Abstract
Ca2+ is a dynamic cellular secondary messenger which mediates a vast array of cellular responses. Control over these processes is achieved via an extensive combination of pumps and channels which regulate the concentration of Ca2+ within not only the cytosol but also all intracellular compartments. Precisely how these pumps and channels are regulated is only partially understood, however, recent investigations have identified members of the Early Growth Response (EGR) family of zinc finger transcription factors as critical players in this process. The roles of several other transcription factors in control of Ca2+ homeostasis have also been demonstrated, including Wilms Tumor Suppressor 1 (WT1), Nuclear Factor of Activated T cells (NFAT) and c-myc. In this review, we will discuss not only how these transcription factors regulate the expression of the major proteins involved in control of Ca2+ homeostasis, but also how this transcriptional remodeling of Ca2+ homeostasis affects Ca2+ dynamics and cellular responses.
Control of Ca2+ homeostasis
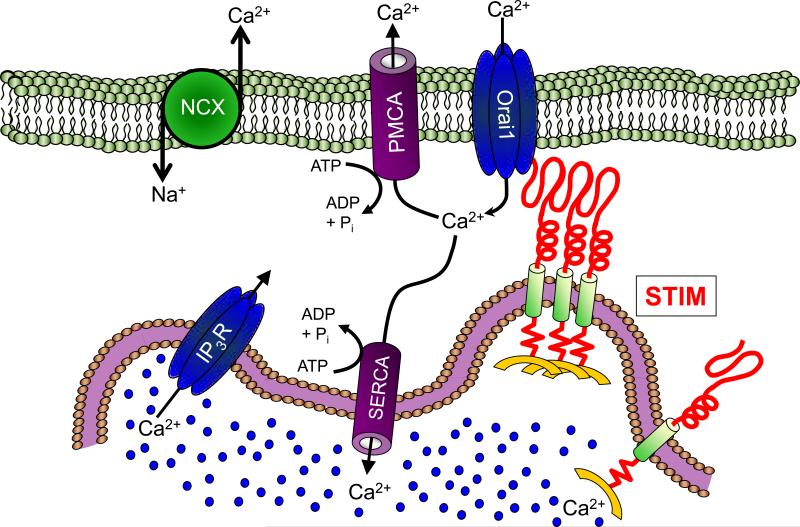
Cytosolic Ca2+ concentration is maintained at a level ~4 orders of magnitude lower than either the extracellular space or the lumen of the endoplasmic reticulum (ER). This remarkably high Ca2+ gradient is maintained via the near constant activity of several highly conserved families of pumps and exchangers located both on the plasma membrane (plasma membrane Ca2+/ATPase; PMCA and the sodium-Ca2+ exchanger; NCX) and the ER (Sarco/endoplasmic reticulum Ca2+/ATPase; SERCA) (Fig 1). The activity of these pumps and exchangers serves to both clear cytosolic Ca2+ and, in the case of SERCA, also to maintain ER Ca2+ concentration at or near 500 μM. This function is, at least in part, assisted by the store-operated Ca2+ entry (SOCe) [1-3]. The ER Ca2+ sensors and activators of SOCe are STIM1 and STIM2, two type 1A transmembrane proteins containing low Ca2+ affinity luminal EF hands [4]; when ER Ca2+ content is high, their EF hands are bound to Ca2+ and the proteins are inactive [5-7]. Decreases in ER Ca2+ concentration cause dissociation of Ca2+ from the STIM EF hands, resulting in a conformational change [7-9] that leads to STIM aggregation in regions of the ER that are adjacent to the PM [10-12], where they interact with and activate the highly Ca2+-selective channel Orai1 [10, 11, 13-20] and, perhaps, members of the TRPC family of cation channels [21-26], although the latter relationship remains a topic of much debate [27-31].
Figure 1. Model depicting control of Ca2+ homeostasis.
The major proteins involved in control of Ca2+ homeostasis are depicted. Resting cytosolic Ca2+ concentration is maintained primarily by the combined action of the sodium/calcium exchanger (NCX) the plasma membrane Ca2+/ATPase (PMCA) and the sarco/endoplasmic calcium ATPase (SERCA). The SERCA pump also serves as the primary regulator of the Ca2+ concentration in the endoplasmic reticulum (ER). Activation of the inositol 1,4,5-triphosphate receptor (IP3R) results in release of Ca2+ from the ER into the cytosol. This depletion of ER Ca2+ content is sensed by STIM proteins, which aggregate near the plasma membrane (PM) to interact with Orai1, thereby initiating store-operated Ca2+ entry.
Recent investigations have revealed that, although constitutively expressed, the level of expression of proteins involved in Ca2+ homeostasis is modulated at the transcriptional level, with significant implications to the nature of Ca2+ signaling. The current review focuses precisely on this type of transcriptional control, with an emphasis on the roles of members of the Early Growth Response (EGR) family of zinc finger transcription factors and the closely related protein Wilms Tumor Suppressor 1 (WT1).
The zinc finger transcription factors EGR1 and WT1
The Early Growth Response (EGR) family of transcription factors consists of 4 closely related members (EGR1-4) that, through conserved zinc finger domains, bind to GC-rich DNA motifs and enhance or repress the expression of wide variety genes which regulate growth, differentiation or cell remodeling [32-36]. While the EGR transcription factors are only transiently induced, the impact of this expression has a relatively long-term impact. Hence, EGR1-induced gene products initiate subsequent waves of gene expression which can ultimately result in cell differentiation, changes in the rate of proliferation or apoptosis [37].
Activation of the EGR transcription factors occurs in nearly all cell types through a wide array of physiological and pathophysiological stimuli which facilitate the activation of the transcription factors Ets-Like gene-1 (ELK-1) and cAMP response element-binding protein (CREB), the primary transcriptional activators of the EGR genes [38-42]. Hence, multiple pathological conditions including hypoxia, dysregulation of redox balance, UV genotoxic stress, viral infection and mechanical strain activate ELK-1/CREB-mediated EGR transcription via ERK1/2, p38 and JNK1 paradigms [40, 43-49]. Similarly, activation of tyrosine-kinase receptors, such as Insulin-like Growth Factor-1 Receptor (IGF-1R) or Epidermal Growth Factor Receptor (EGFR) leads to the induction of EGR transcription via analogous MAPK paradigms and/or the PI3K/Akt cascade [42, 50, 51]. Interestingly, increases in intracellular Ca2+ concentration can also lead to CREB induction, thereby potently inducing EGR transcription [52-54]. Considered collectively then, crosstalk amongst multiple receptor-mediated signal transduction pathways leads to convergence at the promoters of EGR genes, making their induction common components of multiple cellular stimuli.
WT1 is structurally very similar to EGR1 and the array of WT1 and EGR1-regulated genes extensively overlaps, although usually with mutually opposing consequences [55, 56]. Despite these similarities, because W T 1 i s n on-responsive to growth factor stimulation and predominantly a developmentally regulated gene [57, 58], we do not consider it to be a member of the EGR family. A key feature of WT1 is the existence at least 2 sites for alternative splicing, resulting in 4 major splice variants (see Fig 2) [59]. The first and most significant alternative splice donor site results in the addition of the amino acids KTS (Lys-Thr-Ser) between zinc fingers three and four [59]. WT1 variants A and B, which lack this KTS site are the forms best described as transcription factors; the functions served by WT1 variants C and D (KTS+) is a subject of ongoing controversy. Whereas these proteins have been thought to function post-transcriptionally as RNA splicing proteins [59], KTS+ forms of WT1 have been reported to regulate gene transcription, albeit with distinct binding characteristics [60]. The other major alternative splice donor site results in the inclusion of 17 amino acids in the middle of the protein in WT1 B and D, which is thought to regulate interactions between WT1 and cofactors [61].
Figure 2. The domain structure of the 4 WT1 splice variants.
WT1 is a zinc finger transcription factor with 2 distinct sites for RNA splicing. WT1A is the shortest form of WT1, in that it lacks both inserts. WT1B and WT1D contain exon 5 (Ex5), a 17 aa insert located immediately 3’ of the activation domain. In addition, the tripeptide lysine-threonine-serine (KTS) is inserted immediately after zinc finger 3 (ZF3) in both WT1C and WT1D. Amino acids 1-180 are critical for dimerization of WT1, which is a prelude to transcriptional activation; amino acids 84-124 repress WT1 activity by blocking dimerization. Finally, there is a putative RNA Recognition Motif (RRM) near the N-terminus of WT1 thought to contribute to the RNA splicing functions of the KTS+ forms of WT1 (WT1C and WT1D).
WT1 and EGR1 regulate STIM1 expression
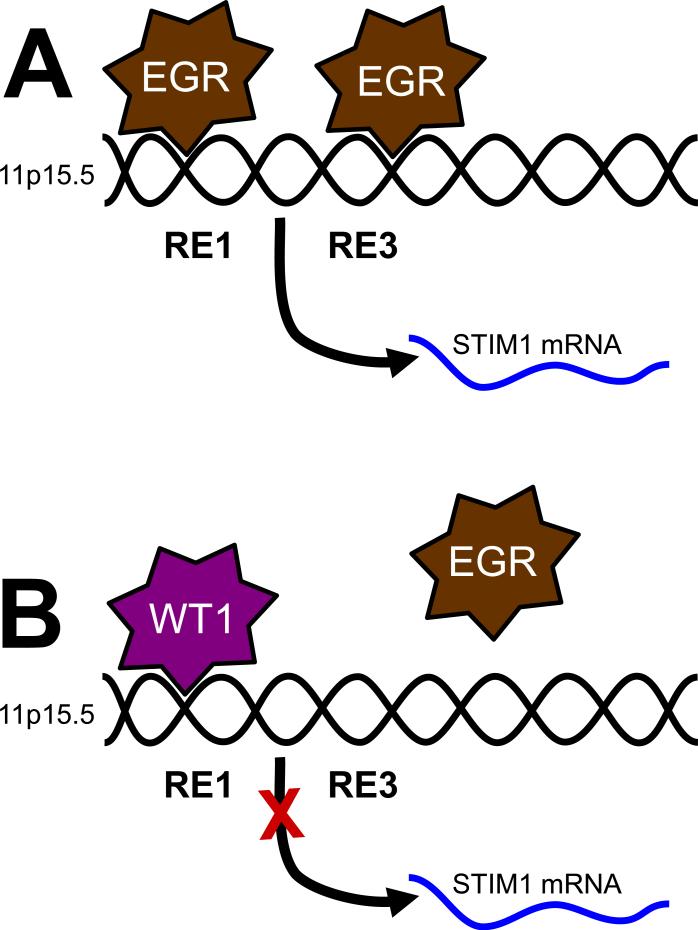
Our interest in WT1 and EGR1 as transcription factors regulating the expression of Ca2+ signaling proteins was initiated with the observation that they regulate the expression of STIM1 [56]. Thus, based on a combination of knockdown and overexpression approaches, we determined that EGR1 drives STIM1 expression, while WT1 inhibits its expression. In an effort to distinguish between direct and indirect roles for WT1 and EGR1, we analyzed the region of the genome at or around the STIM1 transcriptional start site using the Transcriptional Element Search System (TESS; University of Pennsylvania), uncovering 4 potential response elements. We then tested the validity of these sites using chromatin immunoprecipitation (ChIP), comparing the binding efficacy of EGR1 in WT1-null G401 tumor cells with that in WT1-expressing HEK293 cells. Intriguingly, both EGR1 and WT1 bound to response element 1 (RE1; see Fig 3), although binding of EGR1 was considerably weaker in the WT1-expressing cells. Further, EGR1, but not WT1 also bound to RE3, which was only detectable in the WT1-null cells. Based on these observations, we propose that binding of WT1 to RE1 inhibits both the binding of EGR1 to the STIM1 promoter and loss of STIM1 expression (Fig 3).
Figure 3. Model for control of STIM1 expression by WT1 and EGR1.
Functional WT1/EGR1 response elements are depicted relative to the start site for transcription of STIM1 mRNA (marked by the blue arrow). WT1-mediated inhibition of EGR1 binding at RE1 is marked with an arrow.
An important question that remains only partially answered is how WT1/EGR1-dependent control of STIM1 expression modulates Ca2+ signals. In our recent study [56], we showed that loss of STIM1 expression due to either overexpression of WT1 or knockdown of EGR1 resulted in decreased thapsigargin-induced SOCe. However, the physiological implications of the finding were not fully elucidated. As discussed above, transient upregulation of EGR1 is a fairly common signaling event [62, 63], while WT1 is heavily expressed in the developing kidney, mammary glands and hematopoietic system [57, 58]. Considered in combination with our findings then, we would predict STIM1 expression to be upregulated in response to multiple receptor-mediated signaling pathways, although predominantly in mature rather than undifferentiated WT1-expressing cell types. Precisely how STIM1 upregulation would impact Ca2+ signals would likely also be highly dependent on stoichiometry, as STIM1 upregulation does not increase Ca2+ signals unless Orai1 is in excess [14, 64]. Current investigations in our laboratory are focused on defining and characterizing this concept of dynamic receptor-mediated transcriptional control of store-operated Ca2+ entry via upregulation of EGR1.
Transcriptional control of SERCA2 expression
SERCA proteins are Ca2+ pumps found on the ER in every type of eurkaryotic cell. These proteins are critical for the maintenance of both cytosolic and ER Ca2+ levels. The three SERCA paralogs (SERCA 1, 2, and 3) are encoded by separate genes and produce more than 10 isoforms through alternative splicing mechanisms [65]. Whereas SERCA1 isoforms are exclusively expressed in fast-twitch skeletal muscle, SERCA3 can be found in multiple cell types, particularly those of the hematopoietic system, exocrine and endocrine glands [65]. The SERCA2 gene produces two distinct isoforms; SERCA2a which is found in cardiac and slow-twitch skeletal muscle and SERCA2b, a ubiquitously expressed isoform found at varying levels in all cell types [65].
Interestingly, while a relationship between EGR1 and SERCA2 expression has been demonstrated [66, 67], the precise nature of this relationship remains somewhat unclear [68]. The first indication that EGR1 might be involved in control of SERCA2 expression was based on examination of doxorubicin-induced cardiomyopathy [67]. This type of cardiomyopathy is a well documented side effect of this otherwise useful chemotherapeutic agent. In this study, the investigators linked doxorubicin (DOX) treatment of cardiomyocytes to EGR1-mediated repression of the SERCA2 gene. Specifically, incubation of cardiomyocytes with DOX led to a p44/42 MAPK-dependent increase in EGR1 expression (as expected since EGR1 is a redox-activated transcription factor [69, 70]) with correlative decreases in SERCA2 mRNA and protein. This phenomenon was attributed to direct EGR1-mediated transcriptional repression based on the identification of EGR1 binding sites in the 5’ flanking region of the SERCA2 gene. In a more recent study by the same group, they showed that EGR1 also mediates prostaglandin-induced inhibition of SERCA2 expression in cultured neonatal rat cardiomyocytes [66]. However, this issue has also been addressed by the Fuller group (Imperial College London, UK), who came to a contradictory conclusion [68]. Hence, their analysis of a SERCA2 promoter-driven luciferase construct failed to support any functional role for these EGR1-binding sites, leading the authors to conclude that they were inactive [68].
While it is obvious that both conclusions cannot be correct, our own investigations in this area support the conclusion that EGR1 is a negative regulator of SERCA2 expression, although we see little evidence for a direct relationship. Hence, overexpression of EGR1 has a relatively minor effect on SERCA2 mRNA levels in multiple cell types, yet leads to an approximately 90% reduction in SERCA2 protein content (with WT1 having the opposite effect) [71]. Further, we obtained the SERCA2 luciferase vector from Dr. Stephen Fuller (Imperial College London, UK; [68]) and saw no evidence of EGR1-dependent luciferase expression (unpublished observations). Finally, our attempts to perform ChIP using primers targeting EGR1 consensus sequences in the SERCA2 promoter found no evidence of binding (unpublished observations). Based on this combination of findings, we are confident that EGR1 does indeed regulate SERCA2 expression, although it seems likely that this relationship is indirect and quite possibly post-transcriptional.
While EGR1 functions as a negative regulator of SERCA2 expression, SERCA2 transcription is induced via the combination of several other transcription factors. Basal SERCA2 expression is primarily driven by the ubiquitously-expressed transcription factors Sp1 and Sp3, which bind directly to proximal regions of the SERCA2 promoter to transactivate the SERCA2 gene [68]. In addition, there is considerable circumstantial evidence that Nuclear Factor of Activated T cells (NFAT) is a positive regulator of SERCA2 transcription [72-75], despite the lack of evidence of direct transcriptional control. Hence, NFAT activation leads to induction of SERCA2 transcription [72, 74, 76], while induction of glycogen synthase kinase 3β (GSK3β; a negative regulator of NFAT nuclear translocation) leads to downregulation of SERCA2 [77, 78]. Furthermore, GSK3β transgenic mice exhibit impaired post-natal cardiomyocyte growth, abnormal cardiac contractile function and impaired diastolic relaxation [78], all consistent with defective SERCA2 expression.
Interestingly, there are also several examples of SERCA2 regulation by early or upstream signaling molecules. Hence, treating primary β cells with IL-1 or IFN-γ for 24 hours substantially reduces SERCA2 expression and reduces the ER Ca2+ pool enough to induce the ER stress response [79]. Furthermore, direct activation of protein kinase C (PKC) by the addition of phorbol-12-myristate-13-acetate (PMA) to neonatal cardiomyocytes significantly reduces SERCA2 expression [80]. Although these modes of SERCA2 down regulation were not attributed to an EGR-1 dependent mechanism, IL-1, IFN-γ and PKC are all potent activators of EGR1 [81-83]. Thus, given previous investigations on EGR1-dependent inhibition of SERCA expression, these effects may well be dependent on EGR1 activity [67, 84].
Transcriptional Control of PMCA
Although often described simply as PMCA, there are, in fact, 4 different PMCA genes, each of which exists in multiple isoforms [85]. While virtually all cell types express PMCA, these isoforms have distinct expression patterns and functional characteristics. Hence, PMCA1 and PMCA4 are present in virtually every tissue type, while PMCA2 and PMCA3 are expressed predominantly in excitable cells [85-87]. The functional differences among PMCA isoforms correspond to the differences in Ca2+ dynamics between excitable and non-excitable cells. Hence, PMCA2 and PMCA3 are the most quickly activated PMCA isoforms, enabling them to clear Ca2+ within the rapid time course of changes in Ca2+ concentration observed in excitable cells [88]. PMCA4, in contrast, is both activated and inactivated relatively slowly, permitting relatively long changes in cytosolic Ca2+ concentration, as observed in non-excitable cells [89].
Unlike for SERCA, a published relationship between WT1, EGR1 and the expression of PMCA has not yet been elucidated. However, the involvement of several different transcription factors in PMCA expression have been investigated, particularly c-myc. Hence, in B lymphocytes, activation of the B cell receptor (BCR) by agonists such as IL-7 and IL-4 leads to both elevated cytosolic Ca2+ concentration and induction of c-myc [90, 91]. Interestingly, c-myc has been shown to repress PMCA4 expression, resulting in decreased Ca2+ clearance from the cytosol, augmented NFAT and, ultimately, increased B cell activation [92]. Similar observations have been made in pancreatic β cells, in which glucose both induces c-myc expression and downregulates both PMCA1 and PMCA2 [93, 94]. Hence, downregulation of PMCA may be a common component of “activation” signals in a wide variety of different cell types.
While the ability of c-myc to suppress PMCA expression seems clear, precisely what drives their expression has yet to be fully elucidated, although there have been several interesting clues. Thus, vitamin D3 induces expression of PMCA1 in kidney epithelial cells, osteoblasts and duodenom cells [95-97]. Induction of PMCA4b has also been demonstrated in duodenum cells in response to the synthetic anti-inflammatory steroid, dexamethasone [98]. However, in neither case, has direct transcriptional control been demonstrated. Given the dynamic roles that PMCA proteins play in shaping not only Ca2+ dynamics, but also both normal and pathophysiological function of numerous critical cell types, future investigations directed at characterizing the transcriptional mechanisms in control of PMCA expression are clearly a necessity.
EGR1-mediated remodeling of Ca2+ homeostasis in cardiac hypertrophy
In addition to control of SERCA2 expression, EGR1 negatively regulates both NCX [99] and calsequestrin expression [100]. This has led to several studies addressing the role of EGR1 in cardiac remodeling. Hence, not only is EGR1 upregulated during cardiac hypertrophy [99-102], but EGR1-KO mice exhibit increased cardiac damage in response to catecholamine infusion [99, 100, 103]. EGR1-mediated loss of SERCA2 and NCX (the major proteins mediating cytosolic calcium clearance in cardiomyocytes) would lead to attenuated cytosolic Ca2+ clearance and amplification of Nuclear Factor of T cells (NFAT) activation, ultimately the critical mediator of myocardial thickening [104-106]. However, as the major Ca2+ binding protein in the sarcoplasmic reticulum (SR), loss of calsequestrin expression would tend to have the opposite effect, making the role of EGR1 in cardiac remodeling somewhat more difficult to fully quantify.
Interestingly, multiple cell systems, including cardiac models, increase PMCA expression in response to loss of SERCA2 expression [107, 108]. Additionally, there is growing evidence to support the notion that while PMCA plays a limited role in the excitation–contraction coupling process of cardiac systems, it modulates Ca2+ dependent signal transduction pathways [109, 110]. Hence, upregulation of PMCA attenuates the calcineurin-dependent hypertrophic response [111]. Thus, increases in PMCA expression might mitigate hypertrophic Ca2+ signaling cascades in response to SERCA2 deficiency and protect a cell from hypertrophy, although this concept yet to be validated.
In addition to regulation of Ca2+ clearance mechanisms, EGR1 also activates STIM1 expression [56], which could potentially increase Ca2+ influx, thereby further augmenting the aforementioned Ca2+ dynamics. However, while STIM1-mediated Ca2+ entry is observed in neonatal cardiomyocytes, it is downregulated in adult cardiomyocytes [112, 113]. This is somewhat intriguing, in that the reactivation of fetal gene programs is thought to be a component of induction of cardiac hypertrophy [114, 115]. Further, inhibition of STIM1-mediated Ca2+ entry abrogates hypertrophic characteristics in studies performed in vitro in neonatal cardiomyocytes [116, 117].
In summary, induction of EGR1 results in inhibition of Ca2+ clearance, increased Ca2+ entry and decreased ER Ca2+ load. Precisely what role these changes have on cardiac remodeling is not currently clear, however, considered collectively, it is difficult to discount EGR1 as a critical player in this process.
Redox-state regulation of Calcium dynamics: a potential role for EGR1
Reactive oxygen species (ROS) can directly modulate both the activity and expression of several key proteins involved in Ca2+ homeostasis. The primary means whereby ROS affects protein function are via modifications of the sulfhydryl (SH) moieties within cysteine residues leading to formation of disulfide bonds and/or oxidation of reduced glutathione leading to S-glutathionylation. We have recently shown that S-glutathionylation of cysteine 56 on STIM1 leads to constitutive, store-independent Orai1 activation [118]; hence, the initial response to elevates ROS is elevated Ca2+ entry. However, this effect can be compensated in part by direct ROS-induced stimulation of both SERCA [119] and NCX [120] activity. In addition, H2O2-induced decreases L-type Ca2+ channel activity may also partially compensate for ROS-induced Ca2+ elevation in excitable cells [121, 122]. This is particularly relevant in muscle, where elevated ROS is a relatively common event during exercise [123, 124]. Although the mechanism whereby H2O2 changes L-type Ca2+ channel function was not described, recent demonstrations by us and others that STIM1 inhibits L-type channel activity [30, 125] may provide an explanation for this phenomenon.
Interestingly, ROS is well known to induce EGR1 expression [49, 126, 127]. Given the host of different Ca2+ homeostasis proteins regulated by EGR1 described in this review, it seems reasonable to speculate that EGR1-dependent changes in their expression may greatly impact ROS-dependent changes in Ca2+ homeostasis, ultimately determining physiological outcomes. Hence, EGR1-dependent downregulation of SERCA2 and NCX as well as upregulation of STIM1 would exacerbate ROS-dependent Ca2+ elevation and induce cell death via collapse of mitochondrial membrane potential [128, 129] and/or induction of NFAT [130, 131]. However, similar to direct ROS-dependent changes in the activity of Ca2+ regulatory proteins, there are compensatory mechanisms in place to limit damage caused by changes in their expression. For example, acute elevations in cytosolic Ca2+ concentration stimulate cAMP response element-binding protein (CREB) activity [132] which induces the expression of both antioxidants and ROS detoxifying enzymes [133-137]. Similarly, ROS stimulates the expression of Down syndrome critical region gene 1 (DSCR1) [138] which inhibits calcineurin-dependent NFAT activity [139-141]. Finally, as mentioned in preceding sections, decreased SERCA2 expression leads to increased PMCA expression. Interestingly, PMCA recruits calcineurin from the cytoplasm to the plasma membrane and inhibits its ability to effectively activate NFAT [143].
In summary, ROS-mediated changes in Ca2+ homeostasis via either direct or transcriptional mechanisms lead to elevated Ca2+ concentration while, paradoxically stimulating multiple compensatory pathways. Presumably, these observations reflect not only cell type-dependent differences in tolerance to oxidative stress, but also the difference between normal physiological ROS signaling and pathophysiological ROS levels.
Summary and perspective
We have delineated several mechanisms by which transcription factors regulate Ca2+ signaling through modifications in the gene expression of the key families of Ca2+ homeostasis proteins SERCA, STIM1, PMCA and NCX. While most of the investigations demonstrating these relationships were performed in isolation, it is important to recognize that these events do not occur that way. This is particularly true of EGR1, which has been separately demonstrated to modulate the expression of SERCA2 [66, 67, 71], NCX [99] and STIM1 [56] (see Fig 4). These reports point to dramatic EGR1-dependent changes in how Ca2+ dynamics are regulated. Given that EGR1 is a major receptor-dependent transcription factor, it seems reasonable to speculate that receptor-dependent remodeling of Ca2+ signals via transcriptional control of Ca2+ homeostatic proteins is a common event in cellular physiology. However, since EGR1-dependent gene transcription is negatively regulated by WT1, it is likely that these responses are both cell-type dependent and highly contextual. Nevertheless, this remodeling is by no means limited to EGR1; both NFAT and c-Myc regulate SERCA2 and PMCA, respectively, and are also exceedingly common receptor-dependent transcription factors. Considered collectively then, it seems reasonable to propose that transcriptional control of Ca2+ homeostasis proteins represents a new paradigm in receptor-dependent control of Ca2+ signaling. Future investigations directed at characterizing precisely how this receptor-dependent remodeling of Ca2+ homeostasis impacts Ca2+ signals will provide important new insight into Ca2+-dependent changes in cellular physiology.
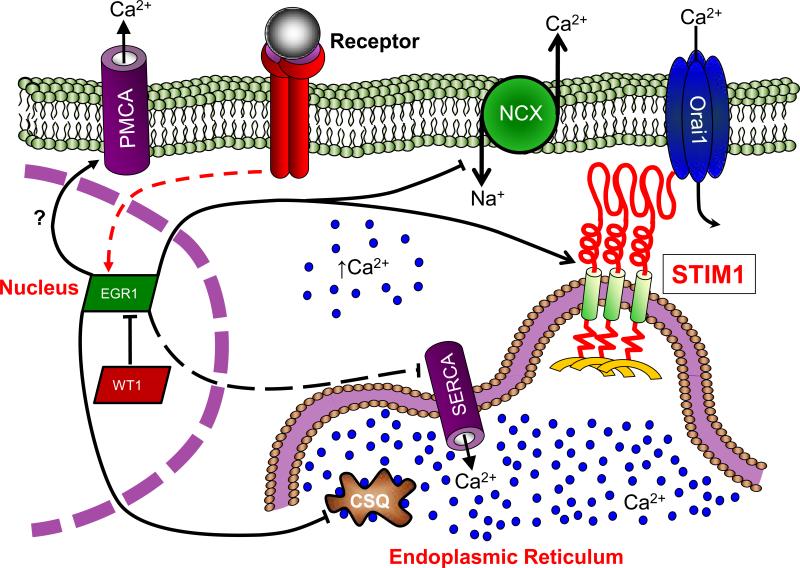
Figure 4. Regulation of Ca2+ homeostasis by WT1 and EGR1.
Early Growth Response 1 (EGR1) activation is achieved through multiple receptor-dependent signal transduction pathways. Activated EGR1 rapidly enters the nucleus where it induces STIM1 expression, yet negatively regulates the expression of regulates the expression of the sodium/calcium exchanger (NCX) and the sarco/endoplasmic calcium ATPase (SERCA); the potential relationship between EGR1 and PMCA expression remains currently undefined. However, in cells expressing Wilms Tumor Suppressor 1 (WT1), EGR1-mediated changes in the expression of these Ca2+ signaling proteins are greatly attenuated.
Acknowledgements
We greatly appreciate support from the American Heart Association (JS) and the Pennsylvania Department of Health (JS). In addition, we thank Dr. Muniswamy Madesh (Temple University) for critical reading of the manuscript.
References
- 1.Jousset H, Frieden M, Demaurex N. STIM1 knockdown reveals that store-operated Ca2+ channels located close to sarco/endoplasmic Ca2+ ATPases (SERCA) pumps silently refill the endoplasmic reticulum. J Biol Chem. 2007;282:11456–64. doi: 10.1074/jbc.M609551200. [DOI] [PubMed] [Google Scholar]
- 2.Soboloff J, Berger SA. Sustained ER Ca2+ depletion suppresses protein synthesis and induces activation-enhanced cell death in mast cells. J Biol Chem. 2002;277:13812–20. doi: 10.1074/jbc.M112129200. [DOI] [PubMed] [Google Scholar]
- 3.Soboloff J, Zhang Y, Minden M, Berger SA. Sensitivity of myeloid leukemia cells to calcium influx blockade: application to bone marrow purging. Exp Hematol. 2002;30:1219–26. doi: 10.1016/s0301-472x(02)00893-7. [DOI] [PubMed] [Google Scholar]
- 4.Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, Smith PJ, Dziadek MA. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J. 2001;357:673–85. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng X, Wang Y, Zhou Y, Soboloff J, Gill DL. STIM and Orai: dynamic intermembrane coupling to control cellular calcium signals. J Biol Chem. 2009;284:22501–5. doi: 10.1074/jbc.R109.018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–22. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–62. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 9.Stathopulos PB, Zheng L, Ikura M. Stromal Interaction Molecule (STIM) 1 and STIM2 Calcium Sensing Regions Exhibit Distinct Unfolding and Oligomerization Kinetics. J Biol Chem. 2009;284:728–32. doi: 10.1074/jbc.C800178200. [DOI] [PubMed] [Google Scholar]
- 10.Hewavitharana T, Deng X, Wang Y, Ritchie MF, Girish GV, Soboloff J, Gill DL. Location and function of STIM1 in the activation of Ca2+ entry signals. J Biol Chem. 2008;283:26252–62. doi: 10.1074/jbc.M802239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–42. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–25. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM Reconstitute Store-operated Calcium Channel Function. J Biol Chem. 2006;281:20661–5. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Deng X, Zhou Y, Hendron E, Mancarella S, Ritchie MF, Tang XD, Baba Y, Kurosaki T, Mori Y, Soboloff J, Gill DL. STIM protein coupling in the activation of Orai channels. Proc Natl Acad Sci U S A. 2009;106:7391–6. doi: 10.1073/pnas.0900293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C. A Cytosolic Homomerization and a Modulatory Domain within STIM1 C Terminus Determine Coupling to ORAI1 Channels. J Biol Chem. 2009;284:8421–6. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–90. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–43. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor. Stim1. J Biol Chem. 2006;281:24979–90. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1). Nat Cell Biol. 2006;8:771–3. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–10. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 22.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104:4682–7. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(-/-) mice. Proc Natl Acad Sci U S A. 2007;104:17542–7. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill D, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–16. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–45. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008;105:2895–900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dehaven W, Jones B, Petranka J, Smyth J, Tomita T, Bird G, Putney J. TRPC channels function independently of STIM1 and Orai1. J Physiol. 2009 doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross SA, Guzman GA, Wissenbach U, Philipp SE, Zhu MX, Bruns D, Cavalie A. TRPC5 is a Ca2+ -activated channel functionally coupled to Ca2+ -selective ion channels. J Biol Chem. 2009 doi: 10.1074/jbc.M109.018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varga-Szabo D, Authi KS, Braun A, Bender M, Ambily A, Hassock SR, Gudermann T, Dietrich A, Nieswandt B. Store-operated Ca(2+) entry in platelets occurs independently of transient receptor potential (TRP) C1. Pflugers Arch. 2008 doi: 10.1007/s00424-008-0531-4. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store-sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010 doi: 10.1126/science.1191086. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietrich A, Kalwa H, Storch U, Mederos YSM, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Arch. 2007;455:465–77. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- 32.Delbridge GJ, Khachigian LM. FGF-1-induced platelet-derived growth factor-A chain gene expression in endothelial cells involves transcriptional activation by early growth response factor-1. Circ Res. 1997;81:282–8. doi: 10.1161/01.res.81.2.282. [DOI] [PubMed] [Google Scholar]
- 33.Sukhatme VP. Early transcriptional events in cell growth: the Egr family. J Am Soc Nephrol. 1990;1:859–66. doi: 10.1681/ASN.V16859. [DOI] [PubMed] [Google Scholar]
- 34.Bae SK, Bae MH, Ahn MY, Son MJ, Lee YM, Bae MK, Lee OH, Park BC, Kim KW. Egr-1 mediates transcriptional activation of IGF-II gene in response to hypoxia. Cancer Res. 1999;59:5989–94. [PubMed] [Google Scholar]
- 35.Mahalingam D, Natoni A, Keane M, Samali A, Szegezdi E. Early growth response-1 is a regulator of DR5-induced apoptosis in colon cancer cells. Br J Cancer. 2010;102:754–64. doi: 10.1038/sj.bjc.6605545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bettini M, Xi H, Milbrandt J, Kersh GJ. Thymocyte development in early growth response gene 1-deficient mice. J Immunol. 2002;169:1713–20. doi: 10.4049/jimmunol.169.4.1713. [DOI] [PubMed] [Google Scholar]
- 37.DeLigio JT, Zorio DA. Early growth response 1 (EGR1): a gene with as many names as biological functions. Cancer Biol Ther. 2009;8:1889–92. doi: 10.4161/cbt.8.20.9804. [DOI] [PubMed] [Google Scholar]
- 38.Mack KJ, Yi SD, Chang S, Millan N, Mack P. NGFI-C expression is affected by physiological stimulation and seizures in the somatosensory cortex. Brain Res Mol Brain Res. 1995;29:140–6. doi: 10.1016/0169-328x(94)00243-8. [DOI] [PubMed] [Google Scholar]
- 39.Khachigian LM, Anderson KR, Halnon NJ, Gimbrone MA, Jr., Resnick N, Collins T. Egr-1 is activated in endothelial cells exposed to fluid shear stress and interacts with a novel shear-stress-response element in the PDGF A-chain promoter. Arterioscler Thromb Vasc Biol. 1997;17:2280–6. doi: 10.1161/01.atv.17.10.2280. [DOI] [PubMed] [Google Scholar]
- 40.Morawietz H, Ma YH, Vives F, Wilson E, Sukhatme VP, Holtz J, Ives HE. Rapid induction and translocation of Egr-1 in response to mechanical strain in vascular smooth muscle cells. Circ Res. 1999;84:678–87. doi: 10.1161/01.res.84.6.678. [DOI] [PubMed] [Google Scholar]
- 41.Lim JH, Jung CR, Lee CH, Im DS. Egr-1 and serum response factor are involved in growth factors- and serum-mediated induction of E2-EPF UCP expression that regulates the VHL-HIF pathway. J Cell Biochem. 2008;105:1117–27. doi: 10.1002/jcb.21914. [DOI] [PubMed] [Google Scholar]
- 42.Cabodi S, Morello V, Masi A, Cicchi R, Broggio C, Distefano P, Brunelli E, Silengo L, Pavone F, Arcangeli A, Turco E, Tarone G, Moro L, Defilippi P. Convergence of integrins and EGF receptor signaling via PI3K/Akt/FoxO pathway in early gene Egr-1 expression. J Cell Physiol. 2009;218:294–303. doi: 10.1002/jcp.21603. [DOI] [PubMed] [Google Scholar]
- 43.Yan SF, Lu J, Zou YS, Soh-Won J, Cohen DM, Buttrick PM, Cooper DR, Steinberg SF, Mackman N, Pinsky DJ, Stern DM. Hypoxia-associated induction of early growth response-1 gene expression. J Biol Chem. 1999;274:15030–40. doi: 10.1074/jbc.274.21.15030. [DOI] [PubMed] [Google Scholar]
- 44.Heiniger CD, Kostadinova RM, Rochat MK, Serra A, Ferrari P, Dick B, Frey BM, Frey FJ. Hypoxia causes down-regulation of 11 beta-hydroxysteroid dehydrogenase type 2 by induction of Egr-1. Faseb J. 2003;17:917–9. doi: 10.1096/fj.02-0582fje. [DOI] [PubMed] [Google Scholar]
- 45.Cai Y, Liu Y, Zhang X. Induction of transcription factor Egr-1 gene expression in astrocytoma cells by Murine coronavirus infection. Virology. 2006;355:152–63. doi: 10.1016/j.virol.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quinones A, Dobberstein KU, Rainov NG. The egr-1 gene is induced by DNA-damaging agents and non-genotoxic drugs in both normal and neoplastic human cells. Life Sci. 2003;72:2975–92. doi: 10.1016/s0024-3205(03)00230-3. [DOI] [PubMed] [Google Scholar]
- 47.Lo LW, Cheng JJ, Chiu JJ, Wung BS, Liu YC, Wang DL. Endothelial exposure to hypoxia induces Egr-1 expression involving PKCalpha-mediated Ras/Raf-1/ERK1/2 pathway. J Cell Physiol. 2001;188:304–12. doi: 10.1002/jcp.1124. [DOI] [PubMed] [Google Scholar]
- 48.Lim CP, Jain N, Cao X. Stress-induced immediate-early gene, egr-1, involves activation of p38/JNK1. Oncogene. 1998;16:2915–26. doi: 10.1038/sj.onc.1201834. [DOI] [PubMed] [Google Scholar]
- 49.Huang RP, Adamson ED. Characterization of the DNA-binding properties of the early growth response-1 (Egr-1) transcription factor: evidence for modulation by a redox mechanism. DNA Cell Biol. 1993;12:265–73. doi: 10.1089/dna.1993.12.265. [DOI] [PubMed] [Google Scholar]
- 50.Abdel-Malak NA, Mofarrahi M, Mayaki D, Khachigian LM, Hussain SN. Early growth response-1 regulates angiopoietin-1-induced endothelial cell proliferation, migration, and differentiation. Arterioscler Thromb Vasc Biol. 2009;29:209–16. doi: 10.1161/ATVBAHA.108.181073. [DOI] [PubMed] [Google Scholar]
- 51.Criswell T, Beman M, Araki S, Leskov K, Cataldo E, Mayo LD, Boothman DA. Delayed activation of insulin-like growth factor-1 receptor/Src/MAPK/Egr-1 signaling regulates clusterin expression, a pro-survival factor. J Biol Chem. 2005;280:14212–21. doi: 10.1074/jbc.M412569200. [DOI] [PubMed] [Google Scholar]
- 52.Ginty DD, Glowacka D, Bader DS, Hidaka H, Wagner JA. Induction of immediate early genes by Ca2+ influx requires cAMP-dependent protein kinase in PC12 cells. J Biol Chem. 1991;266:17454–8. [PubMed] [Google Scholar]
- 53.Mayer SI, Thiel G. Calcium influx into MIN6 insulinoma cells induces expression of Egr-1 involving extracellular signal-regulated protein kinase and the transcription factors Elk-1 and CREB. Eur J Cell Biol. 2009;88:19–33. doi: 10.1016/j.ejcb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Grewal SS, Fass DM, Yao H, Ellig CL, Goodman RH, Stork PJ. Calcium and cAMP signals differentially regulate cAMP-responsive element-binding protein function via a Rap1-extracellular signal-regulated kinase pathway. J Biol Chem. 2000;275:34433–41. doi: 10.1074/jbc.M004728200. [DOI] [PubMed] [Google Scholar]
- 55.Hamilton TB, Borel F, Romaniuk PJ. Comparison of the DNA binding characteristics of the related zinc finger proteins WT1 and EGR1. Biochemistry. 1998;37:2051–8. doi: 10.1021/bi9717993. [DOI] [PubMed] [Google Scholar]
- 56.Ritchie MF, Yue C, Zhou Y, Houghton PJ, Soboloff J. Wilms Tumor Suppressor 1 (WT1) and Early Growth Response 1 (EGR1) Are Regulators of STIM1 Expression. J Biol Chem. 2010;285:10591–6. doi: 10.1074/jbc.M109.083493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivera MN, Haber DA. Wilms’ tumour: connecting tumorigenesis and organ development in the kidney. Nat Rev Cancer. 2005;5:699–712. doi: 10.1038/nrc1696. [DOI] [PubMed] [Google Scholar]
- 58.Yang L, Han Y, Suarez Saiz F, Minden MD. A tumor suppressor and oncogene: the WT1 story. Leukemia. 2007;21:868–76. doi: 10.1038/sj.leu.2404624. [DOI] [PubMed] [Google Scholar]
- 59.Morrison AA, Viney RL, Ladomery MR. The post-transcriptional roles of WT1, a multifunctional zinc-finger protein. Biochim Biophys Acta. 2008;1785:55–62. doi: 10.1016/j.bbcan.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Nurmemmedov E, Yengo RK, Uysal H, Karlsson R, Thunnissen MM. New insights into DNA-binding behavior of Wilms tumor protein (WT1)--a dual study. Biophys Chem. 2009;145:116–25. doi: 10.1016/j.bpc.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Richard DJ, Schumacher V, Royer-Pokora B, Roberts SG. Par4 is a coactivator for a splice isoform-specific transcriptional activation domain in WT1. Genes Dev. 2001;15:328–39. doi: 10.1101/gad.185901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beckmann AM, Matsumoto I, Wilce PA. AP-1 and Egr DNA-binding activities are increased in rat brain during ethanol withdrawal. J Neurochem. 1997;69:306–14. doi: 10.1046/j.1471-4159.1997.69010306.x. [DOI] [PubMed] [Google Scholar]
- 63.Gashler A, Sukhatme VP. Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol. 1995;50:191–224. doi: 10.1016/s0079-6603(08)60815-6. [DOI] [PubMed] [Google Scholar]
- 64.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 Is an Inhibitor of STIM1-Mediated Store-Operated Ca(2+) Entry. Curr Biol. 2006;16:1465–70. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 65.Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–42. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 66.Hara S, Arai M, Tomaru K, Doi H, Koitabashi N, Iso T, Watanabe A, Tanaka T, Maeno T, Suga T, Yokoyama T, Kurabayashi M. Prostaglandin F2alpha inhibits SERCA2 gene transcription through an induction of Egr-1 in cultured neonatal rat cardiac myocytes. Int Heart J. 2008;49:329–42. doi: 10.1536/ihj.49.329. [DOI] [PubMed] [Google Scholar]
- 67.Arai M, Yoguchi A, Takizawa T, Yokoyama T, Kanda T, Kurabayashi M, Nagai R. Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca(2+)-ATPase gene transcription. Circ Res. 2000;86:8–14. doi: 10.1161/01.res.86.1.8. [DOI] [PubMed] [Google Scholar]
- 68.Brady M, Koban MU, Dellow KA, Yacoub M, Boheler KR, Fuller SJ. Sp1 and Sp3 transcription factors are required for trans-activation of the human SERCA2 promoter in cardiomyocytes. Cardiovasc Res. 2003;60:347–54. doi: 10.1016/s0008-6363(03)00529-7. [DOI] [PubMed] [Google Scholar]
- 69.Bijur GN, Davis RE, Jope RS. Rapid activation of heat shock factor-1 DNA binding by H2O2 and modulation by glutathione in human neuroblastoma and Alzheimer's disease cybrid cells. Brain Res Mol Brain Res. 1999;71:69–77. doi: 10.1016/s0169-328x(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 70.Pines A, Bivi N, Romanello M, Damante G, Kelley MR, Adamson ED, D'Andrea P, Quadrifoglio F, Moro L, Tell G. Cross-regulation between Egr-1 and APE/Ref-1 during early response to oxidative stress in the human osteoblastic HOBIT cell line: evidence for an autoregulatory loop. Free Radic Res. 2005;39:269–81. doi: 10.1080/10715760400028423. [DOI] [PubMed] [Google Scholar]
- 71.Zhou Y, Ritchie MF, Yue C, Madesh M, Soboloff J. Targeting Store-operated Ca2+ Entry to Induce Cell Death depends on WT1-Regulated SERCA2 expression. Cell Death Differ. 2010 in revision. [Google Scholar]
- 72.Vlasblom R, Muller A, Musters RJ, Zuidwijk MJ, Van Hardeveld C, Paulus WJ, Simonides WS. Contractile arrest reveals calcium-dependent stimulation of SERCA2a mRNA expression in cultured ventricular cardiomyocytes. Cardiovasc Res. 2004;63:537–44. doi: 10.1016/j.cardiores.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 73.Mufti S, Wenzel S, Euler G, Piper HM, Schluter KD. Angiotensin II-dependent loss of cardiac function: mechanisms and pharmacological targets attenuating this effect. J Cell Physiol. 2008;217:242–9. doi: 10.1002/jcp.21501. [DOI] [PubMed] [Google Scholar]
- 74.Anwar A, Taimor G, Korkususz H, Schreckenberg R, Berndt T, Abdallah Y, Piper HM, Schluter KD. PKC-independent signal transduction pathways increase SERCA2 expression in adult rat cardiomyocytes. J Mol Cell Cardiol. 2005;39:911–9. doi: 10.1016/j.yjmcc.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Prasad AM, Inesi G. Effects of thapsigargin and phenylephrine on calcineurin and protein kinase C signaling functions in cardiac myocytes. Am J Physiol Cell Physiol. 2009;296:C992–C1002. doi: 10.1152/ajpcell.00594.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10:688–97. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.King TD, Gandy JC, Bijur GN. The protein phosphatase-1/inhibitor-2 complex differentially regulates GSK3 dephosphorylation and increases sarcoplasmic/endoplasmic reticulum calcium ATPase 2 levels. Exp Cell Res. 2006;312:3693–700. doi: 10.1016/j.yexcr.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Michael A, Haq S, Chen X, Hsich E, Cui L, Walters B, Shao Z, Bhattacharya K, Kilter H, Huggins G, Andreucci M, Periasamy M, Solomon RN, Liao R, Patten R, Molkentin JD, Force T. Glycogen synthase kinase-3beta regulates growth, calcium homeostasis, and diastolic function in the heart. J Biol Chem. 2004;279:21383–93. doi: 10.1074/jbc.M401413200. [DOI] [PubMed] [Google Scholar]
- 79.Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54:452–61. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- 80.Porter MJ, Heidkamp MC, Scully BT, Patel N, Martin JL, Samarel AM. Isoenzyme-selective regulation of SERCA2 gene expression by protein kinase C in neonatal rat ventricular myocytes. Am J Physiol Cell Physiol. 2003;285:C39–47. doi: 10.1152/ajpcell.00461.2002. [DOI] [PubMed] [Google Scholar]
- 81.McMahon SB, Monroe JG. The role of early growth response gene 1 (egr-1) in regulation of the immune response. J Leukoc Biol. 1996;60:159–66. doi: 10.1002/jlb.60.2.159. [DOI] [PubMed] [Google Scholar]
- 82.Cao XM, Guy GR, Sukhatme VP, Tan YH. Regulation of the Egr-1 gene by tumor necrosis factor and interferons in primary human fibroblasts. J Biol Chem. 1992;267:1345–9. [PubMed] [Google Scholar]
- 83.Coleman DL, Bartiss AH, Sukhatme VP, Liu J, Rupprecht HD. Lipopolysaccharide induces Egr-1 mRNA and protein in murine peritoneal macrophages. J Immunol. 1992;149:3045–51. [PubMed] [Google Scholar]
- 84.Magnier C, Papp B, Corvazier E, Bredoux R, Wuytack F, Eggermont J, Maclouf J, Enouf J. Regulation of sarco-endoplasmic reticulum Ca(2+)-ATPases during platelet-derived growth factor-induced smooth muscle cell proliferation. J Biol Chem. 1992;267:15808–15. [PubMed] [Google Scholar]
- 85.Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 86.Stauffer TP, Guerini D, Carafoli E. Tissue distribution of the four gene products of the plasma membrane Ca2+ pump. A study using specific antibodies. J Biol Chem. 1995;270:12184–90. doi: 10.1074/jbc.270.20.12184. [DOI] [PubMed] [Google Scholar]
- 87.Stauffer TP, Hilfiker H, Carafoli E, Strehler EE. Quantitative analysis of alternative splicing options of human plasma membrane calcium pump genes. J Biol Chem. 1993;268:25993–6003. [PubMed] [Google Scholar]
- 88.Caride AJ, Filoteo AG, Penheiter AR, Paszty K, Enyedi A, Penniston JT. Delayed activation of the plasma membrane calcium pump by a sudden increase in Ca2+: fast pumps reside in fast cells. Cell Calcium. 2001;30:49–57. doi: 10.1054/ceca.2001.0212. [DOI] [PubMed] [Google Scholar]
- 89.Caride AJ, Elwess NL, Verma AK, Filoteo AG, Enyedi A, Bajzer Z, Penniston JT. The rate of activation by calmodulin of isoform 4 of the plasma membrane Ca(2+) pump is slow and is changed by alternative splicing. J Biol Chem. 1999;274:35227–32. doi: 10.1074/jbc.274.49.35227. [DOI] [PubMed] [Google Scholar]
- 90.Klemsz MJ, Justement LB, Palmer E, Cambier JC. Induction of c-fos and c-myc expression during B cell activation by IL-4 and immunoglobulin binding ligands. J Immunol. 1989;143:1032–9. [PubMed] [Google Scholar]
- 91.Morrow MA, Lee G, Gillis S, Yancopoulos GD, Alt FW. Interleukin-7 induces N-myc and c-myc expression in normal precursor B lymphocytes. Genes Dev. 1992;6:61–70. doi: 10.1101/gad.6.1.61. [DOI] [PubMed] [Google Scholar]
- 92.Habib T, Park H, Tsang M, de Alboran IM, Nicks A, Wilson L, Knoepfler PS, Andrews S, Rawlings DJ, Eisenman RN, Iritani BM. Myc stimulates B lymphocyte differentiation and amplifies calcium signaling. J Cell Biol. 2007;179:717–31. doi: 10.1083/jcb.200704173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jonas JC, Laybutt DR, Steil GM, Trivedi N, Pertusa JG, Van de Casteele M, Weir GC, Henquin JC. High glucose stimulates early response gene c-Myc expression in rat pancreatic beta cells. J Biol Chem. 2001;276:35375–81. doi: 10.1074/jbc.M105020200. [DOI] [PubMed] [Google Scholar]
- 94.Ximenes HM, Kamagate A, Van Eylen F, Carpinelli A, Herchuelz A. Opposite effects of glucose on plasma membrane Ca2+-ATPase and Na/Ca exchanger transcription, expression, and activity in rat pancreatic beta-cells. J Biol Chem. 2003;278:22956–63. doi: 10.1074/jbc.M212339200. [DOI] [PubMed] [Google Scholar]
- 95.Kip SN, Strehler EE. Vitamin D3 upregulates plasma membrane Ca2+-ATPase expression and potentiates apico-basal Ca2+ flux in MDCK cells. Am J Physiol Renal Physiol. 2004;286:F363–9. doi: 10.1152/ajprenal.00076.2003. [DOI] [PubMed] [Google Scholar]
- 96.Glendenning P, Ratajczak T, Dick IM, Prince RL. Regulation of the 1b isoform of the plasma membrane calcium pump by 1,25-dihydroxyvitamin D3 in rat osteoblast-like cells. J Bone Miner Res. 2001;16:525–34. doi: 10.1359/jbmr.2001.16.3.525. [DOI] [PubMed] [Google Scholar]
- 97.Pannabecker TL, Chandler JS, Wasserman RH. Vitamin-D-dependent transcriptional regulation of the intestinal plasma membrane calcium pump. Biochem Biophys Res Commun. 1995;213:499–505. doi: 10.1006/bbrc.1995.2159. [DOI] [PubMed] [Google Scholar]
- 98.Kim MH, Lee GS, Jung EM, Choi KC, Jeung EB. The negative effect of dexamethasone on calcium-processing gene expressions is associated with a glucocorticoid-induced calcium-absorbing disorder. Life Sci. 2009;85:146–52. doi: 10.1016/j.lfs.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 99.Wang C, Dostanic S, Servant N, Chalifour LE. Egr-1 negatively regulates expression of the sodium-calcium exchanger-1 in cardiomyocytes in vitro and in vivo. Cardiovasc Res. 2005;65:187–94. doi: 10.1016/j.cardiores.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 100.Kasneci A, Kemeny-Suss NM, Komarova SV, Chalifour LE. Egr-1 negatively regulates calsequestrin expression and calcium dynamics in ventricular cells. Cardiovasc Res. 2009;81:695–702. doi: 10.1093/cvr/cvn357. [DOI] [PubMed] [Google Scholar]
- 101.Saadane N, Alpert L, Chalifour LE. Expression of immediate early genes, GATA-4, and Nkx-2.5 in adrenergic-induced cardiac hypertrophy and during regression in adult mice. Br J Pharmacol. 1999;127:1165–76. doi: 10.1038/sj.bjp.0702676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yan SF, Mackman N, Kisiel W, Stern DM, Pinsky DJ. Hypoxia/Hypoxemia-Induced activation of the procoagulant pathways and the pathogenesis of ischemia-associated thrombosis. Arterioscler Thromb Vasc Biol. 1999;19:2029–35. doi: 10.1161/01.atv.19.9.2029. [DOI] [PubMed] [Google Scholar]
- 103.Saadane N, Alpert L, Chalifour LE. Altered molecular response to adrenoreceptor-induced cardiac hypertrophy in Egr-1-deficient mice. Am J Physiol Heart Circ Physiol. 2000;278:H796–805. doi: 10.1152/ajpheart.2000.278.3.H796. [DOI] [PubMed] [Google Scholar]
- 104.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–75. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 105.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–86. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao XS, Shin DM, Liu LH, Shull GE, Muallem S. Plasticity and adaptation of Ca2+ signaling and Ca2+-dependent exocytosis in SERCA2(+/-) mice. Embo J. 2001;20:2680–9. doi: 10.1093/emboj/20.11.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Andersson KB, Birkeland JA, Finsen AV, Louch WE, Sjaastad I, Wang Y, Chen J, Molkentin JD, Chien KR, Sejersted OM, Christensen G. Moderate heart dysfunction in mice with inducible cardiomyocyte-specific excision of the Serca2 gene. J Mol Cell Cardiol. 2009;47:180–7. doi: 10.1016/j.yjmcc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 109.Strehler EE, Filoteo AG, Penniston JT, Caride AJ. Plasma-membrane Ca(2+) pumps: structural diversity as the basis for functional versatility. Biochem Soc Trans. 2007;35:919–22. doi: 10.1042/BST0350919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oceandy D, Stanley PJ, Cartwright EJ, Neyses L. The regulatory function of plasma-membrane Ca(2+)-ATPase (PMCA) in the heart. Biochem Soc Trans. 2007;35:927–30. doi: 10.1042/BST0350927. [DOI] [PubMed] [Google Scholar]
- 111.Wu X, Chang B, Blair NS, Sargent M, York AJ, Robbins J, Shull GE, Molkentin JD. Plasma membrane Ca2+-ATPase isoform 4 antagonizes cardiac hypertrophy in association with calcineurin inhibition in rodents. J Clin Invest. 2009;119:976–85. doi: 10.1172/JCI36693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luo X, Jiang N, Tandan S, Hojayev B, Rakalin A, Rothermel BA, Hill JA. Activation of Stim1-mediated Store-operated Calcium (SOC) Influx in Calcineurin-dependent Cardiac Hypertrophy. Circulation. 2009;120:S664. [Google Scholar]
- 113.Ji W, Xu P, Li Z, Lu J, Liu L, Zhan Y, Chen Y, Hille B, Xu T, Chen L. Functional stoichiometry of the unitary calcium-release-activated calcium channel. Proc Natl Acad Sci U S A. 2008;105:13668–73. doi: 10.1073/pnas.0806499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marks AR. Calcium and the heart: a question of life and death. J Clin Invest. 2003;111:597–600. doi: 10.1172/JCI18067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McKinsey TA, Olson EN. Cardiac hypertrophy: sorting out the circuitry. Curr Opin Genet Dev. 1999;9:267–74. doi: 10.1016/s0959-437x(99)80040-9. [DOI] [PubMed] [Google Scholar]
- 116.Voelkers M, Salz M, Herzog N, Frank D, Dolatabadi N, Frey N, Gude N, Friedrich O, Koch WJ, Katus HA, Sussman MA, Most P. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol. 2010;48:1329–34. doi: 10.1016/j.yjmcc.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ohba T, Watanabe H, Murakami M, Sato T, Ono K, Ito H. Essential role of STIM1 in the development of cardiomyocyte hypertrophy. Biochem Biophys Res Commun. 2009;389:172–6. doi: 10.1016/j.bbrc.2009.08.117. [DOI] [PubMed] [Google Scholar]
- 118.Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien YC, Wang Y, Bhanumathy CD, Subbiah R, Ritchie MF, Soboloff J, Baba Y, Kurosaki T, Joseph SK, Gill DL, Madesh M. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–7. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 120.Reeves JP, Bailey CA, Hale CC. Redox modification of sodium-calcium exchange activity in cardiac sarcolemmal vesicles. J Biol Chem. 1986;261:4948–55. [PubMed] [Google Scholar]
- 121.Cerbai E, Ambrosio G, Porciatti F, Chiariello M, Giotti A, Mugelli A. Cellular electrophysiological basis for oxygen radical-induced arrhythmias. A patch-clamp study in guinea pig ventricular myocytes. Circulation. 1991;84:1773–82. doi: 10.1161/01.cir.84.4.1773. [DOI] [PubMed] [Google Scholar]
- 122.Matsuura H, Shattock MJ. Membrane potential fluctuations and transient inward currents induced by reactive oxygen intermediates in isolated rabbit ventricular cells. Circ Res. 1991;68:319–29. doi: 10.1161/01.res.68.2.319. [DOI] [PubMed] [Google Scholar]
- 123.Bloomer RJ, Goldfarb AH. Anaerobic exercise and oxidative stress: a review. Can J Appl Physiol. 2004;29:245–63. doi: 10.1139/h04-017. [DOI] [PubMed] [Google Scholar]
- 124.Aoi W, Naito Y, Takanami Y, Kawai Y, Sakuma K, Ichikawa H, Yoshida N, Yoshikawa T. Oxidative stress and delayed-onset muscle damage after exercise. Free Radic Biol Med. 2004;37:480–7. doi: 10.1016/j.freeradbiomed.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 125.Park CY, Shchlegovitov A, Dolmetsch RE. The store operated calcium channel activator STIM1 binds and inhibits L-type voltage gated calcium channels. Science. 2010 doi: 10.1126/science.1191027. in press. [DOI] [PubMed] [Google Scholar]
- 126.Ohba M, Shibanuma M, Kuroki T, Nose K. Production of hydrogen peroxide by transforming growth factor-beta 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J Cell Biol. 1994;126:1079–88. doi: 10.1083/jcb.126.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.FitzGerald UF, Gilbey T, Brodie S, Barnett SC. Transcription factor expression and cellular redox in immature oligodendrocyte cell death: effect of Bcl-2. Mol Cell Neurosci. 2003;22:516–29. doi: 10.1016/s1044-7431(02)00040-4. [DOI] [PubMed] [Google Scholar]
- 128.Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–60. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien YC, Wang Y, Bhanumathy CD, Subbiah R, Ritchie MF, Soboloff J, Baba Y, Kurosaki T, Joseph SK, Gill DL, Madesh M. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin H, Sue YM, Chou Y, Cheng CF, Chang CC, Li HF, Chen CC, Juan SH. Activation of a nuclear factor of activated T-lymphocyte-3 (NFAT3) by oxidative stress in carboplatin-mediated renal apoptosis. Br J Pharmacol. 2010 doi: 10.1111/j.1476-5381.2010.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu Y, Borchert GL, Surazynski A, Hu CA, Phang JM. Proline oxidase activates both intrinsic and extrinsic pathways for apoptosis: the role of ROS/superoxides, NFAT and MEK/ERK signaling. Oncogene. 2006;25:5640–7. doi: 10.1038/sj.onc.1209564. [DOI] [PubMed] [Google Scholar]
- 132.Lee B, Cao R, Choi YS, Cho HY, Rhee AD, Hah CK, Hoyt KR, Obrietan K. The CREB/CRE transcriptional pathway: protection against oxidative stress-mediated neuronal cell death. J Neurochem. 2009;108:1251–65. doi: 10.1111/j.1471-4159.2008.05864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 134.Lee HC, Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. 2005;37:822–34. doi: 10.1016/j.biocel.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 135.Kronke G, Bochkov VN, Huber J, Gruber F, Bluml S, Furnkranz A, Kadl A, Binder BR, Leitinger N. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J Biol Chem. 2003;278:51006–14. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- 136.Bedogni B, Pani G, Colavitti R, Riccio A, Borrello S, Murphy M, Smith R, Eboli ML, Galeotti T. Redox regulation of cAMP-responsive element-binding protein and induction of manganous superoxide dismutase in nerve growth factor-dependent cell survival. J Biol Chem. 2003;278:16510–9. doi: 10.1074/jbc.M301089200. [DOI] [PubMed] [Google Scholar]
- 137.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–83. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 138.Lin HY, Michtalik HJ, Zhang S, Andersen TT, Van Riper DA, Davies KK, Ermak G, Petti LM, Nachod S, Narayan AV, Bhatt N, Crawford DR. Oxidative and calcium stress regulate DSCR1 (Adapt78/MCIP1) protein. Free Radic Biol Med. 2003;35:528–39. doi: 10.1016/s0891-5849(03)00358-7. [DOI] [PubMed] [Google Scholar]
- 139.Gorlach J, Fox DS, Cutler NS, Cox GM, Perfect JR, Heitman J. Identification and characterization of a highly conserved calcineurin binding protein, CBP1/calcipressin, in Cryptococcus neoformans. Embo J. 2000;19:3618–29. doi: 10.1093/emboj/19.14.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fuentes JJ, Genesca L, Kingsbury TJ, Cunningham KW, Perez-Riba M, Estivill X, de la Luna S. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet. 2000;9:1681–90. doi: 10.1093/hmg/9.11.1681. [DOI] [PubMed] [Google Scholar]
- 141.Kingsbury TJ, Cunningham KW. A conserved family of calcineurin regulators. Genes Dev. 2000;14:1595–604. [PMC free article] [PubMed] [Google Scholar]
- 142.Reiter TA, Abraham RT, Choi M, Rusnak F. Redox regulation of calcineurin in T-lymphocytes. J Biol Inorg Chem. 1999;4:632–44. doi: 10.1007/s007750050387. [DOI] [PubMed] [Google Scholar]
- 143.Buch MH, Pickard A, Rodriguez A, Gillies S, Maass AH, Emerson M, Cartwright EJ, Williams JC, Oceandy D, Redondo JM, Neyses L, Armesilla AL. The sarcolemmal calcium pump inhibits the calcineurin/nuclear factor of activated T-cell pathway via interaction with the calcineurin A catalytic subunit. J Biol Chem. 2005;280:29479–87. doi: 10.1074/jbc.M501326200. [DOI] [PubMed] [Google Scholar]
- 144.Sharikabad MN, Ostbye KM, Brors O. Effect of hydrogen peroxide on reoxygenation-induced Ca2+ accumulation in rat cardiomyocytes. Free Radic Biol Med. 2004;37:531–8. doi: 10.1016/j.freeradbiomed.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 145.Temsah RM, Netticadan T, Chapman D, Takeda S, Mochizuki S, Dhalla NS. Alterations in sarcoplasmic reticulum function and gene expression in ischemic-reperfused rat heart. Am J Physiol. 1999;277:H584–94. doi: 10.1152/ajpheart.1999.277.2.H584. [DOI] [PubMed] [Google Scholar]
- 146.Gen W, Tani M, Takeshita J, Ebihara Y, Tamaki K. Mechanisms of Ca2+ overload induced by extracellular H2O2 in quiescent isolated rat cardiomyocytes. Basic Res Cardiol. 2001;96:623–9. doi: 10.1007/s003950170014. [DOI] [PubMed] [Google Scholar]