Abstract
The non-keratinized epithelia of the ocular surface are constantly challenged by environmental insults, such as smoke, dust, and airborne pathogens. Tears are the sole physical protective barrier for the ocular surface. Production of tears in inadequate quantity or of inadequate quality results in constant irritation of the ocular surface, leading to dry eye disease, also referred to as keratoconjunctivitis sicca (KCS). Inflammation of the lacrimal gland, such as occurs in Sjögren’s syndrome, sarcoidosis, chronic graft versus-host disease, and other pathological conditions, results in inadequate secretion of the aqueous layer of the tear film, and is a leading cause of dry eye disease. The hallmarks of lacrimal gland inflammation are the presence of immune cell infiltrates, loss of acinar epithelial cells (the secreting cells), and increased production of proinflammatory cytokines. To date, the mechanisms leading to acinar cell loss and the associated decline in lacrimal gland secretion are still poorly understood. It is also not understood why the remaining lacrimal gland cells are unable to proliferate in order to regenerate a functioning lacrimal gland. This article reviews recent advances in exocrine tissue injury and repair, with emphasis on the roles of programmed cell death and stem/progenitor cells.
Keywords: apoptosis, autophagy, dry eye, inflammation, lacrimal gland, programmed cell death, Sjögren’s (Please note the right spelling for this syndrome) syndrome, stem cells
I. Introduction
The tear film, which is the interface between the external environment and the ocular surface, has several different functions.1,2 It forms a smooth refractive surface over the corneal surface and lubricates the eyelids. Moreover, it maintains an optimal extracellular environment for the epithelial cells of the cornea and conjunctiva, because the electrolyte composition, osmolarity, pH, O2 and CO2 levels, nutrient levels, and concentration of growth factors in the tears are regulated within narrow limits.1,2 Tears dilute and wash away noxious stimuli. They also provide an antibacterial system for the ocular surface and serve as an entry pathway for polymorphonuclear leukocytes, in case of injury to the ocular surface.
Because tears have many and varied functions, it is not surprising that they have a complex structure and are produced by several different sources. The tear film consists of three interacting layers. The inner layer is a mucous layer that coats the cornea and conjunctiva. The middle layer is mainly an aqueous layer, but it contains proteins and soluble mucins. Finally, the outer layer is a lipid layer that floats on the aqueous layer. Each layer of the tear film is secreted by a different set of glands.1,2 The mucous layer is secreted by the cells of the cornea and conjunctiva. The aqueous layer is secreted by the main and accessory lacrimal glands, which secrete electrolytes, water, and proteins, including secretory immunoglobulins.3 The lipid layer is secreted by the meibomian glands embedded in the eyelid.
The lacrimal gland is a compound tubuloacinar gland consisting of acini, ducts, nerves, myoepithelial cells, and plasma cells.3 About 80% of the gland is acini, which secrete electrolytes, water, and proteins to form primary fluid (Figure 1). As the primary fluid moves along the duct system, duct cells modify the primary fluid by secreting or absorbing electrolytes (Figure 1).4 Myoepithelial cells are the third type of cells and are located between the epithelium and basement membrane (Figure 1).3,5 These cells express [ALPHA]α-smooth muscle actin and hence are generally assumed to be able to contract to help expel the secretory products.5 Contraction of myoepithelial cells has been shown to occur in the mammary gland but awaits demonstration in the lacrimal gland.
Figure 1.
Major cellular components of the lacrimal gland. The acinar cells, which account for 80% of the cell types present in the lacrimal gland, and ductal cells were stained with hematoxylin and eosin. The myoepithelial cells were identified immunohistochemicaly (brown stain) using an antibody against [ALPHA]α-smooth muscle actin.
Insufficient or inadequate production of the aqueous layer of the tear film leads to symptoms of dry eye. The lacrimal gland, as discussed in the following sections, can become the target of the immune system and show signs of inflammation that impair the normal function of this tissue. The cellular and molecular mechanisms responsible for impairing lacrimal gland secretion are still poorly understood. Furthermore, the role of programmed cell death (apoptosis) in the cell loss associated with inflammatory diseases of the lacrimal gland and its impact on tissue function are also not well understood. The purpose of this review is to summarize our current knowledge on the impact of inflammation and programmed cell death on lacrimal gland functions and the potential ability of this gland to regenerate following injury.
II. Impact of Inflammation on Lacrimal Gland Functions
A. Inflammatory Disorders of the Lacrimal Gland
In several pathological situations, the lacrimal gland can become a target of the immune system and show signs of inflammation (Figure 2). This can occur as a result of autoimmune diseases (Sjogren syndrome, sarcoidosis), following bone marrow transplants (graft-versus-host-disease [GVHD]), or simply as a result of aging.6 Lacrimal gland inflammation results in insufficient secretion, leading to aqueous-deficient type of dry eye.7,8
Figure 2.
Effects of inflammation on the lacrimal gland and the ocular surface. Schematic diagram summarizing how inflammation of the lacrimal gland can lead to damage of the ocular surface.
In Sjogren syndrome, which affects an estimated 1–4 million North Americans (mostly women), the lacrimal gland is infiltrated by lymphocytes organized in foci.9 Sjögren’s syndrome can present itself as a primary disorder affecting mainly the lacrimal and salivary glands, or it can be secondary to another autoimmune disease, such as rheumatoid arthritis, systemic lupus erythematosus, or systemic sclerosis.9 Besides the lymphocytic infiltrates, another hallmark of Sjögren’s syndrome is the presence of circulating autoantibodies to both organ-specific and non-organ-specific autoantigens. In addition, increased expression of proinflammatory cytokines is common in Sjögren’s syndrome patients.
Sarcoidosis is a chronic systemic disorder of unknown origin, with an estimated prevalence ranging from 1 to 40 cases per 100,000 population.10 It is characterized by the presence of noncaseating granulomas in multiple organs, with the lungs being involved most frequently.11 The vast majority of sarcoidosis patients suffer from dry eye and dry mouth.12
GVHD develops after hematopoietic stem cell transplantation performed on patients suffering from hematologic malignancies.13 Dry eye is the most frequent ocular complication associated with chronic GVHD, occurring in 50% to 70% of patients.14–16 The histopathologic features of the lacrimal gland in chronic GVHD include prominent fibrosis and an increase in stromal fibroblasts.15
The prevalence of the aqueous-deficient type of dry eye is known to be high among the elderly.17–23 Studies in animals have shown that lacrimal gland secretion in response to several neural agonists decreases with increasing age.21–23 Other studies have shown that, with age, the lacrimal gland undergoes dramatic structural changes highlighted by inflammation.22–25 Focal infiltration by T and B cells, numbers of mast cells, and accumulation of lipofuscin in the lacrimal gland increase with aging.22–25
Lacrimal gland deficiency is also associated with several other systemic and autoimmune diseases, including, but not limited to, hepatitis C,26,27 acquired immunodeficiency syndrome (AIDS) due to infection by human immunodeficiency virus (HIV),28,29 thyroid disease,30–32 and diabetes.33–35
B. Mechanisms of Lacrimal Gland Dysfunction
The molecular mechanisms responsible for the impaired secretory function of the inflamed lacrimal gland are still poorly understood. In the case of autoimmune diseases of the lacrimal gland, several autoantibodies have been described. Of these, antibodies against M3 muscarinic receptors have been identified in Sjögren’s syndrome patients. However, the role of these autoantibodies in the impaired function of the lacrimal gland is still controversial, as some studies showed that these autoantibodies have agonistic activity,36–38 whereas others showed that they have antagonistic activity.39–42 In the context of this controversy, it may be noted that Qian et al have presented evidence that even agonistic autoantibodies might impair function by down-regulating post-receptor signal transduction.43
Alterations in signaling molecules causing the acinar cells to become unresponsive to neurotransmitters and hormone action have also been proposed. These include down-regulation of cell surface receptors,44,45 down-regulation of intracellular signaling effector molecules such protein kinase C,46 or defective cellular trafficking of the water channel aquaporin 5.47 In contrast, other studies showed an up-regulation of M3 muscarinic receptors and their signaling pathways in both human and animal models.48,49 The latter findings led to the hypothesis that the supersensitivity observed in diseased lacrimal glands to exogenous stimulation might be due to the inability of their nerves to release their neurotransmitters, which make the gland behave as if denervated. This hypothesis was later verified in animal studies showing that, indeed, diseased lacrimal gland nerves are not able to release their neurotransmitters.50 Such findings have not yet been confirmed in human tissues.
Increased production of proinflammatory cytokines is very common in all inflammatory diseases of the lacrimal gland. Several studies have shown that the amount of proinflammatory cytokines is increased in lacrimal glands from patients with Sjögren’s syndrome, GVHD, or sarcoidosis, and from aging individuals.51–53 Furthermore, increased amounts of proinflammatory cytokines have been described in the tears of Sjögren’s syndrome patients.54–56 Studies in animal models have shown that proinflammatory cytokines inhibit neurotransmitter release leading to insufficient lacrimal gland secretion.57
III. Overview of Lacrimal Gland Development
Remodeling of tissues following injury or trauma usually recapitulate the same cellular events that govern embryonic tissue development. It is therefore not surprising that programmed cell death and a number of growth factors and cytokines known to regulate tissue development were found to also play a role during tissue regeneration. Furthermore, progenitor/stem cells play a pivotal role in tissue repair following injury.
A. Branching Morphogenesis
Development of the lacrimal gland occurs through a process known as branching morphogenesis.58–60 This process of morphogenesis is achieved through epithelial-mesenchymal interactions that include a highly coordinated spatio-temporal release of several growth factors and subsequent activation of critical transcription factors.58,59 In the mouse, lacrimal gland development begins at embryonic age E13.5, when the primary bud invaginates from the conjunctival epithelium at the temporal aspect of the eye.60,61 The primary bud then extends as a tubular sinus into the periorbital, neural crest-derived mesenchyme. Between E15.5 and E16.5, the tubular invagination of the lacrimal gland extends and branches in two locations to form a major extraorbital lobe and a minor intraorbital one. In humans, the lacrimal gland develops from the ectoderm of the superior conjunctival fornix when embryos are 22–24 mm crown-rump in length.62 The human lacrimal gland is made up of two lobes, palpebral and orbital, which are formed through branching and proliferation of buds originating from the conjunctival fornix.62 The orbital lobe originates from five to six epithelial buds, and its formation concludes around the second month of embryonic development.62 Subsequently, other epithelial buds initiate the formation of the palpebral lobe.62
Several growth and transcription factors have been shown to be crucial for branching morphogenesis of the lacrimal gland.63–67 In particular, fibroblast growth factor 10 and bone morphogenetic protein 7 have been shown to play pivotal roles in lacrimal gland development.64–65,67
B. Role of Fibroblast Growth Factors
During embryonic development, fibroblast growth factors (FGFs) play a critical role in morphogenesis by regulating cell proliferation, differentiation, and cell migration.68,69 In the adult organism, FGFs play an important role in the control of the nervous system, in tissue repair, wound healing, and in tumor angiogenesis.68,69 There are presently 23 members of the FGF family that signal through binding to four FGF receptors (FGFRs) designated FGFR1-FGFR4.68,69 FGFRs are single-pass transmembrane proteins with tyrosine kinase activity (Figure 3). Ligand binding to the extracellular domain of the receptor initiates a signal transduction cascade that ultimately results in modification of gene expression.68,69 The extracellular ligand-binding domain of FGFR is composed of three immunoglobulin (Ig)-like domains, designated D1–D3 (Figure 3). A hallmark of the FGFR family is that a variety of FGFR isoforms are generated by alternative splicing of FGFR transcripts.68,69
Figure 3.
Schematic diagram of the FGFR and listing of ligand specificity for FGFR isoforms. FGF7 and 10 are considered to be mesenchymally expressed, and FGF 2, 4, 6, 8, 9 and 17 are considered to be expressed in epithelia.
In the developing lacrimal gland, FGF10 is expressed in the mesenchyme and promotes the proliferation of the epithelium.64,67,70 In FGF10-null mice embryos, the epithelial component of the lacrimal gland is completely absent, although the mesenchyme remains.64 Exogenous addition of FGF10 can induce ectopic lacrimal bud formation in explant cultures, and this induction can be blocked with inhibitors of FGFR2IIIb.64 Similarly, lens-specific expression of FGF10 induces ectopic lacrimal gland within the cornea.67
Lacrimo-auriculo-dento-digital (LADD) syndrome, also known as Levy-Hollister syndrome, is a rare autosomal-dominant disorder.71 It is characterized by pronounced dysplasias in various organ systems.71 The resulting disturbances affect primarily the lacrimal glands, the inner and outer ear, and the salivary glands.71 Aplasia of the lacrimal and salivary gland (ALSG) syndrome is another autosomal-dominant disorder that is characterized by xerophthalmia (keratoconjunctivitis sicca, commonly referred to as dry eye) and xerostomia (dry mouth). Recent genetic studies have linked mutations in the FGF10 and FGFR2 genes to aplasia of the lacrimal gland in both LADD and ASLG, further establishing FGF10 as a crucial growth factor for the lacrimal gland.72–74
Of relevance to inflammation and tissue repair, it has been shown that exogenous delivery of FGF10 accelerated tissue repair in the salivary glands.75 Similarly, FGF10 and its receptor FGFR2IIIb were found to play critical roles in pancreatic development and regeneration.76,77 The role of FGF in repair of the lacrimal gland has not been investigated yet.
C. Role of Bone Morphogenetic Proteins
Bone morphogenetic proteins (BMPs) were originally identified by their ability to cause bone differentiation.78 BMPs are members of the transforming growth factor-beta (TGF-[BETA]β) superfamily. More than 20 BMP-related proteins have been identified, and have been subdivided into several groups based on their structures and functions.79,80 Like other members of the TGF-[BETA]β family, BMPs elicit their effects through activation of different receptor complexes, each composed of two type I and two type II serine/threonine kinase receptors (Figure 4). Seven type I receptors called activin receptor-like kinases (ALKs) have been discovered in mammals.79,80 ALK4 and ALK5 are receptors for activin and TGF-[BETA]β, respectively, whereas ALK2, ALK3 and ALK6 are receptors for BMPs.79,80 The serine/threonine kinase domains of type II receptors are constitutively active and, upon ligand binding, which induces a conformational change, phosphorylate specific residues in the juxtamembrane domains of type I receptors, leading to their activation (Figure 4). The activated type I receptors then propagate the signal by phosphorylating a family of transcription factors, called receptor-activated Smads (R-Smads).79,80 BMP receptors activate Smad1, Smad5 and Smad8, whereas Smad2 and Smad3 are phosphorylated by the activin and the TGF-[BETA]β receptors. In addition to R-Smads, two other types of Smads have been identified, the common mediator Smad (co-Smad: Smad4) and the inhibitory Smads (I-Smads: Smad6 and Smad7). Activated R-Smads assemble into a heteromeric complex with Smad4 in the cytoplasm, which translocates to the nucleus to modulate the expression of target genes (Figure 4).79,80
Figure 4.
Schematic diagram of signaling through BMP receptors. Upon binding of BMP7, type II receptor phosphorylate type I receptors that, in turn, phosphorylate Smads1, 5, and 8. Phosphorylated Smads1/5/8 associate with Smad4 and translocate to the nucleus where, along with cofactors, initiate the transcription of BMP response genes.
In the developing lacrimal gland, BMP7 is expressed in both the epithelium and mesenchymal components.65 However, BMP7 acts primarily on mesenchymal cells, where it stimulates cell division and formation of mesenchymal condensations.65 Exogenous addition of BMP7 to mesenchymal cells induces the formation of cell contacts through increased expression of connexins 43 and cadherins and leads to upregulation of [ALPHA]α-smooth muscle actin that forms well organized stress fibers.65 BMP7-null mice have reduced lacrimal gland size and branching,81 and inhibition of BMP activity with noggin or follistatin (two naturally occurring antagonists of BMPs) was shown to have similar consequences when used in lacrimal gland explant cultures.65
Of relevance to inflammation and tissue repair, BMP7 has been shown to ameliorate the course of injury through a variety of mechanisms, including inhibition of apoptosis, prevention of infarction and cell necrosis, and downregulation of intercellular adhesion molecule expression, thus reducing the accumulation of neutrophils and tissue myeloperoxidase activity.82,83 BMP7 was also shown to inhibit production of the proinflammatory cytokines interleukin (IL)-1 and tumor necrosis factor-alpha (TNF-[ALPHA]α).82,8382–83 It was recently reported that concomitant with lacrimal gland repair, the BMP7 signaling pathway was upregulated.84 However, further studies are needed to investigate the target cells affected by BMP7 during repair of the lacrimal gland.
IV. Injury and Repair of the Lacrimal Gland
A large body of literature exists on the regenerative capacity of the salivary glands. Long-term (7–21 days) ligation of the main excretory ducts of salivary glands leads to atrophy of these glands.85–89 Salivary glands with ligated ducts show signs of inflammation, edema, and death of the acinar cells.85–89 When the duct ligation is released, the salivary glands go through a process of repair through proliferation of acinar, ductal, and/or myoepithelial cells.86,88,90–94 Similarly, studies on the pancreas,95–97 liver,98,99 intestine,100 and mammary glands101 reported self-regenerating capabilities of these tissues. As for the salivary glands, these tissues go through a phase of inflammation/destruction of parenchymal cells followed by a period of proliferation/tissue repair.
Although similar studies on the lacrimal gland are still lacking, it was recently reported that murine lacrimal gland is capable of repair following experimentally induced injury.84,102 In the following sections, the potential roles of programmed cell death and stem cells in tissue repair will be discussed.
A. Role of Apoptosis in Tissue Repair
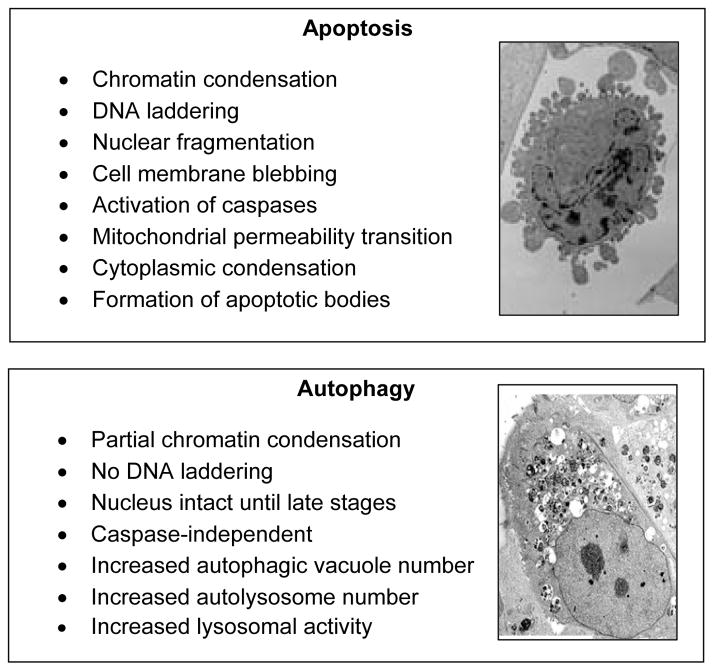
Apoptosis, a form of programmed cell death, is crucial during embryonic development and is equally important to the maintenance of normal cellular homeostasis in adult organs.103 It is worth noting that under normal physiological conditions, the occurrence of apoptosis in tissues is typically a rare event, but apoptotic cells become readily identifiable under pathophysiological conditions, such as inflammation.103 The biochemical hallmark of apoptosis is degradation of DNA by endogenous DNases, which cut the internucleosomal regions into double-stranded DNA fragments of 180–200 base pairs.104 These fragments are detectable as a ladder pattern in electrophoresis of isolated DNA.105 However, they are most commonly detected, in situ, using the terminal transferase mediated DNA nick end labeling (TUNEL) assay.106 Other biochemical hallmarks of apoptosis involve the downregulation of anti-apoptotic molecules (such as Bcl2), upregulation of pro-apoptotic molecules (such as Bax), and activation of several caspases (Figure 5).103,104,107
Figure 5.
Similarities and differences between apoptosis and autophagy. The electron micrographs depict the cellular changes that occur during apoptosis and autophagy, including membrane blebbing and accumulation of autophagic vacuoles.
The role of the tumor suppressor p53 protein in apoptosis is well documented.108,109 p53 signals cell apoptosis byenhancing transcription of pro-apoptotic genes (such as Bax), but it has also been shown to potentiate the apoptotic response through transcriptionally independent activities.109,110 Indeed, p53 binds directly to the anti-apoptotic Bcl2 proteins and activates pro-apoptotic proteins (such as Bax) to induce apoptosis.110 (AU: check changes in previous sentence.)(Changes are OK) p53-null mice appear to develop normally, but are susceptible to spontaneous tumor formation.111 Several studies have shown that p53-null mice have defective apoptotic responses.112,113
The role of apoptosis in tissue atrophy/repair is well documented.85,87,93,114 In fact, it has been demonstrated that apoptosis of the pancreatic acinar cells is necessary for induction of cell proliferation in the regenerating tissues.95 Indeed, Scoggins et al showed that in cells from duct-ligated pancreas of p53-null mice, when compared to wild type mice, not only did apoptosis not occur, but the associated proliferative response was inhibited.95
Apoptosis has been proposed as a possible mechanism responsible for the impairment of lacrimal gland secretory function associated with Sjogren syndrome.115,117 However, other studies found no correlation between apoptosis and the extent of glandular dysfunction.118,119 It is worth noting that the pathophysiological mechanisms discussed in section II.B can account for glandular dysfunction in the absence of apoptosis. It was reported recently that experimentally induced lacrimal gland inflammation led to increased apoptosis of the acinar cells,84 and it was hypothesized that apoptosis is necessary for initiation of lacrimal gland repair.
As discussed above, occurrence of apoptosis in inflammatory diseases of the human lacrimal gland is still controversial. If the hypothesis that apoptosis of the acinar cells is the driving force for lacrimal gland repair, through induction of cell proliferation, then maybe the lack of apoptosis in diseased human lacrimal glands is responsible for lack of repair and conversely, inducing apoptosis might trigger repair. This might sound counterintuitive, but in recent studies in type 1 diabetes that leads to loss of insulin secreting [BETA]β-cells, it was proposed that therapy based on increasing TNF-[ALPHA]α production to induce apoptosis, rather than neutralizing TNF-[ALPHA]α, might lead to increased [BETA]β-cell mass.120–122 Further studies are clearly needed to test this intriguing hypothesis in the lacrimal gland.
B. Role of Autophagy in Tissue Repair
Another form of cell death has also been implicated in tissue repair. Autophagic cell death (also called type II cell death, with apoptosis being type I and necrosis type III) is characterized by the appearance of double- or multiple-membrane delimited cytoplasmic vesicles (autophagic vesicles or vacuoles) engulfing bulk cytoplasm and/or cytoplasmic organelles, such as mitochondria and endoplasmic reticulum (Figure 5).123,124 Autophagic vesicles are then destroyed by the cellular lysosomal system.125 Apoptosis and autophagic cell death are not mutually exclusive phenomena; they may occur simultaneously in tissues or even conjointly in the same cell.123–125
Autophagy is an evolutionarily conserved and dynamic process in which cytoplasmic components are sequestered and delivered to the lysosome for degradation and recycling.123,124 Autophagy starts with the formation of a double membrane-delimited autophagic vacuole (also called autophagosome), which engulfs bulk cytoplasm and/or cytoplasmic organelles such as mitochondria and endoplasmic reticulum.124 In mammalian cells, autophagosomes undergo a maturation process by fusing with endocytic compartments and lysosomes.124
Recently, it was reported that experimentally induced inflammation of the lacrimal gland leads to increased autophagy.84 In this model, injection of IL-1 into the exorbital lacrimal glands results in decreased tear production, inflammation of the lacrimal gland, decreased stimulated protein secretion, increased cell death, and recruitment of mesenchymal stem cells.84,102 All of these pathological changes were transient (1–3 day post-injection) and resolved when lacrimal gland repair occurred (4–7 days post-injection).84,102 As can be seen in Figure 6, compared to saline injected glands, acinar cells from IL-1-injected glands lost their polarity and the cytoplasm contained fewer secretory granules (SGs) and instead was filled with vacuoles (V). Some of the vacuoles contained fragmented cellular structures and were double-membrane-delimited, a structural characteristic of autophagic vacuoles (Figure 6, inset). The electron-dense structures visible in Figure 6 might represent different stages from autophagosomes to lysosomes. This interpretation of the images was further substantiated by showing that the amounts of the lysosomal protein LAMP-1 and the autophagosome marker MAP LC3 were increased in IL-1 injected glands.84 As with apoptosis, it remains to be investigated whether inhibition of autophagy will delay or inhibit lacrimal gland repair following experimentally induced injury.
Figure 6.
Autophagic cell death in injured lacrimal glands. Lacrimal glands from saline (control) and IL-1 injected animals were processed for transmission electron microscopy. In control lacrimal glands, the acinar cells are highly polarized with basally located nuclei (N); they have a very dense, rough endoplasmic reticulum (ER) network, and the cytoplasm is filled with secretory granules (SG). In contrast, in IL-1-injected lacrimal glands, the acinar cells have lost their polarity, the ER network is disorganized, and the cytoplasm is filled with vacuoles (V), some of which are double-membrane-delimited (inset). Arrows point to potential autophagosomes or lysosomes.
C. Stem Cells Versus Transdifferentiation in Tissue Repair
The presence of stem cells in adult tissues is obviously an area of intense research because of their potential clinical benefits. In the field of tissue repair, there are two schools of thought: one that advocates repair through expansion of stem/progenitor cells and another that advocates the transdifferentiation of existing cells.126–128 Several studies on the salivary glands and the pancreas have shown that stem/progenitor cells are present in these tissues and are involved in regeneration of these tissues.128–133 However, a major technical difficulty has been the inability to distinguish between the expansion of stem/progenitor cells and transdifferentiation during tissue repair.128 Using genetic marking for lineage tracing with the insulin promoter, Dor et al showed that during regeneration of the pancreas, new [BETA]β-cells could be generated by replication of existing [BETA]β--cells, arguing against the involvement of stem/progenitor cells.134 Conversely, several investigators have isolated and expanded stem cells from the salivary glands and the pancreas.130–133 These cells are usually associated with the ductal/periductal fraction of these tissues.128,130–132 Of note, in studies on salivary stem/progenitor cells, it was reported that the number of these cells increased dramatically after tissue injury.130 Although the issue of stem/progenitor cells versus transdifferentiation is still hotly debated, it seems most likely that more than one mechanism may apply in tissue repair.126,128,129
Using antibodies against nestin, an intermediate filament protein and a well-studied and accepted marker for stem cells, Zoukhri et al recently reported that stem cells were present in uninjured lacrimal glands and that their number increased during the repair phase of IL-1 injected glands.84 Once the tissue was fully repaired, the number of nestin-positive cells returned to control level. It was also shown that a subpopulation of these cells stained positive for [ALPHA]α-smooth muscle actin, suggesting that myoepithelial cells might be the source of stem cells.84
In the salivary glands and the pancreas, it was shown that stem cells reside in the periductal areas. In Sjogren syndrome lacrimal gland, the immune cells infiltrate and form foci in the periductal areas.9 If this area of the lacrimal gland is indeed populated with stem cells, then one could speculate that these cells are lost during the immune attack, and, hence, repair of the lacrimal gland is compromised. Simplistically, it might be hypothesized that down-regulation of autoimmunity/inflammation may give the lacrimal gland the “breathing space” to regenerate acinar cells. Supplementation with factors known to induce acinar cell replication and neogenesis, such as FGF10 and BMP7, might further augment the regenerative processes. Conversely, if one could isolate and expand lacrimal gland stem cells in vitro, then these could be transplanted into diseased lacrimal glands to trigger tissue repair. This strategy could be combined with those aimed at dampening the autoimmune inflammatory milieu to restore/enhance lacrimal gland functions.
V. Summary and Conclusions
Lacrimal gland insufficiency due to inflammation is a common condition, which results in symptoms of dry eye. Despite the large number of studies to date, multiple questions remain unanswered. For example, our understanding of the molecular mechanisms responsible for the loss of secretory function is still incomplete. Although autoantibodies and proinflammatory cytokines have attracted considerable attention, it is not completely understood how these affect lacrimal gland functions. The mechanisms through which lacrimal gland acinar cells are lost during inflammation are not known. Similarly, the impact cell death has on gland functionality is not known. There is abundant literature on injury and repair of the salivary glands, but little of this knowledge has been applied to the lacrimal gland. The role of programmed cell death, cytokines, growth factors, and stem cells in lacrimal gland injury and repair are areas that deserve further investigations.
Acknowledgments
Grant support: National Institutes of Health/National Eye Institute grant RO1-EY12383.
Footnotes
The author has no proprietary or commercial interests in any concept or product discussed in this article.
References
- 1.Holly FJ. Tear film physiology. Int Ophthalmol Clin. 1987;27:2–6. doi: 10.1097/00004397-198702710-00002. [DOI] [PubMed] [Google Scholar]
- 2.Tiffany JM. The normal tear film. Dev Ophthalmol. 2008;41:1–20. doi: 10.1159/000131066. [DOI] [PubMed] [Google Scholar]
- 3.Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–77. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mircheff AK. Water and electrolyte secretion and fluid modification. In: Albert D, Jakobiec F, editors. Principles and practice of ophthalmology: basic sciences. Philadelphia: WB Saunders; 1994. pp. 466–72. [Google Scholar]
- 5.Lemullois M, Rossignol B, Mauduit P. Immunolocalization of myoepithelial cells in isolated acini of rat exorbital lacrimal gland: cellular distribution of muscarinic receptors. Biol Cell. 1996;86:175–81. doi: 10.1016/0248-4900(96)84782-4. [DOI] [PubMed] [Google Scholar]
- 6.Zoukhri D. Effect of inflammation on lacrimal gland function. Exp Eye Res. 2006;82:885–98. doi: 10.1016/j.exer.2005.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–32. [PubMed] [Google Scholar]
- 8.Stern ME, Gao J, Siemasko KF, et al. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78:409–16. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321–31. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 10.Bresnitz EA, Strom BL. Epidemiology of sarcoidosis. Epidemiol Rev. 1983;5:124–56. doi: 10.1093/oxfordjournals.epirev.a036255. [DOI] [PubMed] [Google Scholar]
- 11.Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet. 2003;361:1111–8. doi: 10.1016/S0140-6736(03)12888-7. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Casals M, Brito-Zeron P, Garcia-Carrasco M, Font J. Sarcoidosis or Sjögren’s syndrome? Clues to defining mimicry or coexistence in 59 cases. Medicine (Baltimore) 2004;83:85–95. doi: 10.1097/01.md.0000121237.98962.1e. [DOI] [PubMed] [Google Scholar]
- 13.Kansu E. The pathophysiology of chronic graft-versus-host disease. Int J Hematol. 2004;79:209–15. doi: 10.1532/ijh97.04015. [DOI] [PubMed] [Google Scholar]
- 14.Mencucci R, Rossi Ferrini C, Bosi A, et al. Ophthalmological aspects in allogenic bone marrow transplantation: Sjogren-like syndrome in graft-versus-host disease. Eur J Ophthalmol. 1997;7:13–8. doi: 10.1177/112067219700700103. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa Y, Kuwana M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Cornea. 2003;22:S19–S27. doi: 10.1097/00003226-200310001-00004. [DOI] [PubMed] [Google Scholar]
- 16.Anderson NG, Regillo C. Ocular manifestations of graft versus host disease. Curr Opin Ophthalmol. 2004;15:503–7. doi: 10.1097/01.icu.0000143684.22362.46. [DOI] [PubMed] [Google Scholar]
- 17.Schaumberg DA, Sullivan DA, Dana MR. Epidemiology of dry eye syndrome. Adv Exp Med Biol. 2002;506 (Pt B):989–98. doi: 10.1007/978-1-4615-0717-8_140. [DOI] [PubMed] [Google Scholar]
- 18.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–26. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 19.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118:1264–8. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 20.Dalzell MD. Dry eye: prevalence, utilization, and economic implications. Manag Care. 2003;12:9–13. [PubMed] [Google Scholar]
- 21.Bromberg BB, Cripps MM, Welch MH. Sympathomimetic protein secretion by young and aged lacrimal gland. Curr Eye Res. 1986;5:217–23. doi: 10.3109/02713688609020046. [DOI] [PubMed] [Google Scholar]
- 22.Draper CE, Adeghate E, Lawrence PA, et al. Age-related changes in morphology and secretory responses of male rat lacrimal gland. J Auton Nerv Syst. 1998;69:173–83. doi: 10.1016/s0165-1838(98)00026-5. [DOI] [PubMed] [Google Scholar]
- 23.Rios JD, Horikawa Y, Chen LL, et al. Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. Exp Eye Res. 2005;80:477–41. doi: 10.1016/j.exer.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benson WR. Acceleration of aging changes in the exorbital lacrimal gland of the female rat. Am J Pathol. 1964;45:587–97. [PMC free article] [PubMed] [Google Scholar]
- 25.Williams RM, Singh J, Sharkey KA. Innervation and mast cells of the rat exorbital lacrimal gland: the effects of age. J Auton Nerv Syst. 1994;47:95–108. doi: 10.1016/0165-1838(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 26.Ramos-Casals M, Garcia-Carrasco M, Brito Zeron MP, et al. Viral etiopathogenesis of Sjögren’s syndrome: role of the hepatitis C virus. Autoimmun Rev. 2002;1:238–43. doi: 10.1016/s1568-9972(02)00057-5. [DOI] [PubMed] [Google Scholar]
- 27.De Vita S, Damato R, De Marchi G, et al. True primary Sjögren’s syndrome in a subset of patients with hepatitis C infection: a model linking chronic infection to chronic sialadenitis. Isr Med Assoc J. 2002;4:1101–5. [PubMed] [Google Scholar]
- 28.DeCarlo DK, Penner SL, Schamerloh RJ, Fullard RJ. Dry eye among males infected with the human immunodeficiency virus. J Am Optom Assoc. 1995;66:533–8. [PubMed] [Google Scholar]
- 29.Chronister CL. Review of external ocular disease associated with aids and HIV infection. Optom Vis Sci. 1996;73:225–30. doi: 10.1097/00006324-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Eckstein AK, Finkenrath A, Heiligenhaus A, et al. Dry eye syndrome in thyroid-associated ophthalmopathy: lacrimal expression of TSH receptor suggests involvement of TSHR-specific autoantibodies. Acta Ophthalmol Scand. 2004;82:291–7. doi: 10.1111/j.1395-3907.2004.00268.x. [DOI] [PubMed] [Google Scholar]
- 31.Patel SJ, Lundy DC. Ocular manifestations of autoimmune disease. Am Fam Physician. 2002;66:991–8. [PubMed] [Google Scholar]
- 32.Punzi L, Betterle C. Chronic autoimmune thyroiditis and rheumatic manifestations. Joint Bone Spine. 2004;71:275–83. doi: 10.1016/j.jbspin.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Goebbels M. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol. 2000;84:19–21. doi: 10.1136/bjo.84.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmowski AM, Ruprecht KW. The cornea and systemic diseases. Curr Opin Ophthalmol. 1995;6:17–20. doi: 10.1097/00055735-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Grus F, Sabuncuo P, Dick H, et al. Changes in the tear proteins of diabetic patients. BMC Ophthalmology. 2002;2:4. doi: 10.1186/1471-2415-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reina S, Sterin-Borda L, Orman B, Borda E. Human mAChR antibodies from Sjögren’s syndrome sera increase cerebral nitric oxide synthase activity and nitric oxide synthase mRNA level. Clin Immunol. 2004;113:193–202. doi: 10.1016/j.clim.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Bacman SR, Berra A, Sterin-Borda L, Borda ES. Human primary Sjögren’s syndrome autoantibodies as mediators of nitric oxide release coupled to lacrimal gland muscarinic acetylcholine receptors. Curr Eye Res. 1998;17:1135–42. doi: 10.1076/ceyr.17.12.1135.5124. [DOI] [PubMed] [Google Scholar]
- 38.Bacman S, Perez Leiros C, Sterin-Borda L, et al. Autoantibodies against lacrimal gland M3 muscarinic acetylcholine receptors in patients with primary Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 1998;39:151–6. [PubMed] [Google Scholar]
- 39.Li J, Ha YM, Ku NY, et al. Inhibitory effects of autoantibodies on the muscarinic receptors in Sjögren’s syndrome. Lab Invest. 2004;84:1430–8. doi: 10.1038/labinvest.3700173. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen KH, Brayer J, Cha S, et al. Evidence for antimuscarinic acetylcholine receptor antibody-mediated secretory dysfunction in NOD mice. Arthritis Rheum. 2000;43:2297–2306. doi: 10.1002/1529-0131(200010)43:10<2297::AID-ANR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 41.Waterman SA, Gordon TP, Rischmueller M. Inhibitory effects of muscarinic receptor autoantibodies on parasympathetic neurotransmission in Sjögren’s syndrome. Arthritis Rheum. 2000;43:1647–54. doi: 10.1002/1529-0131(200007)43:7<1647::AID-ANR31>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 42.Cavill D, Waterman SA, Gordon TP. Antibodies raised against the second extracellular loop of the human muscarinic M3 receptor mimic functional autoantibodies in Sjögren’s syndrome. Scand J Immunol. 2004;59:261–6. doi: 10.1111/j.0300-9475.2004.01395.x. [DOI] [PubMed] [Google Scholar]
- 43.Qian L, Wang Y, Xie J, et al. Biochemical changes contributing to functional quiescence in lacrimal gland acinar cells after chronic ex vivo exposure to a muscarinic agonist. Scand J Immunol. 2003;58:550–65. doi: 10.1046/j.1365-3083.2003.01343.x. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y, Humphreys-Beher MG. Decreased levels of adenylate cyclase contribute to the down-regulation of beta-adrenergic signal transduction in the salivary glands of the non-obese diabetic (NOD) mouse. Autoimmunity. 1995;21:137–42. doi: 10.3109/08916939508993362. [DOI] [PubMed] [Google Scholar]
- 45.Hu Y, Purushotham KR, Wang P, et al. Downregulation of beta-adrenergic receptors and signal transduction response in salivary glands of NOD mice. Am J Physiol. 1994;266:G433–G443. doi: 10.1152/ajpgi.1994.266.3.G433. [DOI] [PubMed] [Google Scholar]
- 46.Törnwall J, Konttinen YT, Tuominen RK, Törnwall M. Protein kinase C expression in salivary gland acinar epithelial cells in Sjögren’s syndrome. Lancet. 1997;349:1814–5. doi: 10.1016/s0140-6736(05)61694-7. [DOI] [PubMed] [Google Scholar]
- 47.Tsubota K, Hirai S, King LS, et al. Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjögren’s syndrome. Lancet. 2001;357:688–9. doi: 10.1016/S0140-6736(00)04140-4. [DOI] [PubMed] [Google Scholar]
- 48.Beroukas D, Goodfellow R, Hiscock J, et al. Up-regulation of M3-muscarinic receptors in labial salivary gland acini in primary Sjögren’s syndrome. Lab Invest. 2002;82:203–10. doi: 10.1038/labinvest.3780412. [DOI] [PubMed] [Google Scholar]
- 49.Zoukhri D, Hodges RR, Rawe IM, Dartt DA. Ca2+ signaling by cholinergic and 1-adrenergic agonists is up-regulated in lacrimal and submandibular glands in a murine model of Sjögren’s syndrome. Clin Immunol Immunopathol. 1998;89:134–40. doi: 10.1006/clin.1998.4598. [DOI] [PubMed] [Google Scholar]
- 50.Zoukhri D, Kublin CL. Impaired neurotransmitter release from lacrimal and salivary gland nerves of a murine model of Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 2001;42:925–32. [PMC free article] [PubMed] [Google Scholar]
- 51.Agostini C, Meneghin A, Semenzato G. T-lymphocytes and cytokines in sarcoidosis. Curr Opin Pulm Med. 2002;8:435–40. doi: 10.1097/00063198-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Antin JH, Ferrara JLM. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–8. [PubMed] [Google Scholar]
- 53.Baughman RP, Iannuzzi M. Tumour necrosis factor in sarcoidosis and its potential for targeted therapy. BioDrugs. 2003;17:425–31. doi: 10.2165/00063030-200317060-00005. [DOI] [PubMed] [Google Scholar]
- 54.Tishler M, Yaron I, Geyer O, et al. Elevated tear interleukin-6 levels in patients with Sjogren syndrome. Ophthalmology. 1998;105:2327–9. doi: 10.1016/S0161-6420(98)91236-2. [DOI] [PubMed] [Google Scholar]
- 55.Pflugfelder SC, Jones D, Ji Z, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–11. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 56.Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–92. [PubMed] [Google Scholar]
- 57.Zoukhri D, Hodges RR, Byon D, Kublin CL. Role of proinflammatory cytokines in the impaired lacrimation associated with autoimmune xerophthalmia. Invest Ophthalmol Vis Sci. 2002;43:1429–36. [PMC free article] [PubMed] [Google Scholar]
- 58.Hogan BL. Morphogenesis. Cell. 1999;96:225–33. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 59.Hogan BL, Yingling JM. Epithelial/mesenchymal interactions and branching morphogenesis of the lung. Curr Opin Genet Dev. 1998;8:481–6. doi: 10.1016/s0959-437x(98)80121-4. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Laurie GW. Organogenesis of the exocrine gland. Dev Biol. 2004;273:1–22. doi: 10.1016/j.ydbio.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 61.Kammandel B, Chowdhury K, Stoykova A, et al. Distinct cis-essential modules direct the time-space pattern of the Pax6 gene activity. Dev Biol. 1999;205:79–97. doi: 10.1006/dbio.1998.9128. [DOI] [PubMed] [Google Scholar]
- 62.de la Cuadra-Blanco C, Peces-Pena MD, Merida-Velasco JR. Morphogenesis of the human lacrimal gland. J Anat. 2003;203:531–6. doi: 10.1046/j.1469-7580.2003.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mattiske D, Sommer P, Kidson SH, Hogan BL. The role of the forkhead transcription factor, Foxc1, in the development of the mouse lacrimal gland. Dev Dyn. 2006;235:1074–80. doi: 10.1002/dvdy.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Makarenkova HP, Ito M, Govindarajan V, et al. FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development. 2000;127:2563–72. doi: 10.1242/dev.127.12.2563. [DOI] [PubMed] [Google Scholar]
- 65.Dean C, Ito M, Makarenkova HP, et al. Bmp7 regulates branching morphogenesis of the lacrimal gland by promoting mesenchymal proliferation and condensation. Development. 2004;131:4155–65. doi: 10.1242/dev.01285. [DOI] [PubMed] [Google Scholar]
- 66.Dean CH, Miller LA, Smith AN, et al. Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev Biol. 2005;286:270–86. doi: 10.1016/j.ydbio.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 67.Govindarajan V, Ito M, Makarenkova HP, et al. Endogenous and ectopic gland induction by FGF-10. Dev Biol. 2000;225:188–200. doi: 10.1006/dbio.2000.9812. [DOI] [PubMed] [Google Scholar]
- 68.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–49. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 69.Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–47. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 70.Pan Y, Carbe C, Powers A, et al. Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development. 2008;135:301–10. doi: 10.1242/dev.014829. [DOI] [PubMed] [Google Scholar]
- 71.Murdoch-Kinch CA, Miles DA. Clinical and radiographic features of the lacrimo-auriculo-dento-digital syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:727–35. doi: 10.1016/s1079-2104(96)80080-1. [DOI] [PubMed] [Google Scholar]
- 72.Milunsky J, Zhao G, Maher T, et al. LADD syndrome is caused by FGF10 mutations. Clin Genet. 2006;69:349–54. doi: 10.1111/j.1399-0004.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 73.Rohmann E, Brunner HG, Kayserili H, et al. Mutations in different components of FGF signaling in LADD syndrome. Nat Genet. 2006;38:414–7. doi: 10.1038/ng1757. [DOI] [PubMed] [Google Scholar]
- 74.Entesarian M, Matsson H, Klar J, et al. Mutations in the gene encoding fibroblast growth factor 10 are associated with aplasia of lacrimal and salivary glands. Nat Genet. 2005;37:125–7. doi: 10.1038/ng1507. [DOI] [PubMed] [Google Scholar]
- 75.Okazaki Y, Kagami H, Hattori T, et al. Acceleration of rat salivary gland tissue repair by basic fibroblast growth factor. Arch Oral Biol. 2000;45:911–9. doi: 10.1016/s0003-9969(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 76.Pulkkinen M-A, Spencer-Dene B, Dickson C, Otonkoski T. The IIIb isoform of fibroblast growth factor receptor 2 is required for proper growth and branching of pancreatic ductal epithelium but not for differentiation of exocrine or endocrine cells. Mech Dev. 2003;120:167–75. doi: 10.1016/s0925-4773(02)00440-9. [DOI] [PubMed] [Google Scholar]
- 77.Miralles F, Lamotte L, Couton D, Joshi RL. Interplay between FGF10 and Notch signalling is required for the self-renewal of pancreatic progenitors. Int J Dev Biol. 2006;50:17–26. doi: 10.1387/ijdb.052080fm. [DOI] [PubMed] [Google Scholar]
- 78.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 79.Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–78. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 80.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–63. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 81.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 82.Yanagita M. BMP antagonists: their roles in development and involvement in pathophysiology. Cytokine Growth Factor Rev. 2005;16:309–17. doi: 10.1016/j.cytogfr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 83.Simic P, Vukicevic S. Bone morphogenetic proteins in development and homeostasis of kidney. Cytokine Growth Factor Rev. 2005;16:299–308. doi: 10.1016/j.cytogfr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 84.Zoukhri D, Fix A, Alroy J, Kublin CL. Mechanisms of murine lacrimal gland repair after experimentally induced inflammation. Invest Ophthalmol Vis Sci. 2008;49:4399–4406. doi: 10.1167/iovs.08-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takahashi S, Shinzato K, Nakamura S, et al. The roles of apoptosis and mitosis in atrophy of the rat sublingual gland. Tissue Cell. 2002;34:297–304. doi: 10.1016/s0040816602000034. [DOI] [PubMed] [Google Scholar]
- 86.Takahashi S, Shinzato K, Domon T, et al. Mitotic proliferation of myoepithelial cells during regeneration of atrophied rat submandibular glands after duct ligation. J Oral Pathol Med. 2004;33:430–4. doi: 10.1111/j.1600-0714.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- 87.Takahashi S, Nakamura S, Suzuki R, et al. Apoptosis and mitosis of parenchymal cells in the duct-ligated rat submandibular gland. Tissue Cell. 2000;32:457–63. doi: 10.1016/s0040-8166(00)80002-6. [DOI] [PubMed] [Google Scholar]
- 88.Burgess KL, Dardick I, Cummins MM, et al. Myoepithelial cells actively proliferate during atrophy of rat parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:674–80. doi: 10.1016/s1079-2104(96)80443-4. [DOI] [PubMed] [Google Scholar]
- 89.Cummins M, Dardick I, Brown D, Burford-Mason A. Obstructive sialadenitis: a rat model. J Otolaryngol. 1994;23:50–6. [PubMed] [Google Scholar]
- 90.Burford-Mason AP, Cummins MM, Brown DH, et al. Immunohistochemical analysis of the proliferative capacity of duct and acinar cells during ligation-induced atrophy and subsequent regeneration of rat parotid gland. J Oral Pathol Med. 1993;22:440–6. doi: 10.1111/j.1600-0714.1993.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 91.Dardick I, Byard RW, Carnegie JA. A review of the proliferative capacity of major salivary glands and the relationship to current concepts of neoplasia in salivary glands. Oral Surg Oral Med Oral Pathol. 1990;69:53–67. doi: 10.1016/0030-4220(90)90269-x. [DOI] [PubMed] [Google Scholar]
- 92.Takahashi S, Domon T, Yamamoto T, Wakita M. Regeneration of myoepithelial cells in rat submandibular glands after yttrium aluminium garnett laser irradiation. Int J Exp Pathol. 1997;78:91–9. doi: 10.1046/j.1365-2613.1997.d01-244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takahashi S, Nakamura S, Shinzato K, et al. Apoptosis and proliferation of myoepithelial cells in atrophic rat submandibular glands. J Histochem Cytochem. 2001;49:1557–64. doi: 10.1177/002215540104901209. [DOI] [PubMed] [Google Scholar]
- 94.Takahashi S, Shinzato K, Domon T, et al. Proliferation and distribution of myoepithelial cells during atrophy of the rat sublingual gland. J Oral Pathol Med. 2003;32:90–4. doi: 10.1034/j.1600-0714.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 95.Scoggins CR, Meszoely IM, Wada M, et al. p53-dependent acinar cell apoptosis triggers epithelial proliferation in duct-ligated murine pancreas. Am J Physiol Gastrointest Liver Physiol. 2000;279:G827–G836. doi: 10.1152/ajpgi.2000.279.4.G827. [DOI] [PubMed] [Google Scholar]
- 96.Walker NI. Ultrastructure of the rat pancreas after experimental duct ligation. I. The role of apoptosis and intraepithelial macrophages in acinar cell deletion. Am J Pathol. 1987;126:439–51. [PMC free article] [PubMed] [Google Scholar]
- 97.Walker NI, Winterford CM, Kerr JF. Ultrastructure of the rat pancreas after experimental duct ligation. II. Duct and stromal cell proliferation, differentiation, and deletion. Pancreas. 1992;7:420–34. doi: 10.1097/00006676-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 98.Kurosawa H, Que FG, Roberts LR, et al. Hepatocytes in the bile duct-ligated rat express Bcl-2. Am J Physiol. 1997;272:G1587–1593. doi: 10.1152/ajpgi.1997.272.6.G1587. [DOI] [PubMed] [Google Scholar]
- 99.Giannelli G, Quaranta V, Antonaci S. Tissue remodelling in liver diseases. Histol Histopathol. 2003;18:1267–74. doi: 10.14670/HH-18.1267. [DOI] [PubMed] [Google Scholar]
- 100.Alison MR, Sarraf CE. The role of growth factors in gastrointestinal cell proliferation. Cell Biol Int. 1994;18:1–10. doi: 10.1006/cbir.1994.1001. [DOI] [PubMed] [Google Scholar]
- 101.Walker NI, Bennett RE, Kerr JF. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am J Anat. 1989;185:19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- 102.Zoukhri D, Macari E, Kublin CL. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp Eye Res. 2007;84:894–904. doi: 10.1016/j.exer.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 104.Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45:528–37. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 105.Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–6. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 106.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 108.Chipuk JE, Green DR. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006;13:994–1002. doi: 10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]
- 109.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 110.Schuler M, Green DR. Transcription, apoptosis and p53: catch-22. Trends Genet. 2005;21:182–7. doi: 10.1016/j.tig.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 111.Donehower LA, Harvey M, Slagle BL, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. 1992;356:215–21. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 112.Botchkarev VA, Komarova EA, Siebenhaar F, et al. p53 involvement in the control of murine hair follicle regression. Am J Pathol. 2001;158:1913–9. doi: 10.1016/S0002-9440(10)64659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dumble ML, Knight B, Quail EA, Yeoh GCT. Hepatoblast-like cells populate the adult p53 knockout mouse liver: evidence for a hyperproliferative maturation-arrested stem cell compartment. Cell Growth Differ. 2001;12:223–31. [PubMed] [Google Scholar]
- 114.Takahashi S, Nakamura S, Domon T, et al. Active participation of apoptosis and mitosis in sublingual gland regeneration of the rat following release from duct ligation. J Mol Histol. 2005;36:199–205. doi: 10.1007/s10735-005-1764-6. [DOI] [PubMed] [Google Scholar]
- 115.Bolstad AI, Jonsson R. The role of apoptosis in Sjögren’s syndrome. Ann Med Interne (Paris) 1998;149:25–9. [PubMed] [Google Scholar]
- 116.Humphreys-Beher MG, Peck AB, Dang H, Talal N. The role of apoptosis in the initiation of the autoimmune response in Sjögren’s syndrome. Clin Exp Immunol. 1999;116:383–7. doi: 10.1046/j.1365-2249.1999.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ishimaru N, Yoneda T, Saegusa K, et al. Severe destructive autoimmune lesions with aging in murine Sjögren’s syndrome through Fas-mediated apoptosis. Am J Pathol. 2000;156:1557–64. doi: 10.1016/S0002-9440(10)65027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohlsson M, Skarstein K, Bolstad AI, et al. Fas-induced apoptosis is a rare event in Sjögren’s syndrome. Lab Invest. 2001;81:95–105. doi: 10.1038/labinvest.3780215. [DOI] [PubMed] [Google Scholar]
- 119.Dawson LJ, Fox PC, Smith PM. Sjogrens syndrome--the non-apoptotic model of glandular hypofunction. Rheumatology (Oxford) 2006;45:792–8. doi: 10.1093/rheumatology/kel067. [DOI] [PubMed] [Google Scholar]
- 120.Kuhtreiber WM, Hayashi T, Dale EA, Faustman DL. Central role of defective apoptosis in autoimmunity. J Mol Endocrinol. 2003;31:373–99. doi: 10.1677/jme.0.0310373. [DOI] [PubMed] [Google Scholar]
- 121.Hayashi T, Faustman DL. Role of defective apoptosis in type 1 diabetes and other autoimmune diseases. Recent Prog Horm Res. 2003;58:131–53. doi: 10.1210/rp.58.1.131. [DOI] [PubMed] [Google Scholar]
- 122.Kodama S, Davis M, Faustman DL. The therapeutic potential of tumor necrosis factor for autoimmune disease: a mechanistically based hypothesis. Cell Mol Life Sci. 2005;62:1850–62. doi: 10.1007/s00018-005-5022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–9. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 124.Galluzzi L, Maiuri MC, Vitale I, et al. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–43. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 125.Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001;8:569–81. doi: 10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- 126.Dor Y, Melton DA. How important are adult stem cells for tissue maintenance? Cell Cycle. 2004;3:1104–6. [PubMed] [Google Scholar]
- 127.Parenteau NL, Rosenberg L, Hardin-Young J. The engineering of tissues using progenitor cells. Curr Top Dev Biol. 2004;64:101–39. doi: 10.1016/S0070-2153(04)64006-3. [DOI] [PubMed] [Google Scholar]
- 128.Kume S. Stem-cell-based approaches for regenerative medicine. Dev Growth Differ. 2005;47:393–402. doi: 10.1111/j.1440-169X.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- 129.Holland AM, Gonez LJ, Harrison LC. Progenitor cells in the adult pancreas. Diabetes Metab Res Rev. 2004;20:13–27. doi: 10.1002/dmrr.430. [DOI] [PubMed] [Google Scholar]
- 130.Hisatomi Y, Okumura K, Nakamura K, et al. Flow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic lineages. Hepatology. 2004;39:667–75. doi: 10.1002/hep.20063. [DOI] [PubMed] [Google Scholar]
- 131.Okumura K, Nakamura K, Hisatomi Y, et al. Salivary gland progenitor cells induced by duct ligation differentiate into hepatic and pancreatic lineages. Hepatology. 2003;38:104–13. doi: 10.1053/jhep.2003.50259. [DOI] [PubMed] [Google Scholar]
- 132.Kishi T, Takao T, Fujita K, Taniguchi H. Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochem Biophys Res Commun. 2006;340:544–52. doi: 10.1016/j.bbrc.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 133.Zhang YQ, Kritzik M, Sarvetnick N. Identification and expansion of pancreatic stem/progenitor cells. J Cell Mol Med. 2005;9:331–44. doi: 10.1111/j.1582-4934.2005.tb00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–6. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]