Abstract
We have performed a genome-wide association study (GWAS) of schizophrenia in a Norwegian discovery sample of 201 cases and 305 controls (TOP study) with a focused replication analysis in a larger European sample of 2663 cases and 13,780 control subjects (SGENE-plus study). Firstly, the discovery sample was genotyped with Affymetrix Genome-Wide Human SNP Array 6.0 and 572,888 markers were tested for schizophrenia association. No SNPs in the discovery sample attained genome-wide significance (P < 8.7 × 10−8). Secondly, based on the GWAS data, we selected 1000 markers with the lowest P values in the discovery TOP sample, and tested these (or HapMap-based surrogates) for association in the replication sample. Sixteen loci were associated with schizophrenia (nominal P value < 0.05 and concurring OR) in the replication sample. As a next step, we performed a combined analysis of the findings from these two studies, and the strongest evidence for association with schizophrenia was provided for markers rs7045881 on 9p21, rs433598 on 16p12 and rs10761482 on 10q21. The markers are located in PLAA, ACSM1 and ANK3, respectively. PLAA has not previously been described as a susceptibility gene, but 9p21 is implied as a schizophrenia linkage region. ACSM1 has been identified as a susceptibility gene in a previous schizophrenia GWAS study. The association of ANK3 with schizophrenia is intriguing in light of recent associations of ANK3 with bipolar disorder, thereby supporting the hypothesis of an overlap in genetic susceptibility between these psychopathological entities.
Keywords: Schizophrenia, Genome-wide association study, PLAA, ACSM1, ANK3, Psychiatric genetics
1. Introduction
Schizophrenia is a severe mental disorder with a life time risk of nearly 1%. It is regarded as a complex disease, where several genes and environmental factors play a role in the pathophysiology (Prasad et al., 2002). It is postulated to be caused by the coactions of several loci, each with a small effect (odds ratio (OR) < 1.5) (Manolio et al., 2008). Genome-wide association studies (GWASs) have successfully identified susceptibility genes for various complex disorders (Craddock et al., 2008). However, despite an estimated heritability of up to 0.8 (Cardno and Gottesman, 2000; Lichtenstein et al., 2009), few common variants have been identified in schizophrenia (Owen et al., 2009; O’Donovan et al., 2008; Stefansson et al., 2009; Shi et al., 2009; Purcell et al., 2009). Scandinavians are generally regarded as well suited for genetic studies, as they form relatively ethnically homogenous populations, since these countries have only recently experienced non-Caucasian immigration. This notion is ensured in our study as all study subjects are born in Norway and the vast majority has two Norwegian-born parents.
In the current study we aimed at identifying SNPs associated with susceptibility to schizophrenia using a Norwegian GWAS discovery sample (n = 506) and tested the best scoring markers in a large North-European multi-centre sample, SGENE-plus (n = 16,443).
2. Methods
2.1. Sample description
2.1.1. Subjects
The present study was performed on two different datasets. Participants received a detailed description of the goal of the study, which was approved by the Ethics Boards, and they signed a written informed consent.
2.1.2. TOP study (discovery sample)
A total of 459 Caucasian individuals with severe mental disorders and 313 healthy controls were recruited and successfully genotyped on Affymetrix Genome-Wide Human SNP Array 6.0 (Affymetrix Inc., Santa Clara, CA, USA). The subjects participated in a large ongoing study on schizophrenia and bipolar disorder, the Thematic Organized Psychosis Research (TOP) Study, and were recruited from out-patient and in-patient psychiatric units at four University Hospitals in Oslo, Norway, from May 2003 through July 2007. The health care system is catchment area based, free of charge, and no other psychiatric health care provider exists. The patients were invited to participate in the study by the clinician responsible for their treatment.
The study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate, and the biobank was approved by the Norwegian Directorate of Health.
In order to participate in the current study subjects needed to be between 18 and 65 years old, obtain an IQ score above 70, meet the DSM-IV criteria for schizophrenia, schizoaffective or schizophreniform disorder and be willing and able to give informed consent.
Diagnosis was established using the Structured Clinical Interview for DSM-IV-TR-axis I disorders (SCID-I) (Spitzer et al., 1992). All interviewers finished a training course in SCID assessment based on a UCLA training program (Ventura et al., 1998), and participated in diagnostic evaluation meetings on regular basis led by an experienced clinical researcher in the field of diagnostics in severe mental disorder. To assess reliability for the actual study interviews, a stratified random sample was drawn, consisting of cases from each of the raters. Anonymous vignettes describing symptoms and development of the illness were then rated by two experts blind to the study ratings. For the 28 vignettes the overall agreement for the DSM-IV diagnostic categories was 82% and the overall K 0.77 (95% CI: 0.60-0.94).
Global Assessment of Functioning Scale (GAF) (Endicott et al., 1976) was utilized to measure psychosocial functioning and split into scales of symptoms (GAF-S) and function (GAF-F) to improve psychometric properties (Endicott et al., 1976; Pedersen et al., 2007). The inter-rater reliability of the investigators was good for the GAF with an intra class correlation, ICC 1.1, of 0.86 (Endicott et al., 1976; Shrout and Fleiss, 1979).
The majority (90%) of the patients were ethnically Norwegian, i.e. the patient and both parents were born in Norway, while in a minor fraction of the cases (10%) one parent was born outside Norway in another North-Western European country.
The healthy subjects were randomly selected using records of people from the same catchment areas as the patient groups. Only subjects born in Norway were contacted by letter and invited to participate. All controls were of Caucasian origin; around 85% had two Norwegian parents, the rest had one parent of other European origin. Moreover, all participants had to have Norwegian as their first language or have received their compulsory schooling in Norway.
The control subjects were screened by interview and with the Primary Care Evaluation of Mental Disorders (PRIME-MD). None of the control subjects had a history of moderate/severe head injury, neurological disorder, mental retardation or an age outside the age range of 18–65 years. Healthy subjects were excluded if they or any of their close relatives had a lifetime history of a severe psychiatric disorder (schizophrenia, bipolar disorder and major depression), a history of medical problems thought to interfere with brain function (hypothyroidism, uncontrolled hypertension and diabetes), or significant illicit drug use.
2.1.3. SGENE-plus study (replication sample)
A total of 1321 schizophrenia cases and 12,277 controls from Germany (Munich), England, Finland, Iceland, Italy and Scotland (The SGENE sample; http://SGENE.eu) with an additional 859 cases and 854 controls from Germany (Munich) and Scotland, 483 cases and 367 controls from Germany (Bonn), and as well as 282 controls from Finland, giving a total of 2663 cases and 13,780 controls (SGENE-plus) were used in the replication analysis. For details see (Stefansson et al. (2009).
2.2. Genotyping
The TOP sample (n = 772) was genotyped at Expression Analysis Inc. (Durham, USA) using the Affymetrix Genome-Wide Human SNP Array 6.0 (Affymetrix Inc., Santa Clara, CA, USA). Samples were excluded if they were low-yield (call rate below 97%), if they were duplicates of other samples included in the study, if they had a sex determined by X chromosome marker homozygosity different from their reported sex or if they were calculated to have other ancestry than European. This resulted in a sample of 506 individuals. Of the 909,622 markers on the Affymetrix 6.0 Array, 336,734 were deemed unusable due to lack of polymorphism, severe deviation from Hardy-Weinberg equilibrium (P < 1 × 10−5), low-yield (<95% in either cases or controls) or with minor allele frequencies below 0.05; following this 572,888 markers remained for analysis. The total genotyping rate in remaining individuals was 0.978 and the inflation factor (θ) was 1.017.
The SGENE-plus sample was genotyped using HumanHap300 Bead Array™ (Illumina, San Diego, CA, USA) at deCODE Genetics (Reykjavik, IS). For the SGENE-plus sample, the subjects from Bonn were typed on HumanHap550v3 Bead Array™ (Illumina, San Diego, CA, USA) at Bonn University, while the additional samples (from Aberdeen and Munich) were typed at Duke University using either the HumanHap300- or the HumanHap550 Bead Array™ (Illumina, San Diego, CA, USA) and the additional controls from Finland were typed at the Broad Institute using the Human CNV 370 Bead Array. For more details, see Stefansson et al. (2009).
2.3. Modeling stratification
In order to detect genetic outliers in the TOP sample, we calculated the pair-wise identity-by-state distance matrix with subsequent principal component clustering using PLINK (Purcell et al., 2007). Eigenvector 1 and 2 were used for visualization. In collaboration with database curator, we excluded 49 individuals that were obvious genetic outliers upon visual inspection of the PCA plot and having a non-Norwegian surname, suggesting a non-Norwegian ancestry.
2.4. SNP selection for replication
We selected 1000 markers that obtained the lowest P values in the discovery sample for analysis in the SGENE-plus sample. This latter sample was genotyped on a different platform than the TOP sample, so surrogate markers had to be found for markers that were not on both platforms. HapMap-based surrogate markers were found for 529 markers, while 141 markers were on both platforms. For 330 of the markers no surrogates could be found, and they therefore had to be excluded from the replication analysis.
2.5. Software
Affymetrix Power Tools (APT), version 1.10.0 and the embedded birdseed-v2 algorithm were used to genotype the raw data. Subsequent output files were modified and combined with marker annotation and so-called transposed .ped and .fam files were created as ready input files for PLINK (Purcell et al., 2007). PLINK was then used for quality control and association analysis. Software used at deCODE was NEMO (Gretarsdottir et al., 2003).
2.6. Association analysis
The TOP-data was analyzed for SNP-schizophrenia association using the allelic test implemented in PLINK (Purcell et al., 2007).
Association analyses were undertaken using a likelihood procedure and, to combine the eight study groups, the Cochrane–Mantel–Haentzel model test in the SGENE-plus sample.
2.7. Combined analysis
We undertook a combined analysis using Fischer’s combined probability test to calculate the combined chi-square values, and R (R Development Core Team, 2008) to obtain a new combined P value for each marker.
3. Results
3.1. Discovery sample
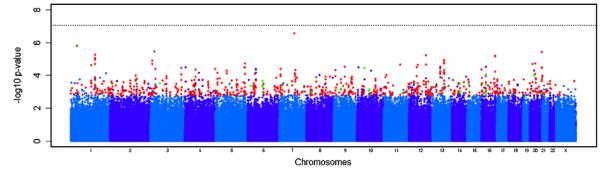
We carried out a genome-wide survey for sequence variants associated with schizophrenia in a Norwegian discovery sample of 201cases and 305 healthy controls that passed our quality and control measures (Supplementary Method online). A total of 572,888 SNPs on the Affymetrix Genome-Wide Human SNP Array 6.0 (Affymetrix Inc., Santa Clara, CA, USA) were analyzed, including 550,511 autosomal SNPs and 22,377 SNPs located on chromosome X (Supplementary Method online). Based on genome-wide allelic tests (Fig. 1), 29,681 SNPs showed signal of association below P < 0.05 in the discovery sample (P values ranging from 2.84 × 10−7 to 0.05). No SNPs reached a P value below 8.7 × 10−8, a threshold that corresponds to P < 0.05 after adjusting for 572,888 independent tests. All markers with P values < 1 × 10−3 (n = 617) are listed in Supplementary Table 1 online.
Fig. 1.
Genome-wide association results for TOP (discovery sample). Manhattan plot showing significance of association of all quality-control-positive SNPs in the discovery sample with schizophrenia as −log10 of the allelic test P value. SNPs are plotted on the x axis according to their position on each chromosome. Chromosomes are shown in alternating colors for clarity. Signals examined in SGENE-plus are shown in red, and signals with OR in concordance are shown in green. Associations with schizophrenia are indicated on the y axis as −log10 P value.
The analysis revealed seven loci, represented by eleven SNPs, that were moderately associated with schizophrenia (P < 1 × 10−5). The strongest signal, rs6979348, was located on 7q21, 12 kb downstream of the 3′ end of the gene piccolo (PCLO), which encodes a presynaptic cytomatrix protein. The other moderately associated loci are located on 1p34.3, 1q24.3, 3p25.1, 12q23.1, 16q23.3 and 20q13.33, and the markers representing these loci are near the RRAGC, FMO3 and FMO6P, WNT7A, ANO4 and CDH13 genes and the expressed sequence tag AL117372.35, respectively.
We also investigated the top 30 genes and gene regions from www.schizophreniaforum.org (accessed June 2009) similar to the procedure of Sullivan et al. (2009). We found the SNPs rs16854954 in DISC1 (P = 7.72 × 10−5), rs10264893 in RELN (P = 1.46 × 10−4), rs10095694 in NRG1 (P = 1.38 10−3), rs1254702 on 10q26.13 (P = 6.82 × 10−4) and rs1941213 in OPCML (P = 3.41 × 10−4) nominally significantly × associated with schizophrenia. The other genes from schizopfreniaforum.org did not show significant association.
3.2. Replication sample
To validate potential associations with schizophrenia from our GWAS discovery sample, we selected the 1000 strongest signals for attempted replication in SGENE-plus. For 330 of the markers, no surrogates in linkage disequilibrium (R2 > 0.8) were identified. For the remaining 670 markers, either the same marker (n = 141) or a surrogate marker (n = 529) was analyzed in SGENE-plus. We replicated the positive associations observed in the discovery sample for 32 markers with an uncorrected P value < 0.05 and concurring OR between the two samples (Table 1). To reduce the risk of false positives, we corrected for 412 independent tests, using the one-sided cut-off P = 0.00024, leaving rs7045881 experiment-wide significant. The suggestive association signals define 16 loci with potential susceptibility genes for schizophrenia.
Table 1.
The most significant association results from the combined analysis.
| Discovery sample Cases n = 201 Controls n = 307 |
Replication sample Cases n = 2663 Controls n = 13,780 |
Combined analysis Cases n = 2864 Controls n = 14,087 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromosome | Affy SNP | Minor allele | SZ | CON | Allelic (P) | Odds ratio | Illumina SNP | R 2 | CMH (P) | Odds ratio | Meta CMH (P) | Locus |
| 9 | rs7045881 | A | 0.06 | 0.13 | 6.39E-04 | 0455 | rs7035755 | 1 | 1.96E-04 | 0.86 | 2.12E-06 | PLAA |
| 16 | rs433598 | T | 0.38 | 0.27 | 1.06E-04 | 1.7 | rs433598 | 1 | 1.88E-03 | 1.13 | 3.27E-06 | ACSM1 |
| 10 | rs10761482 | T | 0.17 | 0.26 | 7.33E-04 | 0.58 | rs4948410 | 0.83 | 6.75E-04 | 0.86 | 7.68E-06 | ANK3 |
| 10 | rs4313476 | C | 0.46 | 0.33 | 3.63E-05 | 1.72 | rs4313476 | 1 | 3.59E-02 | 1.08 | 1.90E-05 | GLUDP5 |
| 16 | rs163274 | C | 0.35 | 0.24 | 3.66E-04 | 1.65 | rs163234 | 0.96 | 4.72E-03 | 1.12 | 2.46E-05 | ACSM1 |
| 15 | rs951442 | C | 0.37 | 0.48 | 3.44E-04 | 0.63 | rs951442 | 1 | 8.55E-03 | 0.91 | 4.04E-05 | RYR3 |
| 6 | rs9296494 | C | 0.24 | 0.35 | 4.40E-04 | 0.6 | rs9296494 | 1 | 9.33E-03 | 0.9 | 5.50E-05 | PHACTR1 |
| 20 | rs4813250 | T | 0.33 | 0.45 | 9.53E-05 | 0.59 | rs6080400 | 0.9 | 4.50E-02 | 0.93 | 5.72E-05 | OTOR |
| 20 | rs6111277 | A | 0.33 | 0.45 | 1.07E-04 | 0.6 | rs6080400 | 0.9 | 4.50E-02 | 0.93 | 6.37E-05 | OTOR |
| 16 | rs163234 | G | 0.36 | 0.26 | 1.19E-03 | 1.58 | rs163234 | 1 | 4.72E-03 | 1.12 | 7.36E-05 | ACSM1 |
| 6 | rs6911854 | C | 0.18 | 0.1 | 2.32E-04 | 1.97 | rs9443543 | 0.8 | 2.73E-02 | 1.13 | 8.22E-05 | AL591500.6> |
| 16 | rs12324972 | T | 0.3 | 0.21 | 7.84E-04 | 1.64 | rs6497501 | 0.86 | 9.02E-03 | 1.12 | 9.09E-05 | ACSM2 |
| 16 | rs163275 | G | 0.33 | 0.24 | 1.53E-03 | 1.57 | rs163234 | 0.96 | 4.72E-03 | 1.12 | 9.28E-05 | ACSM1 |
| 3 | rs2372342 | C | 0.09 | 0.04 | 3.06E-04 | 2.57 | rs2372342 | 1 | 2.70E-02 | 1.04 | 1.05E-04 | C3orf39|SNRK |
| 8 | rs10106755 | A | 0.28 | 0.38 | 1.01E-03 | 0.64 | rs13273158 | 0.9 | 9.97E-03 | 0.9 | 1.26E-04 | PSD3 |
| 6 | rs946138 | T | 0.17 | 0.09 | 4.12E-04 | 1.95 | rs9443543 | 0.86 | 2.73E-02 | 1.13 | 1.40E-04 | AL591500.6> |
| 6 | rs3857446 | C | 0.18 | 0.11 | 4.97E-04 | 1.89 | rs9443543 | 1 | 2.73E-02 | 1.13 | 1.66E-04 | AL591500.6> |
| 14 | rs6573695 | G | 0.03 | 0.09 | 3.16E-04 | 0.34 | rs1952070 | 1 | 4.58E-02 | 0.89 | 1.75E-04 | GPHN |
| 10 | rs4316429 | T | 0.17 | 0.1 | 9.08E-04 | 1.88 | rs7092779 | 0.92 | 2.61E-02 | 1.13 | 2.76E-04 | PCDH15 |
| 18 | rs4264496 | A | 0.4 | 0.5 | 1.54E-03 | 0.66 | rs11662668 | 0.9 | 1.67E-02 | 1.09 | 2.98E-04 | CTAGE1|RBBP8 |
| 6 | rs2050689 | T | 0.17 | 0.1 | 1.01E-03 | 1.85 | rs9443543 | 1 | 2.73E-02 | 1.13 | 3.18E-04 | AL591500.6> |
| 10 | rs1539318 | C | 0.16 | 0.1 | 1.61E-03 | 1.83 | rs7092779 | 0.92 | 2.61E-02 | 1.13 | 4.64E-04 | PCDH15 |
| 14 | rs17247749 | C | 0.04 | 0.1 | 9.48E-04 | 0.39 | rs1952070 | 1 | 4.58E-02 | 0.89 | 4.79E-04 | GPHN |
| 14 | rs6573719 | G | 0.04 | 0.09 | 9.95E-04 | 0.4 | rs1952070 | 1 | 4.58E-02 | 0.89 | 5.01E-04 | GPHN |
| 14 | rs17836572 | C | 0.04 | 0.09 | 1.04E-03 | 0.4 | rs1952070 | 1 | 4.58E-02 | 0.89 | 5.23E-04 | GPHN |
| 14 | rs1885198 | T | 0.04 | 0.09 | 1.04E-03 | 0.4 | rs1952070 | 1 | 4.58E-02 | 0.89 | 5.23E-04 | GPHN |
| 14 | rs6573706 | T | 0.04 | 0.09 | 1.04E-03 | 0.4 | rs1952070 | 1 | 4.58E-02 | 0.89 | 5.23E-04 | GPHN |
| 14 | rs7154017 | C | 0.04 | 0.09 | 1.04E-03 | 0.4 | rs1952070 | 1 | 4.58E-02 | 0.89 | 5.23E-04 | GPHN |
| 14 | rs7157740 | G | 0.04 | 0.09 | 1.04E-03 | 0.4 | rs1952070 | 1 | 4.58E-02 | 0.89 | 5.23E-04 | GPHN |
| 11 | rs500063 | T | 0.39 | 0.49 | 1.24E-03 | 0.66 | rs500063 | 1 | 4.06E-02 | 0.92 | 5.48E-04 | SLC35F2 |
| 14 | rs9783601 | G | 0.04 | 0.09 | 1.12E-03 | 0.4 | rs1952070 | 1 | 4.58E-02 | 0.89 | 5.57E-04 | GPHN |
| 20 | rs6100223 | C | 0.18 | 0.27 | 1.27E-03 | 0.6 | rs6015375 | 0.84 | 4.33E-02 | 0.92 | 5.95E-04 | NPEPL1|GNAS |
Note: Combined analysis of allelic association tests for SNPs with a nominal significant P value in the replication sample with concurring OR between the discovery and the replication sample. The minor allele frequencies are listed in SZ (cases) and CON (controls).
3.3. Combined analysis
The integrated analysis of the combined samples (TOP and SGENE-plus) (Table 1) demonstrated that three markers emerge as associated with schizophrenia, approaching genome-wide significance level (P < 1 × 10−5). The markers are rs7045881 located in the gene PLAA on 9p21 (P = 2.12 × 10−6), rs433598 in the ACSM1 gene on 16p12 (P = 3.27 10−6), and rs10761482 in the gene ANK3 on 10q21 (P = 7.68 × 10−6). Two other markers in the ACSM1 gene, rs163274 and rs163275 show association signals of P = 2.46 × 10−5 and P = 9.28 × 10−5 respectively. These two markers are in complete linkage × disequilibrium (LD), and are correlated with rs433598 (R2 = 0.687). In the discovery sample, the signals of associations in ACSM1 were observed for four markers (rs433598, rs163274, rs163275, and rs163234). In the replication analysis, we only examined rs433598 and rs163234, since the latter marker also tags rs163274 and rs163275.
4. Discussion
We report here GWAS-based nominally significant associations with schizophrenia for 32 SNPs. The odds ratios are in the range of 1.1–1.2 as expected for this complex disease. The markers highlight 16 loci harboring potential susceptibility genes for schizophrenia. Three markers displayed moderately genome-wide significance of association with the disease in the combined analysis.
The strongest association signal (P = 2.12 10−6) in the combined analysis was found with marker rs7045881 × located in the first intron of the PLAA gene on 9p21, encoding phospholipase A2-activating protein (PLAP). This finding reached the experiment-wide significance level of association in the replication sample. This gene has not been previously reported as a susceptibility gene for schizophrenia, but has been linked to inflammatory response (Ruiz et al., 1999). In normal brain cells, phospholipase A2 balances the conversion of arachidonic acid into proinflammatory mediators as well as the reincorporation of arachidonic acid into the membrane. Patients suffering from schizophrenia are reported to have increased levels of cytosolic phospholipase A2 (cPLA2), possibly leading to breakdown of phospholipids in the cell membrane. A polymorphism in the first intron of the cPLA2 gene is associated with schizophrenia (Peet et al., 1998). Thus, alterations in PLAA could lead to altered cytosolic phospholipase A2 activation.
The second strongest signal was seen for marker rs433598 within the ACSM1 locus, (P = 3.27 × 10−6) in the combined analysis. There was also significant association signal (P = 2.46-9.28 × 10−5) from two other markers, rs163274 and rs163275 within the same gene, which are correlated with rs433598 (R2 = 0.687). These markers are located in the fifth intron of ACSM1, encoding acyl-CoA synthetase medium-chain family member 1, which is involved in endocrine function, and has been implicated in dyslipidemia (Haketa et al., 2004). Another locus (ACSM2) on 16p12 also showed association with schizophrenia (P = 9.09 × 10−5) in the combined analysis. This gene has been implicated in metabolic syndrome (Lindner et al., 2006). Both dyslipidemia and metabolic syndrome affect a large proportion of schizophrenia patients (Birkenaes et al., 2007). Both loci are part of a longer linkage region based on a meta-analysis of previous whole-genome scans (Lewis et al., 2003). Two markers (rs234993 and rs151222) in ACSM1 were reported to be among the top 25 markers in the CATIE study (Sullivan et al., 2008). These markers were not in LD with our significant SNPs, but this further support ACSM1 as a schizophrenia susceptibility gene.
The third strongest signal (P = 7.68 × 10−6) was seen with marker rs10761482 in the first intron of ANK3 on 10q21. ANK3 encodes the protein ankyrin 3, node of Ranvier. ANK3 has previously been associated with bipolar disorder (Baum et al., 2008; Ferreira et al., 2008; Schulze et al., 2008). Two polymorphism (rs10994336 and rs9804190) show association of P = 1.7 10−5 (OR = 1.54) and P = 3 × 10−6 (OR = 1.32) respectively in a meta-analysis × consisting of three bipolar samples (Schulze et al., 2008). The markers are not in LD and contribute independently to bipolar disorder. Our signal from the ANK3 gene was obtained for marker rs10761482. The marker is not in LD with the two previously described markers and looks like an independent risk SNP. Ankyrin links the integral membrane proteins to the cytoskeleton and is implied in activation, proliferation, contact, cell mobility and maintenance of specialized membrane domains. The gene was first discovered in the axonal initial segments and nodes of Ranvier of neurons in the central and peripheral nervous system (Kordeli et al., 1995). At present, ANK3 appears to be the gene finding with strongest support in bipolar disorder. Schizophrenia and bipolar disorder were considered to be etiologically distinct entities, but new findings support a common etiology (Lichtenstein et al., 2009; Purcell et al., 2009; Craddock et al., 2005). The association of polymorphisms in ANK3 with schizophrenia could further advocate for possible common genetic susceptibility with bipolar disorder.
Several other markers may be interesting from a biological perspective. The polymorphism rs4316429 within the glutamate dehydrogenase pseudogene 5 (GLUDP5) on 10p11 shows nominal association (P = 3.59 × 10−2) in the replication analysis and in the combined analysis (P = 1.90 × 10−5). Furthermore, marker rs951442 on 15q14 shows nominal association in the replication sample (P = 8.55 × 10−3) and in the combined analysis (P = 4.04 × 10−5). The marker is located within RYR3 encoding ryanodine receptor 3, an intracellular ion channel that mediate release of Ca2+ from intracellular stores subsequent to transduction of several extracellular stimuli. The locus has been previously examined for association with schizophrenia in a Japanese population with a negative result (Yamada et al., 2007). PHACTR1 shows nominally association with schizophrenia in the combined analysis (P = 5.50 × 10−5) and the locus is located in a schizophrenia linkage region based on previous meta-analysis (Lewis et al., 2003). Further on, a marker on 20p12, a region also implied as a schizophrenia linkage region (Lewis et al., 2003), is nominally significant in the combined analysis (P = 5.72 × 10−5). The polymorphism associated is located downstream of otoraplin (OTOR), expressed in the ear and brain.
It is of interest that some previously identified candidate genes and regions for schizophrenia (www.schizophreniaforum.org), DISC1, RELN, NRG1, 10q26.13 and OPCML were nominally significantly associated with schizophrenia in the discovery sample. Also, the strongest signal from the discovery sample was located near PCLO, a gene that is associated to major depressive disorder (Sullivan et al., 2009).
A potential limitation of the present data is the small sample size used in the discovery GWAS. However, the sample derives from an ethnically homogenous population, ensured in our study as all study subjects are born in Norway and the vast majority has two Norwegian-born parents, minimizing the risk of false positive findings due to population stratification.
As expected, potential true association signals which were replicated in the SGENE-plus sample were not necessarily among the apparent top hits in the discovery analysis, but rather “hiding” among noise signals further down on the list. Controlling the family-wise error rate (FWER) requires very strict α adjustment, preventing many true associations with small effect sizes from being followed up. Instead, we used the first GWAS (TOP sample) as a hypothesis generating step and the replication analysis as a hypothesis verification step to separate potential true associations from false positives. The markers typed in a GWAS are not necessarily the causative polymorphism, and further fine-mapping and functional analysis are required to identify biological systems that underpin the clinical syndromes. To further validate these loci as susceptibility genes for schizophrenia, replication in additional populations are needed.
Supplementary Material
Acknowledgments
We thank patients and controls for their participation in the study, and the health professionals who facilitated our work. We also wish to thank Knut-Erik Gylder, Marie J. Skogstad, Thomas Bjella, Eivind Bakken and Elin Inderhaug for skilful technical and administrative assistance. We thank David Goldstein and GSK for genotyping additional samples from Munich and Aberdeen. We are grateful to K.-H. Jöckel and R. Erbel for providing control individuals from the Heinz Nixdorf Recall Study.
Role of funding source
The work was supported by grants from the Research Council of Norway (#167153/V50, #163070/V50, and #175345/V50); South-East Norway Health Authority (#123-2004); Ulleval University Hospital and the University of Oslo to support the Thematic Organized Psychosis Research (TOP) Study group; and support from Sigurd K. Thoresen’s Foundation and E. Lilly Inc. for parts of the genotyping costs of the TOP sample. This work was also supported by the National Genomic Network (NGFN-2 and NGFN-MooDS) of the German Federal Ministry of Education and Research (BMBF), and the EU grant LSHM-CT-2006-037761 (Project SGENE). The funding sources had no further role in study design and execution.
Abbreviations
- ACSM
acyl-CoA synthetase medium-chain family member 1
- ANK3
ankyrin 3
- ANO4
anocatmin 4
- APT
Affymetrix Power Tools
- CDH13
cadherin 13
- cPLA2
cytosolic phospholipase A2
- DISC1
disrupted in schizophrenia 1
- FMO3
flavin-containing monooxygenase 3
- FMO6P
flavin-containing monooxygenase 6
- FWER
family-wise error rate
- GAF
Global Assessment of Function of mental disorders
- GAF-S
Global Assessment of Function of mental disorders symptoms
- GAF-F
Global Assessment of Function of mental disorders functions
- GLUDP5
glutamate dehydrogenase pseudogene 5
- GWAS
genome-wide association study
- LD
linkage disequilibrium
- NRG1
neuregulin 1
- OPCML
opioid binding protein/cell adhesion molecule-like
- OR
odds ratio
- OTOR
otoraplin
- PCA
principal component analysis
- PCLO
piccolo
- PHACTR1
phosphatase and actin regulator 1
- PLAA
phospholipase A2-activating protein
- RRAGC
ras-related GTP binding C
- PRIME-MD
Primary Care Evaluation of Mental Disorders
- RELN
reelin
- RYR3
ryanoid receptor 3
- SCID-I
Structured Clinical Interview for DSM-IV-TR axis I disorders
- SNP
single nucleotide polymorphism
- TOP
Thematic Organized Psychosis Research
- WNT7A
wingless-type MMTV integration site family, member 7A
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jpsychires.2010.02.002.
Footnotes
Conflict of interest
None declared.
References
- Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Molecular Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenaes AB, Opjordsmoen S, Brunborg C, Engh JA, Jonsdottir H, Ringen PA, et al. The level of cardiovascular risk factors in bipolar disorder equals that of schizophrenia: a comparative study. Journal of Clinical Psychiatry. 2007;68:917–23. doi: 10.4088/jcp.v68n0614. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Gottesman II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. American Journal of Medical Genetics. 2000;97:12–7. [PubMed] [Google Scholar]
- Craddock N, O’Donovan MC, Owen MJ. The genetics of schizophrenia and bipolar disorder: dissecting psychosis. Journal of Medical Genetics. 2005;42:193–204. doi: 10.1136/jmg.2005.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, O’Donovan MC, Owen MJ. Genome-wide association studies in psychiatry: lessons from early studies of non-psychiatric and psychiatric phenotypes. Molecular Psychiatry. 2008;13:649–53. doi: 10.1038/mp.2008.45. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry. 1976;33:766–71. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nature Genetics. 2008;40:1056–8. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretarsdottir S, Thorleifsson G, Reynisdottir ST, Manolescu A, Jonsdottir S, Jonsdottir T, et al. The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nature Genetics. 2003;35:131–8. doi: 10.1038/ng1245. [DOI] [PubMed] [Google Scholar]
- Haketa A, Soma M, Nakayama T, Sato M, Kosuge K, Aoi N, et al. Two medium-chain acyl-coenzyme A synthetase genes, SAH and MACS1, are associated with plasma high-density lipoprotein cholesterol levels, but they are not associated with essential hypertension. Journal of Hypertension. 2004;22:1903–7. doi: 10.1097/00004872-200410000-00012. [DOI] [PubMed] [Google Scholar]
- Kordeli E, Lambert S, Bennett V. AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. Journal of Biological Chemistry. 1995;270:2352–9. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Levinson DF, Wise LH, Delisi LE, Straub RE, Hovatta I, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: schizophrenia. American Journal of Human Genetics. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–9. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner I, Rubin D, Helwig U, Nitz I, Hampe J, Schreiber S, et al. The L513S polymorphism in medium-chain acyl-CoA synthetase 2 (MACS2) is associated with risk factors of the metabolic syndrome in a Caucasian study population. Molecular Nutrition and Food Research. 2006;50:270–4. doi: 10.1002/mnfr.200500241. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Brooks LD, Collins FS. A HapMap harvest of insights into the genetics of common disease. Journal of Clinical Investigation. 2008;118:1590–605. doi: 10.1172/JCI34772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nature Genetics. 2008;40:1053–5. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Williams HJ, O’Donovan MC. Schizophrenia genetics: advancing on two fronts. Current Opinion in Genetics and Development. 2009;19:266–70. doi: 10.1016/j.gde.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Pedersen G, Hagtvet KA, Karterud S. Generalizability studies of the global assessment of functioning-split version. Comprehensive Psychiatry. 2007;48:88–94. doi: 10.1016/j.comppsych.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Peet M, Ramchand CN, Lee J, Telang SD, Vankar GK, Shah S, et al. Association of the Ban I dimorphic site at the human cytosolic phospholipase A2 gene with schizophrenia. Psychiatric Genetics. 1998;8:191–2. doi: 10.1097/00041444-199800830-00010. [DOI] [PubMed] [Google Scholar]
- Prasad S, Semwal P, Deshpande S, Bhatia T, Nimgaonkar VL, Thelma BK. Molecular genetics of schizophrenia: past, present and future. Journal of Biosciences. 2002;27:35–52. doi: 10.1007/BF02703682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing. 2008. [Google Scholar]
- Ruiz A, Nadal M, Puig S, Estivill X. Cloning of the human phospholipase A2 activating protein (hPLAP) gene on the chromosome 9p21 melanoma deleted region. Gene. 1999;239:155–61. doi: 10.1016/s0378-1119(99)00354-6. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Tera-Wadleigh SD, Akula N, Gupta A, Kassem L, Steele J, et al. Two variants in Ankyrin 3 (ANK3) are independent genetic risk factors for bipolar disorder. Molecular Psychiatry. 2008;14:487–91. doi: 10.1038/mp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–7. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Archives of General Psychiatry. 1992;49:624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–7. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Lin D, Tzeng JY, van den OE, Perkins D, Stroup TS, et al. Genomewide association for schizophrenia in the CATIE study: results of stage 1. Molecular Psychiatry. 2008;13:570–84. doi: 10.1038/mp.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, DE Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T, et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Molecular Psychiatry. 2009;14:359–75. doi: 10.1038/mp.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Research. 1998;79:163–73. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Yamada K, Gerber DJ, Iwayama Y, Ohnishi T, Ohba H, Toyota T, et al. Genetic analysis of the calcineurin pathway identifies members of the EGR gene family, specifically EGR3, as potential susceptibility candidates in schizophrenia. Proceedings of the National Academy of Science of the United States of America. 2007;104:2815–20. doi: 10.1073/pnas.0610765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.