Abstract
The insulin release after an oral glucose load is both earlier and greater than would be expected from the glycemic stimulus. This augmentation of insulin release has been attributed to humoral factors from the gut.
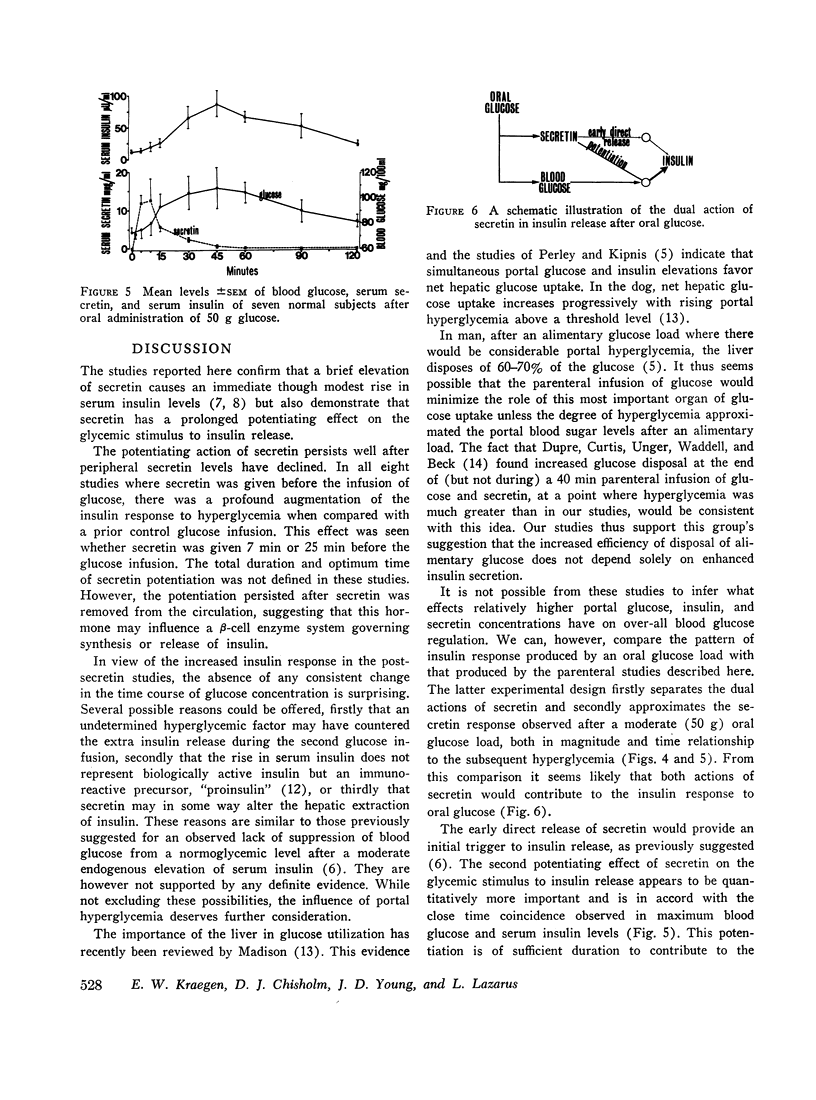
It has been previously demonstrated that secretin is released very rapidly after oral glucose and postulated that it acts as an early trigger to insulin release. This effect would not explain the magnitude of the peak insulin response which occurs about 30 min after peak secretin levels. The present studies, however, demonstrate an additional action of secretin which may explain this.
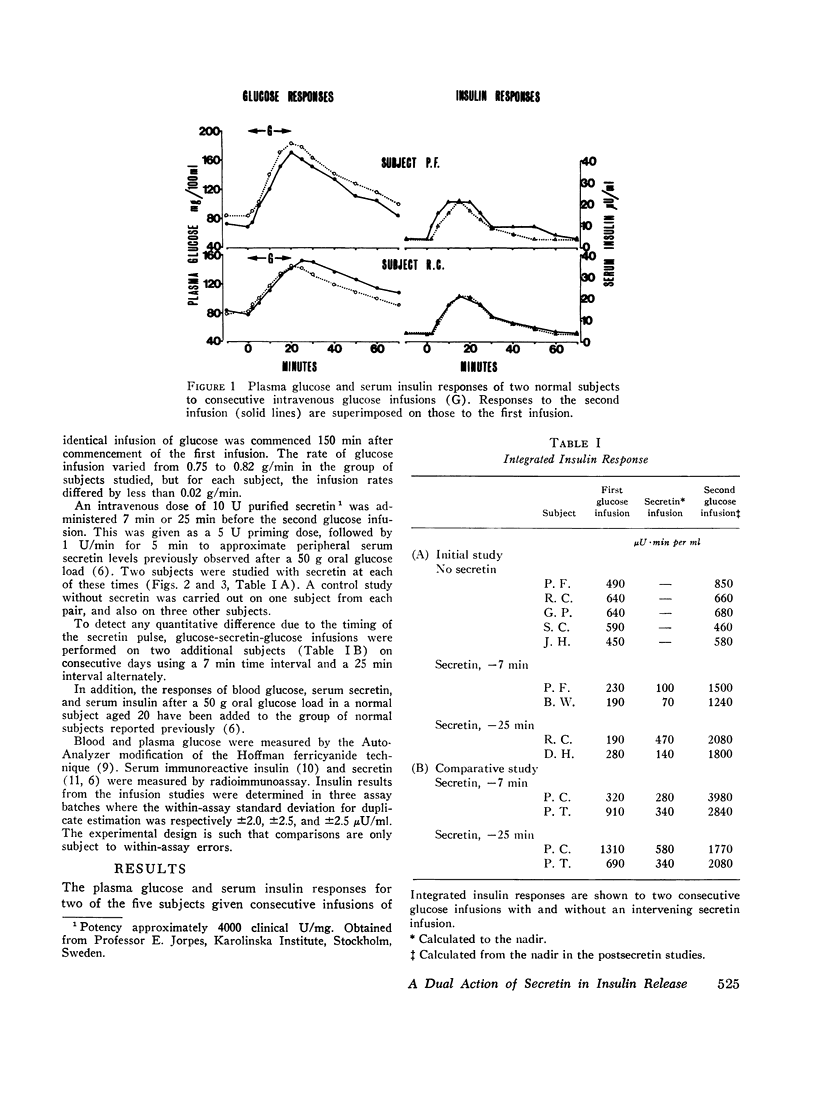
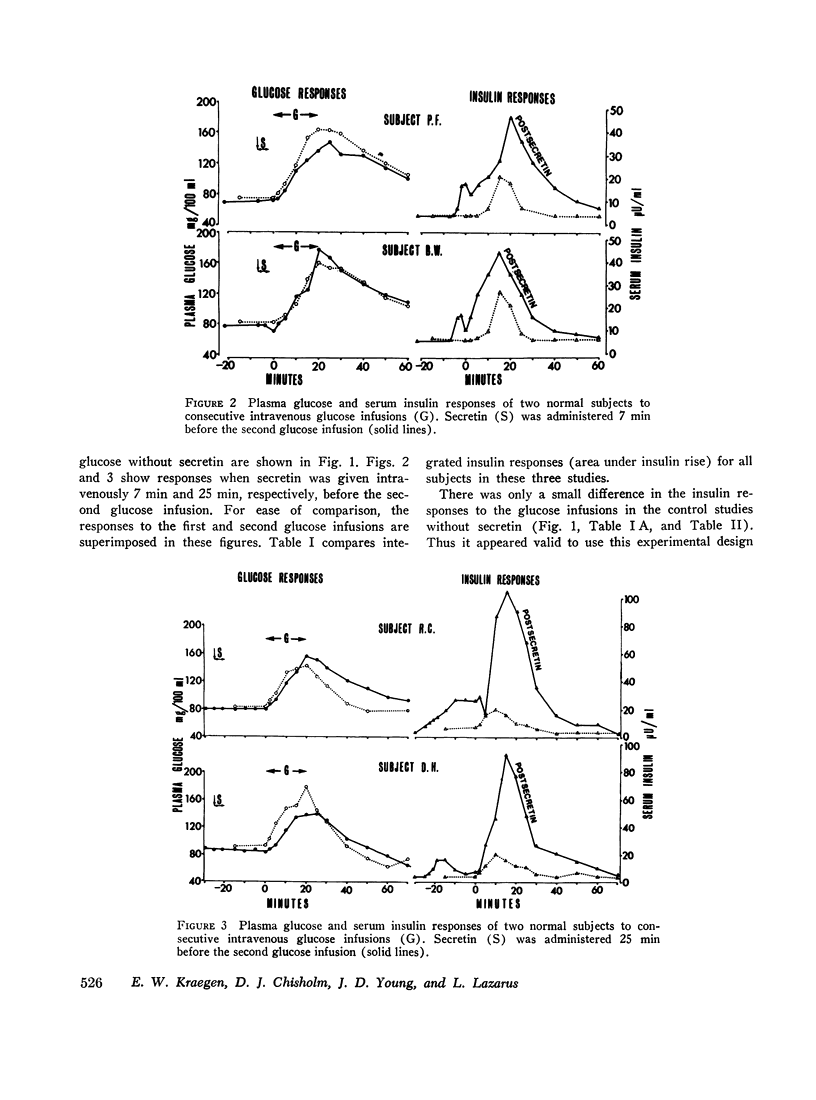
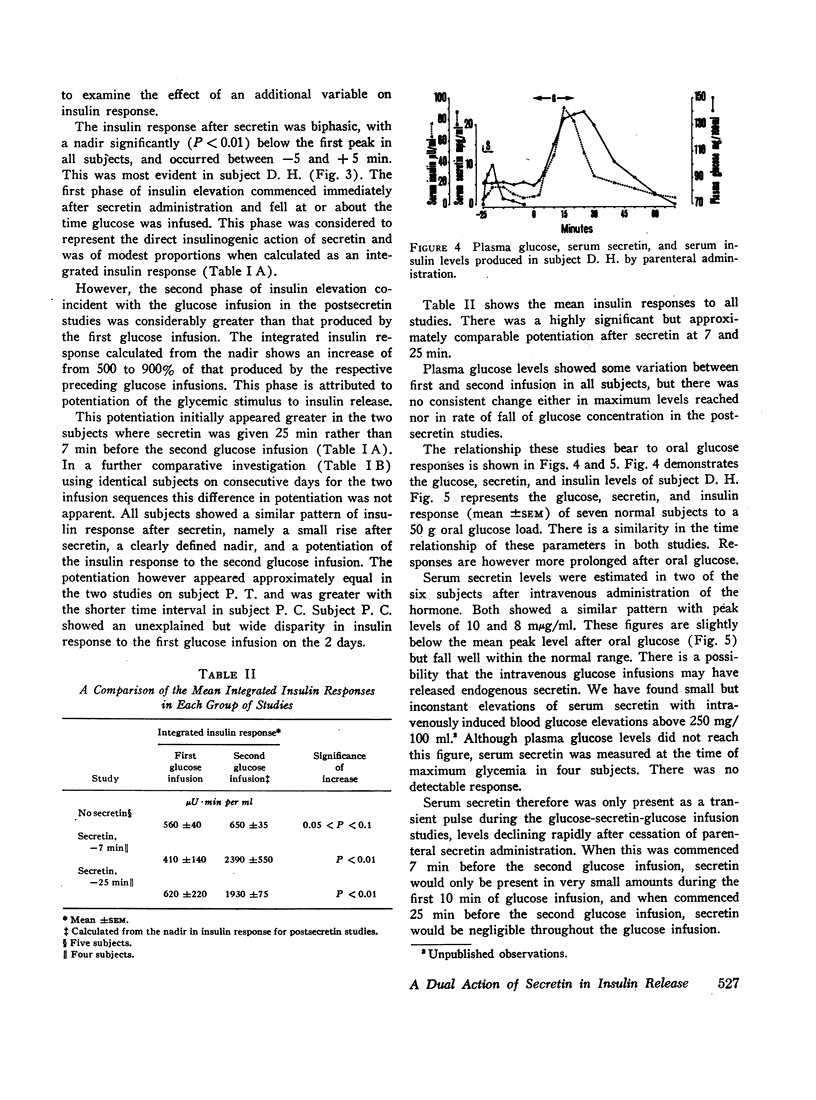
To further study the role of secretin in insulin release in normal subjects, two consecutive 20 min intravenous glucose infusions were administered 150 min apart with and without an intervening secretin infusion (10 U) given to approximate serum secretin levels seen after oral glucose ingestion. A highly significant (P<0.01) potentiation of the insulin response to the post-secretin glucose infusion was observed. This occurred both when secretin was given 7 min or 25 min before glucose. In the latter case, serum secretin was undetectable during the glucose infusion. These studies demonstrate that secretin potentiates the glycemic release of insulin.
Despite the augmented insulin response, no consistent change in blood glucose variation was observed. This is consistent with the suggestion that the facilitated disposal of an alimentary glucose load is not dependent solely on enhanced insulin secretion.
Secretin appears to have a dual role in insulin release, an early direct stimulation followed by a prolonged potentiation of the glycemic stimulus. The potentiating effect is of such magnitude to suggest that secretin is the dominant factor in the enteric component of insulin release after an oral glucose load.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chisholm D. J., Young J. D., Lazarus L. The gastrointestinal stimulus to insulin release. I. Secretin. J Clin Invest. 1969 Aug;48(8):1453–1460. doi: 10.1172/JCI106111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre J., Curtis J. D., Unger R. H., Waddell R. W., Beck J. C. Effects of secretin, pancreozymin, or gastrin on the response of the endocrine pancreas to administration of glucose or arginine in man. J Clin Invest. 1969 Apr;48(4):745–757. doi: 10.1172/JCI106032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupré J., Rojas L., White J. J., Unger R. H., Beck J. C. Effects of secretin on insulin and glucagon in portal and peripheral blood in man. Lancet. 1966 Jul 2;2(7453):26–27. doi: 10.1016/s0140-6736(66)91750-8. [DOI] [PubMed] [Google Scholar]
- ELRICK H., STIMMLER L., HLAD C. J., Jr, ARAI Y. PLASMA INSULIN RESPONSE TO ORAL AND INTRAVENOUS GLUCOSE ADMINISTRATION. J Clin Endocrinol Metab. 1964 Oct;24:1076–1082. doi: 10.1210/jcem-24-10-1076. [DOI] [PubMed] [Google Scholar]
- Lawrence A. M. Radioimmunoassayable glucagon levels in man: effects of starvation, hypoglycemia, and glucose administration. Proc Natl Acad Sci U S A. 1966 Feb;55(2):316–320. doi: 10.1073/pnas.55.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCINTYRE N., HOLDSWORTH C. D., TURNER D. S. NEW INTERPRETATION OF ORAL GLUCOSE TOLERANCE. Lancet. 1964 Jul 4;2(7349):20–21. doi: 10.1016/s0140-6736(64)90011-x. [DOI] [PubMed] [Google Scholar]
- Madison L. L. Role of insulin in the hepatic handling of glucose. Arch Intern Med. 1969 Mar;123(3):284–292. [PubMed] [Google Scholar]
- McIntyre N., Holdsworth C. D., Turner D. S. Intestinal factors in the control of insulin secretion. J Clin Endocrinol Metab. 1965 Oct;25(10):1317–1324. doi: 10.1210/jcem-25-10-1317. [DOI] [PubMed] [Google Scholar]
- Perley M. J., Kipnis D. M. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest. 1967 Dec;46(12):1954–1962. doi: 10.1172/JCI105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Hallund O., Rubenstein A., Cho S., Bayliss C. Isolation and properties of proinsulin, intermediate forms, and other minor components from crystalline bovine insulin. Diabetes. 1968 Dec;17(12):725–736. doi: 10.2337/diab.17.12.725. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Eisentraut A. M. Entero-insular axis. Arch Intern Med. 1969 Mar;123(3):261–266. [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Dupré J., Eisentraut A. M. The effects of secretin, pancreozymin, and gastrin on insulin and glucagon secretion in anesthetized dogs. J Clin Invest. 1967 Apr;46(4):630–645. doi: 10.1172/JCI105565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Kraegen E. W. Simultaneous assay of insulin and glucagon in serum. Aust J Exp Biol Med Sci. 1968 Dec;46(6):697–705. doi: 10.1038/icb.1968.176. [DOI] [PubMed] [Google Scholar]
- Young J. D., Lazarus L., Chisholm D. J., Atkinson F. F. Radioimmunoassay of secretin in human serum. J Nucl Med. 1968 Dec;9(12):641–642. [PubMed] [Google Scholar]
- Young J. D., Lazarus L., Chisholm D. J. Radioimmunoassay of pancreozymin cholecystokinin in human serum. J Nucl Med. 1969 Dec;10(12):743–745. [PubMed] [Google Scholar]
- Young J. D., Lazarus L., Chisholm D. J. Secretin and pancreozymin-cholecystokinin after glucose. Lancet. 1968 Oct 26;2(7574):914–914. doi: 10.1016/s0140-6736(68)91081-7. [DOI] [PubMed] [Google Scholar]



