Abstract
Fasted dogs prepared with catheters in the femoral artery, portal vein, and hepatic vein and infused intravenously with palmitate-1-14C were used to estimate uptake of free fatty acids in liver and their conversion to major metabolic products secreted into hepatic venous blood. Animals were studied under ordinary conditions and when fat mobilization was increased abruptly by infusing norepinephrine or for a prolonged period by withdrawing insulin from depancreatized dogs. 80% of hepatic blood flow was assumed to be derived from the portal vein.
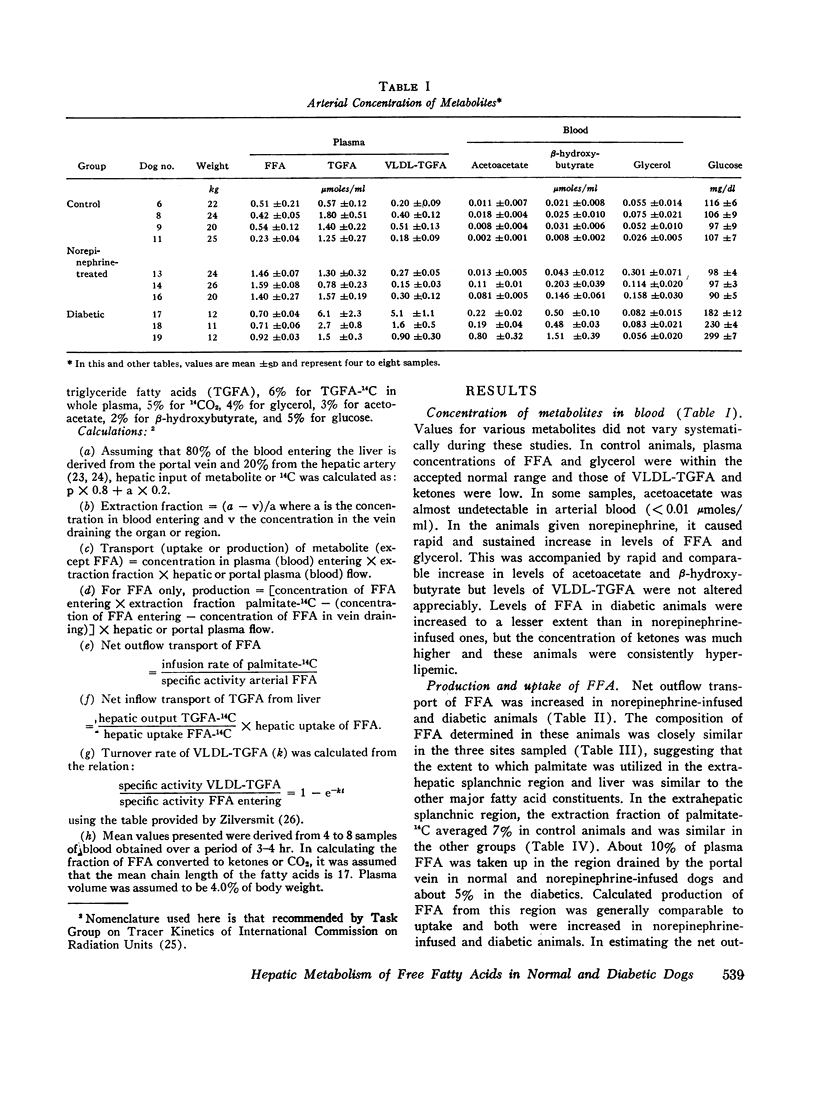
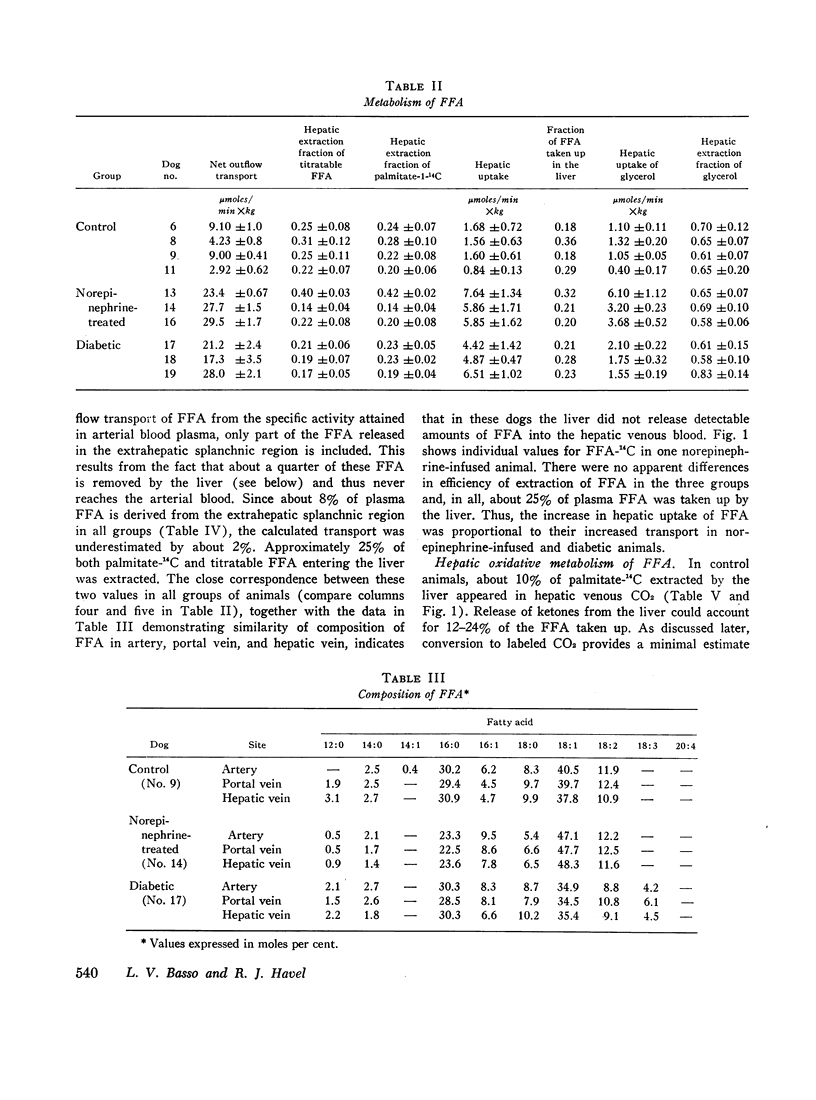
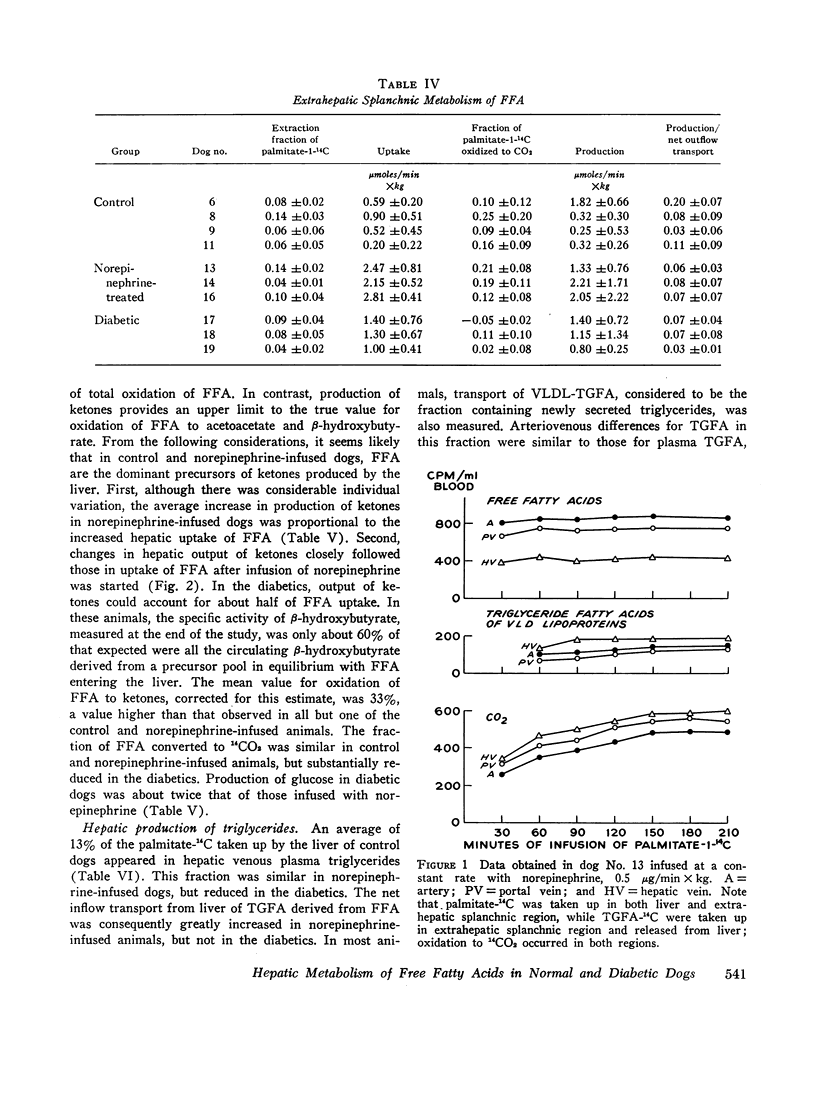
Hepatic uptake was proportional to net outflow transport of plasma free fatty acids in the three groups and, in each, hepatic extraction fraction was about 25%. Since specific activity of free fatty acids entering and leaving the liver was equal and their composition was closely similar in the three sites sampled, it was concluded that palmitate is a representative tracer for free fatty acids entering the liver and that the liver does not release free fatty acids into the blood.
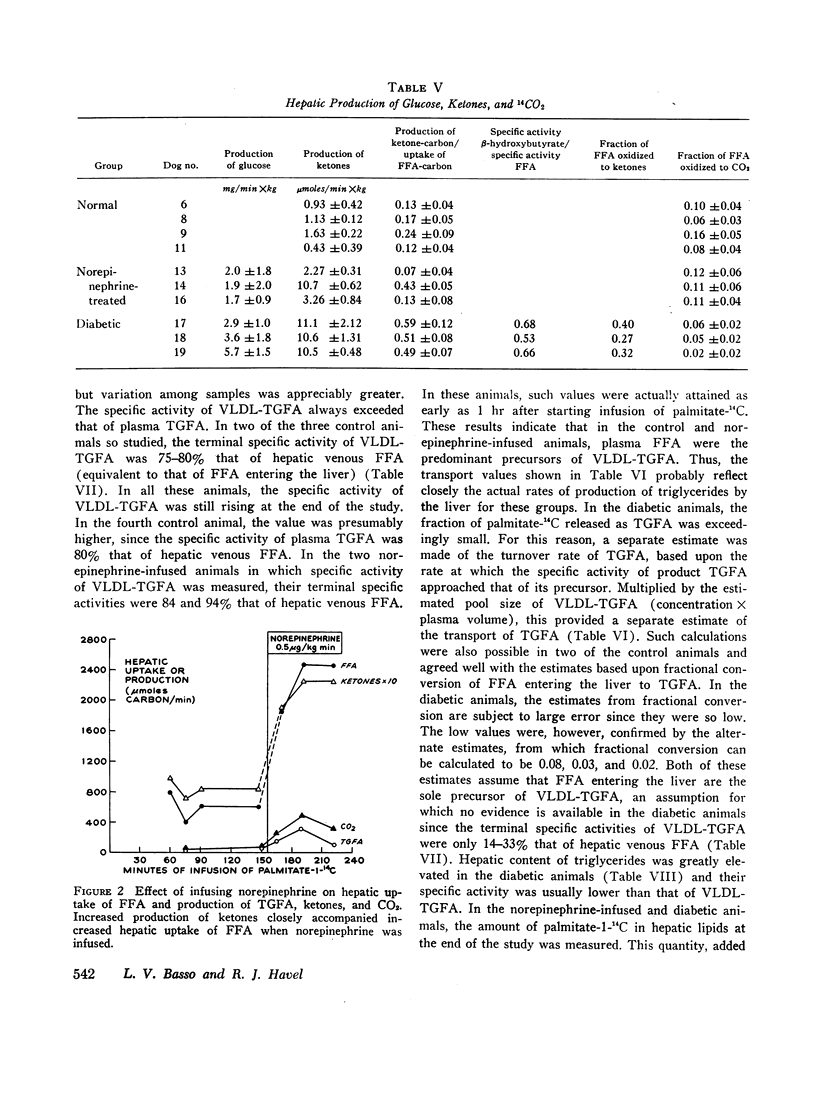
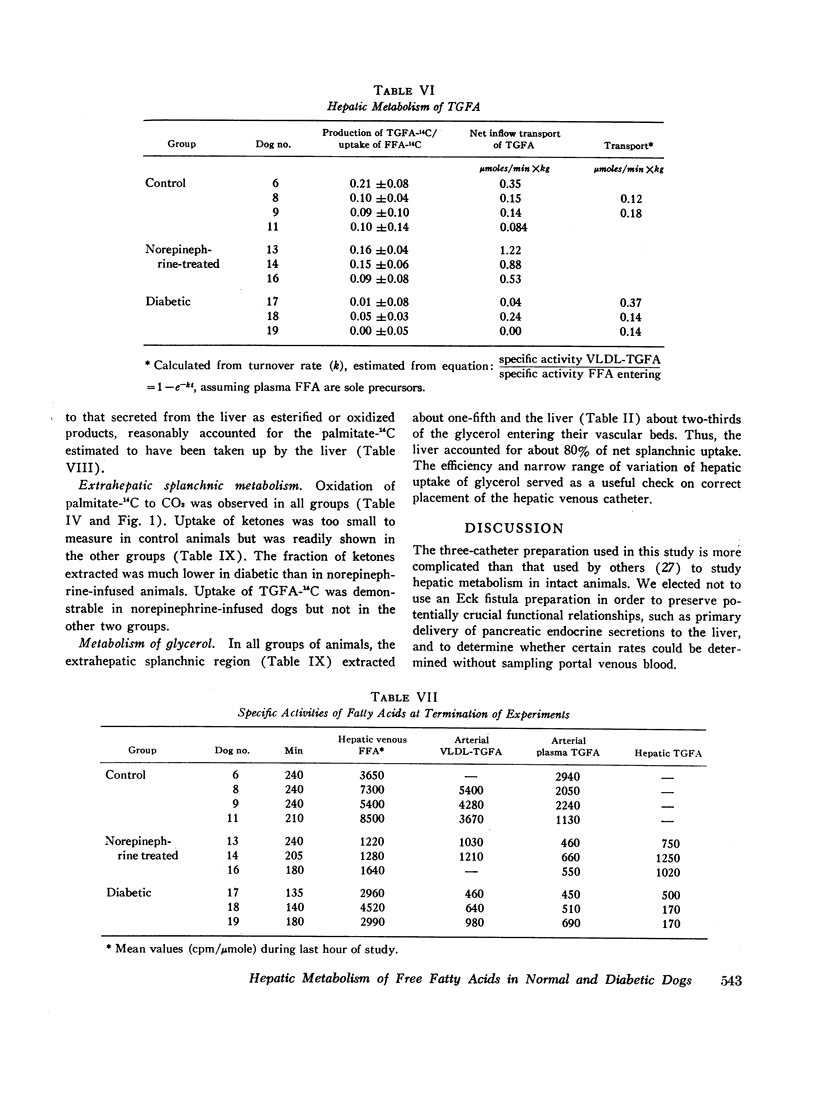
In norepinephrine-infused dogs, the fraction of free fatty acids secreted in triglycerides (13%) was similar to that of control animals, so that transport of triglycerides was increased. In diabetic dogs no increased transport could be demonstrated since an average of only 2% of free fatty acids was converted to plasma triglyceride fatty acids; the hyperlipemia uniformly observed therefore appeared to result from defective removal of triglycerides from the blood.
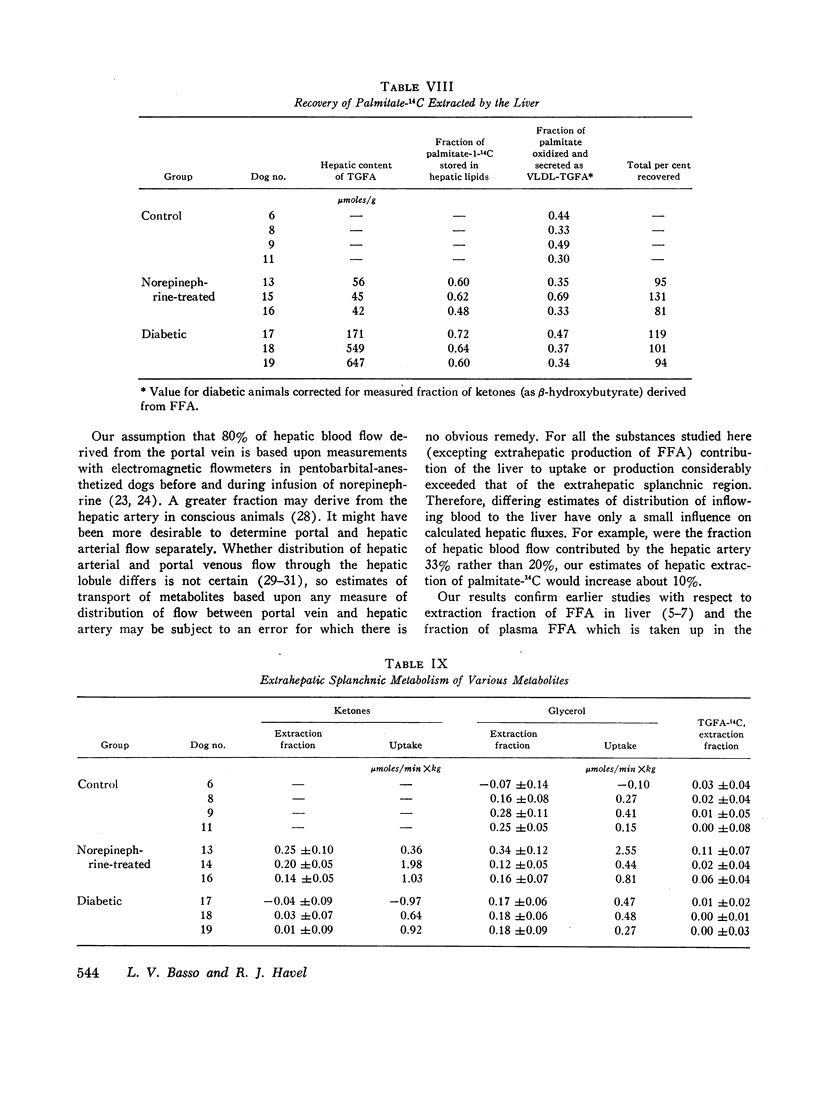
A similar fraction of free fatty acids was converted to ketones in normal and norepinephrine-infused dogs. This fraction was somewhat higher in diabetic animals and, in addition, a substantial quantity of ketones was derived from unlabeled precursors. Fractional conversion of free fatty acids to CO2 was similar in normal and norepinephrine-infused dogs, but reduced in the diabetics.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAGDON J. H., GORDON R. S., Jr Tissue distribution of C14 after the intravenous injection of labeled chylomicrons and unesterified fatty acids in the rat. J Clin Invest. 1958 Apr;37(4):574–578. doi: 10.1172/JCI103640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUHLER D. R. A simple scintillation counting technique for assaying C1402 in a Warburg flask. Anal Biochem. 1962 Nov;4:413–417. doi: 10.1016/0003-2697(62)90143-4. [DOI] [PubMed] [Google Scholar]
- Bagdade J. D., Porte D., Jr, Bierman E. L. Diabetic lipemia. A form of acquired fat-induced lipemia. N Engl J Med. 1967 Feb 23;276(8):427–433. doi: 10.1056/NEJM196702232760802. [DOI] [PubMed] [Google Scholar]
- Bierman E. L., Amaral J. A., Belknap B. H. Hyperlipemia and diabetes mellitus. Diabetes. 1966 Sep;15(9):675–679. doi: 10.2337/diab.15.9.675. [DOI] [PubMed] [Google Scholar]
- Bremer J. Pathogenesis of ketonemia. Scand J Clin Lab Invest. 1969 Apr;23(2):105–108. doi: 10.3109/00365516909077011. [DOI] [PubMed] [Google Scholar]
- Brownell G. L., Berman M., Robertson J. S. Nomenclature for tracer kinetics. Int J Appl Radiat Isot. 1968 Mar;19(3):249–262. doi: 10.1016/0020-708x(68)90022-7. [DOI] [PubMed] [Google Scholar]
- CARLSON L. A. DETERMINATION OF SERUM TRIGLYCERIDES. J Atheroscler Res. 1963 Jul-Aug;3:334–336. doi: 10.1016/s0368-1319(63)80012-5. [DOI] [PubMed] [Google Scholar]
- Cohn J. N., Pinkerson A. L. Intrahepatic distribution of hepatic arterial and portal venous flows in the dog. Am J Physiol. 1969 Feb;216(2):285–289. doi: 10.1152/ajplegacy.1969.216.2.285. [DOI] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEIGELSON E. B., PFAFF W. W., KARMEN A., STEINBERG D. The role of plasma free fatty acids in development of fatty liver. J Clin Invest. 1961 Dec;40:2171–2179. doi: 10.1172/JCI104443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELTS J. M., MASORO E. J. Effects of cold acclimation on hepatic carbohydrate and lipid metabolism. Am J Physiol. 1959 Jul;197(1):34–36. doi: 10.1152/ajplegacy.1959.197.1.34. [DOI] [PubMed] [Google Scholar]
- FINE M. B., WILLIAMS R. H. Effect of fasting, epinephrine and glucose and insulin on hepatic uptake of nonesterified fatty acids. Am J Physiol. 1960 Sep;199:403–406. doi: 10.1152/ajplegacy.1960.199.3.403. [DOI] [PubMed] [Google Scholar]
- FRITZ I. B. Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev. 1961 Jan;41:52–129. doi: 10.1152/physrev.1961.41.1.52. [DOI] [PubMed] [Google Scholar]
- HAESSLER H. A., ISSELBACHER K. J. THE METABOLISM OF GLYCEROL BY INTESTINAL MUCOSA. Biochim Biophys Acta. 1963 Jul 9;73:427–436. doi: 10.1016/0006-3002(63)90444-x. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., CARLSON L. A., EKELUND L. G., HOLMGREN A. TURNOVER RATE AND OXIDATION OF DIFFERENT FREE FATTY ACIDS IN MAN DURING EXERCISE. J Appl Physiol. 1964 Jul;19:613–618. doi: 10.1152/jappl.1964.19.4.613. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., NAIMARK A., BORCHGREVINK C. F. Turnover rate and oxidation of free fatty acids of blood plasma in man during exercise: studies during continuous infusion of palmitate-1-C14. J Clin Invest. 1963 Jul;42:1054–1063. doi: 10.1172/JCI104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M., Dougherty J. Liver blood flow measured by portal venous and hepatic arterial routes with Kr-85. Am J Physiol. 1966 May;210(5):926–932. doi: 10.1152/ajplegacy.1966.210.5.926. [DOI] [PubMed] [Google Scholar]
- Hopkinson B. R., Schenk W. G., Jr The electromagnetic measurement of liver blood flow and cardiac output in conscious dogs during feeding and exercise. Surgery. 1968 Jun;63(6):970–975. [PubMed] [Google Scholar]
- KETTERER S. G., WIEGAND B. D., RAPAPORT E. Hepatic uptake and biliary excretion of indocyanine green and its use in estimation of hepatic blood flow in dogs. Am J Physiol. 1960 Sep;199:481–484. doi: 10.1152/ajplegacy.1960.199.3.481. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. Bovine ketosis. Vet Rec. 1966 Feb 5;78(6):187–192. doi: 10.1136/vr.78.6.187. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Weidemann M. J., Speake R. N. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J. 1966 Oct;101(1):242–249. doi: 10.1042/bj1010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADISON L. L., COMBES B., ADAMS R., STRICKLAND W. The physiological significance of the secretion of endogenous insulin into the portal circulation. III. Evidence for a direct immediate effect of insulin on the balance of glucose across the liver. J Clin Invest. 1960 Mar;39:507–522. doi: 10.1172/JCI104065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRIS B. THE METABOLISM OF FREE FATTY ACIDS AND CHYLOMICRON TRIGLYCERIDES BY THE ISOLATED PERFUSED LIVER OF THE RAT. J Physiol. 1963 Oct;168:564–583. doi: 10.1113/jphysiol.1963.sp007208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Hamilton R. L., Lequire V. S. Characterization of lipoprotein particles isolated from the Golgi apparatus of rat liver. J Lipid Res. 1969 Jul;10(4):433–439. [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Determination of 14C-labelled ketone bodies by liquid-scintillation counting. Biochem J. 1967 Jan;102(1):230–235. doi: 10.1042/bj1020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Regulation of fat metabolism of the liver. Nature. 1967 Aug 12;215(5102):716–718. doi: 10.1038/215716a0. [DOI] [PubMed] [Google Scholar]
- Pinakatt T., Richardson A. W. Distribution of cardiac output in dogs. Am J Physiol. 1967 Oct;213(4):905–909. doi: 10.1152/ajplegacy.1967.213.4.905. [DOI] [PubMed] [Google Scholar]
- REES J. R., REDDING V. J., ASHFIELD R. HEPATIC BLOOD-FLOW MEASUREMENT WITH XENON 133. EVIDENCE FOR SEPARATE HEPATIC-ARTERIAL AND PORTAL-VENOUS PATHWAYS. Lancet. 1964 Sep 12;2(7359):562–563. doi: 10.1016/s0140-6736(64)90623-3. [DOI] [PubMed] [Google Scholar]
- ROHEIM P. S., SPITZER J. J. Metabolism of unesterified fatty acids (UFA) in normal dogs. Am J Physiol. 1958 Nov;195(2):288–290. doi: 10.1152/ajplegacy.1958.195.2.288. [DOI] [PubMed] [Google Scholar]
- SAIFER A., GERSTENFELD S. The photometric microdetermination of blood glucose with glucose oxidase. J Lab Clin Med. 1958 Mar;51(3):448–460. [PubMed] [Google Scholar]
- SHOEMAKER W. C., ASHMORE J., CARRUTHERS P. J., SCHULMAN M. Effect of insulin on rate of hepatic uptake of NEFA. Proc Soc Exp Biol Med. 1960 Mar;103:585–588. doi: 10.3181/00379727-103-25604. [DOI] [PubMed] [Google Scholar]
- SMYTHE C. M., GILMORE J. P., HANDFORD S. W. The effect of levarterenol (L-norepinephrine) on hepatic blood flow in the normal, anesthetized dog. J Pharmacol Exp Ther. 1954 Apr;110(4):398–402. [PubMed] [Google Scholar]
- Scholtholt J., Lochner W., Renn H., Shiraishi T. Die Wirkung von Noradrenalin, Adrenalin, Isoproterenol und Adenosin auf die Durchblutung der Leber und des Splanchnicusgebietes des Hundes. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;293(2):129–154. [PubMed] [Google Scholar]
- TROUT D. L., ESTES E. H., Jr, FRIEDBERG S. J. Titration of free fatty acids of plasma: a study of current methods and a new modification. J Lipid Res. 1960 Apr;1:199–202. [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZILVERSMIT D. B. The design and analysis of isotope experiments. Am J Med. 1960 Nov;29:832–848. doi: 10.1016/0002-9343(60)90117-0. [DOI] [PubMed] [Google Scholar]



