large brain regions used in MRI alter cortical excitability remains an open issue, which deserves further investigation.
In conclusion, application of transcranial static magnetic field stimulation (tSMS) to the motor cortex decreases cortical excitability. tSMS using small magnets may thus be a promising tool to modulate cerebral excitability in a non-invasive, painless and reversible way. Moreover, our results suggest that a deeper knowledge of the mechanisms of how a static magnetic field interacts with cortical excitability is required.
Acknowledgments
We would like to thank Dr Sven Bestmann and Dr Wolfgang Pita-Thomas for useful comments and suggestions and Mrs Arianna Pizzichino for useful technical help. This work was partially supported by the ‘FISCAM’, Gobierno de Castilla La Mancha (Spain).
Glossary
Abbreviations
- AMT
active motor threshold
- MEP
motor evoked potential
- MRI
magnetic resonance imaging
- PAS
paired associative stimulation
- RMT
resting motor threshold
- TBS
theta-burst stimulation
- TES
transcranial electric stimulation
- TMS
transcranial magnetic stimulation
- tSMS
transcranial static magnetic field stimulation
Author contributions
A.O., G.F. & G.F. designed the study. L.M.M., P.A. & I.P. performed the experiments. A.O., L.M.M. & G.F. analyzed the data. A.O. wrote the paper. A.O. & J.A. supervised the study. All authors contributed to the scientific discussion, edited the paper and approved the final version for publication. The work was done in the Hospital Nacional de Parapléjicos, Toledo (Spain).
References
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2006;19:1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Coots A, Shi R, Rosen A. Effects of a 0.5-T static magnetic field on conduction in guinea pig spinal cord. J Neurol Sci. 2004;222:55–57. doi: 10.1016/j.jns.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol. 1998;109:397–401. doi: 10.1016/s0924-980x(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Islam N, Aftabuddin M, Moriwaki A, Hattori Y, Hori Y. Increase in the calcium level following anodal polarization in the rat brain. Brain Res. 1995;684:206–208. doi: 10.1016/0006-8993(95)00434-r. [DOI] [PubMed] [Google Scholar]
- Kirschvink JL, Kobayashi-Kirschvink A, Woodford BJ. Magnetite biomineralization in the human brain. Proc Natl Acad Sci U S A. 1992;89:7683–7687. doi: 10.1073/pnas.89.16.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N, Nitsche MA, Dileone M, Mazzone P, De Andrés-Arés J, Diaz-Jara L, Paulus W, Di Lazzaro V, Oliviero A. Transcranial direct current stimulation effects on I-wave activity in humans. J Neurophysiol. 2011;105:2802–2810. doi: 10.1152/jn.00617.2010. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: State of the art 2008. Brain Stim. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton HD, Amassian VE. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17:345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- Rosen AD. Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem Biophys. 2003;39:163–173. doi: 10.1385/CBB:39:2:163. [DOI] [PubMed] [Google Scholar]
- Rosen AD, Lubowsky J. Magnetic field influence on central nervous system function. Exp Neurol. 1987;95:679–687. doi: 10.1016/0014-4886(87)90308-6. [DOI] [PubMed] [Google Scholar]
- Schlamann M, Yoon MS, Maderwald S, Pietrzyk T, Bitz AK, Gerwig M, Forsting M, Ladd SC, Ladd ME, Kastrup O. Short term effects of magnetic resonance imaging on excitability of the motor cortex at 1.5T and 7T. Acad Radiol. 2010;17:277–281. doi: 10.1016/j.acra.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Zaghi S, Acar M, Hultgren B, Boggio PS, Fregni F. Noninvasive brain stimulation with low-intensity electrical currents: putative mechanisms of action for direct and alternating current stimulation. Neuroscientist. 2010;16:285–307. doi: 10.1177/1073858409336227. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]


