Abstract
Vectors based on the primate-derived adeno-associated virus serotype 8 (AAV8) are being evaluated in preclinical and clinical models. Natural infections with related AAVs activate memory B cells that produce antibodies capable of modulating the efficacy and safety of the vector. We have evaluated the biology of AAV8 gene transfer in macaque liver, with a focus on assessing the impact of pre-existing humoral immunity. Twenty-one macaques with various levels of AAV neutralizing antibody (NAb) were injected intravenously with AAV8 vector expressing green fluorescent protein. Pre-existing antibody titers in excess of 1:10 substantially diminished hepatocyte transduction that, in the absence of NAbs, was highly efficient. Vector-specific NAb diminished liver deposition of genomes and unexpectedly increased genome distribution to the spleen. The majority of animals showed high-level and stable sequestration of vector capsid protein by follicular dendritic cells of splenic germinal centers. These studies illustrate how natural immunity to a virus that is related to a vector can impact the efficacy and potential safety of in vivo gene therapy. We propose to use the in vitro transduction inhibition assay to evaluate research subjects before gene therapy and to preclude from systemic AAV8 trials those that have titers in excess of 1:10.
Wang and colleagues evaluate the impact of preexisting humoral immunity on adeno-associated virus 8 (AAV8)-mediated gene transfer to macaque livers. They injected AAV8 vectors expressing green fluorescent protein into 21 macaques with various levels of AAV-neutralizing antibody (Nab), and found that NAb titers above 1:10 significantly impaired transduction and affected distribution of vector genomes.
Introduction
In vivo delivery of viral vectors has shown promise in a variety of preclinical and clinical models of inherited disorders (Bainbridge et al., 2008; Hauswirth et al., 2008; Maguire et al., 2008). The major challenges to date involve host immune responses and poor transduction efficiency due to limited vector tropism and poor delivery of the vector to the target cell (Manno et al., 2006; Mingozzi et al., 2009; Mendell et al., 2010). Improved transduction efficiencies have been achieved with second-generation vector technologies such as pseudotyped lentiviruses and novel adeno-associated virus (AAV) serotypes (Kobinger et al., 2001; Gao et al., 2002; Zhong et al., 2008). Much is being learned about host–vector interactions, such as the role of innate immunity and the activation of T cells and B cells in response to the vector and its transgene product (Manno et al., 2006; Cao et al., 2007; Mingozzi et al., 2007; Zhu et al., 2009).
One of the most important aspects of host immunity is the impact of neutralizing antibodies (NAbs) on transduction efficiency. This principle was first evaluated in the setting of adenovirus-mediated gene transfer. Preimmunization of mice and rabbits with an adenovirus serotype 5 vector substantially diminished the efficacy of a second vector administration when administered intravenously (Kozarsky et al., 1994; Yang et al., 1996). The promise of AAV-based vectors for in vivo gene transfer was demonstrated in multiple preclinical models including the targeting of liver to express a number of therapeutic genes (Nathwani et al., 2006, 2007; Koeberl et al., 2008; Cunningham et al., 2009). As expected, NAb to the AAV vector capsid resulted in a substantial reduction in transduction when administered systemically (Jiang et al., 2006; Manno et al., 2006; Scallan et al., 2006; Hurlbut et al., 2010; Wang et al., 2010a).
Initial characterizations of NAb-mediated inhibition of AAV transduction in vivo were performed in animals preimmunized with the vector capsid, simulating what would happen if a patient needed a second administration of vector (Xiao et al., 2000). Of even more concern is the impact of pre-existing NAbs due to natural AAV infections, among which AAV serotype 2 is quite prevalent in humans. Discovery of an expanded family of AAVs in human and nonhuman primates has produced a second generation of vectors with improved performance profiles as compared with AAV2 (Gao et al., 2002, 2004). One feature of AAV vectors isolated from nonhuman primates is the relatively low level of NAbs to their capsids in human populations (Calcedo et al., 2009; Boutin et al., 2010). Additional advantages include substantially increased transduction efficiencies and diminished T cell responses to the capsid and transgene product (Wang et al., 2007, 2010a,b). This paper focuses on AAV8 capsid-based vectors, which are being considered for widespread clinical development of diseases that can be treated by targeting hepatocytes, photoreceptors, and skeletal muscle.
Evaluating the impact of host immune memory to natural AAV infections on AAV-mediated gene transfer is best performed in a preclinical model in which the wild-type version of the vector is permissive. Our previous work with AAV infections in primates suggests that macaques are indeed the best model for studying the vector immunobiology of gene therapy. AAVs are widely prevalent in both humans and macaques as latent viral genomes and their capsids are highly conserved, differing by less than 15% (Gao et al., 2004). In fact, the clade E family of AAVs, of which AAV8 is a member, includes isolates from both humans and macaques, some of which differ by only one amino acid (AAVhu·37 and rh.39) in the entire VP1 protein (Gao et al., 2004). A wide spectrum of NAb profiles across AAV serotypes is present in sera from humans and macaques (Calcedo et al., 2009). In general, the prevalence and titers are higher in the species from which the virus was isolated, such as AAV2 in humans and AAV8 in macaques, although there is substantial cross-species neutralizing activity.
The successful clinical development of AAV vectors requires a quantitative assessment of the role of pre-existing NAbs in gene therapy efficacy and safety as well as an assay that can be used to screen humans for the presence of problematic levels of NAbs. These objectives were achieved in the current study through the development of a robust NAb assay and large-scale macaque studies of in vivo gene transfer with AAV8 vectors to target liver.
Materials and Methods
Vector
AAV2/8.TBG.EGFP vector used in the macaque study was produced by a scaled production method based on polyethylenimine (PEI) transfection and purified from supernatant as described (Lock et al., 2010). Genome titers (i.e., genome copies [GC]/ml) of AAV vectors were determined by real-time PCR using a primer–probe set corresponding to the poly(A) region of the vector and linearized plasmid standards. Vectors were subjected to extensive quality control tests including three repeated vector genome titrations, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis for vector purity, Limulus amebocyte lysate (LAL) for endotoxin detection (Cambrex Bio Science, East Rutherford, NJ), and transgene expression analysis in mice.
Macaque experiments
Three groups of macaques were enrolled into this study: eight adult rhesus macaques (Indian origin and captive bred, 6–10 years old, 9.9–14.0 kg; recycled from a previous non-AAV-related study), five juvenile rhesus macaques (Chinese origin and captive bred, 2–3 years old, 2.9–3.6 kg; purchased from Covance Research Products, Alice, TX), and eight adult cynomolgus macaques (Mauritian origin and captive bred, 4–10 years old, 5.2–12.8 kg; recycled from a previous non-AAV-related pharmacokinetic study). Once purchased or obtained, all animals were treated and cared for at the Nonhuman Primate Research Program (NPRP) facility of the Gene Therapy Program of the University of Pennsylvania (Philadelphia, PA) during the study. The study was performed according to a protocol approved by the Environmental Health and Radiation Safety Office, the Institutional Biosafety Committee, and the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. Vectors (3 × 1012 GC/kg) were administered to the study animals via the saphenous vein in a total volume of 10 ml infused at 1 ml/min, using a Harvard infusion pump. Blood samples were taken prestudy and at the time of necropsy (day 7) via venipuncture of the femoral vein. At the time of necropsy, 16 tissues, including the target organ liver and 15 distant tissues (brain, bone marrow, diaphragm, heart, kidney, lung, mesenteric lymph nodes, pancreas, seminal vesicles, skeletal muscle, spinal cord, spleen, stomach, testicles, and urinary bladder), were collected for histopathology and vector biodistribution analysis.
Passive transfer experiments
The passive transfer (PT) assay was performed as previously described (Wang et al., 2010a). C57BL/6 male mice (6–8 weeks old) were purchased from Charles River Laboratories (Wilmington, MA) and kept at the Animal Facility of the Translational Research Laboratories at the University of Pennsylvania. All animal procedures were performed in accordance with protocols approved by the IACUC at the University of Pennsylvania. For a typical PT assay, a group of three mice each received an intravenous injection of 200 μl of test serum or plasma sample (heat inactivated at 56°C for 30 min), or naive mouse serum as control. Two hours after the passive transfer, mice received an intravenous injection of 1 × 109 GC of AAV2/8.LSP.cFIX-W. Canine factor IX (cFIX) expression levels in the plasma 7 days after vector administration were determined by enzyme-linked immunosorbent assay (ELISA) and are shown as the percentage of the cFIX level in control mice passively transferred with naive mouse serum.
AAV neutralizing antibody assay
Macaque serum samples or human plasma samples were heat inactivated at 56°C for 30 min. Precipitates resulting from heat inactivation in the plasma samples were removed by centrifugation at 15,000 g for 10 min. NAb assays were performed on Huh7 cells as previously described (Calcedo et al., 2009), and the limit of detection occurred at a serum dilution of 1:5.
Anti-AAV8 IgG titers
AAV8-specific total IgG titers in the macaque serum samples before vector administration were determined by an ELISA as described (Hurlbut et al., 2010).
Quantification of green fluorescent protein expression in liver
Green fluorescent protein (GFP) transduction in macaque liver was quantified on the basis of three different aspects: the percentage of area expressing GFP (Wang et al., 2010a), the intensity of GFP, and the GFP protein concentration in total liver lysate measured by ELISA and Western analysis. GFP intensity was measured as the total intensity of every image (i.e., the sum of all pixel values per image) determined with ImageJ software (Rasband, 1997–2011; National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/). The intensity values obtained from the liver of untreated control animals were then subtracted from the values obtained from the treated animals. To correct for potential inconsistencies of the ultraviolet lamp the resulting intensity values are reported as a fraction of a fluorescence standard as described (Model and Burkhardt, 2001). We used a 10% (w/v) solution of sodium fluorescein (Sigma-Aldrich, St. Louis, MO) in 0.1 M NaHCO3 as standard and took a series of images of this reference each time before photographing livers. The original GFP intensity values (with background values subtracted) were then divided by the reference values to obtain the final GFP intensity value. For each liver, 10 images were analyzed and mean values are presented.
GFP ELISA and Western blot were done according to standard procedures. Total liver lysate was generated with radioimmunoprecipitation assay (RIPA) buffer (Boston BioProducts, Ashland, MA) containing protease inhibitors (Roche, Indianapolis, IN), and total protein concentration was measured with a micro BCA protein assay kit (Thermo Scientific, Rockford, IL). For GFP ELISA, GFP protein was captured with a goat anti-GFP antibody (1:2000 dilution; Fitzgerald, Acton, MA) and detected with rabbit anti-GFP (1:2000 dilution; Fitzgerald) and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Thermo Scientific). Series dilutions of purified enhanced GFP (EGFP) protein with 12-histidine tags (His-Tags; BioVision, Mountain View, CA) were used as standards (starting at 0.25 ng/ml), and 1 ng–1 μg of liver lysates was analyzed. For Western analysis, 10 μg of liver lysate or purified EGFP protein (1, 5, and 25 ng) was loaded into each lane. Proteins were transferred to polyvinylidene difluoride (PVDF) membrane, blocked, and probed with rabbit anti-GFP antibody (1:2000 dilution; Fitzgerald) and rabbit anti-tubulin antibody (Abcam, Cambridge, MA). Bound primary antibody was detected with HRP-conjugated goat anti-rabbit IgG antibody (1:5000 dilution; Thermo Scientific) and SuperSignal West Pico chemiluminescence substrate (Thermo Scientific).
Immunofluorescence to detect AAV capsid protein in nonhuman primate spleens
Immunofluorescence was performed on frozen spleen sections. Cryosections were air dried, fixed in acetone (–20°C) for 7 min, and blocked with 1% donkey serum in phosphate-buffered saline (PBS) for 20 min. The sections were then incubated with the following primary antibodies diluted in blocking buffer: a rabbit serum raised against AAV8 (1:1000 dilution; made in our laboratory), monoclonal antibody ADK8 directed against a conformational epitope of AAV8 (1:500; provided by J. Kleinschmidt, German Cancer Research Center, Heidelberg, Germany), and monoclonal antibody CNA.42, a marker for follicular dendritic cells (FDCs) (1:50; Sigma-Aldrich). After a 1-hr incubation the sections were washed in PBS and stained for 40 min with the corresponding secondary antibodies labeled with DyLight 649 or tetramethylrhodamine isothiocyanate (TRITC) (Jackson ImmunoResearch Laboratories, West Grove, PA). Fluorescein was not used to avoid confusion with GFP fluorescence. For double immunofluorescence the two primary and corresponding secondary antibodies each were mixed together for simultaneous staining. After final washing the sections were mounted with VECTASHIELD plus 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA) to stain nuclei.
Immunoprecipitation and Western blot to detect AAV capsid protein in nonhuman primate spleens
Immunoprecipitation (IP) was performed with Dynabeads Protein G (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Anti-AAV8 rabbit serum (0.5 μl) was used to pull down AAV capsid proteins from 0.5 to 1.5 mg of spleen lysate. Eluates were separated on SDS–polyacrylamide gels by electrophoresis and probed with mouse anti-AAV VP1, VP2, VP3 monoclonal antibody clone B1 (1:200 dilution; American Research Products, Belmont, MA). Bound primary antibody was detected with HRP-conjugated goat anti-mouse IgG antibody (1:10,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) and SuperSignal West Pico chemiluminescence substrate (Thermo Scientific).
Vector biodistribution analysis
Tissue DNA was extracted with a QIAamp DNA mini kit (Qiagen, Valencia, CA). Detection and quantification of vector genomes in extracted DNA were performed by real-time PCR as described previously (Bell et al., 2006).
Statistical analysis
Statistical differences between sample groups with different in vitro NAbs were determined by analysis of variance (ANOVA) Dunnett test or Student t test. Values of p < 0.05 were considered statistically significant.
Results
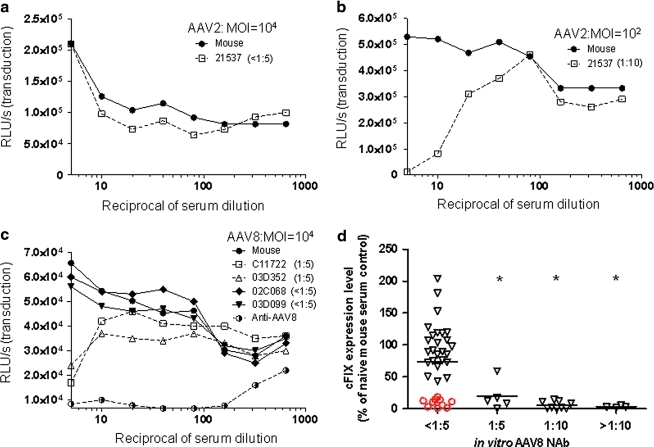
The standard approach for measuring NAbs to viral vectors such as those based on AAV is inhibition of transduction in vitro on permissive cells. A vector expressing a reporter gene is mixed with serial dilutions of the test article (e.g., serum from a human or macaque) and the immune complex is incubated with a cell line that is subsequently evaluated for reporter gene expression. The highest dilution capable of inhibiting transduction is determined to be the titer of the NAb. We and others developed this assay for measuring NAb to AAV2, using 293 cells, with a 50% reduction of LacZ expression serving as the threshold for neutralization. The direct application of this assay toward the measurement of NAbs to the novel serotypes has been difficult because of the limited transduction they show to virtually all cells in vitro despite the superior transduction they show in vivo (Gao et al., 2002, 2004). Supplementary Figure S1 (supplementary data are available online at www.liebertonline.com/hum) shows GFP transduction in 293 cells after incubation with vectors based on various serotypes. The highest transduction we have achieved with AAV8 is on Huh7 cells preincubated with adenovirus. The assays must be performed with multiplicities of infection (MOIs) of 104 or greater in order to achieve sufficient transduction to discern a 50% reduction in the presence of NAb. The high MOI used in this assay limits its sensitivity. To illustrate this point, NAb titrations were performed with macaque serum, using different MOIs of AAV2 [Fig. 1a (MOI, 104) and Fig. 1b (MOI, 102)]. The measured NAb from this macaque serum sample ranged from undetectable (<1:5) to 1:10 when the MOI of the in vitro transduction was decreased from 104 to 102.
FIG. 1.
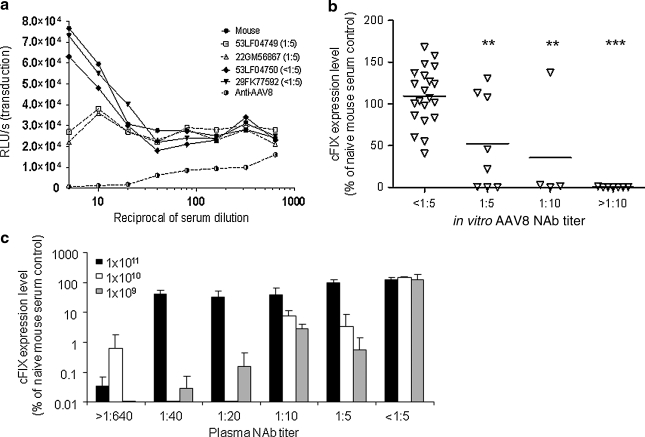
Different sensitivity of in vitro neutralizing antibody (NAb) assay for AAV2 and AAV8 dictated by the in vitro transduction efficiency and a highly sensitive in vivo passive transfer (PT) assay to detect low levels of AAV8 NAb in macaque samples. Serial 2-fold dilutions of macaque and naive mouse serum were incubated with 1 × 109 genome copies (GC) (a) or 1 × 107 GC (b) of AAV2.CMV.LacZ, or with 1 × 109 GC of AAV8.CMV.LacZ (c), and transferred to preseeded 96-well plates containing 1 × 105 Huh7 cells per well. Transduction efficiency at each serum dilution was measured by determining β-galactosidase levels [relative light units (RLU) per second] 24 hr after infection. The AAV neutralization titer for each sample (indicated in parentheses) was determined as the highest serum dilution that inhibited AAV.CMV.LacZ transduction by ≥50%, compared with naive mouse serum control. To obtain a transduction signal within the dynamic range of the assay, AAV2 (MOI, 104) vector–serum mix was diluted 1:50 before transferral to the preseeded 96-well plate. (d) An in vivo PT assay to detect low levels of AAV8 NAb in macaque serum samples. Seven days after PT and vector injection, canine factor IX (cFIX) levels in plasma were measured and shown as the percentage of the cFIX level in control mice passively transferred with naive mouse serum. Open red circles (n = 10) indicate macaque samples with undetectable AAV8 NAb (<1:5) that showed inhibition by the in vivo PT assay. Mean levels for each group are shown by the horizontal lines. *p < 0.05, using an ANOVA Dunnett test when compared with the group with pre-NAb < 1:5. n = 35 (<1:5), n = 5 (1:5), n = 9 (1:10), and n = 4 (>1:10).
Several features of the assay make it difficult to measure low NAb titers. Any concentration of serum greater than 1:5 is toxic to the cells. In addition, dilutions of control serum (i.e., no NAb) equal to or greater than 1:5 result in a dose-dependent enhancement of transduction, creating nonlinear background from which a 50% reduction is measured. Each assay must include a parallel titration with control serum to allow for a calculation of 50% reduction relative to the same dilution of control serum. Examples of NAb assays of sera from four macaques with low titers as well as sera from an immunized rabbit with a high titer are shown in Fig. 1c.
The higher level of transduction achieved with AAV8 in vivo after intravenous administration led us to consider a passive transfer (PT) assay for NAb. In this assay, C57BL/6 mice are infused with 200 μl of the test article 2 hr before vector infusion. Animals were evaluated for transgene expression in the presence of NAb relative to levels achieved in the absence of NAb; the assay uses canine factor IX (cFIX) as the reporter gene and plasma levels of cFIX as the readout. As with the in vitro transduction inhibition assay, the sensitivity of the PT assay is a function of vector dose, with higher sensitivity achieved with lower doses of vector; initial assays were performed with 109 GC/mouse, which is the lowest dose that produces enough cFIX to measure a reduction. Figure 1d shows a comparison of the PT results in terms of cFIX relative to the titer determined by in vitro transduction inhibition. Approximately 29% of macaque samples with in vitro NAb titers less than 1:5 showed significant inhibition in vivo, indicating that the PT assay is more sensitive than the in vitro assay for macaque sera.
Pre-existing neutralizing antibodies at a titer greater than 1:10 substantially inhibit AAV8 gene transfer to macaque liver
One goal of this study was to recruit a diverse cohort of macaques with different levels of pre-existing AAV8 NAb into a gene therapy study in which we measure in vivo gene transfer/transduction, extrahepatic dissemination of vector, and anamnestic NAb responses. These data were correlated with baseline assessments of NAb based on PT and in vitro assays. A total of 21 macaques were enrolled in the study, including 8 adult rhesus, 5 juvenile rhesus, and 8 adult cynomolgus. The animals fell into four serological groups with respect to AAV8 NAb: <1:5 (n = 11), 1:5 (n = 2), 1:10 (n = 3), and >1:10 (n = 5 with 1:20 × 2, 1:40 × 1, and 1:80 × 2). Each animal received, per kilogram, 3 × 1012 GC of AAV8 vector expressing GFP from the liver-specific promoter TBG that was administered intravenously; 7 days later animals were necropsied and samples were collected for analysis. A summary of all data collected from these animals is presented in Table 1.
Table 1.
Summary of Gene Transfer in Macaques After Systemic Vector Administration
| |
|
AAV8 NAb (1:dilution) |
|
GFP transduction in liver |
GFP DNA copies/diploid genomef |
|
PT in mouseg(% of control) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Animal no. | Prea | Day 7 | Capsid in spleenb | Efficiency (%)c (mean ± SD) | Intensityd(mean ± SD) | GFP proteine(ng/μg protein) | Liver | Spleen | Spleen/liver GFP DNA ratio | 1 × 109 GC (200 μl) | 1 × 1010GC (100 μl) |
| Cynomolgus (adult) | 02-07 | <1:5 | 1:80 | + | 4.4 ± 4.6 | 0.19 ± 0.12 | 0.47 | 31.23 | 13.33 | 0.43 | 35.0 | 101.0 |
| 086-356 | <1:5 | 1:80 | ND | 26.2 ± 23.5 | 0.42 ± 0.20 | 0.99 | 33.56 | 6.80 | 0.20 | 51.8 | 97.9 | |
| 086-367 | <1:5 | 1:80 | − | 4.9 ± 6.0 | 0.25 ± 0.12 | 0.60 | 35.41 | 2.90 | 0.08 | 41.6 | 82.1 | |
| C13991 | <1:5 | 1:160 | ND | 10.6 ± 7.8 | 0.69 ± 0.32 | 0.44 | 26.15 | 7.46 | 0.29 | 107.5 | 91.5 | |
| C13992 | <1:5 | 1:80 | − | 40.0 ± 14.9 | 1.03 ± 0.35 | 1.49 | 65.47 | 2.44 | 0.04 | 118.3 | 85.6 | |
| C11722 | 1:5 | 1:5120 | − | 5.5 ± 7.1 | 0.17 ± 0.12 | 0.46 | 15.76 | 7.56 | 0.48 | 0.6 | 8.3 | |
| 054-1040 | 1:10 | 1:2560 | ++ | 1.5 ± 0.9 | 0.06 ± 0.08 | 0.28 | 11.47 | 10.09 | 0.88 | 0.1 | 9.9 | |
| 04-01 | 1:10 | 1:2560 | ++ | 11.1 ± 4.8 | 0.24 ± 0.10 | 0.60 | 7.45 | 11.57 | 1.55 | 0.0 | 3.3 | |
| Rhesus (adult) | 02C068 | <1:5 | 1:80 | ND | 6.3 ± 3.8 | 0.25 ± 0.11 | 0.28 | 48.90 | 5.40 | 0.11 | 106.0 | 83.6 |
| 03D099 | <1:5 | 1:80 | ++ | 11.9 ± 10.3 | 0.28 ± 0.20 | 0.46 | 51.20 | 5.20 | 0.10 | 73.1 | 69.2 | |
| 03D233 | <1:5 | 1:320 | + | 16.0 ± 6.8 | 0.48 ± 0.24 | 0.51 | 35.70 | 1.80 | 0.05 | 0.9 | 50.6 | |
| 03D352 | 1:5 | 1:1280 | ++ | 6.9 ± 5.5 | 0.31 ± 0.16 | 0.26 | 32.80 | 39.40 | 1.20 | 12.3 | 56.9 | |
| 02D276 | 1:20 | 1:1280 | + | 2.9 ± 2.0 | 0.32 ± 0.26 | 0.23 | 5.80 | 31.00 | 5.34 | 2.7 | 0.0 | |
| 03C041 | 1:40 | 1:640 | ND | 0 | 0 | 0 | 0.033 | 24.78 | 743.65 | 0.0 | 0.5 | |
| 21537 | 1:80 | 1:2560 | +/− | 0 | 0.02 ± 0.04 | 0 | 0.010 | 16.32 | 1632.30 | 0.0 | 0.5 | |
| 03C113 | 1:80 | 1:5120 | +/− | 0 | 0 | 0 | 0.007 | 9.64 | 1377.57 | 0.6 | 0.7 | |
| Rhesus (juvenile) | RQ8082 | <1:5 | 1:160 | ND | 23.0 ± 17.6 | 0.56 ± 0.26 | 0.45 | 26.19 | 2.75 | 0.10 | 97.7 | 97.5 |
| RQ8086 | <1:5 | 1:320 | − | 21.6 ± 8.4 | 0.50 ± 0.13 | 0.38 | 42.20 | 1.24 | 0.03 | 149.0 | 140.1 | |
| RQ8083 | <1:5 | 1:160 | − | 20.5 ± 12.7 | 0.68 ± 0.24 | 0.47 | 29.35 | 3.01 | 0.10 | 0.0 | 46.6 | |
| RQ8085 | 1:10 | 1:2560 | +/− | 2.5 ± 1.4 | 0.11 ± 0.07 | 0.19 | 8.50 | 5.63 | 0.66 | 0.6 | 35.2 | |
| RQ8124 | 1:20 | 1:2560 | − | 0 | 0 | 0 | 0.043 | 2.58 | 60.36 | 0.5 | 0.5 | |
GFP, green fluorescent protein; NAb, neutralizing antibody; ND, not determined; PT, passive transfer.
Pre samples were obtained from all animals except C13991 on the same day as vector administration, just before gene transfer. The pre sample for C13991 was obtained 12 days before gene transfer.
Detection of capsid protein in spleen by immunofluorescence.
Measured as the percentage of GFP-positive area per liver section regardless of brightness. Ten sections per animal were analyzed.
Determined as the total intensity of each image, subtracting the background level (see Material and Methods. Ten sections per animal were analyzed.
Determined by an ELISA of liver lysate from the right lobe of each monkey.
Determined by qPCR of transgene EGFP, according to a standard curve generated with linearized plasmid DNA pAAV.CMV.EGFP. The limit of detection is 7 × 10−5 copies/diploid genome.
Determined by the day 7 cFIX expression levels as compared with the control group passively transferred with naïve mouse serum and receiving the same vector dose. The 1 × 109 GC (200 μl) groups received passive transfer of 200 μl of monkey serum followed by intravenous injection of 1 × 109 GC of AAV2/8.LSP.cFIX-W vector; 1 × 1010 GC (100 μl) groups received passive transfer of 100 μl of monkey serum followed by intravenous injection of 1 × 1010 GC of AAV2/8.LSP.cFIX-W vector.
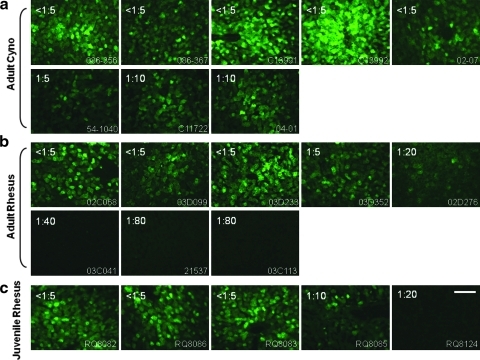
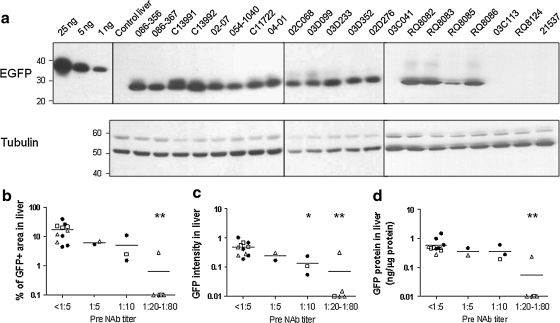
Liver tissue was evaluated for GFP expression using three independent quantitative assays including morphometric analysis of fluorescence micrographs for total fluorescence intensity and efficiency of gene transfer (percentage of hepatocytes expressing GFP) in addition to GFP-specific ELISA and Western blot analysis of liver lysates. Figure 2 shows representative fluorescence micrographs, Fig. 3a presents the Western blots, and Table 1 summarizes the quantitative measurements of GFP expression for each animal. Correlations of expression with serological status are presented in Fig. 3b–d. Transduction efficiency in NAb-negative macaques was slightly lower than that achieved in mice given a similar dose, although there was significant animal-to-animal variation (mean, 17% transduced area; range, 4 to 40%; Table 1 and Fig. 3b). All but one of the animals with NAb > 1:10 failed to demonstrate GFP fluorescence; the mean transduction efficiency was slightly reduced but detectable in animals with low NAb (6.2% transduced area for NAb = 1:5 and 5.0% for NAb = 1:10). The relative trends in transduction efficiency as measured by fluorescence intensity and GFP ELISA/Western blots agreed with the direct measurement of percent transduced area (Fig. 3 and Table 1).
FIG. 2.
Transduction of macaque liver with AAV2/8 vector. EGFP expression in macaque liver 7 days after intravenous infusion of AAV2/8.TBG.EGFP at 3 × 1012 GC/kg. (a) Adult cynomolgus macaques (n = 8). (b) Adult rhesus macaques (n = 8). (c) Juvenile rhesus macaques (n = 5). Representative images of each animal are shown. Preinjection AAV8 NAb titer is indicated at the top left corner, and the animal number is shown at the bottom right corner. Scale bar: 100 μm.
FIG. 3.
Impact of pre-existing AAV8 NAb on GFP expression levels in macaque liver. (a) Western analysis of liver lysate (10 μg of protein per lane) from a control and vector-treated macaques (7 days after vector administration). Purified EGFP protein with 12-His tag was loaded at 25, 5, and 1 ng per lane as controls. (b) Quantitative morphometric analysis of the transduction efficiency based on percent transduction of area. (c) Quantitative morphometric analysis of the transduction efficiency based on relative GFP intensity. (d) Quantification of GFP protein in liver lysate by ELISA: adult cynomolgus macaques (n = 8, solid circles), adult rhesus macaques (n = 8, open triangles), juvenile rhesus macaques (n = 5, open squares). Horizontal lines show the mean levels for each group. *p < 0.05, **p < 0.01 by ANOVA Dunnett test when compared with the group with pre-NAb < 1:5.
Expression data were also correlated with the level of NAb present at baseline as measured by PT. The initial PT studies were performed under the most sensitive conditions, in which animals were injected with 109 GC of AAV2/8.LSP.cFIX-W (Table 1). As predicted by data in Fig. 1d, baseline sera from several animals with NAb < 1:5 demonstrated a nearly complete block of transduction in vivo in the context of the PT assay. Importantly, these macaques did show transduction in vivo in NHPs, suggesting that the PT assay with 109 GC is too sensitive in terms of predicting in vivo transduction in macaques. We repeated the PT assay at a dose of 1010 AAV2/8.LSP.cFIX-W with 100 μl of serum from all the macaques (Table 1). This version of the assay is indeed less sensitive; a number of NAb-negative sera that showed substantial reduction of transduction in the PT assay with 109 GC of vector no longer inhibited in mice when the PT assay was performed with 1010 GC of vector. In fact, the PT assay at the higher dose of vector was more predictive of in vivo gene transfer in macaques, although not any better than the in vitro transduction inhibition assay. We used the in vitro transduction inhibition assays for additional correlations with vector biology.
Antibodies to AAV8 redirect distribution of vector genomes to spleen after systemic administration to macaques and mice
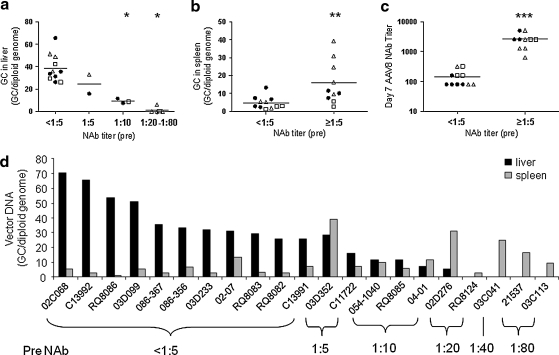
Liver and spleen were analyzed for vector genomes by real-time PCR. We have shown in work with a limited number of animals that pre-existing NAb reduced genome distribution to liver but may have actually increased genome distribution to spleen (Wang et al., 2010a). This was evaluated in all 21 macaques of this study. The actual genome data for each animal are shown as a function of NAb (Fig. 4d and Table 1) as well as in different serological groups in Fig. 4a. There was a clear correlation between NAb and reduction in liver genomes: 41 GC/diploid genome with NAb < 1:5; 22 GC/diploid genome with NAb = 1:5; 10 GC/diploid genome with NAb = 1:10; and 0.02 GC/diploid genome for NAb > 1:10, with one outlier (02D276). In NAb-negative macaques, vector genomes in liver were equal to or higher than that achieved in mice given a similar dose. The abundance of vector DNA in spleen was indeed higher in a subset of NAb-positive animals, although this did not directly correlate with the level of NAb (Fig. 4b and d).
FIG. 4.
Impact of pre-existing AAV8 NAb on vector biodistribution and day 7 NAb in macaques. (a) The amount of vector in liver (day 7) is negatively impacted by pre-existing AAV8 NAb. (b) Vector distribution to spleen is enhanced by pre-existing AAV8 NAb. (c) Dramatic increase in day 7 NAb titer in macaques with pre-existing AAV8 NAb. (d) Vector biodistribution in liver and spleen in individual animals 7 days after vector administration. Adult cynomolgus macaques (n = 8, solid circles), adult rhesus macaques (n = 8, open triangles), juvenile rhesus macaques (n = 5, open squares). Horizontal lines show the mean levels for each group. *p < 0.0001 by ANOVA Dunnett test, compared with the group with pre-NAb < 1:5. **p < 0.01, ***p < 0.0001 by Student t test.
The most direct measure of prior exposure to an AAV8-like capsid is the presence of NAbs. We hypothesized that another measure of capsid exposure would be the presence of memory B cells, which would produce an anamnestic response to vector challenge. Those animals with any detectable pre-existing NAb did show a brisk antibody response to vector as evidenced by titers usually equal to or greater than 1:1280 by day 7 (Fig. 4c).
AAV vector capsids are stably sequestered by follicular dendritic cells after systemic administration in macaques
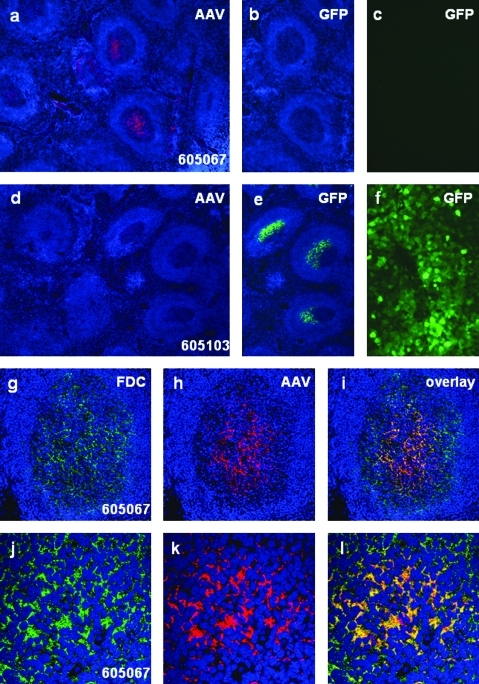
Additional studies were performed to assess the impact of antibody-enhanced uptake of AAV particles in macaque spleen. We first evaluated spleens for the presence of AAV8 protein by immunohistochemistry, using a rabbit polyclonal antibody made to the AAV8 capsid. Spleens from 16 of 21 macaques necropsied on day 7 of this study were analyzed, as were 3 animals from a previous study (Wang et al., 2010a) that were necropsied 35 days after gene transfer (Table 1 and Fig. 5). Initial studies showed staining in germinal centers of spleens from the majority of animals at both time points. High-magnification views of these sections demonstrated a cellular distribution of AAV antigen consistent with follicular dendritic cells (FDCs). All sections were then evaluated for the presence of AAV antigen together with CNA.42, which is a marker of FDCs. Representative histological sections are presented in Fig. 5 showing data from day 35 animals: Fig. 5a–c from an animal with pre-existing NAb = 1:10 and Fig. 5d–f from an animal with NAb < 1:5. In the presence of NAb there was clear localization of AAV capsid in the majority of germinal centers of the spleen, which clearly localizes with the FDC marker (Fig. 5g–l). This was associated with no GFP transduction in liver or spleen (Fig. 5b and c). In contrast, another day 35 animal with no pre-existing NAb showed high level GFP protein in spleen and liver but no AAV protein in the germinal centers (Fig. 6d–f). These studies were extended to include 16 of 21 spleen samples from the macaques that were necropsied 7 days after gene transfer as part of this project. Qualitative results are provided in Table 1. Ten of 16 demonstrated some level of AAV protein localization in FDCs of germinal centers. However, there was no clear association of AAV protein in spleen and pre-existing NAb. Pre-vector serum samples were also evaluated for AAV-binding antibodies, using an ELISA (Supplementary Table S1). Binding AAV antibodies correlated mostly with NAbs; however, there was no clear association with AAV protein in FDCs (Table 1 and Supplementary Table S1). Animals that were naive to AAV vector were analyzed for AAV capsid protein in spleen, using the immunohistochemical assay noted previously. These samples were negative, suggesting that the capsid protein detected from vector-treated animals was from the vector rather than endogenous virus.
FIG. 5.
Detection of AAV8 capsid protein or GFP protein in the germinal centers of macaque spleens. (a–c) Spleen and liver from animal 605067 (pre-NAb titer, 1:10; harvested 35 days after receiving AAV8 vector). Immunostaining shows the presence of capsid protein in germinal centers of the spleen (a). GFP is undetectable in the spleen (shown on the same spleen section) (b) or in the liver (c). (d–f) Spleen and liver from animal 605103 (pre-NAb titer <1:5, harvested on day 35 after vector administration). No capsid protein is detectable in the spleen by immunostaining (d). The germinal centers contain GFP (e) and GFP expression is visible in the liver (f). (g–i) Double immunofluorescence staining of spleen from animal 605067 with antibodies directed against follicular dendritic cells (FDCs) (g) and AAV8 capsid (h), showing colocalization (i) within germinal centers. (j–l) Double staining of FDCs and capsid shown at higher magnification. Spleen images have been counterstained with DAPI (shown in blue) to visualize nuclei.
FIG. 6.
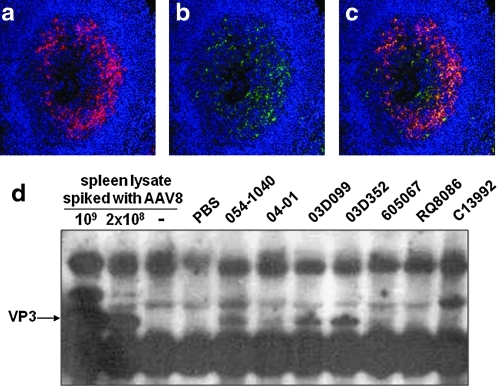
Detection of AAV8 capsid protein in macaque spleen by immunostaining and immunoprecipitation (IP). Double immunofluorescence staining of germinal centers (animal 03D352) with two different antibodies against AAV8: (a) AAV8-immunized rabbit serum, (b) AAV8 capsid-specific monoclonal antibody ADK8, and (c) overlay of both stains. Sections have been stained with DAPI (blue) to show nuclei. (d) Detection of VP3 protein in some nonhuman primate (NHP) spleen lysates by IP and Western blot. IP was performed with anti-AAV8 rabbit serum to pull down AAV capsid proteins from 0.5 mg (animals 605067 and RQ8086) or 1.5 mg (the remaining five animals) of spleen lysate. A negative spleen sample was spiked in with 1 × 109 or 2 × 108 GC of AAV8 to serve as positive controls. The capsid proteins were further detected by Western blot, using the monoclonal B1 antibody.
The specificity of our immunohistochemical assay for AAV8 protein based on the rabbit polyclonal antibody was further evaluated. Using a monoclonal antibody to AAV8 capsid we were able to show detection of AAV8 protein in a distribution consistent with uptake by FDCs and colocalization with signal from the polyclonal antibody (Fig. 6a–c). Splenic extracts were also analyzed for biochemical evidence of persistent AAV8 capsid, using immunoprecipitation/Western analysis. Homogenates were immunoprecipitated with the AAV8 polyclonal serum followed by fractionation on an SDS gel and Western blot analysis using a monoclonal antibody to denatured AAV capsid protein. A representative gel is shown in Fig. 6d. A band that comigrates with VP3 is demonstrated in those spleen samples that were positive by immunohistochemistry.
Levels of antibodies capable of interfering with AAV8 gene transfer are present in less than 25% of humans in North America
The macaque data demonstrated a significant drop-off in gene transfer and transgene expression with NAb titers greater than 1:10. Furthermore, the PT assay added no advantage in terms of predicting in vivo performance in primates. To help facilitate the translation of these results to clinical trials we subjected a number of human sera and plasma to in vitro transduction inhibition and in vivo PT in mice. The basic properties of the in vitro assay illustrated with macaque sera were present with human sera—the limit of sensitivity of the assay was a titer of 1:5 (Fig. 7a). An evaluation of sera from 125 normal subjects showed the prevalence of AAV8 NAb to be as follows: 49% NAb < 1:5, 17% NAb = 1:5, 11% NAb = 1:10, and 23% NAb > 1:10. A subset of these sera was evaluated for NAb using the most sensitive version of the PT assay, which uses 109 GC of vector. Figure 7b shows a correlation of the PT results in terms of cFIX expression (percentage of control with naive serum) with in vitro NAb titers. Human sera showed better correlations than macaque sera in that all humans with NAb < 1:5 showed no PT inhibition and all human samples with NAb > 1:10 showed complete PT inhibition; the picture was mixed at low levels of NAb = 1:5 and 1:10. Additional studies were performed to evaluate the impact of vector dose on the sensitivity of the PT assay (Fig. 7c). As we saw with macaques, the assay loses sensitivity as the dose of vector is increased. This also suggests that low levels of NAb in humans may be overcome with higher doses of vector.
FIG. 7.
In vitro and in vivo assays to detect AAV8 NAb in human plasma samples. (a) In vitro NAb assay. The AAV8 NAb titer for each sample is indicated in parentheses. (b) Correlation of in vitro NAb titer and the outcome of in vivo passive transfer (n = 40 samples, vector dose = 1 × 109 GC). (c) Effect of vector dose on passive transfer. Human plasma samples with various AAV8 NAb titers or naive mouse serum was passively transferred to C57BL/6 mice (200 μl/mouse) via the tail vein. Two hours later, mice were intravenously injected with 1 × 109, 1 × 1010, or 1 × 1011 GC of AAV2/8.LSP.cFIX-W (n = 3 for each dose). Seven days after vector injection, cFIX levels in plasma were measured by ELISA and are shown as the percentage of the cFIX level in control mice passively transferred with naive mouse serum and receiving the same vector dose. Means and SD are shown. **p < 0.01, ***p < 0.001 by ANOVA Dunnett test, compared with the group with pre-NAb < 1:5.
Discussion
Important to the success of in vivo gene therapy is an understanding of host factors that may complicate safe and effective delivery of the gene. As is the case for small-molecule pharmaceuticals, genetic factors such as MHC genotypes and polymorphisms that regulate innate immunity will play a role. In the case of viral vectors, previous exposure of the patient to a virus with immunological relatedness to the vector will substantially impact on outcome. An example of this emerged from an HIV vaccine study with a vector based on a human adenovirus serotype 5. Subjects with pre-existing NAbs to the virus showed an unexpected increase in the acquisition of HIV infection after vaccine as compared with placebo group subjects who did not receive vector (McElrath et al., 2008). Our current study focused on the effect of pre-existing NAb to AAV8 in nonhuman primates on the efficacy and safety of liver-directed gene transfer.
In an earlier report, we noted the impact of pre-existing immunity on liver gene transfer in macaques, focusing on different capsids evaluated in a minimal number of monkeys (i.e., three animals administered AAV8 and necropsied on day 7) (Wang et al., 2010a). Transgene expression was shown to be stable for 35 days in the limited number of animals studied (Wang et al., 2010a). The goal of the current study was to (1) establish a threshold of pre-existing immunity in macaques that impacts on safety and efficacy, (2) develop an assay that best correlates with in vivo transduction inhibition in macaques, and (3) make predictions as to the extent of the problem of confounding antibodies in human populations. To accomplish this goal we enrolled 21 macaques representing a wide range of pre-existing AAV8 antibodies.
Nonhuman primates were selected as the model to address the impact of humoral immunity on gene transfer. AAVs are endogenous viruses in both humans and macaques and they share substantial structural homologies. In fact, AAV8 is in a clade that is composed of isolates from both humans and macaques (Gao et al., 2004). The prevalence of endogenous genomes and profiles of seroreactivity to AAVs are similar in humans and macaques (Gao et al., 2003; Calcedo et al., 2009). Natural infections with AAVs establish a broad scope of neutralizing responses in both human and nonhuman primates. Others have attempted to simulate host antibody responses to natural infections by immunizing animals with AAV capsid (Nathwani et al., 2007). However, the breadth of the humoral response seen in primates after natural infections contrasts with the narrow serotype-specific response elicited in nonprimate animal models after exposure to AAV vectors (Brantly et al., 2009).
An important goal of our study was to establish an assay for AAV antibodies capable of predicting levels of NAb that diminish transduction efficiency. This required direct correlations with in vivo transduction in primate liver. We favor the in vitro transduction inhibition assay rather than the PT assay for several reasons. The in vitro assay provided a better correlation with in vivo transduction in macaques than did the PT assay. Many macaque sera with undetectable NAb in vitro showed substantial inhibition in the PT assay despite the fact that all yielded good transduction in macaque liver. Validation of the in vitro transduction inhibition assay will be much easier than with the PT assay.
A study by Hurlbut and colleagues attempted to address some of the same issues evaluated in this paper by evaluating AAV8 vectors expressing human α-galactosidase (α-Gal) administered intravenously in 12 macaques (Hurlbut et al., 2010). Their studies differed from ours in both content and conclusions. The key to our study was a cohort of 21 macaques with varying levels of pre-existing immunity in which we established direct correlations with quantitative measures of transduction efficiency through GFP analysis at short time points. Hurlbut and colleagues failed to establish this kind of correlation. Expression of α-Gal did differ 9-fold within their cohort of macaques; however, expression did not correlate with pre-existing antibodies (the highest expressing animal had the second highest AAV antibodies and some of the lowest expressing animals had the lowest levels of AAV antibodies). The basis for these differences is unclear although it may relate to several aspects of their study, such as (1) confounding immune responses to the secreted human-derived transgene product, (2) the use of a binding rather than a neutralizing antibody assay, and (3) the limited scope of the study in terms of animals and their corresponding neutralizing antibody assays. In establishing a threshold of pre-existing antibody that would interfere with transduction in humans they had to rely on passive transfer studies in mice, using dilutions of macaque serum, whereas we were able to extrapolate from actual in vivo primate hepatocyte transduction and direct correlation of sera evaluated in several AAV antibody assays. In other words we used the in vivo primate data to validate the legitimacy of the in vitro and passive transfer assays. Our experience with the passive transfer assay is that it is context dependent as it relates to dose of vector and species of the passively transferred antibody. For example, we show lack of inhibition in the most sensitive passive transfer assay in all human samples that are negative for NAb on the basis of in vitro assays. However, 29% of NAb-negative macaque sera do show significant inhibition in the same passive transfer assay. It may not be surprising therefore that estimates of the scale of the problem of pre-existing antibodies in humans are so different between the two groups: Hurlbut and colleagues predict 90% of humans will not be receptive to AAV8 gene therapy at a dose of 2 × 1012 GC/kg whereas we believe only 25% of humans will have interfering antibodies at a similar dose of vector.
On the basis of the in vitro assay we predict that patients with a NAb titer of 1:10 or less will achieve good gene transfer to liver with AAV8 at a dose of 3 × 1012 GC/kg. This suggests that 75% of humans in the United States will be suitable for systemic delivery of AAV8. We and others (Hurlbut et al., 2010) have explored the use of plasmapheresis in diminishing NAb to viruses such as AAV8 and have found that each sequential apheresis session can reduce NAb approximately 2-fold, which could be a strategy for converting some of the AAV8-resistant patients to eligibility for AAV8 gene transfer (i.e., one plasmapheresis would result in an additional 3% of patients being eligible for gene therapy while two sequential treatments would make eligible 9% more patients). The threshold of NAb that leads to reduction of transduction will clearly be dependent on dose and route of administration. NAb should have substantially less effect on transduction when the vector is directly injected into tissue compartments such as in the eye (subretinal or intravitreal), muscle, heart, and brain although a more thorough evaluation of this in nonhuman primates is warranted (Mastakov et al., 2002; Manno et al., 2003; Li et al., 2008; Barker et al., 2009).
We further explored the potential of pre-existing NAb changing the safety profile of systemic AAV. There was no obvious short-term clinical or pathological consequence of NAb; however, we were concerned about the increased targeting of vector into spleen especially as it relates to enhanced innate and adaptive immune responses. In further exploring this result we found that the majority of animals deposited substantial quantities of the vector capsid within germinal centers of the spleen; antigen persisted for at least 35 days. Within the germinal centers the capsids colocalized with markers of FDCs, which are known to function in sequestration of soluble antigens and presentation to B cells. Pre-existing humoral immune responses to the capsid do not correlate with the presence and/or extent of AAV capsid deposition in spleen. It is likely, however, that FDC capsid sequestration will provide a long-term depot of antigen presentation for activation of T and B cells. The implications of this are unclear in terms of vector performance although strategies to thwart these adaptive immune responses through transient immune suppression may be of limited value. It would be useful to understand what influences capsid targeting of FDCs and how it can be predicted and/or avoided.
Clinical trials of AAV8 are being actively pursued in multiple applications, based on its superior performance. Preliminary results in a phase I study in hemophilia B are encouraging (Ponder, 2011). Careful attention needs to be placed on host factors that may impact on outcome. We provide guidance regarding the impact of pre-existing NAb, which should be considered in the design of clinical trials.
Supplementary Material
Acknowledgments
The authors thank Martin Lock and Vector Core (Gene Therapy Program) for supplying vectors; Erin Bote (Gene Therapy Program) for invaluable assistance with macaque studies; Judith Franco, Qiuyue Qin, Surina Boyd, Hongwei Yu, Deirdre McMenamin (Gene Therapy Program), and Rafic Melhem (summer student) for technical assistance; Juergen Kleinschmidt of German Cancer Research Center for providing the ADK8 antibody; and the Blood Bank of the Hospital of the University of Pennsylvania for providing normal human plasma samples and human plasma samples before and after apheresis. This work was supported by NIH grants to J.M.W.: P01-HD057247, P01-HL059407, and P30-DK047757.
Author Disclosure Statement
J.M.W. is a consultant to ReGenX Holdings, and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings.
References
- Bainbridge J.W. Smith A.J. Barker S.S., et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Barker S.E. Broderick C.A. Robbie S.J., et al. Subretinal delivery of adeno-associated virus serotype 2 results in minimal immune responses that allow repeat vector administration in immunocompetent mice. J. Gene Med. 2009;11:486–497. doi: 10.1002/jgm.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P. Moscioni A.D. McCarter R.J., et al. Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol. Ther. 2006;14:34–44. doi: 10.1016/j.ymthe.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Boutin S. Monteilhet V. Veron P., et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- Brantly M.L. Chulay J.D. Wang L., et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R. Vandenberghe L.H. Gao G., et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao O. Dobrzynski E. Wang L., et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham S.C. Spinoulas A. Carpenter K.H., et al. AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal Spfash Mice. Mol. Ther. 2009;17:1340–1346. doi: 10.1038/mt.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L., et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Alvira M.R. Somanathan S., et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6081–6086. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Vandenberghe L.H. Alvira M.R., et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth W.W. Aleman T.S. Kaushal S., et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum. Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut G.D. Ziegler R.J. Nietupski J.B., et al. Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy. Mol. Ther. 2010;18:1983–1994. doi: 10.1038/mt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. Couto L.B. Patarroyo-White S., et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobinger G.P. Weiner D.J. Yu Q.C. Wilson J.M. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat. Biotechnol. 2001;19:225–230. doi: 10.1038/85664. [DOI] [PubMed] [Google Scholar]
- Koeberl D.D. Pinto C. Sun B., et al. AAV vector-mediated reversal of hypoglycemia in canine and murine glycogen storage disease type Ia. Mol. Ther. 2008;16:665–672. doi: 10.1038/mt.2008.15. [DOI] [PubMed] [Google Scholar]
- Kozarsky K.F. McKinley D.R. Austin L.L., et al. In vivo correction of low density lipoprotein receptor deficiency in the Watanabe heritable hyperlipidemic rabbit with recombinant adenoviruses. J. Biol. Chem. 1994;269:13695–13702. [PubMed] [Google Scholar]
- Li Q. Miller R. Han P.Y., et al. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol. Vis. 2008;14:1760–1769. [PMC free article] [PubMed] [Google Scholar]
- Lock M. Alvira M. Vandenberghe L.H., et al. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum. Gene Ther. 2010;21:1259–1271. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M. Simonelli F. Pierce E.A., et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C.S. Chew A.J. Hutchison S., et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mastakov M.Y. Baer K. Symes C.W., et al. Immunological aspects of recombinant adeno-associated virus delivery to the mammalian brain. J. Virol. 2002;76:8446–8454. doi: 10.1128/JVI.76.16.8446-8454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath M.J. De Rosa S.C. Moodie Z., et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: A case–cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J.R. Campbell K. Rodino-Klapac L., et al. Dystrophin immunity in Duchenne's muscular dystrophy. N. Engl. J. Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F. Maus M.V. Hui D.J., et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Mingozzi F. Meulenberg J.J. Hui D.J., et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Model M.A. Burkhardt J.K. A standard for calibration and shading correction of a fluorescence microscope. Cytometry. 2001;44:309–316. doi: 10.1002/1097-0320(20010801)44:4<309::aid-cyto1122>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nathwani A.C. Gray J.T. Ng C.Y., et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani A.C. Gray J.T. McIntosh J., et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder K.P. Hemophilia gene therapy: A holy grail found. Mol. Ther. 2011;19:427–428. doi: 10.1038/mt.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan C.D. Jiang H. Liu T., et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- Wang L. Figueredo J. Calcedo R., et al. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum. Gene Ther. 2007;18:185–194. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- Wang L. Calcedo R. Wang H., et al. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol. Ther. 2010a;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Wang H. Bell P., et al. Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol. Ther. 2010b;18:118–125. doi: 10.1038/mt.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W. Chirmule N. Schnell M.A., et al. Route of administration determines induction of T-cell-independent humoral responses to adeno-associated virus vectors. Mol. Ther. 2000;1:323–329. doi: 10.1006/mthe.2000.0045. [DOI] [PubMed] [Google Scholar]
- Yang Y. Greenough K. Wilson J.M. Transient immune blockade prevents formation of neutralizing antibody to recombinant adenovirus and allows repeated gene transfer to mouse liver. Gene Ther. 1996;3:412–420. [PubMed] [Google Scholar]
- Zhong L. Li B. Mah C.S., et al. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. Huang X. Yang Y. The TLR9–MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Invest. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.