Abstract
Inner ear diseases are common and often result in hearing disability. Sensorineural hearing loss is the main cause of hearing disability. So far, no effective treatment is available although some patients may benefit from a hearing aid equipped with a hearing amplifier or from cochlear implantation. Inner ear gene therapy has become an emerging field of study for the treatment of hearing disability. Numerous new discoveries and tremendous advances have been made in inner ear gene therapy including gene vectors, routes of administration, and therapeutic genes and targets. Gene therapy may become a treatment option for inner ear diseases in the near future. In this review, we summarize the current state of inner ear gene therapy including gene vectors, delivery routes, and therapeutic genes and targets by examining and analyzing publications on inner ear gene therapy from the literature and patent documents, and identify promising patents, novel techniques, and vital research projects. We also discuss the progress and prospects of inner ear gene therapy, the advances and shortcomings, with possible solutions in this field of research.
Sun and colleagues summarize the current state of inner ear gene therapy, including gene vectors, delivery routes, and therapeutic genes and targets. The authors also discuss the progress and prospects for inner ear gene therapy, the advances and shortcomings, with possible solutions.
Introduction
The inner ear is a highly differentiated peripheral sensory organ that lies in the temporal bones of both mammalian animals and human beings. It is composed of the cochlea, comprising the organ of Corti for detecting the mechanical vibrations of sound waves and the vestibular organ comprising the utricle, saccule, and ampullae for detecting linear motion (utricle and saccule) and curvilinear motion (ampullae). The sensory epithelium of the organ of Corti, the utricle and saccule, and the ampullae can transform mechanical stimuli to bioelectrical impulses that can be recognized by the brain. Then, the afferent neurons contacting the bottoms of the hair cells in these sensory epithelia transfer the impulses from hair cells to the auditory or vestibular nerve center located in the temporal lobe of the cerebrum via the brainstem (Brough, 2007). Therefore, the inner ear is the most important organ for perception of hearing and balance stimuli, which is crucial for an individual's responses to the environment, especially in dangerous circumstances. The hair cells and inner ear neurons are common pathological sites of inner ear diseases as well as the major targets for inner ear gene therapy (Brough, 2007).
There are three main types of organic hearing impairment: sensorineural hearing loss (SNHL; it could be further divided into cochlear deafness and retrocochlear deafness [central deafness]), conductive hearing loss, and mixed hearing loss (a combination of sensorineural and conductive loss) (Petersen and Willems, 2006). SNHL is due to a defect in the inner ear or the acoustic nerve whereas conductive hearing loss is due to a defect in the sound-conducting apparatus, that is, the external auditory canal or middle ear. Disabling hearing impairment (deafness) affected 250 million people in the world in 2001, much higher than previously estimated (World Health Organization, 2003). About two-thirds of the 250 million affected people were from developing countries. There are 110 million deaf persons in Southeast Asia, population 1.5 billion, with a 7.3% overall prevalence of deafness (World Health Organization, 2003). A population-based survey of ear and hearing disorders for 6626 persons in Guizhou Province of China demonstrated that the prevalence of hearing impairment is 17.1% (the standardized rate was 17.6% for the whole country) and that of hearing disability is 6.1% (the standardized rate was 6.5% for the whole country), the rate being slightly lower than the global population prevalence of 7.3% (Wang et al., 2007). About 35 million Americans have detectable hearing loss, affecting approximately 1.7% of the people under the age of 18 years, 31.4% over the age of 65, and 40–50% over 75 and older. Two or three of every 1000 children are born deaf or hard-of-hearing in the United States (National Institute on Deafness and Other Communication Disorders, 2007). The main cause of hearing disability is SNHL, usually resulting from hypoxia–ischemia of the cochlea, ototoxicity, noise injury, viral infection, and heredity or gene mutation. SNHL can be inherited as an autosomal dominant or recessive pattern, with 90% as autosomal recessive. Unfortunately, no substantial therapy can reverse SNHL so far. Although hearing loss is not a life-threatening disease it affects more people than any other disease and negatively impacts the quality of life of numerous patients and their families. Therefore, it is urgent that an effective treatment for this disease be found.
The concept of gene therapy was first introduced in the early 1970s (Osterman et al., 1970; Qasba and Aposhian, 1971). Gene therapy may be defined as a technique or approach to treat and/or prevent a disease by introducing a desired foreign gene or gene-regulatory element, such as RNA interference, into the target cells to replace or fix the defective gene (Mulligan, 1993). Because of the risk of degradation of the foreign nucleic acid by nucleases in the body, the therapeutic gene must be carried and protected by a vehicle to help it enter the target cells at an adequate level. The vehicle for carrying the therapeutic gene is now referred to as the gene vector (Zaki et al., 2007). The gene vector is a crucial element among the four major elements of gene therapy: the therapeutic gene, the gene vector, the route of gene administration, and the target cells.
The study of gene therapy for the inner ear started in the mid-1990s (Fujiyoshi et al., 1994). The study of inner ear gene therapy has become an emerging field and it may bring a glimmer of hope for successful treatment of hearing disability. However, it is still at the experimental stage and has a long way to go from translating the success of laboratory research into clinical practice.
Initiation of Inner Ear Gene Therapy
To our knowledge, the first study of gene therapy for hearing disorders was reported in 1994 by Fujiyoshi and colleagues. They generated myelin basic protein (MBP) transgenic mice by microinjected an MBP cosmid clone into the pronucleus of fertilized eggs of shiverer mice to replace the autosomal recessive mutation (deletion) gene for MBP. The MBP transgenic mice were found to recover up to 25% of normal levels of MBP and to have a greater number of myelinated axons than did control mice. In addition, the interpeak latencies of the auditory brainstem response were shortened in the transgenic mice than in the control mice. In 1996 foreign genes were successfully transfected into the inner ear, using replication-deficient viral vectors (Lalwani et al., 1996; Raphael et al., 1996). The inner ear, especially the cochlea, is a highly differentiated and extremely precise electrophysiological organ with a fine-spun anatomical structure, so any improper intervention during the process of gene transfection may result in undesired morphological and/or functional damage to the inner ear. Thus, it would be more difficult to transfer an exogenous gene into the inner ear than into other organs. For example, the blood–labyrinth barrier prevents macromolecules moving from the peripheral blood into the inner ear.
Therapeutic Genes for Inner Ear Gene Therapy
Therapeutic genes encoding proteins/peptides for inner ear gene therapy may be divided into two major groups: inner ear protectors and transdifferentiation activators. These therapeutic molecules have rapidly increased in number as research in this field has advanced. The therapeutic genes used for inner ear gene therapy are summarized in the Table 1.
Table 1.
Therapeutic Genes Used for Inner Ear Gene Therapy
| Gene | Main target | Protective effect |
|---|---|---|
| Neurotrophic factors | Hair cells, neurons | Comprehensive protection, such as antiapoptosis, antioxidation, and modulation of neuronal physiological and biochemical activities |
| 1. Neurotrophin family | ||
| 2. Glial cell line-derived neurotrophic factor family | ||
| 3. Ciliary neurotrophic factor family | ||
| 4. Fibroblast growth factor family | ||
| 5. Other neurotrophically acting factors such as epidermal growth factor (EGF), transforming growth factor (TGF), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), and hepatocyte growth factor (HGF) | ||
| Otospiralin | Nonsensory epithelial cells of the inner ear | Maintaining normal structure and function of the fibrocytes and other nonsensory epithelial cells in inner ear |
| Antiapoptotic agents | Hair cells, neurons, other inner ear cells | Antiapoptosis |
| XIAP, IAPs, and Bcl-2 family | ||
| Connexins | Gap junctions in inner ear | Maintaining normal structure and function of the gap junction of the inner ear |
| Such as Cx26 (GJB2), Cx30 (GJB6), Cx31 (GJB3), and Cx43 (GJA1) | ||
| Atonal-related factors or bHLH transcription factors | Supporting cells of the organ or Corti and its adjacent nonsensory epithelial cells | Inducing a phenotypic trans-differentiation from nonsensory cells to the hair cells |
| Such as Math1, Cath1, Xath1, and Hath1 |
bHLH, basic helix–loop–helix; IAP, inhibitor of apoptosis; XIAP, X-linked IAP.
Genes for protection of the inner ear and hereditary single-gene defects
Neurotrophic factors are a large group of biologically active peptides, most of them able to protect inner ear hair cells and spiral ganglion neurons from the damage caused by various pathogenic factors and to promote recovery from cochlear injury. Normally, neurotrophic factors in the inner ear are produced mainly by hair cells to maintain normal function and the survival of cochlear hair cells and neurons. The neurotrophic factors play an important role in cellular differentiation, proliferation, development, neuronal plasticity, and cellular survival, not only in the embryonic stage but also throughout life. So far, more than 20 neurotrophic factors have been revealed with protective effects on inner ear cells. These factors belong to one of the following groups: (1) the neurotrophin (NT) family including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophins 3–7 (NT-3, NT-4/5, NT-7); (2) the glial cell line-derived neurotrophic factor (GDNF) family; (3) the ciliary neurotrophic factor (CNTF) family; (4) the fibroblast growth factor (FGF) family including at least 17 members, with acidic fibroblast growth factor (aFGF or FGF-1) and basic fibroblast growth factor (bFGF or FGF-2) the most extensively studied factors; and (5) other neurotrophically acting factors including but not limited to epidermal growth factor (EGF), transforming growth factor (TGF), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), hepatocyte growth factor (HGF), and so on (Oshima et al., 2004). It has been reported that infusion of either NT-3, BDNF, or NT-3 plus BDNF into the scala tympani of guinea pigs exposed to ototoxic agents (i.e., ototoxin-exposed cochleae) resulted in 78–90% survival of auditory neurons compared with 14–24% neuronal survival in untreated controls (Staecker et al., 1996). It is well established that neurotrophic factors, especially NT-3 and BDNF, can protect inner ear hair cells and neurons via antiapoptosis, antioxidation, and modulation of neuronal physiological and biochemical activities. A large number of documents have demonstrated the successful transfection and expression of neurotrophic factors in the inner ear, mediated by both viral and nonviral vectors in vitro and in vivo (Staecker et al., 1996; Noushi et al., 2005; Jiang et al., 2007). It is interesting that either BDNF or NT-3 may have protective effects on inner ear hair cells and neurons even 1 month after deafness has occurred. Of course, the earlier the treatment with a therapeutic gene/protein, the better the outcome (Miller et al., 2007). Therefore, neurotrophic factor genes have become the preferred therapeutic genes for inner ear gene therapy. Besides BDNF and NT-3, other neurotrophic factors, such as TGF, GDNF, FGF, CNTF, and HGF, also have protective effects to different extents on inner ear hair cells and neurons (Pickles et al., 1998; Stover et al., 2000; Kawamoto et al., 2003b; Nakaizumi et al., 2004).
Otospiralin is a newly discovered ear-specific protein produced by fibrocytes from the nonsensory epithelial regions of the inner ear, specifically by the spiral ligament and limbus in the cochlea, and the maculae and semicircular canals of the vestibule. These mesenchymal nonsensory epithelial tissues surrounding the neuroepithelium in the inner ear play an important role in maintaining ionic balance, which affects the normal structure and function of the inner ear. Otospiralin is a novel 6.4-kDa protein with unknown function and shares its protein motif with the Gag p30 core shell nucleocapsid protein of type C retroviruses (Delprat et al., 2002). Downregulation of otospiralin by cochlear perfusion with antisense oligonucleotides of otospiralin led to a rapid decrease in compound action potentials and irreversible deafness in guinea pigs (Delprat et al., 2002). The possible mechanism of deafness was hair cell loss and degeneration of the organ of Corti, as revealed by transmission electron microscopy. The result indicated that otospiralin is essential not only for normal function but also for survival of the cochlear sensory epithelium. The same research group further found that knocking out the Otos gene encoding otospiralin in mice led to degeneration of type II and IV fibrocytes and moderate hearing loss (Delprat et al., 2005). These findings suggested that loss of otospiralin would induce fibrocyte damage and lead to both structural and functional impairment of the inner ear. The loss of hair cells also indicated the importance of supporting cells for hair cell survival, and that the function of otospiralin is much more than just for “supporting” the hair cells in the right location. Upregulation of Otos gene expression through adenoviral vector-mediated gene transfection significantly decreased apoptosis induced by cisplatin treatment in cultured spiral ligament fibrocytes (Zhuo et al., 2008). Taken together, these findings indicate that otospiralin may be a potential protective molecule for the inner ear. In addition, it may be possible to employ a specific antibody to otospiralin as the targeting element of a gene vector for inner ear gene therapy, based on the specificity of otospiralin for the inner ear.
In addition to the conventional inhibitors of the families of inhibitor-of-apoptosis proteins (IAPs) and Bcl-2, the most convincing antiapoptotic agent that may be used for inner ear gene therapy is the X-linked inhibitor of apoptosis protein (XIAP), a member of the IAP family. It is an extremely potent suppressor of apoptosis and selectively binds and inhibits caspase-3, caspase-7, and caspase-9 (Deveraux et al., 1997). It was discovered that XIAP inhibited apoptosis of cochlear cells under various conditions such as age-related hearing loss and cisplatin-induced deafness (Cooper et al., 2006; Wang et al., 2008). Studies demonstrated that the severity of hearing loss induced by cisplatin was significantly reduced by XIAP (Cooper et al., 2006). The experiments were carried out by delivering the XIAP gene by an adeno-associated viral (AAV) vector into the inner ear of rats via round window membrane (RWM) injection. At least 2 months later, the rats were treated with cisplatin and the auditory-evoked brainstem response (ABR) threshold and hair cells were investigated 72 hr after cisplatin treatment. The results showed that cochleae transfected with AAV encoding XIAP were significantly protected from cisplatin-induced ototoxicity by the antiapoptosis effects of XIAP, that is, the ABR threshold shift and hair cell loss were significantly decreased (78 and 45%, respectively) in treated cochleae compared with untreated contralateral cochleae. The finding suggested that XIAP may be a promising gene for inner ear gene therapy.
Connexins, the major proteins of gap junctions, have been demonstrated to be crucial for the maintenance of hearing capacity, and may represent a new target for inner ear gene therapy. Mutations of genes encoding connexins may lead to hearing impairment, especially for nonsyndromic hereditary deafness, which accounts for 70% of inherited hearing impairment (Cohen-Salmon et al., 2005). Mutations are most frequently detected in the Cx26 gene encoding connexin-26, which is responsible for as much as 49% of nonsyndromic deafness. More than 100 causative mutations in Cx26 (GJB2) have been found and account for a large proportion of prelingual deafness (Hoang Dinh et al., 2009). Other relatively common mutations have been detected in Cx30 (GJB6), Cx31 (GJB3), and Cx43 (GJA1) (Cohen-Salmon et al., 2005). A susceptibility gene for nonsyndromic sensorineural autosomal deafness (DFNB1) was identified to link to chromosome 13q11-12, where the Cx26 gene is localized (Kelsell et al., 1997). This finding indicates that connexin-26 is an important component in the cochlea. Connexin-26 exists in gap junctions connecting many types of cells in the cochlea, including the epithelial cells and connective tissues (Kikuchi et al., 2000). Therefore, it would certainly interfere with the intracellular and/or intercellular internal environments or signaling pathways if the cochlear gap junctions were damaged by a connexin gene mutation. The lesion pattern and time course of cellular degeneration in the cochlea of conditional Cx26 (cCx26) null and Cx30 null mice has been investigated (Sun et al., 2009). It was observed in that study that cellular degeneration in the cochlea of cCx26 null mice was much more rapid and widespread than in Cx30 null mice. This result suggests that different deafness mechanisms may exist in spite of the coassembly of Cx26 and Cx30 in forming gap junctions in the cochlea. In addition, one of the functions of the gap junction system is to recirculate K+ ions from hair cells to the strial marginal cells. Interruption of the recirculation of K+ ions would disrupt the ionic balance in the cochlea and result in hearing loss (Kikuchi et al., 2000). Considering their importance in maintaining the morphology and function of the inner ear, connexin gene therapy may become a treatment for a large number of cases of hereditary deafness in the embryonic or even early postnatal stage by replacing the mutated connexin gene of the patients with a specific therapeutic gene. Unfortunately, no such successful case has yet been reported.
Genes for cell regeneration of the inner ear
In general, the highly differentiated hair cells and neurons of the mammalian inner ear cannot be replaced through cellular regeneration if they are damaged after birth (Raphael, 2002). There is no evidence of regeneration of the mammalian auditory sensory epithelium in vivo except for a low degree of regeneration of the vestibular epithelium in rodents just after birth. Encouraged by the full regeneration of avian statoacoustic epithelia after inner ear damage, attempts have been made to activate the potential capacity of regenerating functional sensory epithelium of the inner ear after impairment in mammals. Early studies showed that the adult utricular sensory epithelium of mice displayed the characteristic features of stem cells, having the capacity for self-renewal and forming spheres capable of expressing marker genes for the development of the inner ear and nervous system (Li et al., 2003). This experiment provided evidence for the possibility of sensory epithelium regeneration in the mammalian inner ear. The formation of regenerated hair cell-like cells strongly indicates that inner ear stem cells are pluripotent and that damaged hair cells may be replaced by regenerated cells via proper gene transfer (Kawamoto et al., 2003a). Atonal homolog-1 (Atoh1, encoded by Atoh1; also known as Math1) plays an important role in the differentiation of hair cells of the developing inner ear. It was originally isolated from Drosophila chordotonal organs as a proneural gene. The homologs of homolog-1 were called atonal-related factors or basic helix–loop–helix (bHLH) transcription factors, and include Math1, Cath1, Xath1, and Hath1, with Math1 being the most extensively studied factor. Studies demonstrated that mouse atonal homolog-1 (Math1) was essential for the generation of hair cells (Bermingham et al., 1999). Math1 is a positive regulator of hair cell differentiation during cochlear development and is expressed only in the developing stage of the hair cells. It is a bHLH transcription factor homolog of the Drosophila atonal gene. bHLH transcription factors modulate the development of several systems of both vertebrates and invertebrates, and also play an important role in inner ear hair cell differentiation (Kawamoto et al., 2003a). These homologs of bHLH with similar structure and function were called atonal-related factors or bHLH transcription factors, and include Math1, Cath1 (chicken atonal homolog-1), Xath1 (Xenopus atonal homolog-1), and Hath1 (human atonal homolog-1). It has been reported that Math1 was expressed in the supporting cells of the organ of Corti and its adjacent nonsensory epithelial cells by endolymphatic perfusion of adenoviral vector loaded with Math1 gene into mature guinea pig cochlea (Kawamoto et al., 2003a). It led to the appearance of immature hair cells in the organ of Corti and new hair cells within the interdental cells, inner sulcus, and Hensen cell regions. In addition, the axons of ganglion neurons were attached to some of the newly developed hair cells. Therefore, a possible promising strategy for restoring hearing capacity is to induce phenotypic transdifferentiation of nonsensory cells retaining the competence of response to Math1 or other atonal-related factors in the damaged inner ear to regenerate new hair cells or sensory epithelia with normal morphological and functional properties. On the other hand, bHLH-related inhibitors of differentiation and DNA-binding (Id) proteins are known to negatively regulate many bHLH transcription factors, including Math1, in a number of different systems. Id1, Id2, and Id3 are expressed within the cochlear duct and the Ids play an important role in the regulation of expression of Math1 and hair cell differentiation in the developing cochlea (Jones et al., 2006). An interesting study has reported that numerous newly developed hair cells were observed in the outer hair cell region of the cochlea of guinea pigs after the delivery of atonal gene by adenoviral vectors into the cochlea, the hair cells of which were almost totally damaged by injection of kanamycin and ethacrynic acid before the transfection of exogenous gene (Izumikawa et al., 2005). The most significant result of this study was that the average threshold of the auditory brainstem response (ABR) of the treated animals was significantly lower than that of the control group, which indicated that transfection of atonal gene into the inner ear could help hearing recovery. To our knowledge, this is the first report of successful regeneration of hair cells with hearing improvement in the experimentally profoundly deafened mature mammalian cochlea. There is a possibility that transfection of the genes encoding atonal-related factors into the inner ear may lead to an exciting breakthrough in the regeneration of inner ear hair cells in mammals. However, the success of atonal gene transfection is currently debated and discussed with caution. This study would strongly imply the feasibility of hair cell regeneration in the mammalian cochlea if the result could be confirmed by other laboratories. Studies from two separate research groups demonstrated regeneration of vestibular hair cells or ectopic vestibular hair cell-like cells in the rodent by adenoviral vector-mediated delivery of the Math1 gene (Staecker et al., 2007; Huang et al., 2009). However, no other successful experiment has been reported on Atoh1-induced cochlear hair cell regeneration from other laboratories. It will therefore take more time to translate laboratory success into the clinical setting for the generation of cochlear hair cells with natural morphology and function. It should be remembered that regenerated or newly developed hair cells induced by a foreign gene are not the same as natural and functional hair cells in terms of spatial location, histological and ultrastructural morphology, or physiological and biochemical properties. Not all of the cellular signal pathways and regulating elements necessary for natural hair cell regeneration, which have developed during the long process of evolution in the avian inner ear, have been clearly elucidated. Sequences of the Math1 and Hath1 genes are publicly available at GenBank (www.ncbi.nlm.nih.gov/genbank/).
Vectors for Inner Ear Gene Therapy
The success of gene therapy is dependent mainly on the development of a vector or vehicle (Li and Huang, 2000). An ideal gene delivery vector should be able to (1) reach the target tissues/organs in vivo, preferably with recognition of the specific target cells; (2) cross the membranous barriers of cells and deliver its cargo into the cells; (3) easily control the intensity and duration of foreign gene expression with the precondition of high efficiency of expression; (4) biodegrade to ensure biological safety without carcinogenicity, immunogenicity, or cytotoxicity; and (5) be manufactured on a commercial scale for straightforward clinical use. Gene delivery vectors may be divided into viral and nonviral vectors. A virus (polyoma pseudovirus) was used as a vehicle for gene therapy in the earliest studies (Osterman et al., 1970; Qasba and Aposhian, 1971). For purposes of safety, the virus was modified before gene transfection by deleting partial sequences related to its replication, to prevent harming the host. The most significant advantage of the viral vector is its high efficiency of transfection. However, its clinical application is limited by various significant shortcomings including immunogenicity and carcinogenicity, difficulty in production, lack of selectivity for specific target cells, and the possibility of causing disease due to reversion of the engineered replication-deficient virus to a wild-type virus (Bermingham et al., 1999). On the other hand, nonviral vectors have virtually unlimited loading capacity and may be produced on a large scale without the risk of immunogenicity and carcinogenicity; however, low transfection efficiency has limited their use (Bermingham et al., 1999; Li et al., 2003). In this review, the gene vectors described were not developed especially for inner ear gene transfer but can be used for this purpose.
Viral vectors
Numerous replication-deficient viruses have been modified and developed as viral vector systems. The commonly used viruses include, but are not limited to, adenovirus; adeno-associated virus (AAV); retrovirus, including lentivirus; herpes simplex virus (HSV); and hemagglutinating virus of Japan (HVJ, Sendai virus), a member of the paramyxovirus family. Adenovirus-mediated atonal gene transfection of the inner ear may be the most exciting progress in inner ear gene therapy; it effectively activated the regeneration of cochlear hair cells in the mature ear of mammals (Bermingham et al., 1999). After in utero delivery of lentivirus and an array of recombinant AAV serotypes to the developing mouse otocyst, AAV2/1 was found to be the optimal vector for in utero cochlear gene transfer in terms of efficiency and cellular specificity of transgene expression (Bedrosian et al., 2006). It efficiently transduced progenitors, giving rise to both inner and outer hair cells and supporting cells with no adverse effect on cochlear cell differentiation and no pathological effect on differentiated hair cells or the integrity of the auditory nerve or brainstem nuclei as measured by auditory brainstem response testing (Bedrosian et al., 2006).
A modified adenoviral gene delivery vector with several elements was invented by Kadan and colleagues (2001). The vector consists of a genome with an adenoviral 5′ inverted terminal repeat (ITR), 3′ ITR, and encapsidation signal, a DNA sequence (therapeutic gene) encoding a heterologous protein or polypeptide, and a promoter for controlling expression of the DNA sequence. In addition, portions of the E1, E4, E2a and/or E2 DNA sequences were deleted to eliminate harmful elements of the proteins. This vector has the advantages of minimizing the host's immunological response to the vector, prolonging the duration of the vector's existence, and increasing gene expression. A new viral vector was constructed with adenovirus type 11p (Ad 11p) and type 4p (Ad 4p) as the backbone of the vector system (Wadell et al., 2002). This vector was able to deliver transfected gene into cells of neural origin, especially human cells of neural origin. Adenovirus type 11p is suitable as a gene vector because of its relatively low prevalence in the human population, and its capacity for high-affinity and excessive infection. A pharmaceutical preparation for injection was invented for the gene therapy of hearing impairment (Kaneda et al., 2006). It was composed of a viral envelope vector, using HVJ as the backbone, and plasmid DNA into which a hepatocyte growth factor (HGF) gene was inserted as the therapeutic gene. HGF was overexpressed in the cochlea after injection of the preparation into the subarachnoid space of Sprague-Dawley rats made deaf by administration of kanamycin.
Nonviral vectors
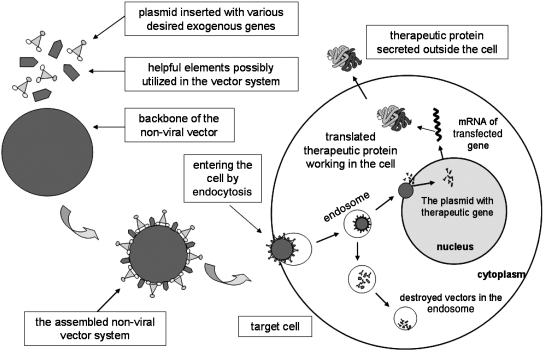
The basic unit of a nonviral vector system consists of a backbone of nonviral materials and plasmid DNA into which is inserted a therapeutic and/or reporter gene. The simplest approach for nonviral delivery system is direct gene transfer with naked plasmid DNA (Li and Huang, 2000). The commonly used nonviral vectors are cationic polymer, cationic liposome, and other inorganic nanoparticles, usually with positive charges on their surfaces. The vector combines with its cargo (the foreign nucleic acid) via negative charges on the surface (electrostatic effect), and compresses it to a smaller size for better protection and transportation. There are several important advantages with nonviral vectors for gene delivery: (1) almost infinite structural variations of the molecules make the vectors easy to create with various functions; (2) the ability to form a complex with a plasmid carrying various desired genes; (3) possibly no immunogenicity, or carcinogenicity, and low or even no toxicity to the host; and (4) possibly becoming a targeting vector when bound to cell-specific ligands (Chattopadhyay et al., 2005). The major disadvantages for all nonviral vectors are the low transfection efficiency and transient expression in the host cells. The transfection process for nonviral gene vectors is illustrated in Fig. 1.
FIG. 1.
Schematic of gene therapy with a nonviral vector system.
The most extensively studied artificial polymers are polyethylenimine (PEI) and polyamidoamine dendrimer (PAMAM-D). The other commonly used nonviral vector is liposome, which was first described in 1965 (Bangham et al., 1965). Liposome became an important vector for drug and gene delivery after the discovery of its ability to protect biological molecules from degradation and increase cellular uptake. The most commonly used liposome formulation for delivering anionic molecules such as DNA is cationic liposome (Vagle et al., 2008). Inorganic nanoparticles may also be used as vectors for gene delivery. Inorganic nanoparticles have lower transfection efficiency compared with liposome or polymer; however, they have the advantages of easy preparation, relative convenience for storage, low cost, and, more importantly, manufacturing on a commercial scale (Mori et al., 2006; Sun et al., 2006, 2008; Son et al., 2007). It could become a more popular or even ideal gene vector if its transfection efficiency could be significantly increased.
The expression and distribution of a foreign gene in the host could be monitored in vivo when a nonviral vector was linked to an appropriate tracer. The key advantage of such a vector is that it allows monitoring of the expression and distribution of a foreign gene in vivo almost in real time (Hanawa, 2006; Sen and Gambhir, 2007). A gene delivery system with the ability to monitor foreign gene expression in vivo may provide an effective approach for gene therapy in the clinic, in a negative feedback fashion.
Inorganic nanoparticle vectors have been constructed for gene therapy. A nonviral gene delivery system was constructed on the basis of multisegment bimetallic nanorods (Salem et al., 2003). It could simultaneously bind with compacted DNA plasmids and targeting ligands in a spatial manner. This approach allows for precise control over the composition, size, and multifunctionality of the gene delivery system. A vector with superparamagnetic iron oxide nanoparticles (SNP) composed of magnetite (Fe3O4) for therapeutic molecule delivery into the inner ear of rats has been reported (Kopke et al., 2006). The embedded SNPs, coated with either dextran, silica, or poly(d,l-lactide-co-glycolide), were placed in the round window membrane (RWM) niche of the rat. The RWM of the experimental rat ear was positioned horizontally upward, and the head was placed on the surface of the center of a 4-inch cube 48-MGOe neodymium–iron–boron (NdFeB48) magnet, with the magnetic pole facing the opposite side of the rat's head. The experiment demonstrated that the forces generated by permanent magnetic fields were sufficient to pull SNPs into and across the RWM, entering the perilymphatic space of the cochlea. No significant toxicity was observed in the tested rats. Our laboratory successfully transferred the NT-3 gene into primary cultures of cochlear spiral ganglion neurons of neonatal mice in vitro and into cochlear spiral ganglion neurons of adult guinea pigs in vivo, using hydroxyapatite nanoparticles as the gene vector (Sun et al., 2006, 2008). Good compatibility was observed by MTT test. To our knowledge, this was the first report of a therapeutic gene being successfully transfected into the mammalian inner ear with an inorganic nanoparticle vector (Sun et al., 2006). Various new gene vectors comprising both viral and nonviral elements, called “multiplex gene vectors,” were developed by several laboratories. These new gene vectors could, at least to some extent, improve the outcomes of gene therapy in both laboratories and clinical settings. A new gene vector called “targeted artificial gene delivery” (TAGD) (Rozenberg et al., 2004) was invented. In addition, multiplex gene vectors were constructed, comprising a cationic polymer containing the desired nucleic acid (e.g., therapeutic gene) and a lipid-based vesicle encapsulating a membrane-active agent, such as viral envelope proteins or membrane-active peptides, to enhance the efficiency of foreign gene transfection into eukaryotic cells (Yu and Matsumoto, 2006). The multiplex gene vectors possess most of the advantages of both viral and nonviral vectors and they may represent the right approach for gene therapy in the future.
Routes of Administration for Inner Ear Gene Therapy
An ideal route for inner ear gene delivery should possess several features. First, the cargo-carrying gene vectors should be able to effectively access the inner ear without harm to the peripheral vestibular or auditory organs. Second, any impairment of the inner ear induced by the process should be minimal and acceptable compared with the benefit from gene therapy. Third, administration to the inner ear should be convenient, with easy operation and control. The routes of administration used for inner ear gene therapy are summarized in the Table 2.
Table 2.
Routes of Administration Used for Inner Ear Gene Therapy
| Route | Advantage | Disadvantage |
|---|---|---|
| Labyrinthine drilling or RWM injection | 1. Maximum drug/gene entering | 1. Higher risks of inducing damage and infection of the inner ear |
| 2. Minimal systemic interference | 2. Difficult manipulation | |
| Intrathecal injection | 1. Relatively convenient | Diffusing effect may harm other parts of central nervous system |
| 2. No harm to inner ear | ||
| Systemic application | 1. Convenient | Difficult to pass blood–labyrinthine barrier |
| 2. No harm to inner ear | ||
| Intratympanic approach or intact RWM permeation | 1. Relatively high drug/gene entering | 1. Difficult manipulation |
| 2. Minimal systemic interference | 2. Risk of infection of middle ear | |
| 3. Minimal harm to inner ear | 3. May do slight harm to RWM and inner ear | |
| Intrauterine approach | Start treatment in embryonic stage | 1. May harm embryo and/or mother |
| 2. Difficult manipulation | ||
| 3. Need for expensive equipment | ||
| Applied with implanted object (cochlear electrode) | Convenient (applied along with the implantation) | — |
RWM, round window membrane.
Transgene expression was observed in the contralateral (untransfected) cochlea and cerebrospinal fluid (CSF) on unilateral transfer of adenovirus-mediated lacZ reporter genes into the cochlea of guinea pigs by intrathecal injection (Stover et al., 2000). Successful transduction of both cochleae was achieved by direct injection of adenovirus-mediated lacZ reporter gene into the CSF intrathecally; no transduction was observed when injecting the reporter gene into the bloodstream. This result indicated that CSF may be a possible approach for inner ear gene transfer, and that the cochlear aqueduct may be the most likely route for transferring genes into the contralateral cochlea. It has been demonstrated that the systemic route of foreign gene administration is infeasible because of the existence of the blood–labyrinth barrier. A novel strategy for inner ear gene therapy was proposed, in which a combination of human hepatocyte growth factor (HGF) gene and HVJ envelope (HVJ-E) vector was delivered into the inner ear of rats by intrathecal injection of the viral vector into the CSF via the cisterna magna (Oshima et al., 2004). Transgene expression was detected in the spiral ganglion cells (SGCs) of rats made deaf by the administration of kanamycin, and hearing impairment was prevented or significantly recovered by HGF gene transfer before or 2 weeks after kanamycin treatment. However, the quantity of vector entering the inner ear is obviously limited via the CSF route, and foreign gene transfection into the CNS may result in unexpected side effects or even harm the CNS. Therefore, this approach should be used with caution.
Perilymphatic or endolymphatic perfusion of gene vectors through a tiny hole drilled in the bony wall of the labyrinth or via RWM injection with or without the help of an osmotic micropump is a conventional route for inner ear drug/gene delivery. It has the advantages of allowing the maximal amount of drug/gene to enter the inner ear with minimal systemic interference. Numerous reports have been published on the successful delivery via this approach of various foreign genes and genetically engineered therapeutic cells into inner ears, mediated by various vectors (Stover et al., 2000; Oshima et al., 2004; Okano et al., 2006; Akin et al., 2007; Kanzaki et al., 2007; Liu et al., 2007; Wenzel et al., 2007). Inner ear perfusion with a osmotic micropump may be a reasonable choice for continuing and steady delivery of a foreign gene (Luebke et al., 2001). An osmotic micropump may provide continuous drug/gene supply for several days to several months, depending on the requirements of the therapy and the capacity of the micropump. It is typically used for perilymphatic infusion. The invasive approach could directly deliver foreign genes into the inner ear with relatively higher efficiency and easier control to compensate for the disadvantage of transient gene expression mediated by nonviral gene vectors. However, it could dramatically increase the risks of damage to and infection of the inner ear. The potential for mechanical impairment and the complexity of manipulation may limit its clinical usage.
The round window is the only membranously sealed window on the bony labyrinth. The RWM is composed of three layers. The outer epithelial layer is nonciliated, but often contains microvilli. The middle layer contains a large amount of collagen and elastic fibers as well as fibrocytes. The inner layer is a continuation of the epithelial layer of the perilymphatic space (Miriszlai et al., 1978). The first study on RWM permeability to macromolecules with tritiated normal human serum albumin was carried out in an experimental cat model of otitis media (Goycoolea et al., 1980). The study demonstrated the feasibility of diffusion of smaller molecules (toxins and enzymes) through an intact RWM. A large number of studies have confirmed that the corresponding exogenous substances can be detected in the perilymph after placing albumin, steroid, antibiotics, anesthetics, and toxins on the RWM (Miriszlai et al., 1978; Anniko et al., 1989; Plontke et al., 2007, 2008). These experiments imply that the RWM is a potential route for delivery of biologically active molecules (drug or toxin) into the inner ear. Higher perilymphatic drug concentrations have been detected in many laboratories after delivering the drugs intratympanically through the intact RWM compared with other routes, such as the peritoneum and bloodstream. The molecules used for studies of RWM delivery include antibiotics, poly(lactic-glycolic acid) (PLGA), methylprednisolone, and so on (Tamura et al., 2005; Bird et al., 2007; Plontke et al., 2007). The intact RWM approach (intratympanic pathway) may provide higher a perilymphatic concentration of exogenous molecules with low drug dosage by bypassing the blood–labyrinth barrier. Meanwhile, it induces slight but acceptable structural impairment of the inner ear with minimal systemic interference, although it is relatively inconvenient for clinical practice compared with the oral or intravenous route. However, the molecular weight of the complexes consisting of exogenous gene, vector, and helper element is much higher than for tested molecules such as antibiotics and PLGA, and therefore it is more difficult to deliver a sufficient quantity of these complexes into the inner ear through the intact RWM. Transgenes were successfully expressed in a variety of cochlear tissues or cells by placing a Gelfoam cube absorbed with liposome or adenoviral vector on the RWM of mice (Jero et al., 2001). Several separate studies have confirmed the feasibility of inner ear gene transfer mediated by viral or nonviral vectors via the intact RWM (Suzuki et al., 2003; Zou et al., 2008). An ultrastructural study of the RWM revealed that the paracellular pathway is the major route by which the gene vector penetrates the RWM (Zou et al., 2010). The efficiency of foreign gene transfection will be significantly increased if the RWM is pretreated or simultaneously treated with a facilitating agent, such as histamine, local anesthetic phenol, or other chemicals (Chandrasekhar et al., 2000; Suzuki et al., 2003). A possible mechanism for the effects of facilitating agents on the RWM may involve damage of the RWM epithelium by the facilitator, thus enlarging the space between cells. The RWM offers an atraumatic route of administration to the inner ear. The intact RWM route may become an ideal approach for inner ear gene transfection compared with invasive gene delivery methods such as labyrinth drilling and RWM injection. Thus, facilitating agents are worthy of further study for promotion of the transfection efficiency of gene vectors.
Another novel method for delivering foreign genes into the inner ear has been proposed. Cochlear implant electrodes were coated with guinea pig fibroblasts transfected by an adenoviral vector carrying a brain-derived neurotrophic factor (BDNF) gene (Rejali et al., 2007). The researchers found that the BDNF-expressing electrodes had preserved more spiral ganglion neurons compared with control electrodes 48 days after implantation. Although the protective effect decreased in the higher cochlear turns, the result demonstrated the feasibility of combining cochlear implant therapy with ex vivo gene transfer to enhance the survival of spiral ganglion neurons. Again, another interesting method for inner ear gene delivery was carried out by delivering foreign genes into inner ear cells in the otocyst of a developing embryonic mouse via the uterus (Gubbels et al., 2008). A plasmid carrying genes encoding atonal homolog-1 and enhanced green fluorescent protein (GFP) was microinjected through the mouse uterus and into the fluid-filled cavity of the embryonic day 11.5 (E11.5) mouse otic vesicle, and then a directional, square-wave pulse train was delivered to electroporate ventral progenitor cells that give rise to the organ of Corti, allowing transfection of the foreign gene. Expression of hair cell marker myosin 7a (Myo7a) by Atoh1/GFP+ cells was detected in the otocyst 24 hr after the electroporation. These Atoh1/GFP+/Myo7a+ cells were present in the base, mid-base, and apex of the transfected cochleae. The cochlear stereotyped pattern of hair cells in the one inner and three outer rows was altered by overexpression of the Atoh1/GFP+/Myo7a+ cells, called supernumerary cells. There were phalloidin-positive epithelial protrusions on the apical surfaces of the cells at E18.5, which resembled immature stereociliary bundles, and lasted for 1 month after the birth. In addition, the experiment demonstrated that cochlear morphology and the hearing ability of transfected mice were not affected by the intrauterine gene transfer. This may be a promising new approach for inner ear gene delivery, especially for the treatment of hereditary hearing loss at the embryonic stage.
Animal Models for Inner Ear Gene Therapy
Animal models can play important roles in auditory research, particularly research aimed at improving cochlear prostheses, understanding central auditory plasticity, and developing strategies for inner ear rescue and repair (McFadden et al., 2002). Gene therapy in the inner ear is applied in animal models of ototoxicity and ischemia–reperfusion injury (Maiorana and Staecker, 2005). The animals used as models of inner ear gene therapy include mice, rats, guinea pigs, cats, and Mongolian gerbils.
As mentioned previously, a mouse model was used for cell–gene therapy with nonviral vectors for delivery of therapeutic molecules into the cochlea of the mouse inner ear. NIH3T3 cells were transfected with the brain-derived neurotrophic factor gene (Bdnf), using lipofection, and then transplanted into the mouse inner ear. Immunohistochemistry and Western blotting demonstrated the survival of grafted cells in the cochlea for up to 4 weeks after transplantation. A BDNF-specific enzyme-linked immunosorbent assay revealed a significant increase in BDNF production in the inner ear after transplantation of engineered cells (Okano et al., 2006). A gene knockout mouse model was used to evaluate the degeneration of type II and IV fibrocytes and hearing loss by knocking out the Otos gene encoding otospiralin (Delprat et al., 2005). Another interesting mouse model involved delivery of a foreign gene into the inner ear cells in the otocyst of a developing embryonic mouse via the uterus (Gubbels et al., 2008).
A rat model was used for the study of gene expression in the organ of Corti (OC) exposed to gentamicin, using DNA microarray technology to compare the expression profile of OC exposed to gentamicin with that of untreated OC of Sprague-Dawley rats (Nagy et al., 2004). In a rat model study for inner ear gene therapy, adenoviral vectors carrying the Math1 gene were constructed to examine their effect on vestibular epithelia in postnatal rats. The new ectopic vestibular hair cell-like cells induced by overexpression of Math1 were found in the nonsensory region of the postnatal rat vestibular epithelia, as observed in the cochlea. The study indicated that the cells could have the ability to differentiate into new hair cells (Huang et al., 2009). Another rat model was used to deliver the XIAP gene into the inner ear of rats by AAV vector via RWM injection to study its ability to protect against cisplatin-induced ototoxicity (Cooper et al., 2006).
A study was carried out by infusion of NT-3, BDNF, or NT-3 plus BDNF into the scala tympani of guinea pigs exposed to ototoxic agents to examine survival of auditory neurons (Staecker et al., 1996). Another guinea pig model was used to study inner ear gene therapy. Transgene expression was observed in the contralateral (untransfected) cochlea and CSF on unilateral, adenovirus-mediated transfer of the lacZ reporter gene into the cochlea of guinea pigs by intrathecal injection (Stover et al., 2000). Successful transduction of both cochleae was achieved by direct injection of adenovirus-mediated lacZ reporter gene into the CSF intrathecally, whereas no transduction was observed when injecting the reporter gene into the bloodstream. A guinea pig model was used to test cochlear implant electrodes coated with guinea pig fibroblasts transfected by an adenoviral vector carrying a BDNF-encoding gene (Rejali et al., 2007). A surgical approach for vector inoculation was developed in a guinea pig model by injecting Ad.RSVntlacz into the guinea pig endolymphatic sac (Yamasoba et al., 1999). The experiment indicated that inoculation of viral vector into the endolymphatic sac could provide efficient gene transfer that is not possible via scala tympani inoculation.
A study of RWM permeability to macromolecules with tritiated normal human serum albumin was carried out in a cat model of otitis media (Goycoolea et al., 1980).
A Mongolian gerbil model was used for inner ear gene therapy with GDNF after ischemia–reperfusion injury, and the study has demonstrated the effect of GDNF in protecting the cochlea from injury (Hakuba et al., 2003).
Prospects for Inner Ear Gene Therapy
Tremendous advances and progress have been made in gene therapy for the inner ear, especially for the development of gene vector systems. As more attention has been paid to nonviral vectors, great achievements and progress related to nonviral gene delivery systems have been made. The organic/inorganic nanoparticle gene vectors, which are relatively easy to prepare and store, have developed rapidly (Sun et al., 2006, 2008; Son et al., 2007; Vagle et al., 2008). A novel nonviral vector was invented by genetic engineering techniques (Zaki et al., 2007). The vector consists of genetically engineered polymer transcribed from a single gene with nucleic acid-binding protein and is called a nucleic acid-binding protein-based polymer (NABP) or amino acid-based polymer. It can be enhanced by linking it to specific elements such as a target ligand, an endosome-disrupting moiety, or a nuclear localization sequence. A novel technique has been developed to deliver cargo-carrying nanoparticles into target cells by intracellular bacteria (Akin et al., 2007). The process of transferring plasmid DNA into target cells, using bacteria as a nonviral carrier, is called “bactofection.” Nanoparticles containing plasmid DNA (carrying the GFP gene) were linked to the surface of Listeria monocytogenes through the specific combination of biotin and avidin, which can penetrate mammalian cells via a nonphagocytic process. The cargo-carrying bacteria, which the inventors named “microbots,” had been successfully transfected and expressed the reporter gene in various cultured cell lines and in mice in vivo (Akin et al., 2007). The microbots may become a new promising approach to deliver different types of cargo (genes, drugs, and other biological active molecules) into a variety of cultured cells and live animals.
Three kinds of vector may have great prospects: (1) The first is the bacterial vector, which mediates bactofection. The vector exerts no carcinogenesis because the bacterial genome will not integrate into the host's genome; (2) the second is the multiplex gene vector, which has the advantages of both viral and nonviral gene delivery systems and is constructed by biochemical and genetic engineering techniques. The capabilities of the so-called multiplex gene vector could be tremendously increased compared with those of a purely nonviral vector. The major advantage of the multiplex gene vector is its almost infinite capacity for structural variation, which may affect the physicochemical and biological properties of the vector. Such vectors may become an ideal tool for gene therapy in the future; and (3) the third is the labeled gene vector, with its obvious commercial value. It could be used as an ex vivo and in vivo gene vector with the capacity for real-time monitoring (Mori et al., 2006; Sen and Gambhir, 2007). On the basis of the progress in gene vector development, it is estimated that breakthrough for transfection efficiency of nonviral vectors or multiplex gene vectors could take place within 5 years. It will take about 15 years for multiplex gene vectors to become routinely used by physicians carrying out gene therapy in patients.
Considering the safety, effectiveness, and easy operation in clinical practice, the intact RWM is the most promising route for inner ear gene delivery. The transfection efficiency with this approach could be further increased after more effective facilitating agents are developed. It is reasonable to believe that the intact RWM approach may finally and permanently replace the commonly used transfection route of labyrinth drilling or RWM injection for inner ear gene delivery. Some portion of inner ear gene therapy may need to be carried out at the embryonic stage for the treatment of hereditary or congenital deafness in the future. In these circumstances, the intrauterine approach may be the best choice, especially when the endoscopic technique and related instruments become more precise and accurate. Considering the large number of hereditary and congenital deafness defects, the in utero approach for inner ear gene delivery is worthy of further study. However, this strategically important project has not been paid enough attention so far.
Neurotrophic factors, especially NT-3 and BDNF, are well-known comprehensive protectors of inner ear hair cells and neurons. In addition to the known major protective factors, otospiralin and XIAP have been shown to be possible protectors for the inner ear cells. The dream of regeneration of functional hair cells in the mammalian cochlea may some day become true via cochlear cell transdifferentiation. Neurotrophic factors, inhibitors of apoptosis, antioxidants, otospiralin, and the atonal-related factors may become the mainstream therapeutic molecules for inner ear gene therapy. As science and technology advance and progress, new therapeutic targets (such as connexins) and novel therapeutic molecules will be rapidly discovered and developed. On the basis of our knowledge of the current status of inner ear gene therapy, we predict that the first successful case of inner ear gene therapy may be reported within 5–8 years. Inner ear gene therapy may become a common treatment of inner ear diseases in about 15 to 20 years.
Acknowledgments
This paper was partially supported by the National Natural Science Foundation of China (30772402) and by the Science and Technology Commission of Hunan Province (grant 2007SK2001).
Author Disclosure Statement
The authors have no conflicts of interest to declare.
References
- Akin D. Sturgis J. Ragheb K., et al. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat. Nanotechnol. 2007;2:441–449. doi: 10.1038/nnano.2007.149. [DOI] [PubMed] [Google Scholar]
- Anniko M. Hellstrom S. Schmidt S.H. Spandow O. Toxic effects on inner ear of noxious agents passing through the round window membrane. Acta Otolaryngol. Suppl. 1989;457:49–56. doi: 10.3109/00016488809138884. [DOI] [PubMed] [Google Scholar]
- Bangham A.D. Standish M.M. Watkins J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- Bedrosian J.C. Gratton M.A. Brigande J.V., et al. In vivo delivery of recombinant viruses to the fetal murine cochlea: Transduction characteristics and long-term effects on auditory function. Mol. Ther. 2006;14:328–335. doi: 10.1016/j.ymthe.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham N.A. Hassan B.A. Price S.D., et al. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bird P.A. Begg E.J. Zhang M., et al. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol. Neurotol. 2007;28:1124–1130. doi: 10.1097/MAO.0b013e31815aee21. [DOI] [PubMed] [Google Scholar]
- Brough D.E. Methods of gene therapy for treating disorders of the ear by administering a vector encoding an atonal-associated factor. U.S. Patent 2007141029. 2007. www.investorvillage.com/mbthread.asp?mb=1195&tid=2586647&showall=1. [Mar;2011 ]. www.investorvillage.com/mbthread.asp?mb=1195&tid=2586647&showall=1
- Chandrasekhar S.S. Rubinstein R.Y. Kwartler J.A., et al. Dexamethasone pharmacokinetics in the inner ear: Comparison of route of administration and use of facilitating agents. Otolaryngol. Head Neck Surg. 2000;122:521–528. doi: 10.1067/mhn.2000.102578. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D. Mitra S. Maitra A. A method using inorganic nanoparticles as non-viral vectors for gene therapy. Patent WO/2005/123142. 2005. www.wipo.int/pctdb/en/wo.jsp?WO=2005123142. [Mar;2011 ]. www.wipo.int/pctdb/en/wo.jsp?WO=2005123142
- Cohen-Salmon M. del Castillo F.J. Petit C. Connexins responsible for hereditary deafness—the tale unfolds. In: Winterhager E., editor. Gap Junctions in Development and Disease. Springer-Verlag; Berlin: 2005. pp. 111–134. [Google Scholar]
- Cooper L.B. Chan D.K. Roediger F.C., et al. AAV-mediated delivery of the caspase inhibitor XIAP protects against cisplatin ototoxicity. Otol. Neurotol. 2006;27:484–490. doi: 10.1097/01.mao.0000202647.19355.6a. [DOI] [PubMed] [Google Scholar]
- Delprat B. Boulanger A. Wang J., et al. Downregulation of otospiralin, a novel inner ear protein, causes hair cell degeneration and deafness. J. Neurosci. 2002;22:1718–1725. doi: 10.1523/JNEUROSCI.22-05-01718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delprat B. Ruel J. Guitton M.J., et al. Deafness and cochlear fibrocyte alterations in mice deficient for the inner ear protein otospiralin. Mol. Cell. Biol. 2005;25:847–853. doi: 10.1128/MCB.25.2.847-853.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux Q.L. Takahashi R. Salvesen G.S. Reed J.C. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- Fujiyoshi T. Hood L. Yoo T.J. Restoration of brain stem auditory-evoked potentials by gene transfer in shiverer mice. Ann. Otol. Rhinol. Laryngol. 1994;103:449–456. doi: 10.1177/000348949410300606. [DOI] [PubMed] [Google Scholar]
- Goycoolea M.V. Paparella M.M. Goldberg B. Carpenter A.M. Permeability of the round window membrane in otitis media. Arch. Otolaryngol. 1980;106:430–433. doi: 10.1001/archotol.1980.00790310054014. [DOI] [PubMed] [Google Scholar]
- Gubbels S.P. Woessner D.W. Mitchell J.C., et al. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakuba N. Watabe K. Hyodo J, et al. Adenovirus-mediated overexpression of a gene prevents hearing loss and progressive inner hair cell loss after transient cochlear ischemia in gerbils. Gene Ther. 2003;10:426–433. doi: 10.1038/sj.gt.3301917. [DOI] [PubMed] [Google Scholar]
- Hanawa H. Vector for gene therapy and method of quantifying target protein in mammal or cultured cells with the administration of the vector for gene therapy. U.S. Patent Application 20060223767. 2006.
- Hoang Dinh E. Ahmad S. Chang Q., et al. Diverse deafness mechanisms of connexin mutations revealed by studies using in vitro approaches and mouse models. Brain Res. 2009;1277:52–69. doi: 10.1016/j.brainres.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Chi F. Han Z., et al. New ectopic vestibular hair cell-like cells induced by Math1 gene transfer in postnatal rats. Brain Res. 2009;1276:31–38. doi: 10.1016/j.brainres.2009.04.036. [DOI] [PubMed] [Google Scholar]
- Izumikawa M. Minoda R. Kawamoto K., et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jero J. Mhatre A.N. Tseng C.J., et al. Cochlear gene delivery through an intact round window membrane in mouse. Hum. Gene Ther. 2001;12:539–548. doi: 10.1089/104303401300042465. [DOI] [PubMed] [Google Scholar]
- Jiang M. Zhang Y.Q. He G.X. Sun H. [Protective effect of NT-3 gene mediated by hydroxyapatite nanoparticle on the cochlea of guinea pigs injured by excitotoxicity] [article in Chinese] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007;32:563–567. [PubMed] [Google Scholar]
- Jones J.M. Montcouquiol M. Dabdoub A., et al. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J. Neurosci. 2006;26:550–558. doi: 10.1523/JNEUROSCI.3859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadan M. Gaziglia M. Trapnell B. Improved adenoviral vectors and produced cells. WO 96/18418, PCT/US95/15947. 1996.
- Kaneda Y. Oshima K. Morishita R. Kubo T. Drug for auditory dysfunction. U.S. Patent 7390482. 2006. www.freepatentsonline.com/7390482.html. [Mar;2011 ]. www.freepatentsonline.com/7390482.html
- Kanzaki S. Shiotani A. Inoue M., et al. Sendai virus vector-mediated transgene expression in the cochlea in vivo. Audiol. Neurootol. 2007;12:119–126. doi: 10.1159/000097798. [DOI] [PubMed] [Google Scholar]
- Kawamoto K. Ishimoto S. Minoda R., et al. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J. Neurosci. 2003a;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K. Yagi M. Stover T., et al. Hearing and hair cells are protected by adenoviral gene therapy with TGF-β1 and GDNF. Mol. Ther. 2003b;7:484–492. doi: 10.1016/s1525-0016(03)00058-3. [DOI] [PubMed] [Google Scholar]
- Kelsell D.P. Dunlop J. Stevens H.P., et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–83. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi T. Adams J.C. Miyabe Y., et al. Potassium ion recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med. Electron Microsc. 2000;33:51–56. doi: 10.1007/s007950070001. [DOI] [PubMed] [Google Scholar]
- Kopke R.D. Wassel R.A. Mondalek F., et al. Magnetic nanoparticles: Inner ear targeted molecule delivery and middle ear implant. Audiol. Neurootol. 2006;11:123–133. doi: 10.1159/000090685. [DOI] [PubMed] [Google Scholar]
- Lalwani A.K. Walsh B.J. Reilly P.G., et al. Development of in vivo gene therapy for hearing disorders: Introduction of adeno-associated virus into the cochlea of the guinea pig. Gene Ther. 1996;3:588–592. [PubMed] [Google Scholar]
- Li S. Huang L. Nonviral gene therapy: Promises and challenges. Gene Ther. 2000;7:31–34. doi: 10.1038/sj.gt.3301110. [DOI] [PubMed] [Google Scholar]
- Li H. Liu H. Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat. Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- Liu Y.H. Ke X.M. Qin Y., et al. Adeno-associated virus-mediated Bcl-xL prevents aminoglycoside-induced hearing loss in mice. Chin. Med. J. (Engl.) 2007;120:1236–1240. [PubMed] [Google Scholar]
- Luebke A.E. Steiger J.D. Hodges B.L. Amalfitano A. A modified adenovirus can transfect cochlear hair cells in vivo without compromising cochlear function. Gene Ther. 2001;8:789–794. doi: 10.1038/sj.gt.3301445. [DOI] [PubMed] [Google Scholar]
- Maiorana C.R. Staecker H. Advances in inner ear gene therapy: Exploring cochlear protection and regeneration. Curr. Opin. Otolaryngol. Head Neck Surg. 2005;13:308–312. doi: 10.1097/01.moo.0000179248.51476.11. [DOI] [PubMed] [Google Scholar]
- McFadden S.L. Ding D. Jiang H, et al. Chinchilla models of selective cochlear hair cell loss. Hear. Res. 2002;174:230–238. doi: 10.1016/s0378-5955(02)00697-4. [DOI] [PubMed] [Google Scholar]
- Miller J.M. Le Prell C.G. Prieskorn D.M., et al. Delayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowth of auditory nerve peripheral processes: Effects of brain-derived neurotrophic factor and fibroblast growth factor. J. Neurosci. Res. 2007;85:1959–1969. doi: 10.1002/jnr.21320. [DOI] [PubMed] [Google Scholar]
- Miriszlai E. Benedeczky I. Csapo S. Bodanszky H. The ultrastructure of the round window membrane of the cat. ORL J. Otorhinolaryngol. Relat. Spec. 1978;40:111–119. doi: 10.1159/000275393. [DOI] [PubMed] [Google Scholar]
- Mori H. Tabata Y. Ando K., et al. Nucleic acid-containing complex. U.S. Patent 20090203768. 2006. www.freepatentsonline.com/y2009/0203768.html. [Mar;2011 ]. www.freepatentsonline.com/y2009/0203768.html
- Mulligan R.C. The basic science of gene therapy. Science. 1993;260:926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Nagy I. Bodmer M. Brors D. Bodmer D. Early gene expression in the organ of Corti exposed to gentamicin. Hear. Res. 2004;195:1–8. doi: 10.1016/j.heares.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Nakaizumi T. Kawamoto K. Minoda R. Raphael Y. Adenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damage. Audiol. Neurootol. 2004;9:135–143. doi: 10.1159/000077264. [DOI] [PubMed] [Google Scholar]
- National Institute on Deafness and Other Communication Disorders, National Institutes of Health. Statistics about hearing disorders, ear infections and deafness. 2007. www.nidcd.nih.gov/health/statistics/hearing.asp. [Mar;2011 ]. www.nidcd.nih.gov/health/statistics/hearing.asp
- Noushi F. Richardson R.T. Hardman J., et al. Delivery of neurotrophin-3 to the cochlea using alginate beads. Otol. Neurotol. 2005;26:528–533. doi: 10.1097/01.mao.0000169780.84588.a5. [DOI] [PubMed] [Google Scholar]
- Okano T. Nakagawa T. Kita T., et al. Cell-gene delivery of brain-derived neurotrophic factor to the mouse inner ear. Mol. Ther. 2006;14:866–871. doi: 10.1016/j.ymthe.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Oshima K. Shimamura M. Mizuno S., et al. Intrathecal injection of HVJ-E containing HGF gene to cerebrospinal fluid can prevent and ameliorate hearing impairment in rats. FASEB J. 2004;18:212–214. doi: 10.1096/fj.03-0567fje. [DOI] [PubMed] [Google Scholar]
- Osterman J.V. Waddell A. Aposhian H.V. DNA and gene therapy: Uncoating of polyoma pseudovirus in mouse embryo cells. Proc. Natl. Acad. Sci. U.S.A. 1970;67:37–40. doi: 10.1073/pnas.67.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M.B. Willems P.J. Non-syndromic, autosomal-recessive deafness. Clin. Genet. 2006;69:371–392. doi: 10.1111/j.1399-0004.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- Pickles J.O. Harter C. Rebillard G. Fibroblast growth factor receptor expression in outer hair cells of rat cochlea. Neuroreport. 1998;9:4093–4095. doi: 10.1097/00001756-199812210-00016. [DOI] [PubMed] [Google Scholar]
- Plontke S.K. Mynatt R. Gill R.M., et al. Concentration gradient along the scala tympani after local application of gentamicin to the round window membrane. Laryngoscope. 2007;117:1191–1198. doi: 10.1097/MLG.0b013e318058a06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plontke S.K. Biegner T. Kammerer B., et al. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol. Neurotol. 2008;29:401–406. doi: 10.1097/MAO.0b013e318161aaae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasba P.K. Aposhian H.V. DNA and gene therapy: Transfer of mouse DNA to human and mouse embryonic cells by polyoma pseudovirions. Proc. Natl. Acad. Sci. U.S.A. 1971;68:2345–2349. doi: 10.1073/pnas.68.10.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael Y. Cochlear pathology, sensory cell death and regeneration. Br. Med. Bull. 2002;63:25–38. doi: 10.1093/bmb/63.1.25. [DOI] [PubMed] [Google Scholar]
- Raphael Y. Frisancho J.C. Roessler B.J. Adenoviral-mediated gene transfer into guinea pig cochlear cells in vivo. Neurosci Lett. 1996;207:137–141. doi: 10.1016/0304-3940(96)12499-x. [DOI] [PubMed] [Google Scholar]
- Rejali D. Lee V.A. Abrashin K.A., et al. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear. Res. 2007;228:180–187. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenberg Y. Medvedkin V. Anderson W.F. Targeted artificial gene delivery. WO 01/12235, PCT/US00/22619. 2001.
- Salem A.K. Searson P.C. Leong K.W. Multifunctional nanorods for gene delivery. Nat. Mater. 2003;2:668–671. doi: 10.1038/nmat974. [DOI] [PubMed] [Google Scholar]
- Sen L. Gambhir S.S. A method for noninvasively and quantitatively monitoring therapeutic and diagnostic transgene expression induced by ex vivo and in vivo gene targeting in organs, tissues and cells. Patent WO/2007/109335. 2007. www.wipo.int/pctdb/en/wo.jsp?WO=2007109335. [Mar;2011 ]. www.wipo.int/pctdb/en/wo.jsp?WO=2007109335
- Son S.J. Bai X. Lee S.B. Inorganic hollow nanoparticles and nanotubes in nanomedicine. 1. Drug/gene delivery applications. Drug Discov. Today. 2007;12:650–656. doi: 10.1016/j.drudis.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Staecker H. Kopke R. Malgrange B., et al. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Staecker H. Praetorius M. Baker K. Brough D.E. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol. Neurotol. 2007;28:223–231. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- Stover T. Yagi M. Raphael Y. Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther. 2000;7:377–383. doi: 10.1038/sj.gt.3301108. [DOI] [PubMed] [Google Scholar]
- Sun H. Jiang M. Li G.Y. Zhu S.H. Neurotrophin-3 gene transfection of cochlear cells with hydroxyapatite nanoparticle vector. Presented at the 34th midwinter meeting of the Association for Research in Otolaryngology; Baltimore, MD. Feb 19–23;2006 ; 2006. [Mar;2011 ]. Abstract 372. [Google Scholar]
- Sun H. Jiang M. Zhu S.H. [In vitro and in vivo studies on hydroxyapatite nanoparticles as a novel vector for inner ear gene therapy] [article in Chinese] Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;43:51–57. [PubMed] [Google Scholar]
- Sun Y. Tang W. Chang Q., et al. Connexin30 null and conditional connexin26 null mice display distinct pattern and time course of cellular degeneration in the cochlea. J. Comp. Neurol. 2009;516:569–579. doi: 10.1002/cne.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M. Yamasoba T. Suzukawa K. Kaga K. Adenoviral vector gene delivery via the round window membrane in guinea pigs. Neuroreport. 2003;14:1951–1955. doi: 10.1097/00001756-200310270-00014. [DOI] [PubMed] [Google Scholar]
- Tamura T. Kita T. Nakagawa T., et al. Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope. 2005;115:2000–2005. doi: 10.1097/01.mlg.0000180174.81036.5a. [DOI] [PubMed] [Google Scholar]
- Vagle K. Wang W. Vargeese C., et al. Lipid nanoparticle based compositions and methods for the delivery of biologically active molecules. U.S. Patent 2008/0020058 A1. 2008. http://ip.com/patapp/US20080020058. [Mar;2011 ]. http://ip.com/patapp/US20080020058
- Wadell G. Mei Y.F. Segerman A., et al. Viral vector for gene therapy. Patent WO/02053759. 2002. www.europatentbox.com/patent/EP1348030B1/abstract/221597.html. [Mar;2011 ]. www.europatentbox.com/patent/EP1348030B1/abstract/221597.html
- Wang Y. Yang C. Xu S., et al. [Report on the study of WHO ear and hearing disorders survey protocol in Guizhou Province][article in Chinese] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;21:731–734. [PubMed] [Google Scholar]
- Wang J. Menchenton T. Yin S., et al. Over-expression of X-linked inhibitor of apoptosis protein slows presbycusis in C57BL/6J mice. Neurobiol. Aging. 2008;37:399–410. doi: 10.1016/j.neurobiolaging.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Wenzel G.I. Xia A. Funk E., et al. Helper-dependent adenovirus-mediated gene transfer into the adult mouse cochlea. Otol. Neurotol. 2007;28:1100–1108. doi: 10.1097/MAO.0b013e318158973f. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) WHO Regional Office for South-East Asia Meeting report: Prevention of deafness and hearing impairment for development of framework of proposed regional collaboration; Bangkok, Thailand. Nov 27–28;2003 ; ICP DPR 001, New Delhi: WHO Project; 2004. Feb, 2004. [Google Scholar]
- Yamasoba T. YaGi M. Roessler B.J., et al. Inner ear transgene expression after adenoviral vector inoculation in the endolymphatic sac. Hum. Gene Ther. 1999;10:769–774. doi: 10.1089/10430349950018526. [DOI] [PubMed] [Google Scholar]
- Yu L. Matsumoto K. Vector for transfection of eukaryotic cells. U.S. Patent 2006/0074045 A1. 2006. http://ip.com/patapp/US20060074045. [Mar;2011 ]. http://ip.com/patapp/US20060074045
- Zaki M. Arash H. Hamidreza G. Recombinant protein polymer vectors for systemic gene delivery. U.S. Patent 2007/0098702. 2007. www.freepatentsonline.com/y2007/0098702.html. [Mar;2011 ]. www.freepatentsonline.com/y2007/0098702.html
- Zhuo X.L. Wang Y. Zhuo W.L., et al. Adenoviral-mediated up-regulation of Otos, a novel specific cochlear gene, decreases cisplatin-induced apoptosis of cultured spiral ligament fibrocytes via MAPK/mitochondrial pathway. Toxicology. 2008;248:33–38. doi: 10.1016/j.tox.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Zou J. Saulnter P. Perrier T., et al. Distribution of lipid nanocapsules in different cochlear cell populations after round window membrane permeation. J. Biomed. Mater. Res. B. Appl. Biomater. 2008;87B:10–18. doi: 10.1002/jbm.b.31058. [DOI] [PubMed] [Google Scholar]
- Zou J. Sood R. Ranjan S., et al. Manufacturing and in vivo inner ear visualization of MRI traceable liposome nanoparticles encapsulating gadolinium. J. Nanobiotechnol. 2010;8:32. doi: 10.1186/1477-3155-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]