Abstract
A sublingual soluble film formulation of buprenorphine and naloxone (B/N) has been approved by the Food and Drug Administration. This preparation provides unit dose child resistant packaging amenable to tracking and accountability, offers more rapid dissolution, and has potentially preferred taste versus tablets. This study compared the ability of buprenorphine (B) and B/N films to suppress spontaneous withdrawal in opioid dependent volunteers.
Methods
Participants were maintained on morphine and underwent challenge sessions to confirm sensitivity to naloxone induced opioid withdrawal. Subjects were randomized onto either B (16mg, n=18) or B/N (16/4 mg, n=16) soluble films for five days. Primary outcome measure was the Clinical Opiate Withdrawal Scale (COWS) score.
Results
Thirty-four subjects completed induction onto soluble films. There was a significant decrease in COWS scores but no significant differences between groups.
Conclusions
Results support use of B and B/N soluble films as safe and effective delivery methods for opioid induction.
Keywords: buprenorphine, buprenorphine/naloxone, precipitated withdrawal, soluble film
2.0 Introduction
The most current Department of Health and Human Services estimates suggest that in the U.S. approximately 213,000 individuals age twelve or older are dependent on or abuse heroin, and that 1,707,000 individuals 12 or older are dependent on or abuse prescription pain medications (1). In 1996 the cost of heroin addiction in the U.S. due to criminal activities, medical care, productivity losses and social welfare was estimated to be US$21.9 billion (2).
In 2000 the U.S. Congress passed the Drug Addiction Treatment Act, which allows qualified physicians to treat opioid addiction with schedule III – V narcotics approved by the FDA for opioid dependence treatment. This legislation expanded options in the U.S. for opioid dependence treatment outside of methadone clinics, allowing patients to receive care in an office based setting. The subsequent FDA approval of buprenorphine (B) and buprenorphine/naloxone (B/N) in 2002 provided physicians with a pharmacotherapy for use within this new system. Buprenorphine is a novel opioid (3) currently marketed in a sublingual tablet formulation for the treatment of opioid dependence (Subutex and a generic form), and a form in combination with naloxone (Suboxone). The addition of naloxone is to discourage diversion and parenteral misuse, throughout all phases of treatment, and is currently the preferred formulation in the U.S. (4).
While the sublingual tablet forms of B have been extensively used, are safe, and effective, there have been concerns noted with these medications. The tablets require several minutes to fully dissolve, and there are concerns regarding diversion of these medications (4, 5, 6). Additionally, unintentional exposure to B tablets in children less than 6 years of age has increased from 53 exposures in 2004 to 907 exposures in 2008, and there were a total of 1,786 childhood exposures to B from 2000 to 2008 (7). This rising number of childhood exposures as well as other liabilities of tablets suggest alternate forms of B would be valuable.
One such alternate formulation for delivery of B is a soluble film. Advantages to soluble films include unit dose packaging, greater mucoadhesion, and more rapid dissolution than the tablet (8). The purpose of this study was to assess the safety and efficacy of B and B/N soluble films in suppressing withdrawal symptoms during induction in persons with active opioid dependence. It was hypothesized that the addition of naloxone would not increase the incidence of precipitated withdrawal relative to buprenorphine alone. A secondary objective was to determine if soluble films would precipitate opioid withdrawal. Previous studies have shown that under certain conditions B can elicit withdrawal in opioid dependent individuals (9, 10). The results of this study should aid in determining the appropriateness and feasibility of using a B/N soluble film for induction in a clinical setting.
3.0 Results
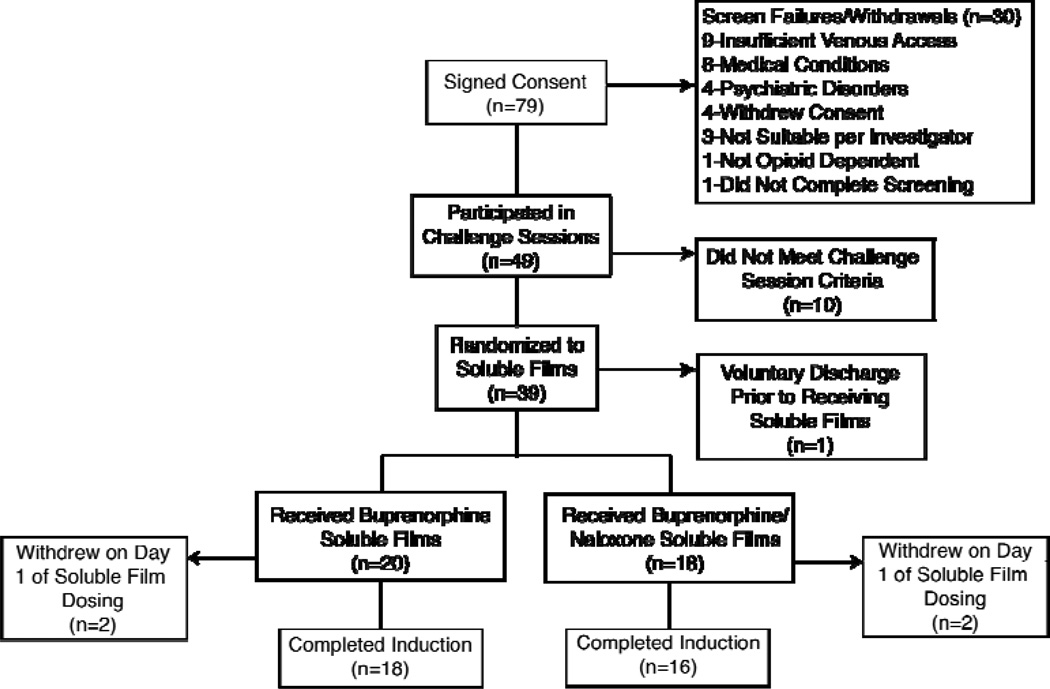
Twenty study participants received at least one B soluble film and 18 received at least one B/N soluble film (Figure 1). Four of the 38 subjects (B = 2; B/N = 2) randomized to soluble films voluntarily discontinued their participation during Day 1 of soluble film induction, reporting continued opioid withdrawal. For subjects who completed the soluble film dosing (the evaluable population, 18 = B and 16 = B/N; Table 1), groups did not differ by mean age (41 yrs), gender or race (74% male, 65% white). Participants had a mean age of 41 years (range 25 to 56), and reported that they had used heroin a mean of 27.5 out of the 30 days prior to study screening (range 2–30). The participant using heroin for 2 days out of 30 had misused prescription opioids 30 days out of 30. Participants reported that they had used heroin a mean of 9.8 years.
Figure 1.
Participant enrollment and disposition.
Table 1.
Participants
| Demographics* | ||
|---|---|---|
|
B (N=18) |
B/N (N=16) |
|
| Mean Age | 40.5 | 40.1 |
| % Male | 77.8 | 68.8 |
| % Female | 22.2 | 31.2 |
| % Caucasion | 61.1 | 68.8 |
| % African American | 38.9 | 25.0 |
| % Other | 0 | 6.3 |
| Mean Height (inches) | 69.1 | 67.8 |
| Mean Weight (pounds) | 163.7 | 168.4 |
| Mean Lifetime Years Heroin Use | 9.0 | 10.6 |
| Mean Heroin Use** | 27.2 | 27.9 |
| Mean Benzodiazepine Use** | 2.1 | 3.5 |
| Mean Cocaine Use** | 2.2 | 2.1 |
| Mean Alcohol Use** | 2.1 | 3.5 |
| Mean Cannabis Use** | 1.8 | .8 |
| Mean Nicotine Use** | 26.7 | 29.1 |
| % IV Heroin Users | 72.2 | 81.2 |
| % Inhalational Heroin Users | 27.8 | 18.8 |
B=buprenorphine, B/N=buprenorphine/naloxone
Number of days out of the last 30
3.1 Challenge Sessions
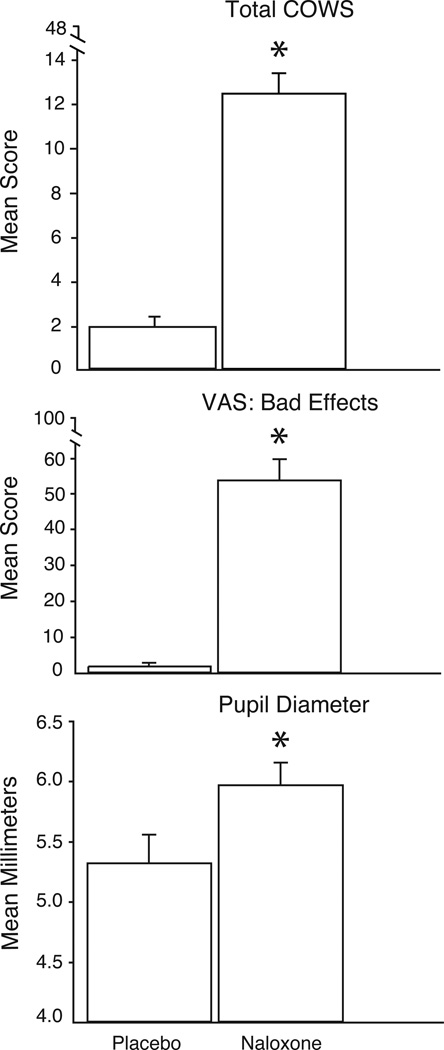
Figure 2 shows the mean peak scores of three representative measures collected from challenge sessions for the 34 subjects in the evaluable population. Results significantly differed between placebo and naloxone for total COWS scores, VAS Bad Effects ratings, and pupil diameter. Results from the challenge sessions are reported more completely elsewhere (11).
Figure 2.
Results from the challenge sessions during the morphine maintenance phase of the study. Participants received an intramuscular injection of either placebo or naloxone (0.4 mg). Mean (SEM) peak ratings for COWS (Clinical Opioid Withdrawal Scale), visual analog scale (VAS) ratings of Bad Effects, and maximum pupil diameter increase are shown. Asterisks indicate a significant difference (p<0.05) between the two conditions.
3.2 Primary Outcome Measure
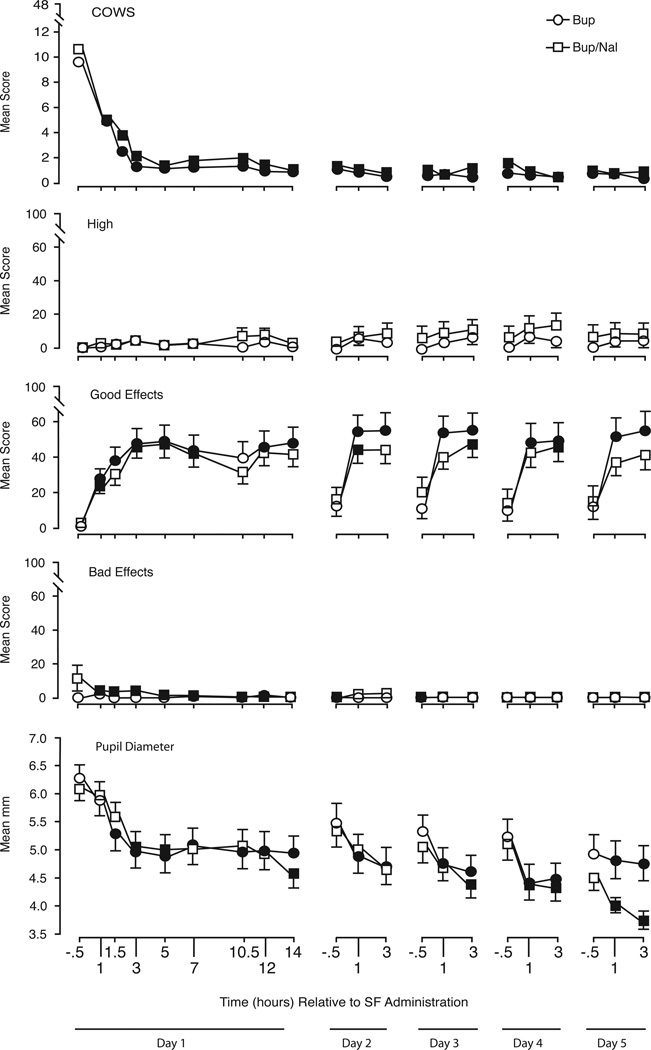
The top panel of Figure 3 shows the time course of ratings for COWS scores (the primary outcome) over the course of soluble film administration for the evaluable population (n=34). Prior to the first soluble film dose, COWS scores were elevated (mean = 9.6), consistent with mild spontaneous opioid withdrawal induced by substituting placebo for the last two morphine injections. There was a significant decrease in COWS scores from the baseline score (pre-first soluble film) to the peak post-soluble film score, but no significant differences between groups in baseline (B = 9.1, B/N = 10.1) or peak post-soluble film COWS scores (B = 4.2, B/N = 5.7). The mean scores for COWS ratings (Figure 3) show that there was a significant decrease in observable withdrawal signs at 1-hour post first soluble film dose relative to baseline. Significant differences from daily baseline evaluations in Figure 3 are indicated by filled versus open symbols. The figure shows that mean COWS scores decreased significantly after the first dose of B or B/N soluble films and remained low throughout the five days.
Figure 3.
Time course results for the first five days of soluble film (SF) administration. Shown are mean (SEM) ratings on the COWS (Clinical Opioid Withdrawal Scale), Visual analog scale ratings of High, Good Effects, and Bad Effects, and pupil diameter. Filled symbols indicate a significant difference from the baseline value (−0.5 hours on each day of assessment).
3.3 Secondary Outcome Measures
Secondary outcome measures included pupillometry, VAS assessments and subjective adjective rating scales. Figure 3 illustrates time course data for VAS ratings of high, good effects and bad effects items as well as pupil diameter. Participants were specifically asked to rate how the drug made them feel, not how they felt in general. Ratings of high, bad effects, and sick (not shown) remained low throughout soluble film administration. There were significant time effects (p<0.0001) for good effects, drug effects, and liking (e.g., peak liking, B = 59.2, B/N = 51.4).
3.4 Safety Evaluation
Safety was evaluated through oral mucosal exams, clinical laboratory outcomes, ECGs and adverse event collection. The most common adverse events experienced during the study were those consistent with opioid withdrawal. One participant was treated at the Johns Hopkins Bayview Medical Center’s emergency department for a non-oral abscess, this was considered unrelated to any of the study treatments.
Of the 38 participants who received soluble films, four (2 in each treatment arm) had mild non-ulcerous irritations of the oral mucosa that were not present at baseline. In three of the four the irritation was attributed to teeth grinding or dental decay. Two participants in the B/N (n=18) group had clinically significant changes from baseline in their clinical chemistry results. One participant had liver function test values within acceptable limits (Normal Ranges: AST 10 – 40 U/L, ALT 9 – 60 U/L) at baseline but at discharge had clinically significant elevations (i.e., values greater than 3 times the upper limit of normal) of alanine transaminase ([ALT]=226 U/L), aspartate transaminase ([AST]=84 U/L), and alkaline phosphatase (66 U/L). Follow up liver function tests showed a slight decrease in ALT (211 U/L) and alkaline phosphate (63U/L) and an increase in AST (105 U/L). Total bilirubin remained within normal limits throughout the study for this participant. The participant reported a history of Hepatitis C. Previous studies have suggested that buprenorphine treatment can elevate liver enzyme levels in individuals with Hepatitis C (12). Vital signs remained stable throughout the study with the exception of mild elevations in blood pressure and heart rate during the first induction day. No serious adverse events occurred during the study. Electrocardiogram results were normal for all participants at screening and at discharge.
Four participants, two in each treatment arm, dropped out on the first induction day after beginning soluble film dosing. The baseline (pre-soluble film) COWS scores for these individuals were higher (mean = 14.5) than the mean score for the evaluable group (mean = 9.6), and this may have contributed to their early discontinuation. These four participants cited inadequate relief of withdrawal symptoms in response to the B and B/N doses used on the first day of induction as the primary reason for discontinuation. One of the four participants additionally cited a family emergency as a reason for early discontinuation.
4.0 Discussion
Development of improved B formulations that allow for increased compliance and reduced risk of diversion could be of significant value. A soluble film formulation of B/N is expected to offer advantages over current tablet formulations. Soluble film formulations can have unit dose packaging, improving the ability to track a dose of the medication and providing more child resistant containment of the product. Its quick dissolution can be an advantage to use in settings where monitored dose ingestion needs to be accomplished quickly. Both the 2 and 8 mg soluble films were identical in size and there did not appear to be any difference in dissolution rates between dosages. In addition, higher doses of the medication can be taken relatively easily compared to the tablet form of B.
The results from this clinical trial suggest that these soluble film formulations were clinically effective in suppressing opioid withdrawal. Neither B nor B/N soluble films produced statistically significant differences from each other on the primary or secondary outcomes. Both soluble film formulations were able to attenuate the signs and symptoms of opioid withdrawal. Significant reduction in the overall COWS scores document the ability of the soluble film formulations to attenuate the opioid withdrawal syndrome, as is seen with clinical use of the tablet formulations of B and B/N (13). These results demonstrate that induction onto B or B/N soluble films was similarly effective.
Like their tablet counterparts, soluble film formulations have the potential to precipitate withdrawal in opioid dependent individuals (9, 10). In this study, four of the study participants (B = 2; B/N = 2) withdrew after beginning induction onto soluble films due to persistent opioid withdrawal symptoms. However, there was no indication that either of the formulations precipitated withdrawal or worsened existing withdrawal symptoms in any of the study participants, including these four. The presence of withdrawal-alleviating effects and the lack of a difference between the B and B/N groups suggests that neither soluble film formulation is likely to precipitate an opioid withdrawal syndrome when given in a manner consistent with the study protocol. The significant decrease in COWS scores from baseline to post-soluble film shows that both soluble film formulations were effective in treating the symptoms of spontaneous opioid withdrawal observed prior to induction.
Subjects in the study found the use of the soluble films acceptable; while not systematically assessed, there were no spontaneous reports that the films as a delivery system were disliked. The soluble films have a lemon-lime flavor (as do B/N tablets). In a prior study of B/N soluble films in which subjects were asked to rate the flavor, 71% of 160 subjects gave the films a score of 5 or better on a 10-point scale (where 1 was extremely unpleasant, and 10 was extremely pleasant; R.E. Johnson, personal communication, August 15, 2010).
The use of a morphine stabilization phase in this study provided some standardization of the level of physical dependence among participants, although a longer period of stable morphine dosing would have strengthened this aspect of the study design. The use of the naloxone challenge ensured that the lack of B or B/N precipitated withdrawal during the induction phase was not due to a population that was insensitive to the detection of opioid withdrawal. Conversely, the lack of withdrawal from the placebo challenge provided assurance that these were not subjects who were overly sensitive to reporting any opioid withdrawal.
Limitations to the present study should be noted. With respect to the apparent absence of precipitated withdrawal, the present context of abstinence-induced spontaneous withdrawal is perhaps not ideally sensitive for detecting additional precipitated withdrawal. Also, conclusions regarding the withdrawal-suppressing efficacy of B and B/N are based on their rapid reversal of spontaneous withdrawal in the absence of a placebo or a full agonist comparison control condition. A placebo control condition was expected to produce sustained spontaneous withdrawal that increased the risk of study dropout, while an agonist control condition would have replicated prior studies that have already demonstrated B’s efficacy to suppress opioid withdrawal (14, 15, 16) and would not have addressed the present study question of comparing B versus B/N for suppressing opioid withdrawal. Finally, the present study utilized a fixed dose schedule, and clinical practice generally uses a more flexible dosing procedure that is responsive to patient needs. The protocol did allow for some flexibility with supplemental soluble film doses up to 24mg per day and it is interesting that neither staff nor participants ever requested such.
The study was designed to examine dosing during the induction period. While the concept of gradual induction for opioid treatment is generally well accepted (17), there is not a well accepted definition of the length or goal of induction onto a medication such as B (or methadone). This study used a two-day period for induction and assumes that the goal of induction is to have the patient successfully transition on to the medication though not necessarily to stop all illicit drug use immediately. Induction should be distinguished from stabilization (which in turn differs from maintenance); stabilization is the period during which a stable dose of medication is determined. This study showed that induction onto B and B/N soluble films could be achieved, and that there was no difference associated with the inclusion of naloxone with B during this critical first period of medication treatment. In summary, this study showed no significant differences in efficacy or tolerability between B and B/N soluble films. Both were effective and safe under the dose induction procedures used here. As with the marketed Suboxone tablets, it is expected that the addition of naloxone to the soluble film formulation will not precipitate withdrawal when taken sublingually as directed. The use of soluble films may have distinct advantages that will facilitate the use of B for the treatment of opioid dependence.
5.0 Methods
5.1 Study Participants
All procedures for this study were conducted at the Behavioral Pharmacology Research Unit (BPRU) of the Johns Hopkins University School of Medicine. The University’s Institutional Review Board approved the study and all participants provided written informed consent prior to participating in any study related activities. This study was registered at www.clinicaltrials.gov under: NCT00637000.
Responders to print advertisements in local newspapers, flyers and word of mouth were initially screened over the telephone for age, occupation, current and historical drug use, health status, date of birth and other general information. Those who appeared to be good study candidates based on the initial telephone interview were scheduled for a more intensive in-person screening. Applicants completed the informed consent process and underwent a physical examination, medical history, demographics, blood and urine collection for clinical laboratory testing, ECG, Structured Clinical Interview for DSM-IV-TR (SCID) (18), Mini Mental Status Exam (MMSE) (19), oral mucosa examination, urine drug screen, and a pregnancy test for females. Participants were required to meet the following criteria for study entry: (a) DSM-IV diagnosis of opioid dependence based on the E module of the SCID; (b) 18 to 65 years of age inclusive; (c) negative pregnancy test result if female; (d) no participation in a clinical trial within 30 days prior to screening; (e) absence of a clinically significant non-substance use psychiatric disorder; (f) MMSE score ≥ 24; (g) absence of physical dependence on alcohol or sedative-hypnotics; (h) no observable evidence of active aphthous stomatitis or oral herpes; (i) absence of dental caries that required immediate medical intervention; and (j) no on-going prescription medications that interact with the P450 3A4 system. Seventy-nine participants signed the study consent form, 49 participated in the challenge sessions (as described below), 38 received the soluble films and 34 completed the study (Figure 1).
Participants who met criteria, with the exception of the clinical laboratory result review which typically required a 24-hour turn around, were admitted on the same screening day to BPRU’s closed, 14 bed residential research unit (RRU). This admission without knowledge of clinical laboratory results was allowed to minimize participant drop out between screening and admission visits. It also met the request of participants who had come to the site with the goal of immediate study enrollment.
Subjects were paid for their participation and informed that they were enrolling in a study that would initially provide morphine to stabilize their opioid dependence, followed by a novel formulation of B and B/N. While they would know the time of transition from morphine to soluble film, information regarding doses or specific formulation content was not provided.
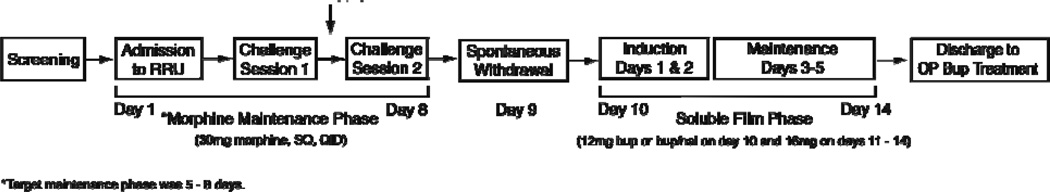
5.2 Study Schedule and Setting (Figure 4)
Figure 4.
Schedule of study procedures. The vertical arrow between challenge sessions 1 and 2 indicates there was at least one day off between the sessions. RRU = Residential Research Unit; OP = outpatient.
Upon admission to the RRU, participants began four times daily dosing of 30 mg subcutaneous (SC) morphine (7am, 12pm, 5pm and 10pm) for a period of 5 – 8 days in order to prevent opioid withdrawal and establish a common baseline of physical dependence across subjects. This dose was selected to approximate daily 60 mg heroin usage. During the maintenance phase subjects participated in two randomized, double-blind challenge sessions, one involving an intramuscular (IM) injection of naloxone 0.4 mg and the other a matching placebo. Challenge sessions were intended to identify individuals who were sensitive to opioid withdrawal (i.e., in response to the naloxone, demonstrating opioid physical dependence) and who had an absence of such in response to placebo. Participants who successfully met criteria based upon challenge session results were randomized onto either B or B/N soluble films for up to five days. Participants were offered treatment at BPRU’s outpatient clinic at the time of study discharge regardless of successful study completion.
5.3 Challenge Sessions
Challenge sessions began at 10:30 am and lasted three hours with at least a twenty-four hour period between sessions. Participants recorded their responses to subjective questionnaires in an iMac computer (Apple Inc., Cupertino, California). Trained research assistants recorded observer assessments on a separate computer terminal. Vital signs, except manually recorded respiration rates, were collected via a Criticare unit (Criticare Systems Inc., Waukesha, Wisconsin). Pupil diameter was measured using a hand-held pupillometer (NeurOptics Inc., San Clemente, California). After 30 minutes of baseline data collection, participants were given 0.4 mg of naloxone IM or a matching placebo and then asked to complete visual analog scale (VAS) items reflecting how they felt at that time. The VASs used during sessions consisted of six items asking participants to rate on a scale of 0 – 100 the degree of their drug experience. Items on the VASs included: “How HIGH are you?”; “Do you feel any DRUG EFFECTS?”; “Does the drug have any GOOD EFFECTS?”; “Does the drug have any BAD EFFECTS?”; “Do you LIKE the drug?”; and “Does this drug make you feel SICK?”. A research assistant completed the COWS at each time point using the standard scoring system (20). Clinical Opioid Withdrawal Scale items are: GI upset, sweating, tremor, restlessness, yawning, pupil size, anxiety or irritability, bone or joint aches, gooseflesh and runny nose or tearing. Additionally, the Clinical Institute Narcotic Assessment (CINA) was completed by the research assistant (21). Data collection occurred at −30 and −15 minutes prior to drug administration for baseline purposes and subsequently every fifteen minutes for 2 ½ hours post injection.
5.4 Challenge Session Eligibility Determination
Results from the two challenge sessions were reviewed to determine each participant’s eligibility to receive soluble films. Participants were required to meet at least two of three inclusion criteria in responses during the active naloxone challenge condition: (1) a peak VAS bad effects rating of 30 or greater, (2) an increase in pupil diameter from baseline of at least 0.4 mm, and (3) an increase from baseline in the total COWS score of at least 5 points. In addition, participants who showed significant evidence of an opioid withdrawal response to the placebo condition, defined as a peak change from baseline COWS score greater than 12, were excluded. These criteria were based on previous studies involving naloxone challenge sessions in opioid dependent volunteers (9).
5.5 Procedures for Induction onto Buprenorphine & Buprenorphine/Naloxone Soluble Films
Participants were assigned to either B or B/N soluble films via an urn randomization program (22). Participants were stratified on naloxone challenge session peak VAS Bad Effects score being < 50 versus ≥ 50. The day prior to beginning soluble film induction, participants received double-blinded, placebo injections in place of their last two scheduled morphine injections (5pm and 10pm) in order to induce a mild opioid withdrawal prior to the first dose of soluble film at 9am (i.e., 21 hours of morphine abstinence). Observation of clear opioid withdrawal signs is generally recommended prior to initiating B therapy (17, 5). On the first day of soluble film induction participants received 4 mg of B or 4/1 mg of B/N at 9am, 11am and 8pm for a total of 12 mg of B or 12/3 mg of B/N. On the subsequent four dosing days participants received one daily 16 mg dose of B or 16/4 mg of B/N at 9am. The protocol allowed flexible dosing of between 12 mg of B (12/3 mg B/N) and up to 24 mg of B (24/6 mg B/N) per day upon participant request to control withdrawal symptoms.
Results from physiological measurements, pupillometry, subjective VAS assessments and observer COWS ratings were used during the soluble film induction and stabilization phase of the study to assess opioid withdrawal and drug effects. On the first day of soluble film administration assessments were performed at −0.5, 1, 1.5, 3, 5, 7, 10.5, 12 and 14 hours relative to the first soluble film dose. On subsequent soluble film administration days assessments were performed at −0.5, 1 and 3 hours. Nursing staff collected vital signs and performed observer ratings and participants completed subjective questionnaires on computer terminals located on the RRU.
5.6 Drug Supplies and Drug Administrations
Buprenorphine, B/N and matching placebo soluble films were supplied by MonoSol Rx LLC (Portage, Indiana) in 8 mg (8/2 mg B/N) and 2 mg (2/.5 mg B/N) dosages to replicate the marketed tablet formulations of Subutex and Suboxone. The rectangular soluble films were 2.2 cm by 1.3 cm in dimension; B films were white in color and B/N films were orange. Both B and B/N films were lemon/lime flavored. Placebo films matched both color and taste for each type of soluble film. Morphine sulfate was from Baxter Healthcare Corp. (Deerfield, Illinois) and naloxone for challenge sessions was from Hospira Inc. (Lake Forest, Illinois). Bacteriostatic saline was used for placebo injections.
All drug administrations (SC morphine and placebo, IM naloxone and placebo, and SL B and B/N) were performed by nursing staff. Nurses had been trained prior to study enrollment on administration of the soluble films and had previous experience with B and B/N tablet administration. Buprenorphine and B/N soluble films have different colors, therefore, placebo soluble films were administered to ensure that the number and color of soluble films were identical and the blind maintained. Study participants always received orange films (B/N or placebo) first, followed by white films (B or placebo). The soluble films were placed by the nurse into the participant’s sublingual space and subjects were instructed to hold the soluble films under their tongue and refrain from speaking or swallowing for three minutes. After three minutes the nurse examined the oral cavity to ensure that the soluble films had been completely dissolved. If remnants of the soluble films remained, participants were instructed to refrain from speaking or swallowing until the soluble films were completely dissolved.
5.7 Data Analysis
Analysis was performed by a statistician blinded to study drug assignment using SAS™ version 9.1 (SAS Institute, Cary, N.C.). SAS PROC Mixed was used to conduct repeated measures regression analyses using an AR(1) covariance structure. Posthoc comparisons were conducted between baseline and subsequent timepoints within each soluble film condition using Tukey's posthoc tests. All statistical comparisons were considered significant at p<=0.05. The evaluable population was defined as those individuals who were randomized and completed the first two days of soluble film administrations and assessments. The primary outcome to assess the effectiveness of the two formulations was total COWS scores during the first two days of soluble film dosing (the predefined induction period). Secondary outcome measures included pupil measurements and VAS assessments.
Acknowledgements
The authors thank Jessica Vanderhoff and Sonia Bansal for their assistance with data collection, John Yingling for his technical support and Linda Felch for her statistical work. We also thank the nursing staff at the Behavioral Pharmacology Research Unit for their assistance.
This study was supported by a contract from Reckitt Benckiser Pharmaceuticals Inc. and NIDA grants DA08045, DA023186.
Footnotes
Some of these data were presented at the annual meeting of the College on Problems of Drug Dependence, held in Reno, Nevada, U.S.A. (June of 2009).
Funding for the study described in this article was provided by Reckitt Benckiser Pharmaceuticals Inc. The company also manufactures drugs used in the study. Dr. Strain is a consultant to and paid member of the Scientific Advisory Board of Reckitt Benckiser Pharmaceuticals. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. Through his university, Dr. Bigelow has received research support from Titan Pharmaceuticals, Inc., developer/manufacturer of a different B formulation.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2008. (Office of Applied Studies, NSDUH Series H-34, DHHS Publication No. SMA 08-4343) [Google Scholar]
- 2.Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug Alcohol Depend. 2001;61:195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- 3.Jasinski D, Pevnik J, Griffith J. Human pharmacology and abuse potential of the analgesic buprenorphine. Arch. Gen. Psychiatry. 35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- 4.Ling W. Buprenorphine for opioid dependence. Expert Rev. Neurother. 2009;9:609–616. doi: 10.1586/ern.09.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones H. Practical considerations for the clinical use of buprenorphine. Sci. Pract. Perspect. 2004;2:4–20. doi: 10.1151/spp04224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strain EC, Stoller K, Walsh SL, Bigelow GE. Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers. Psychopharmacology. 2000;148:374–383. doi: 10.1007/s002130050066. [DOI] [PubMed] [Google Scholar]
- 7.Boyer E, McCance-Katz E, Marcus S. Methadone and buprenorphine toxicity in children. The American Journal on Addictions. 2009;19:89–85. doi: 10.1111/j.1521-0391.2009.00002.x. [DOI] [PubMed] [Google Scholar]
- 8.Das N, Das S. Development of mucoadhesive dosage forms of buprenorphine for sublingual drug delivery. Drug Delivery. 2004;11:89–95. doi: 10.1080/10717540490280688. [DOI] [PubMed] [Google Scholar]
- 9.Rosado J, Walsh SL, Bigelow GE, Strain EC. Sublingual buprenorphine/naloxone precipitated withdrawal in subjects maintained on 100 mg of daily methadone. Drug Alcohol Depend. 2007;90:261–269. doi: 10.1016/j.drugalcdep.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strain E, Preston K, Liebson I, Bigelow G. Buprenorphine effects in methadone-maintained volunteers: effects at two hours after methadone. J. Pharmacol. Exper. Ther. 1995;272:628–638. [PubMed] [Google Scholar]
- 11.Tompkins D, Bigelow G, Harrison J, Johnson R, Fudala P, Strain E. Concurrent validation of the Clinical Opiate Withdrawal Scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrument. Drug Alcohol Depend. 2009;105:154–159. doi: 10.1016/j.drugalcdep.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petry N, Bickel W, Piasecki D, Marsch L, Badger G. Elevated liver enzyme levels in opioid –dependent patients with Hepatitis treated with buprenorphine. Am. J. Addict. 2000;9:265–269. doi: 10.1080/10550490050148099. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug Alcohol Depend. 1995;40:17–25. doi: 10.1016/0376-8716(95)01186-2. [DOI] [PubMed] [Google Scholar]
- 14.Umbricht A, Hoover DR, Tucker MJ, Leslie JM, Chaisson RE, Preston KL. Opioid detoxification with buprenorphine, clonidine, or methadone in hospitalized heroin-dependent patients with HIV infection. Drug Alcohol Depend. 2003;69:263–262. doi: 10.1016/s0376-8716(02)00325-3. [DOI] [PubMed] [Google Scholar]
- 15.Blondell RD, Smith SJ, Servoss TJ, DeVaul SK, Simons RL. Buprenorphine and methadone: a comparison of patient completion rates during inpatient detoxification. J. Addict. Dis. 2007;26:3–11. doi: 10.1300/J069v26n02_02. [DOI] [PubMed] [Google Scholar]
- 16.Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. A clinical trial of buprenorphine comparison with methadone in detoxification in heroin addicts. Clin. Pharmacol. Ther. 1988;43:72–78. doi: 10.1038/clpt.1988.13. [DOI] [PubMed] [Google Scholar]
- 17.Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. [PubMed] [Google Scholar]
- 18.Spitzer R, Williams J. Structured interview for DSM-III-R (SCID) New York: New York State Psychiatric Institute, Biometrics Research; 1987. [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “MINI-MENTAL STATE”, A practical method for grading the cognitive state of patients for the clinician. J. of Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS) J. Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 21.Peachey JE, Lei H. Assessment of opioid dependence with naloxone. Br. J. Addict. 1988;83:193–201. doi: 10.1111/j.1360-0443.1988.tb03981.x. [DOI] [PubMed] [Google Scholar]
- 22.Stout RL, Wirtz PW, Carbonri JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J. Stud. Alcohol. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]