Abstract
c-MET is a receptor tyrosine kinase that, after binding with its ligand, hepatocyte growth factor, activates a wide range of different cellular signaling pathways, including those involved in proliferation, motility, migration and invasion. Although c-MET is important in the control of tissue homeostasis under normal physiological conditions, it has also been found to be aberrantly activated in human cancers via mutation, amplification or protein overexpression. This paper provides an overview of the c-MET signaling pathway, including its role in the development of cancers, and provides a rationale for targeting the pathway as a possible treatment option.
Keywords: cancer, c-MET, hepatocyte growth factor (HGF), MET, signaling, receptor tyrosine kinase
Receptor tyrosine kinases
Receptor tyrosine kinases (RTKs) regulate many essential cellular processes in mammalian development, cell function and tissue homeostasis. However, although RTKs are important in normal physiology, dysregulation of certain RTKs has been implicated in the development and progression of many types of cancer [Krause and Van Etten, 2005]. For example, expression of the c-MET RTK and its ligand, hepatocyte growth factor (HGF), has been observed in tumor biopsies of most solid tumors and c-MET signaling has been documented in a wide range of human malignancies [Peruzzi and Bottaro, 2006; Birchmeier et al. 2003; Comoglio and Trusolino, 2002]. This paper provides an overview of the c-MET signaling pathway, including its role in the development of cancers, and provides a rationale for targeting the pathway as a possible treatment option.
Hepatocyte growth factor and c-MET: structure and function
The c-MET proto-oncogene is located on chromosome 7q21-31. Its transcription is regulated by Ets (E-twenty six), Pax3 (paired box 3), AP2 (activator protein-2) and Tcf-4 (transcription factor 4) [Boon et al. 2002; Epstein et al. 1996; Gambarotta et al. 1996; Boccaccio et al. 1994], and it is expressed as multiple mRNA transcripts of 8, 7, 4.5, 3 and 1.5 kilobases [Park et al. 1986]. The protein product of this gene is the c-MET tyrosine kinase. This cell surface receptor is expressed in epithelial cells of many organs, including the liver, pancreas, prostate, kidney, muscle and bone marrow, during both embryogenesis and adulthood [Comoglio et al. 2008].
The c-MET receptor is formed by proteolytic processing of a common precursor in the post-Golgi compartment into a single-pass, disulphide-linked α/β heterodimer (Figure 1a.) [Trusolino and Comoglio, 2002]. The extracellular portion of c-MET is composed of three domain types. The N-terminal 500 residues fold to form a large semaphorin (Sema) domain, which encompasses the whole α-subunit and part of the β-subunit. The Sema domain shares sequence homology with domains found in the semaphorin and plexin families. The PSI domain (found in plexins, semaphorins and integrins) follows the Sema domain, spans approximately 50 residues and includes four disulphide bonds. This domain is connected to the transmembrane helix via four immunoglobulin–plexin–transcription (IPT) domains, which are related to immunoglobulin-like domains and are found in integrins, plexins and transcription factors. Intracellularly, the c-MET receptor contains a tyrosine kinase catalytic domain flanked by distinctive juxtamembrane and carboxy-terminal sequences.
Figure 1.
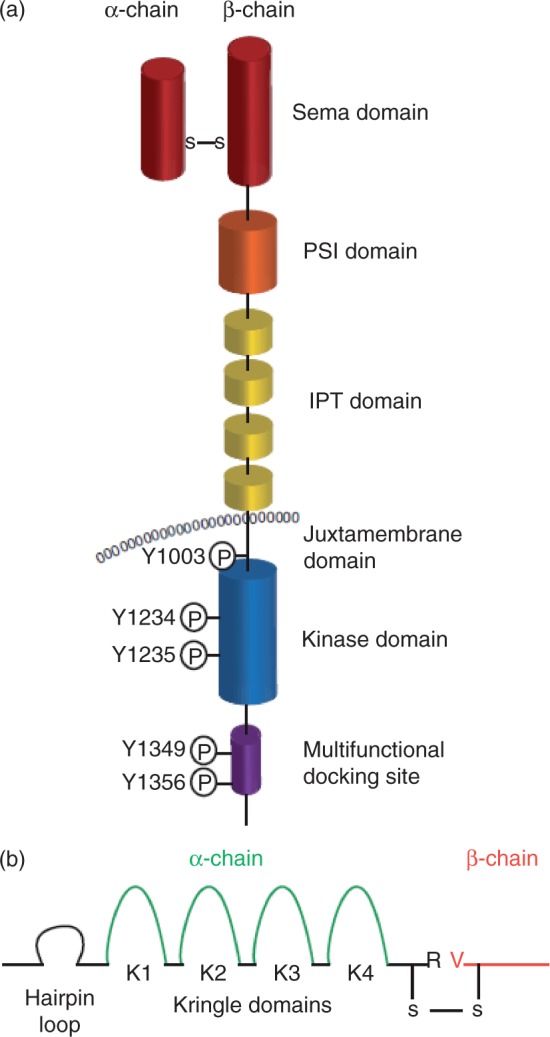
Domain structure of c-MET and hepatocyte growth factor (HGF). (a) The c-MET receptor is formed by proteolytic processing of a common precursor into a single-pass, disulphide-linked α/β heterodimer. The extracellular portion of c-MET is composed of three domain types. The N-terminal 500 residues fold to form a large semaphorin (Sema) domain, which encompasses the whole α-subunit and part of the β-subunit. The plexin–semaphorin–integrin (PSI) domain follows the Sema domain, spans approximately 50 residues and includes four disulphide bonds. This domain is connected to the transmembrane helix via four immunoglobulin–plexin–transcription (IPT) domains, which are related to immunoglobulin-like domains. Intracellularly, the c-MET receptor contains a tyrosine kinase catalytic domain flanked by distinctive juxtamembrane and carboxy-terminal sequences. This portion of c-MET contains the catalytic tyrosines Y1234 and Y1235, which positively modulate enzyme activity, while the juxtamembrane tyrosine 1003 negatively regulates c-MET by recruiting the ubiquitin ligase casitas B-lineage lymphoma (c-CBL). The multifunctional docking site in the C-terminal tail contains tyrosines Y1349 and Y1356, which recruit several transducers and adaptors when c-MET is active. (b) The c-MET ligand, hepatocyte growth factor (HGF), is secreted by mesenchymal cells as a single-chain, biologically inert precursor and is converted into its bioactive form when extracellular proteases cleave the bond between Arg494 and Val495. The mature form of HGF consists of an α- and β-chain, which are held together by a disulphide bond. The α-chain contains an N-terminal hairpin loop followed by four kringle domains (80 amino acid double-looped structures formed by three internal disulphide bridges), K1–4. The β-chain is homologous to the serine proteases of the blood-clotting cascade, but lacks any proteolytic activity. Adapted from Comoglio et al. [2008].
The ligand for c-MET was identified by two independent studies as both a motility factor and a scatter factor for hepatocytes, and this factor was later found to be the same molecule: HGF, also known as scatter factor (SF) [Weidner et al. 1991; Nakamura et al. 1989; Stoker et al. 1987]. HGF acts as a pleiotropic factor and cytokine, promoting cell proliferation, survival, motility, scattering, differentiation and morphogenesis [Basilico et al. 2008; Birchmeier et al. 2003; Trusolino and Comoglio, 2002]. In addition, HGF appears to play a protective role in several diseases, including liver cirrhosis [Ueki et al. 1999], lung fibrosis [Watanabe et al. 2005] and progressive nephropathies [Liu and Yang, 2006; Okada and Kalluri, 2005]. HGF is secreted by mesenchymal cells as a single-chain, biologically inert precursor and is converted into its bioactive form when extracellular proteases cleave the bond between Arg494 and Val495. The mature form of HGF consists of an α- and β-chain, which are held together by a disulphide bond. The α-chain contains an N-terminal hairpin loop followed by four kringle domains (80 amino acid double-looped structures formed by three internal disulphide bridges). The β-chain is homologous to serine proteases of the blood-clotting cascade, but lacks proteolytic activity (Figure 1b).
Physiologically, c-MET is responsible for the cell-scattering phenotype, as first demonstrated with MDCK cells treated with HGF [Zhu et al. 1994]. This process involves the disruption of cadherin-based cell–cell contacts and subsequent cell motility, and is a key epithelial function in embryogenesis and wound repair [Corso et al. 2005]. During embryogenesis, this motility function of c-MET is crucial for the long-range migration of skeletal muscle progenitor cells. Ablation of the MET or Hgf gene in mice results in the complete absence of all muscle groups derived from these cells [Bladt et al. 1995]. During development, c-MET and HGF provide essential signals for survival and proliferation of hepatocytes and placental trophoblast cells; consequently, MET or Hgf knockout embryos show markedly reduced liver size. As well, altered placental development in Hgf and MET knockout mice is responsible for the death of these animals in utero [Schmidt et al. 1995; Uehara et al. 1995].
HGF/c-MET signaling
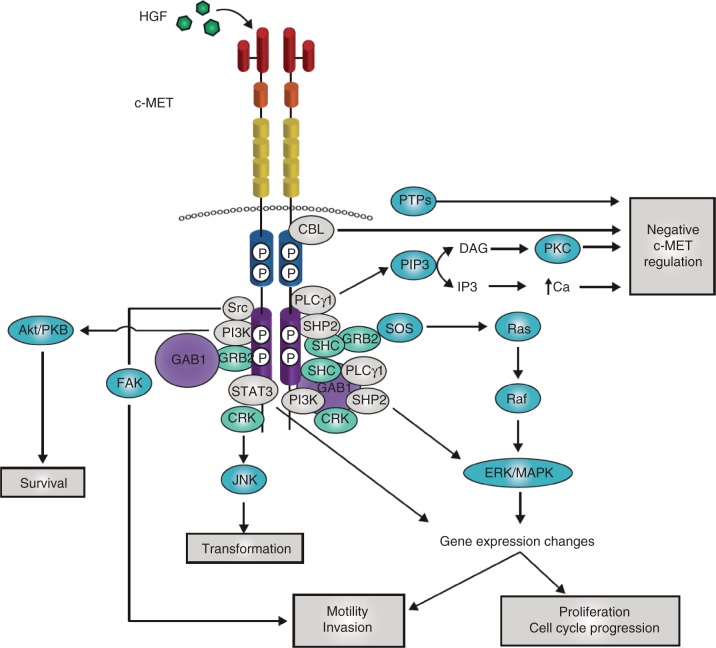
The complex phenotype that results from c-MET signaling involves a number of molecular events, which have been described in detail in previous reviews [Trusolino et al. 2010; Liu et al. 2008; Peruzzi and Bottaro, 2006; Birchmeier et al. 2003; Maulik et al. 2002b]. HGF binding to c-MET results in receptor homodimerization and phosphorylation of two tyrosine residues (Y1234 and Y1235) located within the catalytic loop of the tyrosine kinase domain [Rodrigues and Park, 1994]. Subsequently, tyrosines 1349 and 1356 in the carboxy-terminal tail become phosphorylated. These two tyrosines form a tandem SH2 recognition motif unique to c-MET (Y1349VHVX3Y1356VNV) [Ponzetto et al. 1994]. When these tyrosines become phosphorylated, they recruit signaling effectors that include the adaptor proteins Growth factor receptor-bound protein 2 (GRB2) [Fixman et al. 1996], Src homology-2-containing (SHC) [Pelicci et al. 1995] and v-crk sarcoma virus CT10 oncogene homolog (CRK) and CRK-like (CRKL) [Sakkab et al. 2000; Garcia-Guzman et al. 1999], the effector molecules phosphatidylinositol 3-kinase (PI3K), phospholipase Cγ (PLCγ) and v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (SRC) [Ponzetto et al. 1994], Src homology domain-containing 5' inositol phosphatase (SHIP-2) [Koch et al. 2005] and the transcription factor signal transducer and activator of transcription (STAT-3) [Zhang et al. 2002; Boccaccio et al. 1998] (Figure 2). In addition, unique to c-MET is its association with the adaptor protein GRB2-associated binding protein 1 (GAB1) [Weidner et al. 1996], a multi-adaptor protein that, once bound to and phosphorylated by c-MET, creates binding sites for more downstream adaptors. GAB1 can bind either directly to c-MET or indirectly, through GRB2. Additional tyrosines can also contribute to c-MET signaling. When Y1313 is phosphorylated, it binds and activates PI3K, which probably promotes cell viability and motility [Maulik et al. 2002a]. In addition, Y1365 regulates cell morphogenesis when phosphorylated [Maulik et al. 2002a].
Figure 2.
c-MET signaling adaptors and mediators. When the tyrosines within the multifunctional docking site become phosphorylated they recruit signaling effectors, including the adaptor proteins growth factor receptor-bound protein 2 (GRB2), src homology 2 domain-containing (SHC), v-crk sarcoma virus CT10 oncogene homolog (CRK) and CRK-like (CRKL); the effector molecules phosphatidylinositol 3-kinase (PI3K), phospholipase Cγ (PLCγ) and SRC, the src homology 2 domain-containing 5' inositol phosphatase SHIP-2, and the signal transducer and activator of transcription STAT3. In addition, unique to c-MET is its association with the adaptor protein GRB2-associated binding protein 1 (GAB1), a multi-adaptor protein that, once bound to and phosphorylated by c-MET, creates binding sites for more downstream adaptors. GAB1 can bind either directly to c-MET or indirectly, through GRB2. The downstream response to c-MET activation relies on stereotypical signaling modulators common to many receptor tyrosine kinases. For activation of the mitogen-activated protein kinase (MAPK) cascades, c-MET activation stimulates the activity of the rat sarcoma viral oncogene homolog (RAS) guanine nucleotide exchanger son of sevenless (SOS) via binding with SHC and GRB2 leading to the activation of RAS. This leads to the indirect activation of v-raf murine sarcoma viral oncogene homolog B1 (RAF) kinases, which can subsequently activate MAPK effector kinase (MEK), and finally MAPK, which can then translocate to the nucleus to activate the transcription factors responsible for regulating a large number of genes, including those involved in cell proliferation, cell motility and cell cycle progression. SHP2 can also link c-MET signaling to the MAPK cascade, as sequestration of SHP2 to GAB1 is responsible for extending the duration of MAPK phosphorylation. The p85 subunit of PI3K can bind either directly to c-MET or indirectly through GAB1, which then signals through AKT/protein kinase B. This axis is primarily responsible for the cell survival response to c-MET signaling. Transformation downstream of the c-MET receptor is mediated by the phosphorylation of Janus kinase 1 (JNK), which occurs via binding to CRK. STAT3 has also been implicated in transformation. The direct binding of STAT3 to c-MET results in STAT3 phosphorylation, dimerization and its translocation to the nucleus. This has been shown to result in tubulogenesis and invasion. However, other reports have found that, although STAT3 is required for c-MET-mediated tumorigenesis, it has no effect on proliferation, invasion or branching morphogenesis. Cellular migration is also mediated downstream of c-MET by focal adhesion kinase (FAK), which is localized to cellular adhesion complexes. FAK is activated through phosphorylation by SRC family kinases, which have been shown to directly associate with c-MET. The c-MET–SRC–FAK interaction leads to cell migration and the promotion of anchorage-independent growth. Negative regulation of the c-MET receptor is crucial for its tightly controlled activity. The Y1003 site, located in the juxtamembrane domain, is a negative regulatory site for c-MET signaling that acts by recruiting c-CBL. Regulation of c-MET signaling is also accomplished via its binding to various protein tyrosine phosphatases (PTPs). These PTPs modulate c-MET signaling by dephosphorylation of either the tyrosines in the c-MET kinase or the docking site. Finally, binding of PLCγ to c-MET results in the activation of protein kinase C (PKC), which can then negatively regulate c-MET receptor phosphorylation and activity. Independently of PKC activation, an increase in intracellular calcium levels can also lead to negative c-MET regulation. Adapted from Trusolino et al. [2010] and Birchmeier et al. [2003]. DAG, diacylglycerol; HGF, hepatocyte growth factor; IP3, inositol triphosphate; PIP3, phosphatidylinositol (3,4,5)-triphosphate.
The downstream response to c-MET activation relies on stereotypical signaling modulators common to many RTKs. These pathways have been reviewed in detail [Trusolino et al. 2010], and are summarized in Figure 2. For activation of the Mitogen activated protein kinase (MAPK) cascades, c-MET activation stimulates the activity of the rat sarcoma viral oncogene homolog (RAS) guanine nucleotide exchanger Son of Sevenless (SOS) via binding with SHC and GRB2 [Graziani et al. 1993], leading to the activation of RAS. This leads to the indirect activation of v-raf murine sarcoma viral oncogene homolog B1 (RAF) kinases, which can subsequently activate the MAPK effector kinase MEK and finally MAPK, which can then translocate to the nucleus to activate transcription factors responsible for regulating a large number of genes. In the context of c-MET signaling, this results in phenotypes such as cell proliferation, cell motility and cell cycle progression [Paumelle et al. 2002; Fixman et al. 1996]. Src homology 2 domain-containing phosphatase-2 (SHP2) can also link c-MET signaling to the MAPK cascade, as sequestration of SHP2 to GAB1 is responsible for extending the duration of MAPK phosphorylation [Maroun et al. 2003; Schaeer et al. 2000].
The other major arm of c-MET signaling is the PI3K/Akt signaling axis. The p85 subunit of PI3K can bind either directly to c-MET or indirectly through GAB1, which then signals through AKT/protein kinase B. This axis is primarily responsible for the cell survival response to c-MET signaling [Xiao et al. 2001]. Transformation downstream of the c-MET receptor is mediated by the phosphorylation of Janus kinase 1 (JNK), which occurs via binding to CRK [Garcia-Guzman et al. 1999; Rodrigues et al. 1997]. STAT3 has also been implicated in transformation, although its proposed mechanism is controversial. The direct binding of STAT3 to c-MET results in STAT3 phosphorylation, dimerization and its translocation to the nucleus. This has been shown to result in tubulogenesis [Boccaccio et al. 1998] and invasion [Syed et al. 2011]. However, other reports found that, although it is required for c-MET-mediated tumorigenesis, it has no effect on proliferation, invasion or branching morphogenesis [Zhang et al. 2002]. Therefore, the role of STAT3 in c-MET signaling is probably context- and tissue-dependent.
Cellular migration is also mediated downstream of c-MET by focal adhesion kinase (FAK), which is localized to cellular adhesion complexes. FAK is activated through phosphorylation by SRC family kinases, which have been shown to associate directly with c-MET [Ponzetto et al. 1994]. The c-MET–SRC–FAK interaction leads to cell migration and the promotion of anchorage-independent growth [Hui et al. 2009; Rahimi et al. 1998]. In addition, SRC activation can positively feed back on c-MET activation [Organ et al. 2011; Hui et al. 2009]. Because of this, combinatorial therapies involving both c-MET and SRC inhibitors show promise in the treatment of cancers dependent on either kinase [Sen et al. 2011; Bertotti et al. 2010; Okamoto et al. 2010].
Negative regulation of the c-MET receptor is crucial for its tightly controlled activity, and can occur through a number of mechanisms. The Y1003 site, located in the juxtamembrane domain, is a negative regulatory site for c-MET signaling that acts by recruiting c-CBL (casitas B-lineage lymphoma) [Petrelli et al. 2002; Peschard et al. 2001]. Regulation of c-MET signaling is also accomplished via its binding to various protein tyrosine phosphatases (PTPs), including the receptor-type PTPs density enhanced phosphatase 1 (dEP1) (or PTPrI) and leukocyte common antigen-related molecule (LAR) (or PTPrF) [Machide et al. 2006; Palka et al. 2003], and the nonreceptor PTPs PTP1B and T-cell protein tyrosine phosphatase (TCPTP) [Sangwan et al. 2008]. These PTPs modulate c-MET signaling by dephosphorylation of either the tyrosines in the c-MET kinase domain (in the case of PTP1b and TCPTP) or the docking tyrosines (in the case of dEP1). Finally, binding of PLCγ to c-MET results in the activation of protein kinase C (PKC), which can then negatively regulate c-MET receptor phosphorylation and activity [Gandino et al. 1994; Gandino et al. 1990]. Independently of PKC activation, an increase in intracellular calcium levels can also lead to negative c-MET regulation [Gandino et al. 1991].
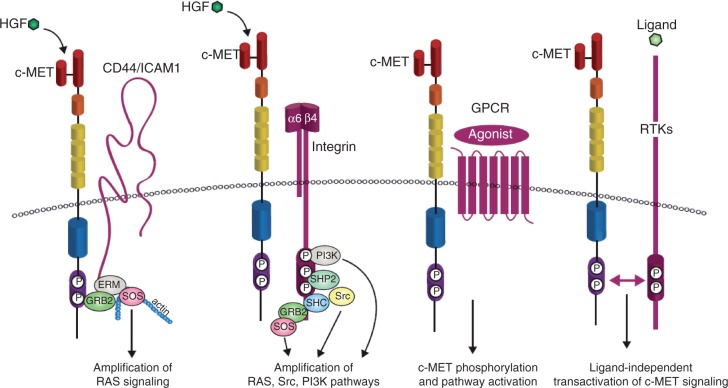
Although the downstream response to c-MET is common to many RTKs, the potency, endurance and specificity of c-MET-triggered pathways is secured by a network of upstream signaling co-receptors that physically associate with c-MET at the cell surface (Figure 3) [Trusolino et al. 2010]. c-MET membrane partners can then amplify and/or diversify c-MET-dependent biochemical inputs and translate them into meaningful (and specific) biological outcomes. For instance, the v6 splice variant of the hyaluronan receptor CD44 links c-MET signaling to the actin cytoskeleton via GRB2 and the ezrin, radixin and moesin (ERM) family of proteins in order to recruit SOS, which then amplifies RAS-ERK signaling [Orian-Rousseau et al. 2007]. Recent work has also shown that intercellular adhesion molecule 1 (ICAM-1) can substitute for CD44v6 as a co-receptor for c-MET in CD44v6 knockout mice, resulting in similar c-MET pathway activation [Olaku et al. 2011]. As another example, c-MET binding to integrin α6β4 creates a supplementary docking platform for binding of signaling adaptors, leading to specific enhancement of PI3K, RAS and SRC activation [Trusolino et al. 2001; Bertotti et al. 2005]. In addition, the G-protein-coupled receptor (GPCR) agonists lysophosphatidic acid (LPA), bradykinin, thrombin and carbachol can induce c-MET phosphorylation [Fischer et al. 2004], although the functional consequences of these interactions are still unclear.
Figure 3.
c-MET transactivation. The potency and endurance of c-MET-triggered pathways is secured by a network of upstream signaling co-receptors that physically associate with c-MET at the cell surface. c-MET membrane partners can then amplify and/or diversify c-MET-dependent biochemical inputs and translate them into meaningful (and specific) biological outcomes. The v6 splice variant of the hyaluronan receptor CD44 links c-MET signaling to the actin cytoskeleton via the growth factor receptor-bound protein 2 (GRB2) and the ezrin, radixin, moesin (ERM) family of proteins in order to recruit son of sevenless (SOS), which then amplifies RAS-ERK signaling. Intercellular adhesion molecule 1 (ICAM-1) can substitute for CD44v6 as a co-receptor for c-MET in CD44v6 knockout mice, resulting in similar c-MET pathway activation. c-MET binding to integrin α6β4 creates a supplementary docking platform for the binding of signaling adaptors, leading to specific enhancement of phosphatidylinositol 3-kinase (PI3K), RAS and SRC activation. c-MET can also be activated by G-protein coupled receptors (GPCRs), although the functional outcome of this interaction is not well characterized. Crosstalk between c-MET and other receptor tyrosine kinases (RTKs) has also been studied in great depth because of its potential importance in the development of resistance to cancer therapeutics. Examples of these RTKs include the semaphorin receptors, the epidermal growth factor receptor (EGFR) family of receptors, the recepteur d'origine nantais (RON), platelet-derived growth factor receptor (PDGFR) and Axl; the list continues to grow. Adapted from Trusolino et al. [2010] and Corso et al. [2005] HGF, hepatocyte growth factor; SHC, src homolgy 2 domain-containing; SHP2, src homology 2 domain-containing phosphatase 2.
Crosstalk between c-MET and other RTKs has also been studied in great depth because of its potential importance in the development of resistance to cancer therapeutics [Lai et al. 2009]. For instance, several members of the family of semaphorin receptors, including the plexins and neuropilins, can transactivate c-MET in the absence of HGF when stimulated by their semaphorin ligands [Sierra et al. 2008; Hu et al. 2007; Conrotto et al. 2004]. c-MET has also been shown by multiple studies to interact directly with the epidermal growth factor receptor (EGFR), allowing activation of c-MET after stimulation of cells with the EGFR ligands EGF or transforming growth factor (TGF-α) [Jo et al. 2000]. Stimulation of cells expressing both c-MET and EGFR with EGF resulted in phosphorylation of c-MET, and stimulation with ligands for both receptors resulted in synergistic activation of downstream modulators, indicating mutual activation of these two pathways [Puri and Salgia, 2008]. Evidence also exists for c-MET interaction with the other EGFR family members ERBB2 and ERBB3 (for erythroblastic leukemia viral oncogene homologs B2 and B3), causing transactivation of both receptors [Bachleitner-Hofmann et al. 2008; Khoury et al. 2005]. Interaction of c-MET with the closely related RON (recepteur d'origine nantais) receptor has also been shown to cause transphosphorylation of the c-MET receptor in the absence of HGF [Follenzi et al. 2000]. Interestingly, it was recently shown that transactivation of RON by c-MET may be a feature of cancer cells that are ‘addicted’ to c-MET signaling [Benvenuti et al. 2011]. Recently, transactivation between c-Met and both platelet-derived growth factor receptor (PDGFR) and Axl was found to play a role in bladder cancer [Yeh et al. 2011]. The list of cell surface receptors that play a role in c-MET signaling is growing constantly, and highlights the importance of personally targeted cancer therapies, depending on the expression of these RTKs in specific patients.
The c-MET receptor relies on its multitude of signaling adaptors and cell surface co-receptors to mediate biological responses unique to the receptor. Recent large-scale phosphoproteomic studies have provided even more insight into the intricacies of the HGF/c-MET signaling axis [Organ et al. 2011; Hammond et al. 2010; Guo et al. 2008]. Although these studies identified the highly conserved, core elements in c-MET signaling, they also identified tissue-specific differences, in addition to activation- compared with inhibition-specific differences, in downstream mediators of c-MET. Although much work has been done since the discovery of the c-MET oncogene to map out the details of c-MET signaling, this suggests that our understanding of the greater c-MET network remains incomplete.
HGF/c-MET signaling in cancer
As described above, c-MET signaling is an intricate and highly regulated process. Mechanisms operating during tumor growth or cancer progression have been identified that can result in constitutive or prolonged activation of c-MET. Data collected from in vitro and in vivo tumor models suggest that these typically take place by means of three mechanisms: the occurrence of specific genetic lesions, including translocations, gene amplifications and activating mutations; by transcriptional upregulation of the c-MET protein in the absence of gene amplification; or via ligand-dependent autocrine or paracrine mechanisms [Danilkovitch-Miagkova and Zbar, 2002].
c-MET was originally identified as an oncogene in the 1980s [Cooper et al. 1984], isolated first from a human osteosarcoma cell line treated with the carcinogen N-methyl-N-nitro-N-nitrosoguanidine. The c-MET identified in this cell line contained a chromosomal rearrangement that fused the tyrosine kinase domain of the c-MET proto-oncogene to an upstream translocating promoter region (TPR). This rearrangement caused constitutive dimerization and therefore activation of the encoded protein [Park et al. 1986]. Expression of TPR-MET in transgenic mice resulted in the development of multiple epithelial-derived tumors [Liang et al. 1996]. In humans, the TPR-MET translocation has been found in both the precursor lesions of gastric cancers and in the adjacent normal mucosa, suggesting that this genetic lesion can predispose to the development of gastric carcinomas [Soman et al. 1991].
Amplification of the c-MET gene, with consequent protein overexpression and constitutive kinase activation, has been reported in a number of human primary tumors. These include gastric and oesophageal carcinomas [Miller et al. 2006; Hara et al. 1998; Kuniyasu et al. 1992; Houldsworth et al. 1990], medulloblastomas [Tong et al. 2004], and liver metastases from colon carcinoma [Di Renzo et al. 1995c]. This last finding suggests that MET gene amplification can be acquired during the course of tumor progression. Interestingly, recent research has shown that non-small cell lung carcinomas with acquired resistance to EGFR inhibitors tend to show amplifications in MET [Bean et al. 2007; Engelman et al. 2007]. This suggests that combined treatment with EGFR and c-MET inhibitors could be necessary in a subset of patients to circumvent the onset of resistance to these drugs.
The most convincing evidence that implicates c-MET in human cancers is provided by the activating mutations that were discovered in the c-MET kinase domain in both sporadic and inherited forms of human renal papillary carcinomas [Olivero et al. 1999; Schmidt et al. 1999]. Activating kinase domain mutations have subsequently been identified in a small number of other cancers. Mutations have also been identified in the c-CBL binding site of the juxtamembrane domain and in the HGF-binding region of the Sema domain [Forbes et al. 2008]. In hereditary cancers, heterozygous mutations are usually accompanied by trisomy of the whole chromosome 7, suggesting that when only a single allele is mutated the mutation must be present in multiple copies to produce the full transformed phenotype [Schmidt et al. 1997].
Increased protein expression as a consequence of transcriptional upregulation in the absence of gene amplification is the most frequent cause of constitutive c-MET activation in human tumors [Comoglio et al. 2008], and has been reported in an ever growing number of carcinomas, including thyroid [Di Renzo et al. 1992; Di Renzo et al. 1995b], colorectal [Hiscox et al. 1997; Di Renzo et al. 1995a; Liu et al. 1992], ovarian [Di Renzo et al. 1994], pancreatic [Di Renzo et al. 1995b; Furukawa et al. 1995], lung [Nakamura et al. 2008; Tsao et al. 1998] and breast [Lengyel et al. 2005], to name a few. Hypoxia, caused by lack of oxygen diffusion to the centre of a growing tumor, is one mechanism that has been demonstrated to activate c-MET transcription in vitro and in vivo [Pennacchietti et al. 2003]. Hypoxia activates the c-MET promoter, via the transcription factor hypoxia inducible factor 1α (HIF1α), which itself is regulated by the concentration of intracellular oxygen [Kitajima et al. 2008].
Although c-MET activation via a ligand-dependent autocrine or paracrine loop will be fully discussed elsewhere in this supplement, we will touch on it briefly here. HGF is expressed ubiquitously within the body and has been found to be frequently overexpressed in the reactive stroma of primary tumors [Matsumoto and Nakamura, 2006]. This supports the formation of paracrine positive feedback loops, which in turn can support the dissemination of cancer cells to distant locations. The autocrine stimulation of c-MET has also been identified in cancer cells [Rahimi et al. 1996; Rong and Vande Woude, 1994], and appears to be indicative of increased aggressiveness of tumors along with poor prognostic signs in cancer patients [Navab et al. 2009; Vadnais et al. 2002; Tuck et al. 1996].
c-MET as a target for therapeutic inhibition
Although the development of c-MET inhibitors will be discussed elsewhere in this supplement, here we consider the dual role c-MET plays in both the development and progression of cancers, and how each could be targeted by c-MET inhibitors.
Some tumors appear to be dependent on (or ‘addicted’ to) sustained c-MET activity for their growth and survival, and this is often associated with MET gene amplification. This phenomenon is known as ‘oncogene addiction’ and applies to all settings where cancer cells appear to be dependent on a single overactive oncogene for their proliferation and survival [Sharma et al. 2007; Sharma and Settleman, 2007]. Oncogene addiction was identified after studies using EGFR tyrosine kinase inhibitors demonstrated that these inhibitors were efficacious only in a small subset of tumors which exhibited genetic alterations of the receptor itself [Sharma et al. 2007]. Although this c-MET-addicted phenotype has only recently been (yet consistently) described in cultured cells from gastric and non-small cell lung carcinomas, it continues to strongly suggest that amplification of the MET gene might be a genetic predictor of therapeutic responsiveness [Lutterbach et al. 2007; Smolen et al. 2006].
‘Oncogene expedience’ is a tumor-specific term that describes the scattering, invasion and survival of cancer cells associated with metastatic spreading [Comoglio et al. 2008]. In contrast to oncogene addiction, the inappropriate activation of c-MET resulting in oncogene expedience is the consequence rather than the cause of the transformed phenotype. Thus, activation of c-MET is a secondary event in various types of tumor, exacerbating the malignant properties of already transformed cells. In these cases, aberrant c-MET activation occurs through a number of possible routes; these include transcriptional upregulation by other oncogenes [Abounader et al. 2004; Ivan et al. 1997], environmental conditions such as hypoxia [Pennacchietti et al. 2003] and agents secreted by reactive stroma such as inflammatory cytokines, proangiogenic factors and HGF itself [Bhowmick et al. 2004; Boccaccio et al. 1994].
As MET is a necessary oncogene for a number of neoplasms, targeted therapies against c-MET could be effective as a front-line intervention to treat a limited subset of c-MET-addicted tumors and subsequent c-MET-addicted metastases [Comoglio et al. 2008]. In addition, as MET also acts as an adjuvant prometastatic gene for many neoplasms, targeted therapies against c-MET could also be used as a secondary approach to hamper the progression of a much wider spectrum of advanced cancers that rely on c-MET activation for metastatic spreading.
Summary and conclusions
The HGF/c-MET pathway comprises a complex and unique signaling network and plays a pivotal role in both normal development and cancer progression. c-MET controls multiple biological functions, including proliferation, survival, motility and invasion, which, when dysregulated by aberrant c-MET activation, can lead to both tumor growth and metastatic progression of cancer cells. Consequently, c-MET is a versatile candidate for targeted therapeutic intervention.
Acknowledgement
Matthew Joynson, a medical writer, assisted with the styling of this manuscript. The authors wrote and revised the main draft of the article.
Funding
This study was supported by the Canadian Institutes of Health Research (grant number MOP-64345) and in part by the Ontario Ministry of Health and Long Term Care. SLO is supported by the CIHR Banting and Best Doctoral Research Award. MST is the M. Qasim Choksi Chair in Lung Cancer Translational Research. Editorial assistance was funded by Daiichi Sankyo Europe GmbH.
Conflict of interest statement
Shawna L. Organ declares no conflict of interest. Dr Ming-Sound Tsao has received honoraria from Daiichi Sankyo Europe GmbH for speaking at scientific symposia.
References
- Abounader R., Reznik T., Colantuoni C., Martinez-Murillo F., Rosen E.M., Laterra J. (2004) Regulation of c-Met-dependent gene expression by PTEN. Oncogene 23: 9173–9182 [DOI] [PubMed] [Google Scholar]
- Bachleitner-Hofmann T., Sun M.Y., Chen C.T., Tang L., Song L., Zeng Z., et al. (2008) HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol Cancer Ther 7: 3499–3508 [DOI] [PubMed] [Google Scholar]
- Basilico C., Arnesano A., Galluzzo M., Comoglio P.M., Michieli P. (2008) A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met. J Biol Chem 283: 21267–21277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean J., Brennan C., Shih J.Y., Riely G., Viale A., Wang L., et al. (2007) MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA 104: 20932–20937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuti S., Lazzari L., Arnesano A., Li Chiavi G., Gentile A., Comoglio P.M. (2011) Ron kinase transphosphorylation sustains MET oncogene addiction. Cancer Res 71: 1945–1955 [DOI] [PubMed] [Google Scholar]
- Bertotti A., Bracco C., Girolami F., Torti D., Gastaldi S., Galimi F., et al. (2010) Inhibition of Src impairs the growth of met-addicted gastric tumors. Clin Cancer Res 16: 3933–3943 [DOI] [PubMed] [Google Scholar]
- Bertotti A., Comoglio P.M., Trusolino L. (2005) Beta4 integrin is a transforming molecule that unleashes Met tyrosine kinase tumorigenesis. Cancer Res 65: 10674–10679 [DOI] [PubMed] [Google Scholar]
- Bhowmick N.A., Neilson E.G., Moses H.L. (2004) Stromal fibroblasts in cancer initiation and progression. Nature 432: 332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier C., Birchmeier W., Gherardi E., Vande Woude G.F. (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4: 915–925 [DOI] [PubMed] [Google Scholar]
- Bladt F., Riethmacher D., Isenmann S., Aguzzi A., Birchmeier C. (1995) Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376: 768–771 [DOI] [PubMed] [Google Scholar]
- Boccaccio C., Ando M., Tamagnone L., Bardelli A., Michieli P., Battistini C., et al. (1998) Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature 391: 285–288 [DOI] [PubMed] [Google Scholar]
- Boccaccio C., Gaudino G., Gambarotta G., Galimi F., Comoglio P.M. (1994) Hepatocyte growth factor (HGF) receptor expression is inducible and is part of the delayed-early response to HGF. J Biol Chem 269: 12846–12851 [PubMed] [Google Scholar]
- Boon E.M., van der Neut R., van de Wetering M., Clevers H., Pals S.T. (2002) Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res 62: 5126–5128 [PubMed] [Google Scholar]
- Comoglio P.M., Giordano S., Trusolino L. (2008) Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 7: 504–516 [DOI] [PubMed] [Google Scholar]
- Comoglio P.M., Trusolino L. (2002) Invasive growth: from development to metastasis. J Clin Invest 109: 857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrotto P., Corso S., Gamberini S., Comoglio P.M., Giordano S. (2004) Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene 23: 5131–5137 [DOI] [PubMed] [Google Scholar]
- Cooper C.S., Park M., Blair D.G., Tainsky M.A., Huebner K., Croce C.M., et al. (1984) Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 311: 29–33 [DOI] [PubMed] [Google Scholar]
- Corso S., Comoglio P.M., Giordano S. (2005) Cancer therapy: can the challenge be MET?. Trends Mol Med 11: 284–292 [DOI] [PubMed] [Google Scholar]
- Danilkovitch-Miagkova A., Zbar B. (2002) Dysregulation of Met receptor tyrosine kinase activity in invasive tumors. J Clin Invest 109: 863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Renzo M.F., Olivero M., Ferro S., Prat M., Bongarzone I., Pilotti S., et al. (1992) Overexpression of the c-MET/HGF receptor gene in human thyroid carcinomas. Oncogene 7: 2549–2553 [PubMed] [Google Scholar]
- Di Renzo M.F., Olivero M., Giacomini A., Porte H., Chastre E., Mirossay L., et al. (1995a) Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clinical Cancer Res 1: 147–154 [PubMed] [Google Scholar]
- Di Renzo M.F., Olivero M., Katsaros D., Crepaldi T., Gaglia P., Zola P., et al. (1994) Overexpression of the Met/HGF receptor in ovarian cancer. Int J Cancer 58: 658–662 [DOI] [PubMed] [Google Scholar]
- Di Renzo M.F., Olivero M., Serini G., Orlandi F., Pilotti S., Belfiore A., et al. (1995b) Overexpression of the c-MET/HGF receptor in human thyroid carcinomas derived from the follicular epithelium. J Endocrinol Invest 18: 134–139 [DOI] [PubMed] [Google Scholar]
- Di Renzo M.F., Poulsom R., Olivero M., Comoglio P.M., Lemoine N.R. (1995c) Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res 55: 1129–1138 [PubMed] [Google Scholar]
- Engelman J.A., Zejnullahu K., Mitsudomi T., Song Y., Hyland C., Park J.O., et al. (2007) MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316: 1039–1043 [DOI] [PubMed] [Google Scholar]
- Epstein J.A., Shapiro D.N., Cheng J., Lam P.Y., Maas R.L. (1996) Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc Natl Acad Sci USA 93: 4213–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer O.M., Giordano S., Comoglio P.M., Ullrich A. (2004) Reactive oxygen species mediate Met receptor transactivation by G protein-coupled receptors and the epidermal growth factor receptor in human carcinoma cells. J Biol Chem 279: 28970–28978 [DOI] [PubMed] [Google Scholar]
- Fixman E.D., Fournier T.M., Kamikura D.M., Naujokas M.A., Park M. (1996) Pathways downstream of Shc and Grb2 are required for cell transformation by the tpr-Met oncoprotein. J Biol Chem 271: 13116–13122 [DOI] [PubMed] [Google Scholar]
- Follenzi A., Bakovic S., Gual P., Stella M.C., Longati P., Comoglio P.M. (2000) Cross-talk between the proto-oncogenes Met and Ron. Oncogene 19: 3041–3049 [DOI] [PubMed] [Google Scholar]
- Forbes S.A., Bhamra G., Bamford S., Dawson E., Kok C., Clements J., et al. (2008) The Catalogue of Somatic Mutations in Cancer (COSMIC). Curr Protoc Hum Genet Chapter 10, Unit 10.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Duguid W.P., Kobari M., Matsuno S., Tsao M.S. (1995) Hepatocyte growth factor and Met receptor expression in human pancreatic carcinogenesis. Am J Pathol 147: 889–895 [PMC free article] [PubMed] [Google Scholar]
- Gambarotta G., Boccaccio C., Giordano S., Ando M., Stella M.C., Comoglio P.M. (1996) Ets up-regulates MET transcription. Oncogene 13: 1911–1917 [PubMed] [Google Scholar]
- Gandino L., Di Renzo M.F., Giordano S., Bussolino F., Comoglio P.M. (1990) Protein kinase-c activation inhibits tyrosine phosphorylation of the c-met protein. Oncogene 5: 721–725 [PubMed] [Google Scholar]
- Gandino L., Longati P., Medico E., Prat M., Comoglio P.M. (1994) Phosphorylation of serine 985 negatively regulates the hepatocyte growth factor receptor kinase. J Biol Chem 269: 1815–1820 [PubMed] [Google Scholar]
- Gandino L., Munaron L., Naldini L., Ferracini R., Magni M., Comoglio P.M. (1991) Intracellular calcium regulates the tyrosine kinase receptor encoded by the MET oncogene. J Biol Chem 266: 16098–16104 [PubMed] [Google Scholar]
- Garcia-Guzman M., Dolfi F., Zeh K., Vuori K. (1999) Met-induced JNK activation is mediated by the adapter protein Crk and correlates with the Gab1 - Crk signaling complex formation. Oncogene 18: 7775–7786 [DOI] [PubMed] [Google Scholar]
- Graziani A., Gramaglia D., dalla Zonca P., Comoglio P.M. (1993) Hepatocyte growth factor/scatter factor stimulates the Ras-guanine nucleotide exchanger. J Biol Chem 268 9165–9168 [PubMed] [Google Scholar]
- Guo A., Villen J., Kornhauser J., Lee K.A., Stokes M.P., Rikova K., et al. (2008) Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci USA 105: 692–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D.E., Hyde R., Kratchmarova I., Beynon R.J., Blagoev B., Clague M.J. (2010) Quantitative analysis of HGF and EGF-dependent phosphotyrosine signaling networks. J Proteome Res 9: 2734–2742 [DOI] [PubMed] [Google Scholar]
- Hara T., Ooi A., Kobayashi M., Mai M., Yanagihara K., Nakanishi I. (1998) Amplification of c-myc, K-sam, and c-met in gastric cancers: detection by fluorescence in situ hybridization. Lab Invest 78: 1143–1153 [PubMed] [Google Scholar]
- Hiscox S.E., Hallett M.B., Puntis M.C., Nakamura T., Jiang W.G. (1997) Expression of the HGF/SF receptor, c-met, and its ligand in human colorectal cancers. Cancer Invest 15: 513–521 [DOI] [PubMed] [Google Scholar]
- Houldsworth J., Cordon-Cardo C., Ladanyi M., Kelsen D.P., Chaganti R.S. (1990) Gene amplification in gastric and esophageal adenocarcinomas. Cancer Res 50: 6417–6422 [PubMed] [Google Scholar]
- Hu B., Guo P., Bar-Joseph I., Imanishi Y., Jarzynka M.J., Bogler O., et al. (2007) Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene 26: 5577–5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui A.Y., Meens J.A., Schick C., Organ S.L., Qiao H., Tremblay E.A., et al. (2009) Src and FAK mediate cell-matrix adhesion-dependent activation of Met during transformation of breast epithelial cells. J Cell Biochem 107: 1168–1181 [DOI] [PubMed] [Google Scholar]
- Ivan M., Bond J.A., Prat M., Comoglio P.M., Wynford-Thomas D. (1997) Activated ras and ret oncogenes induce over-expression of c-met (hepatocyte growth factor receptor) in human thyroid epithelial cells. Oncogene 14: 2417–2423 [DOI] [PubMed] [Google Scholar]
- Jo M., Stolz D.B., Esplen J.E., Dorko K., Michalopoulos G.K., Strom S.C. (2000) Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J Biol Chem 275: 8806–8811 [DOI] [PubMed] [Google Scholar]
- Khoury H., Naujokas M.A., Zuo D., Sangwan V., Frigault M.M., Petkiewicz S., et al. (2005) HGF converts ErbB2/Neu epithelial morphogenesis to cell invasion. Mol Biol Cell 16: 550–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima Y., Ide T., Ohtsuka T., Miyazaki K. (2008) Induction of hepatocyte growth factor activator gene expression under hypoxia activates the hepatocyte growth factor/c-Met system via hypoxia inducible factor-1 in pancreatic cancer. Cancer Science 99: 1341–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A., Mancini A., El Bounkari O., Tamura T. (2005) The SH2-domain-containing inositol 5-phosphatase (SHIP)-2 binds to c-Met directly via tyrosine residue 1356 and involves hepatocyte growth factor (HGF)-induced lamellipodium formation, cell scattering and cell spreading. Oncogene 24: 3436–3447 [DOI] [PubMed] [Google Scholar]
- Krause D.S., Van Etten R.A. (2005) Tyrosine kinases as targets for cancer therapy. N Engl J Med 353: 172–187 [DOI] [PubMed] [Google Scholar]
- Kuniyasu H., Yasui W., Kitadai Y., Yokozaki H., Ito H., Tahara E. (1992) Frequent amplification of the c-met gene in scirrhous type stomach cancer. Biochem Biophys Res Commun 189: 227–232 [DOI] [PubMed] [Google Scholar]
- Lai A.Z., Abella J.V., Park M. (2009) Crosstalk in Met receptor oncogenesis. Trends Cell Biol 19: 542–551 [DOI] [PubMed] [Google Scholar]
- Lengyel E., Prechtel D., Resau J.H., Gauger K., Welk A., Lindemann K., et al. (2005) C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int J Cancer 113: 678–682 [DOI] [PubMed] [Google Scholar]
- Liang T.J., Reid A.E., Xavier R., Cardiff R.D., Wang T.C. (1996) Transgenic expression of tpr-met oncogene leads to development of mammary hyperplasia and tumors. J Clin Invest 97: 2872–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Park M., Tsao M.S. (1992) Overexpression of c-met proto-oncogene but not epidermal growth factor receptor or c-erbB-2 in primary human colorectal carcinomas. Oncogene 7: 181–185 [PubMed] [Google Scholar]
- Liu X., Yao W., Newton R.C., Scherle P.A. (2008) Targeting the c-MET signaling pathway for cancer therapy. Expert Opin Investig Drugs 17: 997–1011 [DOI] [PubMed] [Google Scholar]
- Liu Y., Yang J. (2006) Hepatocyte growth factor: new arsenal in the fights against renal fibrosis?. Kidney Int 70: 238–240 [DOI] [PubMed] [Google Scholar]
- Lutterbach B., Zeng Q., Davis L.J., Hatch H., Hang G., Kohl N.E., et al. (2007) Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res 67: 2081–2088 [DOI] [PubMed] [Google Scholar]
- Machide M., Hashigasako A., Matsumoto K., Nakamura T. (2006) Contact inhibition of hepatocyte growth regulated by functional association of the c-Met/hepatocyte growth factor receptor and LAR protein-tyrosine phosphatase. J Biol Chem 281: 8765–8772 [DOI] [PubMed] [Google Scholar]
- Maroun C.R., Naujokas M.A., Park M. (2003) Membrane targeting of Grb2-associated binder-1 (Gab1) scaffolding protein through Src myristoylation sequence substitutes for Gab1 pleckstrin homology domain and switches an epidermal growth factor response to an invasive morphogenic program. Mol Biol Cell 14: 1691–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Nakamura T. (2006) Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer 119: 477–483 [DOI] [PubMed] [Google Scholar]
- Maulik G., Madhiwala P., Brooks S., Ma P.C., Kijima T., Tibaldi E.V., et al. (2002a) Activated c-Met signals through PI3K with dramatic effects on cytoskeletal functions in small cell lung cancer. J Cell Mol Med 6: 539–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik G., Shrikhande A., Kijima T., Ma P.C., Morrison P.T., Salgia R. (2002b) Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev 13: 41–59 [DOI] [PubMed] [Google Scholar]
- Miller C.T., Lin L., Casper A.M., Lim J., Thomas D.G., Orringer M.B., et al. (2006) Genomic amplification of MET with boundaries within fragile site FRA7G and upregulation of MET pathways in esophageal adenocarcinoma. Oncogene 25: 409–418 [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., et al. (1989) Molecular cloning and expression of human hepatocyte growth factor. Nature 342: 440–443 [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Matsubara D., Goto A., Ota S., Sachiko O., Ishikawa S., et al. (2008) Constitutive activation of c-Met is correlated with c-Met overexpression and dependent on cell-matrix adhesion in lung adenocarcinoma cell lines. Cancer Sci 99: 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab R., Liu J., Seiden-Long I., Shih W., Li M., Bandarchi B., et al. (2009) Co-overexpression of Met and hepatocyte growth factor promotes systemic metastasis in NCI-H460 non-small cell lung carcinoma cells. Neoplasia 11: 1292–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H., Kalluri R. (2005) Cellular and molecular pathways that lead to progression and regression of renal fibrogenesis. Curr Mol Med 5: 467–474 [DOI] [PubMed] [Google Scholar]
- Okamoto W., Okamoto I., Yoshida T., Okamoto K., Takezawa K., Hatashita E., et al. (2010) Identification of c-Src as a potential therapeutic target for gastric cancer and of MET activation as a cause of resistance to c-Src inhibition. Mol Cancer Ther 9: 1188–1197 [DOI] [PubMed] [Google Scholar]
- Olaku V., Matzke A., Mitchell C., Hasenauer S., Sakkaravarthi A., Pace G., et al. (2011) c-Met recruits ICAM-1 as a co-receptor to compensate for the loss of CD44 in the Cd44 null mice. Mol Biol Cell 22: 2777–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivero M., Valente G., Bardelli A., Longati P., Ferrero N., Cracco C., et al. (1999) Novel mutation in the ATP-binding site of the MET oncogene tyrosine kinase in a HPRCC family. Int J Cancer 82: 640–643 [DOI] [PubMed] [Google Scholar]
- Organ S.L., Tong J., Taylor P., St-Germain J.R., Navab R., Moran M.F., et al. (2011) Quantitative phospho-proteomic profiling of hepatocyte growth factor (HGF)-MET signaling in colorectal cancer. J Proteome Res 10: 3200–3211 [DOI] [PubMed] [Google Scholar]
- Orian-Rousseau V., Morrison H., Matzke A., Kastilan T., Pace G., Herrlich P., et al. (2007) Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol Biol Cell 18: 76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palka H.L., Park M., Tonks N.K. (2003) Hepatocyte growth factor receptor tyrosine kinase met is a substrate of the receptor protein-tyrosine phosphatase DEP-1. J Biol Chem 278: 5728–5735 [DOI] [PubMed] [Google Scholar]
- Park M., Dean M., Cooper C.S., Schmidt M., O'Brien S.J., Blair D.G., et al. (1986) Mechanism of met oncogene activation. Cell 45: 895–904 [DOI] [PubMed] [Google Scholar]
- Paumelle R., Tulasne D., Kherrouche Z., Plaza S., Leroy C., Reveneau S., et al. (2002) Hepatocyte growth factor/scatter factor activates the ETS1 transcription factor by a RAS-RAF-MEK-ERK signaling pathway. Oncogene 21: 2309–2319 [DOI] [PubMed] [Google Scholar]
- Pelicci G., Giordano S., Zhen Z., Salcini A.E., Lanfrancone L., Bardelli A., et al. (1995) The motogenic and mitogenic responses to HGF are amplified by the Shc adaptor protein. Oncogene 10: 1631–1638 [PubMed] [Google Scholar]
- Pennacchietti S., Michieli P., Galluzzo M., Mazzone M., Giordano S., Comoglio P.M. (2003) Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3: 347–361 [DOI] [PubMed] [Google Scholar]
- Peruzzi B., Bottaro D.P. (2006) Targeting the c-Met signaling pathway in cancer. Clin Cancer Res 12: 3657–3660 [DOI] [PubMed] [Google Scholar]
- Peschard P., Fournier T.M., Lamorte L., Naujokas M.A., Band H., Langdon W.Y., et al. (2001) Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol Cell 8: 995–1004 [DOI] [PubMed] [Google Scholar]
- Petrelli A., Gilestro G.F., Lanzardo S., Comoglio P.M., Migone N., Giordano S. (2002) The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature 416: 187–190 [DOI] [PubMed] [Google Scholar]
- Ponzetto C., Bardelli A., Zhen Z., Maina F., dalla Zonca P., Giordano S., et al. (1994) A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 77: 261–271 [DOI] [PubMed] [Google Scholar]
- Puri N., Salgia R. (2008) Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J Carcinog 7: 9–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi N., Hung W., Tremblay E., Saulnier R., Elliott B. (1998) c-Src kinase activity is required for hepatocyte growth factor-induced motility and anchorage-independent growth of mammary carcinoma cells. J Biol Chem 273: 33714–33721 [DOI] [PubMed] [Google Scholar]
- Rahimi N., Tremblay E., McAdam L., Park M., Schwall R., Elliott B. (1996) Identification of a hepatocyte growth factor autocrine loop in a murine mammary carcinoma. Cell Growth Differ 7: 263–270 [PubMed] [Google Scholar]
- Rodrigues G.A., Park M. (1994) Autophosphorylation modulates the kinase activity and oncogenic potential of the Met receptor tyrosine kinase. Oncogene 9: 2019–2027 [PubMed] [Google Scholar]
- Rodrigues G.A., Park M., Schlessinger J. (1997) Activation of the JNK pathway is essential for transformation by the Met oncogene. EMBO J 16: 2634–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong S., Vande Woude G.F. (1994) Autocrine mechanism for met proto-oncogene tumorigenicity. Cold Spring Harbor Symp Quant Biol 59: 629–636 [DOI] [PubMed] [Google Scholar]
- Sakkab D., Lewitzky M., Posern G., Schaeper U., Sachs M., Birchmeier W., et al. (2000) Signaling of hepatocyte growth factor/scatter factor (HGF) to the small GTPase Rap1 via the large docking protein Gab1 and the adapter protein CRKL. J Biol Chem 275: 10772–10778 [DOI] [PubMed] [Google Scholar]
- Sangwan V., Paliouras G.N., Abella J.V., Dube N., Monast A., Tremblay M.L., et al. (2008) Regulation of the Met receptor-tyrosine kinase by the protein-tyrosine phosphatase 1B and T-cell phosphatase. J Biol Chem 283: 34374–34383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeper U., Gehring N.H., Fuchs K.P., Sachs M., Kempkes B., Birchmeier W. (2000) Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J Cell Biol 149: 1419–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C., Bladt F., Goedecke S., Brinkmann V., Zschiesche W., Sharpe M., et al. (1995) Scatter factor/hepatocyte growth factor is essential for liver development. Nature 373: 699–702 [DOI] [PubMed] [Google Scholar]
- Schmidt L., Duh F.M., Chen F., Kishida T., Glenn G., Choyke P., et al. (1997) Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet 16: 68–73 [DOI] [PubMed] [Google Scholar]
- Schmidt L., Junker K., Nakaigawa N., Kinjerski T., Weirich G., Miller M., et al. (1999) Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene 18: 2343–2350 [DOI] [PubMed] [Google Scholar]
- Sen B., Peng S., Saigal B., Williams M.D., Johnson F.M. (2011) Distinct interactions between c-Src and c-Met in mediating resistance to c-Src inhibition in head and neck cancer. Clin Cancer Res 17: 514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.V., Bell D.W., Settleman J., Haber D.A. (2007) Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 7: 169–181 [DOI] [PubMed] [Google Scholar]
- Sharma S.V., Settleman J. (2007) Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev 21: 3214–3231 [DOI] [PubMed] [Google Scholar]
- Sierra J.R., Corso S., Caione L., Cepero V., Conrotto P., Cignetti A., et al. (2008) Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med 205: 1673–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen G.A., Sordella R., Muir B., Mohapatra G., Barmettler A., Archibald H., et al. (2006) Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci USA 103: 2316–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soman N.R., Correa P., Ruiz B.A., Wogan G.N. (1991) The TPR-MET oncogenic rearrangement is present and expressed in human gastric carcinoma and precursor lesions. Proc Natl Acad Sci USA 88: 4892–4896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M., Gherardi E., Perryman M., Gray J. (1987) Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 327: 239–242 [DOI] [PubMed] [Google Scholar]
- Syed Z.A., Yin W., Hughes K., Gill J.N., Shi R., Clifford J.L. (2011) HGF/c-met/Stat3 signaling during skin tumor cell invasion: indications for a positive feedback loop. BMC Cancer 11: 180–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C.Y., Hui A.B., Yin X.L., Pang J.C., Zhu X.L., Poon W.S., et al. (2004) Detection of oncogene amplifications in medulloblastomas by comparative genomic hybridization and array-based comparative genomic hybridization. J Neurosurg 100(2 Suppl Pediatrics): 187–193 [DOI] [PubMed] [Google Scholar]
- Trusolino L., Bertotti A., Comoglio P.M. (2001) A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell 107: 643–654 [DOI] [PubMed] [Google Scholar]
- Trusolino L., Bertotti A., Comoglio P.M. (2010) MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 11: 834–848 [DOI] [PubMed] [Google Scholar]
- Trusolino L., Comoglio P.M. (2002) Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer 2: 289–300 [DOI] [PubMed] [Google Scholar]
- Tsao M.S., Liu N., Chen J.R., Pappas J., Ho J., To C., et al. (1998) Differential expression of Met/hepatocyte growth factor receptor in subtypes of non-small cell lung cancers. Lung Cancer 20: 1–16 [DOI] [PubMed] [Google Scholar]
- Tuck A.B., Park M., Sterns E.E., Boag A., Elliott B.E. (1996) Coexpression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am J Pathol 148: 225–232 [PMC free article] [PubMed] [Google Scholar]
- Uehara Y., Minowa O., Mori C., Shiota K., Kuno J., Noda T., et al. (1995) Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 373: 702–705 [DOI] [PubMed] [Google Scholar]
- Ueki T., Kaneda Y., Tsutsui H., Nakanishi K., Sawa Y., Morishita R., et al. (1999) Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med 5: 226–230 [DOI] [PubMed] [Google Scholar]
- Vadnais J., Nault G., Daher Z., Amraei M., Dodier Y., Nabi I.R., et al. (2002) Autocrine activation of the hepatocyte growth factor receptor/met tyrosine kinase induces tumor cell motility by regulating pseudopodial protrusion. J Biol Chem 277: 48342–48350 [DOI] [PubMed] [Google Scholar]
- Watanabe M., Ebina M., Orson F.M., Nakamura A., Kubota K., Koinuma D., et al. (2005) Hepatocyte growth factor gene transfer to alveolar septa for effective suppression of lung fibrosis. Mol Ther 12: 58–67 [DOI] [PubMed] [Google Scholar]
- Weidner K.M., Arakaki N., Hartmann G., Vandekerckhove J., Weingart S., Rieder H., et al. (1991) Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci USA 88: 7001–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner K.M., Di Cesare S., Sachs M., Brinkmann V., Behrens J., Birchmeier W. (1996) Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 384: 173–176 [DOI] [PubMed] [Google Scholar]
- Xiao G.H., Jeffers M., Bellacosa A., Mitsuuchi Y., Vande Woude G.F., Testa J.R. (2001) Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci USA 98: 247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C.Y., Shin S.M., Yeh H.H., Wu T.J., Shin J.W., Chang T.Y., et al. (2011) Transcriptional activation of the Axl and PDGFR-alpha by c-Met through a ras- and Src-independent mechanism in human bladder cancer. BMC Cancer 11: 139–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.W., Wang L.M., Jove R., Vande Woude G.F. (2002) Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene 21: 217–226 [DOI] [PubMed] [Google Scholar]
- Zhu H., Naujokas M.A., Park M. (1994) Receptor chimeras indicate that the met tyrosine kinase mediates the motility and morphogenic responses of hepatocyte growth/scatter factor. Cell Growth Differ 5: 359–366 [PubMed] [Google Scholar]