Abstract
Metazoans employ cytoprotective and regenerative strategies to maintain tissue homeostasis. Understanding the coordination of these strategies is critical to develop accurate models for aging and associated diseases. Here we show that cytoprotective Jun-N-terminal Kinase (JNK) signaling influences regeneration in the Drosophila gut by directing proliferation of intestinal stem cells (ISCs). Interestingly, this function of JNK contributes to the loss of tissue homeostasis in old and stressed intestines by promoting the accumulation of mis-differentiated ISC daughter cells. Ectopic Delta/Notch signaling in these cells causes their abnormal differentiation, but also limits JNK-induced proliferation. Protective JNK signaling, and control of cell proliferation and differentiation by Delta/Notch signaling thus have to be carefully balanced to ensure tissue homeostasis. Our findings suggest that this balance is lost in old animals, increasing the potential for neoplastic transformation.
Introduction
Aging is characterized by the progressive decline of tissue function. Two distinct strategies to allay this degeneration are employed by metazoans: Prevention or repair of stress-induced damage (Finkel and Holbrook, 2000) and regeneration of damaged tissue by cells derived from pluripotent stem cells (Rando, 2006; Sharpless and DePinho, 2007). To develop accurate models for aging, a better understanding of how these protective and regenerative mechanisms are employed and coordinated is critical. Importantly, the causes for their failure in age-related degenerative diseases and cancer have to be established.
Damage by oxidative stress and other environmental challenges is one cause for the age-related decline of tissue homeostasis, contributing to functional degeneration and cancer (Beckman and Ames, 1998; Finkel and Holbrook, 2000; Stadtman, 2001). Accordingly, signaling mechanisms that promote cellular stress tolerance are emerging as crucial regulators of lifespan. One example is the Jun-N-terminal Kinase (JNK) signaling pathway, which is activated by a variety of environmental challenges, including oxidative stress, and increases stress tolerance and longevity in flies and worms (Oh et al., 2005; Wang et al., 2003; Wang et al., 2005).
The replacement of damaged cells by newly formed progeny of pluripotent stem cells is an alternative strategy to delay tissue degeneration in metazoans. The regenerative potential in tissues with high turnover rates declines with age, suggesting that aging is caused by a loss of stem cell function (Rando, 2006; Rossi et al., 2008; Sharpless and DePinho, 2007). Interestingly, this functional decline is not necessarily caused by a loss of the ability of stem cells to self-renew, but may also reflect an inability of stem cell progeny to properly differentiate (Morrison et al., 1996; Rossi et al., 2007b; Rossi et al., 2005; Rossi et al., 2008).
Stress signaling and stem cell maintenance
This decline of regenerative capacity of stem cells in aging animals is likely caused by intrinsic and environmental challenges (Rando, 2006; Rossi et al., 2008; Sharpless and DePinho, 2007). Damage control and repair mechanisms are therefore extensively employed in stem cells (Nijnik et al., 2007; Rossi et al., 2007a; Rossi et al., 2005; Tothova et al., 2007). It remains to be established how these mechanisms are coordinated with processes that control cell proliferation and differentiation, and why protective mechanisms often fail in old animals. As a regulator of stress tolerance, JNK signaling is likely to play an important role in this coordination. The consequences of JNK activation in proliferating cells in vivo remain controversial (Kennedy and Davis, 2003; Weston and Davis, 2007; Whitmarsh and Davis, 2007). While the ability of JNK signaling to induce apoptosis under certain conditions suggests that it can act as a tumor suppressor, various studies have also described potential pro-oncogenic functions for JNK signaling (Tournier et al., 2000; Whitmarsh and Davis, 2007). Evidently, cellular responses to JNK activation are highly context-specific, and in vivo analysis of the function of JNK signaling in pluripotent stem cells is required to establish its influence on tissue regeneration.
Stem cells and aging in Drosophila melanogaster
The recent discovery of regenerative processes in Drosophila permits addressing questions regarding the functional maintenance of stem cells and regeneration in aging animals using the power of Drosophila genetics. Studies identifying a variety of adult stem cells have rejected old notions that implied that adult flies lack regenerative capacity of their somatic tissues. Initial work focused on stem cells in the Drosophila germline (germline stem cells, GSCs), which are maintained by a somatic niche (Li and Xie, 2005; Wong et al., 2005). Interestingly, this stem cell population declines in aging animals due to a loss of trophic support from the GSC niche (Boyle et al., 2007; Pan et al., 2007; Wallenfang et al., 2006).
Other adult stem cell populations have been discovered in the fly posterior midgut (Intestinal Stem Cells; ISCs) and in malpighian tubules (Renal and Nephric Stem Cells; RNSCs) (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Singh et al., 2007). Regeneration in the fly midgut is remarkably similar to regeneration of the mammalian intestinal epithelium (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Radtke and Clevers, 2005). ISCs (marked by expression of the transcription factor escargot, esg, and by expression of the Notch ligand Delta, Dl) give rise to at least two differentiated intestinal cell types: enteroendocrine cells (EEs: small, diploid and marked by expression of the gene prospero) and enterocytes (ECs: large and polyploid; the main cell type of the intestine) (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). Differentiation into these two cell types proceeds through an intermediate cell, termed the enteroblast (EB; marked by expression of esg and by elevated Notch signaling activity as determined by expression of a Su(H)-lacZ reporter). Cell cycle arrest and differentiation of ISC daughter cells is controlled by Notch signaling and is characterized by a progressive relocation of the cell away from the basal membrane towards the lumen of the midgut (Ohlstein and Spradling, 2007). The intestinal epithelium is thus composed of a monolayer of large, polyploid ECs into which ISCs are embedded basally, while EBs are located more apically.
In a recent study, Choi and colleagues have found that proliferation of ISCs is elevated in old flies, resulting in increased numbers of esg+/Dl+ as well as esg+/Su(H)-lacZ+ cells (Choi et al., 2008). These changes were proposed to reflect an increase in the number of stem cells as well as of EBs, and a decline in the number of differentiated ECs.
Here we examine the role of JNK signaling in this age-related deterioration of the intestinal epithelium. Interestingly, we find that the increase in esg+ cells observed in old and stressed intestines is not simply caused by an increase in the number of ISCs, but by a combination of excessive ISC proliferation and aberrant differentiation. Strikingly, our results show that these changes are caused by JNK activation in ISCs and/or EBs of old and stressed intestines, and that JNK and Notch signaling interact in the ISC lineage to coordinate stress-induced cell proliferation and differentiation. Tissue regeneration in the intestinal epithelium thus depends on a sensitive balance of signaling events that regulate stress responses, stem cell proliferation and cell differentiation. This balance is lost in aging flies, resulting in tissue degeneration and tumor formation.
Results
Accumulation of abnormal ISC daughter cells causes age-related deterioration of the intestinal epithelium
To gain insight into potential changes in ISC maintenance and function during aging, we examined the ISC population of aging flies using a marker line in which β-Galactosidase is expressed under the control of the escargot gene (esg-lacZ (Yagi and Hayashi, 1997)). esg is specifically expressed in ISCs and enteroblasts (EBs, the daughter cells of asymmetrically dividing ISCs (Micchelli and Perrimon, 2006)), as well as in other adult stem cells (Singh et al., 2007). Strikingly, we found a marked increase in the number of esg+ cells in aging flies, suggesting significant proliferation and/or differentiation defects in the ISC lineage (Fig. 1A). This phenotype was independent of the genetic background, as it was observed in two different esg-lacZ strains, one carrying a transposon insertion into the esg gene (Fig. 1A) and one carrying a transgenic reporter that expresses lacZ under the control of an esg promoter fragment (Fig. S1 (Yagi and Hayashi, 1997)).
Figure 1. Tissue degeneration in the aging Drosophila gut.
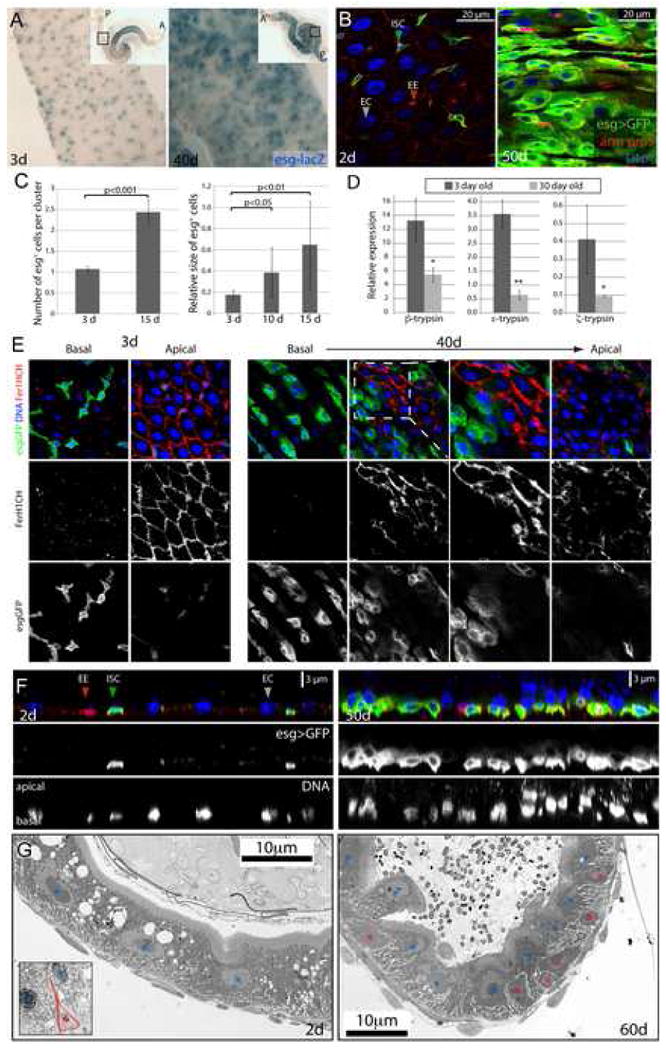
A. ISCs and EBs in young (3 days) and old (40 days) guts labeled by X-Gal staining (genotype: y,w;P{lacW}esgk00606/CyO). Inserts show overview of the depicted guts (A: anterior; P: posterior; endogenous β-Galactosidase Figure S1).
B. Confocal images of aging guts from flies expressing GFP in ISCs and EBs (genotype: w1118; esgGal4, UASGFP). ISCs and EBs are labeled by GFP expression (esgGal4>UASGFP, green) in young flies. Cell boundaries are labeled by immunostaining against Armadillo (membrane red), EE cells are labeled by nuclear pros staining (nuclear red). DNA detected by Hoechst staining (blue). Additional images are shown in Supplementary Figure S2.
C. Age-related changes in the intestinal epithelium quantified by measuring the averages of cell number and size in esg+ cell clusters. Relatively young guts (3, 10 and 15 days) were scored to allow for accurate quantification of the observed parameters. Widespread disorganization (compare with B) prevents accurate identification of individual clusters of cells in older guts. p-values were determined using Student’s t-test.
D. Reduction of trypsin expression in the aging gut. Expression of three different trypsin isoforms was measured by real-time RT-PCR in cDNA prepared from dissected guts from young and old flies. Expression is normalized to the expression of the rp49 gene. p-values were calculated using Student’s t-test: * p<0.05; **p<0.01.
E. Confocal images of guts from young and old flies expressing GFP under the control of esgGal4. Fer1HCH, detected by immunostaining (red), is expressed specifically in ECs in young flies.
F. Cross-sections of guts from old and young esgGal4>UASGFP flies illustrating the loss of the monolayered architecture of the intestinal epithelium in older animals. ISCs and EBs are GFP positive (green), EE cells are labeled by nuclear pros staining (red) and DNA detected by Hoechst staining (blue).
G. Ultrastructural analysis of cross-sections of young and old guts using Transmission Electron Microscopy. Blue asterisks indicate enterocytes, red asterisks mark ISCs or cells accumulating in the basal part of the epithelium. A representative ISC in young intestines is outlined in the insert of the left panel. Additional and higher magnification images are presented in Supplementary Figure S5.
To monitor these changes in the aging intestinal epithelium in more detail, we used an esg-Gal4 line to drive GFP expression in ISCs and EBs (Micchelli and Perrimon, 2006). Interestingly, we observed that not only the number of esg+ cells increases in aging flies, but that their morphology and the overall structure of the gut epithelium is dramatically changed (Fig. 1B, Fig. S2A). In aging intestines, clusters of esg+ cells are increasingly observed. Their accumulation leads to an increase in the total density of cells and a concomitant decrease in the density of normal ECs, without affecting the proportion of EEs among all cells (Fig. S4). The size of these esg+ cells varies significantly, approaching EC-like size in some cases (Fig. 1C). Importantly, their accumulation in old animals is not due to retention of GFP in normally differentiating ECs, as GFP expression is rapidly lost in ISC daughter cells of young intestines (Fig. S3). We further confirmed the unusual nature of these cells using Ferritin 1 Heavy Chain Homologue expression as a marker for ECs (Figure 1E). Fer1HCH is expressed at high levels specifically in ECs of young intestines. In old flies, this expression pattern is disrupted by the accumulation of large esg+ cells that fail to express Fer1HCH. Importantly, these phenotypes correlate with a general functional deterioration of the intestinal mucosa, as indicated by the reduced expression of Trypsin genes in old guts (Figure 1D).
In addition to the accumulation of aberrant cells, the apico-basal organization of the epithelium is disrupted in old intestines, in which cells overlap in the apico-basal axis rather than forming a regular monolayered epithelium (Fig. 1F). We confirmed these findings in wild-type flies, in which ISCs can be detected by their strong expression of the β–catenin homologue armadillo, their small nuclear size and the absence of nuclear pros staining (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). As seen in flies carrying esg reporters, the normal planar architecture of the gut was disrupted in old intestines, which contained overlapping cells of a range of nuclear sizes (Fig. S2B). Analysis of tissue sections using transmission electron microscopy (TEM) further confirmed these findings. Accumulation of basally located cells of unusual morphology can be observed in old animals (Fig. 1G, Fig. S5). Combined, our results show that the intestinal epithelium increasingly deteriorates in aging flies and becomes populated by abnormal clusters of cells that carry stem cell markers, but are polyploid and reach EC-like size without expressing EC marker genes.
Increased proliferation of ISCs in old intestines
The increased number of esg+ cells in the gut of old animals suggested elevated ISC proliferation and/or a failure of ISC daughter cells to correctly differentiate. To test these possibilities, we assessed proliferation of ISCs in the gut of young and old flies. While ISCs are relatively quiescent in young guts, we found widespread BrdU incorporation in old intestines, suggesting that ISCs proliferate at higher rates in older guts (Fig. 2A). BrdU incorporation was found both in large polyploid nuclei as well as in small esg+ cells, reflecting both increased cell division of ISCs and endoreplication of differentiating ISC daughter cells (Fig. 2A, B).
Figure 2. Increased proliferation and deregulation of Notch signaling in old guts.
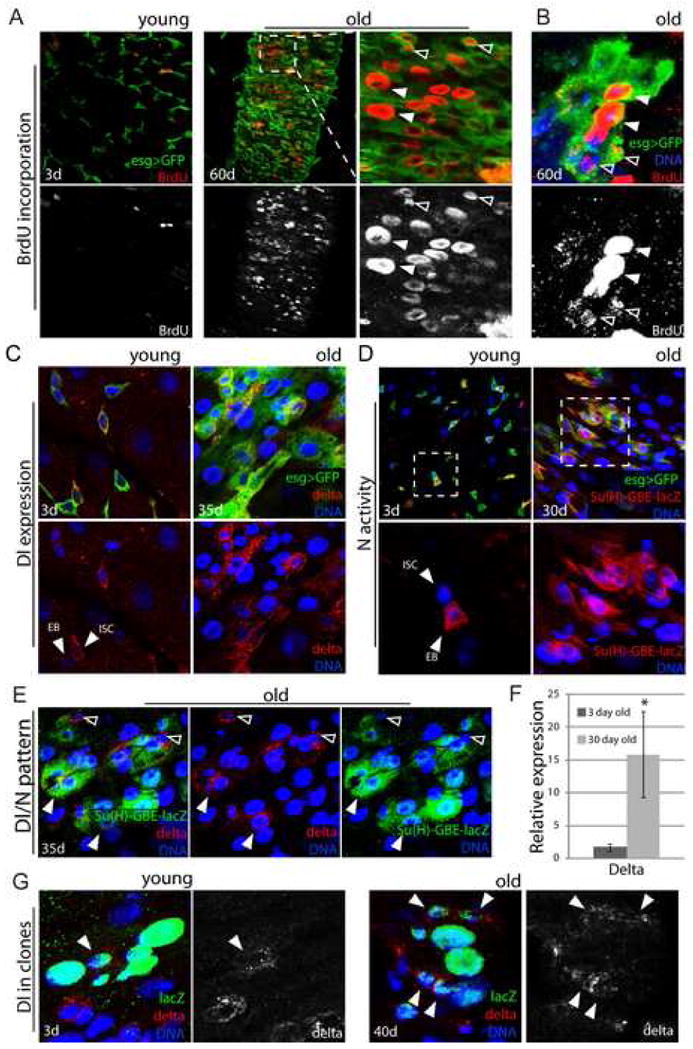
A, B. Increased rate of ISC proliferation in old intestines. Young and old flies were fed BrdU for 4 days. BrdU incorporation can be detected in both small cells (ISCs, EBs; open arrowheads), that divided while BrdU was being provided, and in larger nuclei characterizing ECs that underwent differentiation (including endoreplication; closed arrowheads). Panel B shows an isolated cluster of aberrant esg+ cells in the gut of 60-day-old flies. BrdU labeling of adjacent small and larger nuclei indicate lineage relationship between the labeled cells. Large esg+ cells that were formed before exposure to BrdU, are not labeled with BrdU, highlighting the continued expression of the esg>GFP marker in ISC progeny of old flies.
C. Distribution of Delta protein is aberrant in old guts. Delta is labeled by immunostaining (red), ISCs and EBs are identified by GFP expression (esgGal4>GFP, green) and DNA by Hoechst staining (blue).
D. Activity of the Notch signaling pathway monitored using the Su(H)-GBE-lacZ reporter line, which is specifically expressed in EBs in young guts. β-galactosidase was detected by immunostaining (red). GFP (green) identifies ISCs and EBs.
E. Co-expression of Delta and Notch signaling reporter in the abnormal progeny of ISCs in old flies. Confocal images of posterior midgut from 35-day-old Su(H)-GBE-lacZ/+ flies, Delta is detected by immunostaining (red), β-galactosidase expression identifies cell with elevated Notch signaling (green). Note the presence of cells with large nuclei (blue) that are both Dl- and β-galactosidase-positive (close arrowheads). Cells retaining ISC identity (small nuclei, Dl-positive and β-galactosidase-negative) are also indicated (open arrowheads).
F. Expression of Dl was measured by real-time RT-PCR in cDNA prepared from dissected guts from young and old flies. Expression is normalized to the expression of the rp49 gene. p-values were calculated using Student’s t-test: * p<0.05.
G. Positively marked clones (lacZ+) were generated in the gut of young (left) and old (right) flies as described in (Harrison and Perrimon, 1993). Note the presence of a single Dl+ cell in clone from young animal and multiple Dl+ cells in clone generated in the gut of older animals.
Aberrant Dl/N signaling in aging guts
Increased proliferation of ISCs with age thus contributes to the increase of esg+ cells in old intestines. These cells do not repopulate the intestinal epithelium normally, however, and retain expression of esg, suggesting that differentiation defects also contribute to the observed phenotypes. The differentiation of ISC daughter cells is regulated by Notch signaling (Ohlstein and Spradling, 2007). Expression of the Notch ligand Delta (Dl) in ISCs, and rapid downregulation of Dl expression in EBs, is required to induce EE or EC differentiation. Interestingly, we found that this pattern of Dl expression is disrupted in old guts. In young intestines, Dl protein can only be detected in individual ISCs, or in one of two adjacent esg+ cells (the Dl-negative cell is an EB (Ohlstein and Spradling, 2007), Fig. 2C). In old flies, however, high levels of Dl were found commonly in two or more adjacent esg+ cells of various sizes, supporting the idea that age-related changes in epithelial structure are caused in part by differentiation defects. We confirmed that widespread expression of Dl in old esg+ cells results in increased Notch signaling activity in the intestine, using a Su(H)-GBE-lacZ reporter line (Ohlstein and Spradling, 2006). This line expresses lacZ in response to Dl signals and is active specifically in EBs in young intestines (Ohlstein and Spradling, 2006) (Fig. 2D). In old flies, however, activation of Notch signaling was widespread in esg+ cells. Interestingly, many esg+ cells in the intestine of old flies express Dl and high Notch activity at the same time (Fig. 2E), highlighting their unresolved differentiation. Within most lacZ+ cell clusters, however, a single cell could be detected that expressed Dl, but was Su(H)-lacZ negative (Fig. 2 E), suggesting that this cell retains normal ISC identity. The increase in Dl+ cells in old intestines was further confirmed by real-time RT-PCR, detecting up to 10-fold higher Dl expression in old compared to young guts (Figure 2F).
To confirm that the aberrant Dl-expressing cells are derived from individual ISCs, we performed lineage-tracing analysis by inducing lacZ marked cell clones by mitotic recombination (Harrison and Perrimon, 1993). In 40-day-old flies, multiple Dl expressing cells can be detected in individual clones (Fig. 2G), while in younger flies a single Dl+ ISC is detected in similar clones (Ohlstein and Spradling, 2007).
Disruption of tissue homeostasis by oxidative stress
Chronic exposure to environmental toxins, inflammatory agents and oxidative stress contributes to tissue degeneration and the loss of regenerative capacity in aging stem cells (Finkel and Holbrook, 2000; Rossi et al., 2005; Tothova et al., 2007). To test whether oxidative stress would influence regenerative processes in the Drosophila intestine, we exposed flies to the ROS-inducing compound Paraquat. Strikingly, we found that within 24 to 48 hrs of Paraquat exposure, guts exhibit cellular phenotypes comparable to the defects observed in aging flies. Paraquat exposure induces proliferation of ISCs, producing increased numbers of esg+ cells that increase in size and display aberrant Dl/N signaling patterns (Figure S6).
JNK activation in ISCs causes the age-related deterioration of the intestinal epithelium
The similarity of Paraquat- and aging–induced changes in the intestinal epithelium suggested that signaling mechanisms that regulate the cellular response to stress, such as JNK signaling, are likely to influence age-related tissue degeneration.
To test whether JNK is active in the intestinal epithelium, we used a lacZ reporter line for JNK activity (pucE69 (Martin-Blanco et al., 1998; Wang et al., 2005)). We found that puc-lacZ expression in the gut increases with age, suggesting high levels of stress in old guts (Fig. 3A, B). Strong levels of JNK activity in old intestines are detected exclusively in ECs and EEs, as well as in cells that are esg+, but have lost normal ISC morphology (i.e. they are not small and diploid, Fig. 3C–F). Interestingly, induction of puc-lacZ can be detected in all cell types (including ISCs) of young guts when flies are exposed to Paraquat at levels that induce loss of epithelial homeostasis (Fig. 3G, H).
Figure 3. JNK activity increases with age in the gut and is required for stress tolerance of ECs.
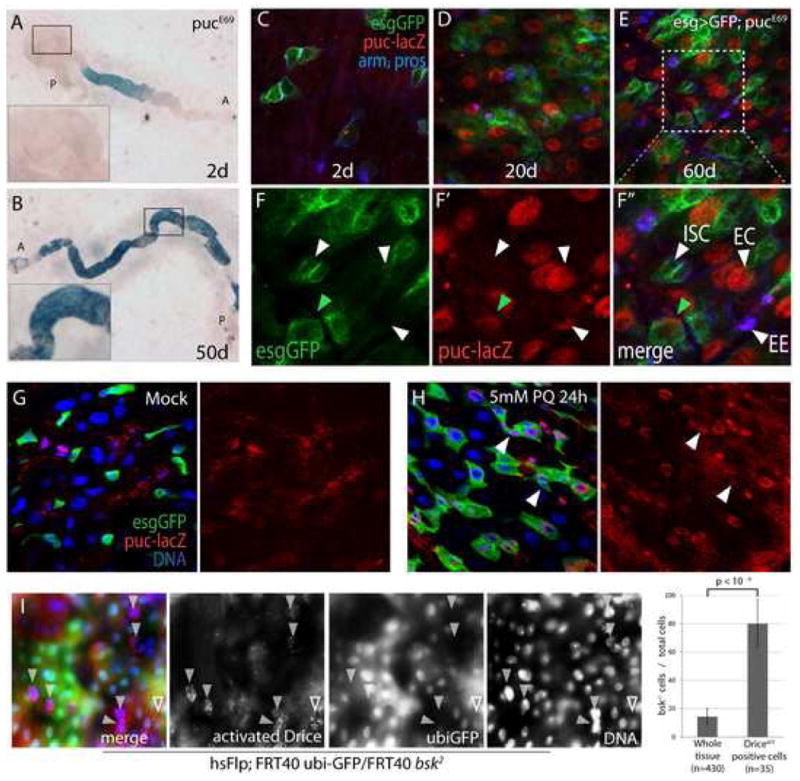
A, B Expression of lacZ in the pucE69 line was monitored to detect JNK activation in young (2 days, A) and old (50 days, B). Inserts show higher magnification of the boxed area (A: anterior, P: posterior).
C-F Nuclear β-galactosidase accumulates in aging pucE69 guts, but is not detected in ISCs (genotype esgGal4, UASGFP; pucE69/+). Projections of confocal Z-stacks. GFP identifies ISCs and EBs (green). β-galactosidase (red) and Arm and Pros (blue) expression detected by immunostaining. (C–E) posterior midguts at 2 days (C), 20 days (D), 60 days (E) of age. (F) Closeup of E. High levels of nuclear lacZ is detected in ECs (closed arrowhead points out one example) and EEs (red arrowhead), but not in ISCs (small GFP+ cells (open arrowhead)). Note that GFP-positive cells with larger nuclei also express lacZ (green arrowhead points out one example).
G, H puc expression is induced by Paraquat exposure in young guts. 5-day-old animals exposed to 5mM Paraquat (PQ, H) or carrier (Mock, G) for 24 hours. Puc-lacZ expression is also observed in ISCs (arrowheads).
I: bsk2 mutant clones marked by the absence of GFP (green; genotype is hsFlp; FRT40 ubi-GFP/FRT40 bsk2). Guts are from 15 day-old flies exposed to 5mM Paraquat for 12 hrs and guts were immunolabeled against the activated form of the Drosophila Caspase3 Drice (red; gift from Bruce Hay). Note elevated Driceact staining in bsk2 mutant ECs (GFP−, closed arrowheads). A Driceact positive wild-type EC is also indicated (open arrowheads). The proportion of bsk mutant cells in the whole tissue and among Driceact positive cells was calculated.
Intestinal cells thus activate JNK signaling in response to stress exposure, presumably to promote cytoprotective gene expression. At the same time, the correlation between activation of JNK and induction of differentiation defects in Paraquat-treated guts, and the absence of puc-lacZ staining in the cells of old intestines that have retained ISC morphology, suggested that JNK activation in ISCs causes the observed differentiation defects.
To test this model, we first confirmed that JNK activity is cytoprotective by exposing flies carrying JNK mutant cell clones in the gut (using the loss-of-function allele bsk2) to Paraquat. This treatment induced apoptotic markers (activated Drosophila Caspase, Drice) more frequently in JNK mutant cells than in wild-type (or heterozygous) cells (Fig. 3I), confirming that JNK activity is required for stress protection in intestinal cells. We then asked whether reducing JNK activity in ISCs would prevent the age-associated changes in gut morphology. Indeed, we found that the accumulation of esg+ cell clusters was significantly reduced in flies mutant for the JNKK hemipterous (hemizygotes for the hypomorphic loss-of-function allele hep1; Fig. 4A–D). hep1 is caused by a P-element insertion into the hep locus. Mutant males are maternally rescued, but show strong defects in JNK-induced stress responses (Glise et al., 1995; Jasper et al., 2001; Wang et al., 2003). Importantly, we obtained similar results when blocking JNK activity specifically in ISCs and EBs (using esgGal4 – mediated expression of a dominant-negative form of Bsk; BskDN (Weber et al., 2000); Fig. 4E, F), demonstrating that cell-autonomous activation of JNK signaling in ISCs and/or EBs is required for the age-associated phenotypes.
Figure 4. JNK-dependent degeneration of epithelial architecture in the intestine.
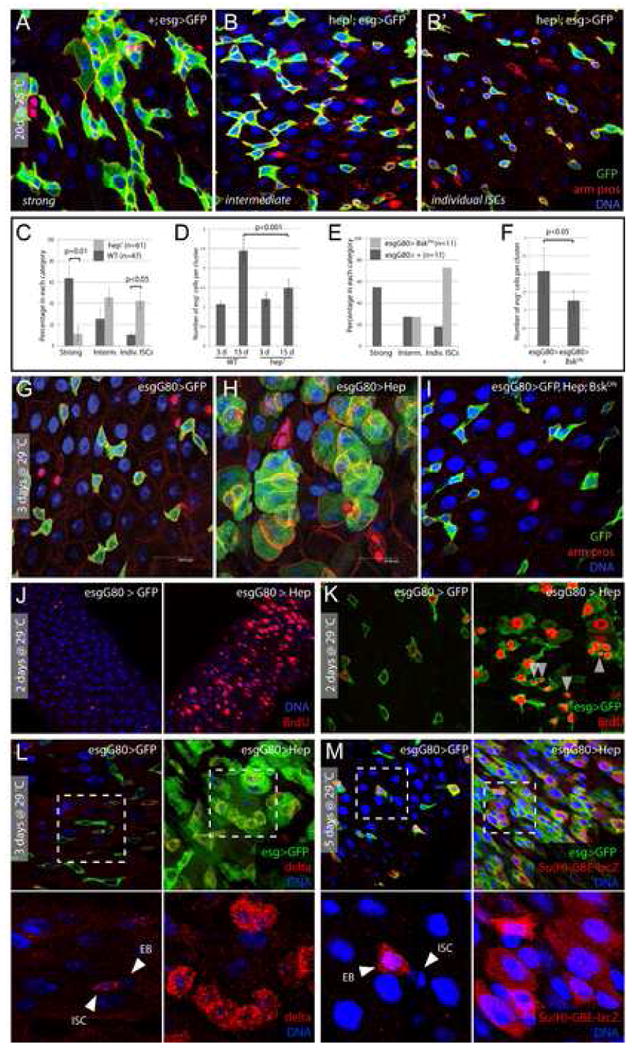
A–D. Age-related changes in the intestinal epithelium are reduced in JNK loss-of-function conditions. 20-day-old males hemizygous for hep1 (B and B′ are independent samples of identical genotypes) are compared to wild-type controls (A) reared under identical conditions. The number of guts containing clusters of esg+ cells is compared between populations of hep1 hemizygotes and wild-type controls in C. Three classes of esg+ overgrowth are represented by panels A (“strong overgrowth”), B (“intermediate”), and B′ (“individual ISCs”). Averages and SEM of three independent double-blind quantifications are shown. P values from Student’s T test. Quantification in D is as described in Figure 1D.
E, F. Similar reduction of age-associated expansion of esg+ population is seen in flies over-expressing dominant-negative Bsk (BskDN) in ISCs. The TARGET system was used to over-express BskDN under the control of esgGal4. Flies were aged for 15 days at 29°C.
G–I. JNK activation is sufficient to induce phenotypes similar to the one observed in old flies. Posterior midguts of WT control flies (esgGal4, UASGFP;tubGal80ts) and flies with moderate activation of JNK (esgGal4, UASGFP/UASHep; tubGal80ts) after 3 days at 29°C. Green: GFP; red: arm, pros; blue: DNA. The phenotype observed in Hep over-expressing flies is reversed when dominant-negative JNK (BskDN) is co-overexpressed (I).
J, K. JNK activation induces ISCs proliferation. Flies were fed BrdU for 2 days and then incubated at 29°C for 2 days. Over-expression of Hep in ISCs and EBs induces widespread BrdU incorporation in esg+ cell clusters, identifying stem cells that recently divided (arrowheads in K), as well as differentiating cells undergoing endoreplication.
L, M. JNK activation induces perturbation in Delta distribution and in Notch signaling. Delta (red in L) and the Notch reporter Su(H)-GBE-lacZ (red in M) are expressed respectively in ISCs and EB in control flies. Moderate activation of JNK (esgGal4, UASGFP/UASHep; tubGal80ts), for 3–5 days, leads to the formation of cluster of esg+ cells expressing high level of delta and showing high Notch signaling activity. Green: GFP; red: delta in L and β-galactosidase in M; blue: DNA.
We then asked whether JNK activation specifically in ISCs and EBs would be sufficient to induce morphological changes in the gut that are reminiscent of the changes observed in stressed and old flies. To activate JNK moderately in these cells, we over-expressed JNKK/Hep under the control of esg-Gal4 using the temperature-sensitive TARGET system (McGuire et al., 2003). Within 3 days of incubation at the restrictive temperature (29°C), we observed an increase in size and number of GFP-labeled cells, ultimately resulting in the formation of clusters of GFP+ cells populating the intestinal epithelium (Fig. 4G, H). Importantly, the observed phenotype was dependent on JNK/Bsk function (tested by co-over-expressing BskDN; Fig. 4I). Further characterization of this phenotype showed that it is indistinguishable from the one observed in aging wild-type guts, as JNK activation induces increased proliferation of ISCs, as measured by BrdU incorporation (Fig. 4J, K), and the formation of clusters of esg+ cells with aberrant Dl/N signaling (fig. 4L, M). As in aging guts, BrdU incorporation was found in small cells (ISCs and EBs) as well as in polyploid daughter cells, which likely underwent differentiation during the period of BrdU exposure. Interestingly, in contrast to the lifespan extension observed when Hep is over-expressed in the brain (Wang et al., 2003), moderate activation of JNK in ISCs and/or EBs (in esgGal4>Hep flies) leads to a significant shortening of lifespan (Fig. S7), suggesting that JNK – mediated disruption of regenerative processes in the gut and other regenerating tissues compromises fitness of the animal (esgGal4 is specifically expressed in ISCs and other stem cell populations of adult flies, (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Singh et al., 2007, and data not shown).
In summary, these results show that, while required for stress protection in differentiated cells, activation of JNK in stem cells induces over-proliferation of ISCs and differentiation defects in their progeny, ultimately leading to age-related deterioration of the intestinal epithelium.
JNK and Notch coordinate stem cell proliferation
The widespread activation of Notch signaling in JNK gain-of-function conditions suggested that this signaling pathway plays an important role in the JNK-mediated accumulation of mis-differentiated cells. To test this, we analyzed the effects of Notch loss-and gain-of-function backgrounds on stress- and JNK-induced phenotypes in the intestinal epithelium.
Complete loss of Delta/Notch signaling leads to ISC tumors within one to three weeks in the gut, indicating that Notch signaling, in addition to promoting daughter cell differentiation, also restricts ISC proliferation (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Ohlstein and Spradling, 2007). To test whether Notch signaling limits JNK – induced ISC proliferation, we co-expressed Hep and a NotchRNAi construct in ISCs and EBs). Strikingly, we found dramatic changes in the gross morphology of the intestine (Fig. 5A), which resulted in death of these animals within a week (not shown). Analysis of intestinal cells showed that the gut of these flies was entirely populated by small esg+ cells, completely disrupting epithelial integrity (Fig. 5B, S8A). These cells also expressed high levels of Delta (Fig. S8B), suggesting that they retain ISC identity. Importantly, these stem cell tumors appeared after 2 days of transgene induction, compared to the delayed effect of expressing NotchRNAi alone (Fig. S8B). We further tested whether N signaling would also restrict ISC proliferation in response to oxidative stress, and found that, as in the JNK gain-of-function conditions, Paraquat exposure resulted in increased tumor formation of NRNAi expressing ISCs compared to wild-type controls (Fig. S9). Expression of Notch in ISCs or EBs is thus required to restrict JNK-and stress-induced cell proliferation.
Figure 5. JNK and Delta/Notch signaling coordinate stem cell proliferation.
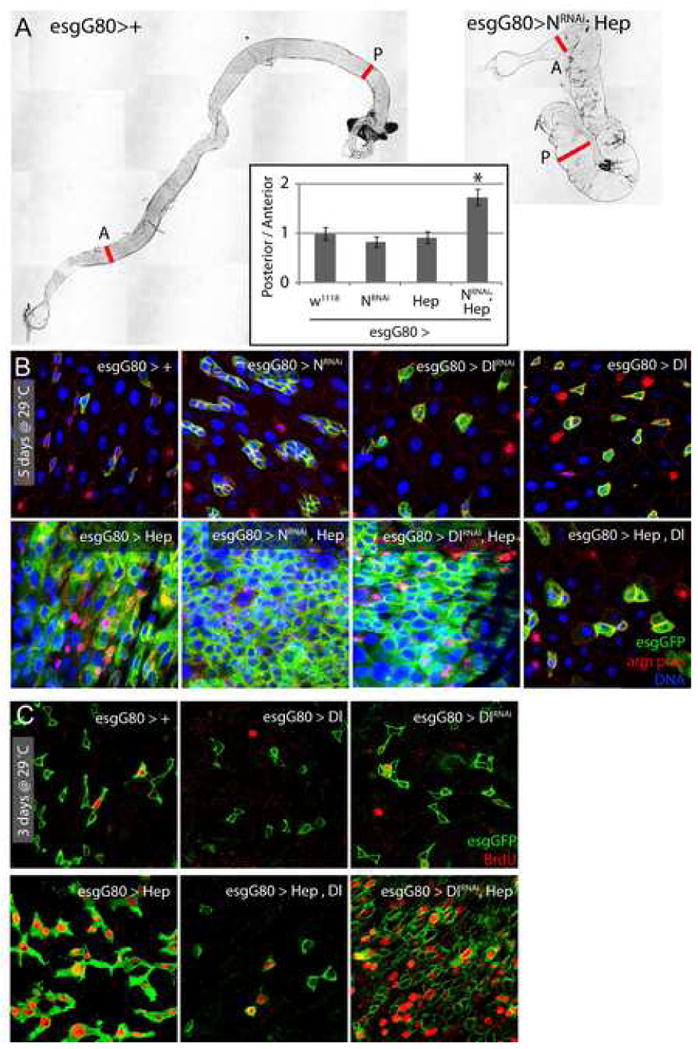
A. Co expression of Hep and NotchRNAi in ISCs and EBs results in dramatic changes in gut morphology. Representative pictures of intestines from wild-type flies and flies expressing Hep and NotchRNAi under the control of the TARGET system (genotype: esgGal4, UASGFP, tub>Gal80ts/UASHep; UASNRNAi). The phenotype was quantified by measuring the ratio between the width of the posterior and anterior midguts. A: anterior, P: posterior; the two red lines indicate where the width of the gut was measured. * p-value <10−4, compared to the 3 other genotypes using Student’s t-test.
B, C. Confocal images of the posterior midgut of flies expressing Hep in combination with Delta/Notch signaling gain- or loss-of-function. After 5 days of induction at 29°C, in flies expressing both Hep+NotchRNAi or Hep+DeltaRNAi, ISCs populate the entire intestinal epithelium, whereas Delta over-expression blocks Hep-induced changes in ISC number and morphology. (C) The effects of co-expression of DeltaRNAi or UASDelta on Hep-induced proliferation were confirmed by assessing BrdU incorporation in intestinal cells. Note that Delta over-expression inhibits JNK-induced proliferation of ISCs.
We tested whether this requirement for Notch signaling could be recapitulated by inhibiting Dl function, and found similar stem cell tumors in flies expressing Hep together with a DlRNAi construct under the control of esgGal4 (Fig. 5B). Widespread BrdU incorporation confirms the high rate of proliferation in these guts (Fig. 5C). Conversely, over-expression of Dl in ISCs and EBs impaired stress- and Hep-mediated over-proliferation (Fig. S9; 5B, D), suggesting that strong Dl/N signaling in ISCs and EBs is sufficient to inhibit JNK-induced proliferation of ISCs.
N signaling induces differentiation defects
Notch signaling in ISCs and their daughter cells thus restricts stress/JNK-induced proliferation of ISCs. The ectopic Dl/N signaling activity in stressed and aging intestines might therefore be induced as a compensatory mechanism to prevent unchecked proliferation of ISCs. Since N signaling directs differentiation of ISC daughter cells, this up-regulation, while restricting ISC proliferation, may also impair normal differentiation of EBs, resulting in the phenotypes described above. This model predicts that moderate reduction of Dl/N signaling would prevent age/stress-induced mis-differentiation.
To test this, we analyzed heterozygotes of the Dl loss-of-function alleles Dl7 and Dl05151. Strikingly, we found that reducing the Dl gene-dose prevents expansion of esg+ cells (Fig. 6A), but does not prevent widespread BrdU incorporation in the intestinal epithelium of flies over-expressing Hep (Fig. 6B). Evidently, Dl heterozygotes retain sufficient N activity to allow normal differentiation to proceed, but do not express enough Dl to either inhibit JNK-induced proliferation or contribute to JNK-induced mis-differentiation.
Figure 6. Reduction of delta gene dose prevents misdifferentiation, but not over-proliferation of ISCs in old flies and in response to JNK activation.
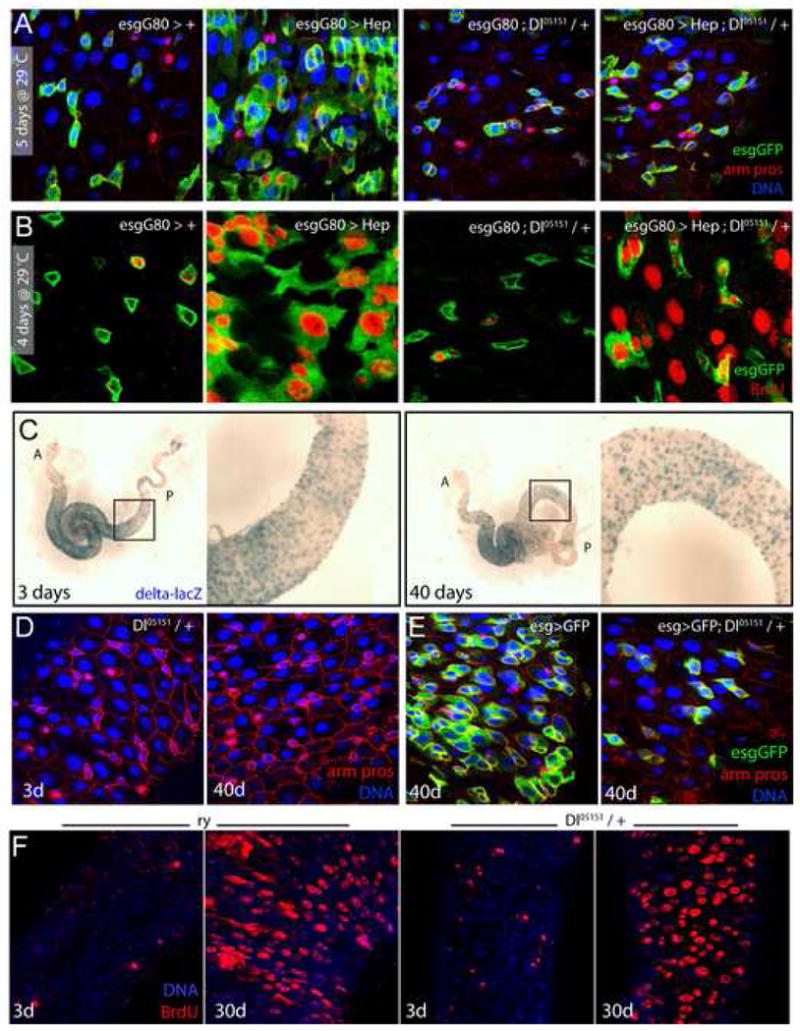
A, B. Reduction of delta gene dose prevents JNK-induced misdifferentiation but not JNK-induced proliferation. (A) Over-expression of Hep in ISCs and EBs, using esgGal4, in a Dl05151/+ background does not result in expansion of the esg+ cell population. (B) The same flies were fed BrdU for 4 days. BrdU incorporation shows that Hep over-expression induces proliferation in Dl05151/+ background. Similar results were observed using another Dl loss of function allele (Dl7; data not shown).
C. The enhancer-trap loss-of-function allele Dl05151 was used to identify Dl expressing cells in the posterior midgut from young and old flies, by X-gal staining. Right panels show higher magnification of boxed area; A: anterior, P: posterior.
D, E. Reduction of delta gene dose (heterozygotes for Dl05151) is sufficient to prevent age-associated loss of epithelial architecture in the gut. Guts from 40-day-old Dl05151/+ (D) or esg>GFP; Dl05151/+ (E) flies do not show the age-induced deterioration of the intestinal epithelium observed in wild-type animals (compare D with Figure S2). green: GFP; red: armadillo and prospero; blue: DNA. Similar results were obtained with an independent Dl allele (Dl7, Figure S11).
F. 3-day-old and 30-day-old Dl05151/+ flies and their isogenic control ry506 were fed BrdU for 4 days. Similar age-related increase in BrdU incorporation is observed in Dl heterozygotes and wild-type flies.
Further supporting a dose-dependent effect of Dl/N signaling on JNK-induced mis-differentiation of the ISC lineage, we found that reducing N signaling by exposure to moderate levels (0.5mM) of the gamma-secretase inhibitor DAPT also prevented JNK-induced misdifferentiation, while occasionally causing ISC and EE tumors (Fig. S10; DAPT exposure results in Notch loss of function phenotypes (Micchelli et al., 2003), and exposure to 2mM DAPT was shown to result in formation of ISC/EE tumors in the Drosophila intestine (Ohlstein and Spradling, 2006)).
To test whether influencing the balance between N-mediated restriction of ISC proliferation and N-mediated differentiation of ISC daughter cells also affects the age-associated deterioration of the intestinal epithelium, we assessed age-related changes in the gut of Dl heterozygotes. Strikingly, we could not identify any age-related changes in Dl-lacZ expression patterns in the gut (Fig. 6C; Dl05151 is caused by insertion of a lacZ-P-element into the promoter of Dl, and expresses β-Galactosidase under the control of delta regulatory sequences (Spradling et al., 1999)), and found no age-related deterioration of gut morphology in Dl05151 or Dl7 heterozygotes (Fig. 6D, S11B). Reducing the Dl gene-dose by half was also sufficient to prevent the formation of aberrant esg+ cell clusters in 40-day-old flies expressing GFP under the control of esgGal4 (Fig. 6E). As seen for Hep over-expressing flies, heterozygosity for Dl did not, however, prevent the wide-spread increase in BrdU incorporation in aging animals (Fig. 6F).
Importantly, JNK activity is still detected in stressed and old Dl heterozygotes, suggesting that Dl acts downstream of JNK signaling in the gut (Fig. S11). This view is supported by experiments in which Hep was co-overexpressed with the N intra-cellular domain (NICD). NICD expression in ISCs results in premature differentiation of these cells (Ohlstein et al., 2007), and co-expression of Hep did not alter this phenotype (Fig. S12A). Similarly, expression of BskDN had no effect on NRNAi-mediated formation of ISC tumors, suggesting that JNK signaling is not required downstream of Dl/N signaling (Fig. S12B).
Discussion
Our results show that elevated JNK activity in ISCs and/or EBs of old flies causes loss of tissue homeostasis in the aging intestinal epithelium. The interaction between JNK and Notch signaling suggests that a critical balance between stress signaling and processes that regulate cell differentiation in the ISC lineage has to be maintained to ensure normal regeneration. This balance is lost in aging and stressed animals, resulting in the accumulation of mis-differentiated cells and an increased potential for neoplastic transformation (Fig. 7).
Figure 7. Model for the effects of JNK and Notch signaling in the aging and stressed intestine.
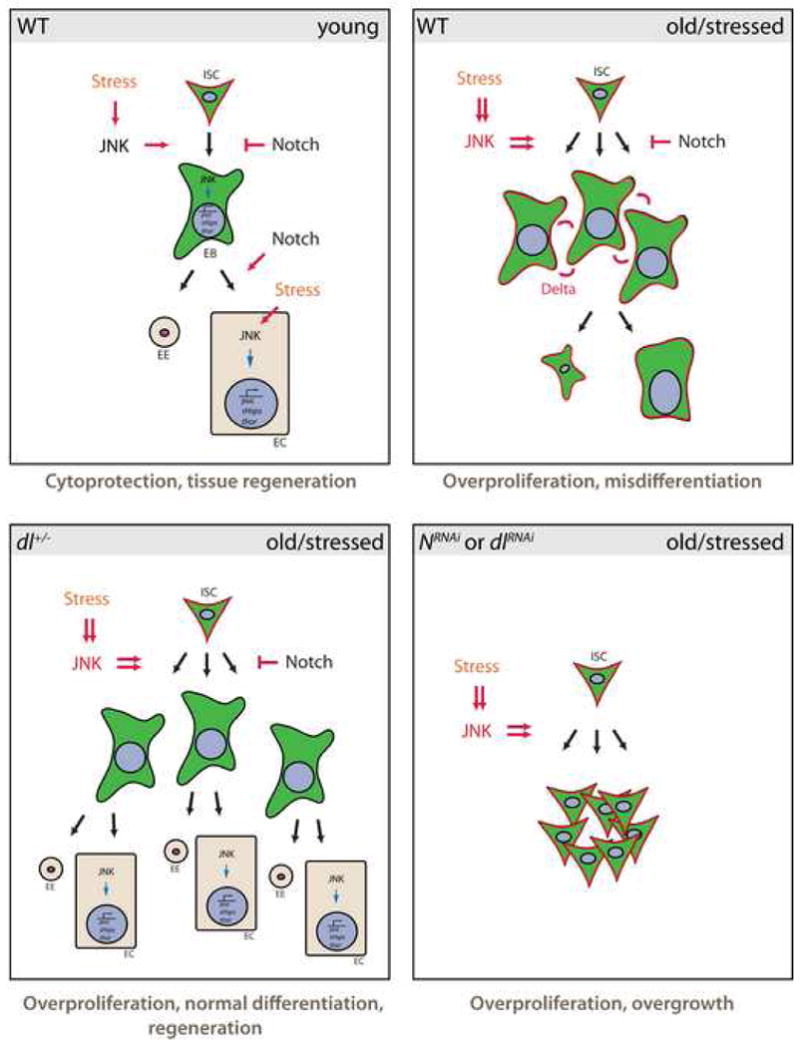
JNK is required for the transcriptional induction of damage repair and stress response genes (such as thor and small heat shock proteins (Wang et al., 2005)), promoting cytoprotection of intestinal cells. At the same time, JNK activation induces proliferation of ISCs. When animals are exposed to high levels or chronic stress, this activity of JNK promotes the accumulation of Dl+ EBs that activate Notch signaling in neighboring cells, resulting in misdifferentiation and loss of tissue homeostasis. In Dl heterozygotes, ectopic Dl expression is reduced, so that differentiation can proceed normally. When Notch signaling is impaired more efficiently by DlRNAi or NRNAi, however, EB differentiation is inhibited, resulting in JNK-mediated over-proliferation of ISCs and loss of epithelial integrity.
Age-related changes in the intestinal epithelium
These findings are consistent with a recent report describing increased proliferative activity of the ISCs and larger numbers of Dl expressing cells in guts of old flies (Choi et al., 2008). Importantly, however, we demonstrate that the increased number of esg+/Dl+ cells in old guts does not reflect an increase in the number of ISCs, but is caused by retention of stem cell markers in cells that also express high levels of Notch signaling activity and have EC-like morphology. These cells fail to express EC markers and accumulate basally in the epithelium. The observed age-related loss of epithelial homeostasis is thus caused by a combination of increased ISC proliferation and misdifferentiation.
Because of the accumulation of cells with unresolved differentiation, it is difficult to accurately quantify changes in the number of specific intestinal cell types during aging. While the number of ECs as a fraction of total cells in the intestine indeed declines (because of an increase in esg+ cells), the increase in EEs reported by Choi et al., for example, is not observable if EE numbers are plotted as percentage of total cell number (counting DAPI+ nuclei) rather than as relative to numbers of ECs (Fig. S4).
JNK and Notch signaling and the stress response of the intestinal epithelium
JNK signaling promotes the expression of cytoprotective genes in flies, resulting in lifespan extension when JNK activity is moderately increased (such as in pucE69 heterozygotes). Importantly, activation of JNK in neurons is sufficient to increase stress tolerance and lifespan, while activation in muscle does not influence overall stress sensitivity of the animal, revealing important tissue-specific effects of JNK signaling (Wang et al., 2003). Our results presented here show that in the highly regenerative intestinal epithelium, JNK signaling is required to protect cells from oxidative stress, but also induces proliferation of ISCs. This function of JNK is likely to contribute to the overall stress-protective function of JNK signaling, since stress-induced division of ISCs is required to replace damaged differentiated cells.
The decline of epithelial homeostasis in the intestine of aging flies is thus likely a pathological consequence of signaling events that are necessary for stress protection and regeneration: While JNK promotes cytoprotection and ISC division, activation of Notch in the ISC lineage is required to limit the proliferation of ISCs and ensure proper differentiation of ISC daughter cells. In conditions of strong or chronic stress, however, these protective mechanisms result in excessive proliferation, caused by high JNK activity, and in defective differentiation, caused by ectopic N activation due to an inability to properly repress Dl expression in ISC daughter cells. Supporting this model, we observe three different phenotypes in JNK gain-of-function conditions, depending on the activity of N (Fig. 7): (i) under normal conditions, JNK activation results in over-proliferation, but misdifferentiation, (ii) when Dl/N signaling is reduced slightly (Dl heterozygotes), JNK induces proliferation, but normal differentiation allows accurate regeneration of the epithelium, (iii) when N signaling is reduced strongly (NRNAi or DlRNAi), proliferation is induced, but no differentiation is observed, resulting in unchecked over-proliferation of ISCs.
Additional work is needed to determine which endogenous stimuli result in the observed widespread activation of JNK in the aging gut. While oxidative stress is a plausible inducer, inflammatory processes and infection, among other stimuli, can also activate JNK. It further remains unclear how Dl/N signaling is disturbed by JNK in stressed or old guts. JNK can affect the transcription of Dl, (as seen in other biological contexts (Weber et al., 2000)), but might also interfere with transport processes required for asymmetric segregation of Dl protein.
JNK signaling and tumor formation
The need to tightly control regenerative and self-renewal capacity of stem cells in high-turnover tissues like the intestinal epithelium is of particular importance for long-lived organisms such as mammals. The failure of this regulation in older individuals is reflected in the high incidence of intestinal adenomatous polyps, which develop in about 50% of the Western population by age 70 (Radtke and Clevers, 2005). Our findings suggest that stress-mediated activation of JNK might be central to this pathology and thus further support the view of the JNK pathway as a potential target of therapeutic intervention (Manning and Davis, 2003).
Based on the evolutionary conservation of structure and function of the JNK pathway (Kockel et al., 2001; Weston and Davis, 2007), it can be expected that its effects on ISC function and intestinal regeneration are also conserved. Interestingly, the intestines of senescent mice contain increased numbers of clonogenic cells, while displaying defects in regenerative potential (Martin et al., 1998). These phenotypes are reminiscent of the changes described here for the intestinal epithelium of flies. Furthermore, recent findings suggest that JNK signaling contributes to intestinal cancer in mouse models for the disease by regulating the interaction between the JNK-responsive transcription factor Jun and the Wnt-responsive transcription factor TCF4 (Nateri et al., 2005). The recent discovery of ISCs in mammals (Barker et al., 2007) will allow directly testing whether the effect of JNK on regeneration of the intestinal epithelium is conserved.
Experimental Procedures
Drosophila stocks and culture
The following strains were used: w1118, OreR, P{lacW}esgk00606, Dl05151, Dl7, and tub-Gal80ts (Bloomington Drosophila Stock Center). UAS-DlRNAi (transformant ID 3720) was obtained from the Vienna Drosophila RNAi Center. esg-Gal4 and esg-LacZC4-1 were kindly provided by S. Hayashi (Yagi and Hayashi, 1997); Su(H)-GBE-lacZ by S. Bray (Furriols and Bray, 2001); pucE69 by E. Martín-Blanco (Martin-Blanco et al., 1998); bsk2 by E. Hafen (Riesgo-Escovar et al., 1996); UAS-Hep and UAS-BskDN by M. Mlodzik (Weber et al., 2000); hep1/FM6 by S. Noselli (Glise et al., 1995); UAS-NRNAi by N. Perrimon; UAS-Dl and UAS-NICD by Y. Jan.
All flies were raised on standard yeast and molasses - based food, at 25°C, on a 12 h light/dark cycle, unless otherwise indicated. For paraquat exposure, flies were starved in empty vials for 6 hours and fed with a 5% sucrose solution +/− 5mM Paraquat.
Conditional expression of UAS-linked transgenes
Conditional expression of UAS-linked transgenes in ISCs was achieved using the TARGET system (McGuire et al., 2003); esg-Gal4 was combined with a ubiquitously expressed temperature-sensitive Gal80 inhibitor (esg-Gal4;tub-Gal80ts). Crosses and flies were kept at room temperature (permissive temperature), then shifted to 29°C for 3 to 7 days to allow expression of the transgenes in ISCs and EBs.
Generation of clones
JNK/bsk mutant clones were generated by somatic recombination in hsFlp; FRT40 ubi-GFP/FRT40 bsk2 flies. Flies were heat-shocked as lat pupae or young adults twice for 1h at 37°C and clones resulting from mitotic recombination were allowed to expand for 15 days.
LacZ marked clones were generated in hsFlp; X-15-29/X-15-33 flies (Harrison and Perrimon, 1993). Young and old flies were heat-shocked for 1h at 37°C and then kept at room temperature. Clones were observed after 5–7 days.
Quantification of age-related changes in the esg+ cell population
Number of esg+ cells per cluster was determined for about 250 cells in 5 different guts. Quantification of the cell size of esg+ cells was performed using the Histogram function in Adobe Photoshop and values are given relative to the size of neighboring enterocytes. The size of at least 12 cells in 3 different guts was determined for each condition. P-values were determined using Student’s T test.
Detection of β-galactosidase activity
Intact guts were dissected in PBS + 2mM MgCl2 and fixed for 10 minutes in 0.5% glutaraldehyde. Detection of β-galactosidase activity was carried out at room temperature in staining buffer (PBS, 2 mM MgCl2, 5 mM K4(Fe[CN]6), 5 mM K3(Fe[CN]6), 0.1% X-gal).
Immunostaining and Microscopy
Intact guts were fixed at room temperature for 45 minutes in 100 mM glutamic acid, 25 mM KCl, 20 mM MgSO4, 4 mM Sodium Phosphate, 1 mM MgCl2, 4% formaldehyde. All subsequent incubations were done in PBS, 0.5% BSA, 0.1% TritonX-100 at 4°C.
The following primary antibodies were used: rabbit anti-activated Drice (gift from Bruce Hay) 1:2000; rabbit anti-β-galactosidase (Cappel) 1:500; rabbit anti-GFP (Invitrogen) 1:500; mouse anti-BrdU (Becton Dickson) 1:100; rabbit anti-Fer1HCH (gift from Fanis Missirlis) 1:500; mouse anti-Prospero, anti-Armadillo and anti-Delta (Developmental Studies Hybridoma Bank) 1:250, 1:100 and 1:100, respectively. Fluorescent secondary antibodies were obtained from Jackson Immunoresearch. Hoechst was used to stain DNA.
Confocal images were collected using a Leica TCS system and processed using ImageJ and Adobe Photoshop.
BrdU incorporation
Flies were cultured on standard food supplemented with BrdU (final concentration 0.2 mg/ml) for 4 days. Intact guts were fixed as previously described and DNA was denatured by incubating tissue in 3M HCl for 30 minutes. Samples were then processed for immunostaining as described above.
Supplementary Material
Acknowledgments
We thank Dirk Bohmann and Julie Hull-Thompson for comments and Karen Bentley at the URMC Electron Microscopy Core Facility for technical assistance. This work was funded in part by the NIA (RO1 AG028127) and the French Cancer Research Association (fellowship to B.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008 doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Furriols M, Bray S. A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr Biol. 2001;11:60–64. doi: 10.1016/s0960-9822(00)00044-0. [DOI] [PubMed] [Google Scholar]
- Glise B, Bourbon H, Noselli S. hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell. 1995;83:451–461. doi: 10.1016/0092-8674(95)90123-x. [DOI] [PubMed] [Google Scholar]
- Harrison DA, Perrimon N. Simple and efficient generation of marked clones in Drosophila. Curr Biol. 1993;3:424–433. doi: 10.1016/0960-9822(93)90349-s. [DOI] [PubMed] [Google Scholar]
- Jasper H, Benes V, Schwager C, Sauer S, Clauder-Munster S, Ansorge W, Bohmann D. The genomic response of the Drosophila embryo to JNK signaling. Dev Cell. 2001;1:579–586. doi: 10.1016/s1534-5807(01)00045-4. [DOI] [PubMed] [Google Scholar]
- Kennedy NJ, Davis RJ. Role of JNK in tumor development. Cell Cycle. 2003;2:199–201. [PubMed] [Google Scholar]
- Kockel L, Homsy JG, Bohmann D. Drosophila AP-1: lessons from an invertebrate. Oncogene. 2001;20:2347–2364. doi: 10.1038/sj.onc.1204300. [DOI] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–565. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- Martin K, Potten CS, Roberts SA, Kirkwood TB. Altered stem cell regeneration in irradiated intestinal crypts of senescent mice. J Cell Sci. 1998;111(Pt 16):2297–2303. doi: 10.1242/jcs.111.16.2297. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Esler WP, Kimberly WT, Jack C, Berezovska O, Kornilova A, Hyman BT, Perrimon N, Wolfe MS. Gamma-secretase/presenilin inhibitors for Alzheimer’s disease phenocopy Notch mutations in Drosophila. Faseb J. 2003;17:79–81. doi: 10.1096/fj.02-0394fje. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- Nateri AS, Spencer-Dene B, Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;437:281–285. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, Crockford TL, Cabuy E, Vindigni A, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Pan L, Chen S, Weng C, Call G, Zhu D, Tang H, Zhang N, Xie T. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1:458–469. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- Riesgo-Escovar JR, Jenni M, Fritz A, Hafen E. The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes Dev. 1996;10:2759–2768. doi: 10.1101/gad.10.21.2759. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007a;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Weissman IL. Hematopoietic stem cell aging: mechanism and consequence. Exp Gerontol. 2007b;42:385–390. doi: 10.1016/j.exger.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- Singh SR, Liu W, Hou SX. The adult Drosophila Malpighian Tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1:191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC, Stern D, Beaton A, Rhem EJ, Laverty T, Mozden N, Misra S, Rubin GM. The Berkeley Drosophila Genome Project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999;153:135–177. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c- mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- Wallenfang MR, Nayak R, DiNardo S. Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell. 2006;5:297–304. doi: 10.1111/j.1474-9726.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Weber U, Paricio N, Mlodzik M. Jun mediates Frizzled-induced R3/R4 cell fate distinction and planar polarity determination in the Drosophila eye. Development. 2000;127:3619–3629. doi: 10.1242/dev.127.16.3619. [DOI] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Role of mitogen-activated protein kinase kinase 4 in cancer. Oncogene. 2007;26:3172–3184. doi: 10.1038/sj.onc.1210410. [DOI] [PubMed] [Google Scholar]
- Wong MD, Jin Z, Xie T. Molecular mechanisms of germline stem cell regulation. Annu Rev Genet. 2005;39:173–195. doi: 10.1146/annurev.genet.39.073003.105855. [DOI] [PubMed] [Google Scholar]
- Yagi Y, Hayashi S. Role of the Drosophila EGF receptor in determination of the dorsoventral domains of escargot expression during primary neurogenesis. Genes Cells. 1997;2:41–53. doi: 10.1046/j.1365-2443.1997.d01-282.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


