Abstract
Biological differences between recombinant adeno-associated virus (rAAV) serotypes define their efficiencies in expressing a transgene in a particular target cell. Few studies have directly compared how differences in viral entry, intracellular trafficking, and nuclear import of rAAV serotypes influence the effectiveness of transduction in the same cell type. We evaluated these characteristics for three rAAV serotypes in HeLa cells, using biochemical techniques and fluorescence-based detection of multiple serotypes in the same cell. Although rAAV2 exhibited the slowest entry, intracellular trafficking, and nuclear import among the three serotypes, it elicited the highest levels of transduction. Conversely, rAAV1 exhibited more rapid entry and nuclear import than the other serotypes, yet was ineffective at transducing HeLa cells due to impaired capsid disassembly in the nucleus. rAAV5, which entered the cell less rapidly than rAAV1, was imported efficiently into the nucleus, but then rapidly degraded, resulting in poor transduction of HeLa cells. We conclude that rAAV1, 2, and 5 utilize distinct mechanisms for intracellular trafficking, and that post-nuclear events play an important role in determining the efficiency of HeLa cell transduction by these serotypes. Thus, overcoming post-nuclear barriers that limit uncoating and/or promote virion degradation may enhance the efficiency of certain AAV serotypes.
Keiser and colleagues investigate differences in viral entry, intracellular trafficking, and nuclear import between recombinant adeno-associated virus (AAV) serotypes 1, 2, and 5 that might define their transduction efficiency in HeLa cells. The authors found that the three serotypes use distinct mechanisms for intracellular trafficking, and that post-nuclear entry events such as uncoating or virion degradation are important in determining transduction efficiency.
Introduction
Recombinant adeno-associated virus (rAAV) is a commonly used gene therapy vector (Wu et al., 2006). Initially, most clinical and nonclinical studies with rAAV focused on rAAV serotype 2 (rAAV2/2) (Carter, 2005). More recently, many additional AAV serotypes have been isolated, each having capsid proteins with unique biological properties with respect to infecting particular cell types (Gao et al., 2005; Wu et al., 2006). Additionally, directed evolution approaches and hybrid capsid construction have greatly expanded the capabilities of the rAAV vectors (Choi et al., 2005; Vandenberghe et al., 2009).
The capsid of AAV consists of three separate proteins (VP1, VP2, and VP3) at a 1:1:18 ratio, arranged in an icosohedral structure (Wu et al., 2006). Sequence differences in the capsid proteins of various AAV serotypes can range from infrequent amino acid changes spread throughout the capsid to longer stretches of amino acid changes. These differences in capsid sequence are thought to control variations in tropism among the hundreds of serotypes that exist. In the case of a handful of AAV serotypes for which different binding receptors and co-receptors have been identified, the observed differences in tropism are thought to be controlled by the unique entry pathways for the virus, by limiting binding of certain serotypes while creating ideal docking and entry platforms for others (Wu et al., 2006). However, receptor/co-receptor interaction is likely not the sole determinant of efficiency of transduction in a particular cell type, as differences in AAV tropism can also be controlled within the cell following endocytosis of the virus.
rAAV2 is a perfect example of a serotype that is subject to multiple levels of regulation with respect to its ability to effectively transduce cells. It enters the cell via clathrin-mediated endocytosis (Duan et al., 1999; Bartlett et al., 2000) and can traffic through both late and recycling endosomal compartments (Ding et al., 2006). The precise route of rAAV2 movement through the endosomal network is thought to influence the processing of its capsid and/or the ability of the virus to escape from the endosome into the cytoplasm, which enables it to efficiently move into the nucleus (Ding et al., 2005). Endosomal escape of rAAV2 is thought to involve a change in the conformation of the viral capsid, such that an otherwise buried phospholipase A2 domain in VP1 becomes exposed (Girod et al., 2002; Kronenberg et al., 2005; Mani et al., 2006). This conformational change is also thought to expose a nuclear localization signal within the capsid, promoting import of the virus into the nucleus following its release into the cytoplasm (Grieger et al., 2007). These endosomal processing steps represent a significant barrier for rAAV2-mediated transduction, and differences in endosomal routing can lead to significant differences in the efficiency of this process (Ding et al., 2006). In airway epithelial cells, for example, rAAV2 is capable of entering via both the apical and basolateral membranes, yet inefficiencies in endosomal processing in the apical trafficking pathway results in vastly lower transduction of this virus compared to the basolateral pathway (Duan et al., 2000). After rAAV2/2, rAAV2/5 is likely the next best characterized serotype in terms of its trafficking biology. It can transduce cells following entry via several different mechanisms (Bantel-Schaal et al., 2009), and appears to predominantly traffic through the Golgi (Bantel-Schaal et al., 2002).
In addition to endosomal trafficking, entry into the nucleus is a significant barrier to efficient gene transfer by AAV. It is likely that uncoating of the AAV capsid takes place following import of the virus into the nucleus, although data on this from different groups are contradictory (Bartlett et al., 2000; Sanlioglu et al., 2000; Seisenberger et al., 2001; Xiao et al., 2002; Lux et al., 2005). Specific regions of the nucleus have been shown to affect rAAV transduction; virus accumulating in the nucleolus is not uncoated properly and is thus not infectious (Johnson and Samulski, 2009). Once the virus is uncoated, second-strand synthesis can initiate, leading to transgene expression. Differences in uncoating efficiency have been tied to variations in the transduction of particular cell types (Thomas et al., 2004; Zhong et al., 2004; Sipo et al., 2007). In hepatocytes, for example, both rAAV2/2 and rAAV2/8 efficiently enter the nucleus, but rAAV2/8 appears to uncoat much more rapidly than rAAV2/2, and this is thought to account for greater levels of hepatic transduction by rAAV2/8 (Thomas et al., 2004).
Despite the extensive number of studies utilizing various rAAV serotypes, much remains unknown about how differences in the trafficking of various rAAV serotypes from the cell membrane to the nucleus influence their efficiency of transduction. Although differences in trafficking biology between rAAV2/2 and rAAV2/5 have been reported (as discussed above), very little is known about other commonly used serotypes, such as rAAV2/1. Furthermore, interpreting the results of comparative studies of trafficking biology between various serotypes is often complicated by fact that the infection conditions used in various studies (i.e., the temperature and multiplicity of infection [MOI]), as well as the methods of virus purification, can influence both the entry and intracellular trafficking pathways of rAAV (Bantel-Schaal et al., 2009; Ding et al., 2006).
In the current study, we directly compared the intracellular trafficking of three commonly used rAAV serotypes (rAAV2/1, rAAV2/2, and rAAV2/5) in HeLa, a cell line in which the majority of rAAV biology has been studied. We hypothesized that the differing capsid sequences of these three serotypes direct virions to distinct intracellular trafficking pathways, and that the transduction efficiency of these vectors would thus directly correlate with the efficiency of trafficking to the nucleus. Specifically, we compared the trafficking of rAAV2/1 and rAAV2/5 to that of rAAV2/2 using fluorescently labeled virions co-infected into the same cell. We observed vastly different levels of HeLa cell transduction (10- to 100-fold) with these three serotypes, and so we hypothesized that both rAAV2/1 and rAAV2/5 would use trafficking pathways distinct from those previously described for rAAV2/2. Indeed, we observed significant differences in the rate of entry, the intracellular trafficking pathways used, and the rate of nuclear uptake for these three serotypes. In contrast to our original hypothesis, the rate of nuclear uptake did not correlate with more efficient transduction. For example, rAAV2/1 entered HeLa cells much more rapidly than the other two serotypes, and trafficked most rapidly through the cytoplasm and into the nucleus, but did not transduce the cell as well as the other serotypes. rAAV2/2 was the slowest of the three serotypes to move into the nucleus, yet was more efficient at transducing HeLa cells than either rAAV2/1 or rAAV2/5. Based on the turnover of fluorescent capsid signal and nuclear genomes in the nucleus, rAAV2/1 appeared to be impaired in nuclear processing/uncoating, and the rAAV2/5 capsid and genome appeared to be rapidly degraded once it entered the nucleus. In contrast, rAAV2/2 appeared to be most effectively uncoated in the nucleus without undergoing significant genome degradation. We conclude that serotype-dependent differences in capsid structure lead to vast differences in intracellular trafficking and processing of the virion, and that this in turn influences post-nuclear events that control the effectiveness of viral uncoating and stability of the viral genome.
Materials and Methods
Vector production and cell culture
Recombinant adeno-associated virus (rAAV) encoding a luciferase transgene within a type-2 genome (rAAV-luciferase) was pseudotyped with type 1, 2, or 5 capsids. All viral vectors were generated using a standard triple plasmid transfection protocol and purified using ion-exchange high-performance liquid chromatography as previously described (Yan et al., 2006). HeLa cells were used for all infection experiments and were maintained on plastic or lysine-coated glass-bottom dishes at 37°C and at 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics (penicillin and streptomycin).
Transduction, cell entry, and nuclear import assays with unlabeled virus
For transduction experiments with unlabeled virus, HeLa cells were grown to 90% confluency on 24-well plates. Cells were incubated with 2.5×108 vector genome copies (VGC) of rAAV-luciferase per well (MOI=1000 VGC/cell) in DMEM containing 2% FBS for 2 hr at 37°C and at 5% CO2. Subsequently, one volume of DMEM containing 18% FBS was added to the wells to increase the final concentration of FBS to 10%, and the cells were returned to normal culture conditions for 24 hr. Cells were washed twice in 1× phosphate-buffered saline (PBS) and lysed for 20 min in 100 μl of Cell Lysis Buffer (Promega) at room temperature. Luciferase assays were performed on cell lysates using the Luciferase Assay System (Promega) according to the manufacturer's instructions. For each cell lysate, protein concentration was determined by Bradford assay and used to calculate the number of relative light units per milligram of protein for each sample (three independent experiments were performed).
For cell entry experiments with unlabeled virus, HeLa cells were grown to 90% confluency on 150-mm plates. The cells were chilled to 4°C with cold PBS and placed on ice, after which rAAV-luciferase virus was added to the cells (MOI=1000 VGC/cell) in 4°C PBS. Virus was bound to the cell surface at 4°C for 1 hr, and unbound virus was washed off with cold PBS. Warm medium was then added to the cells to stimulate entry, and the cells were returned to regular culture conditions for 1 hr. The cells were then removed from the plates via trypsinization, and cells from three replicate plates were pooled. Cells were washed with PBS and resuspended in cold homogenization buffer (0.25 M sucrose, 10 mM triethanolamine, 1 mM EDTA with protease inhibitor tablets [Roche Diagnostics]). Homogenization was performed using a glass dounce homogenizer. Cell homogenates were centrifuged at 1000 g for 10 min to isolate the post-nuclear supernatant (PNS). Samples were boiled at 100°C for 10 min to denature the AAV capsid, and were then centrifuged for 10 min at 9300 g. Viral genomes in the PNS were quantified using TaqMan PCR for the luciferase transgene as previously described (three independent experiments were performed; Ding et al., 2006). PNS was also obtained from cells not shifted to 37°C, and the number of viral genomes in this sample was determined by TaqMan polymerase chain reaction (PCR). This number was subtracted from values obtained for the samples subjected to the 1-hr temperature shift in order to control for any cell-surface bound virions that were not removed by incubation with trypsin.
For nuclear import assays using unlabeled virus, HeLa cells at 90% confluency in six-well plates (MOI=1000 VGC/cell) were infected with rAAV-luciferase virus in DMEM with 2% FBS for 2 hr. Virus was washed off with 1×PBS, and cells were either harvested by trypsinization or further incubated in medium containing 10% FBS and harvested at the 24-hr time point. The cells were lysed and fractionated into nuclear and cytoplasmic fractions as previously described (Yan et al., 2006). Boiled nuclear and cytoplasmic fractions were subjected to TaqMan PCR specific for the luciferase transgene to quantify the total number of viral genomes present in each sample (three independent experiments were performed). Copy numbers were normalized to standard curves created by TaqMan PCR proceeded by spiking in known copy numbers of AAV2.luciferase proviral plasmids into HeLa cytoplasmic or nuclear extract, since these two different fractions have a differential effect on viral genome detection.
Fluorescence labeling of virus and cell infection with labeled virus
rAAV2/1, rAAV2/2, and rAAV2/5 vectors were labeled with Alexa 488 and Alexa 568 dyes using the corresponding Protein Labeling Kits purchased from Invitrogen. From 1×1012 to 5×1012 VGC of rAAV were incubated with one tube of dye and magnetically stirred for 2 hr at room temperature in the dark. Purification was performed by column chromatography, using a resin supplied by the manufacturer. Fractions were collected, and the number of viral genomes in each fraction was determined by TaqMan PCR (Ding et al., 2006). Peak fractions were pooled and stored as frozen aliquots at −80°C until use.
Infection with labeled rAAV was performed on HeLa cells grown on lysine-coated glass-bottom 35-mm dishes (MatTek). Cells were incubated at an MOI of 105 VGC/cell of fluorescently labeled rAAV at 4°C for 1 hr. Unbound virions were removed with two washes of 4°C PBS. The cells were then shifted to 37°C with the addition of warm DMEM and further incubated for various times. The cells were then washed with cold PBS and fixed with cold 4% paraformaldehyde for 15 min. For the 0 min time point, the cells were fixed immediately after washing off unbound labeled rAAV. Cells were washed three times in 4°C PBS, and then the cell surfaces were labeled with wheat germ agglutinin (WGA)-Alexa 350 (Invitrogen) for 1 hr at 4°C. The cells were then washed twice with PBS, and Vectashield (Vector Laboratories) was added to the dishes prior to imaging.
Imaging and image analysis
Confocal fluorescence microscopy was performed using a Leica DMI 6000 microscope, a CARV II Confocal Imager (BD Biosystems), and an EM-CCD digital camera (Model C-9100, Hammamatsu) operating in normal (non-EM) mode. Two-dimensional confocal images were captured of cells from five distinct areas of each culture dish with a 63× objective lens (oil immersion, 1.4 lens NA) using IPLab software (BioVision Technologies) and the following exposure times: Alexa 488 (green)=2 sec; Alexa 568 (red)=2 sec; Alexa 350 (blue)=1 sec; DAPI (blue)=200 msec. Image processing and quantification were performed using Metamorph software (Molecular Devices). Images were captured and processed using the 2D Deconvolution application (Nearest Neighbors setting). Images from separate channels were combined to create merged images, and individual color channels were separated and thresholded (green=20, red=20, blue=70) for quantification. For analysis of virus overlap, regions were drawn around individual cells, and the percent overlap between viral signals was determined for 20 cells per experimental time point, using the Measure Colocalization function (three independent experiments were performed). Nuclear import and stability of fluorescent virus was evaluated by calculating the integrated intensity of the viral signal inside the nuclei of 20 cells, as identified by overlap with DAPI staining (three independent experiments were performed). For fluorescence-based assays of cell entry, WGA staining was used to demarcate the plasma membrane, and 20 cells were evaluated for total cellular fluorescence (i.e., regions on and within the blue plasma membrane) as well as regions within the cells only (i.e., inside the area of blue membrane staining). The integrated fluorescence intensity for each viral signal was then determined for each region, allowing for the assessment of fluorescence associated with endocytosed virus (three independent experiments were performed).
Statistical analysis
All statistical tests were performed using Prism software (GraphPad Software, Inc.). All p values were calculated using post-tests. One-way ANOVA was followed by either Tukey's or Dunnet's multiple comparison post-test depending on whether the comparison was between all data sets or with a control set, respectively. Two-way ANOVA was succeeded by Bonferroni post-test.
Results
rAAV serotypes 1, 2, and 5 display distinct transduction and entry profiles in HeLa cells
Intracellular trafficking and endosomal processing of rAAV have been implicated as important steps that influence the efficiency of transduction (i.e., expression of an encoded transgene). The intracellular trafficking of rAAV2/2 has been shown to be quite slow (Duan et al., 2000; Hansen et al., 2000), and enhancement of this process with proteasome inhibitors can facilitate viral movement to the nucleus and enhance transduction (Duan et al., 2000; Yan et al., 2002, 2004). This process has also been shown to involve diverse vesicular compartments such as the Golgi (Pajusola et al., 2002), late endosomes (Ding et al., 2006), and recycling endosomes (Ding et al., 2006). In spite of this knowledge, little is known about how the various steps of AAV infection differ for the various serotypes, and about how such differences impact the efficiency of transduction. We sought to directly compare these biological events following infection of HeLa cells by rAAV2/1, rAAV2/2, and rAAV2/5.
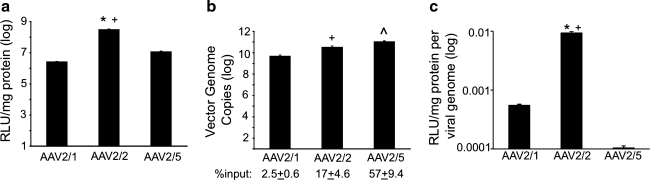
Pseudotyped rAAV2/1, rAAV2/2, and rAAV2/5 luciferase vectors encoding identical AAV2-based genomes demonstrated considerable differences in the efficiency of transduction following infection in HeLa cells (Fig. 1a). Vectors containing the rAAV2 capsid were the most efficient at transducing HeLa cells, at levels approximately 10-fold higher than transduction achieved by rAAV2/5 (p<0.01) and approximately 100-fold higher than that achieved by rAAV2/1 (p<0.01). In order to examine whether these differences were solely due to the efficiency of uptake of each serotype, we quantified the total number of internalized vector genomes following a 1-hr incubation at 4°C (to allow virus to bind the cell) and a 1-hr incubation at 37°C (to enable internalization). As shown in Fig. 1b, the three serotypes had significantly different entry profiles. rAAV2/5 demonstrated the most efficient internalization into HeLa cells, at a level approximately 10-fold (p<0.01) and 100-fold (p<0.01) greater than of rAAV2/2 and rAAV2/1, respectively. This corresponded to greater than 50% of the total input rAAV2/5 virions entering the cells, as compared with 17% for rAAV2/2 and less than 3% for rAAV2/1. In order to determine the efficiency of viral transduction with respect to the number of internalized viral genomes, we calculated the number of transduction units produced per viral genome (relative light units per milligram of protein per viral genome) (Fig. 1c). rAAV2/2 was the most efficient serotype; it was about 17-fold more efficient than rAAV2/1 (p<0.01) and about 100-fold more efficient than of rAAV2/5 (p<0.01). Thus, the 100-fold lower transduction of HeLa cells by rAAV2/1 versus rAAV2/2 is only partially explained by less efficient viral entry by this serotype. Similarly, although rAAV2/5 is significantly more efficient at entering HeLa cells than the other two serotypes, this vector is the least efficient at expressing its transgene. These results demonstrated a clear discordance in the efficiency of transduction and the number of viral particles that entered the cells, suggesting that intracellular processing of the three tested rAAV serotypes must be quite different.
FIG. 1.
Recombinant adeno-associated virus (rAAV) transduction and entry profiles in HeLa cells. (a) Relative transduction efficiencies of the rAAV2/1, rAAV2/2, and rAAV2/5 vectors as assessed by luciferase expression. rAAV vectors pseudotyped with type 1, 2, or 5 capsids were used to transduce HeLa cells at a multiplicity of infection (MOI) of 1000 vector genome copies (VGC)/cell. The cells were infected for 24 hr, and luciferase assays were conducted on cell lysates. The relative light units (RLUs) in each sample were normalized to the total amount of protein in the lysate. Average values for each vector were calculated and plotted on a logarithmic scale (error bars=mean±SEM, n=4 independent experimental samples). (b) Relative entry efficiencies as assessed by virus genome content. rAAV-luciferase vectors were incubated with HeLa cells (MOI=1000 VGC/cell) at 4°C for 1 hour. Unbound virions were washed off with cold PBS, and the cells were further incubated with attached virus at 37°C for 1 hr to allow for virus entry. Cells were then collected by trypsinization, and post-nuclear supernatants (PNS) were isolated from cell homogenates. The total number of VGC in each sample was determined by TaqMan PCR and normalized to the value obtained for the corresponding vector in cells not shifted to 37°C. Average total VGC were calculated and plotted on a logarithmic scale (error bars=mean±SEM, n=3 independent experimental samples). The percent input was determined by calculating the ratio of viral genomes detected by TaqMan PCR to the total viral genomes applied to each well. (c) Efficiency of AAV transduction was calculated as the level of transduction (RLU/mg protein) divided by the VGC in cells at the time of harvest. The resulting average values were then plotted (error bars=mean±SEM, n=4 independent experimental samples). Statistical analysis was performed using one-way ANOVA with post-test (Tukey's multiple comparison): AAV2/1 vs. AAV2/2, *p<0.01; AAV2/2 vs. AAV2/5, +p<0.01; AAV2/1 vs. AAV2/5, ^p<0.01.
rAAV2/1 and rAAV2/5 utilize routes of intracellular trafficking distinct from that utilized by rAAV2/2
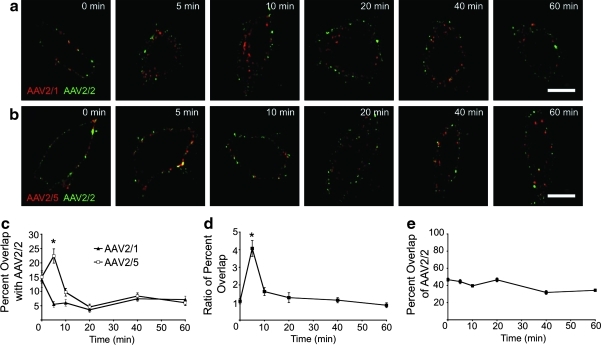
We hypothesized that the observed 17- to 100-fold differences in the specific activities of the rAAV2/1, rAAV2/2, and rAAV2/5 viral genomes following infection of HeLa cells was due to altered intracellular trafficking and processing of internalized virions. Thus, we directly compared the intracellular trafficking of Alexa 568-labeled rAAV2/1 and rAAV2/5 to that of Alexa 488-labeled rAAV2/2 following co-infection. Following co-incubation of cells with rAAV2/2 and either rAAV2/1 or rAAV2/5 at 4°C, they were shifted to 37°C for 0–60 min to allow for entry and intracellular trafficking toward the nucleus. Confocal images were then captured (Fig. 2a and b). In order to determine the precise amount of co-localization between virions, we quantified the percent overlap in signal from rAAV2/1 and rAAV2/2, or from rAAV2/5 and rAAV2/2, at each time point following infection (Fig. 2c). Results from these analyses indicated that moderate co-localization (about 14%) of rAAV2/1 and rAAV2/2 at the cell membrane (at 0 min, 4°C binding) rapidly declined (to about 5%) by 5 min after the cells were shifted to 37°C. This low level of overlap between these two serotypes continued through 60 min post-infection. Specifically, rAAV2/1 entered the cells very rapidly, by 5 min post-infection, whereas rAAV2/2 appeared to remain on the cell surface at this time point. At 10–20 min post-infection, rAAV2/1 continued to traffic toward the perinuclear region, whereas rAAV2/2 remained more peripheral. These findings suggested that the two viruses likely enter HeLa cells at different rates through different endosomal compartments and that they do not likely converge at a similar endosomal destination.
FIG. 2.
Intracellular trafficking of distinct fluorescently labeled rAAV serotypes following co-infection of HeLa cells. rAAV particles were labeled as follows: rAAV2/1 and rAAV2/5 with Alexa-568 dye, and rAAV2/2 with Alexa-488 dye. rAAV2/1-Alexa-568+rAAV2/2-Alexa-488 and rAAV2/5-Alexa-568+rAAV2/2-Alexa-488 co-infection experiments were conducted by first incubating the labeled virions with HeLa cells (MOI=105 VGC/cell) at 4°C to allow them to bind to the cells, and then shifting the cells to 37°C for various times to allow the viruses to enter the cells. Cells were fixed and imaged by confocal fluorescence microscopy using a 63× objective lens (oil immersion). (a) Representative deconvoluted images of cells co-infected with rAAV2/1-Alexa-568 (red) and rAAV2/2-Alexa-488 (green) at each time point. Yellow signal indicates co-localization of rAAV2/1 and rAAV2/2. (b) Representative deconvoluted images of cells co-infected with rAAV2/5-Alexa-568 (red) and rAAV2/2-Alexa-88 (green) at each time point. Yellow signal indicates co-localization of rAAV5 and rAAV2. (c) Percent overlap between red and green signals in each cell was calculated using Metamorph software, as a measure of co-localization between rAAV2/1 or rAAV2/5 and rAAV2/2 at each time point. The average percent overlap of rAAV2/1 (red) or rAAV2/5 (red) with rAAV2/2 (green) was calculated and plotted as a function of time (error bars=mean±SEM, n=20). *p<0.001 by two-way ANOVA with post-test (Bonferroni). (d) The difference between rAAV2/5 and rAAV2/1 in their overlap with rAAV2/2 was determined by dividing the percent overlap for rAAV2/5 and rAAV2/2 by the average percent overlap between rAAV2/1 and rAAV2/2 for each time point. *p<0.05 by one-way ANOVA with post-test (Dunnett's multiple comparison) (as compared to 0-min time point). (e) Maximal measurable signal overlap was determined for rAAV2/2 by co-infecting HeLa cells with Alexa-568- and Alexa-488-labeled rAAV2/2. The plot represents average percent overlap of rAAV2/2-568 (red) and rAAV2/2-488 (green) per cell from 0 to 60 min. Error bars=mean±SEM, n=20 cells. Scale bar=10 μm.
In contrast, we noted an increase in the percent overlap between rAAV2/5 and rAAV2/2 early during infection (at 5 min), followed by a sharp decrease in overlap to minimal levels for the duration of the experiment (Fig. 2b and c). This finding suggests that rAAV2/5 and rAAV2/2 enter through a similar endosomal compartment at similar rates, but then are quickly separated into different compartments. The level of overlap between rAAV2/5 and rAAV2/2 at 5 min was significantly higher than that between rAAV2/1 and rAAV2/2 (p<0.001) at this point in time; however, at later time points the overlap for the two pairs was similar. In order to compare differences in the extent of co-localization between rAAV2/1 and rAAV2/5 with rAAV2/2, we calculated the ratio of rAAV2/2:rAAV2/5 overlap to that of rAAV2/2:rAAV2/1 overlap (Fig. 2d). This analysis indicated that the only significant difference in virus co-localization between the two co-infection experiments occurred at 5 min post-infection (p<0.05).
In order to assess the maximum percent overlap possible with rAAV2/2-Alexa 488 in each cell, we performed control experiments in which HeLa cells were co-infected with rAAV2/2-Alexa 568 and rAAV2/2-Alexa 488. In these experiments, the average percent overlap was approximately 45% for the first half of the time course, and approximately 35% for the remainder of the experiment (Fig. 2e). These results demonstrate that the percent overlap between rAAV2/5 and rAAV2/2 observed at 5 min (22.4%) was a significant value in the context of our particular experimental design. In summary, this series of experiments revealed that rAAV2/1 and rAAV2/2 take vastly divergent paths from the cell membrane to the nucleus, whereas subpopulations of rAAV2/5 and rAAV2/2 use the same trafficking route early in infection but take divergent paths during later stages of trafficking toward the perinuclear space.
The use of co-infection approaches to evaluate biologic differences in serotypes is powerful. However, one potential caveat to studying serotype differences in the setting of co-infection is the potential for viral inference. To determine the extent to which viral interference may alter the transduction efficiency, we performed a viral interference assay. In these studies, rAAV2/1, rAAV2/2, and rAAV2/5 luciferase vectors were co-infected into HeLa cells along with a non-luciferase virus of each serotype, representing the various combinations used to evaluate intracellular trafficking. Results from these studies demonstrated only marginal (25%–50%) interference in the efficiency of transduction (i.e., transduction units per vector genome) for certain combination of serotypes (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/hum). Specifically, rAAV2/2 did not significantly inhibit rAAV2/1 transduction efficiency (Supplementary Fig. S1a), while rAAV2/1 marginally inhibited rAAV2/2 transduction efficiency by about 25% (Supplementary Fig. S1b). Similarly, rAAV2/2 did not significantly inhibit rAAV2/5 transduction efficiency (Supplementary Fig. S1c), but rAAV2/5 inhibited rAAV2/2 transduction efficiency by approximately 50% (Supplementary Fig. S1b). Given the 17- to 100-fold reduced transduction efficiency for rAAV2/5 and rAAV2/1, as compared with rAAV2/2, under single viral infection conditions (Fig. 1c), these changes in transduction efficiency following co-infection are negligible.
rAAV2/1 is internalized from the plasma membrane more rapidly than rAAV2/2 and rAAV2/5
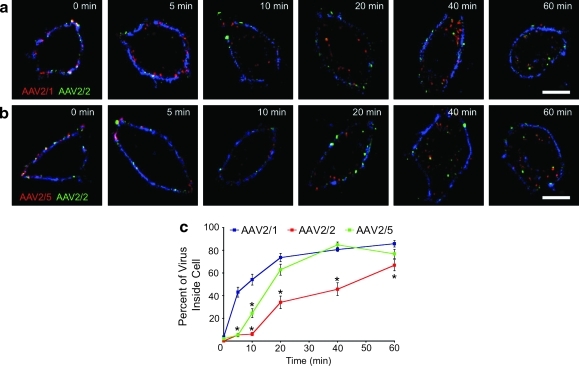
The image analysis shown in Fig. 2 suggests that rAAV2/1 enters cells much more rapidly than rAAV2/2 and rAAV2/5. In order to better quantify these differences, we directly examined the rates of entry of fluorescently labeled virions into HeLa cells, while using Alexa 350-labeled WGA to stain the plasma membrane. As shown in Fig. 3a, both rAAV2/1 and rAAV2/2 are present on the plasma membrane at 0 min. However, whereas most rAAV2/2 remains bound to the plasma membrane at 5 min post-infection, rAAV2/1 has already entered the cell at this early time point. Movement of rAAV2/1 away from the WGA-labeled plasma membrane toward the perinuclear space continued to be more rapid than that of rAAV2/2 throughout the experiment. The same images show that significant amounts of rAAV2/2 remain at the plasma membrane for up to 20 min post-infection, finally entering the cell at 40–60 min post-infection (Fig. 3a). The rate at which rAAV2/5 entered the cell appeared to be similar to that of rAAV2/2 (Fig. 3b). At 5 min post-infection, the viruses co-localized on the WGA-labeled plasma membrane, after which endocytosis occurred from 10 to 60 min post-infection.
FIG. 3.
Internalization of rAAV from the plasma membrane. Fixed, unpermeabilized HeLa cells were co-infected with labeled rAAV vectors as in Fig. 2 (MOI=105 VGC/cell) and also labeled with wheat germ agglutinin (WGA)-Alexa-350 (blue channel) to mark the plasma membrane. The cells were then imaged by confocal fluorescence microscopy. (a) Representative deconvoluted images of cells co-infected with rAAV2/1-Alexa-568 (red) and rAAV2/2-Alexa-488 (green) at each time point. (b) Representative deconvoluted images of cells co-infected with rAAV2/5-Alexa-568 (red) and rAAV2/2-Alexa-488 (green) at each time point. (c) Plot of internalization rate for each labeled vector. Metamorph software was used to calculate total fluorescence intensity for each vector, for regions comprising the entire cell and regions corresponding to the cytoplasm (i.e., intracellular virus that did not co-localize with the WGA-labeled plasma membrane). These intensity values were used to calculate the percentage of virus-associated fluorescence intensity inside each cell. These percentages were averaged and plotted as a function of time (x-axis) for each labeled vector (blue=AAV2/1, red=AAV2/2, green=AAV2/5). Error bars=mean±SEM, n=20 cells. Scale bar=10 μm. *p<0.05 (vs. AAV2/1) by two-way ANOVA with Bonferroni post-test.
The above-described studies were quantified by evaluating the fraction of virion fluorescence intensity per cell that was within the boundaries of the WGA signal (indicative of plasma membrane; Fig. 3c). This analysis confirmed the rapid rate of entry for rAAV2/1 across the plasma membrane—greater than 40% of the virus had moved inside the cell within 5 min of the start of infection, and maximal entry was reached by 20 min post-infection. The rate of entry for both rAAV2/5 and rAAV2/2 as compared with rAAV2/1 was much slower (p<0.001). Both rAAV2/5 and rAAV2/2 demonstrated a lag in endocytosis during the first 5 min; however, by 10 min, the rate of movement of rAAV2/5 into the cell was significantly greater than that of rAAV2/2. By 20 min, the percentage of rAAV2/5 inside the cell had increased to more than 60%, and did not differ statistically from that of rAAV2/1 from this time point through 60 min post-infection. rAAV2/2 exhibited the slowest rate of entry, with only a small fraction of virions present inside the cell until 40 min post-infection. From 5 to 60 min post-infection, the percentage of rAAV2/1 inside the cells was significantly greater than that of rAAV2/2 (p<0.001). These data clearly indicate that rAAV2/1 enters HeLa cells at a much more rapid rate than either rAAV2/2 or rAAV2/5, and that rAAV2/2 is the slowest of these serotypes to internalize.
rAAV2/1 enters the nucleus more rapidly than rAAV2/2 and rAAV2/5
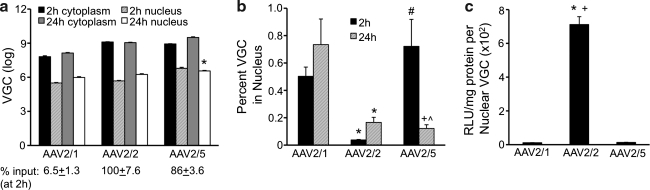
The data presented thus far demonstrate that rAAV2/1 crosses the plasma membrane and traffics to the perinuclear region of HeLa cells more rapidly than rAAV2/2 or rAAV2/5. Additionally, although rAAV2/5 and rAAV2/2 co-localize early during infection, rAAV2/5 appears to be more rapidly endocytosed from the plasma membrane than rAAV2/2. Given that rAAV2/1 and rAAV2/5 are significantly less effective at transducing HeLa cells than rAAV2/2 (Fig. 1), we hypothesized that rAAV2/2 would be imported into the nucleus rapidly, while rAAV2/1 and rAAV2/5 would remain sequestered in a cytoplasmic compartment. To test this hypothesis, we infected HeLa cells with rAAV2/1, rAAV2/2, or rAAV2/5 for either 2 hr or 24 hr, and then quantified the number of VGC in the cytoplasm and nucleus by TaqMan PCR (Fig. 4a and b). In this experiment, less than 7% of the total input rAAV2/1 genomes were detected intracellularly after a 2-hr infection, as compared with 100% of genomes for rAAV2/2 and 86% for rAAV2/5 at the same time point (Fig. 4a). We found that only a small fraction of viral genomes of all three serotypes (less than 1%) reach the nucleus even after 24 hr (Fig. 4b). However, in contrast to our expectations, rAAV2/2 was significantly less efficient at mobilizing genomes from the cytoplasm to the nucleus than rAAV2/1 (p<0.001) and rAAV2/5 (p<0.01) (Fig. 4b), despite the fact it was the most efficient at transducing HeLa cells.
FIG. 4.
Biochemical nuclear import profiles for different rAAV serotypes. In order to determine the rate of nuclear import of rAAV2/1, rAAV2/2, and rAAV2/5 in HeLa cells, we carried out infections using pseudotyped luciferase vectors with capsids from each serotype for 2 or 24 hr (MOI=1000 VGC/cell). Following isolation by trypsinization, the cells were fractionated into cytoplasmic and nuclear compartments. TaqMan PCR was used to determine the number of VGC in each fraction. (a) Average total VGC in the nuclear and cytoplasmic fractions from cells infected for 2 and 24 hr. The percent input below the graph was determined by calculating the ratio of total viral genomes detected in cell lysates by TaqMan PCR (cytoplasmic+nuclear) at 2 hr following infection divided by the total viral genomes applied to cells at the time of infection. This value represents the efficiency of viral infection. Error bars=mean±SEM, n=3 independent experimental samples. Bonferroni post-test results (two-way ANOVA): 2-hr nuclear VGC vs. 24-hr nuclear VGC, *p<0.05. (b) Percent cellular VGC contained within the nucleus. For each sample, the percentage of VGC in the nuclei was calculated based on the total number of VGC in each fraction, as determined by TaqMan PCR; these values were then averaged and plotted (error bars=mean±SEM, n=3 independent experimental samples). Bonferroni post-test results (two-way ANOVA): AAV2/1 vs. AAV2/2, *p<0.05; AAV2/1 vs. AAV2/5, +p<0.01; AAV2/2 vs. AAV2/5, #p<0.01; 2 h vs. 24 h, ^p<0.01. (c) The transducing units per nuclear viral genome. These numbers represent the effectiveness of a nuclear viral genome to express its transgene, and were determined by dividing the level of transduction seen at 24 hr by the number of nuclear VGC at this time point. The resulting average values were then plotted (error bars=mean±SEM, n=4 independent experimental samples). Post-test (Tukey's multiple comparison) results: AAV2/1 vs. AAV2/2, *p<0.001; AAV2/2 vs. AAV2/5, +p<0.001.
Several additional interesting findings emerged from this analysis (Fig. 4a and b). First, rAAV2/1 moved into the nucleus at a faster rate than either rAAV2/2 (p<0.001) or rAAV2/5 (Fig. 4b, p<0.01) by 2 hr, and its nuclear genomes were quite stable through 24 hr post-infection, with little change in the total (cytoplasmic plus nuclear) genome abundance during this period (Fig. 4a). Second, rAAV2/5 also accumulated in the nucleus by 2 hr; however, the number of nuclear genomes for this serotype declined significantly by 24 hr post-infection (Fig. 4a, p<0.05), whereas the number of cytoplasmic genomes remained stable over this period, resulting in a significant decline in the percentage of nuclear genomes (Fig. 4b, p<0.001). Third, rAAV2/2 was the only serotype for which the percentage of viral genomes in the nucleus rose significantly during the period from 2 to 24 hr (Fig. 4b). Interestingly, at the end of the 24-hr infection, the difference in abundance of total nuclear viral genomes between the three serotypes varied by less than 10-fold (Fig. 4a), despite the 10- to 100-fold difference in transduction efficiency between these serotypes (Fig. 1a). Calculations of the number of transduction units produced per nuclear viral genome (relative light units per milligram of protein per nuclear viral genome) reinforce the notion that rAAV2/2 nuclear genomes are much more (40- to 80-fold) effective at expressing their transgene in comparison to those of the other serotypes (Fig. 4c).
In summary, these subcellular fractionation studies revealed the following about each serotype: rAAV2/1 genomes rapidly move to the nucleus by 2 hr after which nuclear genomes remain stable; rAAV2/5 genomes also quickly move to the nucleus, but then appear to be rapidly degraded; rAAV/2/2 genomes move less inefficiently in the nucleus, but accumulate over a 24-hr period.
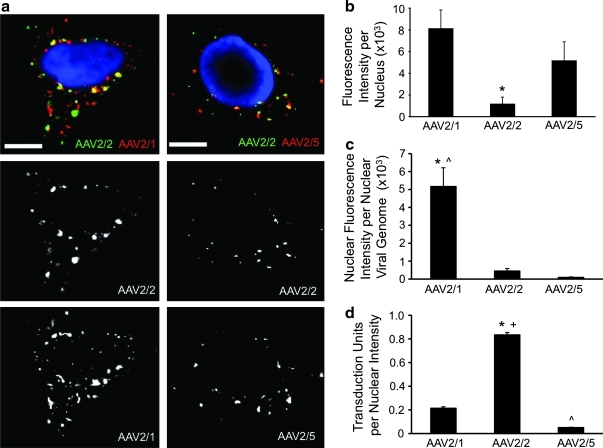
We also used fluorescently labeled rAAV particles to confirm differences in the rate of nuclear import between the serotypes. HeLa cells were co-infected with either rAAV2/1-Alexa 568 and rAAV2/2-Alexa 488, or rAAV2/5-Alexa 568 and rAAV2/2-Alexa 488, by prebinding the virions at 4°C for 1 hr, followed by a 2-hr incubation at 37°C (Fig. 5a). The total nuclear fluorescence intensity of each serotype was then quantified by co-localization with DAPI (Fig. 5b). Results from this analysis demonstrated significantly greater (p<0.05) nuclear rAAV2/1 fluorescence than nuclear rAAV2/2 fluorescence at 2 hr post-infection. Although this finding is consistent with a faster rate of entry (Fig. 3c) and more rapid movement of viral genomes into the nucleus (Fig. 4b) in the case of rAAV2/1, it did not support our findings with unlabeled virus with continuous 2-hr infection, in which total nuclear viral genomes in the nucleus were similar for rAAV2/1 and rAAV2/2 (Fig. 4a). Given that the fluorescent signal of the labeled rAAV capsid would be influenced by the rate of capsid processing in the nucleus, the findings presented in Fig. 5b suggest that nuclear uncoating may be much more efficient in the case of rAAV2/2 than in that of rAAV2/1. Quantification of the ratio of fluorescent nuclear capsid signal to the abundance of viral nuclear genomes supported this hypothesis, demonstrating that rAAV2/1 virions in the nucleus retain 10-fold more capsid fluorescence per genome than rAAV2/2 at 2 hr post-infection (Fig. 5c). MOI had no effect on this pattern of accumulation of nuclear fluorescence signal, as more rAAV2/1 fluorescence was present in the nucleus of HeLa cells than rAAV2/2 and rAAV2/5 at both low MOI (1000 VGC/cell, Supplementary Fig. S2d) at high MOI (100,000 VGC/cell, Supplementary Fig. S2h) in single infection experiments with Qdot-labeled virions. Qdot-labeled virus was necessary to achieve sensitivities for detection of rAAV at MOIs of 1000 VGC/cell; however, the patterns of virion accumulation in the nucleus were similar for the various serotypes using Alexa and Qdot labeling.
FIG. 5.
Nuclear import profiles of fluorescently labeled rAAV particles. (a) Labeled rAAV particles were co-infected into HeLa cells as in the experiments outlined in Figs. 2 and 3 (MOI=105 VGC/cell). Cells were fixed at 2 hr post-infection and imaged by confocal fluorescence microscopy. Shown are representative images of cells co-infected with rAAV2/1 (red) and rAAV2/2 (green) (right panels), and with rAAV2/5 (red) and rAAV2/2 (green) (left panels). Nuclei were stained blue with DAPI to allow for co-localization of the virions with the nucleus. (b) Efficiency of nuclear import at 2 hr post-infection, as determined from quantification of data from images in a. Total fluorescence signal intensity of each viral serotype overlapping with blue DAPI signal was calculated for each cell using Metamorph software, and the average integrated intensity overlap was plotted. (c) Uncoating efficiency, indirectly evaluated as the fluorescence intensity of unprocessed nuclear virions that retained label (as depicted in b) divided by the total number of nuclear viral genomes at 2 hr (shown in Fig. 4a) and adjusted to the number of cells per well. (d) Relative transduction units at 24 hr divided by the average nuclear fluorescence intensity of AAV virions at 2 hr (from b) following infection at MOI=100,000 VGC/cell in both assays. For all panels, error bars=mean±SEM, n=4 independent experimental samples. Scale bar=10 μm. Post-test (Tukey's multiple comparison) results: AAV2/1 vs. AAV2/2, *p<0.001; AAV2/2 vs. AAV2/5, +p<0.001; AAV2/1 vs. AAV2/5, ^p<0.001.
Levels of rAAV2/5 fluorescence in the nucleus by 2 hr were only slightly lower than those of rAAV2/1 (Fig. 5b), despite the fact that fivefold more rAAV2/5 genomes were present in the nucleus at this time point (Fig. 4a). As in the case of rAAV2/2, the ratio of fluorescent nuclear capsid signal to the abundance of nuclear viral genomes for rAAV2/5 was 16-fold lower than that of rAAV2/1 (Fig. 5c). This reduced specific activity of fluorescently labeled rAAV2/5 in the nucleus is consistent with rapid capsid uncoating and degradation in the nucleus. Thus, our findings suggest that rAAV2/2 and rAAV2/5 likely uncoat much more rapidly than rAAV2/1, leading to a rapid decline in fluorescence signal from the capsid.
In order to correlate the entry of the virus into the nucleus at 2 hr to its transduction efficiency, we calculated the number of transduction units following infection with an MOI of 100,000 VGC/cell at 24 hr as a function of nuclear fluorescence intensity of capsid following infection at the same MOI at 2 hr. The results of this analysis (Fig. 5d) demonstrate that nuclear rAAV2/2 virions are significantly more efficient in expressing transgene in HeLa cells than rAAV2/1 (about 4-fold) or rAAV2/5 (approximately 16-fold) (p<0.001). This analysis, which likely takes into account uncoating rates, resulted in a similar trend between serotypes to that obtained by biochemical analysis of viral genomes (Fig. 4c). Taken together, the results shown in Figs. 4 and 5 support the notion that that rAAV2/1 enters the nucleus more rapidly than rAAV2/2 and rAAV2/5, where the virions are stable and fail to uncoat efficiently, and thus its transgene is poorly expressed. In the case of rAAV2/5, both the virion and genome are efficiently degraded, leading to relatively inefficient expression of the encoded transgene. rAAV2/2 is least efficient at entering the nucleus, but appears to be productively uncoated without degradation of its genome—and thus the level of transgene expression is greater than for the other two serotypes.
rAAV serotypes differ dramatically in capsid disassembly in the nucleus
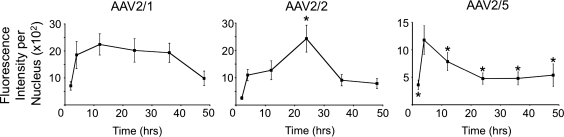
In order to better evaluate the potential differences in capsid uncoating, we compared the kinetics of accumulation and disappearance of fluorescently labeled capsid protein in the HeLa cell nucleus. Cells were infected by binding Alexa 488-labeled virions at 4°C for 1 hr, followed by shifting the cells to 37°C for 2–48 hr. As shown in Fig. 6, the total fluorescence intensity of rAAV2/2 in the nucleus steadily increased from 2 to 24 hr post-infection and then declined, as the virions were presumably disassembled while vector genomes remained stably in the nucleus (Fig. 4b). In the case of rAAV2/5, the intensity of the nuclear signal peaked by 4 hr and rapidly declined thereafter. This finding supported our previous results demonstrating that nuclear rAAV2/5 genomes are very unstable and rapidly cleared from the nucleus (Fig. 4b). Fluorescently labeled AAV2/1 entered the nucleus more rapidly (by 4 hr), but in this case the capsid signal persisted through 36 hr. This stability of rAAV2/1 viral capsids in the nucleus corresponded to the stability of its nuclear viral genome (Fig. 4b). To evaluate whether the Alexa tag on the AAV virion correlated with persistence of intact virions in the nucleus, we performed control experiments co-localizing Alex 488-AAV2/2 with A20 antibody staining against the intact AAV2 capsid. These experiments demonstrated that the presence of Alexa 488-AAV2/2 signal in the nuclei of cells correlated with intact capsid antibody staining and remained stable throughout the experiment (Supplementary Fig. S3). Although it was not possible to perform similar experiments with rAAV2/1 and rAAV2/5, due to the lack of specificity of antibodies against the intact virions of these serotypes (data not shown), the mechanism of Alexa 488 clearance from the nucleus during uncoating of virions is most likely independent of serotype. These results support the hypothesis that rAAV2/1 virions rapidly move to the nucleus where they are inefficiently disassembled, rendering their genomic DNA inaccessible to cellular factors needed for second-strand synthesis and transduction. They also support the hypothesis that rAAV2/5 virions and their genomes are rapidly degraded in the nucleus, leading to poor transduction of HeLa cells. Finally, they are consistent with our finding that rAAV2/2 processing from the cytoplasm to the nucleus is slow, but more effective in rendering the virus a suitable substrate for uncoating and subsequent second-strand synthesis.
FIG. 6.
Persistence of rAAV capsid fluorescence in the nucleus. HeLa cells were infected with rAAV2/1, rAAV2/2, or rAAV2/5 labeled with Alexa-488 by incubating them with virus at 4°C for 1 hr and then shifting them to 37°C for 2–48 hr (MOI=105 VGC/cell). Cells were fixed and imaged by confocal fluorescence microscopy. For each nucleus, the total fluorescence intensity of viral capsids was determined using Metamorph software. Statistical comparisons were performed by one-way ANOVA with post-test (Dunnett's multiple comparison). The asterisk marks statistically significant differences as compared to values at the 4-hr time point (*p<0.05). Error bars=mean±SEM, n=20 cells.
Discussion
The ability to pseudotype rAAV2 vector genomes with a wide array of capsid serotypes has significantly expanded the repertoire of gene therapy vectors. In most cases, the choice of a serotype for a particular gene therapy application is an empirical one, and is based on the ability of the serotype to transduce cells of the target organ efficiently. However, little is known about the biological characteristics that make a particular serotype more effective. Previous work has suggested that for several rAAV serotypes, the rate of intracellular trafficking to the nucleus is a major barrier to efficient transduction (Ding et al., 2005). Additionally, post-nuclear events controlled by the nucleolus have been implicated in the transduction efficiency of rAAV2/2 (Johnson and Samulski, 2009). While these and other processing events have most often been evaluated for single serotypes, few studies have directly compared several different serotypes in a single system to determine how different steps in the infectious process influence transduction. In the present study, we utilized fluorescence imaging and biochemical approaches to evaluate how entry, intracellular trafficking, nuclear uptake, and stability of the nuclear capsid and genome differ for three commonly used AAV serotypes. Our findings suggest that the steps of the infectious process for rAAV2/2, rAAV2/1, and rAAV2/5 differ in unique ways that influence their ability to efficiently transduce HeLa cells.
Our co-infection studies demonstrate that rAAV2/1 enters HeLa cells through a mechanism distinct from that used by rAAV2/2, and that rAAV2/2 and rAAV2/5 co-localize on the cell membrane prior to entry through an common endosomal compartment. This finding was somewhat surprising given that rAAV2/1 and rAAV2/5 have been shown to both utilize α-2,3-N-linked sialic acid as a binding receptor, whereas rAAV2/2 binds to heparan sulfate proteoglycans and several other co-receptors that are apparently not shared by rAAV2/1 and rAAV2/5 (Wu et al., 2006). Although our current study did not directly test the receptors utilized for entry into HeLa cells, it does suggest that partitioning of critical receptors/co-receptors to different parts of the plasma membrane may determine the pathway and rate of entry for a particular serotype. In the case of rAAV2/1, entry from plasma membrane was extremely rapid relative to that for the other two serotypes. Although rAAV2/2 and rAAV2/5 both entered at a slower rate than rAAV2/1, the rapid separation of the two virions suggests that early sorting events occur as early as 5–10 min into infection. To our knowledge, this is the first time differentially labeled rAAV virions have been used to directly compare and contrast serotype-specific trafficking events at the single-cell level, and this strategy has uncovered previously unknown differences between these serotypes.
Perhaps the most profound differences observed between the three serotypes evaluated were related to their rates of accumulation within the nucleus and the stability of the nuclear capsids and genomes. rAAV2/1 clearly underwent the most rapid endocytosis and movement into the HeLa cell nucleus. However, surprisingly, this feature did not translate into efficient transduction. The stability of rAAV2/1 viral genomes and capsids within the nucleus, coupled with their low transduction efficiency, suggests that this serotype uncoats inefficiently in HeLa cells. We did not observe clustering of rAAV2/1 within the nucleus that would be suggestive of nucleolar sequestration, an event previously suggested to potentially limit uncoating (Johnson and Samulski, 2009). rAAV2/5 also appeared to enter the nucleus much more rapidly than rAAV2/2. However, its less efficient transduction compared to that of rAAV2/2 appears to be a consequence of its instability within the nucleus. Both the viral genome and capsids of nuclear rAAV2/5 appear to turn over much more rapidly than those of rAAV2/1, suggesting that the reason for poor transduction by rAAV2/5 is nucleus-specific degradation of the virion. Although rAAV2/2 was the slowest of the serotypes to traffic to the nucleus, its genome was quite stable following nuclear import, even though its viral capsid was eventually lost. These findings suggest that the high level of transduction by rAAV2/2 relative to those of the other serotypes is due to more efficient and productive virus uncoating in the nucleus.
The reason for differences in post-nuclear processing of virions and genomes between the three serotypes tested here is currently unknown. It is possible that differences in endosomal processing of these three serotypes influence presentation of the virus in the nucleus and subsequent uncoating events. In this scenario, the slower rate of endosomal processing of rAAV2/2 may allow for more complete proteolytic processing of the capsid, and thus for more efficient/productive uncoating in the nucleus. This, in turn, would be conducive to productive second-strand synthesis and genome stabilization. It is also possible that the serotype 5 capsid specifically recruits nuclear factors that lead to the degradation of both the capsid proteins and the viral genome, while the capsid of rAAV2/2 fails to do so and is therefore more stable in the nucleus. This may explain why rAAV2/2 and rAAV2/5, while encoding an identical genome, display different viral DNA stability profiles. Few studies have addressed how nuclear uncoating events influence the efficiency of rAAV transduction. However, one comparison between rAAV2/8 and rAAV2/2 has demonstrated that higher levels of transduction of hepatocytes with rAAV2/8 correlated with more rapid uncoating (Thomas et al., 2004). Further insights into the cell-specific mechanisms that regulate rAAV post-nuclear events, such as uncoating and genome degradation, will likely lead to methods whereby transduction may be enhanced.
Although the current study was limited to the biology of rAAV transduction in HeLa cells, our findings may also have relevance to in vivo findings with several rAAV serotypes that have suggested viral entry is not the sole limiting factor in rAAV transduction. For example, in polarized airway epithelia, large differences in transduction efficiency following apical infection with rAAV2/1 and rAAV2/2 are observed despite similar efficiencies of endocytosis. These differences in transduction appear to be due to more efficient intracellular trafficking of rAAV2/1 to the nucleus (Duan et al., 2000; Yan et al., 2006), but may also involve components of uncoating that were not directly investigated. In the liver, rAAV2/2 nuclear processing of the capsid is quite slow, whereas it is more efficient with other serotypes such as rAAV2/8 (Thomas et al., 2004). In this regard, it is interesting that rAAV2/2 appears to uncoat most efficiently in HeLa cells of the three serotypes studied. We hypothesize these differences are a reflection of altered receptor entry pathways for rAAV2/2 in HeLa cells and hepatocytes, which may impact the efficiency of intracellular processing of the virion in these to cell types. The use of co-infection strategies with differentially labeled rAAVs may help to sort out the mechanisms (entry, intracellular trafficking, nuclear import, and uncoating) that limit transduction in vivo with various serotypes.
Our comparative analyses evaluating the rate of viral entry, intracellular trafficking, and nuclear import for three distinct rAAV serotypes have demonstrated unique serotype-specific differences that may influence the efficiency of transduction. In contrast to our original hypothesis, rapid and efficient movement of the virion to the nucleus was not the most important determinant of efficient transgene expression by rAAV. It is likely that the distinct trafficking pathways observed for rAAV2/1, rAAV2/2, and rAAV2/5 in HeLa cells influence virion processing once the virus arrives in the nucleus. Such processing events will likely be cell-type specific and controlled by specific sorting pathways within the endosomal, cytoplasmic, and nuclear compartments. Further studies focusing on the identification of cellular factors involved in these processes would shed light on the precise relationship between viral trafficking, uncoating, and transduction. Knowledge of the underlying mechanisms will make it possible to enhance the effectiveness of rAAV vectors for gene therapy.
Supplementary Material
Acknowledgments
This work was supported by NIH grants HL058340 and HL108902 (to JFE), the University of Iowa Center for Gene Therapy (DK54759), and a Carver Chair in Molecular Medicine (to JFE).
Author Disclosure Statement
No competing financial interests exist.
References
- Bantel-Schaal U. Hub B. Kartenbeck J. Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment. J. Virol. 2002;76:2340–2349. doi: 10.1128/jvi.76.5.2340-2349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantel-Schaal U. Braspenning-Wesch I. Kartenbeck J. Adeno-associated virus type 5 exploits two different entry pathways in human embryo fibroblasts. J. Gen. Virol. 2009;90:317–322. doi: 10.1099/vir.0.005595-0. [DOI] [PubMed] [Google Scholar]
- Bartlett J.S. Wilcher R. Samulski R.J. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 2000;74:2777–2785. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B.J. Adeno-associated virus vectors in clinical trials. Hum. Gene Ther. 2005;16:541–550. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- Choi V.W. McCarty D.M. Samulski R.J. AAV hybrid serotypes: improved vectors for gene delivery. Curr. Gene Ther. 2005;5:299–310. doi: 10.2174/1566523054064968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W. Zhang L. Yan Z. Engelhardt J.F. Intracellular trafficking of adeno-associated viral vectors. Gene Ther. 2005;12:873–80. doi: 10.1038/sj.gt.3302527. [DOI] [PubMed] [Google Scholar]
- Ding W. Zhang L.N. Yeaman C. Engelhardt J.F. rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion. Mol. Ther. 2006;13:671–682. doi: 10.1016/j.ymthe.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D. Li Q. Kao A.W., et al. Dynamin is required for recombinant adeno-associated virus type 2 infection. J. Virol. 1999;73:10371–10376. doi: 10.1128/jvi.73.12.10371-10376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D. Yue Y. Yan Z. Yang J., et al. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Vandenberghe L.H. Wilson J.M. New recombinant serotypes of AAV vectors. Curr. Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Girod A. Wobus C.E. Zadori Z., et al. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J. Gen. Virol. 2002;83:973–978. doi: 10.1099/0022-1317-83-5-973. [DOI] [PubMed] [Google Scholar]
- Grieger J.C. Johnson J.S. Gurda-Whitaker B., et al. Surface-exposed adeno-associated virus Vp1-Nls capsid fusion protein rescues infectivity of noninfectious wild-type Vp2/Vp3 and Vp3-only capsids but not that of fivefold pore mutant virions. J. Virol. 2007;81:7833–7843. doi: 10.1128/JVI.00580-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. Qing K. Kwon H.J., et al. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J. Virol. 2000;74:992–996. doi: 10.1128/jvi.74.2.992-996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.S. Samulski R.J. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J. Virol. 2009;83:2632–2644. doi: 10.1128/JVI.02309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg S. Bottcher B. Von Der Lieth C.W., et al. A conformational change in the adeno-associated virus type 2 capsid leads to the exposure of hidden VP1 N termini. J. Virol. 2005;79:5296–5303. doi: 10.1128/JVI.79.9.5296-5303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux K. Goerlitz N. Schlemminger S., et al. Green fluorescent protein-tagged adeno-associated virus particles allow the study of cytosolic and nuclear trafficking. J. Virol. 2005;79:11776–11787. doi: 10.1128/JVI.79.18.11776-11787.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani B. Baltzer C. Valle N., et al. Low pH-dependent endosomal processing of the incoming parvovirus minute virus of mice virion leads to externalization of the VP1 N-terminal sequence (N-VP1), N-VP2 cleavage, and uncoating of the full-length genome. J. Virol. 2006;80:1015–1024. doi: 10.1128/JVI.80.2.1015-1024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajusola K. Gruchala M. Joch H., et al. Cell-type-specific characteristics modulate the transduction efficiency of adeno-associated virus type 2 and restrain infection of endothelial cells. J. Virol. 2002;76:11530–11540. doi: 10.1128/JVI.76.22.11530-11540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlioglu S. Benson P.K. Yang J., et al. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation. J. Virol. 2000;74:9184–9196. doi: 10.1128/jvi.74.19.9184-9196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seisenberger G. Ried M.U. Endress T., et al. Real-time single-molecule imaging of the infection pathway of an adeno-associated virus. Science. 2001;294:1929–1932. doi: 10.1126/science.1064103. [DOI] [PubMed] [Google Scholar]
- Sipo I. Fechner H. Pinkert S., et al. Differential internalization and nuclear uncoating of self-complementary adeno-associated virus pseudotype vectors as determinants of cardiac cell transduction. Gene Ther. 2007;14:1319–1329. doi: 10.1038/sj.gt.3302987. [DOI] [PubMed] [Google Scholar]
- Thomas C.E. Storm T.A. Huang Z. Kay M.A. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L.H. Wilson J.M. Gao G. Tailoring the AAV vector capsid for gene therapy. Gene Ther. 2009;16:311–319. doi: 10.1038/gt.2008.170. [DOI] [PubMed] [Google Scholar]
- Wu Z. Asokan A. Samulski R.J. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol. Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Xiao W. Warrington K.H., Jr. Hearing P., et al. Adenovirus-facilitated nuclear translocation of adeno-associated virus type 2. J. Virol. 2002;76:11505–11517. doi: 10.1128/JVI.76.22.11505-11517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z. Zak R. Luxton G.W., et al. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J. Virol. 2002;76:2043–2053. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z. Zak R. Zhang Y., et al. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J. Virol. 2004;78:2863–2874. doi: 10.1128/JVI.78.6.2863-2874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z. Lei-Butters D.C. Liu X., et al. Unique biologic properties of recombinant AAV1 transduction in polarized human airway epithelia. J. Biol. Chem. 2006;281:29684–29692. doi: 10.1074/jbc.M604099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L. Li W. Yang Z., et al. Impaired nuclear transport and uncoating limit recombinant adeno-associated virus 2 vector-mediated transduction of primary murine hematopoietic cells. Hum. Gene Ther. 2004;15:1207–1218. doi: 10.1089/hum.2004.15.1207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.