Abstract
Background
More patients in resource-limited settings are starting 2nd-line ART following 1st-line ART failure. We aimed to describe predictors of lack of virologic suppression in HIV-infected patients on 2nd-line ART in a roll-out program in South Africa.
Methods
Retrospective analysis was performed on an adult HIV treatment cohort who started 2nd-line ART (lopinavir/ritonavir, didanosine, and zidovudine) after virologic failure of 1st-line ART (2 consecutive HIV RNA >1000 copies/ml). Predictors of week-24 lack of suppression (HIV RNA > 400 copies/ml) on 2nd-line ART were determined by bivariate analysis where missing equals failure. A multivariable model adjusted for gender, age, and time to ART switch. We tested these findings in sensitivity analyses defining lack of suppression at week-24 as HIV RNA > 1000 and > 5000 copies/ml.
Results
Of 6,339 patients on ART, 202 started 2nd-line ART. At week-24 an estimated 41% (95% CI 34–47%) did not achieve virologic suppression. Female sex (adjusted OR=2.25; 95% CI, 1.03–4.88) and time to ART switch, (adjusted OR=1.07; 95% CI, 1.01–1.14 for each additional month) increased the risk of lack of virologic suppression. Age, CD4 count, and HIV RNA at 2nd-line ART initiation did not predict this outcome. In multivariate models, these findings were insensitive to the definition of lack of virologic suppression.
Conclusions
A substantial number of HIV-infected patients do not achieve virologic suppression by week-24 of 2nd-line ART. Women and patients with delayed start of 2nd-line ART after 1st-line ART failure were at an increased risk of lack of virologic suppression.
Keywords: 2nd-line antiretroviral therapy, HIV outcomes, resource-limited settings, South Africa
Introduction
Enrollment in antiretroviral treatment (ART) programs has escalated dramatically due to expanded global efforts towards universal ART access in the last several years [1]. World Health Organization (WHO) estimates suggest that global ART coverage rose from 400,000 to nearly 3 million people from 2003 to 2007. The focus of this increase has been in low and middle-income countries, predominantly in sub-Saharan Africa, where the number of patients enrolled on ART doubled between 2006 and 2007, to total nearly 2 million [2]. Worldwide, an estimated 180,000 individuals were on 2nd-line ART in 2008, based on a 3% treatment switch per year [3].
There has been an urgent interest among HIV providers and policy-makers for a rational approach to 2nd-line ART use [3–6]. As ART options are limited in resource-limited settings, the consequences of inappropriate treatment switches are greater than in well-resourced areas [5, 7]. There have been few trials testing 2nd-line ART efficacy after 1st-line virologic failure on stavudine or zidovudine-based regimens, those most commonly used in resource-limited settings [8].
Furthermore, consideration of the timing of treatment switch after 1st-line ART failure is particularly important in resource-limited settings where salvage regimens are scarce and costly. Prospective data from two clinical cohorts suggest that treatment delay after virologic failure on a non-protease inhibitor based-regimen had an increased risk of mortality [9]. A single randomized-controlled trial did not complete enrollment and did not detect a difference in timing of treatment switch to 2nd-line ART on the outcome of either drug resistance or CD4 change [10]. The objective of the current study was to describe early outcomes on 2nd-line ART after 1st-line virologic failure in a community-based setting in South Africa and to examine predictors of lack of virologic suppression on 2nd-line ART. We hypothesized that delayed switching from confirmed 1st-line ART failure to 2nd-line ART initiation would adversely impact the effectiveness of 2nd-line ART.
Methods
Study setting
The Gugulethu Clinic is an HIV referral center for Nyanga, a periurban township of Cape Town, South Africa. The clinical site has been described in detail and provides HIV-related care for more than 6,000 patients [11–15]. The district population is roughly 300,000, with antenatal HIV prevalence at 29% in 2006 [15]. Low socioeconomic status predominates, with the majority of inhabitants living in high density housing. Nearly 50% report no personal monthly income [15].
Study population and design
All patients received initial therapy per South African national antiretroviral and WHO guidelines [16, 17]. First-line ART consisted of three drug therapy with two nucleoside reverse transcriptase inhibitors (NRTIs) and a non-nucleoside reverse transcriptase inhibitors (NNRTIs) (i.e., efavirenz or nevirapine) in the majority of cases. Second-line ART consisted of protease inhibitor-based ART with lopinavir/ritonavir, didanosine, and zidovudine.
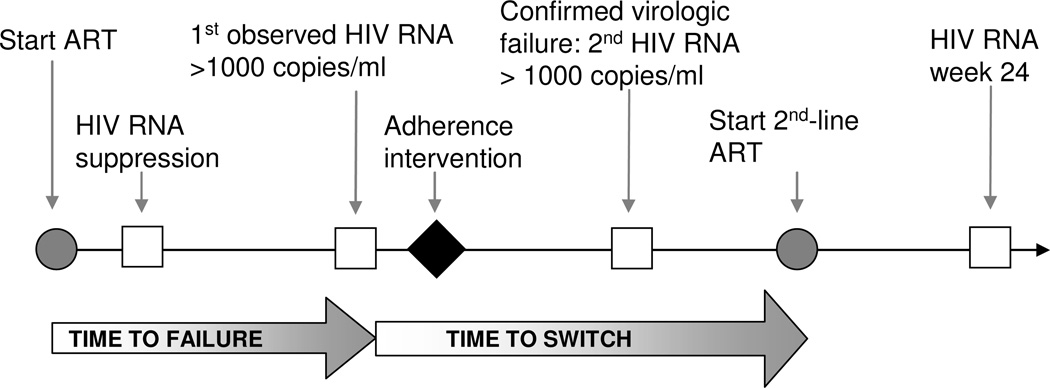
Clinical practice at this site involves patient laboratory testing with CD4 count and HIV RNA every 16 weeks (Figure 1). Any patient without virologic suppression (HIV RNA > 1000 copies/ml) after baseline or if tablet count at clinic visit is < 85% of expected tablet consumption, receives adherence counseling until virologic suppression is achieved and tablet adherence is improved. Patients with virologic suppression and subsequent viral rebound (i.e., first HIV RNA measurement >1000 copies/ml after virologic suppression) undergo a targeted adherence intervention over one month, which includes a pill dosing diary, pill counts, home visits, and patient education sessions. Within 6–8 weeks of the first observed HIV RNA >1000 copies/ml, the HIV RNA assay is repeated [11, 18]. A second HIV RNA >1000 copies/ml confirms the diagnosis of virologic failure and eligibility for switching to 2nd-line ART. CD4 count and HIV RNA are measured every 16 weeks during 2nd-line ART. Local laboratories perform HIV RNA assays by branch DNA hybridization techniques (HIV-1 RNA 3.0 assay®, Bayer Healthcare, Leverkusen, Germany) and CD4 count by flow cytometry (FACS Count™, Beckton Dickinson, NJ, USA). Clinical records were maintained for each patient prospectively from clinic entry and transferred weekly into an electronic database.
Figure 1.
Schematic overview of initiation of antiretroviral therapy (ART), laboratory monitoring, detection of 1st-line ART failure, and treatment switch in a community-based HIV treatment center in South Africa.  = laboratory test
= laboratory test  = ART
= ART  = intervention
= intervention
We included HIV-infected patients (age ≥ 15 years) who were part of a prospective HIV treatment cohort, and were eligible for at least 6 months follow-up on 2nd-line ART. To assure validation of patient treatment history and to limit patient heterogeneity, we excluded from the analysis patients who started 1st-line ART elsewhere and transferred into the study site.
Data elements
Data elements available for this analysis were clinical, immunologic, and virologic characteristics, including calendar dates of ART initiation and switch.
We evaluated patients who were on 2nd-line ART after virologic failure of 1st-line ART, defined as 2 consecutive HIV RNA levels >1000 copies/ml or the last available HIV RNA > 1000 copies/ml prior to 2nd-line ART initiation. These criteria are consistent with South Africa national guidelines for monitoring of ART failure and treatment switching.
To assess early predictors of lack of virologic suppression on 2nd-line ART, we defined the primary study outcome as week-24 HIV RNA > 400 copies/ml. We included HIV RNA data obtained within 12 weeks (before or after) the week-24 date.
Potential predictors of the primary outcome related to demographic, clinical, and programmatic characteristics, here defined as the calendar year of 2nd-line ART initiation. The time to 1st-line ART failure was measured from the time of ART initiation to the first observed HIV RNA >1000 copies/ml in patients in patients who had confirmed virologic failure, or the time to the last available HIV RNA >1000 copies/ml if a second HIV RNA was not available. Time to switch was defined as the time from detected virologic failure (i.e., first HIV RNA > 1000 copies/ml) to 2nd-line ART initiation. To assess if initial lack of virologic suppression on 1st-line ART predicted lack of virologic suppression on 2nd-line ART at week-24, we measured if patients on 2nd-line ART had experienced a 1 log10 decrease in HIV RNA at week–24 while on 1st-line ART.
Statistical Analysis
We conducted a conservative analysis assuming that those with missing HIV RNA at week-24 on 2nd-line ART lacked virologic suppression. Mean proportion of week-24 lack of virologic suppression was reported with standard deviations calculated from a normal approximation. Demographic, clinical, and programmatic characteristics of subjects on 2nd-line ART were tested for association with the primary outcome by bivariate analysis. Immunologic and virologic co-variates were examined as categorical variables to facilitate clinical interpretation. Time to 1st-line ART failure and time to switch were examined as continuous variables. Student’s t-test and the chi-square tests were used to compare continuous and categorical data, respectively.
To assess if the group that started 2nd-line ART was representative of treatment failures we compared the demographic and clinical characteristics of those who did and did not switch to 2nd-line ART after 1st-line ART failure. To further evaluate the comparability of the sample on which the analysis has been conducted with the patients excluded from the analysis, characteristics of excluded and included patients on 2nd-line ART were compared.
An a priori multivariate model included those co-variates (i.e. gender, age at 2nd-line ART initiation, and time to 2nd-line ART initiation) that were hypothesized as related to the outcome of week-24 lack of virologic suppression. We performed logistic regression to assess if these factors predicted week-24 lack of virologic suppression on 2nd-line ART. Statistical tests were two-sided with a significance level of 0.05.
Several sensitivity analyses examined the impact of 3 major areas (e.g. the definition of lack of virologic suppression, co-variates related to timing of virologic non-suppression, and the analytic methodology) on the outcome to assure the robustness of conclusions.
With respect to the first, we examined the group in-care with available week-24 HIV RNA and defined lack of virologic suppression as HIV RNA > 1000 copies/ml and > 5000 copies/ml. These cut-points were to test treatment failure thresholds proposed by the literature. The WHO 2010 Rapid Advice suggested a persistent HIV RNA greater than 5,000 copies/ml as confirmation of ART failure [19]. Prior 2006 guidelines suggested a range of 5,000–10,000 copies/ml [20]. South Africa national guidelines have used a range of > 400 – > 5,000 copies/ml as criteria for ART failure [17].
The second sensitivity analysis varied the definition of two co-variates. The variable that represented a 1 log10 decrease in HIV RNA at week-24 on 1st-line ART, now considered missing HIV RNA as failure (HIV RNA > 1000 copies/ml). To assess whether a proxy for week-24 HIV RNA obtained between weeks-12 and 24 (i.e. “early”) had a differential risk of lack of virologic suppression at 6 months than a proxy obtained after week-24 and before week-36 (i.e. “late”), we performed chi-square and multivariate analysis including this co-variate in the model.
The third sensitivity analysis used a proportional hazards model with survival analysis to estimate predictors of week-24 lack of virologic suppression. Patients who died, went out of care in the first 24 week of 2nd-line therapy or had less than 6 months of follow-up were included. The multivariate model adjusted for age, time to switch to 2nd-line ART, and gender. All statistical analyses were performed using SAS 9.1 software (Cary, North Carolina).
Results
Cohort Description
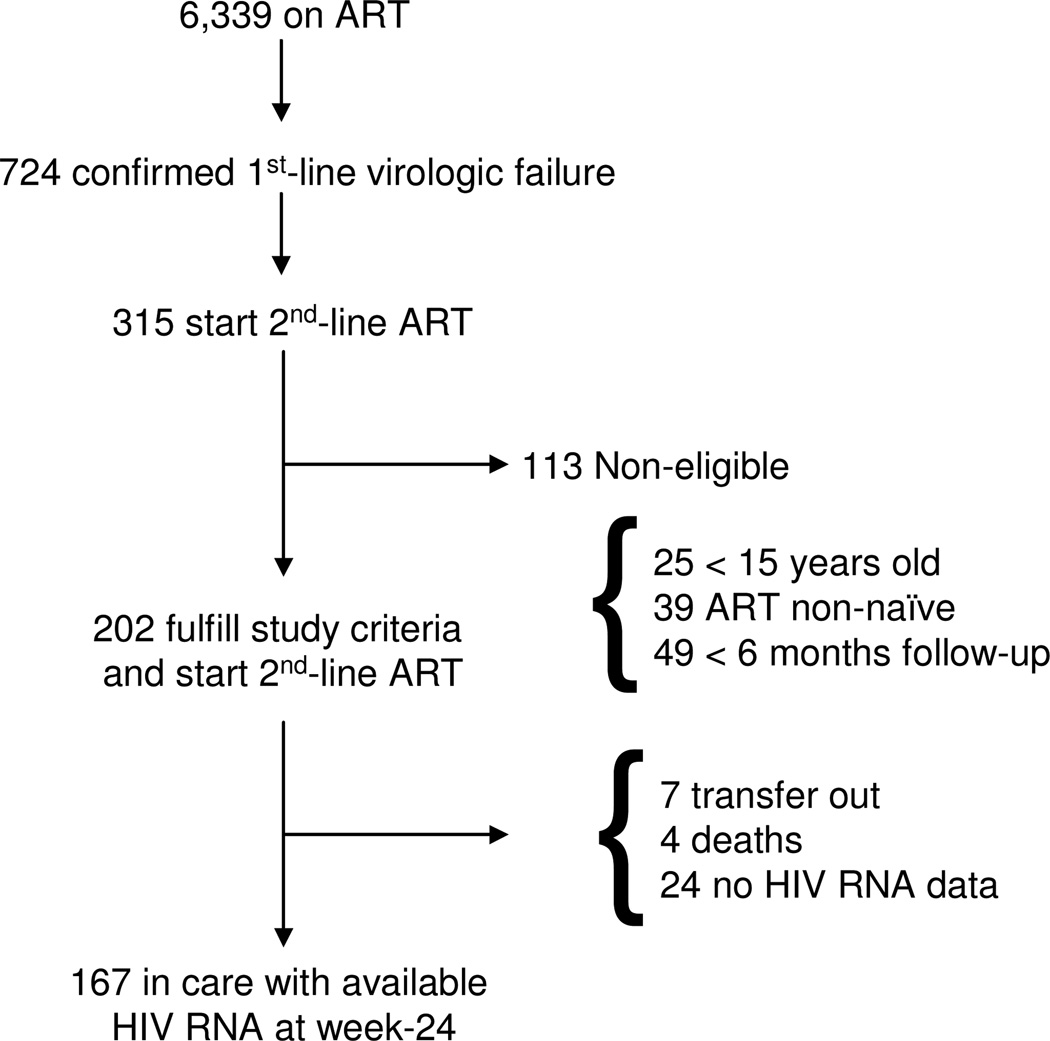
Of the cohort of 6,339 patients on ART, 724 had confirmed virologic failure on 1st-line ART (Figure 2). Of these, 315 started 2nd-line ART between October 23, 2002 and March 12, 2010, and 409 remained on 1st-line ART. Compared with individuals who failed 1st-line ART and switched to 2nd-line ART, individuals who failed 1st-line ART but did not switch to 2nd-line were less likely to be female (63% vs. 76%, p=0.0004), had a longer median time to detection of 1st-line ART failure (16 months [IQR 8–30 months] vs. 12 months [IQR 7–23 months], p=0.002) and failed 1st-line ART with a lower median CD4 count (202 cells/µl [IQR 112–330 cells/µl] vs. 246 cells/µl [IQR 153–349 cells/µl], p=0.003).
Figure 2.
Study flow diagram.
One hundred thirteen of 315 on 2nd-line ART were ineligible for analysis due to age < 15 years (n=25), ART non-naïve at baseline ART initiation (n=39), and had < 6 months follow-up on 2nd-line ART (n=49). Compared with the eligible group, those on 2nd-line ART who were excluded from the analysis were younger but did not differ by gender. The ineligible group had a higher median CD4 count at 1st-line ART failure at 312 cells/µl [IQR 190–460 cells/µl] and 2nd-line ART initiation at 281 cells/µl, [IQR 176–416 cells/µl], both p <0.0001.
The remaining 202 patients were analyzed. One hundred ninety-eight of these individuals were switched after two consecutive HIV RNA > 1000 copies/ml on 1st-line ART, and n=4 switched with the last available HIV RNA > 1000 copies/ml. At 2nd-line ART initiation, mean age was 34 years and almost 44% of the cohort initiated 2nd-line ART between 2008 and 2009, where the remainder started between 2004 and 2007 (Table 1). A majority of the cohort was female (75%).
Table 1.
Demographic and laboratory characteristics of patients resource-limited community of Cape Town, South Africa.
| Characteristic | Total Cohort N=202 (%) |
|---|---|
| Age, Mean years ± SD | 34 ± 7 |
| Female sex | 152 (75) |
| First-line ART Results | |
| Time to virologic failure, median months [IQR] | 11 [7–18] |
| Time to switch, median months [IQR] | 5 [3–8] |
| Nadir CD4 count prior to 2nd-line ART | |
| ≤50 cells/µl | 82 (41) |
| > 50 cells/µl | 120 (59) |
| CD4 count at 1st-line ART failure a | |
| ≤ 200 cells/µl | 87 (46) |
| 201–350 cells/µl | 71 (37) |
| > 350 cells/µl | 32 (17) |
| No 1 log10 decrease in HIV RNA at week–24b | 25 (14) |
| Second-line ART Results | |
| Year of 2nd-line ART start | |
| 2004–2007 | 114 (56) |
| 2008–2009 | 88 (44) |
| CD4 count at 2nd-line ART start, median cells/µl [IQR] | 212 [133–289] |
| ≤ 200 cells/µl | 95 (47) |
| 201–350 cells/µl | 82 (41) |
| > 350 cells/µl | 25 (12) |
| HIV RNA at 2nd-line ART start, median log copies/ml [IQR] | 3.97 [3.63–4.38] |
| 0–3000 copies/ml | 38 (19) |
| 3001–10,000 copies/ml | 65 (32) |
| >10,000 copies/ml | 99 (49) |
| Lack of week-24 suppression, HIV RNA > 400 copies/ml (%, 95% CI) | 82 (41%, 95% CI, 34–47) |
Available data for 190 patients.
Available data for 173 patients.
ART= antiretroviral therapy; SD= standard deviation; IQR= interquartile range; CI= confidence intervals; wk=week.
First-line ART for all patients included lamivudine. The second NRTI component was stavudine in 86% (n=173) and zidovudine in 14% (n=29). The non-nucleoside component was efavirenz in 63% (n=128) and nevirapine in 36% (n=72).
Clinical outcomes prior to 2nd-line ART
On 1st-line ART, 82 patients (41%) had a nadir CD4 count ≤ 50/µl. Twenty-five patients (14%) of 173 patients with available HIV RNA data at week-24 on 1st-line ART never experienced a 1 log10 decline in HIV RNA within the first 24 weeks of 1st-line ART. Median time to 1st-line ART failure was 11 months [IQR 7–18 months] and the median time to switch to 2nd-line ART was 5 [IQR 3–8 months]. The median CD4 count change between 1st-line ART failure and 2nd-line ART initiation was 0/µl [IQR −44 to + 21 /µl].
Early outcomes on 2nd-line ART
Between 2nd-line ART initiation and the week-24 endpoint, there were 4 deaths, 7 patients transferred out of care, and 24 did not have available virologic data, either because they were lost to follow-up or did not return for clinic within the 24 week follow-up period. One hundred sixty-seven patients were in care at week-24 on 2nd-line ART (Figure 2). Between 2nd-line ART initiation and week-24, median CD4 count increase was 90 cells/µl [IQR −4 to 177/µl].
Lack of virologic suppression on 2nd-line ART
In the primary analysis, lack of virologic suppression (HIV RNA > 400 copies/ml) at week-24 was 41% (n=82, 95% CI, 34–47). Women (OR 2.08; 95% CI, 1.04–4.17) and those who waited to switch from failed 1st-line ART, per each month delay (OR 1.06; 95% CI, 1.00–1.12) were at increased odds of lack of virologic suppression on 2nd-line ART at week-24 (Table 2). In the multivariate analysis, the odds ratio for lack of virologic suppression in women compared with men was 2.25 (95% CI, 1.03–4.88) and per each month delay in switching was 1.07 (95% CI, 1.01–1.14). The data did not suggest that age, CD4 count, or HIV RNA at 2nd-line ART initiation significantly predicted week-24 lack of virologic suppression.
Table 2.
Bivariate and multivariate logistic regression analysis of predictors of 24-week lack of virologic suppression in 202 patients on protease inhibitor-based 2nd-line ART in a community-based cohort in South Africa.
| Bivariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| Characteristic | Odds Ratio (95% CI) |
P | Odds Ratio (95% CI) |
P |
| Female sex | 2.08 (1.04–4.17) | 0.04 | 2.25 (1.03–4.88) | 0.04 |
| Factors related to First-line ART | ||||
| Time to virologic failure, per 1 month increase | 1.00 (0.98–1.03) | 0.75 | -- | |
| Time to switch, per 1 month increase | 1.06 (1.00–1.12) | 0.04 | 1.07 (1.01–1.14) | 0.02 |
| Nadir CD4 count, > 50/μl | 1.45 (0.81–2.58) | 0.21 | -- | |
| Absent 1 Log10 decrease in HIV RNA, wk-24 | 1.25 (0.53–2.95) | 0.60 | -- | |
| Factors related to 2nd-line ART | ||||
| Age at 2nd-line initiation | 0.98 (0.95–1.02) | 0.38 | 0.99 (0.95–1.04) | 0.67 |
| Calendar year 2nd-line ART start, 2004–2007 | 0.83 (0.47–1.46) | 0.51 | -- | |
| CD4 count at 2nd-line ART start | ||||
| ≤ 200/µl | Reference | -- | ||
| > 200/µl | 0.58 (0.33–1.03) | 0.06 | -- | |
| HIV RNA at 2nd-line ART start | ||||
| ≤10,000 copies/ml | Reference | -- | ||
| >10,000 copies/ml | 1.49 (0.85–2.61) | 0.17 | -- | |
Sensitivity Analysis
In the secondary analysis where missing data were excluded, lack of virologic suppression (HIV RNA > 400 copies/ml) at week-24 was 28% (n=47, 95% CI, 21–35%). When lack of suppression at week-24 was defined as HIV RNA > 1000, 25% (n=42, 95% CI, 19–32%) did not suppress; and when lack of suppression was defined as HIV RNA > 5000 copies/ml, 22% (n=36, 95% CI, 16–28%) did not achieve virological suppression at week-24.
We examined the impact of alternative definitions of lack of virologic suppression on results. The results were robust across these definitions.
In a chi-square analysis, an “early” proxy for week-24 HIV RNA incurred a higher odds of the week-24 HIV RNA > 400 copies/ml, OR 7.36 (95% CI 3.92–13.85), compared with a “late” proxy. The findings of the multivariate model were insensitive to the addition of this covariate.
A proportional hazards model of the probability of lack of virologic suppression on 2nd-line ART showed a similar trend as the logistic regression model but female sex was no longer statistically significant.
Discussion
The consequences of 2nd-line ART selection after HIV treatment failure are magnified in resource-limited settings because of the scarcity and higher costs of salvage ART regimens. Despite the gravity surrounding the clinical decision to start protease inhibitor-based 2nd-line ART, there is a paucity of evidence on the determinants of lack of virologic suppression on this regimen due to the relative recent expansion of ART delivery and the challenges in identifying treatment failures with scarce resources for laboratory monitoring. We analyzed week-24 virologic outcomes and determined predictors of lack of virologic suppression on protease-inhibitor based 2nd-line ART in a public sector HIV-infected treatment cohort in South Africa. Reported deaths were low and a majority of patients (83%) remained in care and on-ART at week-24. Despite this, a significant proportion of patients failed to achieve virologic suppression at week-24. Our data suggested that delays in switch to 2nd-line ART after the recognition of 1st-line ART failure and female sex may have contributed to these virologic outcomes.
Of individuals who switched to 2nd-line ART, we found that for every month waiting on failed 1st-line ART, there was a 7% increased risk of lack of virologic suppression in the primary analysis, a finding that was insensitive to wide thresholds in the definition of lack of virologic suppression. This observation likely relates to the negative impact on 2nd-line ART effectiveness of time-dependent acquisition of reverse transcriptase mutations during persistent HIV viremia and immunosuppression on 1st-line ART [21, 22], and is consistent with results from the Development of Antiretroviral Therapy in Africa Trial (DART) in Uganda and Zimbabwe, a trial of laboratory and clinical monitoring compared with clinically-driven monitoring. In the DART study, switching to 2nd-line ART was more frequent in the laboratory monitoring arm, which had a lower relative difference in death rates than the arm with clinical monitoring alone. While the absolute number of deaths in clinical monitoring arm was small, this finding also supports a negative impact in treatment outcomes from delayed switching from NNRTI-based 1st-line ART.
Heterogeneity in the calculation and definition of time to 1st-line ART failure and time to switch limits comparisons of the rate of treatment switching to 2nd-line ART among ART roll-out programs. Clinical and immunologic monitoring for detection of treatment failure predominates in resource-limited settings and likely impacts the longer intervals between treatment failure and switch in these programs compared with programs with virologic monitoring [23, 24].
The risks associated with delays in switching to 2nd-line ART were also reflected in the HIV-infected individuals who failed 1st-line ART but did not switch to 2nd-line ART within the follow-up period. This group had more advanced immunosuppression and had a longer time to detection of 1st-line ART failure compared with those who switched to 2nd-line ART. These observations are corroborated by an analysis of 11 HIV-treatment programs in nine countries in sub-Saharan Africa; in this study, patients who remained on failing 1st-line ART, compared with those who failed and switched to 2nd-line ART and those who never failed 1st-line ART, had greater immunosuppression and a higher cumulative 1-year mortality [25]. Together, these findings highlight the potential long-term clinical risks for individuals who remain on failing 1st-line ART.
There are patient-, clinician-, and clinic-level factors that may delay switch to 2nd-line ART after treatment failure. Patient-level factors include missed or delayed clinic visits or treatment preparedness sessions [26]. Clinic-level factors that impede clinic attendance and medication adherence include ART stock outs or inaccessible clinic location [27]. Pharmacy refill and supply data and clinic attendance records, which were unavailable for this analysis, can corroborate suspected medication non-adherence [28]. For individuals co-infected with HIV and tuberculosis and in settings where rifabutin is unavailable, clinicians may delay protease inhibitor-based 2nd-line ART until tuberculosis treatment is completed. This is because rifampin significantly lowers protease inhibitor levels through induction of metabolic enzymes and cellular drug transporters [29]. In this study setting and consistent with WHO guidelines, clinicians double the dose of lopinavir/kaletra to counteract the anticipated decrement in protease inhibitor levels by rifampin, rather than delay 2nd-line ART initiation [20]. Since elevated ritonavir levels can increase the risk of gastrointestinal side effects, this course of action may cause long-term tolerability issues [30]. This risk must be balanced against the benefits of integrated treatment of HIV and tuberculosis.
Women were more likely than men to switch to 2nd-line ART after 1st-line ART failure and they had a two-fold increased odds of lack of virologic suppression at week-24 on 2nd-line ART. While our study was not designed to examine predictors of sex-based differences in virologic outcomes, the large effect size of female sex on risk of lack of virologic suppression may recognize an important confluence of risk factors for ART failure: youth, female status, and adherence if women enter care at a younger age with competing social and financial responsibilities in addition to HIV care [15, 31, 32]. The large effect size persisted in sensitivity analyses on the definition of lack of virologic suppression. The non-significant statistical association at the higher HIV RNA thresholds may relate that more women had missing HIV RNA data at week-24.
Overall, we found a significant proportion of patients lacked virologic suppression (HIV RNA > 400 copies/ml). This finding was lower than other public sector cohorts in South Africa, Uganda, and Malawi on protease inhibitor-based 2nd-line ART who report ranges of suppression (defined as HIV RNA < 50 or 400 copies/ml) between 65–85% [23, 33–36]. Comparisons of ART effectiveness between clinical sites are limited by heterogeneity in patient populations, rates of loss to follow-up, monitoring strategies to detect ART failure, and definitions of treatment failure. One potential explanation for our finding of lower rates of 2nd-line ART effectiveness include the development of nucleoside resistance to zidovudine/didansoine compared with other sites that supplement the nucleoside backbone with the non-thymidine analogue, tenofivir [37, 38].
Furthermore, 2nd-line ART effectiveness was lower when compared to prior reports of virologic suppression on NNRTI-based 1st-line ART published from this and other South African cohorts [12, 39]. While this study was not intended to compare 2nd-line with 1st-line ART, this observation of lower 2nd-line ART effectiveness has been substantiated in the literature. A recent South Africa-based study compared patients switched to 2nd-line ART with matched controls on 1st-line ART. Here patients on 2nd-line ART were less likely to be alive and in-care than there 1st-line ART [33]. Possible explanations for this difference in clinical outcomes between 1st- and 2nd-line ART include poorer adherence on 2nd-line ART, as we found that of patients on 2nd-line ART, 25 patients (14%) did not experience a 1 log10 drop in HIV RNA within the first 6 months of 1st-line ART. We hypothesize that preexisting lopinavir resistance was not a significant contributor to 2nd-line ART effectiveness since a prior analysis of drug resistance mutations from this and other cohorts failing NNRTI-based combination 1st-line ART have reported negligible rates of major protease inhibitor resistance at failure of NNRTI-based 1st-line ART and at failure of lopinavir-ritonavir regimens in previously lopinavir-naïve patients [40, 41].
The strengths of this study were that virologic and immunologic data, which is uncommonly available in public health ART roll-out programs, here provides insight into outcomes on the rising population on 2nd-line ART. Patient characteristics at baseline and 1st-line ART failure are also comparable to other published cohorts with the majority of participants as women [23, 39, 42–47]. Thus, our findings may by generalizable to 2nd-line ART community-based cohorts in resource-limited populations both in South Africa and elsewhere.
There are several limitations to this study. Data on potential key socioeconomic determinants that affect gender-related outcomes such as neighborhood effects, employment, family structure and social support were not available for this analysis. We attempted to partially account for this by excluding patients transferred in to the clinic and the introduction of potential unmeasured patient-level differences. Potential confounding by indication also existed since patients were selected for 2nd-line ART in a non-random fashion. Odds ratios may overestimate effect size compared with relative risk at when the event size is large.
In summary, a substantial number of patients lacked virologic suppression on boosted-lopinavir in a large ART roll-out program in South Africa. Delays in switching to 2nd-line ART increased the odds of lack of virologic suppression. The impact of timing of treatment switch will likely depend on the potency of 2nd-line ART regimens, as well as on the increased availability of additional ART options. Routine laboratory monitoring with HIV RNA and targeted drug resistance testing may prevent the negative clinical impact of the delay in 2nd-line ART initiation.
Acknowledgments
The authors gratefully acknowledge the dedicated staff of the Hannan Crusaid ART clinic and the Desmond Tutu HIV Centre.
Financial Support
US National Institute of Allergy and Infectious Diseases (NIAID T32 AI007433, RO1 AI58072, K24 A1062476); Harvard Center for AIDS Research (P30-AI060354)
Footnotes
Author contributions include formulation of the research question (Levison, Freedberg, Losina, Orrell, Wood), design of the analytic plan (Levison, Freedberg, Losina, Wood), data collection (Orrell, Wood), and preparation (Levison) and critical editing (Levison, Freedberg, Losina, Yu Orrell, Wood) of the manuscript.
Conflicts of Interest Statement
No authors declare a conflict of interest.
References
- 1.World Health Organization. Progress on Global Access to HIV Antiretroviral Therapy A Report on "3×5" and Beyond. Geneva: World Health Organization; 2006. [Google Scholar]
- 2.World Health Organization. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report 2008. Geneva: WHO, UNICEF, UNAIDS; 2008. [Google Scholar]
- 3.World Health Organization. Prioritizing Second-Line Antiretroviral Drugs for Adults and Adolescents: A Public Health Approach. Geneva: World Health Organization, HIV Department; 2007. [Google Scholar]
- 4.Gallant JE. Drug resistance after failure of initial antiretroviral therapy in resource-limited countries. Clin Infect Dis. 2007;44:453–455. doi: 10.1086/510752. [DOI] [PubMed] [Google Scholar]
- 5.Boyd MA, Cooper DA. Second-line combination antiretroviral therapy in resource-limited settings: facing the challenges through clinical research. AIDS. 2007;21 Suppl 4:S55–S63. doi: 10.1097/01.aids.0000279707.01557.b2. [DOI] [PubMed] [Google Scholar]
- 6.Elliott JH, Lynen L, Calmy A, et al. Rational use of antiretroviral therapy in low-income and middle-income countries: optimizing regimen sequencing and switching. AIDS. 2008;22:2053–2067. doi: 10.1097/QAD.0b013e328309520d. [DOI] [PubMed] [Google Scholar]
- 7.Long L, Fox M, Sanne I, Rosen S. The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS. 2010;24:915–919. doi: 10.1097/QAD.0b013e3283360976. [DOI] [PubMed] [Google Scholar]
- 8.Humphreys EH, Hernandez LB, Rutherford GW. Antiretroviral regimens for patients with HIV who fail first-line antiretroviral therapy. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD006517.pub2. CD006517. [DOI] [PubMed] [Google Scholar]
- 9.Petersen ML, van der Laan MJ, Napravnik S, et al. Long-term consequences of the delay between virologic failure of highly active antiretroviral therapy and regimen modification. AIDS. 2008;22:2097–2106. doi: 10.1097/QAD.0b013e32830f97e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riddler SA, Jiang H, Tenorio A, et al. A randomized study of antiviral medication switch at lower- versus higher-switch thresholds: AIDS Clinical Trials Group Study A5115. Antivir Ther. 2007;12:531–541. doi: 10.1177/135965350701200415. [DOI] [PubMed] [Google Scholar]
- 11.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 12.Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Gugulethu, South Africa. S Afr Med J. 2006;96:315–320. [PubMed] [Google Scholar]
- 13.Lawn SD, Myer L, Harling G, et al. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 14.Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther. 2007;12:83–88. [PubMed] [Google Scholar]
- 15.Cornell M, Myer L, Kaplan R, Bekker LG, Wood R. The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Int Health. 2009;17:722–731. doi: 10.1111/j.1365-3156.2009.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Treat 3 million by 2005: Scaling up antiretroviral therapy in resource limited settings. A public health approach. World Health Organization; 2004. [Google Scholar]
- 17.Department of Health South Africa. National Antiretroviral Treatment Guidelines. Jacana: 2004. [Google Scholar]
- 18.Orrell C, Bekker LG, Wood R. Adherence to antiretroviral therapy--achievable in the South African context? S Afr Med J. 2001;91:483–484. [PubMed] [Google Scholar]
- 19.World Health Organization. Rapid Advice: Antiretroviral therapy for HIV infection in adults and adolescents. Geneva: 2009. [PubMed] [Google Scholar]
- 20.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach (2006 revision) Geneva: 2006. [PubMed] [Google Scholar]
- 21.Gupta RK, Pillay D. HIV resistance and the developing world. Int J Antimicrob Agents. 2007;29:510–517. doi: 10.1016/j.ijantimicag.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Seyler C, Adje-Toure C, Messou E, et al. Impact of genotypic drug resistance mutations on clinical and immunological outcomes in HIV-infected adults on HAART in West Africa. AIDS. 2007;21:1157–1164. doi: 10.1097/QAD.0b013e3281c615da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pujades-Rodriguez M, O'Brien D, Humblet P, Calmy A. Second-line antiretroviral therapy in resource-limited settings: the experience of Medecins Sans Frontieres. AIDS. 2008;22:1305–1312. doi: 10.1097/QAD.0b013e3282fa75b9. [DOI] [PubMed] [Google Scholar]
- 24.ART-LINC of IeDEA Study Group. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009;23:1867–1874. doi: 10.1097/QAD.0b013e32832e05b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keiser O, Tweya H, Braitstein P, et al. Mortality after failure of antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health. 2009;15:251–258. doi: 10.1111/j.1365-3156.2009.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller CM, Ketlhapile M, Rybasack-Smith H, Rosen S. Why are antiretroviral treatment patients lost to follow-up? A qualitative study from South Africa. Trop Med Int Health. 15 Suppl 1:48–54. doi: 10.1111/j.1365-3156.2010.02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasquet A, Messou E, Gabillard D, et al. Impact of drug stock-outs on death and retention to care among HIV-infected patients on combination antiretroviral therapy in Abidjan, Cote d'Ivoire. PLoS One. 5:e13414. doi: 10.1371/journal.pone.0013414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalker J, Wagner A, Tomson G, et al. Urgent need for coordination in adopting standardized antiretroviral adherence performance indicators. J Acquir Immune Defic Syndr. 53:159–161. doi: 10.1097/QAI.0b013e3181befa12. [DOI] [PubMed] [Google Scholar]
- 29.Maartens G, Decloedt E, Cohen K. Effectiveness and safety of antiretrovirals with rifampicin: crucial issues for high-burden countries. Antivir Ther. 2009;14:1039–1043. doi: 10.3851/IMP1455. [DOI] [PubMed] [Google Scholar]
- 30.L'Homme RF, Nijland HM, Gras L, et al. Clinical experience with the combined use of lopinavir/ritonavir and rifampicin. AIDS. 2009;23:863–865. doi: 10.1097/QAD.0b013e328329148e. [DOI] [PubMed] [Google Scholar]
- 31.Orrell C, Bangsberg DR, Badri M, Wood R. Adherence is not a barrier to successful antiretroviral therapy in South Africa. AIDS. 2003;17:1369–1375. doi: 10.1097/00002030-200306130-00011. [DOI] [PubMed] [Google Scholar]
- 32.Charles M, Noel F, Leger P, et al. Survival, plasma HIV-1 RNA concentrations and drug resistance in HIV-1-infected Haitian adolescents and young adults on antiretrovirals. Bull World Health Organ. 2008;86:970–977. doi: 10.2471/BLT.07.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox MP, Ive P, Long L, Maskew M, Sanne I. High Rates of Survival, Immune Reconstitution and Virologic Suppression on Second-Line Antiretroviral Therapy in South Africa. J Acquir Immune Defic Syndr. 2009 doi: 10.1097/QAI.0b013e3181bcdac1. [DOI] [PubMed] [Google Scholar]
- 34.Murphy RA, Sunpath H, Lu Z, et al. Outcomes after virologic failure of first-line ART in South Africa. AIDS. 2010;24:1007–1012. doi: 10.1097/QAD.0b013e3283333639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castelnuovo B, John L, Lutwama F, et al. Three-year outcome data of second-line antiretroviral therapy in Ugandan adults: good virological response but high rate of toxicity. J Int Assoc Physicians AIDS Care. 2009;8:52–59. doi: 10.1177/1545109708328538. [DOI] [PubMed] [Google Scholar]
- 36.Hosseinipour MC, Kumwenda JJ, Weigel R, et al. Second-line treatment in the Malawi antiretroviral programme: high early mortality, but good outcomes in survivors, despite extensive drug resistance at baseline. HIV Med. 2010 doi: 10.1111/j.1468-1293.2010.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White KL, Chen JM, Feng JY, et al. The K65R reverse transcriptase mutation in HIV-1 reverses the excision phenotype of zidovudine resistance mutations. Antivir Ther. 2006;11:155–163. doi: 10.1177/135965350601100209. [DOI] [PubMed] [Google Scholar]
- 38.Sungkanuparph S, Manosuthi W, Kiertiburanakul S, et al. Options for a second-line antiretroviral regimen for HIV type 1-infected patients whose initial regimen of a fixed-dose combination of stavudine, lamivudine, and nevirapine fails. Clin Infect Dis. 2007;44:447–452. doi: 10.1086/510745. [DOI] [PubMed] [Google Scholar]
- 39.Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 40.Orrell C, Walensky RP, Losina E, et al. HIV type-1 clade C resistance genotypes in treatment-naive patients and after first virological failure in a large community antiretroviral therapy programme. Antivir Ther. 2009;14:523–531. [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta R, Hill A, Sawyer AW, Pillay D. Emergence of drug resistance in HIV type 1-infected patients after receipt of first-line highly active antiretroviral therapy: a systematic review of clinical trials. Clin Infect Dis. 2008;47:712–722. doi: 10.1086/590943. [DOI] [PubMed] [Google Scholar]
- 42.Virology Group and Trial Team D. Virological response to a triple nucleoside/nucleotide analogue regimen over 48 weeks in HIV-1-infected adults in Africa. AIDS. 2006;20:1391–1399. doi: 10.1097/01.aids.0000233572.59522.45. [DOI] [PubMed] [Google Scholar]
- 43.Marconi VC, Sunpath H, Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008;46:1589–1597. doi: 10.1086/587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosseinipour MC, van Oosterhout JJ, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009;23:1127–1134. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muula AS, Ngulube TJ, Siziya S, et al. Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic review. BMC Public Health. 2007;7:63. doi: 10.1186/1471-2458-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 47.Keiser O, Anastos K, Schechter M, et al. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13:870–879. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]