Abstract
Cell survival, growth, differentiation, and homeostasis all rely on exquisite control over the abundance of particular cell surface membrane proteins. Cell surface proteins must respond appropriately to environmental as well as intracellular cues, often undergoing regulated internalization and lysosomal degradation. In addition, cell surface proteins can sustain damage and must be recognized and removed. A unifying mechanism has now emerged for the trafficking of damaged and downregulated proteins to the lysosome by their attachment to ubiquitin, which serves as a sorting signal for clathrin-mediated internalization and sorting into the lumen of late endosomes. Major questions remain as to how this broad system is governed, how it is adapted to meet the needs of particular cell surface proteins, and whether Ub serves as more than a one-way ticket to the lysosome for degradation. Here we highlight recent insights into these questions and the challenges that remain.
Ubiquitin as a sorting signal for lysosomes
Selective removal of membrane proteins from the plasma membrane serves as a broad mechanism to control many cellular processes, such as dampening signaling output from receptors or slowing the transport of metabolites into the cell. This strategy is also used to eliminate proteins that have sustained damage due to stresses such as an oxidative environment. Removal is mediated by internalization from the cell surface, followed by sorting into intralumenal vesicles of late endosomes/multi-vesicular bodies (MVBs), and subsequent delivery to the lysosomal lumen for degradation. This process needs to both distinguish the correct subset of cell surface proteins for sorting, and be regulated at multiple steps along the endocytic pathway. Ubiquitin (Ub) mediates this process by acting as a sorting tag for internalization and MVB sorting. Ub is a 76 amino acid protein, which can be covalently attached to substrate proteins post translationally and removed from substrates by deubiquitinating enzymes (Figure 1). As a sorting signal, Ub offers several advantages since it is modular, transient, and transferable, and thus can be readily regulated.
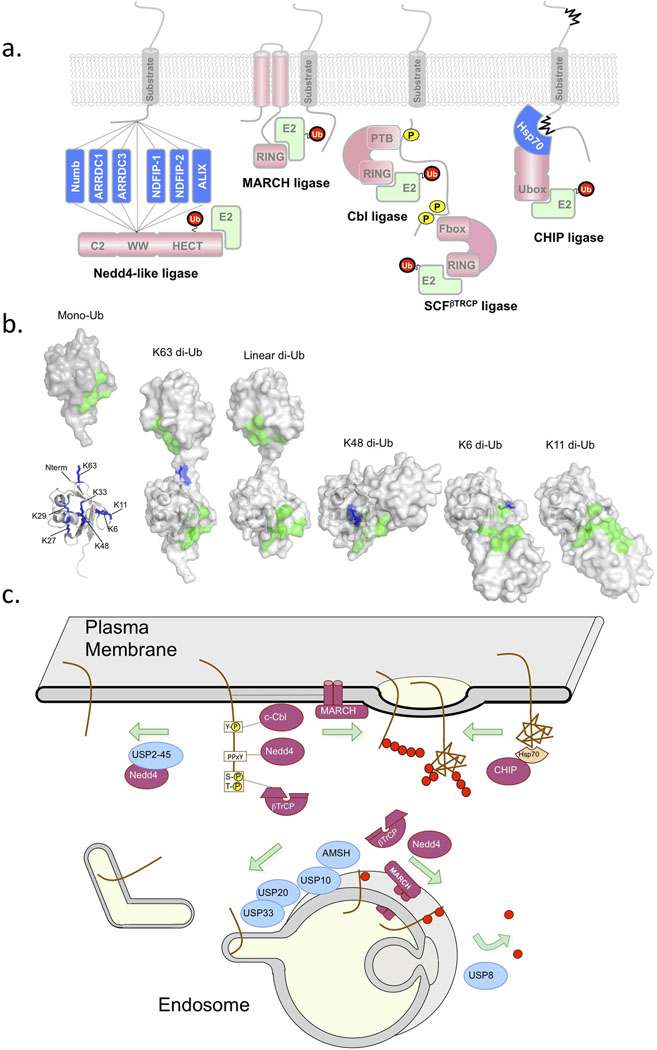
Figure 1. Ubiquitination and Deubiquitination for Lysosomal Sorting.
Several key enzymes have been found to play major roles in controlling the ubiquitination and deubiquitination of cargo. a) Shows a schematic of Ub ligases. Nedd4 family ligases bind to substrates or substrate adaptors via their WW domains [69]. Nedd4 family ligases use the HECT catalytic domain for Ub transfer, which covalently carries Ub via a thioester bond before transfer to substrates [68]. Other Ub ligases have RING domains or Ubox domains that associate with E2 conjugating enzymes and that position the E2 conjugating enzyme which bears the thioester-linked Ub near the substrate. Many RING-containing MARCH Ub-ligases have transmembrane domains, and may use these domains as means to recognize membrane protein substrates. Other RING ligases such as Cbl and Tripartate Skp1/Cullen/Fbox complexes such as SCFβTrCP can recognize phosphorylated cell surface via a phosphotyrosine binding domain (PTB) or other modules that bind phosphorylated serine/threonine residues [92, 93]. The ligase CHIP uses a Ubox to associate with an E2 conjugating enzyme. CHIP forms a complex with the chaperone Hsp70 to recognize and ubiquitinate unfolded damaged proteins fulfilling an important role in peripheral quality control [94]. b) Mono-Ub (left) has an interaction surface (green) centered around L8, I44, R42, H68, and V70 which is involved in binding the majority of Ub-binding domains [1]. Ub also has 7 lysines (blue: 6,11,27,29,33,48,63) as well as the N-terminus that can act as conjugation sites for other Ub molecules via an isopeptide bond with the C-terminus G76 residue [2]. Structures of di-Ub linked via different lysines show dramatically different conformations and position the tandem interaction surfaces (green) within each Ub moiety in different orientations that might be differentially recognized by Ub-binding domains and/or DUbs. c) shows a schematic of where key E3 Ub-ligases and counteracting DUbs are thought act in the endocytic pathway. Either exerting their control at the cell surface to determine whether a cell surface protein undergoes clathrin mediated internalization or whether it undergoes sorting into intralumenal vesicles of MVB/endosomes.
Several components of the endocytic sorting machinery feature Ub-binding domains (UBD) with low affinities, comparable to those of other sorting motifs. Cargo proteins carrying the Ub signal may undergo multiple rounds of ubiquitination and remodeling (by E3 ligases and deubiquitinating peptidases, Dubs, respectively), which provides ample opportunity to regulate their trafficking [1]. Ubiquitin exists in various guises – conjugated to an individual lysine (mono-Ub), conjugated to multiple individual lysines in a tagged protein (multiple mono-Ub), or in multimeric chains (poly-Ub) linked by lysine residues within the Ub moiety (Figure 1) [2]. Non-lysine sites can also be ubiquitinated, significantly broadening the potential biological influence of Ub – while also complicating the design of definitive experiments because it is difficult to eliminate ubiquitination or change the topology of the appended Ub [3-5]. In addition, over 100 DUbs have been identified, each with the potential to remove or remodel the Ub chain according to unique regulatory rules [6]. These features together with the specificity conferred by multiple Ub-binding proteins, provides a system of combinatorial complexity of which even the manufacturers of Lego would be proud.
How these components and regulatory circuits can fit together and how they are configured for different physiological processes and individual cargo proteins represents the overarching enterprise of the field. Some of the key questions now faced are: What are all the components in the endocytic sorting apparatus that bind Ub and do they have direct roles in sorting ubiquitinated cargo? Are there other Ub-dependent trafficking steps within the endocytic pathway besides internalization and MVB sorting and might they lead to other fates besides lysosomal degradation? And how might the complexity of the Ub system, replete with enzymes that specifically build or dismantle distinct poly-Ub chains, be harnessed by the endocytic system as a regulatory device?.
Ub contributes to the downregulation of cell-surface proteins
The first compelling description of Ub-dependent sorting came from yeast studies showing a close correlation between ubiquitination and vacuolar/lysosomal degradation of cell-surface proteins such as GPCRs and transporters [7]. Mutations in either cargo or E3 ligases that decreased ubiquitination markedly impaired cargo delivery to, and degradation in, lysosomes. Notably, although ubiquitination clearly increased the internalization of certain receptors from the surface even proteins that did not rely on Ub for its delivery to endosomes needed Ub as a sorting signal to complete their journey to the vacuole, specifically as a sorting signal for incorporation into MVBs [8]. Studies in mammalian cells with well-characterized cell surface proteins such as the EGFR (Epidermal Growth Factor Receptor) and Epithelial Sodium Channel (ENaC), which bind directly to Ub ligases, all helped support the standard model for how Ub acts as a trafficking signal in the endocytic pathway. In this model, Ub acts as a self-contained sorting signal, which presents a binding surface to a series of endosomal receptors that ultimately deliver the ubiquitinated cargo to lysosomes.
Certainly, the number of cell-surface proteins known to use Ub to mediate their internalization and/or intracellular sorting to lysosomes is continually increasing. However, it is important to remember that our current understanding for how Ub works as a sorting signal is founded on studies involving only a small subset of proteins. It is likely that many additional permutations and surprises still await discovery. One non-canonical example comes from studies of the interferon alpha receptor 1 (IFNAR). Following ligand binding, the Tyk2 and PKD2 kinase pathway is activated leading to receptor phosphorylation and recruitment of the βTrCP Ub-ligase [9]. This leads to receptor internalization and degradation in the lysosomes [10]. Although ubiquitination of IFNAR is required for internalization, the AP2 clathrin adaptor is actually engaged by a tyrosine-based motif, which either becomes exposed after site-specific ubiquitination of the IFNAR cytosolic tail, or works cooperatively with the sorting motifs on the Ub surface to engage the clathrin machinery [11].
A second curious twist to the role of ubiquitination in receptor downregulation is exemplified by PAR-1, a proteinase-activated G-protein-coupled receptor that regulates vascular function. PAR-1 cleavage by thrombin and removal of its N-terminal domain leads to its activation, internalization, and subsequent lysosomal degradation. Curiously, PAR-1 is ubiquitinated in its basal state; receptor activation leads to the loss of ubiquitination and initiates the receptor’s endocytic journey [12]. PAR-1 also undergoes basal constitutive internalization, a phenomenon enhanced in receptor mutants which lacked the major ubiquitinated lysine residues and suppressed in receptor mutants which carried an in-frame Ub fusion.
The Ub sorting machinery
For Ub to work as a modular sorting signal for membrane proteins, it has to be recognized by different sets of endosomal sorting machineries that can incorporate those cargoes into transport intermediates. Efforts to find sorting machinery capable of binding Ub has produced an expanding plethora of Ub-binding domains, all with low binding affinity, scattered amongst of variety of endosomal sorting machines [1]. The best understood Ub-dependent sorting steps are clathrin mediated internalization and ESCRT-dependent MVB sorting (Figure 2). Yet, the likelihood that many more endosomal proteins harbor Ub-binding domains begs the questions of whether we have identified all of the significant Ub-sorting receptors as well as all of the Ub-dependent sorting steps that might exist. In addition, the multifaceted roles now emerging for Ub caution against the simple interpretation that Ub-binding domains within the endocytic sorting machinery function solely as a means to bind ubiquitinated cargo.
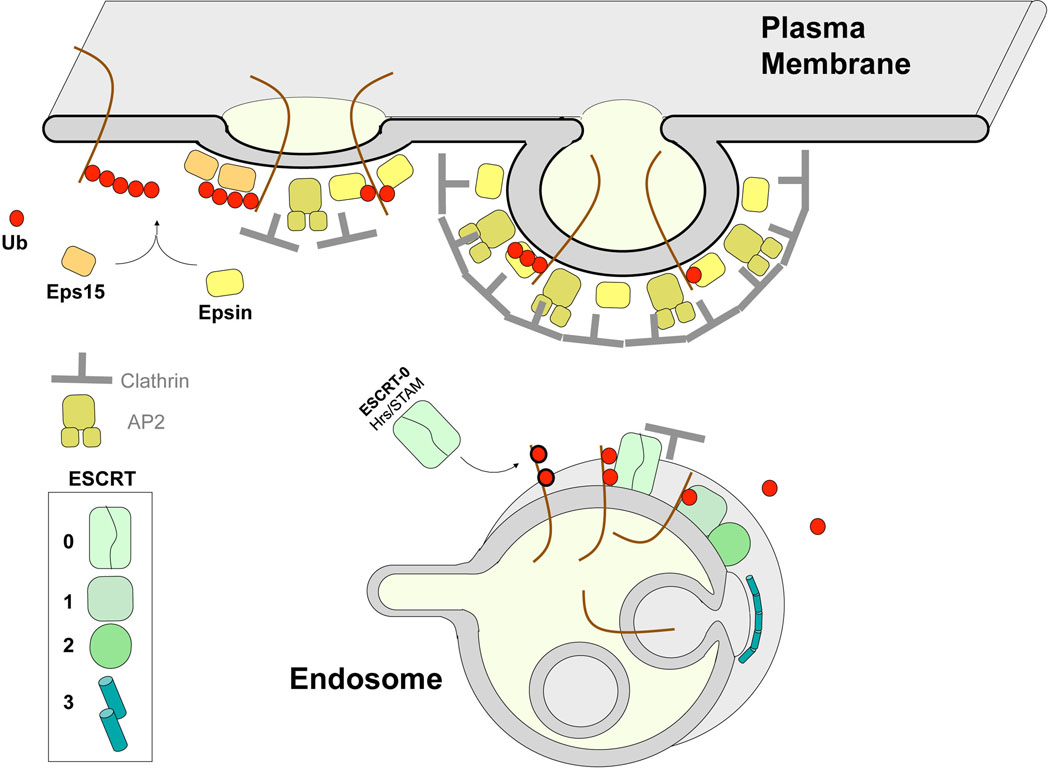
Figure 2. Endosomal Ub-Sorting Machinery.
Overview of the machinery that mediates Ub-dependent endosomal trafficking steps. Eps15 and Epsin are early-acting clathrin associated proteins that are thought to bind and concentrate ubiquitinated cargo in clathrin coated pits. Epsin and Eps15 associate with Ub using their UIM (Ub-interaction motifs). Other Ub-binding proteins that also associate with clathrin may also be present in clathrin-coated pits and could potentially act as additional cargo receptors or instead by regulated by the presence of Ub-cargo or ubiquitinated internalization machinery [1, 50]. Later, ubiquitinated cargo is recognized by the ESCRT-0 subunits Hrs and STAM as well other Ub-binding ESCRT components to usher ubiquitinated cargo into intralumenal vesicles that fill multivesicular bodies/late endosomes [31]. Additional Ub-binding domains are found within ESCRT-I and ESCRT-II, whose activities are also coupled with membrane deformation and scission with the additional ESCRT-III polymer thought to constrict the neck of intralumenal vesicles [30]. Other ESCRT-associated proteins (eg GGAs, TOM1, ALIX not pictured here) also bind Ub [31]. Collectively, these endosomal Ub-binding proteins may fulfill the function of redundant receptors for ubiquitinated-cargo or may be required for sorting of specific types of ubiquitinated cargo. Alternatively, only some of these Ub-binding ESCRT components may be directly involved in gathering ubiquitinated cargo, leaving other UBDs to fulfill other Ub-dependent modes of regulation.
Clathrin-mediated internalization
Ubiquitinated proteins such as the EGFR, the class I Major Histocompatability Complex (MHC-I), and Prolactin receptor, as well as model proteins fused to Ub, undergo Ub-dependent internalization through clathrin-coated pits [13–18]. The clathrin internalization apparatus relies on several accessory adaptors to gather cargo and promote vesicle formation (Figure 2). Two clathrin-associated proteins, Eps15 and Epsin, are excellent candidate Ub-sorting receptors; proteins that could bind ubiquitinated cell-surface proteins and confine them to the site of clathrin-vesicle formation [19]. Both Eps15 and Epsin have multiple Ub-Interaction Motifs (UIM), which bind Ub with a Kd in the high micromolar range [20, 21] and are required for association with ubiquitinated cargo [22–24]. Eps15 and Epsin are recruited into clathrin-coated pits early in the process of internalization, consistent with proposed roles as adaptors for ubiquitinated cargo [25]. Knockdown of either Eps15 or Epsin is sufficient to inhibit Ub-dependent internalization and overexpression of mutant Eps15 or Epsin lacking UIMs also perturbs cargo internalization [13, 14, 17, 18, 23, 26, 27]. These data make a strong case that Eps15 and Epsin are major Ub-sorting receptors for clathrin-meidated internalization. However, Eps15 and Epsin play additional roles in the biogenesis of clathrin vesicles, such as bending membranes or recruiting other factors to the clathrin coat [19]. Moreover, the UIMs within Eps15 and Epsin mediate their own ubiquitination, an effect that might regulate their activity since it appears to influence the repertoire of protein interactions in which they can participate [28, 29]. Finally, other clathrin adaptors may have as yet undiscovered Ub-binding domains and act as Ub-sorting receptors for clathrin-mediated internalization. Thus, clear tests of necessity and sufficiency (i.e. replacing endogenous Epsin, Eps15, and other components with versions that cannot bind Ub) will be required to identify the components of the clathrin internalization apparatus that can directly recognize ubiquitinated cargo proteins.
ESCRT-mediated MVB sorting
Sorting ubiquitinated cargo into intralumenal vesicles of MVBs relies on the ESCRT protein complexes (Endosomal Sorting Complex Required for Transport) [30]. These complexes (ESCRTs 0, I, II, III and the Vps4 complex) bind cargo, deform the membrane, and pinch off vesicles as they form from the limiting membrane of the endosome (Figure 2). Components of ESCRT-0, I, and II have UBDs, and ESCRT-0 is the strongest candidate for an endosomal Ub-sorting receptor based on the available experimental evidence [31]. ESCRT-0 has multiple UBDs that are housed in UIMs and VHS domains (Vps27/Hrs/STAM homology) within its two subunits, Hrs and STAM (Hepatocyte growth factor Receptor Substrate and Signal Transducing Adaptor Molecule). Loss of some of these UBDs blocks the sorting of ubiquitinated cargo without compromising other endosomal functions that depend on ESCRT-0 [31], supporting the idea that the major function provided by UBDs within ESCRT-0 is the binding and sorting of Ub-cargo. In contrast, inactivating the known UBDs within yeast ESCRT-I and/or ESCRT-II does not block MVB sorting of ubiquitinated cargo, thus compromising the simple model in which they too bind and help move cargo into intralumenal vesicles. One possibility is that there are yet undiscovered and partially functionally redundant UBDs within the ESCRTs or ESCRT-associated proteins and that their function will only be revealed when multiple UBDs are systematically eliminated. Indeed, the recent identification of even more Ub-binding domains within mammalian ESCRT-I subunits underscores this possibility [32]. Notably, however, combining mutations that inactivate some of the known UBDs of ESCRT-I with mutations that compromise its association with other ESCRTs causes complete loss of ESCRT function [31]. In addition, inactivating all of the known UBDs of ESCRT-0 leads to complete loss of function by this complex. These results imply that the UBDs promote the function of the ESCRT apparatus in a way that is more general than simply gathering cargo. Ubiquitinated cargo might itself provide an additional binding platform to recruit ESCRT components to the endosomal membrane or a means to stimulate ESCRT activity towards vesicle production. The internalization apparatus may similarly rely on functional Ub-binding domains to sustain normal operation. In yeast, inactivating the Epsin UBDs (those of both yeast Epsins, Ent1 and Ent2) along with those in the Eps15 yeast homolog, Ede1, compromises endocytosis of all proteins, regardless of whether they use Ub as an internalization signal [33].
Nature of the Ubiquitin signal
The Ub-binding domains found within the endocytic sorting machinery are capable of binding a single Ub, making it possible that a single “mono” Ub can impart sorting function. However, Ub is often found in poly-Ub chains in which either the N-terminus or Ub or the 7 lysines of Ub are attached to another Ub moiety to form chains of different topologies (Figure 1) [2]. Poly-Ub chains of different topologies that could promote the formation of differential binding platforms that are recognized by a particular set of Ub-binding proteins. This would impact Ub-sorting receptors, which might bind cargo with only specific Ub linkages, as well as DUbs and the so-called E4 ligases, which are capable of both dismantling and extending poly-Ub chains of a given topology, respectively. Alternatively, polyUb chains may offer a simple means of concatamerizing several weak mono-Ub sorting signals to either increase their avidity for Ub-sorting receptors, or provide a protective ‘hedge’ against non-specific trimming by DUbs.
PolyUb linked via K63 of Ub is the chain linkage most implicated in the endocytic pathway [34]. The debate as to whether mono or K63-polyUb is involved in internalization from the plasma membrane appears to be settling in favor of the latter. Many of the proteins that undergo Ub-dependent internalization are modified by K63-polyUb and some of the key cargo-modifying Ub ligases favor the formation of K63 -linked chains. In addition, a single Ub appended to a model protein serves as a rather poor internalization signal. Proteins that undergo internalization once attached to a K63polyUb chain include: TrkA (tropomyosin receptor kinase A, the nerve growth factor [NGF] receptor), which is modified by TRAF6 (Tumor Necrosis Factor Receptor associated factor), an enzyme that favors formation of K63 chains [35]; MHC-I, which is modified by the Karposi Sarcoma virus encoded K3-MARCH (membrane-associated RING-CH) ligase and the Ub-conjugating enzymes UbcH5/Ubc13, the latter of which favor K63-linked chains; CAT1 (Cationic Amino acid Transporter 1) and DAT (dopamine transporter), which are modified by Nedd4-2 [36, 37]; and the Aquaporin-2 channel [38] and Prolactin receptor, which are modified with both K63 and K48 chains by the Skp1/Cullin/βTrCP-Fbox Ub ligase [16]. Importantly, perturbing the Ub system so as to inhibit the formation of K63 chains diminishes internalization. For instance, the overexpression of mutant UbK63R, which interferes with the formation of K63-polyUb, attenuates internalization of the Prolactin receptor, TrkA, and MHC-I [14, 16, 35, 39]. In addition, siRNA-mediated suppression of Ubc13, the E2 enzyme which extends K63-polyUb chains, blocks K3- and K5-MARCH ligase-induced endocytosis of MHC-I [14, 39]. Finally, engineered cell-surface reporter proteins internalize poorly when fused in frame to a single UbK0 moiety that lacks lysines and C-terminal glycines to prevent further ubiquitination, but does internalize efficiently when lysine residues are reintroduced or when fused to several linearly-linked Ub moieties [13, 17, 26, 39].
These data make it clear that the more Ub a cargo carries, the more effective its internalization – most likely because this promotes the interaction of cargo with Ub-sorting receptors, which typically have poor affinity for single Ubs. Formation of a polyUb chain may simply be the most facile mechanism for achieving good binding, especially when a limited number of lysine residues are available on relevant cargo. It is more difficult to assess whether there is something unique about a given polyUb chain topology that allows it to function as an internalization signal. Are multiple mono-Ub moieties directly attached to cargo as effective? Support for the idea that K63-polyUb is the operative Ub-dependent internalization signal comes from studies showing that Epsin, the best candidate clathrin-associated Ub-sorting receptor, has a uniquely high affinity for K63-polyUb, but not for mono-Ub or other polyUb topologies [40]. One line of evidence that argues against this model comes from earlier studies in yeast examining the internalization of the Ste2 (STErile 2) GPCR receptor. Strains with mutant UbK63R as their sole source of Ub remain efficient at Ub-dependent internalization of Ste2, and fusion of a single Ub onto ubiquitination-deficient Ste2 is sufficient to restore internalization [41]. The apparent discrepancies between these data and those supporting a predominant role for K63-polyUb may be reconciled by studies showing that Ste2 also contains an NPxY-based sorting motif that binds Sla1 (Synthetic Lethal with abp1 1, which is the yeast homolog of mammalian CIN85, Cbl interacting protein of 85 kDa) [42]; it is possible that the NPxY motif and mono-Ub signal work cooperatively as a sorting signal in this context, simultaneously engaging the endocytic machinery.
Interestingly, other polyUb linkages also operate in the endocytic pathway. For example, K29-linked chains form on the Notch-regulator Deltex by the AIP4 (atrophin interacting protein 4) Nedd4-family ligase [43]. The Karposi Sarcoma virus encoded K5-MARCH ligase modifies MHC-I with either K63 or K11 Ub linkages, with some chains bearing mixed linkages that are required for efficient internalization [26, 39]. Intriguingly, K11-polyUb does not bind the Ub-binding domain of the clathrin adaptor Epsin, implying that K11-polyUb may work through a distinct class of Ub-sorting receptors [26].
The nature of the Ub signal mediating sorting into MVBs may differ from that required for internalization. In addition to correlative studies identifying K63 linkages on lysosomally targeted proteins, studies in yeast clearly show that strains lacking the ability to form K63-polyUb are impaired for the sorting of cargo into intralumenal vesicles of the MVB [34]. Attaching a single mutant UbK63R onto cargo in a yeast strain unable to make K63-polyUb is sufficient to restore sorting of that cargo into MVBs [44]. In addition, sorting of ubiquitinated proteins into MVBs was dramatically blocked in cells where ESCRT proteins were fused to deubiquitinating enzymes, but was restored when those cargos were covalently linked to a single Ub (which could not be removed by the ESCRT-DUb fusions) [44]. These results suggest that ESCRTs can sort cargo bearing only a single Ub, indicating that the role for K63-polyUb in endosomal sorting steps may be to defend against endosomal DUb activity, or potentially, to mediate other Ub-dependent endosomal sorting events that precede ESCRT activity. A critical role for the K63 -polyUb chain linkage in MVB cargo would be considerably strengthened by the identification of endosomal Ub-sorting receptors with unique specificity to K63-linked polyUb chains. The ESCRT apparatus has many subunits that bind polyUb better than mono-Ub. In the case of ESCRT-0, which serves a prominent role for a Ub-sorting receptor thought to work early in the process of gathering cargo, the preference for K63-linked polyUb is likely due to increased avidity for multiple Ubs, however, rather than specifically for a K63-linked chain per se [31]. A bias for K63-linked polyUb has been found for the NZF Ub-binding domain within the yeast ESCRT-II subunit Vps36 [45]. However, in the context of other binding assays, the bias Vps36 demonstrates towards K63-linked polyUb over linkages or mono-Ub is modest and loss of Ub-binding by the Vps36 NZF domain in vivo does not hamper sorting of cargo into MVBs [46, 47]. Of course, the strict demonstration that mono-Ub is capable of conferring sorting into MVBs does not diminish the possibility that particular cargo may be greatly influenced by a particular polyUb chain or that polyUb chain indeed does provide a set of powerful regulatory opportunities. The identification of not only new Ub-binding domains within ESCRTs and ESCRT-associated proteins that may have a preference for particular poly-Ub topologies but also regulatory ligases and DUbs that have preference those linkages is accelerating and should clarify how polyUb is exploited during endocytic sorting.
Ubiquitin contributes to the regulation of cell-surface protein quality control
The majority of studies examining Ub-dependent lysosomal sorting have focused on cell-surface proteins whose localization is selectively regulated, usually in response to external stimuli. These proteins are essentially behaving normally, and are likely devoid of any damage. Nonetheless, any protein exposed to the extracellular milieu is vulnerable to damage as a consequence of a wide range of insults thus necessitating a quality-control mechanism that removes and transports damaged proteins to lysosomes for degradation. This process is less well-studied since it is harder to simulate “damage” than it is to induce other regulatory responses. Recent studies have circumvented this problem by developing model “damaged” proteins that bypass ER quality control to reveal a “peripheral” quality control machinery at the plasma membrane. Such model proteins have been generated from CFTR (Cystic Fibrosis Transmembrane conductance Regulator) as well as two GPCRs, DRD4 (Dopamine Receptor D4) and the V2R (Vasopressin Receptor 2), with the latter designed to misfold in a temperature-sensitive manner [48, 49]. In all these cases, protein unfolding at the plasma membrane stimulates recruitment of the Hsp40, Hsc70 and Hsp90 chaperones, and the CHIP (Carboxy terminus of Hsp70-interacting protein) Ub ligase (Figure 1). This recruitment stimulated the ubiquitination of damaged receptors with K48-polyUb, K63-polyUb, and mono-Ub and accelerated receptor internalization. In parallel with ER quality-control mechanisms, the propensity of CFTR to undergo ubiquitination was enhanced by conformational destabilization in post-Golgi compartments. These studies highlight the role of chaperone-dependent CHIP activity in ubiquitination in the context of peripheral quality control (QC), though other Ub ligases may also be involved. This peripheral QC system likely contributes to many triage decisions involving a wide range conformationally defective plasma-membrane proteins, given that Hsc70 and Hsp90 have broad specificity for unfolded polypeptide substrates. The described selectivity of the peripheral QC machinery appears to differ from that used for ligand-induced desensitization of cell-surface receptors in that the former always uses CHIP whereas the latter uses a wide array of ligases. Reassuringly, regardless of which system is used to ubiquitinate a receptor, however, routing for lysosomal degradation occurs via the same ESCRT machinery.
Ubiquitin may act as a non-degradative sorting signal
Ub may also be used as a sorting signal that gives proteins access to parts of the endocytic system without necessarily being degraded in lysosomes. For example, Ub might label receptors for transport to ‘signaling endosomes’, which would allow them to efficiently stimulate downstream signaling pathways [50]. This could work by allowing DUbs to intervene along the trafficking pathway to prevent efficient transport into lysosomes. Access of EGFR to certain intracellular compartments is important for some arms of EGFR signal transduction, and when endocytosis is inhibited or EGFR lacks ubiquitinatable lysine residues, these pathways are not fully activated [15, 51]. Although the ubiquitination and degradation of TrkA ultimately limits its signal transduction [52, 53], other findings suggest that ubiquitination may guide TrkA to a signaling endosome in which it can signal effectively [35, 54]. Loss of lysine residues within the cytosolic domain of erythropoietin receptors not only attenuates ubiquitination and degradation, but also the ability to signal efficiently [55]. Aside from controlling localization, receptor ubiquitination may also promote signaling by enhancing interactions with effector components. Conglomeration of signal transduction components followed by their activation is beautifully illustrated in several of the pathways that lead to activation of NFκB [56]. This conceptual template may apply to other receptors as well. For example, the abilities of EGFR and the Hepatocyte growth factor receptor to interact with and phosphorylate ESCRT components depend on Ub interactions [57]. This in turn may regulate certain aspects of endosome function that could then regulate receptor output. Furthermore, a variety of downstream effectors of plasma membrane receptors also have UBDs; it is possible that receptor ubiquitination enhances their interaction and, therefore, effector activation [58–61].
Another context in which Ub could serve as a transient signal for intracellular sorting is GLUT4, the glucose transporter isoform 4 that is sequestered in specialized endosomal compartments within insulin-sensitive cells, such as myocytes and adipocytes. Insulin stimulates GLUT4 translocation from these compartments to the cell surface, and increases glucose uptake. GLUT4 is ubiquitinated in adipocytes, and removal of its ubiquitinatable lysines prevents sorting to specialized insulin-sensitive compartments. Insulin-stimulated translocation and ubiquitination of GLUT4 can be restored by adding back lysines, independent of their position. These findings suggest that Ub may serve as a signal for entry into an insulin-responsive endosomal compartment. Intriguingly, formation of this compartment depends on GGA proteins [62], which bind Ub and mediate Ub-sorting within the TGN and endosomes [63, 64]. One caveat to these studies is that in addition to being ubiquitinated, GLUT4 is covalently modified by SUMO (small ubiquitin-like modifier) on its lysine residues, and the SUMO E2 conjugating enzyme Ubc9 fosters sorting of GLUT4 into glucose transporter vesicles, which prevents its degradation in lysosomes [65]. Thus, it will be important to identify the exact contributions of Ub and SUMO-dependent processes in GLUT4 sorting. An intriguing parallel to the translocation of GLUT4 is that of the Robo1 receptor from its predominant localization in endosomal compartments to the cell surface. This shift in localization is triggered by cellular exposure to the Robo1 ligand, Slit, a guidance factor for cell migration [66, 67]. This positive-feedback loop, which stimulates the mobilization of stored Robo receptors to the cell surface, depends on interaction between Robo1 and the DUb USP33. These data support the possibility that Robo1 is the target of USP33 deubiquitination, and that ubiquitination of Robo1 is responsible for sequestering it into endosomal compartments.
Conclusions
The general features for how Ub can work as a trafficking signal for lysosomal degradation have now been revealed. Different Ub-binding domains within various endosomal protein sorting machines as well as the potential to assemble, modify, and recognize different sorting signals using Ub as a building block that forms various polyUbs add to the complexity and adaptability of this pathway. How these potential mechanisms and regulatory capabilities are put into practice for particular proteins responding to various physiological conditions will be fascinating and critically important. However, the full range for how Ub works as a sorting signal has yet to be fully defined. Not everything is likely to fit within the simple paradigm of lysosomal sorting mediated by a small set of Ub-binding proteins controling a limited set of sorting steps. Perhaps the most exciting prospects are to discover new Ub-dependent trafficking pathways, sorting steps, and the machinery that controls them.
BOX 1 Key ligases controlling Ub-dependent sorting
A number of important Ub-ligases have been discovered that modify a wide variety of membrane protein cargos (Figure 1). Here we discuss a few that have prominent emerging roles on endocytic membrane trafficking.
Nedd4-family ligases
Rsp5, a member of the Nedd4 family of HECT (homologous to E6 carboxy terminus) E3 ligases, is the major ligase controlling traffic to the yeast lysosome [68]. Humans have nine Nedd4-family ligases bearing an N-terminal C2 lipid-binding domain and WW domains, which bind PPxY (PY) motifs. These ligases may bind their substrates directly (exemplified by ENaC that contains PY motifs) or indirectly via adaptor proteins that contain both PY motifs and other protein-interaction modules [69]. These adaptors may also activate Nedd4-family ligases by interfering with intramolecular interactions that cause autoinhibition [70].
One class of adaptors is the Arrestin Domain Containing (ADC) proteins that contain either an arrestin fold or simply have a motif derived from a conserved portion of the arrestin domain, although what functionality the arrestin fold or motif confers remains unclear [71]. Substrates for some ADCs have been identified. For example, human ARRDC3 targets both β-Adrenergic Receptor and Integrin-β4 for lysosomal degradation [72, 73]. In yeast, the ADCs have overlapping sets of substrates, which implies that they either act at different steps of the endocytic pathway or respond to different physiological stimuli [74]. Many ADCs are themselves ubiquitinated, which may target them for degradation [75, 76] or stimulate their activity [71]. How ubiquitination promotes ADC function is unclear. The appended Ub may be recognized by a Ub-binding domain in a downstream sorting complex, for example an ESCRT component, improving recruitment of the substrate protein [77]. Alternatively, the ADC-Ub complex may activate the ligase by binding a Ub-binding region in the HECT-domain of Nedd4 ligases [78, 79].
MARCH ligases
The membrane associated RING-CH (MARCH) family of Ub ligases are the cellular orthologues of the Kaposi sarcoma-associated herpesvirus (KSHV)-encoded K3 and K5 ligases. Most MARCH ligases have two transmembrane domains [80]. MARCH1, the best characterized family member, ubiquitinates and downregulates MHC-II from the cell surface of antigen-presenting dendritic cells [81]. The KSHV-encoded K3 ligase polyubiquitinates MHC I as part of an overall strategy to evade immune detection. K3-dependent ubiquitination stimulates both the internalization of MHC I and its ultimate sorting to lysosomes [14]. Overexpression of other MARCH family members downregulates a number of cell-surface receptors including CD44, CD81, CD4, ICAM, CD86, Fas and the Transferrin receptor; however, whether these are true substrates requires confirmation [80]. No recognition motif has yet been identified for MARCH ligases. One possibility is that MARCH ligases recognize their substrates through interactions between transmembrane domains. Interestingly CD83, a single-pass membrane protein, interferes with the ability of MARCH1 to bind, ubiquitinate and downregulate MHC II, and this effect is conferred by the CD83 transmembrane domain. This suggests that the CD83 transmembrane domain may displace MARCH1 [82]. MARCH ligases may also be subject to spatial constraints imposed by their membrane association. Indeed, the Ub-acceptor lysine for all defined substrates of the K5 viral ligase lie within the membrane-proximal, positively charged stop-transfer region [80].
BOX 2 Multiple roles for Deubiquitinating enzymes in endocytic trafficking
Ubiquitin removal is catalyzed by DUbs, and several are implicated in the control of Ub-dependent sorting within endosomes. Some DUbs associate with specific membrane proteins thereby allowing them to regulate lysosomal sorting in a cargo-specific manner. Other DUbs can associate with the machinery that sorts ubiquitinated proteins and thus may have more global effects on Ub-dependent trafficking (Figure 1).
Overexpression and siRNA suppression studies demonstrate a role for the DUbs UCH-L3, Usp10, and Usp2–45 in maintaining cell surface levels of ENaC [83–85]; a role for USP10 in fostering CFTR recycling [86], and the pair of homologous DUbs USP33 and USP20 in controlling recycling of β-AR (beta-adrenergic receptor) [87]. One way these DUbs could operate is to remove of Ub from cargo itself to enable recycling back to the cell surface as proposed for the effect of USP10 on CFTR or USP33/USP20 on β-AR. However, these DUbs may have a variety of targets, allow them to fulfill complex cellular roles that belies one dimensional models. For instance, cell surface ENaC levels are increased by elevated expression of USP10, a condition that is physiologically mediated by vasopressin. Yet, overexpressed USP10 does not deubiquitinate the bulk of the ENaC pool. Rather, USP10 interacts with and stabilizes the retromer subunit SNX3 thereby protecting it against degradation and elevating its ability to mediate recycling from endosomal compartments [85]. Usp2–45 may play a similarly complex role in that it not only can directly interact with ENaC, but also directly with the ligase Nedd4–2 allowing it the capacity to regulate a variety of Nedd4-dependent processes [88].
DUbs also associate with the ESCRT apparatus, which may impart global control of the MVB sorting steps they execute. However, the protein-interaction profiles between these components are complex, as are the consequences of their perturbation. These findings suggest that the ESCRT-associated DUbs play a variety of roles at distinct points. Elucidating the roles of ESCRT-associated DUbs is probably simplest in yeast. In yeast, Doa4/Ubp4, associates with ESCRT-III, which works late in the process of cargo sorting to recycle Ub from cargo, just prior to its entry into the MVB lumen [89]. In mammalian cells, USP8/UBPY and AMSH associate with ESCRTs. However, both of these enzymes can interact directly with ESCRT-0 and ESCRT-III [90]. USP8 is a broad-specificity enzyme, whereas AMSH is specific for K63-polyUb. Depending on the system examined, these enzymes have been proposed to deubiquitinate cargo early in the sorting process to foster cargo recycling, deubiquitinate cargo late in the process to rescue Ub from lysosomal degradation, deubiquitinate and stabilize key Ub-ligases that mediate cargo ubiquitination, or deubiquitinate the ESCRT machinery itself to enhance its activity by stabilizing it against proteasome-mediate degradation or Ub-induced conformational changes [90, 91].
Contributor Information
Robert C. Piper, Molecular Physiology and Biophysics, University of Iowa, Iowa City, IA 52242, Robert-Piper@uiowa.edu
Paul J. Lehner, Cambridge Institute for Medical Research, Addenbrooke's Hospital, Hills Road, Cambridge, CB2 0XY, pjl30@cam.ac.uk
References
- 1.Hurley JH, Stenmark H. Molecular mechanisms of ubiquitin-dependent membrane traffic. Annu Rev Biophys. 2011;40:119–142. doi: 10.1146/annurev-biophys-042910-155404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komander D. The emerging complexity of protein ubiquitination. Biochem Soc Trans. 2009;37:937–953. doi: 10.1042/BST0370937. [DOI] [PubMed] [Google Scholar]
- 3.Cadwell K, Coscoy L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science. 2005;309:127–130. doi: 10.1126/science.1110340. [DOI] [PubMed] [Google Scholar]
- 4.Ciechanover A, Ben-Saadon R. N-terminal ubiquitination: more protein substrates join in. Trends Cell Biol. 2004;14:103–106. doi: 10.1016/j.tcb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, et al. Ubiquitination of serine, threonine, or lysine residues on the cytoplasmic tail can induce ERAD of MHC-I by viral E3 ligase mK3. The Journal of cell biology. 2007;177:613–624. doi: 10.1083/jcb.200611063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sowa ME, et al. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138:389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hicke L. Getting' down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends in cell biology. 1999;9:107–112. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- 8.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng H, et al. Ligand-stimulated downregulation of the alpha interferon receptor: role of protein kinase D2. Mol Cell Biol. 2011;31:710–720. doi: 10.1128/MCB.01154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar KG, et al. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. EMBO J. 2003;22:5480–5490. doi: 10.1093/emboj/cdg524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar KG, et al. Site-specific ubiquitination exposes a linear motif to promote interferon-alpha receptor endocytosis. J Cell Biol. 2007;179:935–950. doi: 10.1083/jcb.200706034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe BL, et al. Ubiquitination differentially regulates clathrin-dependent internalization of protease-activated receptor-1. J Cell Biol. 2007;177:905–916. doi: 10.1083/jcb.200610154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barriere H et al. Molecular basis of oligoubiquitin-dependent internalization of membrane proteins in Mammalian cells. Traffic. 2006;7:282–297. doi: 10.1111/j.1600-0854.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 14.Duncan LM et al. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J. 2006;25:1635–1645. doi: 10.1038/sj.emboj.7601056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goh LK et al. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J Cell Biol. 2010;189:871–883. doi: 10.1083/jcb.201001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varghese B et al. Polyubiquitination of prolactin receptor stimulates its internalization, postinternalization sorting, and degradation via the lysosomal pathway. Mol Cell Biol. 2008;28:5275–5287. doi: 10.1128/MCB.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertelsen V et al. A chimeric pre-ubiquitinated EGF receptor is constitutively endocytosed in a clathrin-dependent, but kinase-independent manner. Traffic. 2011;12:507–520. doi: 10.1111/j.1600-0854.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 18.Hawryluk MJ et al. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic. 2006;7:262–281. doi: 10.1111/j.1600-0854.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 19.Traub LM, Lukacs GL. Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs. J Cell Sci. 2007;120:543–553. doi: 10.1242/jcs.03385. [DOI] [PubMed] [Google Scholar]
- 20.Dikic I et al. Ubiquitin-binding domains - from structures to functions. Nat Rev Mol Cell Biol. 2009;10:659–671. doi: 10.1038/nrm2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann K, Falquet L. A ubiquitin-interacting motif conserved in components of the proteasomal and lysosomal protein degradation systems. Trends Biochem Sci. 2001;26:347–350. doi: 10.1016/s0968-0004(01)01835-7. [DOI] [PubMed] [Google Scholar]
- 22.Girao H et al. Eps15 interacts with ubiquitinated Cx43 and mediates its internalization. Exp Cell Res. 2009;315:3587–3597. doi: 10.1016/j.yexcr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Kazazic M et al. Epsin 1 is involved in recruitment of ubiquitinated EGF receptors into clathrin-coated pits. Traffic. 2009;10:235–245. doi: 10.1111/j.1600-0854.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 24.Sigismund S et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor MJ et al. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 2011;9:e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goto E et al. Contribution of lysine 11-linked ubiquitination to MIR2-mediated major histocompatibility complex class I internalization. J Biol Chem. 2010;285:35311–35319. doi: 10.1074/jbc.M110.112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Melker AA et al. Ubiquitin ligase activity of c-Cbl guides the epidermal growth factor receptor into clathrin-coated pits by two distinct modes of Eps15 recruitment. J Biol Chem. 2004;279:55465–55473. doi: 10.1074/jbc.M409765200. [DOI] [PubMed] [Google Scholar]
- 28.Chen H et al. Rapid Ca2+-dependent decrease of protein ubiquitination at synapses. Proc Natl Acad Sci U S A. 2003;100:14908–14913. doi: 10.1073/pnas.2136625100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polo S et al. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–455. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- 30.Henne WM et al. The ESCRT Pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Shields SB, Piper RC. How Ubiquitin Functions with ESCRTs. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefani F et al. UBAP1 Is a Component of an Endosome-Specific ESCRT-I Complex that Is Essential for MVB Sorting. Curr Biol. 2011;21:1245–1250. doi: 10.1016/j.cub.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 33.Dores MR et al. The function of yeast epsin and Ede1 ubiquitin-binding domains during receptor internalization. Traffic. 2010;11:151–160. doi: 10.1111/j.1600-0854.2009.01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauwers E et al. The ubiquitin code of yeast permease trafficking. Trends Cell Biol. 2010;20:196–204. doi: 10.1016/j.tcb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Geetha T et al. Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol Cell. 2005;20:301–312. doi: 10.1016/j.molcel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Vina-Vilaseca A et al. Protein kinase C-dependent ubiquitination and clathrin-mediated endocytosis of the cationic amino acid transporter CAT-1. J Biol Chem. 2011;286:8697–8706. doi: 10.1074/jbc.M110.186858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vina-Vilaseca A, Sorkin A. Lysine 63-linked polyubiquitination of the dopamine transporter requires WW3 and WW4 domains of Nedd4-2 and UBE2D ubiquitin-conjugating enzymes. J Biol Chem. 2010;285:7645–7656. doi: 10.1074/jbc.M109.058990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamsteeg EJ et al. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci U S A. 2006;103:18344–18349. doi: 10.1073/pnas.0604073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boname JM et al. Efficient internalization of MHC I requires lysine-11 and lysine-63 mixed linkage polyubiquitin chains. Traffic. 2010;11:210–220. doi: 10.1111/j.1600-0854.2009.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato Y et al. Structural basis for specific recognition of Lys 63-linked polyubiquitin chains by tandem UIMs of RAP80. EMBO J. 2009;28:2461–2468. doi: 10.1038/emboj.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terrell J et al. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol Cell. 1998;1:193–202. doi: 10.1016/s1097-2765(00)80020-9. [DOI] [PubMed] [Google Scholar]
- 42.Howard JP et al. Sla1p serves as the targeting signal recognition factor for NPFX(1,2)D-mediated endocytosis. J Cell Biol. 2002;157:315–326. doi: 10.1083/jcb.200110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chastagner P et al. Itch/AIP4 mediates Deltex degradation through the formation of K29-linked polyubiquitin chains. EMBO Rep. 2006;7:1147–1153. doi: 10.1038/sj.embor.7400822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stringer DK, Piper RC. A single ubiquitin is sufficient for cargo protein entry into MVBs in the absence of ESCRT ubiquitination. J Cell Biol. 2011;192:229–242. doi: 10.1083/jcb.201008121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulathu Y et al. Two-sided ubiquitin binding explains specificity of the TAB2 NZF domain. Nat Struct Mol Biol. 2009;16:1328–1330. doi: 10.1038/nsmb.1731. [DOI] [PubMed] [Google Scholar]
- 46.Sato Y et al. Structural basis for specific recognition of Lys 63-linked polyubiquitin chains by NZF domains of TAB2 and TAB3. The EMBO journal. 2009;28:3903–3909. doi: 10.1038/emboj.2009.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shields SB et al. ESCRT ubiquitin-binding domains function cooperatively during MVB cargo sorting. J Cell Biol. 2009;185:213–224. doi: 10.1083/jcb.200811130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okiyoneda T et al. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science. 2010;329:805–810. doi: 10.1126/science.1191542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Apaja PM et al. Quality control for unfolded proteins at the plasma membrane. J Cell Biol. 2010;191:553–570. doi: 10.1083/jcb.201006012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 51.Vieira AV et al. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 52.Arevalo JC et al. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron. 2006;50:549–559. doi: 10.1016/j.neuron.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 53.Yu T et al. Regulation of trafficking of activated TrkA is critical for NGF-mediated functions. Traffic. 2011;12:521–534. doi: 10.1111/j.1600-0854.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Georgieva MV et al. Ubiquitination of TrkA by Nedd4-2 regulates receptor lysosomal targeting and mediates receptor signaling. J Neurochem. 2011;117:479–493. doi: 10.1111/j.1471-4159.2011.07218.x. [DOI] [PubMed] [Google Scholar]
- 55.Yosha L et al. Cytosolic lysine residues enhance anterograde transport and activation of the erythropoietin receptor. Biochem J. 2011 doi: 10.1042/BJ20101876. [DOI] [PubMed] [Google Scholar]
- 56.Skaug B et al. The role of ubiquitin in NF-kappaB regulatory pathways. Annu Rev Biochem. 2009;78:769–796. doi: 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- 57.Urbe S et al. The UIM domain of Hrs couples receptor sorting to vesicle formation. J Cell Sci. 2003;116:4169–4179. doi: 10.1242/jcs.00723. [DOI] [PubMed] [Google Scholar]
- 58.Erneux C et al. SHIP2 multiple functions: a balance between a negative control of PtdIns(3,4,5)P(3) level, a positive control of PtdIns(3,4)P(2) production and intrinsic docking properties. J Cell Biochem. 2011 doi: 10.1002/jcb.23146. [DOI] [PubMed] [Google Scholar]
- 59.Lin Q et al. HECT E3 ubiquitin ligase Nedd4-1 ubiquitinates ACK and regulates epidermal growth factor (EGF)-induced degradation of EGF receptor and ACK. Mol Cell Biol. 2010;30:1541–1554. doi: 10.1128/MCB.00013-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duex JE et al. Recruitment of Uev1B to Hrs-containing endosomes and its effect on endosomal trafficking. Exp Cell Res. 2010;316:2136–2151. doi: 10.1016/j.yexcr.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J et al. The SAM domains of Anks family proteins are critically involved in modulating the degradation of EphA receptors. Mol Cell Biol. 2010;30:1582–1592. doi: 10.1128/MCB.01605-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watson RT et al. Entry of newly synthesized GLUT4 into the insulin-responsive storage compartment is GGA dependent. EMBO J. 2004;23:2059–2070. doi: 10.1038/sj.emboj.7600159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scott PM et al. GGA proteins bind ubiquitin to facilitate sorting at the trans-Golgi network. Nat Cell Biol. 2004;6:252–259. doi: 10.1038/ncb1107. [DOI] [PubMed] [Google Scholar]
- 64.Puertollano R, Bonifacino JS. Interactions of GGA3 with the ubiquitin sorting machinery. Nat Cell Biol. 2004;6:244–251. doi: 10.1038/ncb1106. [DOI] [PubMed] [Google Scholar]
- 65.Liu LB et al. The SUMO conjugating enzyme Ubc9 is a regulator of GLUT4 turnover and targeting to the insulin-responsive storage compartment in 3T3-L1 adipocytes. Diabetes. 2007;56:1977–1985. doi: 10.2337/db06-1100. [DOI] [PubMed] [Google Scholar]
- 66.Yuasa-Kawada J et al. Midline crossing and Slit responsiveness of commissural axons require USP33. Nat Neurosci. 2009;12:1087–1089. doi: 10.1038/nn.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuasa-Kawada J et al. Deubiquitinating enzyme USP33/VDU1 is required for Slit signaling in inhibiting breast cancer cell migration. Proc Natl Acad Sci U S A. 2009;106:14530–14535. doi: 10.1073/pnas.0801262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10:398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 69.Leon S, Haguenauer-Tsapis R. Ubiquitin ligase adaptors: regulators of ubiquitylation and endocytosis of plasma membrane proteins. Experimental cell research. 2009;315:1574–1583. doi: 10.1016/j.yexcr.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 70.Mund T, Pelham HR. Control of the activity of WW-HECT domain E3 ubiquitin ligases by NDFIP proteins. EMBO Rep. 2009;10:501–507. doi: 10.1038/embor.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin CH et al. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135:714–725. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 72.Nabhan JF et al. Arrestin domain-containing protein 3 recruits the NEDD4 E3 ligase to mediate ubiquitination of the beta2-adrenergic receptor. EMBO Rep. 2010;11:605–611. doi: 10.1038/embor.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Draheim KM et al. ARRDC3 suppresses breast cancer progression by negatively regulating integrin beta4. Oncogene. 2010;29:5032–5047. doi: 10.1038/onc.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nikko E, Pelham HR. Arrestin-mediated endocytosis of yeast plasma membrane transporters. Traffic. 2009;10:1856–1867. doi: 10.1111/j.1600-0854.2009.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stimpson HE et al. Transferrin receptor-like proteins control the degradation of a yeast metal transporter. EMBO J. 2006;25:662–672. doi: 10.1038/sj.emboj.7600984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leon S et al. Ear1p and Ssh4p are new adaptors of the ubiquitin ligase Rsp5p for cargo ubiquitylation and sorting at multivesicular bodies. Mol Biol Cell. 2008;19:2379–2388. doi: 10.1091/mbc.E08-01-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herrador A et al. Recruitment of the ESCRT machinery to a putative seven-transmembrane-domain receptor is mediated by an arrestin-related protein. Mol Cell Biol. 2010;30:897–907. doi: 10.1128/MCB.00132-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maspero E et al. Structure of the HECT:ubiquitin complex and its role in ubiquitin chain elongation. EMBO Rep. 2011;12:342–349. doi: 10.1038/embor.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim HC et al. Structure and function of a HECT domain ubiquitin-binding site. EMBO Rep. 2011;12:334–341. doi: 10.1038/embor.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nathan JA, Lehner PJ. The trafficking and regulation of membrane receptors by the RING-CH ubiquitin E3 ligases. Exp Cell Res. 2009;315:1593–1600. doi: 10.1016/j.yexcr.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 81.Ishido S et al. MARCH-I: a new regulator of dendritic cell function. Mol Cells. 2010;29:229–232. doi: 10.1007/s10059-010-0051-x. [DOI] [PubMed] [Google Scholar]
- 82.Tze LE et al. CD83 increases MHC II and CD86 on dendritic cells by opposing IL-10-driven MARCH1-mediated ubiquitination and degradation. J Exp Med. 2011;208:149–165. doi: 10.1084/jem.20092203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruffieux-Daidie D et al. Deubiquitylation regulates activation and proteolytic cleavage of ENaC. J Am Soc Nephrol. 2008;19:2170–2180. doi: 10.1681/ASN.2007101130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Butterworth MB et al. The deubiquitinating enzyme UCH-L3 regulates the apical membrane recycling of the epithelial sodium channel. J Biol Chem. 2007;282:37885–37893. doi: 10.1074/jbc.M707989200. [DOI] [PubMed] [Google Scholar]
- 85.Boulkroun S et al. Vasopressin-inducible ubiquitin-specific protease 10 increases ENaC cell surface expression by deubiquitylating and stabilizing sorting nexin 3. Am J Physiol Renal Physiol. 2008;295:F889–F900. doi: 10.1152/ajprenal.00001.2008. [DOI] [PubMed] [Google Scholar]
- 86.Bomberger JM et al. The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J Biol Chem. 2009;284:18778–18789. doi: 10.1074/jbc.M109.001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berthouze M et al. The deubiquitinases USP33 and USP20 coordinate beta2 adrenergic receptor recycling and resensitization. EMBO J. 2009;28:1684–1696. doi: 10.1038/emboj.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oberfeld B et al. Ubiquitin-specific protease 2-45 (Usp2-45) binds to epithelial Na+ channel (ENaC) ubiquitylating enzyme Nedd4-2. Am J Physiol Renal Physiol. 2011 doi: 10.1152/ajprenal.00487.2010. [DOI] [PubMed] [Google Scholar]
- 89.Luhtala N, Odorizzi G. Bro1 coordinates deubiquitination in the multivesicular body pathway by recruiting Doa4 to endosomes. J Cell Biol. 2004;166:717–729. doi: 10.1083/jcb.200403139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wright MH et al. Regulation of Endocytic Sorting by ESCRT-DUB-Mediated Deubiquitination. Cell Biochem Biophys. 2011;60:39–46. doi: 10.1007/s12013-011-9181-9. [DOI] [PubMed] [Google Scholar]
- 91.Avvakumov GV et al. Amino-terminal dimerization, NRDP1-rhodanese interaction, and inhibited catalytic domain conformation of the ubiquitin-specific protease 8 (USP8) J Biol Chem. 2006;281:38061–38070. doi: 10.1074/jbc.M606704200. [DOI] [PubMed] [Google Scholar]
- 92.Swaminathan G, Tsygankov AY. The Cbl family proteins: ring leaders in regulation of cell signaling. J Cell Physiol. 2006;209:21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- 93.Frank SJ, Fuchs SY. Modulation of growth hormone receptor abundance and function: roles for the ubiquitin-proteasome system. Biochim Biophys Acta. 2008;1782:785–794. doi: 10.1016/j.bbadis.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nature reviews. Molecular cell biology. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]