Abstract
Background
Brassica rapa is an economically important crop and a model plant for studies concerning polyploidization and the evolution of extreme morphology. The multinational B. rapa Genome Sequencing Project (BrGSP) was launched in 2003. In 2008, next generation sequencing technology was used to sequence the B. rapa genome. Several maps concerning B. rapa pseudochromosome assembly have been published but their coverage of the genome is incomplete, anchoring approximately 73.6% of the scaffolds on to chromosomes. Therefore, a new genetic map to aid pseudochromosome assembly is required.
Results
This study concerns the construction of a reference genetic linkage map for Brassica rapa, forming the backbone for anchoring sequence scaffolds of the B. rapa genome resulting from recent sequencing efforts. One hundred and nineteen doubled haploid (DH) lines derived from microspore cultures of an F1 cross between a Chinese cabbage (B. rapa ssp. pekinensis) DH line (Z16) and a rapid cycling inbred line (L144) were used to construct the linkage map. PCR-based insertion/deletion (InDel) markers were developed by re-sequencing the two parental lines. The map comprises a total of 507 markers including 415 InDels and 92 SSRs. Alignment and orientation using SSR markers in common with existing B. rapa linkage maps allowed ten linkage groups to be identified, designated A01-A10. The total length of the linkage map was 1234.2 cM, with an average distance of 2.43 cM between adjacent marker loci. The lengths of linkage groups ranged from 71.5 cM to 188.5 cM for A08 and A09, respectively. Using the developed linkage map, 152 scaffolds were anchored on to the chromosomes, encompassing more than 82.9% of the B. rapa genome. Taken together with the previously available linkage maps, 183 scaffolds were anchored on to the chromosomes and the total coverage of the genome was 88.9%.
Conclusions
The development of this linkage map is vital for the integration of genome sequences and genetic information, and provides a useful resource for the international Brassica research community.
Background
The genus Brassica is one of the core genera within the tribe Brassicea. It comprises a large number of crops with a wide spectrum of morphological variation that can be cultivated under a variety of agro-climatic conditions. Brassicas provide vegetable oil, fresh and preserved vegetables, fodder and condiments, as well as being important sources of dietary fibre, vitamin C and nutritionally beneficial factors including anti-cancer compounds [1]. There are six representative species in the Brassica genus including three diploid species B. rapa (AA, 2n = 20), B. nigra (BB, 2n = 16) and B. oleracea (CC, 2n = 18), and three amphidiploids B. juncea (AABB, 2n = 36), B. napus (AACC, 2n = 38) and B. carinata (BBCC, 2n = 34). The genetic relationships among these Brassica species are well defined in U's triangle [2]. One of the diploid species, B. rapa, comprises a variety of morphologically diverse cultivated types including Chinese cabbage, misuna, aburana, flowering cabbage, turnip, turnip rape, yellow sarson, tatsoi and komatsuna, and these provide leaf heads, leaves, flowering stems, turnips and seeds, the productive organs for economical consumption [3,4]. Furthermore, B. rapa is an excellent model for studying polyploidy genome evolution owing to its paleohexaploid ancestry and its close evolutionary relationships with Arabidopsis thaliana [5].
The multi-national B. rapa Genome Sequencing Project (BrGSP) was launched in 2003 owing to the economical and biological importance of B. rapa, and the A3 chromosome was sequenced using traditional Sanger technology [5]. In 2008, rapid next generation sequencing technology was employed for B. rapa genome sequencing and a high density genetic map based on sequence-tagged markers is necessary to anchor the assembled scaffolds to chromosomes. Several maps concerning B. rapa have been published to be used as reference genetic maps for pseudochromosome assembly (http://www.brassica-rapa.org) [6-8] despite their coverage only allowing in total 73.6% of the scaffolds being anchored on to chromosomes [The Brassica rapa Genome Sequencing Project Consortium: The genome of the mesohexaploid crop species Brassica rapa, submitted].
Genetic mapping is important for understanding the origin of and relationships among the genomes of Brassica species. Genetic linkage maps can also provide improved insight into genome organization and evolution through comparative mapping, and serve as the basis for genetic studies concerning various agronomic traits through the localization of major genes and quantitative trait loci (QTLs). Furthermore, they can aid breeding programs with the development of marker assisted selection (MAS) [9]. More than 20 genetic linkage maps have been constructed for B. rapa using a range of marker types including Restriction Fragment Length Polymorphisms (RFLPs), random amplified polymorphic DNA (RAPD), amplified fragment length polymorphisms (AFLPs), sequence-related amplified polymorphisms (SRAPs) and simple sequence repeats (SSRs) [6-8,10-20]. However, there are limited published data concerning sequence-tagged PCR-markers, predominantly SSRs, mapped in B. rapa [6-8,15,19], particularly markers that could provide anchors for the B. rapa genome that are transferable to other mapping populations.
Recent developments in sequencing technology have simplified and accelerated the discovery of sequence variants, enabling development of sequence-based markers including single nucleotide polymorphisms (SNPs) and insertion/deletion polymorphisms (InDels) [21]. InDels and SNPs are the markers of choice for high-resolution genetic mapping and association studies owing to their abundance and distribution throughout the genome [22,23]. For example, a study investigating genetic variation on human chromosome 22 suggested that InDels represent 18% of the polymorphisms on this chromosome [24]. Studies concerning genetic variation in A. thaliana have demonstrated that InDels represent 34% of all genetic polymorphisms [25]. Furthermore, InDels can contribute directly to a phenotype [26], or can associate with a phenotype as a result of linkage disequilibrium [27]. By re-sequencing 1,398 sequence-tagged sites (STSs) in eight B. rapa genotypes, Park et al. identified and characterized 6,753 InDels in the gene space of the B. rapa genome [28]. InDel polymorphisms are the second most frequent type of polymorphism in the genome, and can be genotyped using simple procedures including the analysis of size polymorphisms of polymerase chain reaction (PCR) products on agarose gels [29,30]. Another advantage of InDel markers is the improbability of two InDel mutations being exactly the same length and at the same genomic position. Therefore, shared InDels represent identity-by-descent [31]. Very few InDel markers have been used to construct genetic linkage maps of B. rapa. With the recent completion of the sequencing of the B. rapa genome [The Brassica rapa Genome Sequencing Project Consortium: The genome of the mesohexaploid crop species Brassica rapa, submitted], the development of whole genome-wide InDel markers based on re-sequencing has become feasible, and this will be a useful resource for the international research community.
In this study, InDel and SSR markers, both of which are sequence-tagged PCR markers, were used to construct a high resolution genetic map of B. rapa. The map was used as a reference linkage map to anchor and orient sequence scaffolds for B. rapa genome assembly.
Results
Generation of markers and polymorphism survey
The process of genotyping InDel polymorphisms was optimised. A range of annealing temperatures from 55°C to 63°C for 16 primer pairs were tested, and the results demonstrated that annealing at 57°C produced favourable results for all primer pairs. Therefore, amplification of InDels was accomplished using a single, uniform set of conditions with a denaturing temperature of 57°C. The size of amplified DNA fragments was within the range of 80-200 bp and 4-10 bp insertion/deletions were used as markers. Therefore, the PCR products could be separated using PAGE (polyacrylamide gel electrophoresis).
A population named RCZ16_DH with 119 doubled haploid (DH) lines derived from an F1 cross between a Chinese cabbage DH line (Z16) and a rapid cycling inbred line (L144) was used for genetic map construction. To construct the RCZ16_DH map, 520 unique PCR-based InDel markers for 'Z16' and 'L144' were designed. Of these, 427 (82.1%) yielded single PCR fragments and demonstrated polymorphism, eight (1.6%) did not amplify any products and 85 (16.3%) had no polymorphism. Among the 427 polymorphic InDels, 411 yielded single PCR products with different lengths for the two parental lines, while 16 had amplicons in one of the parents only. These 16 primer pairs were discarded to prevent false negatives when carrying out PCR. An additional 333 InDel markers were screened, and 163 were designed on the basis of the InDel variations between 'Chiifu-401-42' and 'Kenshin'; 170 pairs were designed on the basis of variations between 'Chiifu-401-42' and 'L144'. In total, 415 polymorphic InDel primer pairs including 323 from 'Z16' and 'L144', 40 from 'Chiifu-401-42' and 'Kenshin', and 52 from 'Chiifu-401-42' and 'L144', were scored and used to generate the RCZ16_DH genetic linkage map using 119 DH lines.
For assessment of SSRs, 1309 SSRs from a range of sources were tested [6,7,16,32-37]; 130 presented with polymorphic banding patterns between the parental lines and 92 easily scored SSRs were screened for the 119 DH lines. Of these, three SSR marker assays (BoE347, BoE974 and KBRH139B23) detected more than one segregating locus. The information concerning all mapped InDel and SSR loci is presented in Additional File 1.
Construction of the RCZ16_DH linkage map
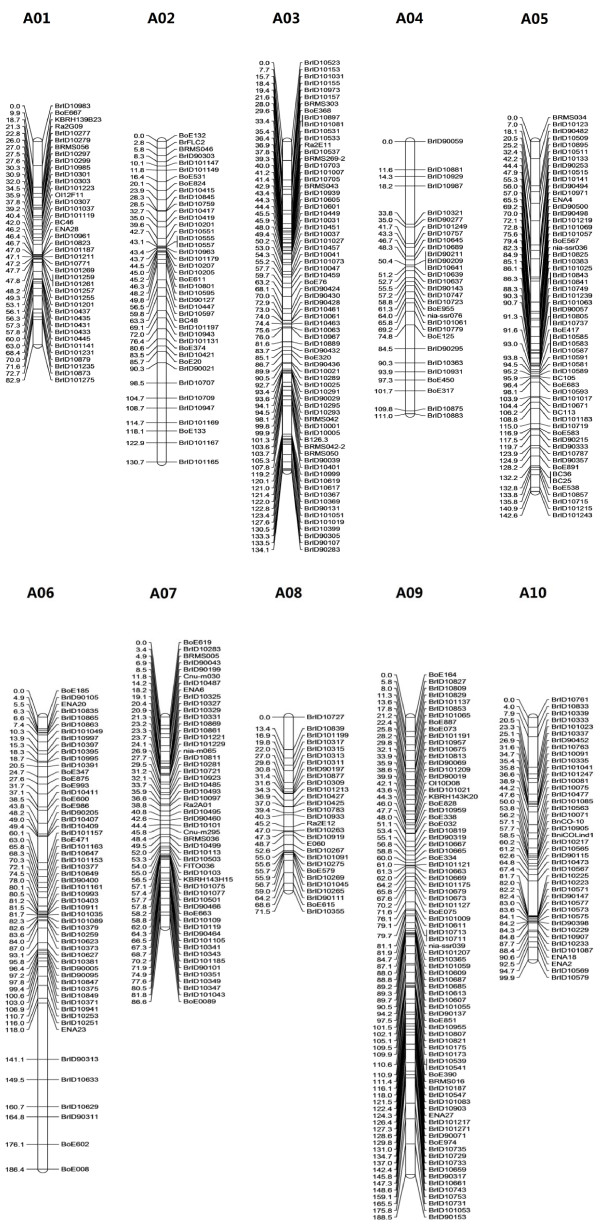
A total of 507 markers including 415 InDel markers and 92 SSR markers were assigned to 10 linkage groups (Figure 1) and designated as A01-A10, corresponding to the previously published linkage maps [6,8,15,19,20,38]. Each of the ten linkage groups contained at least two previously published SSR markers that provided anchors to previously published maps, with the exception of chromosome A04 (Additional File 1). Anchoring A04 by the two InDels (BrID90277 and BrID10363) located at the scaffolds on which the two BACs (KBrB068A13 and KBrB033O04) were positioned was confirmed using three SSR markers, locating at A04 in the maps of VCS_DH, JWF3P and CKDH (Additional File 1). The linkage map covered a genetic distance of 1234.2 cM, with an average distance of 2.43 cM between ordered adjacent markers (Table 1). The largest linkage group contained the maximum number of markers (81) for A09 and spanned 188.5 cM, while the smallest contained the minimum number of markers (29) for A08, with a length of 71.5 cM. The distribution of InDels along the linkage groups ranged from 25 on A04 and A08 to 65 on A09, and the distribution of SSRs ranged from 3 on A10 to 16 on A09. The physical length of the B. rapa genome is approximately 283.8 Mbp [The Brassica rapa Genome Sequencing Project Consortium: The genome of the mesohexaploid crop species Brassica rapa, submitted]. The map defined herein represents average genetic and physical intervals of 2.43 cM and 559.8 Kbp per marker, respectively. Therefore, it is currently the most saturated linkage map for B. rapa. In the final linkage map, 90% of the covered genome had a marker within 5 cM. However, there were still two gaps (>15 cM) [7] of 23 cM and 16 cM on A06 and A04, respectively.
Figure 1.
Linkage map of B. rapa L. ssp. pekinensis. Recombination distances are indicated on the left hand side of each linkage group in centimorgans (cM), and the locus names are depicted on the right side of each linkage group. The map demonstrates the distribution of 507 loci along ten linkage groups (A01-A10).
Table 1.
Summary of the genetic linkage map of B. rapa constructed using sequence-based markers
| Linkage group |
Length (cM) | Markers (no.) | Density (cM/marker) |
Intervals a (no.) | Gaps b (no.) | Ave. interval (cM) |
|---|---|---|---|---|---|---|
| A01 | 82.9 | 41 | 2.02 | 28 | 0 | 2.96 |
| A02 | 130.7 | 42 | 3.11 | 34 | 0 | 3.84 |
| A03 | 134.1 | 72 | 1.86 | 41 | 0 | 3.27 |
| A04 | 111.0 | 30 | 3.70 | 27 | 1 | 4.11 |
| A05 | 142.6 | 59 | 2.42 | 35 | 0 | 4.07 |
| A06 | 186.4 | 57 | 3.27 | 39 | 1 | 4.78 |
| A07 | 86.6 | 54 | 1.60 | 36 | 0 | 2.41 |
| A08 | 71.5 | 29 | 2.46 | 20 | 0 | 3.57 |
| A09 | 188.5 | 81 | 2.33 | 59 | 0 | 3.20 |
| A10 | 99.9 | 42 | 2.38 | 26 | 0 | 3.84 |
| Total | 1234.2 | 507 | 2.43 | 345 | 2 | 3.61 |
a Adjacent markers > 1 cM; b Interval distance between adjacent markers ≥15 cM [7].
Alignment of the linkage groups to B. rapa pseudochromosomes
The high-resolution RCZ16_DH genetic linkage map with 507 sequence-based markers was successfully used to anchor and orientate scaffolds for the genome assembly of B. rapa together with the three publicly available B. rapa genetic maps, VCS_DH, JWF3P (http://www.brassica-rapa.org) and CKDH [6-8]. The markers of the RCZ16_DH genetic linkage map were aligned to the B. rapa genome sequence using their primer sequences; 66 SSR markers and the 415 InDel markers were mapped to a total of 152 scaffolds. Among these 481 positioned markers, three SSR markers (BoE347, BoE974 and KBRH139B23) were detected on more than one segregating locus. Therefore, only 478 unique markers were used for anchoring 152 scaffolds of B. rapa, covering 82.9% of the assembled genome. The uniquely aligned markers used to anchor scaffolds ranged from 76 for A09 to 27 for A04. The number of scaffolds anchored on to the chromosomes by these markers ranged from 6 for A10 to 32 for A09. In 417 cases, more than one marker was located on a single scaffold, allowing 91 scaffolds to be oriented throughout the 10 chromosomes. The details of the markers used to anchor the scaffolds are presented in Table 2.
Table 2.
Summary of sequence-based markers used to anchor and orient the scaffolds to linkage groups of B. rapa
| Linkage group |
Markers (no.) | InDels (no.) | SSRs (no.) | Anchored InDels (no.) |
Anchored SSRs (no.) |
Anchored Scaff. no. |
Anchored Scaff.lg (bp) a |
Anchored Scaff.cv (%) b |
|---|---|---|---|---|---|---|---|---|
| A01 | 41 | 34 | 7 | 34 | 5 | 21 | 20867750 | 7.35 |
| A02 | 42 | 32 | 10 | 32 | 8 | 18 | 27594208 | 9.72 |
| A03 | 72 | 61 | 11 | 61 | 9 | 9 | 31313061 | 11.03 |
| A04 | 30 | 25 | 5 | 25 | 2 | 14 | 16383005 | 5.77 |
| A05 | 59 | 46 | 13 | 46 | 8 | 17 | 21627037 | 7.62 |
| A06 | 57 | 46 | 11 | 46 | 8 | 14 | 25300054 | 8.91 |
| A07 | 54 | 42 | 12 | 42 | 9 | 10 | 21622174 | 7.62 |
| A08 | 29 | 25 | 4 | 25 | 3 | 11 | 16765946 | 5.91 |
| A09 | 81 | 65 | 16 | 65 | 11 | 32 | 36142156 | 12.73 |
| A10 | 42 | 39 | 3 | 39 | 3 | 6 | 17594535 | 6.20 |
| Total | 507 | 415 | 92 | 415 | 66 | 152 | 235209926 | 82.86 |
a anchored scaffold length; b anchored scaffold coverage.
To compare the RCZ16_DH map to the three publicly available genetic linkage maps, VCS_DH (354 markers), JWF3P (498 markers) and CK_DH (719 markers), the BACs where the markers were located were used as the genetic loci. These BACs were aligned to scaffold sequences and regarded as common genetic loci if they were within a range of 100 Kbp in distance to the position of InDels or SSRs on the RCZ16_DH map. Two hundred (39.4%) of the 507 markers on the RCZ16_DH map located common loci on at least one of the three previously published maps. Using VCS_DH, JWF3P and CK_DH maps, there were 84 (171 Mb), 97 (185 Mb) and 91 (175 Mb) scaffolds anchored to the corresponding chromosomes, respectively. Combining these three maps, the total coverage was 73.6% of the B. rapa genome. However, when taken together with the RCZ16_DH map, the anchored scaffold number and the total coverage of the B. rapa genome increased to 183 and 88.9%, respectively (Table 3).
Table 3.
Information relating to scaffold anchoring using four linkage maps of B. rapa
| RCZ16_DH | VCS_DH | JWF3P | CK_DH | Total | |
|---|---|---|---|---|---|
| Marker No. | 507 | 354 | 498 | 719 | 2078 |
| Sequenced-based marker No. | 507 | 354 | 498 | 428 (191)a | 1787(1550)a |
| Anchored scaffold No. | 152 | 84 | 97 | 91 | 183 |
| Oriented scaffold No. | 91 | 55 | 62 | 54 | 114 |
| Physical distance (Mb) | 235 | 171 | 185 | 175 | 252 |
a The number of markers with genetic distance publicly available.
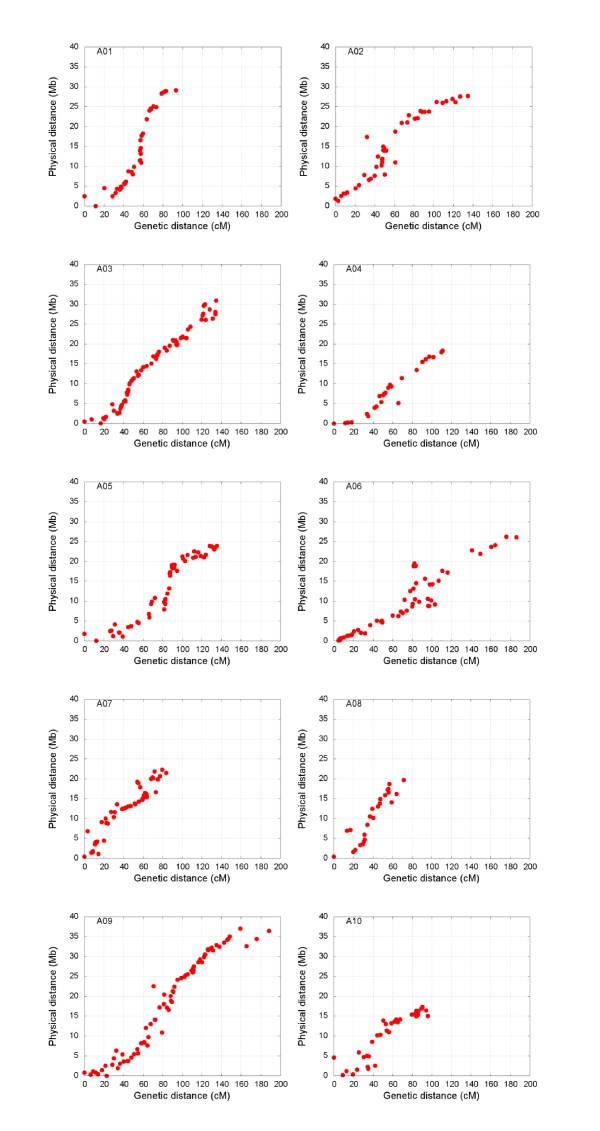
Alignment of the RCZ16_DH linkage map with the constructed pseudochromosomes verified the accuracy of the scaffold order and orientation (Figure 2). The InDel markers were developed from re-sequencing data, and were selected to develop InDel markers from scaffold regions where there had been no marker in previous linkage maps. As a result, the map enhanced the evaluation of the quality of sequence assembly. The majority of the markers (93%) were collinear with the sequence assembly. In several assembly iterations, scaffold misplacement was visible as discontinuity or negative slopes.
Figure 2.
RCZ16_DH genetic map versus physical distance map of the ten B. rapa pseudochromosomes. The pseudochromosomes were constructed using markers from four genetic maps namely RCZ16_DH, VCS_DH, JWF3P (http://www.brassica-rapa.org) [6] and CK_DH [7,8].
Discussion
This study concerns the comprehensive linkage analysis of B. rapa. The map spans 1234.2 cM and is divided into ten linkage groups corresponding to the number of B. rapa chromosomes, with an average distance of 2.43 cM between adjacent markers. A conspicuous characteristic of the present map is that 415 markers, accounting for 81.7% of the total mapped markers, are novel InDel markers, which increases the number of sequence-based markers for B. rapa.
One of the main purposes of this linkage map is to support the B. rapa genome sequencing project to anchor and orient scaffolds onto the chromosomes. Using publically available genetic linkage maps for B. rapa including VCS_DH (354 markers), JWF3P (498 markers) (http://www.brassica-rapa.org) [6] and CK_DH (313 markers and 719 markers) [7,8], 84 (171 Mb), 97 (185 Mb) and 91 (175 Mb) scaffolds were anchored to chromosomes, respectively. However, selecting markers that were evenly distributed along the genome and complementary to the previously reported maps, using the RCZ16_DH map, 152 (235 Mb) scaffolds were anchored on to the chromosomes. The RCZ16_DH map alone covers more than 82.9% of the B. rapa genome, indicating the potential of this sequence-based linkage map for linkage and QTL analysis.
In B. rapa, polymorphic DNA loci are relatively frequent. Park et al. demonstrated that the frequency of SNPs and InDels were 15.3 SNPs/kb and 4.83 InDels/kb by resequencing 1,398 STSs based on the 557 BAC sequences of B. rapa ssp. pekinensis cv Chiifu-401-42 [28]. Among the 28,222 sequence variants of B. rapa, approximately 24% were InDels. This high frequency of InDels has also been reported for other species including maize [39,40], sugar beet [41], barley [42] and Arabidopsis [25]. In the present study, PCR-based InDel markers were developed using 80 to 200 bp PCR products, and the insertion/deletion size varied between 4 to 10 bp. The InDel polymorphisms were genotyped using a simple procedure that analyzed size polymorphisms of PCR products.
The diploid Brassica genomes contain large replicated blocks of collinear segments within and between linkage groups. These are thought to have derived from a polyploid ancestor, although the exact mechanism by which this occurred is debatable [43]. In the linkage map generated herein, only three SSR markers detected multiple loci and no evidence of conserved blocks of synteny can be deduced from this. This low level of detection of the replication within the genome could be due to the marker types used to construct the map. The InDel markers were developed directly from scaffold sequences by selecting unique InDels, and ambiguous markers were excluded from the marker data set. Furthermore, SSR markers are usually located in non-coding sequences, which are less well conserved between replicated blocks than coding regions.
A total of 92 SSR markers are present in the map developed in this study, and 66 of these could be aligned to the scaffold sequences using stringent criterion of 100% match of the primer sequences. Among the 26 unmapped markers, 20 were designed on the basis of EST sequences of B. oleracea (BoE set and Ol set). For these markers, mismatches could exist within the primer sequences preventing them from being mapped to the scaffold sequence of B. rapa. Five of the six unmapped SSRs derived from B. rapa sequences were mapped to scaffolds with only one side primer, and this could be due to the gaps in the sequence assembly.
Alignment of the RCZ16_DH map to pseudochromosomes indicated high collinearity between the genetic and physical distance (Figure 2). However, the alignment result demonstrated that there were outliers distributed on the linkage groups A02, A04, A06, A08 and A10. This could be due to scaffolds being too small or the genomic region having too little recombination to allow precise placements or orientations, as a relatively small mapping population was used in the present study. The small population size could also lead to the relatively high statistical errors when there were missing marker data for some DH lines. The other possible reason for the inconsistency of genetic and physical distances could be assembling errors as the InDels were designed on the basis of the assembled scaffold sequences.
Conclusions
This study describes the use of sequence-based and highly polymorphic InDel markers to construct a highly resolute reference genetic map of B. rapa. The result is an improved resource for fine mapping of quantitative trait loci, identifying candidate genes and map-based gene isolation.
Methods
RCZ16_DH mapping population
The RCZ16_DH mapping population of 119 doubled haploid (DH) lines was derived from a cross between a DH Chinese cabbage (Brassica rapa ssp. pekinensis) line (Z16) and an inbred rapid cycling line (L144). A wide range of variation exits in terms of morphological traits among the individual lines. DNA from the parents and the DH plants was isolated from mature leaves as described by Wang et al. [44].
Molecular marker analysis
InDel markers
The L144 and Z16 lines were re-sequenced using Illumina GAII with depths of 40 X and 2.5 X genome coverage, respectively. In addition, a Chinese cabbage line, Kenshin, was re-sequenced at 0.1 X using 454 sequencing technology (provided by Dr. David Edwards). InDels were detected by the alignment of reads to reference sequences [31]. The 4-10 bp insertion/deletions were used to develop markers. Primer 3 online software (Whitehead Institute, Cambridge, MA) was used to design primers for amplification of InDels. The criteria used for designing the primers included the following: (1) the amplified DNA fragments were within the range 80-200 bp; (2) Tm ranged from 55 to 63°C, and the difference in Tms within a primer pair was less than 3°C; (3) the GC content was greater than 35%. Each PCR was performed in a 15 μl reaction volume containing 0.4 units of Taq DNA polymerase with 1 × PCR buffer (Tiangen, Beijing, China), 0.5 μM of each primer, 300 μM of each dNTP, 1.5-2.0 mM MgCl2 and approximately 30 ng genome DNA as templates. Thermocycling began at 94°C for 7 min, followed by 35 cycles of 94°C for 40 s, 57°C for 40 s and 72°C for 1 min, and a final extension at 72°C for 10 min. PCR products were separated on 8% polyacrylamide gels and visualized using silver staining.
SSR markers
A total of 1309 SSR markers [6,7,15,32-37] were used to screen for polymorphisms between the two parental lines. The PCR reaction was same as that used for InDels. Thermocycling began at 95°C for 10 min, followed by 35 cycles of 94°C for 1 min, 55°C for 1 min, 72°C for 1.5 min and a final extension at 72°C for 10 min before holding at 12°C. PCR products were separated on 8% polyacrylamide gels and visualized using silver staining.
Linkage map construction
Linkage analysis and genetic map construction were performed using JoinMap 4.0 software (http://www.kyazma.nl) [45]. Initial linkage groups were established on the basis of a LOD value ≥ 7 and the Haldane's [46,47] mapping function was used to convert recombination data into map distances.
Alignment of linkage groups to the physical map
To reconile the linkage groups with the ten B. rapa chromosomes, the genetic map was aligned to the pseudochromosomes [The Brassica rapa Genome Sequencing Project Consortium: The genome of the mesohexaploid crop species Brassica rapa, submitted] on the basis of the primer sequence of the markers. The InDel markers were developed directly from scaffold sequences, and the SSRs were considered anchored if the sequence of both primers matched the scaffold sequence perfectly.
Authors' contributions
YW generated the InDel markers, analyzed marker data and drafted the manuscript. SS carried out linkage analysis and anchoring of scaffolds to the linkage map and compared the genetic map with a physical map. BL analyzed the re-sequencing data and extracted all InDel positions and designed the InDel primers. HW, JD and YL participated in the InDel marker survey. WQ generated all SSR markers. FC participated in comparative analysis of the genetic map with the physical map. WX designed the study and participated in coordination of the study. WJ participated in linkage analysis and coordinated the study. All authors read and approved the final manuscript.
Supplementary Material
Details of the 507 sequence-based markers on the RCZ16_DH map, and information relating to anchored scaffolds using the RCZ16_DH map and the other three publicly available genetic linkage maps.
Contributor Information
Yan Wang, Email: wycaas@yahoo.cn.
Silong Sun, Email: mycaas@163.com.
Bo Liu, Email: lb_bobo@yahoo.cn.
Hui Wang, Email: fromstick@163.com.
Jie Deng, Email: 115425193@163.com.
Yongcui Liao, Email: liao.yong.cui@163.com.
Qian Wang, Email: wqian198203@163.com.
Feng Cheng, Email: chengfeng@caas.net.cn.
Xiaowu Wang, Email: wangxw@mail.caas.net.cn.
Jian Wu, Email: wujian@caas.net.cn.
Acknowledgements
We are grateful to Dr. David Edwards from the University of Queensland for kindly providing re-sequencing data for Kenshin. The work was supported by grants from the National Basic Research Program of China (2007CB108803), Core Research Budget of the Non-profit Governmental Research Institution (ICS, CAAS,201011), and European Community financial participation under the Seventh Framework Programme for Research, Technological Development and Demonstration Activities, for the Integrated Project NUE-CROPS FP7-CP-IP 222645. The authors thank Dr. John Snyder and BioMedES Ltd for assistance on manuscript editing. The research was conducted at the Key Lab of Vegetable Genetics and Physiology of Ministry of Agriculture P.R. China and Sino-Dutch Joint Lab of Horticultural Genomics Technology in Beijing.
References
- Fahey JW, Talalay P. In: Phytochemicals and Health. Gustin DL, Flores HE, editor. Rockville: American Society of Plant Physiologists; 1995. The role of crucifers in cancer chemoprotection; pp. 87–93. [Google Scholar]
- U N. Genome-analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Japan J Bot. 1935;7:389–452. [Google Scholar]
- Gomez-Campo C, Prakash S. In: Biology of Brassica Coenospecies. Gomez-Campo C, editor. Elsevier Amsterdam; 1999. Origin and domestication; pp. 33–58. [Google Scholar]
- Li F, Kitashiba H, Inaba K, Nishio T. A Brassica rapa linkage map of EST-based SNP markers for identification of candidate genes controlling flowering time and leaf morphological traits. DNA Res. 2009;16:311–323. doi: 10.1093/dnares/dsp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun J-H, Kwon S-J, Seol Y-J, Kim JA, Jin M, Kim JS, Lim M-H, Lee S-I, Hong JK, Park T-H, Lee S-C, Kim B-J, Seo M-S, Baek S, Lee M-J, Shin JY, Hahn J-H, Hwang Y-J, Lim K-B, Park JY, Lee J, Yang T-J, Yu H-J, Choi I-Y, Choi B-S, Choi SR, Ramchiary N, Lim YP, Fraser F, Drou N, Soumpourou E, Trick M, Bancroft I, Sharpe AG, Parkin IAP, Batley J, Edwards D, Park B-S. Sequence and structure of Brassica rapa chromosome A3. Genome Biology. 2010;11:R94. doi: 10.1186/gb-2010-11-9-r94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Chung TY, King GJ, Jin M, Yang T-J, Jin Y-M, Kim H-I, Park B-S. A sequence-tagged linkage map of Brassica rapa. Genetics. 2006;174:29–39. doi: 10.1534/genetics.106.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SR, Teakle GR, Plaha P, Kim JH, Allender CJ, Beynon E, Piao ZY, Soengas P, Han TH, King GJ, Barker GC, Hand P, Lydiate DJ, Batley J, Edwards D, Koo DH, Bang JW, Park B-S, Lim YP. The reference genetic linkage map for the multinational Brassica rapa genome sequencing project. Theor Appl Genet. 2007;115:777–792. doi: 10.1007/s00122-007-0608-z. [DOI] [PubMed] [Google Scholar]
- Kim H, Choi S, Bae J, Hong C, Lee S, Hossain M, Nguyen D, Jin M, Park B, Bang J. Sequenced BAC anchored reference genetic map that reconciles the ten individual chromosomes of Brassica rapa. BMC Genomics. 2009;10:432–446. doi: 10.1186/1471-2164-10-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi IV, McMullen MD, Sanchez-Villeda H, Schroeder S, Gardiner J, Polacco M, Soderlund C, Wing R, Fang Z, Coe EH. Single nucleotide polymorphisms and insertion-deletions for genetic markers and anchoring the maize fingerprint contig physical map. Crop Sci. 2006;46:12–21. doi: 10.2135/cropsci2004.0706. [DOI] [Google Scholar]
- Song KM, Suzuki JY, Slocum MK, Williams PH, Osborn TC. A linkage map of Brassica rapa (syn. campestris) based on restriction fragment length polymorphism loci. Theor Appl Genet. 1991;82:296–304. doi: 10.1007/BF02190615. [DOI] [PubMed] [Google Scholar]
- Chyi Y-S, Hoenecke ME, Sernyk JL. A genetic linkage map of restriction fragment length polymorphism loci for Brassica rapa (syn. campestris) Genome. 1992;35:746–757. doi: 10.1139/g92-115. [DOI] [Google Scholar]
- Teutonico RA, Osborn TC. Mapping of RFLP and qualitative trait loci in Brassica rapa and comparison to the linkage maps of B. napus, B. oleracea, and Arabidopsis thaliana. Theor Appl Genet. 1994;89:885–894. doi: 10.1007/BF00224514. [DOI] [PubMed] [Google Scholar]
- Nozaki T, Kumazaki A, Koba T, Ishikawa K, Ikehashi H. Linkage analysis among loci for RAPDs, isozymes and some agronomic traits in Brassica campestris L. Euphytica. 1997;95:115–123. doi: 10.1023/A:1002981208509. [DOI] [Google Scholar]
- Kole C, Kole P, Vogelzang R, Osborn TC. Genetic linkage map of a Brassica rapa recombinant inbred population. J Hered. 1997;88:553–556. [Google Scholar]
- Suwabe K, Tsukazaki H, Iketani H, Hatakeyama K, Kondo M, Fujimura M, Nunome T, Fukuoka H, Hirai M, Matsumoto M. SSR-based comparative genomics between Brassica rapa and Arabidopsis thaliana: the genetic origin of clubroot resistance. Genetics. 2006;173:309–319. doi: 10.1534/genetics.104.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soengas P, Hand P, Vicente JG, Pole JM, Pink DAC. Identification of quantitative trait loci for resistance to Xanthomonas campestris pv. campestris in Brassica rapa. Theor Appl Genet. 2007;114:637–645. doi: 10.1007/s00122-006-0464-2. [DOI] [PubMed] [Google Scholar]
- Cheng XM, Xu JS, Xia S. Development and genetic mapping of microsatellite markers from genome survey sequences in Brassica napus. Theor Appl Genet. 2009;118:1121–1131. doi: 10.1007/s00122-009-0967-8. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Banga SS, Banga SK. A microsatellite (SSR) based linkage map of Brassica rapa. New Biotechnology. 2009;26:239–243. doi: 10.1016/j.nbt.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Wu J, Yuan YX, Zhang XW, Zhao JJ, Song XF, Li Y, Li XN, Sun RF, Koornneef M, Aarts MGM, Wang XW. Mapping QTLs for mineral accumulation and shoot dry biomass under different Zn nutritional conditions in Chinese cabbage (Brassica rapa L. ssp. pekinensis) Plant and Soil. 2008;310:25–40. doi: 10.1007/s11104-008-9625-1. [DOI] [Google Scholar]
- Lou P, Zhao J, Kim JS, Shen S, Carpio DPD, Song X, Jin M, Vreugdenhill D, Wang XW, Koornneef M, Bonnema G. Quantitative trait loci for flowering time and morphological traits in multiple populations of Brassica rapa. J Exp Bot. 2007;58:4005–4016. doi: 10.1093/jxb/erm255. [DOI] [PubMed] [Google Scholar]
- Hyten DL, Cannon SB, Song QJ. High-throughput SNP discovery through deep resequencing of a reduced representation library to anchor and orient scaffolds in the soybean whole genome sequence. BMC Genomics. 2010;11:38. doi: 10.1186/1471-2164-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalski A. Applications of single nucleotide polymorphisms in crop genetics. Curr Opin Plant Biol. 2002;5:94–100. doi: 10.1016/S1369-5266(02)00240-6. [DOI] [PubMed] [Google Scholar]
- Mills RE, Luttig CT, Larkins CE, Beauchamp A, Tsui C, Pittard WS, Devine SE. An initial map of insertion and deletion (INDEL) variation in the human genome. Genome Res. 2006;16:1182–1190. doi: 10.1101/gr.4565806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson E, Chen Y, Hunt S, Smink LJ, Hunt A, Rice K, Livingston S, Bumpstead S, Bruskiewich R, Sham P, Ganske R, Adams M, Kawasaki K, Shimizu N, Minoshima S, Roe B, Bentley B, Dunham I. A SNP resource for human chromosome 22: Extracting dense clusters of SNPs from the genomic sequence. Genome Res. 2001;11:170–178. doi: 10.1101/gr.156901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL. Arabidopsis map-based cloning in the post-genome era. Plant Physiol. 2002;129:440–450. doi: 10.1104/pp.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry J, Goodman MM, Doebley JF, Kresovich S, Nielsen D, Buckler E. Dwarf8 polymorphisms associate with variation in flowering time. Nat Genet. 2001;28:286–289. doi: 10.1038/90135. [DOI] [PubMed] [Google Scholar]
- Daly MJ, Rioux JD, Schaffner SF, Hudson TJ, Lander ES. High-resolution haplotype structure in the human genome. Nat Genet. 2001;29:229–232. doi: 10.1038/ng1001-229. [DOI] [PubMed] [Google Scholar]
- Park S, Yu H-J, Mun J-H, Lee S-C. Genome-wide discovery of DNA polymorphism in Brassica rapa. Mol Genet Genomics. 2010;283:135–145. doi: 10.1007/s00438-009-0504-0. [DOI] [PubMed] [Google Scholar]
- Kwok PY. High-throughput genotyping assay approaches. Pharcogenomics. 2000;1:95–100. doi: 10.1517/14622416.1.1.95. [DOI] [PubMed] [Google Scholar]
- Väli Ü, Brandström M, Johansson M, Ellegren H. Insertion-deletion polymorphisms (indels) as genetic markers in natural populations. BMC Genetics. 2008;9:8. doi: 10.1186/1471-2156-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlock AM, Okada N. SINE insertions: Powerful tools for molecular systematics. Bioessays. 2000;22:148–160. doi: 10.1002/(SICI)1521-1878(200002)22:2<148::AID-BIES6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Suwabe K, Iketani H, Nunome T, Kage T, Hirai M. Isolation and characterization of microsatellites in Brassica rapa L. Theor Appl Genet. 2002;104:1092–1098. doi: 10.1007/s00122-002-0875-7. [DOI] [PubMed] [Google Scholar]
- Suwabe K, Iketani H, Nunome T, Ohyama A, Hirai M, Fukuoka H. Characteristics of microsatellites in Brassica rapa genome and their potential utilisation for comparative genomics in cruciferae. Breed Res. 2004;54:85–90. [Google Scholar]
- Lowe AJ, Moule C, Trick M, Edwards KJ. Efficient large-scale development of microsatellites for marker and mapping applications in Brassica crop species. Theor Appl Genet. 2004;108:1103–1112. doi: 10.1007/s00122-003-1522-7. [DOI] [PubMed] [Google Scholar]
- Cui XM, Dong YX, Hou XL, Cheng Y, Zhang JY, Jin MF. Development and characterization of microsatellite markers in Brassica rapa ssp. chinensis and transferability among related species. Agric Sci China. 2008;7:19–31. [Google Scholar]
- Chen C, Zhuang M, Li KN, Liu YM, Yang LM, Zhang YY, Cheng F, Sun PT, Fang ZY. Development of EST-SSR markers for Brassica oleracea. Acta Horticulturae Sinica. 2010;37:221–228. [Google Scholar]
- Ge J, Xie H, Cui CS, Hong JM, Ma RC. Analysis of expressed sequence tags (ESTs) derived SSR markers in Chinese cabbage (Brassica campestris L. ssp. pekinensis) J Agri Biotech. 2005;13:423–428. [Google Scholar]
- Parkin IA, Sharpe AG, Keith DJ, Lydiate DJ. Identification of the A and C genomes of the amphidiploid Brassica napus (oilseed rape) Genome. 1995;38:1122–1131. doi: 10.1139/g95-149. [DOI] [PubMed] [Google Scholar]
- Ching A, Caldwell KS, Jung M, Dolan M, Smith OS, Tingey S, Morgante M, Rafalski AJ. SNP frequency, haplotype structure and linkage disequilibrium in elite maize inbred lines. BMC Genet. 2002;3:19. doi: 10.1186/1471-2156-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SI, Bi IV, Schroeder SG, Yamasaki M, Doebley JF, McMullen MD, Gaut BS. The effects of artificial selection on the maize genome. Science. 2005;308:1310–1314. doi: 10.1126/science.1107891. [DOI] [PubMed] [Google Scholar]
- Schneider K, Weisshaar B, Borchardt DC, Salamini F. SNP frequency and allelic haplotype structure of Beta vulgaris expressed genes. Mol Breed. 2001;8:63–74. doi: 10.1023/A:1011902916194. [DOI] [Google Scholar]
- Kanazin V, Talbert H, See D, DeCamp P, Nevo E, Blake T. Discovery and assay of single-nucleotide polymorphisms in barley (Hordeum vulgare) Plant Mol Biol. 2002;48:529–537. doi: 10.1023/A:1014859031781. [DOI] [PubMed] [Google Scholar]
- Parkin IA, Gulden SM, Sharpe AG, Lukens L, Trick M, Osborn TC, Lydiate DJ. Segmental structure of the Brassica napus genome based on comparative analysis with Arabidopsis thaliana. Genetics. 2005;171:765–781. doi: 10.1534/genetics.105.042093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XW, Lou P, Bonnema G, Yang BJ, He HJ, Zhang YG, Fang ZY. Linkage mapping of a dominant male sterility gene Ms-cd1 in Brasscia oleracea. Genome. 2005;48:848–854. doi: 10.1139/g05-044. [DOI] [PubMed] [Google Scholar]
- Van Ooijen JW. JoinMap 4.0, Software for the calculation of genetic linkage maps in experimental populations. Wageningen, Netherlands: Kyazma BV; 2006. [Google Scholar]
- Haldane JBS. The combination of linkage values, and the calculation of distances between the loci of linked factors. J Genet. 1919;8:299–309. [Google Scholar]
- Huehn M. Random variability of map distances based on Kosambi's and Haldane's mapping functions. J Appl Genet. 2010;51:27–31. doi: 10.1007/BF03195707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of the 507 sequence-based markers on the RCZ16_DH map, and information relating to anchored scaffolds using the RCZ16_DH map and the other three publicly available genetic linkage maps.