Abstract
Objective
Due to significant individual variability in Attention Deficit/Hyperactivity Disorder (ADHD) medication response, there is increasing interest in identifying genetic predictors of treatment effects. This study examines the role of 4 catecholamine-related candidate genes in moderating methylphenidate (MPH) dose-response.
Method
89 stimulant-naïve children with ADHD aged 7–11 participated in a randomized, double-blind, crossover trial of long-acting MPH. Parents and teachers assessed each child’s response on placebo and three MPH dosage levels using the Vanderbilt ADHD rating scales. Children were genotyped for polymorphisms in the dopamine transporter’s (DAT) 3’ untranslated region, dopamine receptor D4‘s (DRD4) exon 3, catechol-O-methyltransferase’s (COMT) codon 158, and adrenergic α2A-receptor’s (ADRA2A) promoter. Linear mixed models evaluated gene, dose (mg/kg/day), and gene*dose effects on inattentive and hyperactive-impulsive domain outcomes.
Results
The most statistically significant gene*dose interactions were observed on hyperactive-impulsive symptoms for DRD4 and DAT polymorphisms, with participants lacking the DAT 10-repeat allele experiencing greater improvements in symptoms with increasing dose compared to 10-repeat carriers (p=0.008), and those lacking the DRD4 4-repeat allele showing less improvement across MPH doses compared to 4-repeat carriers (p=0.02).
Conclusions
This study suggests that DAT and DRD4 polymorphisms may be associated with individual variability in methylphenidate dose-response, although further research in larger samples is required to confirm these findings and their clinical utility.
Keywords: ADHD, pharmacogenetics, methylphenidate, dopamine receptor D4, dopamine transporter
Introduction
Although abundant data indicate that stimulant medications improve symptoms of Attention Deficit/Hyperactivity Disorder (ADHD),1 notable variability exists in their optimal dosage and duration of effect.2–4 No consistent predictors of ADHD medication response have been identified.5 Hence, there is growing interest in investigating the role of genetics in predicting treatment response.5–7 To date, ADHD pharmacogenetic studies have focused on catecholamine-related polymorphisms.8,9 The majority of studies have investigated the role of polymorphisms in a variable number tandem repeat (VNTR) in the dopamine transporter (DAT) gene’s 3’ untranslated region,10 as DAT inhibition is a primary mechanism of action for methylphenidate (MPH),11 and some studies have implicated the DAT 10-repeat allele in ADHD susceptibility.12 However, prior DAT studies have yielded disparate findings. Some suggest diminished13–16 and others improved17 MPH response with 10-repeat homozygosity, diminished MPH response with 9-repeat homozygosity,18,19 or no effect,20–26 with Cohen’s d effect sizes of 0.41–2.14.16 Many studies have also evaluated polymorphisms in the dopamine receptor D4 (DRD4), which regulates dopamine synthesis, release, and neuron firing rate.27 Although the association between ADHD susceptibility and the 7-repeat VNTR in DRD4’s exon III is one of the most replicated findings in psychiatric genetics,28 outcomes of DRD4 pharmacogenetic studies have been variable, with some showing diminished,29,30 improved,22,31 or no effect13,24–26,32 on MPH response with the 7-repeat allele, and others documenting significant effects20,33 or no effect20,24 on MPH response for the 4-repeat allele, with Cohen’s d effect sizes of 0.39–1.56.30,32
Other genes of increasing interest in ADHD pharmacogenetic studies include the adrenergic α2A-receptor (ADRA2A) and catechol-O-methyltransferase (COMT). ADRA2A encodes a norepinephrine autoreceptor whose activation limits norepinephrine release.34 To date, three pharmacogenetic studies have evaluated the role of a −1291 C>G single nucleotide polymorphism (SNP) in the ADRA2A promoter region,35 and have associated the G allele with improved MPH response, with Cohen’s d effect sizes of 0.42–0.68.36–38 Other studies have focused on COMT, which catabolizes dopamine and norepinephrine. A functional polymorphism at COMT’s codon 158 results in a single amino acid change (met for val) which changes enzyme activity levels.39 Two of three studies have suggested a significant association between MPH effects on ADHD symptoms and this COMT polymorphism20,25,40 (Cohen’s d effect sizes not available).
Thus, inconsistent findings have emerged regarding the effects on methylphenidate response for polymorphisms in DAT and DRD4, the most well-studied genes, and there is emerging interest in ADRA2A and COMT. There are concerns that small sample sizes, study design variations (open-label vs. randomized controlled trials), outcome measure differences, sample differences, and varying dosing regimens may be partially responsible for the disparate findings to date.8,9,41,42 For example, only three ADHD pharmacogenetic studies in school-age children have been randomized, placebo-controlled, and double-blinded.18–20 Only two school-age19,33 and one preschool trial32 have used parent and teacher outcome ratings. Given that most children spend the period of peak stimulant blood levels with teachers rather than parents, and that the association between genotype and psychostimulant response has varied by outcome informant in several studies,19,32 the inclusion of both parent and teacher ratings is important in ADHD pharmacogenetic studies.43 In addition, few studies have evaluated response at more than one dose,18,20,32 and little attention has been given to possible gene*dose interactions,20,32 despite the potential of such knowledge to guide MPH dosing practices in the clinical setting. Furthermore, prior ADHD pharmacogenetic samples were recruited primarily from specialty clinics,14–16,18,19,21,24,25,30,31,33,36,38,40,44 and included children with a history of previous stimulant treatment (see McGough et al32 and Cheon et al40 for exceptions). As a result, prior samples may contain higher numbers of treatment-refractory children, and may not be representative of newly diagnosed or previously untreated children.
Using a randomized, double-blind, placebo-controlled crossover trial of multiple methylphenidate doses in stimulant-naïve school-age children, we investigated the role of four catecholamine-related candidate genes in moderating ADHD treatment response. Outcome measures included parent and teacher ADHD symptom ratings, with gene*dose interactions serving as our primary predictor of interest in models. We hypothesized that individual candidate genes would be associated with variability in methylphenidate dose-response curves.
Method
Participants and Procedures
Stimulant-naïve children ages 7–11 were recruited for a study on methylphenidate effects on neuropsychological outcomes. Recruitment materials were sent to local schools, pediatricians’ offices and hospitals, and email and print advertisements were distributed. Written informed consent/assent was obtained from all parents/caregivers and participants according to the Cincinnati Children’s Hospital Institutional Review Board-approved protocol. ADHD diagnosis was determined using methodology similar to the Multimodal Treatment Study of ADHD.45 Children were considered to meet criteria for a symptom domain (i.e., inattention and/or hyperactivity/impulsivity) if the parent/caregiver on the Diagnostic Interview Schedule for Children (DISC46) and the teacher on the Vanderbilt ADHD Teacher Rating Scale47 reported 6 non-overlapping symptoms in a symptom domain and both parent and teacher reported at least 4 symptoms in that domain. Based on the neuropsychological outcome study specifications, inattentive and combined type participants were recruited in equal ratio and hyperactive-impulsive type was excluded. Participants were required to meet ADHD DSM-IV criteria for onset age, pervasiveness, and impairment as reported on the DISC. Furthermore, families were interviewed by a trained clinician (pediatrician or psychologist) to confirm the diagnosis and obtain a Clinical Global Impression of functional severity (CGI-S). In addition, participants were administered the Wechsler Abbreviated Scale of Intelligence and the Wechsler Individual Achievement Test—2nd edition word reading and numerical operations subtests and required to achieve standard scores of ≥80 on these measures in order to exclude possible learning disorders.
Children were evaluated for psychiatric comorbidities using the DISC. Those with mania/hypomania were excluded; comorbid oppositional defiant disorder, conduct disorder, depression, and anxiety disorders were allowed unless determined to be the primary cause of ADHD symptomatology or requiring different treatment. Children were also excluded if their medical history suggested significant brain injury.
A total of 162 children were assessed for study participation, and 105 met all inclusion criteria. Among those meeting inclusion criteria, 5 dropped out due to no longer wanting to try ADHD medications, 3 could not swallow pills, 3 lost contact with study staff, 3 did not consent to provide a genetic sample, and 2 did not provide enough DNA for analysis. Thus, 89 children participated in the medication trial and genetic analyses. Among the 89 participants, mean age was 8.1 years, and 28% were female. Table 1 details sample characteristics.
Table 1.
Sample Characteristics (N=89)
| Variable | |
|---|---|
| Age, year, mean (SD) | 8.13 (1.21) |
| Weight, kilograms, mean (SD) | 33.30 (10.15) |
| Female, no. (%) | 24 (27) |
| Race/ethnicity,a no. (%) | |
| White | 70 (79) |
| Black | 16 (18) |
| Hispanic/Latino | 2 (2) |
| Other | 1 (1) |
| ADHD subtype, no. (%) | |
| Inattentive | 46 (52) |
| Combined | 43 (48) |
| Abbreviated IQ, mean (SD) | 105.34 (12.65) |
| Anxiety Disorder,b no. (%) | 15 (17) |
| Mood Disorder,c no. (%) | 2 (2) |
| Disruptive Behavior Disorder,d no. (%) | 32 (36) |
Note: ADHD = Attention Deficit/Hyperactivity Disorder; IQ = Intelligence Quotient.
Reported by parent/caregiver.
Social phobia, separation anxiety, panic, agoraphobia, generalized anxiety disorder, obsessive compulsive disorder, and/or post-traumatic stress disorder.
Major depressive episode/dysthymia
Oppositional defiant or conduct disorder.
Medication Trial
Subjects participated in a four-week, double-blind, crossover trial of long-acting osmotic-release oral system (OROS) methylphenidate (Concerta®), during which children were randomly assigned to one of six dosing schedules that included three active dosage weeks (18mg, 27mg, 36mg for children ≤25kg; 18mg, 36mg or 54mg for children >25kg; sample mean maximum dose=1.57 mg/kg/day ) and one week of placebo (see Table S1, available online). Study medication consisted of identical capsules filled with either an inert white powder (placebo) or the prescribed dose of Concerta® over-encapsulated to preserve double-blind.
Outcomes
The Vanderbilt ADHD Parent Rating Scales (VADPRS)48 and Vanderbilt ADHD Teacher Rating Scales (VADTRS)47 were completed at baseline and each week of the trial.47,48 An inattentive domain score was generated by totaling scores from the nine inattention symptoms; a hyperactive-impulsive domain score was derived by totaling scores from the nine hyperactive-impulsive symptoms. Internal consistency reliability is good to excellent for each of the VADTRS subscales47 and excellent for the VADPRS subscales;48 concurrent validity with the DISC is high (r=0.79).48 Correlations between the VADPRS and VADTRS are small to medium for both the inattentive (r=0.33–0.34)49,50 and hyperactive-impulsive (r=0.27–0.36)49,50 domains.
Primary Predictors
We evaluated the association between methylphenidate effects on ADHD symptom domains and polymorphisms in DAT, DRD4, ADRA2A, and COMT.
Participants provided saliva samples for DNA extraction by spitting into an Oragene cup, with DNA extracted using the manufacturer's protocol (DNA Genotek, Ottawa, Canada). Genotyping success rate was 98% as two subjects provided an insufficient saliva sample for genetic analysis. For VNTR genotyping, established assays as described by Hamarman et al30 and Stein et al18 were used for the DRD4 exon III and DAT 3’ untranslated region VNTRs respectively. Genotyping was performed using theTaqMan allelic discrimination system (Applied Biosystem, Forest City, CA) for the ADRA2A -1291 C>G SNP and COMT codon 158 SNP. An ABI-7500 real-time PCR system was used for post-PCR-read allelic discrimination.
Statistical Analysis
Genotype frequencies were calculated and chi-square statistics were used to test them against expected counts according to Hardy-Weinberg equilibrium principles. Given the lack of consensus regarding the relevant genotyping groupings in ADHD pharmacogenetic studies,9 in order to minimize multiple testing on non-independent genotypes, we defined genotypes empirically based on allele frequencies as suggested by McGough et al.20 Participant genotypes were categorized using a three-level variable: homozygous(+/+), heterozygous(+/−), or absent(−/−), with reference to the most common allele.
Linear mixed models, including all four polymorphisms in each model, evaluated gene, dose (mg/kg/day), and gene*dose (mg/kg/day) effects on ADHD symptom scores (SAS Proc MIXED, SAS Version 9.2, SAS Institute, Cary, NC). The inattention and hyperactive-impulsive domain outcomes were evaluated in separate models, as some studies have found genetic effects on medication response in one but not both ADHD symptom domains.25,36,44 Gene*dose effects serve as our primary predictor to determine if the genetic variants display unique MPH dose-response curves. Main gene effects (which show that the genotypes display consistently different symptom levels on placebo and across doses) are of lesser interest as they imply differential risk for ADHD symptoms independent of methylphenidate treatment. We included covariates in the model that have been variably associated with ADHD medication response, including IQ,51 ADHD subtype,52–54 and mental health comorbidities,51 such as disruptive behavior disorders (DISC-diagnosed oppositional defiant disorder and conduct disorder) and anxiety disorders (DISC-diagnosed social phobia, separation anxiety, panic, agoraphobia, generalized anxiety disorder, obsessive compulsive disorder, and/or post-traumatic stress disorder). Models also included baseline parent and teacher ratings for each domain to account for any genotype differences in initial symptom severity,55 and to minimize false positive results associated with regression to the mean. In order to accommodate both parent and teacher ratings in the same models,43 a rater variable was also included in the models to control and assess for effects of parent versus teacher ratings. In recognition that we utilized two primary outcomes (inattention scores and hyperactive-impulsive scores), as per McGough et al,20 α was set at .025 based on the Bonferroni correction. All tests were two-tailed. Given their respective minor allele frequencies, we had 80% power to detect gene*dose effect sizes of 0.61 for DAT, 0.45 for DRD4, 0.81 for ADRA2A, and 1.03 for COMT.
For significant gene*dose interactions, post-hoc tests of differences between groups were conducted to further explore interaction effects, and effect sizes were calculated based on Cohen’s d. Although race/ethnicity has not been a significant predictor of MPH response,56–58 concerns in candidate gene studies have been raised about false positive results due to population stratification (i.e., confounding due to ancestry-associated differences in allele frequencies in sample subpopulations). Hence, for models with significant gene*dose interactions, we conducted secondary analyses to evaluate potential confounding by race as suggested by Kleinbaum et al.59 Race was modeled as a dichotomous variable comparing those with parent/caregiver-reported African American ancestry to all others. Percent change in the gene*dose beta coefficient was then assessed in models that lacked adjustment for race compared to those that included race as a covariate, with >10% considered evidence of potential confounding. Further, we conducted stratified analyses which included only Caucasian participants to determine if the pattern of results differed from the full sample models.
Results
Genotype Frequencies
Candidate genes, allele frequencies, and analyzed genotype groupings appear in Table 2. Genotypes met criteria for Hardy-Weinberg equilibrium (all p>0.3).
Table 2.
Allele Frequencies and Genotype Classifications (N=89)
| Gene Description | Allele | Allele Frequency | Genotype | Genotype Frequency(N) |
|---|---|---|---|---|
| Adrenergic α2A–receptor −1291 C>G SNP | C | 67% | CC | 47%(42) |
| G | 33% | CG | 39%(35) | |
| GG | 13%(12) | |||
|
| ||||
| Catechol-O-methyltransferase Val158Met SNP | Val | 56% | Val/Val | 34%(30) |
| Met | 44% | Val/Met | 45%(40) | |
| Met/Met | 21%(19) | |||
|
| ||||
| Dopamine transporter 3’ untranslated region VNTR | +10 | 77% | +10/+10 | 61%(54) |
| 9 | 20% | +10/−10 | 34%(30) | |
| 8,11 | 3% | −10/−10 | 6%(5) | |
|
| ||||
| Dopamine receptor D4 exon 3 VNTR | 4 | 58% | +4/+4 | 31%(28) |
| 7 | 20% | +4/−4 | 54%(48) | |
| 2,3,5,6,8,10 | 22% | −4/−4 | 15%(13) | |
Note: SNP=Single Nucleotide Polymorphism; VNTR=Variable Nucleotide Tandem Repeat
Inattentive Domain Findings
A linear main effect of dose on inattentive scores, indicating greater symptom reductions as dosages increased, was observed across genotypes (p<0.001), but no significant main gene effects or gene*dose interactions were observed (all p>0.025, Table 3).
Table 3.
Gene, Gene*Dose (G*D), and Dose Effectsa (N=89)
| Inattentive Symptoms | Hyperactive-Impulsive Symptoms | |||||
|---|---|---|---|---|---|---|
| Gene | Effect | β | P value | β | P value | |
| ADRA2A | Gene | CC | Ref | 0.03 | Ref | 0.003 |
| CG | −2.52 | −2.64 | ||||
| GG | −0.23 | 1.05 | ||||
| G*D | CC | Ref | 0.09 | Ref | 0.03 | |
| CG | 1.49 | 1.38 | ||||
| GG | 0.67 | −0.14 | ||||
| COMT | Gene | Val/Val | Ref | 0.35 | Ref | 0.35 |
| Val/Met | −0.52 | −0.86 | ||||
| Met/Met | −1.90 | −1.75 | ||||
| G*D | Val/Val | Ref | 0.35 | Ref | 0.09 | |
| Val/Met | −0.07 | 1.05 | ||||
| Met/Met | 1.23 | 1.64 | ||||
| DAT | Gene | +10/+10 | Ref | 0.19 | Ref | 0.03 |
| +10/−10 | −0.38 | −1.40 | ||||
| −10/−10 | 3.26 | 3.58 | ||||
| G*D | +10/+10 | Ref | 0.04 | Ref | 0.008 | |
| +10/−10 | 1.49 | 0.57 | ||||
| −10/−10 | −1.49 | −3.34 | ||||
| DRD4 | Gene | +4/+4 | Ref | 0.39 | Ref | 0.82 |
| +4/−4 | −1.38 | −0.01 | ||||
| −4/−4 | −1.19 | 0.74 | ||||
| G*D | +4/+4 | Ref | 0.25 | Ref | 0.02 | |
| +4/−4 | 0.58 | −0.69 | ||||
| −4/−4 | 1.75 | 1.47 | ||||
| Dose | −3.94 | <0.0001 | −3.88 | <0.0001 | ||
Note: ADRA2A = adrenergic α2A-receptor; COMT = catechol-O-methyltransferase; DAT = dopamine transporter; DRD4 = dopamine receptor D4 ; Ref=Reference group
See Table S2, available online, for β coefficients and p-values of model covariates.
Hyperactive-Impulsive Domain Findings
Significant linear main dose effects on hyperactive-impulsive scores were observed across genotypes (p<0.001, Table 3), indicating greater reductions in hyperactive-impulsive symptoms as dosages increased.
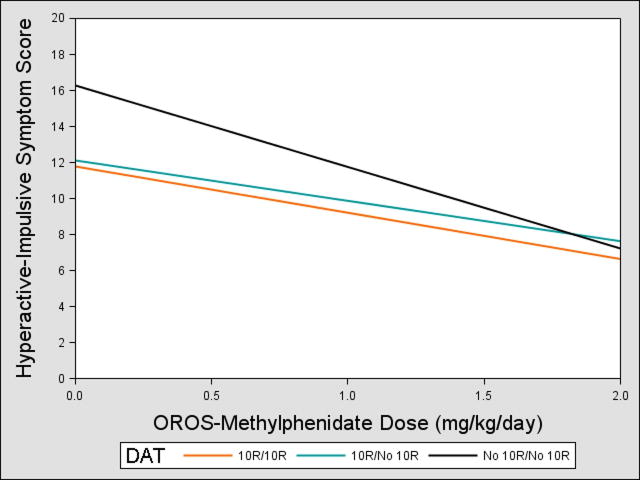
We observed DAT*dose effects on hyperactive-impulsive scores (p=0.008, Table 3). Those lacking the 10-repeat allele showed greater improvement across MPH doses compared to 10-repeat carriers (Figure 1), with effect sizes of 0.59–0.64. Post hoc comparisons showed that 10-repeat heterozygotes and homozygotes did not differ in their gene*dose response (β=0.56, p=0.32). Thus, individuals with no 10-repeat alleles would have a mean reduction of 56% in their hyperactive-impulsive scores at 2mg/kg/day of methylphenidate compared to placebo, while 10-repeat carriers would have a less robust reduction of 37–44%.
Figure 1.
Dopamine transporter (DAT)*Dose Effects on Parent- and Teacher-Rated Hyperactive-Impulsive Scores. Note: Participants with no copies of the 10-repeat (10R) allele had greater reduction in symptoms as methylphenidate dose increased compared with 10R carriers. The 0 mg/kg/day dose corresponds to the placebo condition. OROS = osmotic-release oral system.
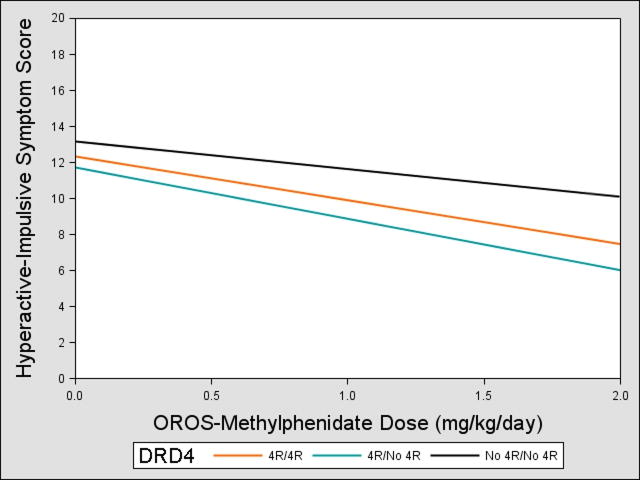
DRD4*dose effects on hyperactive-impulsive scores were also identified (p=0.02, Table 3). Those lacking the 4-repeat allele showed less improvement across MPH doses compared to 4-repeat carriers (Figure 2), with effect sizes of 0.40–0.61. Post hoc comparisons showed that 4-repeat heterozygotes and homozygotes did not differ in their gene*dose response (β=−0.69, p=0.26). Our findings suggest that individuals with no 4-repeat alleles would have a mean reduction of 23% in their hyperactive-impulsive scores at 2 mg/kg/day of methylphenidate compared to placebo, while 4-repeat carriers would have larger reductions of 40–49%.
Figure 2.
Dopamine receptor D4 (DRD4)*Dose Effects on Parent- and Teacher-Rated Hyperactive-Impulsive Scores. Note: Participants with no copies of the 4-repeat (4R) allele experienced less reduction in symptoms as methylphenidate dose increased compared with 4R carriers. The 0 mg/kg/day dose corresponds to the placebo condition. OROS = osmotic-release oral system
In secondary analyses, the inclusion of race as a covariate did not affect the DAT*dose and DRD4*dose hyperactive-impulsive domain findings, as their beta coefficients’ percent change was ≤2% when comparing models that included race as a covariate versus those that did not. In analyses limited to Caucasian participants, the DAT*dose effects were somewhat and the DRD4*dose effects more markedly attenuated (p=0.02 and p=0.51 respectively) in these models of reduced sample size (N=71).
A main effect on hyperactive-impulsive domain scores was detected for the ADRA2A polymorphism (p=0.003), with G homozygotes displaying higher symptom levels on placebo and continuing at these higher levels as MPH doses increased. The ADRA2A*dose interaction fell short of our threshold for statistical significance (p>0.025), and there were no notable main gene or gene*dose effects observed for COMT (Table 3).
Discussion
Results of this double-blind, placebo-controlled ADHD pharmacogenetic trial of psychostimulant-naïve school-aged children suggest DAT and DRD4 variations may be associated with unique MPH dose-response curves. Children lacking the DAT 10-repeat allele and those with the DRD4 4-repeat allele had a more robust methylphenidate response compared to alternate genotypes, consistent with an improved response for the ADHD susceptibility low risk alleles.
Our findings suggest possible MPH dose-response differences based on DAT genotype, with those lacking the 10-repeat showing improved MPH effects on hyperactive-impulsive symptoms and a trend toward improvements in inattentive scores across doses. This is consistent with a prior meta-analysis and several individual studies13–16 showing diminished rates of MPH response for 10-repeat homozygotes, although other studies have linked the 9-repeat allele to blunted response.17–19 Our findings are in line with studies demonstrating functional effects of the DAT VNTR polymorphisms after methylphenidate administration, including greater increases in basal ganglia DAT density15 and a failure to increase short interval cortical inhibition (measured via transcranial magnetic stimulation)60 for 10-repeat homozygotes compared to other groups, although it should be noted that, unlike these studies, we did not observe differences between 10-repeat homozygotes and heterozygotes.
We found that the DRD4 4-repeat allele may be associated with improved MPH response across doses compared to other VNTR lengths, congruent with observations of the 4-repeat’s enhanced receptor expression61 and increased sensitivity to dopamine62 in some studies, although other studies have not demonstrated major pharmacological differences for the DRD4 variants.63 Our findings echo Cheon et al33 and McGough et al,32 who also used teacher ratings for outcome assessment. In contrast, two additional studies found no relationship between the DRD4 4-repeat and MPH effects on ADHD symptoms, but unlike our study, neither considered teacher ratings.20,24 McGough et al studied the effects of MPH on math performance,20 and found that those lacking a 4-repeat allele showed an improved response to methylphenidate at lower doses but deterioration at higher doses. Although the DRD4 variants’ math performance MPH dose-response curves in this sample differed from our findings regarding hyperactive-impulsive symptoms, this is not unexpected given studies identifying MPH dose-response differences for learning compared to social behavior outcomes.64 It is uncertain why the DRD4 effects on MPH response that we observed were limited to the hyperactive-impulsive domain, as some ADHD etiology studies have suggested a predominant effect of this VNTR on inattentive symptoms,65 while others have suggested a preferential effect on hyperactive-impulsive symptoms.66
We observed a main effect of ADRA2A genetic variants on MPH response such that the G allele was associated with significantly higher ratings of hyperactive-impulsive symptoms on placebo and across doses. This is consistent with studies showing an association between increased ADHD symptomatology and the G allele, although prior studies found a stronger association with inattentive rather than hyperactive-impulsive ratings.67–70 ADRA2A*dose interactions did not meet our threshold for significance. Prior studies observing a significant association between the G allele and improved MPH response on inattentive symptoms36,37 or on total symptoms (but not inattentive symptoms alone, with borderline significant effects on hyperactive-impulsive symptoms)38 evaluated response to a single MPH dose and did not evaluate ADRA2A*dose interactions.36–38
COMT was not significantly associated with MPH response, although our pattern of results suggest that val homozygotes experienced greater improvements in hyperactive-impulsive symptoms with increasing doses compared to other groups (p=0.09). This pattern is consistent with 2 prior studies25,40 which found significant effects of the COMT genotypes on responder/nonresponder status25,40 or change in ADHD symptom scores after MPH treatment,25 but did not evaluate gene*dose interactions.
Our study has a number of limitations, including that we adjusted our level of significance by a factor of two (p=0.025) in recognition that we ran two analytic models (one for each outcome), but did not correct for the number of polymorphisms evaluated. Had we made Bonferroni corrections for both the number of models/outcomes and polymorphisms evaluated, the adjusted α would be 0.006. None of our gene*dose interactions met the 0.006 level of significance, although the DAT*dose effect on hyperactive-impulsive symptoms would have been borderline (p=0.008). Additionally, our sample size, while larger than three18,20,32 of the four19 prior pediatric ADHD pharmacogenetic controlled trials, is modest. Due to sample size considerations, we were unable to conduct a genome-wide association study, which can be used to identify genetic variants that have not previously been hypothesized to influence stimulant response (e.g., the glutamate receptor 7 gene).71 Although we recognize the possibility of elevated false positives when using a candidate gene approach,20 corroboration of our DRD432,33 and DAT13–16 findings in prior studies provides some reassurance that our results are not false positives. However, we cannot exclude the possibility that other polymorphisms in linkage disequilibrium with the DRD4 and/or DAT variants may be responsible for the observed effects.
Additional limitations include the restricted duration of follow-up and the heterogeneity of our sample due to recruitment from a variety of sources. Further, our sample had more inattentive (52%) than combined subtype (48%) participants. Although consistent with the subtype distribution in many epidemiologic samples,72–76 this differs from clinic settings where combined type is most common.
Our study is further limited because we did not evaluate the relationship between the catecholamine-related genes and MPH side effects despite increasing evidence of the important role that allelic variants may play in predicting methylphenidate tolerability.20,32 There is also growing recognition that genetic factors are unlikely to act in isolation, but limited sample size precluded our evaluation of gene-gene and gene-environment interactions on methylphenidate dose-response. We found effects of moderate size for the individual DRD4*dose and DAT*dose interactions, but were unable to determine the magnitude of their likely larger combined effects. As we move toward personalized ADHD treatment, pharmacogenetic studies with larger samples and a range of outcomes (i.e., efficacy and side effects) are needed to determine the clinical utility of genomic information.
Acknowledgments
The project described was supported by the National Institute of Mental Health (NIMH) grants R01MH074770 (JNE), K23 MH083881 (TEF), K23MH083027 (WBB), K24 MH064478 (JNE), and MH070564-01 (MAS), and a Cincinnati Children’s Hospital Center for Education and Research Therapeutics Award.
Footnotes
The content is solely the responsibility of the authors and does not represent the official views of the NIMH or the National Institutes of Health.
Supplemental material cited in this article is available online.
Disclosure: Dr. Epstein receives funding from Eli Lilly and Co. as an investigative site for a pharmaceutical trial. Dr. Stein has received research support from Eli Lilly and Co., McNeil Pharmaceuticals, Novartis, and Shire. He has served on the speakers’ bureau for Novartis. He has served as a consultant to Novartis, Shire, and Shinogi Pharmaceuticals. Drs. Froehlich, Brinkman, Langberg, Kahn, and Nick, and Ms. Melguizo Castro and Ms. Graham report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001 Oct;108(4):1033–1044. doi: 10.1542/peds.108.4.1033. [DOI] [PubMed] [Google Scholar]
- 2.Greenhill LL, Swanson JM, Vitiello B, et al. Impairment and deportment responses to different methylphenidate doses in children with ADHD: the MTA titration trial. J Am Acad Child Adolesc Psychiatry. 2001 Feb;40(2):180–187. doi: 10.1097/00004583-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Wolraich ML, Doffing MA. Pharmacokinetic considerations in the treatment of attention-deficit hyperactivity disorder with methylphenidate. CNS drugs. 2004;18(4):243–250. doi: 10.2165/00023210-200418040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Rapport MD, Stoner G, DuPaul GJ, Birmingham BK, Tucker S. Methylphenidate in hyperactive children: differential effects of dose on academic, learning, and social behavior. Journal of abnormal child psychology. 1985 Jun;13(2):227–243. doi: 10.1007/BF00910644. [DOI] [PubMed] [Google Scholar]
- 5.Lowe N, Barry E, Gill M, Hawi Z. An Overview of the Pharmacogenetics and Molecular Genetics of ADHD. Current Pharmacogenomics. 2006;4:231–243. [Google Scholar]
- 6.Polanczyk G, Zeni C, Genro JP, Roman T, Hutz MH, Rohde LA. Attention-deficit/hyperactivity disorder: advancing on pharmacogenomics. Pharmacogenomics. 2005 Apr;6(3):225–234. doi: 10.1517/14622416.6.3.225. [DOI] [PubMed] [Google Scholar]
- 7.McGough JJ. Attention-deficit/hyperactivity disorder pharmacogenomics. Biol Psychiatry. 2005 Jun 1;57(11):1367–1373. doi: 10.1016/j.biopsych.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Froehlich TE, McGough JJ, Stein MA. Progress and promise of attention-deficit hyperactivity disorder pharmacogenetics. CNS drugs. 2010 Feb 1;24(2):99–117. doi: 10.2165/11530290-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein MA, McGough JJ. The pharmacogenomic era: promise for personalizing attention deficit hyperactivity disorder therapy. Child Adolesc Psychiatr Clin N Am. 2008 Apr;17(2):475–490. xi–xii. doi: 10.1016/j.chc.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellgrove MA, Hawi Z, Kirley A, Fitzgerald M, Gill M, Robertson IH. Association between dopamine transporter (DAT1) genotype, left-sided inattention, and an enhanced response to methylphenidate in attention-deficit hyperactivity disorder. Neuropsychopharmacology. 2005 Dec;30(12):2290–2297. doi: 10.1038/sj.npp.1300839. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ. Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord. 2002;6 (Suppl 1):S31–43. doi: 10.1177/070674370200601s05. [DOI] [PubMed] [Google Scholar]
- 12.Roman T, Rohde LA, Hutz MH. Polymorphisms of the dopamine transporter gene: influence on response to methylphenidate in attention deficit-hyperactivity disorder. Am J Pharmacogenomics. 2004;4(2):83–92. doi: 10.2165/00129785-200404020-00003. [DOI] [PubMed] [Google Scholar]
- 13.Winsberg BG, Comings DE. Association of the dopamine transporter gene (DAT1) with poor methylphenidate response. J Am Acad Child Adolesc Psychiatry. 1999 Dec;38(12):1474–1477. doi: 10.1097/00004583-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Roman T, Szobot C, Martins S, Biederman J, Rohde LA, Hutz MH. Dopamine transporter gene and response to methylphenidate in attention-deficit/hyperactivity disorder. Pharmacogenetics. 2002 Aug;12(6):497–499. doi: 10.1097/00008571-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Cheon KA, Ryu YH, Kim JW, Cho DY. The homozygosity for 10-repeat allele at dopamine transporter gene and dopamine transporter density in Korean children with attention deficit hyperactivity disorder: relating to treatment response to methylphenidate. Eur Neuropsychopharmacol. 2005 Jan;15(1):95–101. doi: 10.1016/j.euroneuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Purper-Ouakil D, Wohl M, Orejarena S, et al. Pharmacogenetics of methylphenidate response in attention deficit/hyperactivity disorder: Association with the dopamine transporter gene (SLC6A3) Am J Med Genet B Neuropsychiatr Genet. 2008 Dec 5;147B(8):1425–1430. doi: 10.1002/ajmg.b.30809. [DOI] [PubMed] [Google Scholar]
- 17.Kirley A, Lowe N, Hawi Z, et al. Association of the 480 bp DAT1 allele with methylphenidate response in a sample of Irish children with ADHD. Am J Med Genet B Neuropsychiatr Genet. 2003 Aug 15;121(1):50–54. doi: 10.1002/ajmg.b.20071. [DOI] [PubMed] [Google Scholar]
- 18.Stein MA, Waldman ID, Sarampote CS, et al. Dopamine transporter genotype and methylphenidate dose response in children with ADHD. Neuropsychopharmacology. 2005 Jul;30(7):1374–1382. doi: 10.1038/sj.npp.1300718. [DOI] [PubMed] [Google Scholar]
- 19.Joober R, Grizenko N, Sengupta S, et al. Dopamine transporter 3'-UTR VNTR genotype and ADHD: a pharmaco-behavioural genetic study with methylphenidate. Neuropsychopharmacology. 2007 Jun;32(6):1370–1376. doi: 10.1038/sj.npp.1301240. [DOI] [PubMed] [Google Scholar]
- 20.McGough JJ, McCracken JT, Loo SK, et al. A Candidate Gene Analysis of Methylphenidate Response in Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2009 Dec;48(12):1155–64. doi: 10.1097/CHI.0b013e3181bc72e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langley K, Turic D, Peirce TR, et al. No support for association between the dopamine transporter (DAT1) gene and ADHD. Am J Med Genet B Neuropsychiatr Genet. 2005 Nov 5;139(1):7–10. doi: 10.1002/ajmg.b.30206. [DOI] [PubMed] [Google Scholar]
- 22.van der Meulen EM, Bakker SC, Pauls DL, et al. High sibling correlation on methylphenidate response but no association with DAT1-10R homozygosity in Dutch sibpairs with ADHD. J Child Psychol Psychiatry. 2005 Oct;46(10):1074–1080. doi: 10.1111/j.1469-7610.2005.01521.x. [DOI] [PubMed] [Google Scholar]
- 23.Bellgrove MA, Barry E, Johnson KA, et al. Spatial Attentional Bias as a Marker of Genetic Risk, Symptom Severity, and Stimulant Response in ADHD. Neuropsychopharmacology. 2008 Sep;33(10):2536–45. doi: 10.1038/sj.npp.1301637. [DOI] [PubMed] [Google Scholar]
- 24.Zeni CP, Guimaraes AP, Polanczyk GV, et al. No significant association between response to methylphenidate and genes of the dopaminergic and serotonergic systems in a sample of Brazilian children with attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2007 Apr 5;144(3):391–394. doi: 10.1002/ajmg.b.30474. [DOI] [PubMed] [Google Scholar]
- 25.Kereszturi E, Tarnok Z, Bognar E, et al. Catechol-O-methyltransferase Val158Met polymorphism is associated with methylphenidate response in ADHD children. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1431–1435. doi: 10.1002/ajmg.b.30704. [DOI] [PubMed] [Google Scholar]
- 26.Tharoor H, Lobos EA, Todd RD, Reiersen AM. Association of dopamine, serotonin, and nicotinic gene polymorphisms with methylphenidate response in ADHD. Am J Med Genet B Neuropsychiatr Genet. 2008 Jun 5;147B(4):527–530. doi: 10.1002/ajmg.b.30637. [DOI] [PubMed] [Google Scholar]
- 27.Heckers S, Konradi C. Synaptic function and biochemical neuroanatomy. In: Martin A, Scahill L, Charney DS, Leckman JF, editors. Pediatric Psychopharmacology: Principles and Practice. New York: Oxford University Press; 2003. pp. 20–32. [Google Scholar]
- 28.Faraone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005 Jun 1;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 29.Seeger G, Schloss P, Schmidt MH. Marker gene polymorphisms in hyperkinetic disorder--predictors of clinical response to treatment with methylphenidate? Neurosci Lett. 2001 Nov 2;313(1–2):45–48. doi: 10.1016/s0304-3940(01)02253-4. [DOI] [PubMed] [Google Scholar]
- 30.Hamarman S, Fossella J, Ulger C, Brimacombe M, Dermody J. Dopamine receptor 4 (DRD4) 7-repeat allele predicts methylphenidate dose response in children with attention deficit hyperactivity disorder: a pharmacogenetic study. J Child Adolesc Psychopharmacol. 2004 Winter;14(4):564–574. doi: 10.1089/cap.2004.14.564. [DOI] [PubMed] [Google Scholar]
- 31.Tahir E, Yazgan Y, Cirakoglu B, Ozbay F, Waldman I, Asherson PJ. Association and linkage of DRD4 and DRD5 with attention deficit hyperactivity disorder (ADHD) in a sample of Turkish children. Mol Psychiatry. 2000 Jul;5(4):396–404. doi: 10.1038/sj.mp.4000744. [DOI] [PubMed] [Google Scholar]
- 32.McGough J, McCracken J, Swanson J, et al. Pharmacogenetics of methylphenidate response in preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry. 2006 Nov;45(11):1314–1322. doi: 10.1097/01.chi.0000235083.40285.08. [DOI] [PubMed] [Google Scholar]
- 33.Cheon KA, Kim BN, Cho SC. Association of 4-repeat allele of the dopamine D4 receptor gene exon III polymorphism and response to methylphenidate treatment in Korean ADHD children. Neuropsychopharmacology. 2007 Jun;32(6):1377–1383. doi: 10.1038/sj.npp.1301244. [DOI] [PubMed] [Google Scholar]
- 34.Nestler EJ, Hyman SE, Malenka RC. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience. New York: The McGraw-Hill Companies, Inc; 2001. [Google Scholar]
- 35.Lario S, Calls J, Cases A, Oriola J, Torras A, Rivera F. MspI identifies a biallelic polymorphism in the promoter region of the alpha 2A-adrenergic receptor gene. Clinical genetics. 1997 Feb;51(2):129–130. [PubMed] [Google Scholar]
- 36.Polanczyk G, Zeni C, Genro JP, et al. Association of the adrenergic alpha2A receptor gene with methylphenidate improvement of inattentive symptoms in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007 Feb;64(2):218–224. doi: 10.1001/archpsyc.64.2.218. [DOI] [PubMed] [Google Scholar]
- 37.da Silva TL, Pianca TG, Roman T, et al. Adrenergic alpha2A receptor gene and response to methylphenidate in attention-deficit/hyperactivity disorder-predominantly inattentive type. J Neural Transm. 2008;115(2):341–345. doi: 10.1007/s00702-007-0835-0. [DOI] [PubMed] [Google Scholar]
- 38.Cheon KA, Cho DY, Koo MS, Song DH, Namkoong K. Association between homozygosity of a G allele of the alpha-2a-adrenergic receptor gene and methylphenidate response in Korean children and adolescents with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009 Apr 1;65(7):564–570. doi: 10.1016/j.biopsych.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996 Jun;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Cheon KA, Jun JY, Cho DY. Association of the catechol-O-methyltransferase polymorphism with methylphenidate response in a classroom setting in children with attention-deficit hyperactivity disorder. Int Clin Psychopharmacol. 2008 Sep;23(5):291–298. doi: 10.1097/YIC.0b013e328306a977. [DOI] [PubMed] [Google Scholar]
- 41.Polanczyk G, Faraone SV, Bau CH, et al. The impact of individual and methodological factors in the variability of response to methylphenidate in ADHD pharmacogenetic studies from four different continents. Am J Med Genet B Neuropsychiatr Genet. 2008 Dec 5;147B(8):1419–1424. doi: 10.1002/ajmg.b.30855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kieling C, Genro JP, Hutz MH, Rohde LA. A current update on ADHD pharmacogenomics. Pharmacogenomics. 2010;11:407–419. doi: 10.2217/pgs.10.28. [DOI] [PubMed] [Google Scholar]
- 43.Froehlich TE, Stein MA. Pharmacogenomics of ADHD. In: Schwab M, Kaschka W, Spina E, editors. Pharmacogenomics in Psychiatry. Basil, Switzerland: Karger Medical and Scientific Publishers; 2010. [Google Scholar]
- 44.Yang L, Wang YF, Li J, Faraone SV. Association of norepinephrine transporter gene with methylphenidate response. J Am Acad Child Adolesc Psychiatry. 2004 Sep;43(9):1154–1158. doi: 10.1097/01.chi.0000131134.63368.46. [DOI] [PubMed] [Google Scholar]
- 45.A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999 Dec;56(12):1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 46.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000 Jan;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 47.Wolraich ML, Feurer ID, Hannah JN, Baumgaertel A, Pinnock TY. Obtaining systematic teacher reports of disruptive behavior disorders utilizing DSM-IV. Journal of abnormal child psychology. 1998 Apr;26(2):141–152. doi: 10.1023/a:1022673906401. [DOI] [PubMed] [Google Scholar]
- 48.Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley K. Psychometric properties of the Vanderbilt ADHD diagnostic parent rating scale in a referred population. J Pediatr Psychol. 2003 Dec;28(8):559–567. doi: 10.1093/jpepsy/jsg046. [DOI] [PubMed] [Google Scholar]
- 49.Wolraich ML, Lambert EW, Bickman L, Simmons T, Doffing MA, Worley KA. Assessing the impact of parent and teacher agreement on diagnosing attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2004 Feb;25(1):41–47. doi: 10.1097/00004703-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Malhi P, Singhi P, Sidhu M. Impact of parent and teacher concordance on diagnosing attention deficit hyperactivity disorder and its sub-types. Indian J Pediatr. 2008 Mar;75(3):223–228. doi: 10.1007/s12098-008-0049-y. [DOI] [PubMed] [Google Scholar]
- 51.Buitelaar JK, Van der Gaag RJ, Swaab-Barneveld H, Kuiper M. Prediction of clinical response to methylphenidate in children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995 Aug;34(8):1025–1032. doi: 10.1097/00004583-199508000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Stein MA, Sarampote CS, Waldman ID, et al. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003 Nov;112(5):e404. doi: 10.1542/peds.112.5.e404. [DOI] [PubMed] [Google Scholar]
- 53.Barkley RA, DuPaul GJ, McMurray MB. Attention deficit disorder with and without hyperactivity: clinical response to three dose levels of methylphenidate. Pediatrics. 1991 Apr;87(4):519–531. [PubMed] [Google Scholar]
- 54.Gorman EB, Klorman R, Thatcher JE, Borgstedt AD. Effects of methylphenidate on subtypes of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006 Jul;45(7):808–816. doi: 10.1097/01.chi.0000214191.57993.dd. [DOI] [PubMed] [Google Scholar]
- 55.Laird N. Further comparative analyses of prettest-posttest research designs. The American Statistician. 1983;37(4):329–330. [Google Scholar]
- 56.Barkley RA. Predicting the response of hyperkinetic children to stimulant drugs: a review. Journal of abnormal child psychology. 1976;4(4):327–348. doi: 10.1007/BF00922531. [DOI] [PubMed] [Google Scholar]
- 57.Arnold LE, Elliot M, Sachs L, et al. Effects of ethnicity on treatment attendance, stimulant response/dose, and 14-month outcome in ADHD. Journal of consulting and clinical psychology. 2003 Aug;71(4):713–727. doi: 10.1037/0022-006x.71.4.713. [DOI] [PubMed] [Google Scholar]
- 58.Starr HL, Kemner J. Multicenter, randomized, open-label study of OROS methylphenidate versus atomoxetine: treatment outcomes in African-American children with ADHD. Journal of the National Medical Association. 2005 Oct;97(10 Suppl):11S–16S. [PMC free article] [PubMed] [Google Scholar]
- 59.Kleinbaum DG, Kupper LL, Muller KE, Nizam A. Applied Regression Analysis and Other Multivariable Methods. 3. Pacific Grove, CA: Brooks/Cole Publishing Company; 1998. [Google Scholar]
- 60.Gilbert DL, Wang Z, Sallee FR, et al. Dopamine transporter genotype influences the physiological response to medication in ADHD. Brain. 2006 Aug;129(Pt 8):2038–2046. doi: 10.1093/brain/awl147. [DOI] [PubMed] [Google Scholar]
- 61.Schoots O, Van Tol HH. The human dopamine D4 receptor repeat sequences modulate expression. The pharmacogenomics journal. 2003;3(6):343–348. doi: 10.1038/sj.tpj.6500208. [DOI] [PubMed] [Google Scholar]
- 62.Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995 Sep;65(3):1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- 63.Jovanovic V, Guan HC, Van Tol HH. Comparative pharmacological and functional analysis of the human dopamine D4.2 and D4.10 receptor variants. Pharmacogenetics. 1999 Oct;9(5):561–568. [PubMed] [Google Scholar]
- 64.Sprague RL, Sleator EK. Methylphenidate in hyperkinetic children: differences in dose effects on learning and social behavior. Science. 1977 Dec 23;198(4323):1274–1276. doi: 10.1126/science.337493. [DOI] [PubMed] [Google Scholar]
- 65.Rowe DC, Stever C, Giedinghagen LN, et al. Dopamine DRD4 receptor polymorphism and attention deficit hyperactivity disorder. Mol Psychiatry. 1998 Sep;3(5):419–426. doi: 10.1038/sj.mp.4000432. [DOI] [PubMed] [Google Scholar]
- 66.Todd RD, Huang H, Smalley SL, et al. Collaborative analysis of DRD4 and DAT genotypes in population-defined ADHD subtypes. J Child Psychol Psychiatry. 2005 Oct;46(10):1067–1073. doi: 10.1111/j.1469-7610.2005.01517.x. [DOI] [PubMed] [Google Scholar]
- 67.Roman T, Schmitz M, Polanczyk GV, Eizirik M, Rohde LA, Hutz MH. Is the alpha-2A adrenergic receptor gene (ADRA2A) associated with attention-deficit/hyperactivity disorder? Am J Med Genet B Neuropsychiatr Genet. 2003 Jul 1;120(1):116–120. doi: 10.1002/ajmg.b.20018. [DOI] [PubMed] [Google Scholar]
- 68.Roman T, Polanczyk GV, Zeni C, Genro JP, Rohde LA, Hutz MH. Further evidence of the involvement of alpha-2A-adrenergic receptor gene (ADRA2A) in inattentive dimensional scores of attention-deficit/hyperactivity disorder. Mol Psychiatry. 2006 Jan;11(1):8–10. doi: 10.1038/sj.mp.4001743. [DOI] [PubMed] [Google Scholar]
- 69.Park L, Nigg JT, Waldman ID, et al. Association and linkage of alpha-2A adrenergic receptor gene polymorphisms with childhood ADHD. Mol Psychiatry. 2005 Jun;10(6):572–580. doi: 10.1038/sj.mp.4001605. [DOI] [PubMed] [Google Scholar]
- 70.Comings DE, Gade-Andavolu R, Gonzalez N, Blake H, Wu S, MacMurray JP. Additive effect of three noradrenergic genes (ADRA2a, ADRA2C, DBH) on attention-deficit hyperactivity disorder and learning disabilities in Tourette syndrome subjects. Clinical genetics. 1999 Mar;55(3):160–172. doi: 10.1034/j.1399-0004.1999.550304.x. [DOI] [PubMed] [Google Scholar]
- 71.Mick E, Neale B, Middleton FA, McGough JJ, Faraone SV. Genome-wide association study of response to methylphenidate in 187 children with attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008 Dec 5;147B(8):1412–1418. doi: 10.1002/ajmg.b.30865. [DOI] [PubMed] [Google Scholar]
- 72.Graetz BW, Sawyer MG, Baghurst P. Gender differences among children with DSM-IV ADHD in Australia. J Am Acad Child Adolesc Psychiatry. 2005 Feb;44(2):159–168. doi: 10.1097/00004583-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, Recognition, and Treatment of Attention-Deficit/Hyperactivity Disorder in a National Sample of U.S. Children. Archives of Pediatrics and Adolescent Medicine. 2007;161:857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- 74.Wolraich ML, Hannah JN, Pinnock TY, Baumgaertel A, Brown J. Comparison of diagnostic criteria for attention-deficit hyperactivity disorder in a county-wide sample. J Am Acad Child Adolesc Psychiatry. 1996 Mar;35(3):319–324. doi: 10.1097/00004583-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 75.Gaub M, Carlson CL. Behavioral characteristics of DSM-IV ADHD subtypes in a school-based population. Journal of abnormal child psychology. 1997 Apr;25(2):103–111. doi: 10.1023/a:1025775311259. [DOI] [PubMed] [Google Scholar]
- 76.Baumgaertel A, Wolraich ML, Dietrich M. Comparison of diagnostic criteria for attention deficit disorders in a German elementary school sample. J Am Acad Child Adolesc Psychiatry. 1995 May;34(5):629–638. doi: 10.1097/00004583-199505000-00015. [DOI] [PubMed] [Google Scholar]