Abstract
Using a triple-lumen constant perfusion system, the following observations were made in normal subjects. First, chloride, bicarbonate, and sodium were found to exhibit net movement across ileal mucosa against electrochemical gradients. Second, during perfusion with a balanced electrolyte solution simulating plasma, the ileum generally absorbed, but sometimes secreted fluid. A reciprocal net movement of chloride and bicarbonate was noted when sodium movement was zero. Increasing rates of sodium absorption were associated with decreasing bicarbonate secretion rates and finally bicarbonate absorption. Even when bicarbonate was absorbed ileal contents were alkalinized (by contraction of luminal volume). Third, net chloride movement was found to be sensitive to bicarbonate concentration in ileal fluid. For instance, chloride was absorbed from solutions containing 14 or 44 mEq/liter of bicarbonate, but was secreted when ileal fluid contained 87 mEq/liter of bicarbonate. Fourth, when chloridefree (sulfate) solutions were infused, the ileum absorbed sodium bicarbonate and the ileal contents were acidified. Fifth, when plasma-like solutions were infused, the potential difference (PD) between skin and ileal lumen was near zero and did not change when chloride was replaced by sulfate in the perfusion solution.
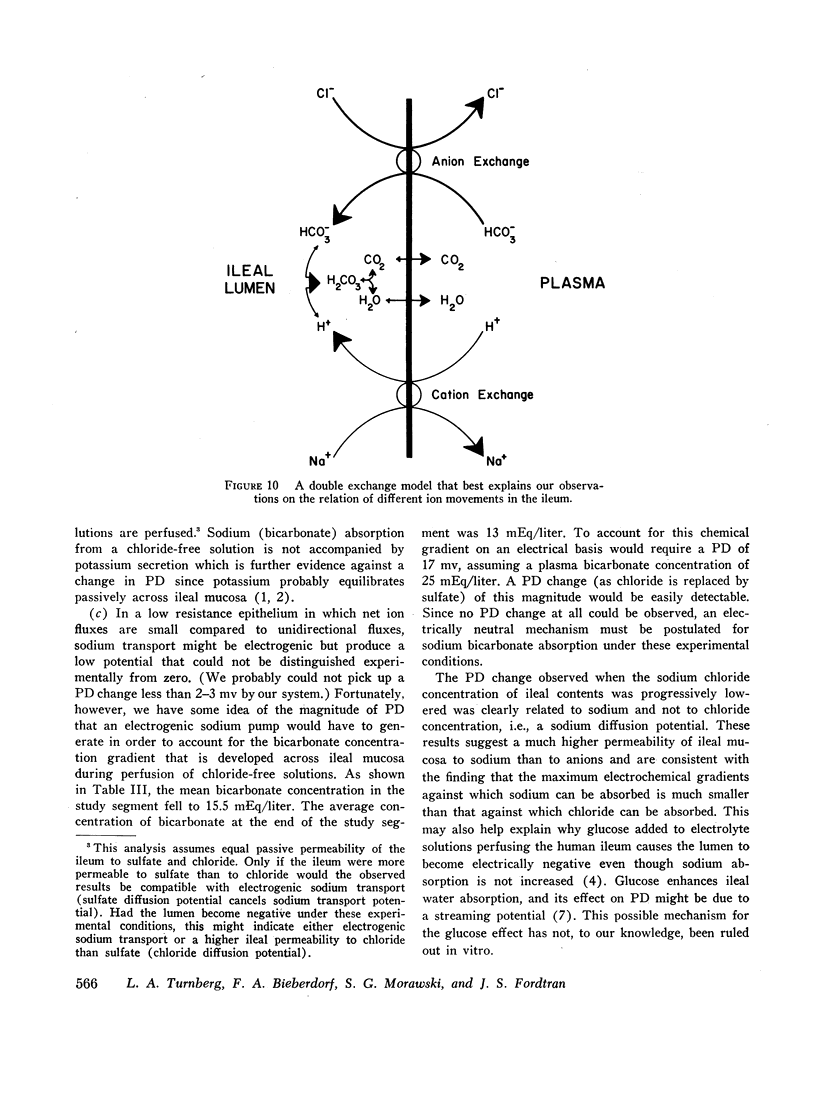
These results suggest that ileal electrolyte transport occurs via a simultaneous double exchange, Cl/HCO2 and Na/H. In this model neither the anion nor the cation exchange causes net ion movement; net movement results from the chemical reaction between hydrogen and bicarbonate. No other unitary model explains all of the following observations: (a) human ileal transport in vivo is essentially nonelectrogenic even though Na, Cl, and HCO3 are transported against electrochemical gradients, (b) the ileum can secrete as well as absorb, (c) ileal contents are alkalinized during absorption of or during secretion into a plasma-like solution, and (d) the ileum acidifies its contents when sulfate replaces chloride. Data obtained with a carbonic anhydrase inhibitor support the proposed model.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter M. J., Parsons D. S. Carbonic anhydrase activity of mucosa of small intestine and colon. Nature. 1968 Jul 13;219(5150):176–177. doi: 10.1038/219176a0. [DOI] [PubMed] [Google Scholar]
- DIETSCHY J. M. WATER AND SOLUTE MOVEMENT ACROSS THE WALL OF THE EVERTED RABBIT GALL BLADDER. Gastroenterology. 1964 Oct;47:395–408. [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Jr, Carter N. W. The mechanisms of sodium absorption in the human small intestine. J Clin Invest. 1968 Apr;47(4):884–900. doi: 10.1172/JCI105781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara S., Fox K. R., Hogben C. A. Depression of chloride transport by carbonic anhydrase inhibitors in the absence of carbonic anhydrase. Nature. 1967 May 20;214(5090):836–837. doi: 10.1038/214836a0. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Wright E. M. Diffusion potentials across the small intestine. Nature. 1966 Oct 8;212(5058):189–190. doi: 10.1038/212189a0. [DOI] [PubMed] [Google Scholar]



