Abstract
The broad-spectrum benzoxaborole antifungal AN2690 blocks protein synthesis by inhibiting leucyl-tRNA synthetase (LeuRS) via a novel oxaborole tRNA trapping mechanism in the editing site. Herein, one set of resistance mutations is at Asp487 outside the LeuRS hydrolytic editing pocket, in a region of unknown function. It is located within a eukaryote/archaea specific insert I4, which forms part of a cap over a benzoxaborole-AMP that is bound in the LeuRS CP1 domain editing active site. Mutational and biochemical analysis at Asp487 identified a salt bridge between Asp487 and Arg316 in the hinge region of the I4 cap of yeast LeuRS that is critical for tRNA deacylation. We hypothesize that this electrostatic interaction stabilizes the cap during binding of the editing substrate for hydrolysis.
Keywords: AN2690, drug resistance, fidelity, translation, antibiotic
1. Introduction
Aminoacyl-tRNA synthetases (AARSs) are essential to all organisms and have been pursued as promising pharmaceutical drug targets [1]. Pseudomonic acid (Mupirocin) has been used for years as a topical antibiotic to selectively inhibit isoleucyl-tRNA synthetase (IleRS) via interactions with its aminoacylation active site. Recently, a novel benzoxaborole called AN2690 was discovered to block protein synthesis in yeast by binding to the editing site of cytoplasmic LeuRS [2].
Each of the AARSs establishes the genetic code via an aminoacylation reaction that attaches a specific amino acid to its cognate tRNA, which is then delivered to the ribosome. To enhance fidelity, about half of the AARSs have evolved ‘editing’ or ‘proofreading’ mechanisms [3]. A ‘post-transfer’ editing mechanism hydrolyzes the misacylated tRNA to release free uncharged tRNA and amino acid. This correction mechanism is carried out by AARSs that have hydrolytic domains or by free-standing tRNA deacylases [4, 5]. Alternatively, ‘pre-transfer’ editing clears misactivated aminoacyl-adenylate [6].
The class 1 AARS canonical core of LeuRS houses the synthetic aminoacylation site. A discretely folded polypeptide insertion, called the connective polypeptide 1 (CP1) [7] contains the hydrolytic editing active site [8]. The antifungal AN2690 forms an adduct to tRNALeu in the editing pocket to trap the enzyme in a non-productive conformation (Fig. 1). A series of AN2690-resistant mutations were isolated within the CP1 domain of yeast LeuRS that localized to the hydrolytic editing active site [2]. Here, we focused on two resistance mutations at Asp487 that lie outside the hydrolytic editing active site in LeuRS, where AN2690 (Fig. 1) binds. This resistance site is downstream of conserved motifs in the primary sequence that define the editing pocket.
Figure 1.
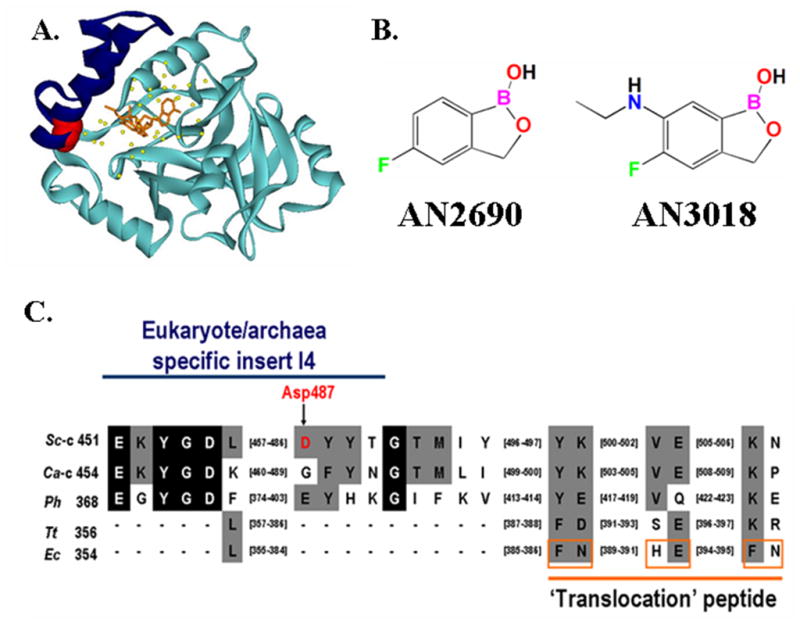
Primary and tertiary structures of LeuRS I4 ‘cap’ insert within the CP1 editing domain. (A) Crystal structure of Candida albicans cytoplasmic LeuRS CP1 domain in complex with AN3018-AMP (orange) (PDB entry 2WFG) [9]. The I4 insert (Glu454 to Asn493 in Candida albicans) is highlighted in blue with Gly490 (which corresponds to the Asp487 in yeast cytoplasmic LeuRS) shown in red. Yellow balls denote ordered water molecules in the editing pocket. (B) Chemical structures of AN2690 and AN3018. (C) Multiple sequence alignment of the I4 insert within the LeuRS CP1 domain. The blue line highlights the I4 insert. The Asp487 in yeast LeuRS is indicated in red and by the arrow. The orange line indicates the putative ‘translocation’ peptide proposed in Escherichia coli LeuRS [14]. The residues boxed in orange within this peptide are proposed to impair translocation. Abbreviations are: Thermus thermophilus (Tt), E. coli (Ec), S. cerevisiae cytoplasmic (Sc-c), C. albicans cytoplasmic (Ca-c) and P. horikoshii (Ph).
Sequence alignments show that Asp487 is located within an insert called I4 that is specifically found in eukaryotes and archae, but is absent in the bacteria (Fig. 1C). An X-ray crystal structure of the CP1 domain from C. albicans LeuRS suggests that the I4 insert forms part of a cap over the hydrolytic active site [9]. When closed, the I4 cap in the C. albicans structure sequesters an AN2690-benzoxaborole analog called AN3018 (Fig. 1) that is covalently linked to AMP. In this case, the AMP portion of the bound small molecule is proposed to mimic the 3’ end of a tRNA molecule that is bound in the editing site [9].
Since the benzoxaborole binds in the editing site where the mischarged tRNA editing substrate binds in LeuRS [2, 10], we postulated that resistance mutations at Asp487 in the I4 cap would impact hydrolytic editing. Biochemical analysis of mutations at Asp487 showed that a negatively charged residue was essential to hydrolytic editing activity. We hypothesize that Asp487 plays a critical role in facilitating the I4 cap closure over the editing active pocket during substrate binding. Our results support that electrostatic interactions between Asp487 and an arginine residue within the editing pocket facilitates the lid-like orientation of the I4 cap to stabilize bound substrate and promote editing.
2. Materials and Methods
Crude Saccharomyces cerevisiae tRNA was acquired from Sigma Chemical Co. (St Louis, MO) and deacylated by incubation in 100 mM Tris, pH 8.5 at 37 oC for 1 h. The concentration of chargeable tRNALeu in crude (total) yeast tRNA was measured based on the plateau or maximal charging levels using standard aminoacylation assays as described below to determine that 1 mg/mL crude (total) tRNA contained 400 nM chargeable tRNALeu.
Mutations that are resistant to AN2690 were selected using the haploid S. cerevisiae strain (ATCC 201388) via methods that have been previously described [2]. Spontaneous ethylmethane sulfonate (EMS)-induced resistant mutants were isolated from YPD agar plates that contained concentrations of 2 μg/mL, 4 μg/mL, and 8 μg/mL AN2690.
Site directed mutagenesis was carried out via the polymerase chain reaction (PCR). The plasmid p32YL-2-3 [10] encoding the wild type S. cerevisiae cytoplasmic LeuRS was used as the template to introduce the desired mutations to generate the plasmids pJSycD487G , pJSycD487N, pJSycD487A, pJSycD487K, pJSycD487R and pJSycD487E encoding the mutant D487G, D487N, D487A, D487K, D487R and D487E LeuRS enzymes, respectively. Plasmids pJSycD487G, pJSycD487N and pJSycD487A were then used as templates in separate PCR reactions to introduce a second T319A mutation in each mutant protein. This created the plasmids pJSycD487G/T319A, pJSycD487N/T319A and pJSycD487A/T319A, encoding the double mutant D487G/T319A, D487N/T319A and D487A/T319A LeuRSs respectively. Plasmid pJSycD487K was utilized as the template to introduce the second mutation R316D or R316E generating the mutant D487K/R316D or D487K/R316E LeuRSs, respectively. Similarly, plasmid pJSycD487R was utilized as the template to introduce the second mutation R316D or R316E generating the mutant D487R/R316D or D487R/R316E LeuRSs, respectively. The N-terminal six-histidine tagged wild type and mutant yeast cytoplasmic LeuRSs were expressed in E. coli strain Rosetta (DE3) (Novagen) and purified via metal affinity chromatography [10]. The final protein concentration was determined spectrophotometrically at 280 nm using an extinction coefficient of 155,310 M−1cm−1, estimated by the ExPASy Protparam tool (http://ca.expasy.org/tools/protparam.html).
The aminoacylation reactions contained 60 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.5, 30 mM MgCl2, 30 mM KCl, 1 mM dithiothreitol (DTT), 4 mg/mL S. cerevisiae crude tRNA, 21 μM [3H]-leucine (150 μCi/mL) and catalytic concentrations of enzyme. Misaminoacylation reactions were carried out similarly, except that the reaction mixture contained higher concentrations of 23.5 μM [3H]-isoleucine (150 μCi/mL) and 1 μM enzyme. Each reaction was initiated with 4 mM ATP. At desired time points, reaction aliquots of 10 μL were quenched and processed as described previously [8]. Kinetic parameters for the aminoacylation reaction were measured using 0.5 μM enzyme. A relatively high enzyme concentration was used for the kinetic assays due to low activity of the yeast LeuRS enzyme at lower concentrations. A range of 0.1 to 100 mg/mL crude yeast tRNA (Sigma) was used which corresponded to 0.05 to 40 μM tRNALeu. The ‘Enzyme Kinetics analysis’ module of Sigma Plot (Systat Software, Inc.) was used to calculate the kinetic parameters and error.
Crude tRNA from S. cerevisiae (8 mg/mL) was misaminoacylated with isoleucine by incubating the following reaction mixture at 30 °C for 3 h in 60 mM HEPES, pH 7.5, 30 mM MgCl2, 30 mM KCl, 1 mM DTT, 50 μM [14C]-isoleucine (15.9 μCi/mL) or 25 μM [3H]-isoleucine (500 μCi/mL), 1 to 5 μM editing defective LeuRS and 4 mM ATP. Mischarged Ile-tRNALeu was isolated as described previously [8]. Deacylation reactions were carried out at pH 7.5 in 60 mM Tris or HEPES, 10 mM MgCl2, 10 mM KCl, and approximately 2 mg/mL [14C]-Ile-tRNALeu or [3H]-Ile-tRNALeu. The reactions were initiated with 0.5 μM enzyme and reaction aliquots of 5 μL were quenched, processed, and analyzed as described above.
Inorganic pyrophosphate (PPi) exchange assays were carried out with reaction mixtures containing 50 mM HEPES, pH 8.0, 10 mM MgCl2, 10 mM KCl, 1 mM DTT, 1 mM [32P]-PPi (100 μCi/mL), 1 mM ATP, and 1 mM leucine or 10 mM isoleucine were initiated with 1 μM enzyme. Aliquots of 2 μL at specific time points were analyzed via thin-layer chromatography [8].
3. RESULTS
The antifungal LeuRS inhibitor, AN2690 binds in the CP1 domain-based editing active site of LeuRS [2]. The boron atom of AN2690 forms bonds with the cis-diol groups of the ribose ring at the 3′ end of tRNALeu to yield a tRNA-AN2690 adduct in the editing site of LeuRS [2] (Fig. 1). The AN2690-tRNA-protein complex prevents enzyme turnover, ultimately leading to an arrest in protein synthesis.
In yeast LeuRS, spontaneous resistant mutations were isolated in the presence of concentrations of AN2690 that were 4-, 8-, and 16-fold of its 0.5 μg/mL minimal inhibitory concentration (MIC) [2]. The mutants were dominant and showed an 8 to 64-fold increase in the yeast MIC. As would be expected based on target interactions, the mutant LeuRSs lacked resistance to several other known antifungal agents including amphotericin B, cerulenin, itraconazole, aculeacin A, terbinafine, tunicamycin, ciclopirox, cyclohexamide, and nikkomycin Z (data not shown).
Previously, AN2690 spontaneous-resistance mutants were localized to the editing active site of yeast LeuRS, where AN2690 binds directly [2]. Herein, we focused on missense mutations to Asp487 where mutants with a cytoplasmic LeuRS bearing a glycine (D487G) or asparagine (D487N) substitution conferred an increase in the MIC of AN2690 to 32 μg/mL compared to 0.5 μg/mL for the wild-type strain. Because Asp487 is at a site that is distal to the hydrolytic editing active site, its effect on editing or alternate mechanisms of action on the target LeuRS were not clear. The Asp487 site is separated from the primary sequence that defines the editing pocket/AN2690 binding site and it is located in an insert called I4 that is specific to the archaeal and eukaryotic enzymes (Fig. 1).
Comparison of the apo crystal structure of the isolated CP1 domain from Candida albicans LeuRS and the co-crystal structure containing AN3018, an analog of AN2690 (Fig. 1B), [9] suggests that the yeast/archeal-specific I4 helix is flexible and can close in a ‘cap’ like fashion over the editing site that is bound to the benzoxaborale inhibitor. The space enclosed over the editing pocket by this I4 ‘cap’ accommodates both the substrate and a fixed network of water molecules. We hypothesized that Asp487 of the I4 insert orients the cap for substrate binding and stabilization in the editing site. Halo assays that incorporated 20 μL of norvaline (40 mg/mL) in the central well [2] support that the LeuRS D487G and D487N are editing defective resulting in norvaline toxicities (Fig. 2).
Figure 2.
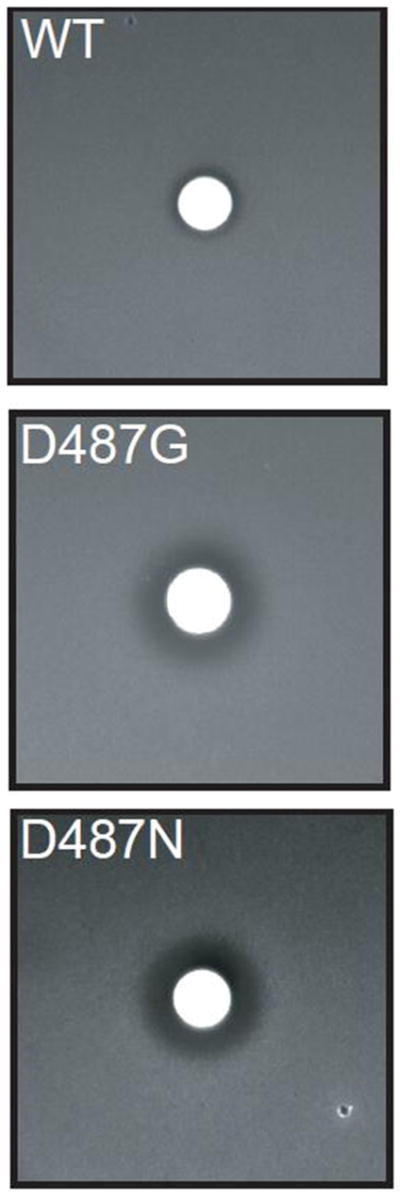
Norvaline sensitivity of AN2690 resistant mutations at Asp487. The parental S. cerevisiae strain ATCC 201388 [2] is labeled wild type (WT). The mutations D487G and D487N correspond to the S. cerevisiae resistant strains ANA325 and ANA359, respectively. The ‘halo’ of cell death around the central well, which incorporates 20 μL of norvaline (40 mg/mL), indicates that the resistant mutants are editing defective.
We re-created the AN2690 resistant mutations, D487G and D487N, as well as a D487A mutation in the yeast cytoplasmic LeuRS. To test the importance of a charged polar side chain in the I4 insert, we also replaced Asp487 with lysine, arginine and glutamic acid, generating the positively charged mutant D487K and D487R as well as the homologous mutant D487E LeuRSs, respectively. Leucine-dependent PPi exchange assays showed that the resistant mutants, D487G and D487N (Fig. S1A) as well as the positively charged D487K and D487R LeuRS mutants (Fig. S1B) activate cognate leucine similar to the wild type enzyme. Based on its lowered plateau, the D487A mutant LeuRS exhibited slightly reduced ATP formation compared to the wild type enzyme (Fig. S1A).
We tested each yeast mutant LeuRS using commercially available crude yeast tRNA, since we previously determined that an in vitro generated yeast tRNALeu transcript is a very poor substrate for its cognate LeuRS [10]. We hypothesize that modifications are critical in stabilizing the structure of yeast tRNALeu. Alternatively, they may provide an important determinant for aminoacylation by yeast LeuRS [11]. These mutant LeuRSs aminoacylate tRNALeu (Fig. S1C, D) with approximately 2-fold reduction in kcat/KM values (Table 1) as compared to the wild type enzyme. The decrease in the kcat/KM for the D487A and the positively charged D487 mutant LeuRSs is primarily due to an increase in KM (Table 1).
Table 1.
Apparent kinetic parameters for tRNALeu aminoacylation.1
| LeuRS | KM (μM) | kcat (sec−1) | kcat/KM (μM−1sec−1) |
|---|---|---|---|
| Wild type | 0.6 ± 0.05 | 0.4 ± 0.01 | 0.7 ± 0.05 |
| D487G | 0.7 ± 0.10 | 0.2 ± 0.06 | 0.3 ± 0.10 |
| D487N | 0.4 ± 0.06 | 0.1 ± 0.01 | 0.3 ± 0.01 |
| D487A | 3.5 ± 0.8 | 0.3 ± 0.1 | 0.1 ± 0.01 |
| D487K | 1.2 ± 0.2 | 0.2 ± 0.01 | 0.2 ± 0.06 |
| D487R | 2.7 ± 1.2 | 0.3 ± 0.1 | 0.1 ± 0.06 |
tRNALeu concentrations were varied as described in the Materials and Methods.
The Asp487 mutant LeuRSs were tested for their amino acid editing activity with yeast crude tRNA that was misaminoacylated with isoleucine using an editing-defective LeuRS. The AN2690-resistant mutants, D487G, D487N and also the D487A mutant exhibited significantly reduced deacylation activity for Ile-tRNALeu (Fig. 3A). The positively charged Asp487 mutant LeuRSs are completely defective in hydrolyzing misacylated tRNA (Fig. 3B). Interestingly however, a homologous substitution at Asp487 by glutamic acid retains substantial editing activity (Fig. 3B). Also as expected, the aminoacylation activities of the LeuRS Asp487 resistance mutants were not inhibited by AN2690 (Fig. S2). The mechanism of action for AN2690 inhibition requires tRNALeu binding in the editing active site for it to be trapped by the benzoxaborole.
Figure 3.
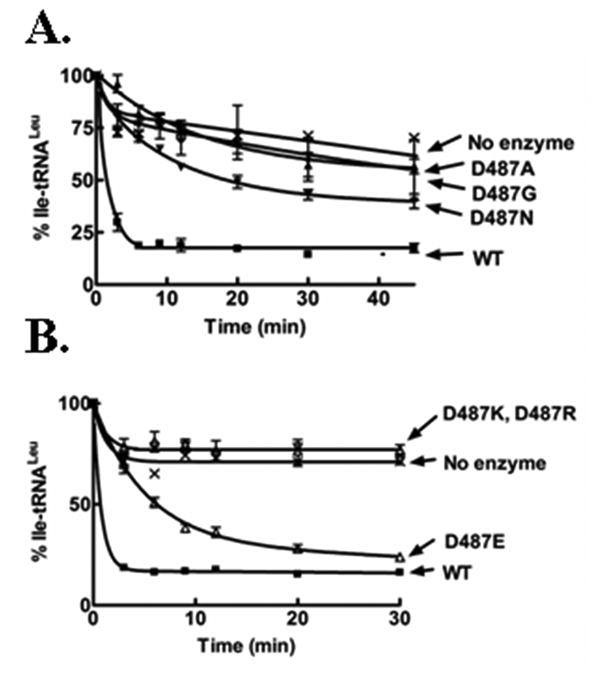
Isoleucine editing activities of AN2690-resistant and charged Asp487 mutants of LeuRS. Deacylation reactions included approximately 2 mg/ml [14C]-Ile-tRNAleu and 0.5 μM enzyme. Symbols are: wild type (■), D487G (▲), D487N (▼), D487A (◆), D487K (□), D487R (◇), D487E (△) and no enzyme (x). Error bars for each time point are a result of each reaction repeated in triplicate.
The yeast cytoplasmic Asp487 LeuRS mutant editing deficiencies resulted in low accumulations of mischarged Ile-tRNALeu in misaminoacylation assays using crude yeast tRNA (Fig. S3A, B). These mischarging plateaus are about 8-fold lower than a strong mischarging LeuRS mutant, such as D419A (data not shown) which substitutes a universally conserved residue in the editing pocket that stabilizes the editing substrate via a hydrogen bond [10] However, these Asp487 LeuRS mutants exhibited similar activation of isoleucine as compared to the wild type enzyme in isoleucine-dependent PPi exchange assays (Fig. S3C, D). As would be expected, the wild type yeast LeuRS did not mischarge the crude yeast tRNA with isoleucine since LeuRS, like all other AARSs, is specific for its cognate tRNA. Recognition of tRNALeu by LeuRS is facilitated by specific determinants found within the tRNA [11] as well as by tertiary structure formed between the D and TΨC-arms of tRNA [12]. The yeast cytoplasmic enzyme is also unusual amongst the LeuRSs in that it relies on the anticodon of tRNALeu for recognition [13].
Since a homologous substitution at Asp487 could restore the enzyme’s hydrolytic activity, we wondered how this negative charge that is outside the editing pocket contributed towards amino acid editing. We scrutinized the X-ray crystal structure of the C. albicans CP1 domain in complex with the benzoxaborale AN3018 [9]. The C. albicans LeuRS CP1 domain has a glycine residue (Gly490) at the position comparable to Asp487 in S. cerevisiae LeuRS (Fig. 1C). Ironically, a glycine at this site renders the yeast LeuRS enzyme resistant to AN2690. Nevertheless, we relied on the AN3018-bound C. albicans LeuRS structure as an opportunity to guide our investigation into the structure-function role of Asp487 in editing by yeast LeuRS.
When the I4 cap is closed over the AN3018-AMP bound editing pocket in the X-ray crystal structure of the C. albicans LeuRS CP1 domain, the α-carbon of Gly490 is in close proximity (~4 Å) to the guanidinium group of the arginine at position 318 (Fig. 4A). We identified Arg316 in S. cerevisiae LeuRS, (corresponding to Arg318 of C. albicans LeuRS in the primary sequence) and hypothesized that it was also located within or near the editing pocket. Interestingly, R316I has been reported to be resistant to AN2690 in S. cerevisiae [2]. Although the yeast LeuRS Asp487 has a longer side chain than the Gly 490 found in C. albicans LeuRS that could place it closer than 4 Å to the arginine, we wondered if local rearrangements within the dynamic and diverse structures might accommodate a salt bridge between Asp487 and Arg316 in the yeast enzyme. If Asp487 at the base of the I4 cap forms an electrostatic salt-bridge with Arg316 of the yeast LeuRS editing pocket, then we postulated that it could stabilize closure of the I4 cap to sequester the bound substrate in the editing pocket.
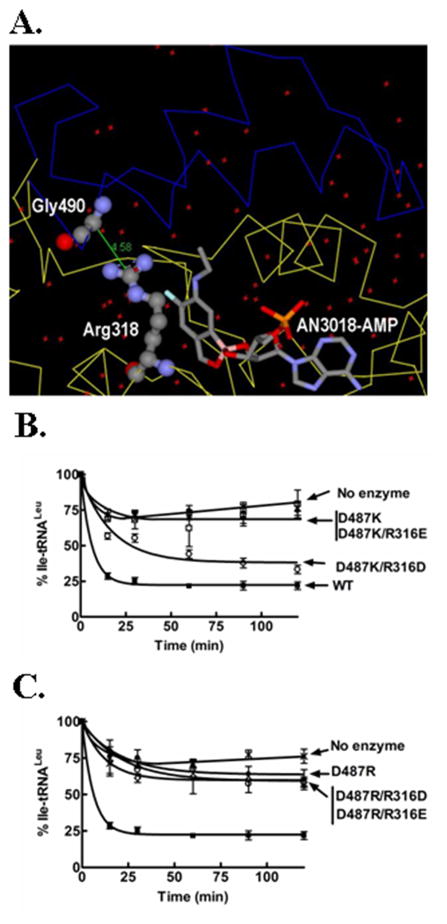
Figure 4.
Putative electrostatic interaction between the I4 insert and the editing pocket of fungal cytoplasmic LeuRS. (A) Crystal structure of C. albicans cytoplasmic LeuRS CP1 domain in complex with AN3018-AMP (PDB entry 2WFG) [9], highlighting the I4 insert (blue), Gly490 in the I4 insert and Arg318 in the editing pocket (analogous to Asp487 and Arg316, respectively in yeast LeuRS). (B)-(C) Hydrolytic editing activities of single charged Asp487 mutants and Asp487/Arg316 double mutants. Deacylation reactions included approximately 2 mg/ml [14C]-Ile-tRNAleu and 0.5 μM enzyme. Symbols are: wild type (■), D487K (□), D487R (◇), D487K/R316D and D487R/R316D (○), D487K/R316E and D487R/R316E (●) and no enzyme (x). Error bars for each time point are a result of each reaction repeated in triplicate.
To test this hypothesis, we mutationally swapped charges at Asp487 and Arg316 to construct the double mutants D487K/R316D, D487K/R316E and D487R/R316D, D487R/R316E. The D487K/R316D double mutant rescued hydrolytic activity of the editing-defective positively-charged D487K mutant to levels that were close to the wild type enzyme (Fig. 4B). The D487K/R316E double mutant did not recover any hydrolytic editing activity (Fig. 4B). We also observed that the double mutants D487R/R316D and D487R/R316E did not rescue hydrolytic editing of the editing-defective D487R mutant LeuRS (Fig. 4C). This suggests that the longer side chains of glutamic acid and arginine may juxtapose the residues too close to form an effective electrostatic interaction. In lieu of an aspartate corresponding to position 487 in C. albicans LeuRS, (Fig. 1C), the enzyme may depend on alternate residues to form an electrostatic bridge. It is possible that Glu489, which neighbors Gly490 (Fig. 1C), could form a salt bridge with Arg318.
Post-transfer editing is dependent on an intra-enzyme translocation event that moves the tRNA between the main body and the CP1 domain that are respectively responsible for aminoacylation and editing. Mutations in the E. coli LeuRS ‘translocation peptide’ (Fig. 1C) appear to cause the mischarged tRNA to bypass the editing active site [14]. The eukaryotic LeuRSs lack this bacterial LeuRS translocation peptide. However, in a primary sequence alignment, Asp487 of the yeast cytoplasmic LeuRS is upstream to the N-terminus of the site that would correspond to the E. coli LeuRS translocation peptide. We wondered if the Asp487 region of the I4 cap could facilitate tRNA translocation via a distinct mechanism. Previously, to test for disruptions in translocation, we relied on a conserved threonine in E. coli LeuRS (Thr252) which is an amino acid specificity determinant in the editing pocket that blocks leucine from binding [8]. When mutated to alanine, loss of specificity confers deacylation of Leu-tRNALeu, resulting in a phenotype of weak charging activity for the T252A mutant LeuRS. Disruption of translocation would restore Leu-tRNALeu accumulation in the LeuRS mutant.
Mutation of the threonine specificity determinant in yeast LeuRS (Thr319) also results in low charging activity (Fig. 5). We combined each of the resistant mutations, D487G and D487N, with the editing site mutation T319A that uncouples specificity in the editing site of yeast LeuRS, resulting in the double mutants D487G/T319A and D487N/T319A, respectively. The Asp487 double mutants rescued aminoacylation of the T319A mutant, resulting in accumulation of Leu-tRNALeu up to wild type levels (Fig. 5). In this case, it is possible that the charged 3′-end of tRNALeu may not be stably binding at the editing pocket or alternatively, has reduced access to the editing site. We determined that the hydrolytic editing activity of the Asp487/Thr319 double mutants was significantly decreased for the correctly charged product Leu-tRNALeu (Fig. S4A) as well as the mischarged product Ile-tRNALeu (Fig. S4B) in comparison to the single T319A mutant. These results suggest that mutation of Asp487 destabilizes binding of the 3′ end of the tRNA in the editing pocket of the CP1 domain, rather than disrupting a tRNA translocation mechanism.
Figure 5.
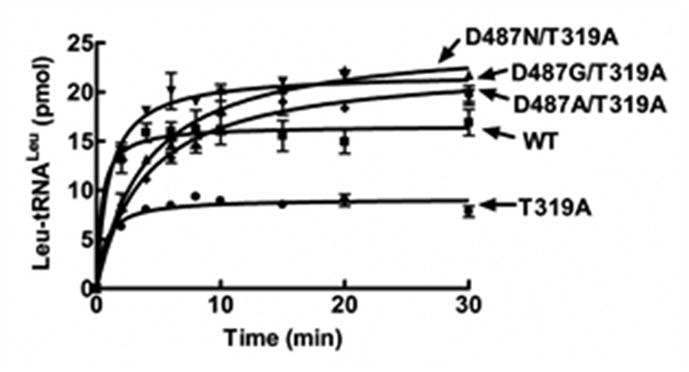
Rescue of aminoacylation of editing site mutation (T319A) by Asp487 LeuRS mutants. Reactions consisted of 4 mg/ml S. cerevisiae crude tRNA, 0.5 μM enzyme and 22 μM [3H]-leucine (150 μCi/ml) and were initiated with 4 mM ATP. Symbols are: wild type (■), D487G/T319A (▲), D487N/T319A (▼), D487A/T319A (◆), T319A (●) and no enzyme (x). Error bars for respective time points represent each reaction repeated in triplicate.
4. Discussion
The AARS family of enzymes by virtue of their essentiality and diversity, have been a focus of drug discovery to identify new antimicrobial agents. A traditional strategy has been to find compounds that selectively mimic the pathogen AARS substrates, intermediates and transition state analogs, while sparing the human counterpart [1]. By screening libraries of synthetic boron-containing small molecules, a new class of antifungals, the benzoxaboroles, were discovered [15]. These antifungal benzoxaboroles inhibited LeuRS in a novel way by binding in the editing pocket of the CP1 domain and trapping the tRNA in the editing conformation via an adduct with the cis-diols of the terminal nucleotide [2, 9].
Isolation of spontaneous and EMS-generated resistant mutants to AN2690 have been reported within the editing pocket of LeuRS [2], where the drug interacts directly with the enzyme. Each of these resistant mutants is editing defective in norvaline-dependent complementation assays [2]. Herein, we characterized resistant mutants D487G and D487N, which lie outside the editing active site. The C. albicans and yeast cytoplasmic CP1 domain have acquired species-specific peripheral insertions, one of which (I4) appears to form a cap over the bound editing substrate (Fig. 1A). The Asp487 lies within the I4 peripheral insert. The crystal structure of the C. albicans CP1 domain shows that a network of water molecules, as well as the editing substrate, are sequestered within the space enclosed by the I4 cap over the editing pocket [9]. We hypothesize that Asp487 in the species-specific I4 insert of the yeast LeuRS could be mechanistically important to the cap’s hinge motion.
Resistance mutations highlighted that this I4 insert may be idiosyncratic, albeit an essential component of the editing mechanism in LeuRS for lower eukaryotes. Mutations at Asp487 in yeast LeuRS directly impact editing with minimal effects on aminoacylation. Charge reversals at Asp487 completely destabilize the editing activity. We scrutinized the available crystal structure information to identify a plausible electrostatic salt bridge interaction between Asp487 at the base of the I4 cap and Arg316 in the editing pocket. Significantly, a double mutation to reverse these charges rescued editing activity of the D487K LeuRS mutant, which suggests that the salt bridge was restored.
There are relatively few synthetase-tRNA contacts in the editing complex compared to the aminoacylation complex [16]. In addition, the yeast LeuRS active site has been suggested to be more open and accessible to water compared to its bacterial counterpart that lacks the I4 insert The I4 cap and this salt bridge between the cap and the editing active site could stabilize substrate and inhibitor binding in a more open editing site, as well as orient enclosed water molecules. Ultimately, AN2690 crosslinks to the tRNA in the editing site resulting in a long half-life. [2]. Although the fit of the AMP-benzoxaborole adduct in the editing pocket of LeuRS is complementary, only a few hydrogen bonds within the LeuRS editing active site stabilize it [2]. The AMP-benzoxaborole adduct is superimposable with a post-transfer editing substrate analog [2]. It also directly contacts the universally conserved aspartic acid residue (Asp419 in yeast LeuRS) in the editing active site that stabilizes the post-transfer editing substrate via a hydrogen bond with the α-amino group of the amino acid. However, the benzoxaborole adduct lacks a key site analogous to the amino group of an aminoacylated tRNA. Thus, a cap over the editing pocket could significantly and critically stabilize the bound editing substrate by providing or orienting either direct or water-mediated contacts between the enzyme and the substrate.
We hypothesize that, in the Asp487 mutants, stabilization of the charged 3′ end of the tRNA is compromised. De-stabilization of the bound substrate (the inhibitor or the charged 3′ end of the tRNA) at the editing site would also, at least in part, explain the resistance of D487G and D487N to AN2690, which binds in the editing pocket. We propose that Asp487, located within the I4 insert, is instrumental in inducing segmental flexibility of the I4 insert and enables the cap to sequester the mischarged tRNA substrate and/or AN2690.
Supplementary Material
Highlights.
A resistance mutation to a novel boron-containing inhibitor that targets the leucyl-tRNA synthetase editing site is isolated and is located outside of the active site.
The resistance mutation is localized to the hinge of a cap-like structure that is unique to some eukaryotic leucyl-tRNA synthetases and is hypothesized to close over the editing active site.
The mutation site is involved in a salt bridge that is hypothesized to electrostatically stabilize the hinge of the editing site cap.
Acknowledgments
We thank an anonymous reviewer for careful analysis of our manuscript and for providing important insight into the results of our electrostatic mutant swaps. This work was supported by a grant from the National Institutes of Health (GM063789).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schimmel P, Tao J, Hill J. Aminoacyl tRNA synthetases as targets for anti-infectives. FASEB J. 1998;12:1599–1609. [PubMed] [Google Scholar]
- 2.Rock FL, Mao W, Yaremchuk A, Tukalo M, Crepin T, Zhou H, Zhang YK, Hernandez V, Akama T, Baker SJ, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759–1761. doi: 10.1126/science.1142189. [DOI] [PubMed] [Google Scholar]
- 3.Mascarenhas AP, Martinis SA, An S, Rosen AE, Musier-Forsyth K. In: Fidelity mechanisms of the aminoacyl-tRNA synthetases in: Protein engineering. Rajbhandary UL, Koehrer C, editors. Springer-Verlag; 2008. pp. 153–200. [Google Scholar]
- 4.An S, Musier-Forsyth K. Cys-tRNA(Pro) editing by Haemophilus influenzae YbaK via a novel synthetase ‘YbaK’ tRNA ternary complex. J Biol Chem. 2005;280:34465–34472. doi: 10.1074/jbc.M507550200. [DOI] [PubMed] [Google Scholar]
- 5.Ruan B, Söll D. The bacterial YbaK protein is a Cys-tRNAPro and Cys-tRNACys deacylase. J Biol Chem. 2005;280:25887–25891. doi: 10.1074/jbc.M502174200. [DOI] [PubMed] [Google Scholar]
- 6.Fersht AR. Editing mechanisms in protein synthesis. Rejection of valine by the isoleucyl-tRNA synthetase. Biochemistry. 1977;16:1025–1030. doi: 10.1021/bi00624a034. [DOI] [PubMed] [Google Scholar]
- 7.Starzyk RM, Webster TA, Schimmel P. Evidence for dispensable sequences inserted into a nucleotide fold. Science. 1987;237:1614–1618. doi: 10.1126/science.3306924. [DOI] [PubMed] [Google Scholar]
- 8.Mursinna RS, Lincecum TL, Jr, Martinis SA. A conserved threonine within Escherichia coli leucyl-tRNA synthetase prevents hydrolytic editing of leucyl-tRNALeu. Biochemistry. 2001;40:5376–5381. doi: 10.1021/bi002915w. [DOI] [PubMed] [Google Scholar]
- 9.Seiradake E, Mao W, Hernandez V, Baker SJ, Plattner JJ, Alley MR, Cusack S. Crystal structures of the human and fungal cytosolic Leucyl-tRNA synthetase editing domains: A structural basis for the rational design of antifungal benzoxaboroles. J Mol Biol. 2009;390:196–207. doi: 10.1016/j.jmb.2009.04.073. [DOI] [PubMed] [Google Scholar]
- 10.Lincecum TL, Jr, Tukalo M, Yaremchuk A, Mursinna RS, Williams AM, Sproat BS, Van Den Eynde W, Link A, Van Calenbergh S, Grötli M, et al. Structural and mechanistic basis of pre- and posttransfer editing by leucyl-tRNA synthetase. Mol Cell. 2003;11:951–963. doi: 10.1016/s1097-2765(03)00098-4. [DOI] [PubMed] [Google Scholar]
- 11.Giegé R, Sissler M, Florentz C. Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res. 1998;26:5017–5035. doi: 10.1093/nar/26.22.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larkin DC, Williams AM, Martinis SA, Fox GE. Identification of essential domains for Escherichia coli tRNALeu aminoacylation and amino acid editing using minimalist RNA molecules. Nucleic Acids Res. 2002;30:2103–2113. doi: 10.1093/nar/30.10.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soma A, Kumagai R, Nishikawa K, Himeno H. The anticodon loop is a major identity determinant of Saccharomyces cerevisiae tRNALeu. J Mol Biol. 1996;263:707–714. doi: 10.1006/jmbi.1996.0610. [DOI] [PubMed] [Google Scholar]
- 14.Hellmann RA, Martinis SA. Defects in transient tRNA translocation bypass tRNA synthetase quality control mechanisms. J Biol Chem. 2009;284:11478–11484. doi: 10.1074/jbc.M807395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker SJ, Zhang YK, Akama T, Lau A, Zhou H, Hernandez V, Mao W, Alley MR, Sanders V, Plattner JJ. Discovery of a new boron-containing antifungal agent, 5-fluoro-1,3-dihydro-1-hydroxy-2,1- benzoxaborole (AN2690), for the potential treatment of onychomycosis. J Med Chem. 2006;49:4447–4450. doi: 10.1021/jm0603724. [DOI] [PubMed] [Google Scholar]
- 16.Tukalo M, Yaremchuk A, Fukunaga R, Yokoyama S, Cusack S. The crystal structure of leucyl-tRNA synthetase complexed with tRNALeu in the post-transfer-editing conformation. Nat Struct Mol Biol. 2005;12:923–930. doi: 10.1038/nsmb986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.