Abstract
We have shown that the antitumor activity of vesicular stomatitis virus (VSV) against B16ova tumors in C57BL/6 mice is predominantly due to innate antiviral immune effectors. We have also shown that the innate immune-activating properties of VSV can be harnessed to prime adaptive T-cell responses against a tumor-associated antigen (TAA) if the virus is engineered to express the cDNA of the antigen. Here, we show that the combination of VSV expressing OVA as a model tumor antigen, along with adoptive T-cell therapy targeted against the same antigen, is superior to either treatment alone and induces systemic antitumor activity. In addition, we extend our findings with the OVA model to the therapeutic use of VSV expressing hgp100, a self TAA against which tolerance is well established in C57BL/6 mice. In contrast to VSV-ova, T-cell responses raised by VSV-hgp100 were insufficient to improve therapy against B16ova tumors compared with VSV-GFP alone. However, in combination with adoptive transfer of gp100-specific pmel T cells, intratumoral VSV-hgp100 cured significantly more mice than either virus or T cells alone. Even in an aggressive model of metastatic disease, antitumor therapy was generated at levels similar to those observed in the VSV-ova/OT-I model in which a potently immunogenic, nonself TAA was targeted. Therefore, individual poorly effective virotherapies and T-cell therapies that target self TAA of low immunogenicity, which reflects the situation in patients, can be combined to generate very effective antitumor therapy.
Wongthida and colleagues demonstrate that a combination of vesicular stomatitis virus expressing a tumor-associated antigen (TAA), along with adoptive T-cell therapy targeted against the same antigen, is superior to either treatment alone and induces systemic antitumor activity in a mouse model. In addition, they show that the therapeutic use of combination oncolytic virotherapy with adoptive T-cell therapy to target a self-TAA, against which tolerance is well established in C57BL/6 mice, is effective in the context of an aggressive model of metastatic disease.
Introduction
Injection of a potent viral immunogen such as an oncolytic virus into a tumor will activate an innate immune reactivity that will limit viral replication and clear the infection (Pecora et al., 2002; Chiocca et al., 2004; Fulci et al., 2006; Breitbach et al., 2007; Galivo et al., 2010b; Le Boeuf et al., 2010; Wongthida et al., 2010a). However, the innate immune response to the virus may also have significant antitumor bystander effects through the direct and indirect activity of both antiviral cytokines (Saloura et al., 2010; Wongthida et al., 2010b) and immune effector cells such as natural killer cells (Diaz et al., 2007; Kottke et al., 2008; Prestwich et al., 2009). Moreover, the innate antiviral immune response is also critical in shaping the subsequent adaptive immune response against both viral and tumor-associated antigens (TAA) (Ghiringhelli et al., 2007). Therefore, as it relates to influencing the efficacy of oncolytic virotherapy, the immune response can be viewed either as a potent inhibitor or as an essential effector of antitumor therapy—or anywhere in between.
Recently, we have shown that the antitumor therapy of intratumoral vesicular stomatitis virus (VSV) in the B16ova model derives predominantly from immune bystander effects resulting from the innate antiviral immune response against the viral immunogen at the tumor site. Thus, therapy was dependent upon viral infection of tumor cells, but not ongoing replication (Galivo et al., 2010a, 2010b), as well as on intact innate immune signaling through MyD88 and innate cytokines and effector cells (Diaz et al., 2007; Wongthida et al., 2010a, 2010b). In particular, even though improved levels of intratumoral viral replication were observed when innate immune signaling was inhibited in vivo in MyD88−/− mice, therapy was lost because of the lack of intact innate immune effectors, which are absent in MyD88−/− mice (Wongthida et al., 2010a). Therefore, in this model at least, the antitumor activity of innate antiviral immune effectors predominate in vivo over the therapeutic effects of viral replication or adaptive T-cell immunity.
We reasoned, therefore, that by recruiting the effector mechanisms associated with adaptive T-cell responses against tumors, it would be possible to enhance the therapeutic effects of oncolytic virotherapy with VSV. This would have two major benefits by enhancing T-cell–mediated clearance of the local virus-injected tumor and by generating systemic therapy against metastatic tumors (Prestwich et al., 2008). In this respect, we and others (Vigil et al., 2008; Bridle et al., 2010; Castelo-Branco et al., 2010; Zhang et al., 2010) have enhanced priming of adaptive CD8+ T-cell responses against TAA by incorporating specific TAA into the oncolytic virus itself (Diaz et al., 2007). Thus, injection of a VSV, engineered to encode the OVA antigen into established B16ova tumors, primed significantly increased numbers of ova-specific T cells in vivo compared with VSV-GFP (Diaz et al., 2007) due in large part to escape of the VSV-ova from the injected tumor into the tumor-draining lymph nodes (TDLN), where it became an extremely effective vaccine for both VSV-specific and OVA-specific immune responses (Diaz et al., 2007).
However, in those studies (Diaz et al., 2007), no tolerance existed in the C57BL/6 mice against the foreign OVA antigen and the frequency of endogenous T-cell precursors with potential reactivity against OVA was high. Therefore, our goal in the current study was to test whether it would be possible to combine the innate immune antitumor activity of intratumoral VSV, with mobilization of specific T-cell responses against a truly self TAA, against which tolerance is in place in the immune-competent host and against which there exists only a very low frequency of T-cell precursors. We show here that, unlike with VSV-ova, intratumoral injections of VSV expressing an altered-self version of the endogenous TAA gp100 were insufficient to improve on therapy with VSV-GFP. However, an alternative way to provide large numbers of potentially tumor-reactive T cells in vivo is through the adoptive transfer of antitumor T cells, such as tumor-infiltrating lymphocytes or receptor-engineered peripheral blood lymphocytes (Rosenberg et al., 1988; Wang and Rosenberg, 1996; Hawkins et al., 2010; Leisegang et al., 2010). Efficacy of adoptive therapy has been restricted by inadequate persistence and expansion of transferred T cells in vivo (Yee et al., 2002; Dudley and Rosenberg, 2003), limitations that have been addressed by combination with host lymphodepletion (Maine and Mule, 2002; Gattinoni et al., 2005), active immunization with TAA (Wang et al., 1995; Parmiani et al., 2002; Smith et al., 2008), or local pro-inflammatory killing of the tumor to enhance T-cell trafficking and activation (Sanchez-Perez et al., 2007; Prestwich et al., 2008; Qiao et al., 2008). Therefore, we hypothesized that it would be possible to combine oncolytic virotherapy with the antitumor potency associated with mobilization of adaptive T-cell immune responses against truly self TAA. We show here that the combination of intratumoral VSV-hgp100, with adoptive transfer of pmel T cells, generated significantly improved therapy, including cures, against both local and distant tumors compared with treatment with either virus or T cells alone. This approach therefore combines direct viral oncolysis, effective tumor vaccination, and adoptive T-cell transfer to mobilize a therapeutic T-cell response through the use of oncolytic virotherapy.
Materials and Methods
Cell lines
Murine B16ova melanoma cells (H2-Kb) were derived from B16 cells transduced with a cDNA encoding the chicken ovalbumin gene (Linardakis et al., 2002). Cell lines were grown in Dulbecco's modified Eagle's medium (DMEM; Life Technologies) supplemented with 10% (v/v) fetal calf serum (FCS; Life Technologies), l-glutamine (Life Technologies), and 5 mg/ml G418 to select for retention of the ova gene. All cell lines were monitored routinely and found to be free of mycoplasma infection.
Naïve OT-I and pmel-1 T cells were isolated from spleen and lymph nodes of OT-I and pmel-1 transgenic mice, respectively. Single cell suspensions were prepared by crushing tissues through a 100-μm filter, and red blood cells were removed by incubation in ACK buffer (sterile distilled H2O containing 0.15 M NH4Cl, 1.0 mM KHCO3, and 0.1 mM EDTA adjusted to pH 7.2–7.4) for 2 min. CD8+ T cells were isolated using the MACS CD8a(Ly-2) microbead magnetic cell sorting system (Miltenyi Biotec).
Mice
C57BL/6 mice (Thy1.1+ and Thy1.2+) were purchased from the Jackson Laboratory at 6–8 weeks of age. The OT-I mouse strain is on a C57BL/6 background (H-2Kb) and expresses a transgenic T-cell receptor Vα2 specific for the SIINFEKL peptide of ovalbumin in the context of MHC class I, H-2Kb (Hogquist et al., 1994). Pmel-1 breeding colonies were purchased from the Jackson Laboratory at 6–8 weeks of age. The pmel-1 mouse expresses a transgenic T-cell receptor αV1/βV13 recognizing an epitope of pmel-17 corresponding to amino acids 25–33 of gp100 presented by H-2Db class I molecules (Overwijk et al., 2003).
Viruses
VSV-GFP and VSV-ova (Indiana serotype) were generated by cloning the cDNA for the green fluorescence protein (GFP) or chicken ovalbumin into the plasmid pVSV-XN2 as described previously (Fernandez et al., 2002). pVSV-hgp100 was constructed by PCR amplifying the human gp100 gene cDNA prepared from Mel88 cells using forward (5′-ATCTCGAGATGGATCTGGTGCTAAAAAGATGC-3′) and reverse (5′-ATGCTAGCTCAGACCTGCTGCCCACT-3′) primers. The PCR product was digested and inserted into the XhoI and NheI sites of the VSV-XN2 vector, genomic plasmid of VSV Indiana serotype (a kind gift from Dr. John Rose, Yale University) to yield pVSV-hgp100. Recombinant VSV-hgp100 was recovered based on the method described previously (Lawson et al., 1995; Ramsburg et al., 2005). Bulk amplification of plaque-purified VSV was performed by infecting BHK-21 cells (multiplicity of infection = 0.01) for 24 hr. Filtered supernatants were harvested and subjected to two rounds of 10% sucrose (10% w/v) in 1 × phosphate-buffered saline (PBS; Mediatech) cushion centrifugation at 100,000 × g for 1 hr at 4°C. The pelleted virus was resuspended in 1 × PBS, aliquoted, and stored at −80°C. VSV stocks were titrated on BHK-21 cells using standard plaque assay (Diaz et al., 2007).
In vivo studies
All procedures were approved by the Mayo Foundation Institutional Animal Care and Use Committee. To establish subcutaneous tumors, 5 × 105 B16-derived tumor cells in 100 μl of PBS were injected into the animals' flanks. Viral injections (50 μl) were administered intratumorally at days 7, 9, 11, 13, 15, and 17 after tumor establishment.
For adoptive transfer experiments, mice were administered naïve (106 cells in 100 μl PBS) or activated (107 cells in 100 μl) OT-I T cells intravenously at day 7 after tumor implantation. Animals were examined daily, and tumor sizes were measured thrice weekly using calipers. Animals were euthanized when tumor size was greater than 1.0 by 1.0 cm in two perpendicular directions.
For in vivo proliferation and activation of naïve OT-I cells, OT-I T cells (Thy1.2+) were adoptively transferred to mice (Thy1.1+) harboring 7-day established B16ova. VSV stocks were injected intratumorally on day 8, and TDLN, tumors, and spleens were harvested for analysis.
In vitro T-cell activation and co-cultures
OT-I mice have been previously described (Hogquist et al., 1994) and were bred at Mayo Clinic. Spleens and peripheral lymph nodes were harvested from OT-I mice and dissociated to obtain a single cell suspension. Red blood cells were lysed with ACK lysis buffer. Cells were resuspended at 1 × 106 cells/ml in Iscove's modified Dulbecco's medium (Gibco) +5% FBS + 1% Pen/Strep + 40 μM 2-ME. Media was supplemented with SIINFEKL peptide at 1 μg/ml and hIL-2 at 50 U/ml. After 2 days, cells were split into new media supplemented with IL-2. Cells were used for adoptive transfer or in vitro assays following 4 days of activation.
Dendritic cell isolation and co-culture
Inguinal TDLN were recovered from mice and dissociated in vitro to achieve single-cell suspensions. Dendritic cell populations (CD11C+ or CD11C+CD8α+) were isolated using MACS microbead magnetic cell-sorting system kits following the manufacturer's protocol (>90% purity) (Miltenyi Biotec). Isolated cells were co-cultured with 1 × 106 naïve OT-I cells (1:10 ratio) for 60 hr at 37°C in a 10% CO2 incubator. Cell-free supernatants and cells were harvested for ELISA and FACS analysis, respectively.
ELISPOT and ELISA analysis for interferon gamma secretion
Cell-free supernatants were tested for interferon gamma (IFN-γ) production by ELISA as directed in the manufacturer's instructions (BD OptEIA Mouse IFN-γ ELISA Set; BD Biosciences Pharmingen). For ELISPOT, spleens were harvested from mice, 1 × 105 cells/well were plated in a 96-well plate in triplicate and restimulated for 48 hr at 37°C with stimulating peptides at 5 μg/ml. Peptide-specific IFN-γ–positive spots were detected according to the manufacturer's protocol (Mabtech Inc.) and were quantified by computer-assisted image analyzer.
The synthetic H-2Kb–restricted peptides hgp10025–33, KVPRNQDWL, ova, SIINFEKL, and VSV N protein–derived RGYVYQGL were synthesized at the Mayo Foundation Core Facility.
Flow cytometry and IFN-γ intracellular staining assay
Cells (1 × 106) were washed and resuspended in PBS containing 0.1% bovine serum albumin (wash buffer) and incubated with directly conjugated primary antibodies for 30 min at 4°C. Cells were then washed and resuspended in 500 μl PBS containing 4% formaldehyde. Cells were analyzed by flow cytometry, and data were analyzed using Flowjo software. For intracellular staining, cell suspensions were incubated for 4 hr in the presence of Golgi Plug reagent, stained, fixed, and permeabilized using a Cytofix/Cytoperm kit from BD Biosciences according to the manufacturer's instructions. All antibodies were obtained from BD Biosciences.
Statistics
Survival data from the animal studies were analyzed by Log-rank test using GraphPad Prism 4 (GraphPad Software). In vitro experiments were analyzed using JMP Software (SAS Institute, Inc.). Statistical significance was determined at the level of p < 0.05 (Diaz et al., 2007).
Results
Virus-expressed antigen activates TAA-specific T cells that traffic to tumors
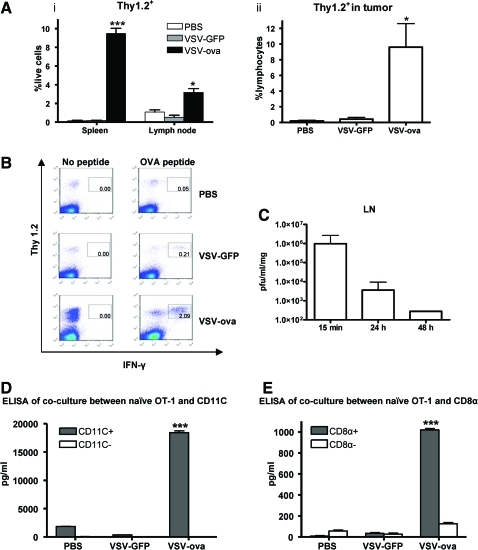
Intratumoral injection of VSV-GFP generates significant antitumor therapy in the B16ova model (Diaz et al., 2007; Galivo et al., 2010b; Wongthida et al., 2010a), suggesting that appreciable amounts of OVA antigen will be released from dying B16ova tumor cells in vivo. Despite this, VSV-GFP was largely ineffective at activating naïve ova-specific OT-I T cells adoptively transferred into mice before intratumoral injection of virus (Fig. 1A, B). In contrast, significantly increased levels of adoptively transferred OT-I T cells (specific for the tumor-associated OVA antigen) persisted in spleens, TDLN (Fig. 1Ai), and tumors (Fig. 1Aii) following intratumoral injection of VSV-ova. In addition, VSV-ova led to significantly higher levels of activation of adoptively transferred OT-I cells, as measured by IFN-γ secretion in the TDLN (Fig. 1B) as well as in other sites (data not shown), compared with VSV-GFP or PBS treatments. Despite being injected directly into the tumor, VSV was detected at high levels within 15 min of injection in the TDLN (Fig. 1C). Therefore, we believe that the increased ability of VSV-ova, compared with VSV-GFP, to activate naïve OT-I T cells in vivo was due primarily to presentation of virus-expressed OVA antigen by lymph node resident APC, themselves activated by the presence of immunogenic virus particles and proteins (Galivo et al., 2010b). Consistent with this hypothesis, only the CD11C+ component of TDLN, recovered from B16ova tumor–bearing mice injected with VSV-ova, induced the proliferation of IFN-γ (data not shown) and the secretion of IFN-γ (T-cell activation) (Fig. 1D) from naïve OT-I T cells in vitro. Further purification of the CD11C+ cells recovered from TDLN showed that CD11C+CD8α+, but not CD11C+CD8α−, dendritic cells were responsible for the in vivo uptake and presentation of the VSV-ova–derived SIINFEKL epitope to naïve OT-I T cells in vitro (Fig. 1E).
FIG. 1.
Vesicular stomatitis virus (VSV) encoding a tumor-associated antigen (TAA) induces antigen-specific T-cell activation. Naïve OT-I (Thy1.2+) T cells were adoptively transferred on day 7 after implantation of B16ova tumors into C57BL/6 (Thy1.1+) mice (three mice/group). Twenty-four hours later, phosphate-buffered saline (PBS), VSV-GFP, or VSV-ova were injected intratumorally. Three days later, (Ai) spleens and tumor-draining lymph nodes (TDLN) or (Aii) tumors were harvested and analyzed by flow cytometry for (A) Thy1.2+ cells or (B) interferon gamma (IFN-γ)–producing Thy1.2+ cells after pulsing with or without SIINFEKL peptide in TDLN. (C) 15 min, 24 hr, or 48 hr following intratumoral injection of B16ova tumors with VSV, TDLN were harvested and viral titers determined by plaque assay (three mice/group). (D, E) Seven days following implantation of B16ova tumors in C57BL/6 mice (three mice/group), tumors were injected intratumorally with PBS, VSV-GFP, or VSV-ova. Twenty-four hours later, inguinal TDLN were fractionated using magnetic beads into CD11C+ and CD11C− populations, which were subsequently co-cultured with naïve OT-I T cells for 60 hr. (D) T-cell activation was measured by IFN-γ secretion by ELISA. The CD11C+ population of D was further fractionated into CD11C+CD8α+ or CD11C+CD8α− populations and co-cultured with naïve OT-I. (E) T-cell activation was measured by IFN-γ secretion by ELISA. *p < 0.05, ***p < 0.001. Color images available online at www.liebertonline.com/hum
VSV-ova activates naïve OT-I T cells in vivo and induces systemic antitumor activity
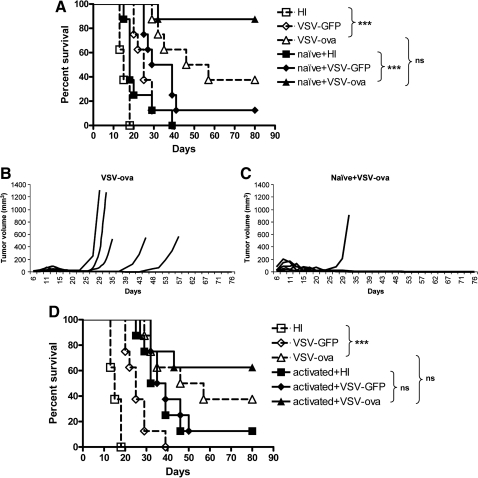
Consistent with an added therapeutic value of in vivo priming of endogenous antitumor T-cell responses in addition to direct oncolytic therapy of B16ova tumors, intratumoral injection of VSV expressing the OVA TAA was consistently more effective than the non–OVA-expressing counterpart VSV-GFP (p = 0.001 compared with VSV-GFP and p < 0.0001 compared with heat-inactivated [HI] virus) (Fig. 2A) (Diaz et al., 2007). However, therapy with VSV-ova was still not complete, probably because of the rapid growth of the subcutaneous B16ova tumors during development of the anti-ova T-cell response. Increasing the frequency of potentially reactive T cells at the time of T-cell priming by VSV-ova in vivo by adoptive transfer of naïve OT-I T cells prior to intratumoral virus injection markedly decreased the rate of tumor progression (Fig. 2B, C) and increased survival of B16ova tumor–bearing mice compared with injection of VSV-ova or adoptive transfer alone (Fig. 2A–C). Thus, the combination of oncolytic virotherapy with VSV-ova and adoptive transfer of naïve OT-I T cells induced tumor regressions and cures in seven of eight mice, compared with long-term cures in only two of eight mice treated with VSV-ova alone (Fig. 2B).
FIG. 2.
Combination oncolytic virotherapy and adoptive T-cell therapy for local disease. (A) 1 × 106 naïve or (D) 1 × 107 activated OT-I T cells were adoptively transferred into C57BL/6 mice bearing 7-day established B16ova tumors (eight mice/group). On the same day, mice were injected intratumorally with 5 × 108 plaque-forming units (PFU) of heat-inactivated (HI) virus, VSV-GFP or VSV-ova every other day for six injections. (A, D) Survival (tumor less than 1.0 cm in any diameter) or (B, C) growth of individual tumors is shown.
In the absence of intratumoral VSV treatment, the adoptive transfer of naïve OT-I was consistently less effective than transfer of activated OT-I against B16ova tumors (p = 0.008) (Fig. 2A, D). In contrast, in combination with intratumoral VSV-ova treatment, adoptive transfer of naïve OT-I was consistently and significantly more effective than treatment with virus or naïve T cells alone, but was not significantly (p = 0.25) superior to the use of virus in combination with activated OT-I.
These data are consistent with a model in which adoptive transfer, prior to intratumoral virus injection, sufficiently increased the frequency of antigen-specific T cells available for rapid in vivo activation to generate significantly improved therapy of B16ova tumors compared with oncolytic virotherapy or T-cell–mediated therapy alone.
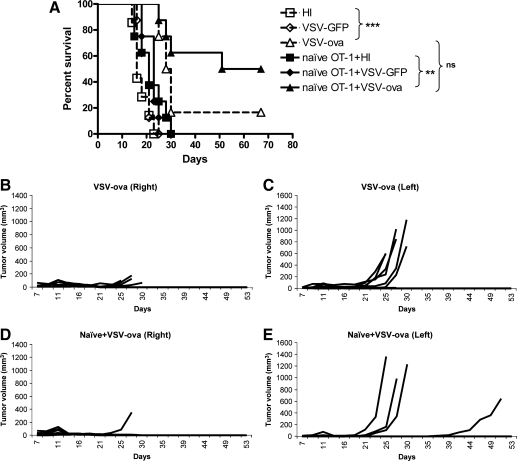
A major goal of combining the activation of tumor antigen–specific T-cell responses with the local efficacy of oncolytic virotherapy was to generate systemic immunity against tumors that cannot be directly accessed with virus injection. When mice bearing established B16ova tumors on both right and left flanks were treated with intratumoral injection of VSV on the right-sided tumor as before, VSV-ova was significantly more effective than VSV-GFP at controlling growth of the injected tumors (Fig. 3A). This also translated into a significant enhancement in control of the contralateral, left-sided (uninjected) tumor, which was reflected in improved survival (p < 0.00001 compared with HI virus and p = 0.0003 compared with VSV-GFP). However, adoptive transfer of naïve OT-I T cells prior to intratumoral injection of VSV-ova, generated significantly improved control of the uninjected tumor compared with combination of VSV-GFP with adoptive OT-I therapy (p = 0.0009) (Fig. 3B–E), resulting in long-term cures in 50% of the mice in Fig. 3A. Taken together, these data show that the combination of antigen-expressing oncolytic virotherapy with adoptive transfer of antigen-specific T cells generated significantly improved therapy against distant established tumors compared with either modality alone.
FIG. 3.
Combination oncolytic virotherapy and adoptive T-cell therapy for metastatic disease. Naïve OT-I T cells (1 × 106) were adoptively transferred into C57BL/6 mice bearing two B16ova tumors that had been established on both flanks 7 days previously (eight mice/group). On the same day, tumors on the right flank were injected intratumorally with 5 × 108 PFU of HI virus, VSV-GFP, or VSV-ova every other day for six injections. (A) Survival (tumor less than 1.0 cm in any diameter) or (B–E) growth of individual tumors is shown.
Antigen-expressing VSV can break tolerance to a self antigen
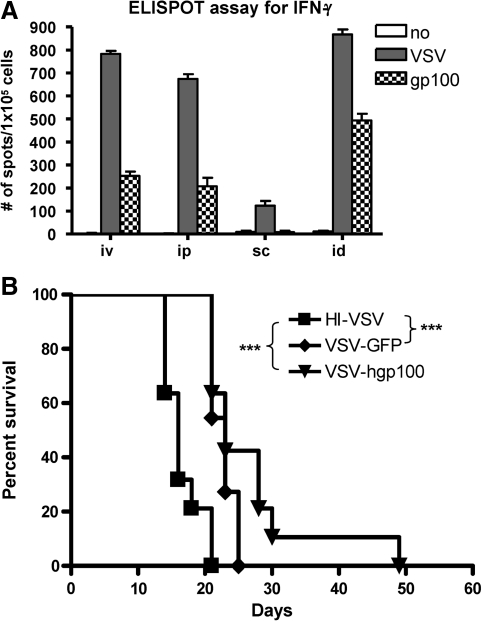
Our main goal was to compare how the results of the VSV-ova/OT-I model, used in mice with no intrinsic immunological tolerance to the (foreign) OVA TAA, would compare with the use of antigen-expressing VSV to target a truly self TAA, against which tolerance is well established in C57BL/6 mice. To do this, we constructed VSV-hgp100 in which the human homologue of the murine melanocyte/melanoma antigen gp100 (Kwon et al., 1991; Schreurs et al., 1997; Zhai et al., 1997) is expressed from the virus. The homologous murine and human epitopes of gp100 that are presented in the context of H-2Db (amino acids 25–33) differ in the three NH2-terminal amino acids, a difference that confers greater avidity on MHC class I binding of the human peptide—thereby increasing its ability to break tolerance to the murine self antigen in C57BL/6 mice (Overwijk et al., 1998). To verify the immunological function of the VSV-hgp100 virus, we confirmed that vaccination with VSV-hgp100 could effectively break tolerance to gp100 in C57BL/6 mice (Fig. 4A). However, despite the fact that B16 tumor–bearing mice treated by intratumoral injection of either VSV-GFP or VSV-hgp100 survived significantly longer than those treated with HI virus, there was no difference in survival or tumor growth between VSV-GFP– or VSV-hgp100–treated mice (Fig. 4B).
FIG. 4.
VSV expressing a poorly immunogenic self antigen can break tolerance to that antigen. (A) C57BL/6 mice bearing 7-day established B16 tumors (three mice/group) were injected intravenously (iv), intraperitoneally (ip), subcutaneously (sc), or intradermally (id) with 5 × 106 PFU of VSV-hgp100. Seven days later, the number of IFN-γ–producing splenocytes were measured by ELISPOT following stimulation by no peptide, VSV-specific peptide, or gp100 specific peptide. (B) C57BL/6 mice bearing 7-day established B16 tumors (eight mice/group) were injected intratumorally every 2 days with 5 × 108 PFU with HI virus, VSV, or VSV-hgp100 for a total of three injections. Survival with time is shown.
Combination viro-/adoptive T-cell therapy is effective against local injected tumors
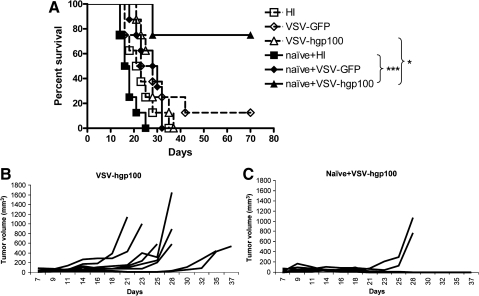
It seemed probable that the inability of VSV-hgp100 to improve therapy of B16ova tumors relative to VSV-GFP (Fig. 4B), unlike the improvement observed with VSV-ova (Fig. 2A), was due to a lower frequency of endogenous gp100-reactive T cells compared with ova-reactive, non–self-reactive T cells. Therefore, to increase this precursor frequency in vivo, gp100-specific pmel T cells (Overwijk et al., 2003) were adoptively transferred prior to intratumoral VSV treatment. Adoptive transfer of naïve pmel cells alone had no therapeutic effects in this model (Fig. 5A). However, the combination of pmel cells with VSV-hgp100 generated significant regressions of B16ova tumors, compared with virus alone (Fig. 5B, C), which also translated into long-term cures in 70%–80% of treated mice (Fig. 5A–C) (p = 0.016 VSV-hgp100 + naïve pmel compared with VSV-hgp100 alone). Therefore, a combination of adoptive transfer of naïve antigen-specific cells and antigen-expressing oncolytic virotherapy generated significant increases in antitumor therapy, including long-term cures, even when targeted against an endogenous self TAA.
FIG. 5.
Combination oncolytic virotherapy and adoptive T-cell therapy is effective against endogenous TAA, local disease. Naïve pmel T cells (1 × 106) were adoptively transferred into C57BL/6 mice bearing subcutaneous B16ova (8 mice/group). Four to 6 hr later, tumors were injected with 5 × 108 PFU of HI virus, VSV-GFP, or VSV-hgp100 every other day for total of six injections. (A) Survival with time or (B, C) growth of individual tumors are shown.
Combination therapy is effective against systemic tumors
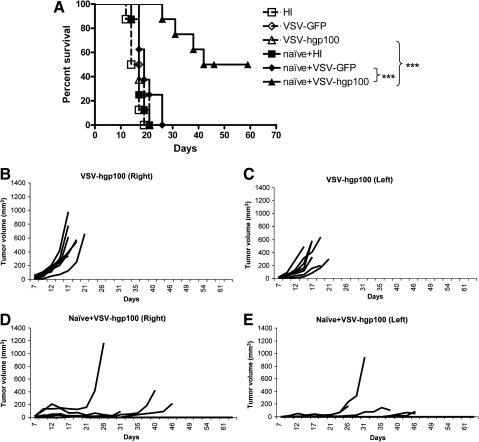
Next, we tested the combination of VSV-hgp100 and adoptive transfer of pmel T cells for activity against systemic, noninjected tumors in the metastatic model of Fig. 3. As before, expression of hgp100 by VSV did not improve therapy compared with VSV-GFP (Fig. 6A); in addition, the combination of systemically administered naïve pmel with intratumoral VSV-GFP was not significantly more effective than either naïve pmel or intratumoral VSV-GFP alone. In contrast, the combination of VSV-hgp100 with pmel generated highly significant improvements in both numbers of long-term cures, as well as delay of tumor progression of both the injected and noninjected tumors (Fig. 6A–E). Similar results were obtained using the combination of intratumoral VSV with activated pmel T cells (data not shown), although, as for the VSV-ova/OT-I model, naïve T cells tended to generate better therapy in combination with VSV-hgp100 than activated T cells. These data show that self TAA can be targeted effectively using a combination of both oncolytic virotherapy and adoptive T-cell therapy even in a stringent model of systemic disease.
FIG. 6.
Combination therapy targeted against endogenous TAA treats systemic disease. Naïve pmel T cells (1 × 106) were adoptively transferred into C57BL/6 mice bearing two B16ova tumors that had been established on both flanks 7 days previously (eight mice/group). On the same day, tumors on the right flank were injected intratumorally with 5 × 108 PFU of VSV-GFP or VSV-hgp100 every other day for six injections. (A) Survival (tumor less than 1.0 cm in any diameter) or (B–E) growth of individual tumors is shown.
Discussion
In the B16ova model used in this study, the therapeutic effects of VSV are predominantly due to the antitumor activity of antiviral innate immune effectors in response to VSV at the tumor site (Diaz et al., 2007; Galivo et al., 2010a, 2010b; Wongthida et al., 2010a, 2010b). In contrast, ongoing viral replication (as opposed to infection) and adaptive immune responses to either virus or tumor play, at most, only minor roles in antitumor therapy (Diaz et al., 2007; Galivo et al., 2010a, 2010b; Wongthida et al., 2010a, 2010b). We have shown previously that it is possible to mobilize antigen-specific T-cell responses against TAA in combination with oncolytic virotherapy by expressing the OVA TAA from VSV (Diaz et al., 2007). As we confirm here, VSV-ova was shown to be a more potent oncolytic agent than VSV-GFP (Diaz et al., 2007). Moreover, we showed that intratumoral VSV-GFP significantly enhanced the efficacy of adoptive transfer of OVA-specific OT-I T cells, but only in the context in which the T-cell–targeted TAA was expressed endogenously by the tumor cells and not by the intratumorally injected virus (Diaz et al., 2007). However, from these previous studies, it was not clear how the efficacy of VSV-ova in C57BL/6 mice would compare with that of a VSV expressing a truly self TAA, against which tolerance is in place in an immune-competent host, either alone or in combination with adoptive T-cell transfer. Therefore, the goal of this current study was to investigate how oncolytic virotherapy with VSV could be used to activate or mobilize antigen-specific T-cell responses against self TAA.
We show here that expression of the OVA TAA by VSV significantly enhanced T-cell activation and accumulation in vivo in the lymphoid organs and in the tumor (Fig. 1). These data suggest a model in which VSV-expressed OVA antigen is carried directly to the TDLN, probably by free virus escaping from the tumor. Upon reaching the TDLN, OVA is released following viral infection of lymph node cells and cross-presented to naïve T cells by activated CD11C+CD8α+ dendritic cells in the context of a highly inflammatory environment induced by the presence of virus. This model is consistent with our observation of induction of a highly pro-inflammatory cytokine environment in the TDLN within 6 hr of intratumoral injection of VSV in vivo (Galivo et al., 2010b).
The expression of the foreign, nonself OVA TAA by the oncolytic virus was itself sufficient to enhance therapeutic efficacy compared with VSV-GFP, consistent with the generation in vivo of anti-ova T-cell responses (Fig. 2) (Diaz et al., 2007). In addition, generation of cytotoxic T lymphocyte (CTL) responses against tumor cells may have been promoted by the induction of tumor cell apoptosis by VSV matrix leading to cross-priming of antigen-presenting cells with tumor antigens, as reported by Zhao and colleagues (Zhao et al., 2008). In contrast, treatment with VSV expressing even an immunogenicity-enhanced, altered-self version of gp100 was not able to improve antitumor efficacy compared with VSV-GFP alone (Fig. 4B). This was despite the fact that VSV-hgp100 could break tolerance to gp100 following vaccination (Fig. 4A). This lack of therapeutic improvement by VSV-gp100 presumably reflects the paucity of (low affinity) gp100-reactive T cells, which can be activated in vivo, compared with the plentiful numbers of high-affinity, anti-OVA T cells activated by VSV-ova.
These data also suggest, therefore, that the use of VSV expressing several different self TAA may still be able to have an impact on tumor growth in the absence of adoptive transfer of antigen-specific T cells. Thus, it may be that VSV expressing a range of TAA will be able to prime individually low levels of tumor-reactive T cells against any given TAA. However, cumulatively, sufficient supra-threshold numbers of T cells, with specificity for multiple antigenic targets, may be generated in vivo to achieve tumor rejection.
However, even the anti-ova T-cell response activated de novo by VSV-ova was unable to cure all the treated mice (Fig. 2A). This was probably, at least in part, because of the rapid growth of the B16ova tumors during the time taken for T-cell expansion following virus injection. Therefore, we reasoned that the T-cell–activating properties of VSV expressing even a self TAA, such as gp100, could be optimally exploited in combination with adoptive transfer of TAA-specific T cells. This would provide a pre-existing pool of T cells that could then be rapidly mobilized in vivo against the tumor by VSV-gp100-mediated immune activation. Consistent with this hypothesis, combination of adoptive transfer of T cells with specificity for either the foreign OVA or the endogenous gp100 tumor-associated antigens with intratumoral injection of VSV expressing the target TAA generated significantly increased numbers of long-term cures compared with the virus or T-cell therapies alone for treatment of both local injectable tumors (Figs. 2, 5) as well as in much more stringent models of metastatic, uninjected tumors (Fig. 3, 6).
Our data also show that antigen-expressing oncolytic virus is more effective in combination with adoptive transfer of naïve antigen-specific T cells than with pre-activated T cells (Fig. 2D and data not shown). We believe that adoptive transfer of a substantial reservoir of naïve antigen specific T cells is functionally equivalent to prior immunological priming. This population of T cells is then immediately available in lymphoid organs to be rapidly activated by the immunological boost provided by viral-mediated TAA expression in the TDLN. In contrast, adoptive transfer of pre-activated T cells (OT-I or pmel) allows for substantially less expansion of the T-cell response following boosting by VSV-TAA. In addition, VSV infection is associated with a generalized immune activation, characterized by increased levels of IFN-γ secretion by splenocytes (Galivo et al., 2010b). This viral-induced immune activation may differentially affect the expansion and/or survival of pre-activated T cells (such as through the induction of activation-induced cell death) relative to naïve T cells. Finally, the better therapeutic efficacy of naïve, as opposed to activated, T cells may also be due to the recently reported activity of intratumoral VSV to affect rapid vascular shutdown within the tumor (Breitbach et al., 2007). Such viral-induced vascular shutdown may occlude circulating T cells from the tumor at early periods following virus and T-cell treatments; hence, although adoptively transferred activated T cells may have reduced in numbers significantly by the time such effects have diminished in vivo, longer lasting/circulating naïve T cells may still be available at higher numbers. If this hypothesis is true, then the relative timing of virus and naïve/activated T cells is likely to be very important for the final therapeutic outcome.
Despite efficient activation of anti-ova T cells in vivo (Fig. 1) (Diaz et al., 2007), intratumoral VSV-ova could not induce large numbers of regressions of uninjected B16ova tumors on the contralateral flank (Fig. 3). This is probably, at least in part, due to the ongoing growth of the contralateral B16ova tumors during development of the antitumor T-cell response. However, by providing a pool of TAA-specific T cells using adoptive transfer, intratumoral injection of VSV-ova or VSV-hgp100 was able to activate a potent T-cell response that was effective systemically against uninjected, well-established B16ova tumors leading to significant numbers of tumor cures (Figs. 3, 6). Therefore, combination therapy of adoptive T-cell transfer with oncolytic virus expressing a self antigen enables the mobilization of antigen-specific T-cell responses against systemic tumors that cannot be accessed directly with virus injection. Of particular significance, we observed that both VSV-hgp100 and pmel T cells as single agents were significantly less effective than either VSV-ova or OT-I, respectively (Figs. 2, 5). However, when used in combination, VSV-hgp100 and pmel T-cell therapy routinely generated similar levels of long-term cures in the aggressive two-sided tumor model as did the combination of VSV-ova and OT-I (Figs. 3, 6).
Overall, our results suggest several novel methods by which the efficacy of oncolytic virotherapy can be improved clinically by recruiting effector mechanisms associated with antigen-specific T-cell responses. Thus, oncolytic viruses engineered to express (multiple) TAA known to be expressed by a specific tumor type can be generated for intratumoral injection. In addition to direct activity against the local tumor, this will also provide direct in vivo vaccinating effects, provided that supra-threshold levels of TAA-specific T-cell responses can be achieved. In addition, these VSV-TAA could be used in patients who have previously undergone adoptive transfer of their own T cells with specificity for their tumor cells, either with or without preconditioning chemotherapy (Mansoor et al., 2005; Morgan et al., 2006; Powell et al., 2006; Rosenberg et al., 2008; Hawkins et al., 2010). This would allow for the activation and expansion of antitumor T cells in the same way that we have observed in our preclinical models here. Finally, alternative conventional prior priming vaccinations against known TAA may be sufficient to expand the numbers of potentially tumor-reactive T cells in vivo to levels similar to those provided by the adoptive transfer step. Subsequent intratumoral oncolytic virus expressing the same range of TAA as used in the priming stage would then expand the T-cell response against tumors. In addition, our studies here use multiple injections of virus, which necessitates repeated clinical access to a tumor site. To make the approach more clinically practical, we are currently investigating the efficacy of combining adoptive T-cell therapy with truly systemic injections of VSV expressing a range of TAA, a strategy in which the oncolytic virus serves also or even predominantly as a prime/boost vehicle to support the expansion and activation of the adoptively transferred T cells. Validation of this approach, using a prime/boost strategy targeted to a defined melanoma-specific antigen and VSV virotherapy, was recently reported by Bridle and colleagues with impressive therapeutic results against brain metastases of B16 (Bridle et al., 2010).
In summary, these current data extend our previous findings with the OVA model (Diaz et al., 2007) to show that VSV expressing a TAA (ova or gp100) can significantly enhance the therapeutic efficacy of either virotherapy or adoptive T-cell therapy alone. In addition, our data here demonstrate the therapeutic use of combination oncolytic virotherapy with adoptive T-cell therapy to target a self TAA against which tolerance is well established in C57BL/6 mice, and that this combination therapy is effective in the context of an aggressive model of metastatic disease. Finally, we show that poorly effective virotherapies and T-cell therapies, which target self TAA of low immunogenicity, as would be the situation in patients, can be combined to generate very effective antitumor therapy. These data indicate new approaches by which the antitumor effector mechanisms associated with tumor antigen–specific T-cell immunity can be recruited in vivo to combine with, and further enhance, the potency of oncolytic virotherapy alone.
Acknowledgments
We thank Toni Higgins for expert secretarial assistance. This work was supported by the Richard M. Schulze Family Foundation, the Mayo Foundation, and by National Institutes of Health grants CA107082, CA130878, and CA132734.
Author Disclosure Statement
The authors declare no conflict of interest.
References
- Breitbach C.J. Paterson J.M. Lemay C.G., et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol. Ther. 2007;15:1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- Bridle B.W. Stephenson K.B. Boudreau J.E., et al. Potentiating cancer immunotherapy using an oncolytic virus. Mol. Ther. 2010;18:1430–1439. doi: 10.1038/mt.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco P. Passer B.J. Buhrman J.S., et al. Oncolytic herpes simplex virus armed with xenogeneic homologue of prostatic acid phosphatase enhances antitumor efficacy in prostate cancer. Gene Ther. 2010;17:805–810. doi: 10.1038/gt.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocca E.A. Abbed K.M. Tatter S., et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol. Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Diaz R.M. Galivo F. Kottke T., et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- Dudley M.E. Rosenberg S.A. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat. Rev. Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M. Porosnicu M. Markovic D. Barber G.N. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J. Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulci G. Breymann L. Gianni D., et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galivo F. Diaz R.M. Thanarajasingam U., et al. Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus. Hum. Gene Ther. 2010a;21:439–450. doi: 10.1089/hum.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galivo F. Diaz R.M. Wongthida P., et al. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2010b;17:158–170. doi: 10.1038/gt.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L. Finkelstein S.E. Klebanoff C.A., et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F. Menard C. Puig P.E., et al. Metronomic cyclophosphamide regimen selectively depletes CD4+ CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R.E. Gilham D.E. Debets R., et al. Development of adoptive cell therapy for cancer: a clinical perspective. Hum. Gene Ther. 2010;21:665–672. doi: 10.1089/hum.2010.086. [DOI] [PubMed] [Google Scholar]
- Hogquist K.A. Jameson S.C. Heath W.R., et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Kottke T. Galivo F. Wongthida P., et al. Treg depletion-enhanced IL-2 treatment facilitates therapy of established tumors using systemically delivered oncolytic virus. Mol. Ther. 2008;16:1217–1226. doi: 10.1038/mt.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B.S. Chintamaneni C. Kozak C.A., et al. A melanocyte-specific gene, Pmel 17, maps near the silver coat color locus on mouse chromosome 10 and is in a syntenic region on human chromosome 12. Proc. Natl. Acad. Sci. U. S. A. 1991;88:9228–9232. doi: 10.1073/pnas.88.20.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson N.D. Stillman E.A. Whitt M.A. Rose J.K. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. U. S. A. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boeuf F. Diallo J.S. McCart J.A., et al. Synergistic interaction between oncolytic viruses augments tumor killing. Mol. Ther. 2010;18:888–895. doi: 10.1038/mt.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisegang M. Turqueti-Neves A. Engels B., et al. T-cell receptor gene-modified T cells with shared renal cell carcinoma specificity for adoptive T-cell therapy. Clin. Cancer Res. 2010;16:2333–2343. doi: 10.1158/1078-0432.CCR-09-2897. [DOI] [PubMed] [Google Scholar]
- Linardakis E. Bateman A. Phan V., et al. Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell-tumor cell fusion. Cancer Res. 2002;62:5495–5504. [PubMed] [Google Scholar]
- Maine G.N. Mule J.J. Making room for T cells. J Clin Invest. 2002;110:157–159. doi: 10.1172/JCI16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor W. Gilham D.E. Thistlethwaite F.C. Hawkins R.E. Engineering T cells for cancer therapy. Br. J. Cancer. 2005;93:1085–1091. doi: 10.1038/sj.bjc.6602839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.A. Dudley M.E. Wunderlich J.R., et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk W.W. Theoret M.R. Finkelstein S.E., et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk W.W. Tsung A. Irvine K.R., et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmiani G. Castelli C. Dalerba P., et al. Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J. Natl. Cancer Inst. 2002;94:805–818. doi: 10.1093/jnci/94.11.805. [DOI] [PubMed] [Google Scholar]
- Pecora A.L. Rizvi N. Cohen G.I., et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J. Clin. Oncol. 2002;20:2251–2266. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- Powell D.J., Jr. Dudley M.E. Hogan K.A., et al. Adoptive transfer of vaccine-induced peripheral blood mononuclear cells to patients with metastatic melanoma following lymphodepletion. J. Immunol. 2006;177:6527–6539. doi: 10.4049/jimmunol.177.9.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich R.J. Errington F. Ilett E.J., et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin. Cancer Res. 2008;14:7358–7366. doi: 10.1158/1078-0432.CCR-08-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich R.J. Errington F. Steele L.P., et al. Reciprocal human dendritic cell-natural killer cell interactions induce antitumor activity following tumor cell infection by oncolytic reovirus. J. Immunol. 2009;183:4312–4321. doi: 10.4049/jimmunol.0901074. [DOI] [PubMed] [Google Scholar]
- Qiao J. Wang H. Kottke T., et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008;15:604–616. doi: 10.1038/sj.gt.3303098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsburg E. Publicover J. Buonocore L., et al. A vesicular stomatitis virus recombinant expressing granulocyte-macrophage colony-stimulating factor induces enhanced T-cell responses and is highly attenuated for replication in animals. J. Virol. 2005;79:15043–15053. doi: 10.1128/JVI.79.24.15043-15053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A. Packard B.S. Aebersold P.M., et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A. Restifo N.P. Yang J.C., et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloura V. Wang L.C. Fridlender Z.G., et al. Evaluation of an attenuated vesicular stomatitis virus vector expressing interferon-beta for use in malignant pleural mesothelioma: heterogeneity in interferon responsiveness defines potential efficacy. Hum. Gene Ther. 2010;21:51–64. doi: 10.1089/hum.2009.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Perez L. Gough M. Qiao J., et al. Synergy of adoptive T-cell therapy and intratumoral suicide gene therapy is mediated by host NK cells. Gene Ther. 2007;14:998–1009. doi: 10.1038/sj.gt.3302935. [DOI] [PubMed] [Google Scholar]
- Schreurs M.W. De Boer A.J. Schmidt A., et al. Cloning, expression and tissue distribution of the murine homologue of the melanocyte lineage-specific antigen gp100. Melanoma Res. 1997;7:463–470. doi: 10.1097/00008390-199712000-00004. [DOI] [PubMed] [Google Scholar]
- Smith F.O. Downey S.G. Klapper J.A., et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin. Cancer Res. 2008;14:5610–5618. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil A. Martinez O. Chua M.A. Garcia-Sastre A. Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol. Ther. 2008;16:1883–1890. doi: 10.1038/mt.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. Bronte V. Chen P.W., et al. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J. Immunol. 1995;154:4685–4692. [PMC free article] [PubMed] [Google Scholar]
- Wang R.F. Rosenberg S.A. Human tumor antigens recognized by T lymphocytes: implications for cancer therapy. J. Leukoc. Biol. 1996;60:296–309. doi: 10.1002/jlb.60.3.296. [DOI] [PubMed] [Google Scholar]
- Wongthida P. Diaz R.M. Galivo F., et al. VSV oncolytic virotherapy in the B16 model depends upon intact MyD88 signaling. Mol. Ther. Submitted. 2010a doi: 10.1038/mt.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongthida P. Diaz R.M. Galivo F., et al. Type III IFN interleukin-28 mediates the antitumor efficacy of oncolytic virus VSV in immune-competent mouse models of cancer. Cancer Res. 2010b;70:4539–4549. doi: 10.1158/0008-5472.CAN-09-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C. Thompson J.A. Byrd D., et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y. Yang J.C. Spiess P., et al. Cloning and characterization of the genes encoding the murine homologues of the human melanoma antigens MART1 and gp100. J. Immunother. 1997;20:15–25. doi: 10.1097/00002371-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.Q. Tsai Y.C. Monie A., et al. Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy. Mol. Ther. 2010;18:692–699. doi: 10.1038/mt.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.M. Wen Y.J. Li Q., et al. A promising cancer gene therapy agent based on the matrix protein of vesicular stomatitis virus. FASEB J. 2008;22:4272–4280. doi: 10.1096/fj.08-110049. [DOI] [PubMed] [Google Scholar]