Abstract
It is well established that the cannabinoid and dopamine systems interact at various levels to regulate basal ganglia function. While it is well known that acute administration of cannabinoids to mice can modify dopamine-dependent behaviors, an understanding of the intraneuronal signaling pathways employed by these agents in the striatum is not well understood. Here we use knockout (KO) mouse models to examine the regulation of striatal ERK1/2 signaling by behaviorally relevant doses of cannabinoids. This cellular pathway has been implicated as a central mediator of drug reward and synaptic plasticity. In C57BL/6J mice, acute administration of cannabinoid agonists, HU-210 and Δ9-THC, promotes a dose- and time-dependent decrease in the phosphorylation of ERK1/2 in dorsal striatum. Co-administration of the CB1 cannabinoid receptor (CB1R) antagonist AM251 with HU-210 prevents ERK1/2 inactivation, indicating a requirement for activation of this receptor. In dopamine D1 receptor (D1R) KO animals treated with HU-210, the magnitude of the HU-210-dependent decrease in striatal ERK1/2 signaling is greater than in wild-type controls. In contrast, the HU-210 administration to NMDA receptor knockdown mice (NR1-Kd) was ineffective at promoting striatal ERK1/2 inactivation. Genetic deletion of other potential ERK1/2 mediators, the dopamine D2 receptors (D2R)s or βarrestin-1 or -2, did not affect HU-210-induced modulation of ERK1/2 signaling in the striatum. These results support the hypothesis that dopamine D1 receptors and NMDA receptors act in an opposite manner to regulate striatal CB1R signal transduction.
Keywords: MAP kinase, Δ9-THC, CB1, βarrestin
Introduction
Cannabinoids constitute a diverse class of compounds that include Δ9-THC, the principal psychoactive constituent of marijuana, and synthetic compounds such as HU-210. Administration of cannabinoids in animal models produces various behavioral changes, many of which are mediated by the centrally expressed CB1 cannabinoid receptor (CB1R) (Ledent et al., 1999). CB1 receptors are expressed at high levels throughout the basal ganglia – a group of subcortical structures, including the striatum and nucleus accumbens, that regulate motor output, as well as drug and natural reward (Mackie, 2005; Matyas et al., 2006). In the striatum, CB1 receptor activation has been shown to promote long-lasting changes in synaptic activity at both corticostriatal and striatonigral/pallidal synapses (Szabo et al., 1998; Gerdeman et al., 2002; Kreitzer & Malenka, 2007), as well as increase both the levels of extracellular dopamine and the activity of dopamine neurons (French, 1997; Tanda et al., 1997). Although many effects of cannabinoids on the glutamate and dopamine systems have been described, information regarding the ability of components of the dopamine and glutamate systems to regulate cannabinoid-mediated cellular effects in vivo is still limited.
Cannabinoids have been shown to activate the PI3K/Akt and ERK1/2 signaling pathways primarily through coupling to Gαi/o G-proteins in heterologous expression systems (Bouaboula et al., 1995; Gomez Del Pulgar et al., 2002). In agreement with these in vitro findings, administration of a low dose of Δ9-THC (1 mg/kg i.p.) to mice has been shown to increase the number of pERK1/2 immunoreactive cells in the striatum and the hippocampus (Valjent et al., 2001; Derkinderen et al., 2003; Valjent et al., 2004). Thus, it has been proposed that CB1 receptor activity positively regulates ERK1/2 signaling in vivo. However, the effects of a dose of Δ9-THC that produces the well-accepted “tetrad” of CB1 receptor-dependent behaviors (≥ 3 mg/kg i.p.) in mice i.e. hypothermia, catalepsy, hypolocomotion and antinociception, (Monory et al., 2007) has never been examined in the context of ERK1/2 signaling in the brain. Additionally, the effects of synthetic CB1 receptor agonists at doses relevant for the behavioral tetrad have not been investigated.
Striatal ERK1/2 signaling is associated with several physiological processes. Notable examples include the critical role for ERK1/2 signaling in the actions of psychostimulant drugs (Valjent et al., 2000; Valjent et al., 2006b) and the direct correlation of this pathway with the development of motor abnormalities induced by L-DOPA (Santini et al.; Santini et al., 2007). Thus, gaining a better understanding of the regulation of striatal ERK1/2 signaling by cannabinoids may provide new insights into the role of this pathway on a variety of neural processes.
Here we use gene-deletion mouse models to examine the effects of cannabinoids on striatal ERK1/2 signaling and to assess the influence of different receptor and protein systems on CB1 receptor-mediated signal transduction. We report that tetrad-relevant doses of both Δ9-THC and HU-210 promote ERK1/2 inactivation in the dorsal striatum in a CB1 receptor-dependent manner. Our results also demonstrate that elimination of dopamine D1 and reduction of NMDA receptors have opposite effects on cannabinoid-mediated ERK1/2 signaling.
Materials and Methods
Experimental Animals
All animal studies were conducted with an approved protocol from the Duke University Institutional Animal Care and Use Committee in accordance with National Institutes of Health guidelines. C57BL/6J (Jackson Labs, Bar Harbor, ME), dopamine receptor 1 knockout (D1R KO) (Drago et al., 1994), dopamine receptor 2 KO (D2R KO) (Kelly et al., 1997), dopamine transporter KO (DAT KO) (Giros et al., 1996), NR1 knockdown (NR1-Kd) (Mohn et al., 1999), βarrestin-1 KO (Conner et al., 1997) and βarrestin-2 KO (Bohn et al., 1999) mice have been previously described. Experimental animals were generated by crossing heterozygotes to obtain wild-type (WT) and KO littermates for all but the NR1-Kd line. The breeding strategy for the generation of these animals has been described (Mohn et al., 1999). Drug naïve mice 2–6 months of age and of mixed sex were utilized for all studies. Animals were housed no more than five to a cage in a temperature- and humidity-controlled room on a 12 h light/dark cycle with ad libitum access to food and water.
Drugs
HU-210 was purchased from Tocris Biosciences (Ellisville, MO). Δ9-THC and AM251 were purchased from Sigma (St. Louis, MO). HU-210 was initially sonicated in a minimal amount of Tween-80 (Sigma) and then diluted to volume with water. Δ9-THC (Sigma) was dissolved in a 1:1:18 ratio of ethanol:cremophor EL:saline. AM251 was initially sonicated in a 1:1 ratio of DMSO:Tween-80 and then brought up to volume with saline. All drugs and the corresponding vehicle solutions were injected as described at a volume of 10 ml/kg body weight.
Measurement of Phosphoprotein Levels by Western Analyses
Mice were injected with the indicated vehicle or drug and then euthanized either by focused microwave irradiation (4.2–5.0 kW for 1.22s) using a small animal microwave (Muromachi Kikai, Tokyo, Japan) or by cervical dislocation followed by rapid decapitation. Both are well-accepted methods to preserve phosphoproteins in vivo. Subsequently, the heads of the decapitated animals were rapidly cooled by immersion in liquid nitrogen for 6 s as previously described (Beaulieu et al., 2006), and the indicated brain regions were dissected out on an ice-cold surface. To isolate the dorsal or ventral striatum, we made two slightly angled cuts (approximately 15° deviated from the coronal plane) using three white matter landmarks (corpus callosum, fornix of the hippocampus and the anterior commissure) as visualized from the midline sagittal view of each half brain for reference. The tissue section (of approximately 1.0–1.25 mm thickness) was placed flat against the cut surface and the dorsal striatum or ventral striatum was dissected in a rapid manner away from the extrastriatal tissue. The frontal cortex or hippocampus was also rapidly dissected from the same hemisphere in subsequent steps. Tissue samples were “snap-frozen” in liquid nitrogen and then sonicated in boiling 1% SDS solution supplemented with protease (Complete Cocktail; Roche Applied Science, Indianapolis, IN) and phosphatase inhibitors (Halt Cocktail; Thermo Fisher, Rockford IL). Protein levels were determined with the BCA kit (Thermo Fisher) and 25 µg of protein per lane was resolved on 10% Tris-Glycine gels (Invitrogen, Carlsbad, CA). Proteins were transferred to nitrocellulose membranes and immunoblotted for pERK1/2 (#9101, 1:500; Cell Signaling Technologies, Danvers, MA), ERK1/2 (#9107, 1:500; Cell Signaling Technologies), pT34 DARPP-32 (p1025-34, 1:300; Phosphosolutions, Aurora, CO), DARPP-32 (#611520, 1:1000; BD Biosciences, San Jose, CA), or actin (MAB1501R, 1:5000; Millipore Corporation, Billerica, MA). Primary antibodies were revealed with infrared dye-conjugated secondary antibodies (Invitrogen and Rockland Immunochemicals, Gilbersville, PA). Direct fluorescence was detected using an Odyssey Infrared imaging system (LI-COR Biosciences, Lincoln, NE) and the band intensity on the individual blots was quantified using ImageJ analysis software (NIH). Phosphoprotein levels for each sample were normalized to the corresponding total protein levels and the fold stimulation was determined by normalizing these values to the average of the vehicle treatment group for each separate experiment.
Behavioral Analyses
Animals were habituated to the behavioral testing room for several days prior to the experiment. All behavioral tests were performed during the light phase of the cycle (between 10:00 a.m. to 5:00 p.m.) and in the following order. Basal body temperature was measured in all animals prior to injection. Mice were then injected with the indicated vehicle or drug solution and placed immediately into the open field. After 30 min, mice were removed from the open field and placed onto the hot-plate meter. Body temperature was then measured, followed shortly thereafter by the test for catalepsy.
Locomotor activity
Spontaneous open field locomotion was evaluated under illuminated conditions in an automated Omnitech Digiscan apparatus (Accuscan Instruments). Non-habituated mice were injected with vehicle or the indicated drug, placed into the open field, and monitored for 30 min after injection. Locomotor activity was measured at 5 min intervals in terms of the total distance traveled (cm/5 min).
Analgesia
Antinociceptive responses were measured using a hot plate analgesia meter maintained at 56 ± 0.5°C (Columbus Instruments). The time that elapsed before the animal “responds” was recorded as response latency (s). The response was defined as either licking or clenching the fore- or hindpaws or flicking the hindpaws. The most prominent “response” in these studies was forepaw licking and clenching. To avoid tissue damage, animals were exposed to the hot plate for a maximum of 30 s.
Catalepsy
Catalepsy was measured using the conventional fixed bar method. For this test, the forepaws of the animal were placed on a 1 cm diameter wooden bar fixed horizontally at 3.5 cm from the bench surface. Descent latency was recorded for an observation period of 60 s and was defined as the time until the animal removed either one or both forepaws from the bar.
Body temperature
Rectal temperature was measured with a digital thermometer (TH8 Physitemp). The probe was inserted into the rectum and maintained until the temperature reading stabilized.
Immunohistochemistry
Mice were injected with vehicle or Δ9-THC (1 mg/kg i.p.) and placed back in their home cage. Mice were then removed 16 min after the injection of vehicle or drug and injected with chloral hydrate (500 mg/kg i.p.). After an additional 4 min (20 min total Δ9-THC treatment), brains were fixed by transcardial perfusion of ice-cold phosphate buffered saline (PBS) followed by 10% formalin solution (Sigma). Brains were removed and postfixed in the same fixative solution overnight at 4°C, sectioned (sagittal sections; 50 µm) on a vibratome and immediately processed for immunohistochemistry. Detection of pERK1/2 was performed in a similar fashion to that previously described (Valjent et al., 2001; Derkinderen et al., 2003; Valjent et al., 2004). Briefly, free-floating brain sections were washed 3X in PBS and then incubated for 5 min in PBS containing 10% MeOH, 3% H202 and 0.5% NaBH2. Sections were then washed 3X in PBS followed by a 15 min incubation in 1.2% Triton-X 100. After an additional 3X washes in PBS, the sections were blocked for 1 hr in PBS containing 5% normal goat serum, 2% bovine serum albumin and 0.2% Triton-X 100 and then incubated overnight at 4°C in the pERK1/2 primary antibody (#9101; 1:500). Following the overnight incubation, sections were washed 3X in PBS and then incubated for 1 hr in blocking solution containing an anti-rabbit Alexa488 secondary antibody (1:1500; Invitrogen). Finally, sections were washed 3X in PBS and mounted in Vectashield (Vector Laboratories, Burlingame, CA). Images (1024 × 1024) were collected on a Zeiss LSM-510 laser-scanning microscope. Confocal images from vehicle and drug-treated mouse sections were acquired using the same instrument settings.
Statistical Analyses
All statistical analyses were performed using GraphPad Prism 4.0 software (GraphPad Software, La Jolla, CA). The results are displayed as means and standard errors of the mean (SEM) and the data were analyzed one-way ANOVA with a Dunnett post-test for comparison of the drug effect relative to a vehicle treatment group or by an unpaired Student’s, two-tailed t-test as indicated in the figure legends. A two-way ANOVA test with a post hoc Bonferroni test was used for comparisons between genotypes and drug treatments. A p < 0.05 was considered significant.
Results
Cannabinoids disrupt ERK1/2 signaling in the striatum and frontal cortex in a CB1R-dependent manner
There have been no studies to date that have examined the influence of CB1 receptor activity on striatal ERK1/2 signaling using doses of cannabinoid that elicit the well-accepted tetrad of behavioral effects in rodent models (≥ 0.001 mg/kg for HU-210 and ≥ 3 mg/kg for Δ9-THC i.p.) (Fox et al., 2001; Monory et al., 2007). To investigate the effects of cannabinoids on striatal ERK1/2 signaling with tetrad-relevant doses of cannabinoids, we initially administered HU-210, a synthetic agonist analog of Δ9-THC and potent CB1 receptor agonist, to C57BL/6J mice. Systemic administration of HU-210 for 1 h dose-dependently decreased (F3,25 = 7.70, p < 0.001, one-way ANOVA) the levels of pERK1/2 in cellular extracts prepared from the dorsal striatum (Fig. 1A). The HU-210-mediated reduction in pERK1/2 levels became significant within 30 min post-injection and remained significantly depressed (F4,19 = 16.42, p < 0.0001, one-way ANOVA) at all time points evaluated past this time-point (Fig. 1B). To determine if CB1 receptor activation was required for the disruption of ERK1/2 signaling by HU-210, we utilized AM251, a CB1 receptor antagonist. Co-administration of AM251 with the maximum dose of HU-210 (0.25 mg/kg) used in this study prevented the decrease in pERK1/2 levels (F3,16 = 5.00, p < 0.05, one-way ANOVA) by HU-210 (Fig. 1C). These results confirm the specific involvement of CB1 receptors in the disruption of ERK1/2 signaling in the dorsal striatum. Interestingly, the administration of AM251 alone did not alter the levels of pERK1/2 suggesting that the constitutive activity of CB1 receptors is negligible in this preparation (Fig. 1C). To preclude the possibility of agonist-specific signaling, we next examined the effects of the CB1 receptor agonist Δ9-THC on ERK1/2 signaling in the dorsal striatum. Acute administration of Δ9-THC (10 mg/kg) to C57BL/6J mice also resulted in a significant decrease (p = 0.02, unpaired t-test) in pERK1/2 levels in extracts from dorsal striatum (Fig. 1D). Taken together, our results indicate that the inactivation of ERK1/2 signaling in the dorsal striatum is a common biochemical outcome of CB1 receptor stimulation by behaviorally relevant doses of cannabinoids.
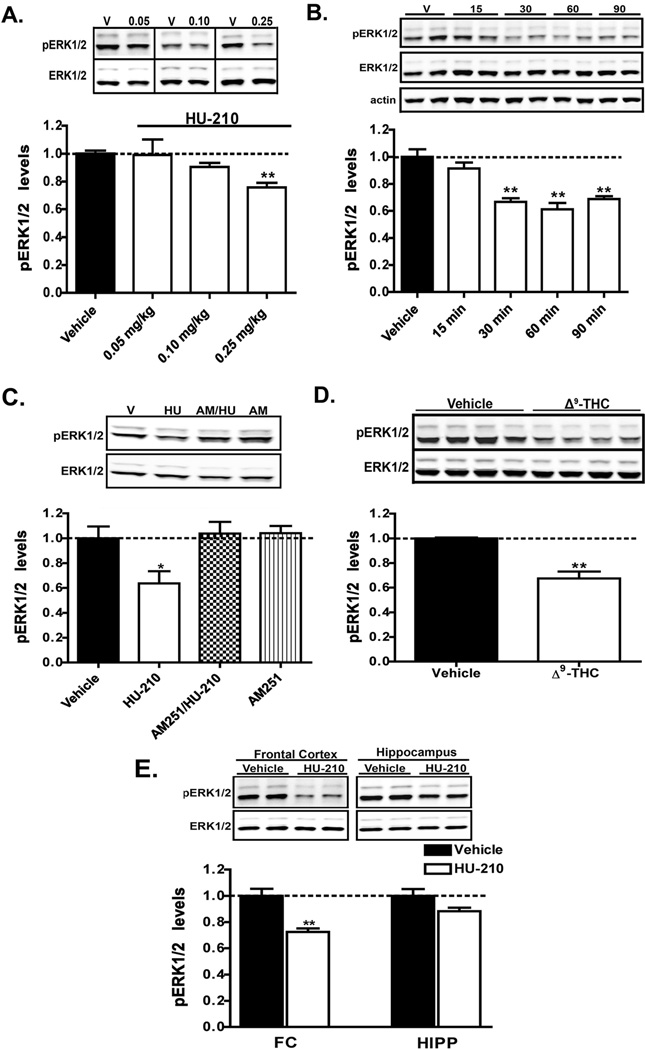
Figure 1. Administration of HU-210 or Δ9-THC promotes ERK1/2 inactivation in the dorsal striatum.
A) C57Bl/6J mice were injected with either vehicle or the indicated doses HU-210, the CB1R agonist. pERK1/2 levels 60 min post-injection were measured in extracts prepared from the dorsal striatum. B) C57Bl/6J mice were administered HU-210 (0.25 mg/kg i.p.) and pERK1/2 levels in striatal extracts were measured at the indicated time-points post-injection. C) C57Bl/6J mice were administered either vehicle, HU-210 alone (0.25 mg/kg i.p), the CB1R antagonist AM251 alone (5 mg/kg i.p.) or given AM251 (5 mg/kg i.p.) 15 min prior to injection of HU-210 (0.25 mg/kg i.p.). pERK1/2 levels were assessed 60 min post-injection of either vehicle, HU-210 or AM251 alone. D) C57Bl/6J mice were injected with either vehicle or Δ9-THC (10 mg/kg i.p.) and euthanized 60 min post-injection. pERK1/2 levels were measured in extracts from dorsal striatum. E) HU-210 (0.25 mg/kg i.p.) was administered to C57Bl/6J mice and the levels of pERK1/2 in extracts from the frontal cortex (FC) or hippocampus (HIPP) were measured 60 min post-injection. Representative western blots and the corresponding densitometric analyses are shown. Quantification is presented as the mean ± S.E.M.; n = 5–10 mice for each treatment group. pERK1/2 levels were normalized to the levels of ERK1/2 in the respective striatal extracts. **p < 0.01 and *p < 0.05, vehicle versus HU-210 by one-way ANOVA or unpaired t-test.
Since CB1 receptors are also expressed at functionally relevant levels in the frontal cortex and hippocampus, we next examined the effect of acute HU-210 administration on ERK1/2 signaling in these brain regions (Mackie, 2005). As shown in Fig. 1E, administration of HU-210 resulted in an inhibition of ERK1/2 signaling in frontal cortex extracts in a magnitude comparable to that observed in the dorsal striatum (p = 0.009, unpaired t-test), whereas pERK1/2 levels in the hippocampus were essentially unmodified (p = 0.63, unpaired t-test). These results highlight a region-specific role for CB1 receptors in the disruption of ERK1/2 signaling.
To confirm that the doses of HU-210 and Δ9-THC utilized in the biochemical studies promote the tetrad of CB1 receptor-dependent behaviors, we next examined the acute effects of these agonists on locomotor activity, nociception, catalepsy and body temperature (Fig. 2). Administration of HU-210 (0.25 mg/kg) to C57BL/6J mice resulted in a significant decrease (F3,32 = 7.10, p < 0.001, one-way ANOVA) in locomotor activity (Fig. 2A) and body temperature (F3,32 = 41.32, p < 0.0001, one-way ANOVA) relative to vehicle treated animals (Fig. 2D). Additionally, we observed an increase in catalepsy (F3,32 = 75.28, p < 0.0001, one-way ANOVA) (Fig. 2B) and HU-210-induced analgesia (F3,31 = 40.65, p < 0.0001, one-way ANOVA) (Fig. 2C). Co-administration of AM251 (3 mg/kg or 5 mg/kg) with HU-210 (0.25 mg/kg) prevented the decrease in locomotor activity (Fig. 2A) and both increases in catalepsy (Fig. 2B) and antinociception (Fig. 2C). HU-210 treatment did promote hypothermia even when co-administered with AM251 (F3,32 = 41.32, p < 0.05, one-way ANOVA), but the magnitude change (−1.9 ± 0.33 for HU-210 vs. 0.21 ± 0.14 for AM251/HU-210) was significantly reduced (p < 0.0001, HU-210 versus AM251/HU-210 by unpaired t-test) relative to the effect of HU-210 alone (Fig. 2D). Similar to our results with HU-210, we found that the acute administration of Δ9-THC (10 mg/kg) to C57BL/6J mice promoted hypolocomotion (Fig. 2E), catalepsy (Fig. 2F), antinociception (Fig. 2G) and hypothermia (Fig. 2H) (all significant effects relative to vehicle control by unpaired t-test). These results confirm that the doses of HU-210 and Δ9-THC utilized in this study promotes the tetrad of behavioral effects. Furthermore, the blockade of HU-210-mediated effects by AM251 indicates that CB1 receptors mediate these behaviors.
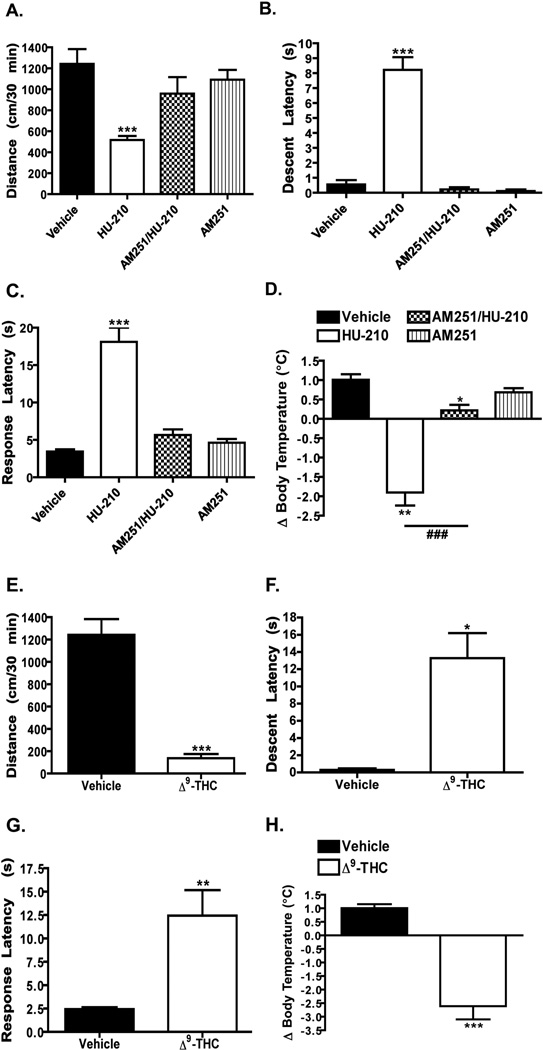
Figure 2. Acute administration of HU-210 or Δ9-THC promotes the “tetrad” of CB1 receptor-dependent behaviors.
A–D) C57Bl/6J mice were administered vehicle, HU-210 alone (0.25 mg/kg i.p.), AM251 alone (3 mg/kg i.p. (A) or 5 mg/kg i.p. (B–D)) or AM251 at the same dose 15 min prior to injection of HU-210. Locomotor activity (A), catalepsy (B), analgesia (C) and body temperature (D) were measured as described in the “Materials and Methods” section. Quantification is presented as the mean ± S.E.M.; n = 9 mice for each treatment group. ***p < 0.001, **p < 0.01 and *p < 0.05, vehicle versus treatment groups by one-way ANOVA. # # #p < 0.001, HU-210 versus AM251/HU-210 by unpaired t-test. E–H) C57Bl/6J mice were administered vehicle or Δ9-THC alone (10 mg/kg i.p.). Locomotor activity (D), catalepsy (E), analgesia (F) and body temperature (H) were measured. Quantification is presented as the mean ± S.E.M.; n = 7–8 mice for each treatment group. ***p < 0.001, **p < 0.01 and *p < 0.05, vehicle versus Δ9-THC by unpaired t-test.
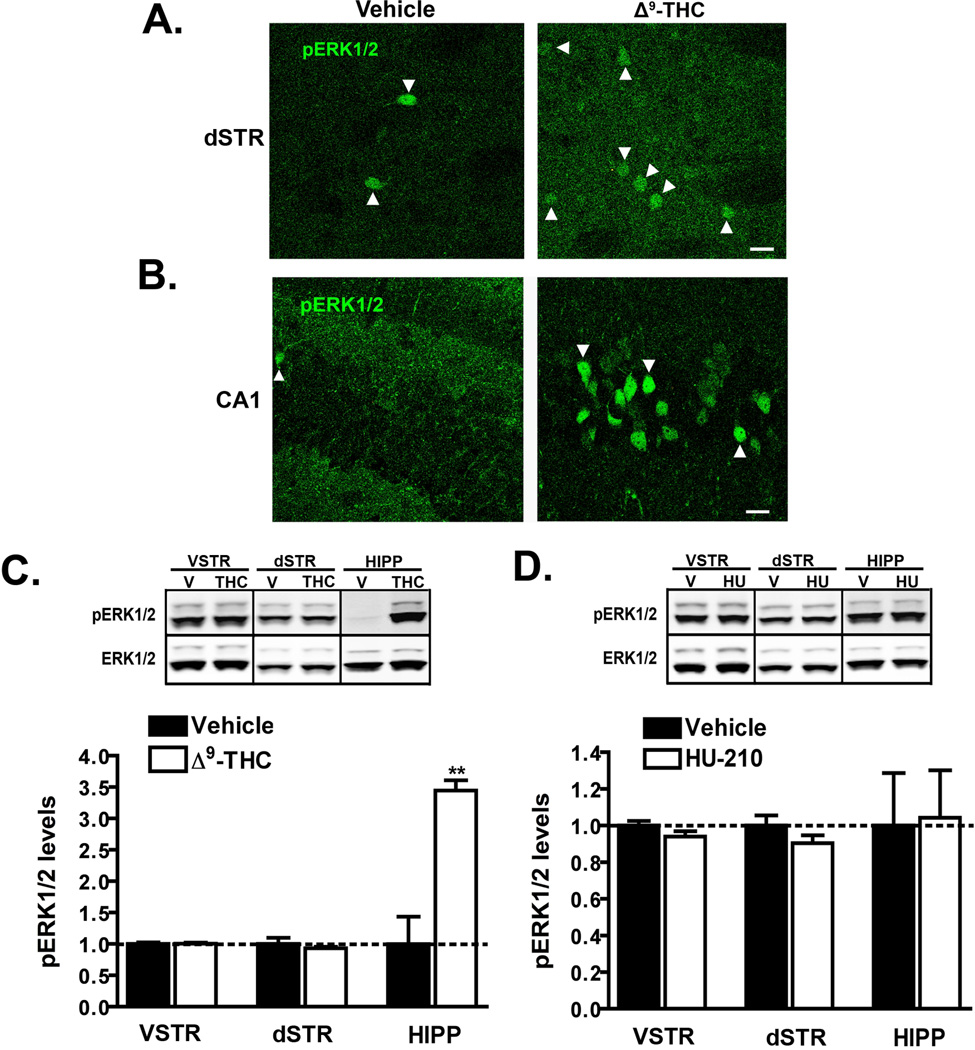
Since previous studies have reported that a low dose of Δ9-THC (1 mg/kg i.p.) transiently increases the number of pERK1/2 positive cells in the dorsal striatum and the CA1 region of the hippocampus (Valjent et al., 2001; Derkinderen et al., 2003; Valjent et al., 2004), we next examined the effects of this same dose and duration of treatment using two different methods. We first analyzed ERK1/2 activation by immunohistochemistry in both of these brain regions (Fig. 3A, B). Under basal conditions, we observed only a few strongly labeled pERK1/2 immunoreactive cell bodies per field in both the dorsal lateral region of the striatum (Fig. 3A) and CA1 (Fig. 3B). Administration of Δ9-THC (1 mg/kg i.p.) for 20 min resulted in an increase in the number of pERK1/2 positive cells relative to vehicle in the same region of the dorsal striatum (2 versus 7 cells in Fig. 3A). Interestingly, the strongly labeled pERK1/2 positive cells clearly evident in the vehicle treated mice, were absent following Δ9-THC treatment. We observed a similar number of pERK1/2 positive cells in different subregions of the dorsal striatum in both vehicle and Δ9-THC treated animals (data not shown). In contrast, administration of the low dose of Δ9-THC (1 mg/kg i.p.) promoted a robust increase in both the number and intensity of pERK1/2 immunoreactive cells in the hippocampus in the very same sagittal brain sections (prepared from the same animal) used to quantify striatal pERK1/2 immunoreactivity (Fig. 3B). Strong labeling was detected in the pyramidal cell layers of CA1 (Fig. 3B) and also in the dentate gyrus and CA3 (data not shown). To determine if treatment with the low dose of Δ9-THC promotes a concomitant quantitative change in ERK1/2 activity, we analyzed pERK1/2 levels by western blot (Fig. 3C). This analysis revealed no significant difference (p = 0.52 by unpaired t-test) in pERK1/2 levels following Δ9-THC administration in either the dorsal or ventral striatum (Fig. 3C). This low dose of Δ9-THC did however promote a very robust increase in pERK1/2 levels (344 ± 16% of control; p = 0.001 by unpaired t-test) in the hippocampus of the same animals. We also analyzed the effects of a low dose of HU-210 (0.05 mg/kg i.p.) on ERK1/2 signaling (Fig. 3D). At this dose, we did not detect any significant differences (p > 0.05 in all cases by unpaired t-test) in pERK1/2 levels in all three brain regions suggesting that a lower dose may be needed to promote ERK1/2 activation in the hippocampus. Collectively, these results suggest that despite the observed increase in the number of pERK1/2 immunoreactive cells in the dorsal lateral striatum following administration of 1 mg/kg Δ9-THC, this does not promote an overall quantitative change in pERK1/2 levels.
Figure 3. Administration of a low dose of Δ9-THC does not promote a quantitative increase in pERK1/2 levels in the striatum.
A–B) C57Bl/6J mice were administered vehicle or Δ9-THC (1 mg/kg i.p.) and euthanized 20 min post-injection. pERK1/2 immunoreactivity in the dorsal lateral striatum (A) and the CA1 region of the hippocampus (B) was assessed in both treatment groups by immunohistochemistry. Representative confocal images of pERK1/2 immunoreactivity at 40X magnification from the two different brain regions in the same mouse are shown. Scale bar denotes a distance of 20 µm. Arrowheads denote all pERK1/2 positive cell bodies in the dorsal striatum and a small subset of the positively labeled in the CA1 region. n = 3 mice for each treatment group. C, D) pERK1/2 levels were measured in extracts from the dorsal striatum (dSTR), ventral striatum (VSTR) or the hippocampus (HIPP) 20 min post-injection of Δ9-THC (1 mg/kg i.p.) (C) or HU-210 (0.05 mg/kg i.p.) (D). Representative western blots and the corresponding densitometric analyses are shown. Data are mean ± S.E.M.; n = 4–5 mice for each treatment group. **p < 0.01, vehicle versus Δ9-THC by unpaired t-test.
D1 receptors attenuate CB1R-mediated signaling
Previous evidence suggests that D1 and D2 dopamine receptors may be involved in CB1 receptor signaling in the striatum (Valjent et al., 2001; Valjent et al., 2004; Andersson et al., 2005). To examine the relationship between these two distinct receptor systems, we measured pERK1/2 levels in dorsal striatum following HU-210 administration in dopamine receptor KO mice. As shown in Fig. 4A, CB1 receptor activation in D1R KO mice resulted in a statistically significant more pronounced inactivation (F1,27 = 5.96, p = 0.02 by two-way ANOVA) of ERK1/2 (58 ± 3.6% of control), relative to that observed in wild-type littermates (80 ± 3.4% of control). The effect of HU-210 treatment in both genotypes was considered highly significant (F1,27 =45.90, p < 0.001 by two-way ANOVA). Importantly, since the levels of pERK1/2 and ERK1/2 in the dorsal striatum of drug-naïve animals were not different (p = 0.02 by unpaired t-test) (Fig. 4B), the HU-210-induced reduction of pERK1/2 levels cannot be attributable to a decrease in the basal state. In contrast, a similar level of ERK1/2 inactivation (F1,14 = 25.76, p = 0.0002 by two-way ANOVA) was observed in both genotypes when HU-210 was administered to D2R KO mice (Fig. 4C). The genetic deletion of D2 receptors did not alter (p = 0.27 by unpaired t-test) the basal levels of striatal pERK1/2 (Supp. Fig. 1A). Since inactivation of ERK1/2 was more pronounced in D1R KO mice than wild-type controls and because differential inactivation was not observed between D2R KO mice, these findings collectively suggest that D1 receptors may play a more important role in negatively modulating this pathway.
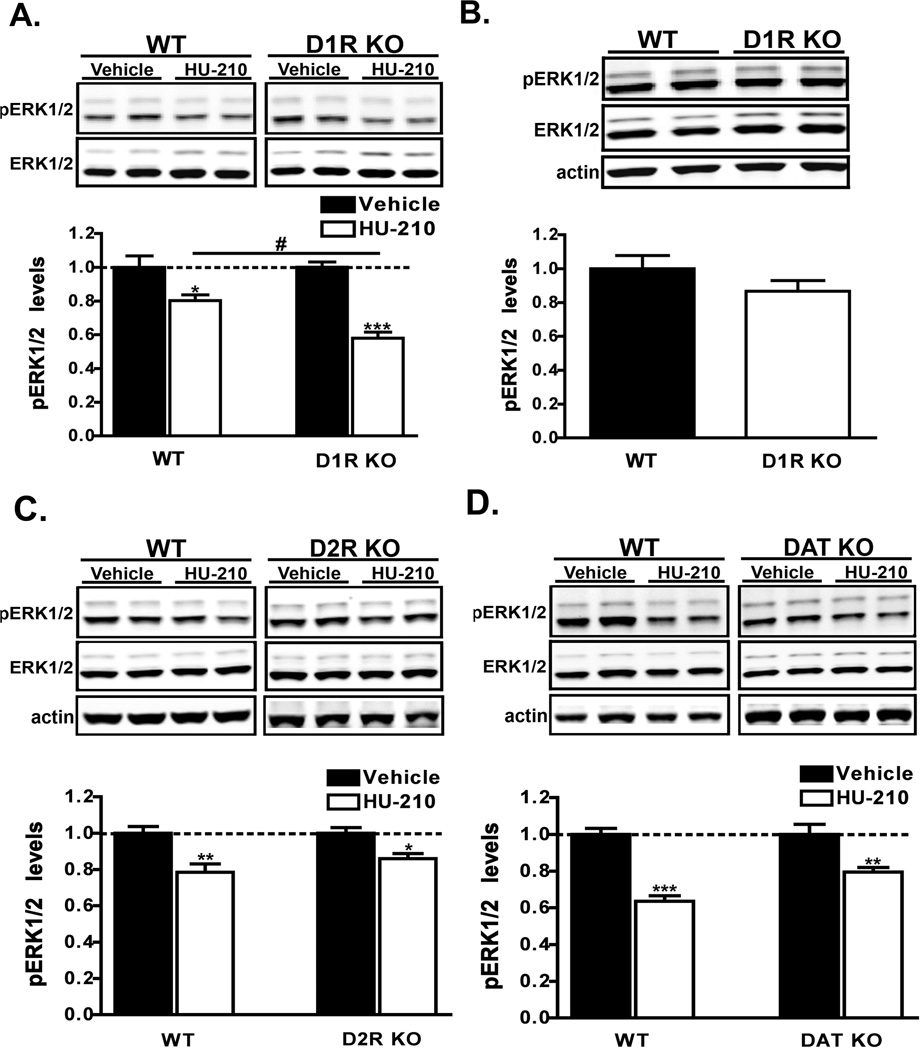
Figure 4. HU-210-mediated ERK1/2 inactivation is augmented in D1R KO mice.
A) Western analyses of pERK1/2 levels in extracts from dorsal striatum of wild-type (WT) or D1R KO mice that were administered HU-210 (0.25 mg/kg i.p.) and euthanized 60 min post-injection. n = 9–10 mice for each treatment group. B) Measurement of basal pERK1/2 levels between WT and D1R KO drug-naïve mice. pERK1/2 levels were normalized to the levels of ERK1/2 in dorsal striatal extracts. Data are mean ± S.E.M.; n = 5 mice for each group. An unpaired t-test found no significant difference between genotypes. C–D) HU-210 (0.25 mg/kg i.p.) was administered to WT, D2R KO (C) or DAT KO (D) mice and the levels of pERK1/2 in striatal extracts was measured by western blot 60 min post-injection. Data are mean ± S.E.M.; n = 4–5 mice for each treatment group. ***p < 0.001 and **p < 0.01 and *p < 0.05, vehicle versus HU-210 treatment within genotypes and #p < 0.05 for comparison between genotypes of the drug effect by two-way ANOVA.
To further investigate the relationship between the dopamine system and cannabinoid-mediated signaling we utilized the dopamine transporter knockout (DAT KO) mice, an animal model with elevated extracellular levels of dopamine (Giros et al., 1996). Interestingly, these mice also have altered striatal levels of the endocannabinoid anandamide suggesting that hyperdopaminergia may promote alterations in the cannabinoid system (Tzavara et al., 2006). We therefore next asked if CB1 receptor-mediated signaling was altered in these animals. As shown in Figure 4D, treatment of DAT KO mice and wild-type littermates with HU-210 resulted in similar extents (F1,14 = 58.65, p < 0.0001 by two-way ANOVA) of ERK1/2 inactivation in striatal extracts between genotypes. Acute Δ9-THC (10 mg/kg) treatment also resulted in similar responses in DAT KO mice (data not shown). The genetic deletion of dopamine transporters did not alter (p = 0.27 by unpaired t-test) the basal levels of striatal pERK1/2 (Supp. Fig. 1B). These data suggest that elevated levels of extracellular dopamine and the concomitant activation of both D1 and D2 dopamine receptors do not modulate CB1 receptor-mediated inactivation of ERK1/2 signaling. Additionally, the lack of regulation by D2 receptors (Fig. 4C) suggests that additional mechanisms in the DAT KO mice, such as D3 dopamine receptors, may regulate the antagonism of HU-210-mediated ERK1/2 signaling by D1 receptors.
NMDA receptors are required HU-210-mediated ERK1/2 inactivation
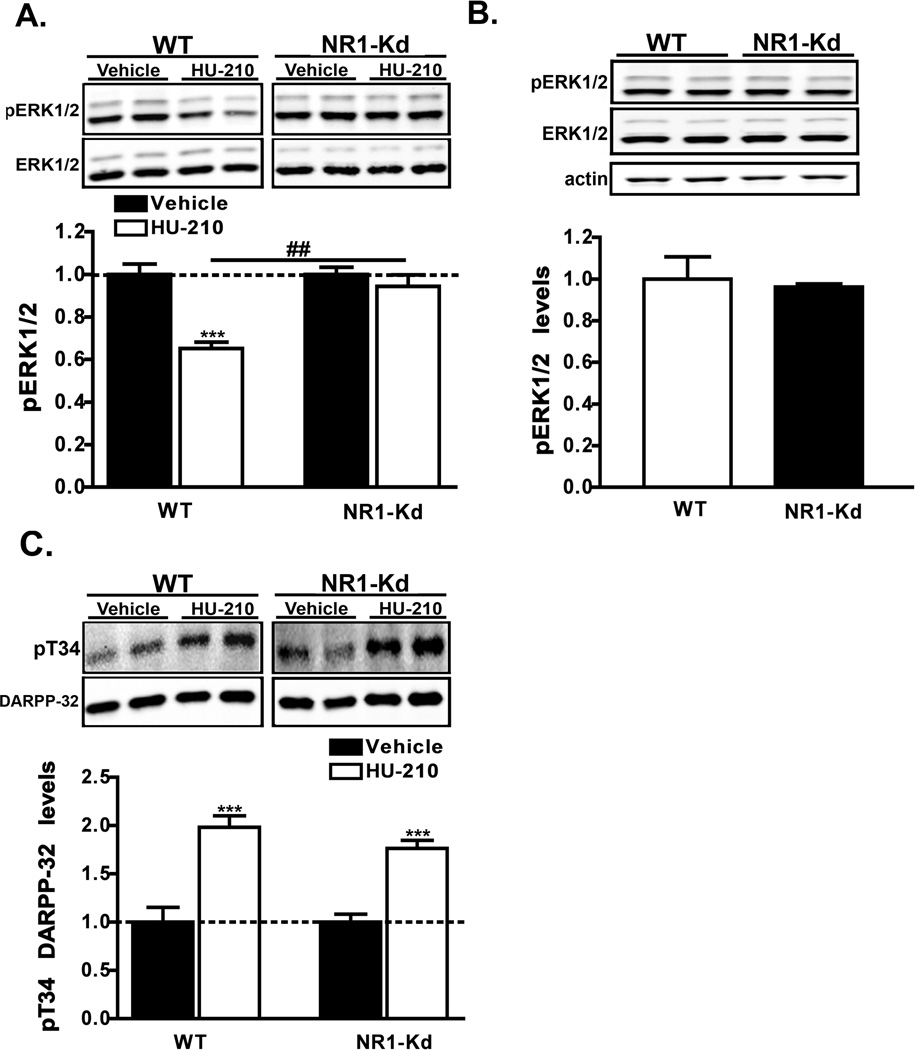
Previous work has demonstrated that CB1 receptor activation causes a reduction in glutamate release which alters synaptic plasticity in the striatum (reviewed in (van der Stelt & Di Marzo, 2003)). Since ERK1/2 activation in response to glutamatergic signaling via NMDA receptors is implicated in the control of long-term synaptic plasticity (Thomas & Huganir, 2004), we hypothesized that NMDAR receptors may be involved in cannabinoid-mediated ERK1/2 signaling. To test this possibility we analyzed the effects of HU-210 in NMDA receptor subunit-1 knockdown (NR1-Kd) mice. These animals have a global reduction (~90%) of NMDA receptors and thus make them a useful genetic model to study the role of NMDA receptors in vivo (Mohn et al., 1999). While the administration of HU-210 to wild-type littermate controls resulted in markedly reduced pERK1/2 levels (65 ± 3%), HU-210-induced ERK1/2 inactivation was completely prevented (94 ± 5.4%) in NR1-Kd mice (F1,31 = 11.41, p = 0.0020 for interaction and F1,31 = 22.03, p < 0.0001 for treatment by two-way ANOVA) (Fig. 5A). Evaluation of basal pERK1/2 levels in drug naïve animals revealed no significant differences between genotypes (Fig. 5B), demonstrating that the genetic reduction of NMDA receptors does not alter (p = 0.73 by unpaired t-test) basal pERK1/2 levels alone in the dorsal striatum. CB1 receptor activation has been reported to increase the phosphorylation of the dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) at the protein kinase A site, Threonine34 (T34), in mouse striatal extracts (Andersson et al., 2005; Borgkvist et al., 2008). To preclude the possibility of altered CB1 receptor sensitivity in NR1-Kd mice, we also evaluated the ability of HU-210 to activate T34 DARPP-32 signaling. As previously reported, CB1 receptor activation resulted in a robust increase (198 ± 12%) in pT34 DARPP-32 phosphophorylation in striatal extracts (Fig. 5C). A similar increase (176 ± 8.2%) in pT34 DARPP-32 phosphorylation was observed (F1,12 = 58.97, p < 0.0001 by two-way ANOVA for treatment) in HU-210-treated NR1-Kd mice suggesting that CB1 receptor sensitivity is preserved in this animal model (Fig. 5C). Collectively, these results reveal a central requirement for post-synaptic NMDA receptors in the inhibition of ERK1/2 signaling by cannabinoids.
Figure 5. HU-210-mediated ERK1/2 inactivation is prevented in NR1-Kd mice.
A, C) WT or NR1-Kd mice were injected with HU-210 (0.25 mg/kg, i.p.) and euthanized 60 min post-injection. Striatal levels of pERK1/2 (A) or pT34 DARPP-32 (C) were measured. Representative western blots and the corresponding densitometric analyses are shown. pERK1/2 and pT34 DARPP-32 levels were normalized to the levels of ERK1/2 and DARPP-32 respectively. Data are mean ± S.E.M.; n = 5–10 mice for each treatment group. ***p < 0.001, vehicle versus HU-210 treatment within genotypes and ##p < 0.05 for comparison between genotypes of the drug effect by two-way ANOVA. B) Basal levels of pERK1/2 in striatal extracts from drug-naïve WT and NR1-Kd were compared. Data are mean ± S.E.M.; n = 5 mice for each group.
βarrestin-1 and βarrestin-2 do not modulate CB1R signaling
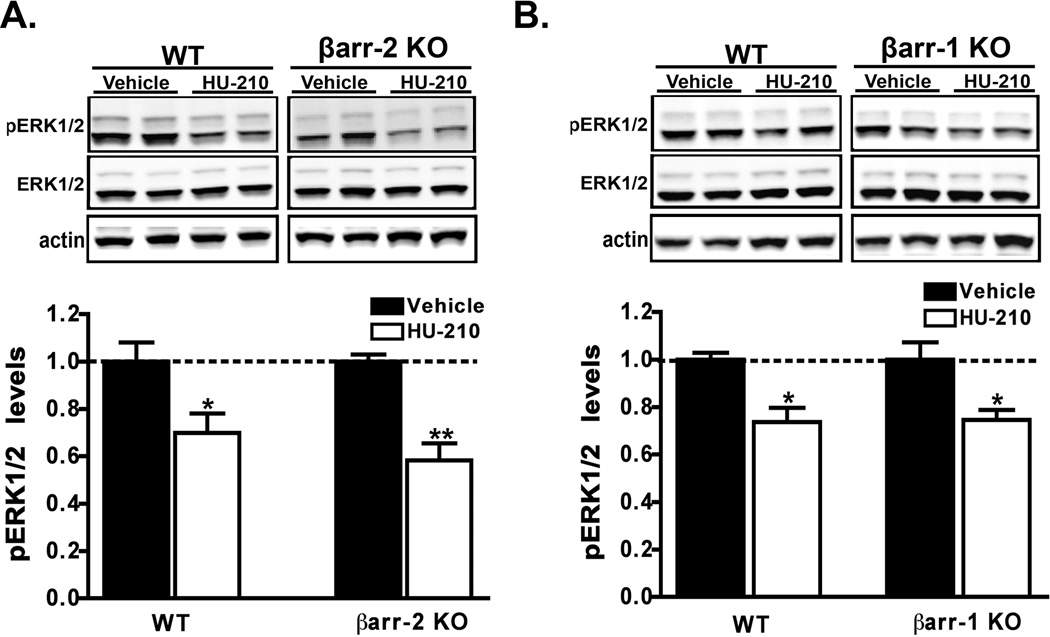
Previous investigations in vitro have suggested that the multifunctional adaptor protein, βarrestin-2, may regulate CB1 receptor desensitization and trafficking (Jin et al., 1999; Kouznetsova et al., 2002; Daigle et al., 2008a; Daigle et al., 2008b). Additionally, select behavioral responses to acute Δ9-THC administration were recently reported to be enhanced in βarrestin-2 deficient mice (Breivogel et al., 2008). To examine the possibility that βarrestin-2 may modulate CB1 receptor signaling in vivo we examined the effects of HU-210 in βarrestin-2 KO mice. Systemic administration of HU-210 to βarrestin-2 KO mice and wild-type controls resulted in similar extent (F1,16 = 27.13, p < 0.0001 by two-way ANOVA) of ERK1/2 inactivation in dorsal striatum (Fig. 6A). Both genotypes also responded in a similar fashion to acute Δ9-THC treatment (data not shown). Since the βarrestin-1 isoform is expressed at higher levels than βarrestin-2 in the dorsal striatum (unpublished observations) and is reportedly modulated by chronic Δ9-THC treatment (Rubino et al., 2006), we next examined the effect of HU-210 in βarrestin-1 deficient mice. Similar to what we observed in βarrestin-2 KO mice, HU-210 treatment promoted ERK1/2 inactivation to similar extents (F1,12 = 21.59, p = 0.0007 by two-way ANOVA) in the βarrestin-1 KO mice (Fig. 6B). The genetic deletion of βarrestin-2 or βarrestin-1 did not alter (p = 1.0 and p = 0.07 respectively, by unpaired t-test) the basal levels of striatal pERK1/2 (Supp. Fig. 1C,D). Collectively, these data suggest that βarrestins are not required for HU-210-mediated ERK1/2 inactivation in the striatum and that βarrestins do not modulate the magnitude of CB1 receptor signaling.
Figure 6. βarrestin-1 or 2 deficiency does not alter HU-210-mediated ERK1/2 inactivation.
A, B) βarrestin-1 or 2 mice were administered HU-210 (0.25 mg/kg i.p.) and euthanized 60 min post-injection. The levels of pERK1/2 in dorsal striatal extracts prepared from either βarrestin-2 or βarrestin-1 KO mice or WT littermates were assessed by western analyses. Representative western blots and the corresponding densitometric analyses are shown. Data are mean ± S.E.M.; n = 4–5 mice for each treatment group. **p < 0.01 and *p < 0.05, vehicle versus HU-210 treatment within genotypes by two-way ANOVA.
Discussion
The present study demonstrates that cannabinoids negatively regulate ERK1/2 signaling in vivo. Through the use of genetic mouse models, we have found that the acute administration of tetrad-relevant doses of cannabinoid receptor agonists, inhibit ERK1/2 signaling in the dorsal striatum and frontal cortex. CB1 receptor activation is required for this phenomenon as the acute blockade of these receptors by AM251 effectively prevented the HU-210-mediated decrease in pERK1/2. Additionally, we have found that ERK1/2 inactivation was enhanced in D1R KO mice and was prevented in NR1-Kd mice, suggesting an opposite role for these two receptor systems in the regulation of striatal CB1R-mediated signaling.
Our results are consistent with the earlier studies that used immunohistochemical techniques to investigate Δ9-THC-mediated ERK1/2 signaling in the striatum and the hippocampus. In agreement with Valjent and colleagues (Valjent et al., 2001; Valjent et al., 2004), we find that the acute administration of a low dose of Δ9-THC (1 mg/kg i.p.) to wild-type mice increases the number of pERK1/2 immunoreactive cell bodies in the dorsal striatum. Additionally, the robust increase of intensely labeled pERK1/2 immunoreactive cell bodies that we observe in the hippocampus is consistent with the findings of Derkinderen and coworkers (Derkinderen et al., 2003). Although the authors of these previous studies concluded that the differences in pERK1/2 immunoreactive cell bodies would translate into quantitative differences within these brain regions, this was never formally investigated using appropriate quantitative methods. Here we report that the administration of a low dose of Δ9-THC to wild-type mice selectively increases pERK1/2 levels in the hippocampus, but does not alter overall striatal pERK1/2 levels. Therefore, the apparent increase in the number of immunoreactive cell bodies does not translate into a global increase in striatal ERK1/2 activity. This may be reflective of the small percentage of all striatal cells exhibiting immunoreactivity with this treatment (in contrast to the relatively large number of immunoreactive cells in the hippocampus). Alternatively, perhaps the modest increase in pERK1/2 immunoreactivity in the cell bodies is accompanied by a decrease in the axonal and/or dendritic compartments (i.e. cellular redistribution) at this dose. More advanced quantitative methods for measuring phosphoprotein levels in a cell type and intracellular region-specific manner (e.g. dendrites versus cell bodies) would be necessary to resolve this issue.
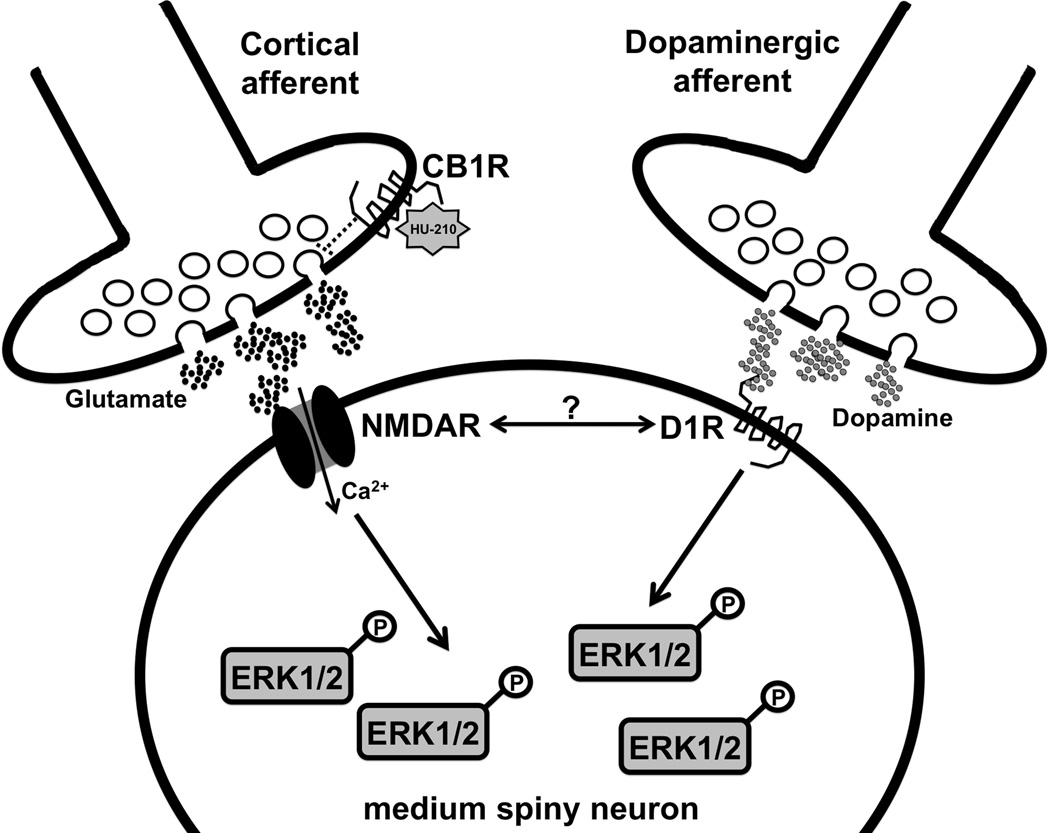
There are several plausible mechanisms by which CB1 receptor activation in the striatum leads to the inactivation of ERK1/2. Many studies have shown that CB1 receptor stimulation decreases corticostriatal glutamatergic transmission (Hoffman & Lupica, 2001; Huang et al., 2001; Robbe et al., 2001; Gerdeman et al., 2002). Therefore, one possibility is that the transient reduction of glutamate at this synapse promotes a decrease in ERK1/2 phosphorylation in postsynaptic GABAergic medium spiny neurons (MSNs) (Fig. 7). This scenario suggests that glutamatergic tone or potentially endogenous dopamine release – directly or indirectly, through the modulation of corticostriatal plasticity – may regulate ERK1/2 phosphorylation under basal conditions. The capacity of both systems to work in concert is well supported by the observation that NMDA and D1 receptors positively regulate ERK1/2 signaling in the striatum in response to various psychostimulants (Valjent et al., 2005; Beaulieu et al., 2006). An equally likely mechanism by which cannabinoids negatively regulate ERK1/2 in the striatum is through the direct activation of CB1 receptors expressed on the terminals (Matyas et al., 2006) or dendrites of both direct- and indirect-projecting MSNs (Rodriguez et al., 2001). This raises the possibility that CB1, D1 and NMDA receptors expressed in the same neuronal type interact at a functional level to control intracellular signaling. Given the importance of ERK1/2 signaling in the regulation of immediate-early genes and behavioral responses to drugs of abuse for example (Valjent et al., 2006a; Valjent et al., 2006b; Girault et al., 2007), future studies investigating ERK1/2 inactivation by cannabinoids in conditional CB1 mutant mice will be of great interest.
Figure 7. Schematic representation of one potential mechanism by which presynaptic CB1 receptors regulate ERK1/2 activity in MSNs.
Under basal conditions, endogenous glutamate and dopamine tone regulates pERK1/2 levels via NMDA and D1 receptors, respectively. Administration of HU-210 or Δ9-THC activates CB1 receptors on corticostriatal synapses thereby decreasing glutamate release and subsequent NMDA receptor activity. This results in a concomitant decrease in pERK1/2 levels in MSNs. D1 receptors may negatively regulate NMDA receptor-mediated signaling directly (possibly via hetero-oligomerization) or indirectly via the modulation of participating kinases downstream.
The contribution of NMDA receptors to CB1-mediated signaling has never been investigated in a genetic mouse model. Here we show that a global reduction in NMDA receptors leads to the complete blockade of HU-210-mediated ERK1/2 dephosphorylation in the dorsal striatum. Since long-term bouts of synaptic activity are intimately connected to ERK1/2 signaling under physiological conditions (Thomas & Huganir, 2004), it was somewhat surprising that the genetic reduction of NMDA receptors alone did not alter the basal levels of pERK1/2. This may be due to compensatory mechanisms that have developed in these animals over time or potentially suggest that the remaining 10% of functional NMDA receptors in these animals are sufficient to maintain striatal pERK1/2 levels. Regardless, the absence of a baseline pERK1/2 deficit supports the conclusion that the lack of effect of HU-210 (Robbe et al., 2002) in NR1-Kd animals is not a consequence of altered basal ERK1/2 phosphoprotein levels. While it is possible that the reduced expression of NMDA receptors alters CB1 receptor levels or responsiveness, this condition is unlikely since DARPP-32 phosphorylation was increased to similar extents in wild-type and NR1-Kd mice. Furthermore, while the cannabinoid system has not been extensively characterized in the NR1-Kd mice, numerous components of the dopamine system (i.e. dopamine receptor, dopamine transporter and extracellular dopamine levels) are not altered in these mutants (Ramsey et al., 2008), suggesting that NR1 deficiency does not produce gross alterations in important striatal components. While further characterization of the NR1-Kd mice is needed, at present, our results suggest that NMDA receptors are required for striatal CB1 receptor signaling.
Acute effects of HU-210 administration were not uniform across different brain areas. HU-210 disrupted ERK1/2 signaling in the dorsal striatum and frontal cortex, but exerted no effect in the hippocampus. These findings indicate that the engagement of different signaling pathways by CB1 receptors in vivo is dependent on the local cellular environment and may reflect the expression of different protein modulators. Additionally, the similar extent of inhibition of ERK by HU-210 in both the striatum and frontal cortex raise the possibility that common molecular mechanisms underlie this phenomenon between regions. These region-specific effects of cannabinoids may be exploited in future efforts to develop more effective cannabinoid-based therapies with minimal side effects.
A somewhat unexpected finding in this study was the lack of regulation by βarrestin proteins, especially given the existing functional data that have suggested a regulatory role for these proteins in various systems. For example, Jin and coworkers reported that overexpression of βarrestin-2 in Xenopus oocytes causes profound homologous CB1 receptor desensitization (Jin et al., 1999). Furthermore, Kouznetsova and colleagues showed that the expression of a dominant negative βarrestin-2 truncation mutant (Δ319–418) inhibited CB1 receptor desensitization in cultured hippocampal neurons (Kouznetsova et al., 2002). The physiological actions of βarrestin-2 in cannabinoid-mediated behavior have also been investigated recently (Breivogel et al., 2008). Breivogel et al. found that Δ9-THC-induced antinociception and hypothermia were selectively enhanced in βarrestin-2 KO mice (Breivogel et al., 2008). Collectively, these results suggest that CB1 receptor function may be regulated by βarrestin-2 in vivo and therefore we hypothesized that CB1 receptor desensitization may be impaired in βarrestin-2 deficient mice, leading to enhanced cannabinoid-mediated ERK1/2 signaling. However our results revealed no genotype differences in striatal or cortical (T. Daigle unpublished observations) ERK1/2 signaling in either βarrestin-2 or βarrestin-1 KO mice. It is possible however that βarrestins may regulate different signaling pathways engaged by CB1 receptors in the striatum, such as Akt/GSK3 signaling (Ozaita et al., 2007) or may potentially regulate cannabinoid effects in different brain regions. Additionally, we cannot exclude the possibility that βarrestins may play redundant roles in CB1 receptor signaling. Therefore, an examination of βarrestin function in a region and/or cell-type specific manner and with isoform-specific pharmacological inhibitors in future research may yield new insights into cannabinoid actions in the brain.
In conclusion, we report a novel effect of cannabinoids on ERK1/2 signaling in vivo – one that is regulated by dopamine D1 and NMDA receptor expression. These findings implicate cannabinoids as negative regulators of cell survival signaling in the brain.
Supplementary Material
A–D) Basal levels of striatal pERK1/2 were compared from drug naïve WT littermates and either D2 KO (A), DAT KO (B), βarr-2 KO (C) or βarr-1 KO (D) mice. Representative western blots and the corresponding densitometric analyses are shown. Data are mean ± S.E.M.; n = 5 mice for each treatment group. No significant differences were found between genotypes by unpaired t-test.
Acknowledgements
We are grateful to Drs. John Drago, Malcolm Low, and Beverley Koller for providing D1R KO, D2R KO and NR1-Kd mice respectively. We would like to thank Xiu Qin Zhang, Katherine Harley and Wendy Roberts for excellent assistance in the maintenance of the mouse colonies, Dr. Jonathan Ting for critically reading the manuscript and Dr. Martin Beaulieu for performing preliminary experiments with WIN 55,212-2 and for providing comments on the manuscript. This work was supported by the National Institutes of Health Grants DA030026 (T.L.D.), MH073853 (M.G.C.) and MH082441 (M.G.C.).
Abbreviations
- CB1R
CB1 cannabinoid receptor
- CPu
Caudate putamen
- DARPP-32
Dopamine- and cAMP-regulated phosphoprotein of 32 kDA
- D1R
Dopamine D1 receptor
- D2R
Dopamine D2 receptor
- DAT KO
Dopamine transporter KO
- KO
Knockout
- NR1-Kd
NMDA receptor knockdown
- WT
Wild-type
References
- Andersson M, Usiello A, Borgkvist A, Pozzi L, Dominguez C, Fienberg AA, Svenningsson P, Fredholm BB, Borrelli E, Greengard P, Fisone G. Cannabinoid action depends on phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa at the protein kinase A site in striatal projection neurons. J Neurosci. 2005;25:8432–8438. doi: 10.1523/JNEUROSCI.1289-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice. J Biol Chem. 2006;281:32072–32080. doi: 10.1074/jbc.M606062200. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Marcellino D, Fuxe K, Greengard P, Fisone G. Regulation of DARPP-32 phosphorylation by Delta9-tetrahydrocannabinol. Neuropharmacology. 2008;54:31–35. doi: 10.1016/j.neuropharm.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312(Pt 2):637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel CS, Lambert JM, Gerfin S, Huffman JW, Razdan RK. Sensitivity to delta9-tetrahydrocannabinol is selectively enhanced in beta-arrestin2 −/− mice. Behav Pharmacol. 2008;19:298–307. doi: 10.1097/FBP.0b013e328308f1e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, Seidman JG. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res. 1997;81:1021–1026. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- Daigle TL, Kearn CS, Mackie K. Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology. 2008a;54:36–44. doi: 10.1016/j.neuropharm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle TL, Kwok ML, Mackie K. Regulation of CB1 cannabinoid receptor internalization by a promiscuous phosphorylation-dependent mechanism. J Neurochem. 2008b;106:70–82. doi: 10.1111/j.1471-4159.2008.05336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, Trzaskos J, Caboche J, Girault JA. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23:2371–2382. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, Ooi GT, Grinberg A, Lee EJ, Huang SP, et al. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci U S A. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- French ED. delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett. 1997;226:159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gomez Del Pulgar T, De Ceballos ML, Guzman M, Velasco G. Cannabinoids protect astrocytes from ceramide-induced apoptosis through the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2002;277:36527–36533. doi: 10.1074/jbc.M205797200. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J Neurophysiol. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Brown S, Roche JP, Hsieh C, Celver JP, Kovoor A, Chavkin C, Mackie K. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J Neurosci. 1999;19:3773–3780. doi: 10.1523/JNEUROSCI.19-10-03773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, Allen RG, Hnasko R, Ben-Jonathan N, Grandy DK, Low MJ. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron. 1997;19:103–113. doi: 10.1016/s0896-6273(00)80351-7. [DOI] [PubMed] [Google Scholar]
- Kouznetsova M, Kelley B, Shen M, Thayer SA. Desensitization of cannabinoid-mediated presynaptic inhibition of neurotransmission between rat hippocampal neurons in culture. Mol Pharmacol. 2002;61:477–485. doi: 10.1124/mol.61.3.477. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schutz G, Wotjak CT, Lutz B, Marsicano G. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5:e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaita A, Puighermanal E, Maldonado R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem. 2007;102:1105–1114. doi: 10.1111/j.1471-4159.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- Ramsey AJ, Laakso A, Cyr M, Sotnikova TD, Salahpour A, Medvedev IO, Dykstra LA, Gainetdinov RR, Caron MG. Genetic NMDA receptor deficiency disrupts acute and chronic effects of cocaine but not amphetamine. Neuropsychopharmacology. 2008;33:2701–2714. doi: 10.1038/sj.npp.1301663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Premoli F, Castiglioni C, Bianchessi S, Zippel R, Parolaro D. Changes in the expression of G protein-coupled receptor kinases and beta-arrestins in mouse brain during cannabinoid tolerance: a role for RAS-ERK cascade. Mol Neurobiol. 2006;33:199–213. doi: 10.1385/MN:33:3:199. [DOI] [PubMed] [Google Scholar]
- Santini E, Sgambato-Faure V, Li Q, Savasta M, Dovero S, Fisone G, Bezard E. Distinct changes in cAMP and extracellular signal-regulated protein kinase signalling in L-DOPA-induced dyskinesia. PLoS One. 5 doi: 10.1371/journal.pone.0012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, Herve D, Greengard P, Fisone G. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Dorner L, Pfreundtner C, Norenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403. doi: 10.1016/s0306-4522(97)00597-6. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Li DL, Moutsimilli L, Bisogno T, Di Marzo V, Phebus LA, Nomikos GG, Giros B. Endocannabinoids activate transient receptor potential vanilloid 1 receptors to reduce hyperdopaminergia-related hyperactivity: therapeutic implications. Biol Psychiatry. 2006;59:508–515. doi: 10.1016/j.biopsych.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A. 2006a;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006b;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pages C, Rogard M, Besson MJ, Maldonado R, Caboche J. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur J Neurosci. 2001;14:342–352. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. Eur J Pharmacol. 2003;480:133–150. doi: 10.1016/j.ejphar.2003.08.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A–D) Basal levels of striatal pERK1/2 were compared from drug naïve WT littermates and either D2 KO (A), DAT KO (B), βarr-2 KO (C) or βarr-1 KO (D) mice. Representative western blots and the corresponding densitometric analyses are shown. Data are mean ± S.E.M.; n = 5 mice for each treatment group. No significant differences were found between genotypes by unpaired t-test.