Abstract
The hallmark of a vaccine is to induce long-term protective immunity against the pathogen. The use of Mycobacterium bovis BCG as a vaccine against tuberculosis has been problematic in that immunity induced by BCG wanes over time and it may be less effective against more virulent strains of M. tuberculosis. Thus it is important to determine what factors might be associated with waning or inefficient immunity. One such factor has been associated with the difference in many types of BCG that are used around the world, or more specifically due to the loss of genomic material in the various sub-strains used in vaccination programs. To address this issue we investigated the long-term immune response generated by 3 sub-strains BCG in the C57BL/6 mouse model of experimental tuberculosis. Mice vaccinated with these diverse strains of BCG were assessed at 6 and 12 months post-vaccination. All BCG sub-strain induced elevated levels of IFN-γ-producing cells at each time point as determined by ELISpot assay. However, when mice were challenged at 6 and 12 months with either M. tuberculosis H37Rv or HN878 the ability of the BCG sub-strains to protect vaccinated mice varied, depending on the time of challenge and on the strain used to infect mice. BCG Pasteur was then used to vaccinate guinea pigs, which were subsequently infected with either H37Rv or HN878. Data showed that BCG Pasteur prolonged the survival of guinea pigs against infection with both strains. Taken together these data suggest that longevity of the immune response generated by BCG is not related to the loss of genetic material and that BCG can induce a protective immune response to infection with a clinical strain of M. tuberculosis.
Keywords: tuberculosis, BCG vaccine, immune response, animal models
Introduction
Mycobacterium bovis bacillus Calmette Guérin (BCG) has been used extensively as a vaccine throughout the world to combat tuberculosis. However it is now recognized that vaccination with BCG is not sufficient to protect humans against tuberculosis and thus novel vaccines are currently being developed. BCG is a complex, live attenuated vaccine that can induce strong innate and adaptive immune responses [1]. In humans, meta-analysis of clinical trials performed throughout the world has shown that immunity generated by BCG may be compromised by factors that result in variable efficacy [2]. Several theories have been put forth to explain why BCG may fail, including interference/masking by non-tuberculous mycobacteria, and the production of various sub-strains that are "impotent" due to serial passage leading to the loss of genetic and thus antigenic components required for generating effective anti-tuberculosis immunity [3, 4]. In addition, it has been postulated that the use of BCG has resulted in the emergence of more virulent strains of M. tuberculosis that are currently in circulation throughout the world today [5]. Therefore, knowing how BCG might fail at inducing effective long-term immunity may provide us with insights into developing new vaccines, for example in developing novel vaccines that can boost BCG induced immunity or combining BCG with novel vaccines that can supplement deficiencies of BCG.
Our previous study in the mouse model examined the immune response generated by three sub-strains of BCG (Connaught, Sweden and Pasteur), each with varying deletions of genetic material [6]. These studies showed that the quality of the effector immune response was not directly related to the genomic content of the sub-strains of BCG used for vaccination. These sub-strains induced varying levels of immunity within 7 days post-vaccination, after which there was a reduction of the effector immune response, probably associated with BCG clearance. In spite of this varied response, the ability of each strain to reduce the mycobacterial burden within 30 days after vaccination was similar for all the sub-strains, suggesting that a threshold level of immunity had been attained by each sub-strain and that anything more had no greater effect. To further understand the immune response generated by BCG we chose to investigate the long-term immune response after vaccination in the mouse model of tuberculosis, and to examine the capacity of these strains to induce a protective response against two strains of M. tuberculosis, a laboratory isolate H37Rv and a clinical isolate HN878. In addition, a sub-strain of BCG was selected, based on its ability to induce protective immunity against both M. tuberculosis strains in the mouse model and then tested in the guinea pig model to determine if it could prolong the survival of guinea pigs after infection with either of the two M. tuberculosis strains.
In C57BL/6 mice, immunity to vaccination with each sub-strain was assessed at 6 and 12 months using an IFN-γ ELISpot assay to assess antigen-specific T cell reactivity. Mice were also infected at these time points with a low dose aerosol with either M. tuberculosis H37Rv or HN878. Guinea pigs were vaccinated with BCG Pasteur, infected with each strain and their body temperature and survival monitored. Our data show that vaccination with BCG induced long-term antigen-specific T cells that provided variable protective immunity against both laboratory and clinical isolates in the mouse model, although surprisingly better against the clinical isolate. In the guinea pig, BCG Pasteur was able to significantly prolong the survival in both H37Rv and HN878 infected animals.
Materials and Methods
Animals
Pathogen free, female, 6-8 week old C57BL/6 mice and out-bred Hartley guinea pigs, weighing 450–500 grams were purchased from Charles River Laboratory (Wilmington, MA). All mice and guinea pigs were maintained in the Animal Biosafety Level 3 facility at Colorado State University with sterile chow and water ad libitum. The pathogen free nature of the mice was determined by routine screening of sentinel mice. Colorado State University Animal Care and Use Committee approved all experimental procedures.
BCG Vaccination
M. bovis, Connaught, Sweden and Pasteur were grown on potato soaked in Sauton’s medium and then grown as a surface pellicle on Sauton’s liquid medium at 37.5°C [7]. Concentrated stocks were stored in Sauton’s liquid at −20°C or in glycerol at −80°C. At CSU, each sub-strain was passaged through Proskauer Beck (P&B) medium containing 0.05% Tween 80 to mid-log phase. Stock aliquots of 1ml were frozen at −80°C until they were used. In preparation for vaccination, the three sub-strains were diluted in pyrogen-free, sterile saline. Mice were vaccinated subcutaneously once with 106 CFU of the BCG sub-strain in the scruff of the neck. Guinea pigs were vaccinated intradermally with 103 CFU. The concentration of each sub-strain was confirmed by plating 10-fold serial dilutions on Middlebrook 7H11 agar (Difco Laboratories, Detroit Ml). The plates were incubated at 37°C and counted after 14-21 days.
Infection with M. tuberculosis
Mice and guinea pigs were infected with Mycobacterium tuberculosis, H37Rv (TMC #102), obtained from the Trudeau Mycobacterial Culture Collection and was passed three times through pellicle and frozen as seed stock. Working stocks were made by passage through liquid culture of Proskauer-Beck medium containing 0.01% Tween 80, and aliquots were made during mid-log phase growth and stored at −80°C until needed. HN878 (W-Beijing strain) was obtained from the TB Vaccine Testing and Research Materials contract (NIH/NIAID NO1-AI-40091) and grown in an identical manner to the H37Rv strain. Mice were infected using the Middlebrook Aerosol Exposure chamber (GlasCol, LLC, Terre Haute, IN). Briefly, bacterial stocks were diluted to 2 × 106 CFU/ml and placed in a nebulizer attached to an airborne infection system. Mice were exposed to 40 minutes of aerosol, during which approximately 50-100 bacteria were deposited in the lungs of each animal. Actual infectious dose was confirmed by homogenizing whole lungs from four mice at 24 hours post aerosol and plating the homogenates onto nutrient 7H11 agar supplemented with oleic acid, albumin, catalase and dextrose (OADC). Colonies were counted after incubation at 37°C for 14-21 days. All infected mice were sacrificed at 30 days post challenge; and the lung and spleens were excised, homogenized in sterile saline, and plated on Middlebrook 7H11 agar (Difco Laboratories, Detroit Ml). Colonies were counted after incubation at 37°C for 14-21 days. Guinea pigs were exposed via the respiratory route to 10-20 CFU of virulent M. tuberculosis H37Rv using a Madison Aerosol Chamber (Madison, Wl). Working stocks of M. tuberculosis H37Rv were diluted to 106 CFU/ml in sterile distilled water and placed in the nebulizer jar and animals were exposed to the aerosol for 5 min. For survival studies, guinea pigs were monitored daily and weighed once each week. Guinea pigs were euthanized when they reached set criteria established by the animal care and use committee such as being moribund or exceeding acceptable weight loss, and/or respiratory rate (labored/heavy breathing). Time to euthanasia is used as time to death. Body temperature was measured to track clinical progression of disease using a microchip implanted subcutaneously (IPT-300, Bio Medic Data Systems Inc., Seaford, DE) that allowed measurement of temperature and also carried information about experiment number and animal number. Body temperature of individual guinea pigs was assessed each day at approximately the same time in the afternoon using a DAS-6006/7 scanner transponder (BMDS). Previous studies had shown that the body temperature of guinea pigs did not vary significantly during the day and that deviations from normal temperature curves were indicative of disease progression. Experiments were performed with 10 guinea pigs per group. Determination of post-infection colony forming units: To assess colony forming units in the lungs of infected animals, the right lung from mice at each sacrifice time-point was excised. Lungs were homogenized with a PRO250® homogenizer (PRO Scientific, Oxford, CT) in sterile tubes containing 4.5 ml of isotonic saline. Four 10-fold dilutions of each homogenate were spread onto plates containing Middlebrook 7H11 agar (Difco Laboratories) enriched with OADC. Plates were incubated at 37°C, 5%CO2 and the number of colonies was counted 14-21 days.
Enzyme-linked immunospot assay (ELISpot) for IFN-γ-producing cells
Cells were prepared from spleens of BCG-vaccinated mice at 6 and 12 months post vaccination. The cells were cultured in complete RPMI-1640 medium (10% fetal bovine serum, penicillin-streptomycin, and L-glutamine) in 96-well sterile plates pre-coated with anti IFN-γ, according to the manufacturer’s protocol (BD Bioscience, San Jose, CA). Cells were plated at 5×103, 5×104 and 5×105 cells per well in duplicate. To determine if there was a difference in the ability of cells to produce IFN-γ from mice vaccinated with different BCG sub-strains, spleen cells were stimulated with either 5μg of Culture Filtrate Protein (CFP), or BCG Connaught, Sweden or Pasteur depending on the vaccine the mice had received. The plates were developed with horseradish peroxidase-conjugated-anti-IFN-γ-detecting-antibody, followed by AEC substrate solution, according to the manufacturer’s protocol (BD Bioscience, San Jose, CA). The spots were counted with an Immunospot reader (Cellular Technology Limited, Cleveland, OH).
Cytometric Bead Array
A Cytometric Bead Array Mouse Th1/Th2 kit (BD Biosciences, San Jose, CA) was used to measure IL-2, IL-4, IL-5, IFN-γ, and TNF-α. The assay procedure was completed according to kit instructions, and beads were analyzed on a FACSCalibur flow cytometer (BD Bioscience). Lung homogenates were centrifuged to remove tissue debris prior to performing assay.
Histology
The right caudal lobe of the guinea pig lung was utilized to analyze pathological lesions. The excised lobe was inflated with formalin and placed in total into formalin. For processing the lobe was embedded in paraffin and sections cut and stained with Hematoxylin and Eosin (H&E; IHC Tech, Aurora, CO). Photomicrographs were taken using an Olympus BX41 microscope attached to a Dell Precision computer with DP2-BSW software for image capture.
Statistical Analysis
Two Way Analysis of Variance with Bonferroni multiple comparisons was performed for the ELISpot data. Student t-test analysis was performed on log10 CFU transformed data and from cytokine data. Guinea pig survival was plotted using the Kaplan-Meier method, and differences between curves were analyzed using the log-rank test.
Results
Induction of long-term IFN-γ producing cells after BCG vaccination
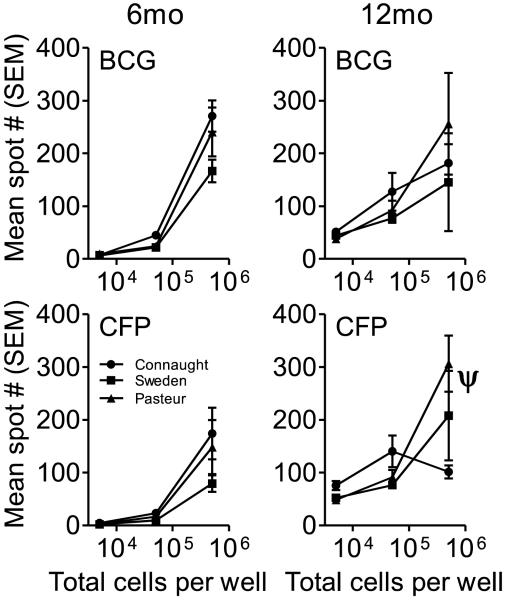
Mice were evaluated at 6 and 12 months post-vaccination for the number of IFN-γ producing cells by ELISpot assay. At 6 and 12 months post-vaccination all three sub-strain induced elevated levels of IFN-γ producing cells in the spleens of mice, when stimulated ex vivo with either the same BCG with which they were vaccinated or with CFP when compared to cells that were not stimulated in vitro (Figure 1). In general, the percentage of IFN-γ producing cells was less than 1% for both of the time points regardless of the stimulant. At 6 months post-vaccination there was no significant difference in the number of IFN-γ-producing splenocytes between groups for both CFP and BCG stimulated cells. Similarly at 12 months post-vaccination there was no significant difference in the number of IFN-γ-producing cells between groups when stimulated with either CFP or BCG except in the cells cultured at 5×105 cells/ml and stimulated with CFP, in which the number of spots induced by BCG Pasteur vaccination was significantly greater than those observed with BCG Connaught (p<0.001). In addition, there was no significant difference when comparing the number of IFN-γ producing cells at 6 and 12 months post-vaccination. Overall the data suggest that vaccination of C57BL/6 mice with BCG, regardless of the sub-strain, generated a population of persisting antigen-specific T cells that were capable of responding to antigenic stimulation for up to 12 months. Given the fact that there was no difference between the 6 and 12 month time points, it may be presumed that this level of antigen-specific T cells represents a baseline of persisting T cells capable of responding to antigenic stimuli.
Figure 1.
C57BL/6 mice were vaccinated with BCG Connaught, Sweden and Pasteur and the number of antigen-specific T cells in the spleen determined at 6 and 12 months post-vaccination by IFN-γ ELISpot. Spleen cells from vaccinated mice were cultured at three concentrations with either 5μg of CFP or the BCG with which they were vaccinated for 24 hours and then processed according to the manufacturer’s protocol. N = 5 mice per group per time point, ψ = p<0.001.
Pulmonary infection with virulent M. tuberculosis H37Rv at 6 and 12 months post-vaccination
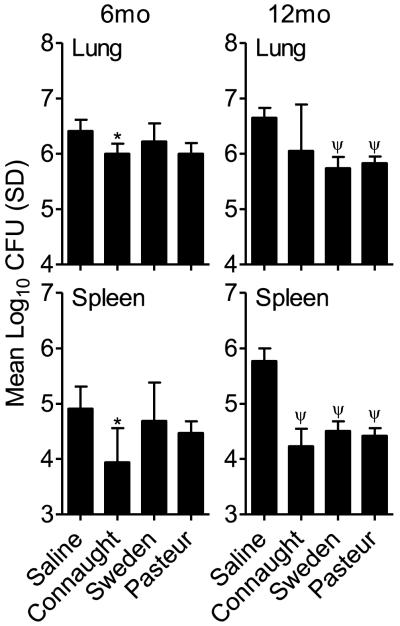
To determine if there was a correlation between the persistence of antigen specific T cells and the ability to reduce the mycobacterial burden, mice were infected with a low dose aerosol of virulent M. tuberculosis H37Rv. At day 30 post-infection, the number of CFU in the lung and spleen was determined. At 6 months post-vaccination there was a significant reduction in the lung and spleen CFU of BCG Connaught vaccinated mice when compared to Saline-treated animals (p<0.05; Figure 2A), which was not observed in mice vaccinated with the other two sub-strains. In contrast, at 12 months there were significant reductions in the lung and spleen CFU for all the groups except those vaccinated with BCG Connaught (p<0.001; Figure 2B). There was a trend, although not significant, in the Connaught vaccinated group, for a reduction in lung CFU. The CFU in the spleens of mice in all groups was significantly reduced compared to the saline treated group.
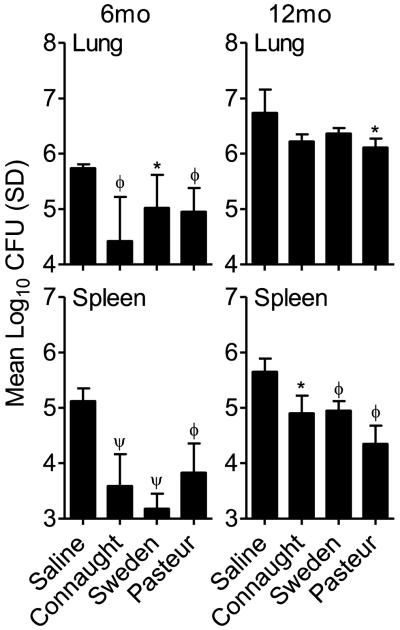
Figure 2.
Colony forming units (CFU) in the lungs and spleens of mice vaccinated with three sub-strains of BCG, Connaught, Sweden and Pasteur at 6 and 12 months prior to low dose aerosol infection with M. tuberculosis H37Rv. CFU were determined at 30 days post-infection by plating organ homogenates on 7H11 agar. N = 5 mice per group per time point. * = p<0.05 and ψ = p<0.001.
Post-infection cytokine profiles
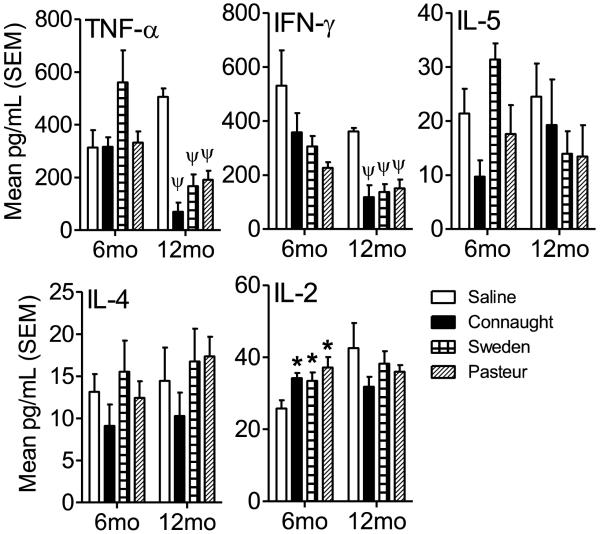
Lung homogenates from day 30 post-infection were assessed for Th1 and Th2 cytokines to determine if there was a significant shift in the cytokine profile at 6 and 12 months post-vaccination. The lung milieu was assayed for the presence of TNF-α, IFN-γ, IL-5, IL-4 and IL-2. At 6 months post-vaccination the concentration of IL-2 was the only cytokine significantly elevated in the lungs of BCG vaccinated mice compared to the Saline-treated group (p<0.05; Figure 3). Although the post-infection immune response reflected that predominantly of a Th1 immune response in all groups, it was insufficient in all but the Connaught BCG-vaccinated groups to cause a significant reduction in CFU (Figure 2). Interestingly, the lung cytokine milieu in the Sweden and Pasteur vaccinated mice had levels of IL-4 and IL-5 similar to or greater than in the Saline-treated mice (although not statistically significant), which may have contributed to their inability to significantly reduce the mycobacterial burden. At 12 months post-vaccination the concentration of TNF-α and IFN-γ were significantly decreased in the lungs of all BCG-vaccinated mice when compared to the Saline-treated group (p<0.001; Figure 3). The reduced levels of TNF-α and IFN-γ at this time point may reflect the reduced numbers of organisms in the lung compared to the Saline-treated group. Overall, the data suggest that there was a change in the quality of the immune response between 6 months and 12 months post-vaccination and that the ability to reduce the mycobacterial burden was similar amongst the BCG sub-strains in the long term.
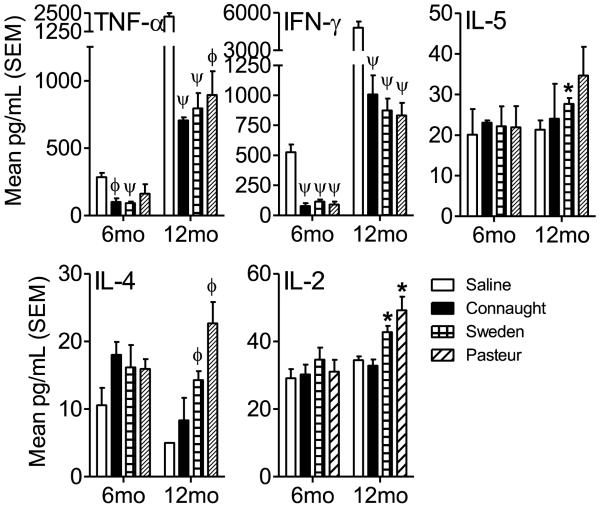
Figure 3.
To determine the cytokine milieu after infection with M. tuberculosis H37Rv in the lungs of vaccinated mice, lung homogenates were analyzed by Cytometric Bead Array assay for TNF-α, IFN-γ, IL-5, IL-4 and IL-2 according to the manufacturer’s protocol. Data is expressed as the mean and standard error of the mean (SEM) in pg/ml for each cytokine. N = 5 mice per group per time point. * = p<0.05 and ψ = p<0.001.
Pulmonary infection with the “hyper-virulent” HN878 strain of M. tuberculosis
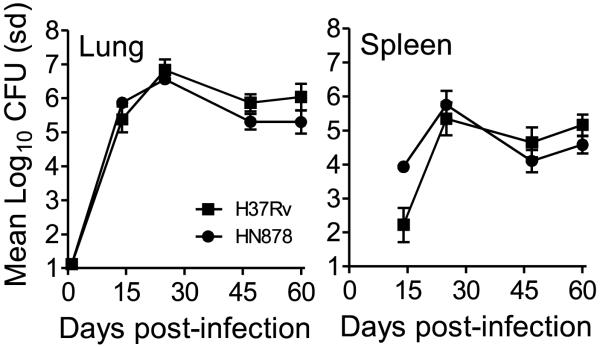
To determine if the ability of the immune response generated by the BCG sub-strains to affect the growth after infection with virulent M. tuberculosis H37Rv was the same with other strains of M. tuberculosis, we infected mice that were vaccinated with the BCG sub-strains at 6 and 12 month with a low dose aerosol infection of the M. tuberculosis HN878. Prior to this infection however, the growth of HN878 after a low dose aerosol was assessed in C57BL/6 mice. The organism grew in a similar manner in the lungs of mice to that observed with H37Rv during the first 60 days of infection (Figure 4) although HN878 seeded the spleen at a higher CFU than H37Rv at day 14, but beyond this time point the two strains grew at the same rate in the spleen.
Figure 4.
Growth of M. tuberculosis H37Rv and HN878 after low dose aerosol infection in C57BL/6 mice at 14, 28, 45 and 60 days post-infection in the lung and spleen. Data is expressed as the mean Log10 CFU and standard deviation (sd).
Mice vaccinated with BCG for 6 months were able to significantly reduce the mycobacterial burden in the lungs and spleens of HN878 infected mice when compared to the Saline-treated group (Figure 5). In contrast at 12 months post-vaccination, only BCG Pasteur-vaccinated mice had significantly fewer organisms in their lungs, although all groups had significantly reduced mycobacteria in their spleens (Figure 5). These observations contrasted those obtained with H37Rv and may reflect the difference between the two strains of M. tuberculosis. The post-infection Th1 and Th2 cytokine profile in the lung milieu was then examined using lung homogenates to determine if there was a shift in the immune response. At 6 and 12 months post-vaccination the immune phenotype in response to infection was predominantly Th1, but varied between groups (Figure 6). TNF-α and IFN-γ concentrations were significantly reduced at both time points in vaccinated mice when compared to the Saline-treated group. However at 12 months, mice vaccinated with BCG Pasteur and Sweden also had significantly elevated levels of IL-4 and IL-2, and the latter also had elevated IL-5 concentrations compared to Saline-treated mice.
Figure 5.
Colony forming units (CFU) in the lungs and spleens of mice vaccinated with three sub-strains of BCG, Connaught, Sweden and Pasteur at 6 and 12 months prior to low dose aerosol infection with M. tuberculosis HN878. CFU were determined at 30 days post-infection by plating organ homogenates on 7H11 agar. N = 5 mice per group per time point. * = p<0.05, ϕ = p<0.01 and ψ = p<0.001.
Figure 6.
To determine the cytokine milieu after infection with M. tuberculosis HN878 in the lungs of vaccinated mice, lung homogenates were analyzed by Cytometric Bead Array assay for TNF-α, IFN-γ, IL-5, IL-4 and IL-2 according to the manufacturer’s protocol. Data is expressed as the mean and standard error of the mean (SEM) in pg/ml for each cytokine. N = 5 mice per group per time point. * = p<0.05, ϕ = p<0.01 and ψ = p<0.001.
Taken together the data from the C57BL/6 mouse model suggest that the strain used to assess BCG vaccine efficacy and the time post-vaccination can be factors that affect the outcome.
Infection of guinea pigs after vaccination with BCG Pasteur
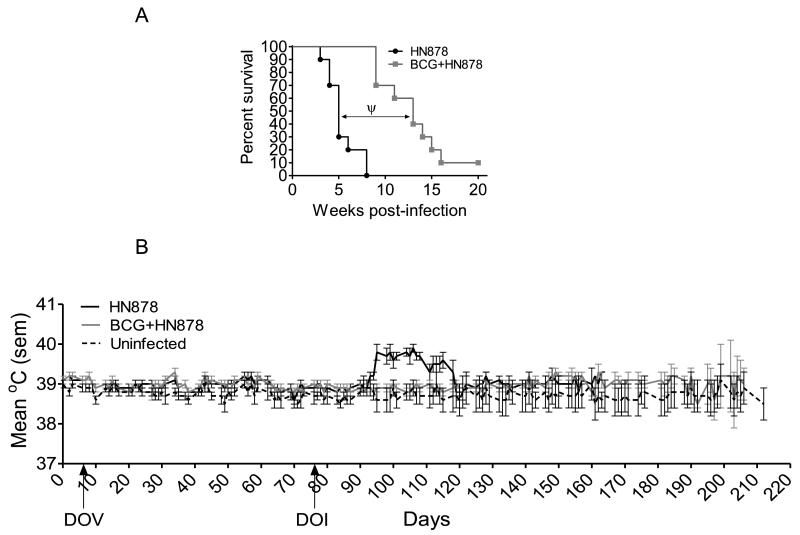
To confirm whether BCG was able to mount an effective immune response against infection with a hypervirulent clinical isolate, BCG Pasteur was used to vaccinate guinea pigs, which were then challenged with a low dose of M. tuberculosis HN878. BCG Pasteur was chosen because it significantly reduced the mycobacterial burden in the mouse model at 12 months post-vaccination for both strains of M. tuberculosis. When guinea pigs were infected with HN878, the median survival time (MST) was approximately 5 weeks (Figure 7A), compared to that of H37Rv, which was approximately 15 weeks post-infection (data not shown), indicating that HN878 was of greater virulence in the guinea pig. Guinea pigs vaccinated with BCG, 10 weeks previously and then infected with HN878 had a significantly prolonged survival time (p<0.001) when compared to non-vaccinated animals, suggesting that BCG was an effective vaccine against the clinical strain. Assessment of body temperatures confirmed the fact that BCG vaccination was effective against the pulmonary challenge, in that it prevented the characteristic increase in temperature at 25-30 days post-vaccination that was observed in the HN878 infected group (Figure 7B).
Figure 7.
(A) Kaplan-Meier plot of the survival of non-vaccinated and BCG-vaccinated guinea pigs after low dose aerosol infection with M. tuberculosis HN878. Guinea pigs were vaccinated with 103 CFU BCG Pasteur or with an equal volume of pyrogen-free sterile saline. Guinea pigs were monitored daily and weighed weekly to assess their health status. n=10 guinea pigs per group. (B) Body temperature measurement of guinea pigs after low dose aerosol infection with virulent M. tuberculosis via implanted temperature measuring microchips. Animals were monitored daily at approximately the same time of day. n=10 guinea pigs per group. Arrows indicate day of vaccination (DOV) and day of low dose aerosol infection (DOI).
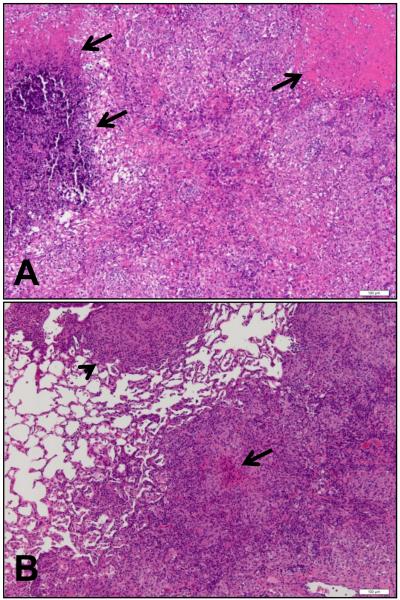
Histological examination of lungs from infected animals showed that HN878 caused significant pulmonary pathology, as demonstrated by the presence of large lesions throughout the lungs. These lesions contained areas of necrosis (Figure 8A, arrows) that were poorly organized and contained areas of randomly accumulated cells. In contrast, lesions in the BCG vaccinated, infected animals consisted of tight accumulations of cells in granulomatous structures, which occupied smaller areas of the lungs (Figure 8B, arrowhead). Some lesions in vaccinated animals also contained small areas of necrosis (Figure 8B, arrow) and a histological assessment and scoring of lesions over a number of experiments, in which guinea pigs were infected with HN878, indicated that there was a trend for BCG-vaccinated group to have smaller lesions with less necrosis (data not shown).
Figure 8.
Representative photomicrographs of lungs from non-vaccinated (A) and BCG-vaccinated (B) guinea pigs at the time of necropsy after Low dose aerosol infection with M. tuberculosis HN878. Lung lobes from guinea pigs were processed and stained with hematoxylin and eosin. Bar = 100μm.
Discussion
The current study was designed to determine if BCG vaccination induced long-term immunity against a laboratory and clinical strain of M. tuberculosis and whether this immunity was related to the amount of genomic deletion in the different sub-strains. Three sub-strains of BCG and two strains of M. tuberculosis (H37Rv and HN878), were used in the C57BL/6 model of tuberculosis to determine whether BCG was able to induce long term immunity that could cause a significant reduction in bacterial burden. Our studies highlight the fact that regardless of the genetic composition, BCG when used as a vaccine was able to induce IFN-γ-producing T cells for up to 12 months after subcutaneous vaccination. Thus BCG, regardless of the genetic deletions, produced long-lived immune T cells that were reactive to antigen stimulation and produced an important cytokine know to be a factor required for the control of tuberculosis infection. The ability of the BCG sub-strains to cause a reduction in the mycobacterial burden after infection at 6 and 12 months varied and was dependent on the M. tuberculosis strain used to infect mice. Interestingly, vaccinated mice infected with the laboratory strain, H37Rv were overall, less capable of controlling infection than mice infected with the clinical isolate, HN878. In this regard there was variation amongst the BCG sub-strains to reduce the mycobacterial burden. In response to infection with H37Rv, the immunity induced by BCG Connaught was the most effective at 6 months, but less so at 12 months post-vaccination, while BCG Sweden and Pasteur were less effective at 6 months than at 12 months. After low dose infection with HN878, all BCG sub-strains caused a significant reduction in CFU at 6 months post-vaccination, but this was not evident at 12 months, except for BCG Pasteur. In relating these outcomes to the known genetic deletions for each sub-strain Sweden is the least, while Connaught and Pasteur have comparable, but different deletions [8]. Similar to our previous data, the ability of each sub-strain was not related to the extent of genomic deletion. In addition, analysis of the known single nucleotide polymorphisms (SNPs) shows that Sweden is the least divergent from the ancestral strain followed by Connaught and then Pasteur [9], There did not seem to be a relationship in the number of SNPs with the ability to cause a reduction in mycobacteria at either 6 or 12 months. In general, there was good agreement in CFU reduction between the lung and spleen, although at 12 month vaccinated mice infected with HN878 there was a difference between these organs. This may be due to T cells present at the being incapable of migrating to the lung or the infection being more overwhelming in the lung and may be a feature of the enhanced virulence of HN878. Further studies are currently in progress to investigate this difference. Taken together the data suggest that although antigen-specific T cells are present throughout the 12 months after infection, other factors may play a significant role in determining the outcome of infection and that the infecting strain may also play a significant role.
Results from the current studies do not support the hypothesis that Beijing strains are resistant to BCG induced immunity [10], but in contrast support the findings of Jeon et al [11] who showed that BCG was able to induce a significant reduction in mycobacterial burden for 6 months after vaccination and a partial reduction after 12 months post-vaccination. Indeed it seemed that H37Rv was more resistant than HN878 to BCG induced immunity at both time points as the reduction in CFU was consistently less significant than that observed for HN878. To date these are the only studies that have examined the ability of BCG to induce long-term immunity against a Beijing strain of M. tuberculosis.
An examination of the post-infection cytokine profile demonstrated that infection after BCG Pasteur vaccination induced a Th2 response. The induction of Th2 immunity may also be related to the outcome observed in the guinea pig model after vaccination with BCG Pasteur, in which there was dampening of pulmonary pathology after infection. In general, infection with H37Rv induced both a Th1 and Th2 response as indicated by the presence of IFN-γ/TNF-α and IL-4 in the non-vaccinated groups, while HN878 infection induced a strong Th2 response (i.e. no IL-4 in the non-vaccinated group). Therefore, since BCG Pasteur and Sweden both seem to induce Th2 responses, it is possible that this is what is needed to overcome the strong damaging effects of Th1 immunity in the HN878 infected mice (and guinea pigs). The concentration of IFN-γ/TNF-α was lower in the BCG-vaccinated animals when compared to the non-vaccinated groups however the opposite was true for IL-4 concentrations. In non-vaccinated mice, where organism numbers are highest, there was very little IL-4 when compared to the BCG-vaccinated groups, which had increased IL-4 and lower organism numbers and may be dependent on the sub-strain of BCG used for vaccination. Therefore the data suggest that induction of Th2 immunity may be beneficial to the overall host immune response to infection, although the need for a stronger Th1 versus Th2 immunity will need to be achieved so as to limit growth of the mycobacteria.
Clearly vaccination altered the quality of the immune response after infection with both H37Rv and HN878. After infection with H37Rv there was a significant decrease in the level of TNF-α and IFN-γ in the lungs at 12 months post-vaccination that corresponded with a decrease in mycobacterial burden. In contrast the concentration of IL-2 in the lungs of vaccinated mice after infection at 6 months post-vaccination was significantly greater than in non-vaccinated mice, suggesting enhanced proliferation due to the presence of activated antigen-specific T cells. After HN878 infection, BCG-vaccination resulted in reduced concentrations of TNF-α and IFN-γ at both 6 and 12 months. It was interesting that IL-4 cytokine levels were elevated in the lungs of HN878 infected mice at 12 months in mice previously receiving BCG Sweden and Pasteur. These data provide strong evidence to suggest that in the mouse model, BCG can induce long-lived antigen-specific T cells that are both Th1 and Th2. The presence of these cells, whether due to the persistence of extremely low numbers of BCG or persistence of BCG-derived antigens is unclear, however our previous study showed that BCG CFU decline to undetectable levels in the mouse within several weeks post-vaccination [6]. A study by Silva et al [12] in which BALB/c mice were vaccinated with BCG Pasteur demonstrated the presence of antigen-specific T cell at 8 and 15 months post-vaccination, that were capable of reducing the pulmonary H37Rv mycobacterial burden by greater than 0.8 Log-10 CFU in an intravenous infection model. These data are somewhat comparable to those obtained in the present study in which BCG Pasteur reduced the mycobacterial burden approximately 0.4 to 0.8 Log10 CFU at 6 and 12 months post-vaccination for infection with H37Rv. The fact that there was variation in the reduction in CFU at the two points, despite the presence of antigen reactive T cells may suggest that other factors may also play a significant role in the ability of the immune response to combat infection. Such possibilities include the presence of regulatory T cell that can inhibit T cells responses in general, or the induction of a dominant Th2 response that can dampen the magnitude of the Th1 response. This latter situation may have been a factor in mice vaccinated with BCG Sweden and Pasteur, where at 12 months post-vaccination, these mice displayed significant increases in IL4 and IL-5, which may have contributed to a diminished level of CFU reduction.
The two strains of M. tuberculosis used in this study were grown in an identical manner and displayed similar growth characteristics in the C57BL/6 mouse after low dose aerosol exposure. When these strains were used to infect guinea pigs with a low dose aerosol, HN878 was significantly more virulent than H37Rv in that mean survival times was 5 weeks for the former compared to a mean survival time of 15 weeks for the latter (data not shown and [13]). When guinea pigs were vaccinated with BCG prior to infection, survival was significantly prolonged when compared to non-vaccinated animals, suggesting that BCG could indeed provide a protective immune response against clinical strains of M. tuberculosis in the guinea pig model. To date few studies have been published in which guinea pigs have been infected with clinical isolates. Palanisamy et al [14] showed, in a similar guinea pig infection model to that used in this study, that HN878 was more virulent than H37Rv, although in that study the MST for HN878 was approximately 16 weeks, whereas the MST for the HN878 strain used in this study was 5 weeks. Why such a difference in MST should exist is not clear and may be related to several factors. Another study used several Beijing strains and showed that they varied in their ability to kill guinea pigs, but all did so better than the H37Rv strain used [15]. The current studies are in agreement with the previous studies, in that HN878, a Beijing strain isolate was more virulent than the laboratory strain. It should also be noted that the H37Rv strain used in the current study is highly virulent and a recent genomic analysis of this strain demonstrated that its sequence was closely related to the H37Rv strain obtained from the Trudeau Institute in 1969 [16]. Therefore, the propensity for BCG-vaccination to fail against H37Rv in the current study may be due to the maintained high virulence of the strain. A review of the literature shows that there are several outcomes associated with BCG vaccination prior to infection with a Beijing strain of M. tuberculosis. Castanon-Arreola et al [17] demonstrated that there was transient protection induced by BCG Tice in a high dose intra-tracheal BALB/c model of tuberculosis and Lopez et al [18] using a similar model demonstrated that BCG Pasteur caused a significant reduction in CFU after infection with a Beijing strain of M. tuberculosis. Grode et al [19] demonstrated using a moderately low dose aerosol infection model in BALB/C mice, that at 120 days post-inoculation, BCG Danish less effective at maintaining a reduced burden of M. tuberculosis Beijing than H37Rv. Tsenova et al [10, 20] using a CNS infection with HN878 in the rabbit model resulted in higher bacillary loads in the cerebrospinal fluid and brain, increased dissemination of bacilli to other organs when compared to other strains. The disparity in the published data and in that presented may be due to various factors including the strain of mouse used, the animal model used, the route of vaccination and infection or the strain of M. tuberculosis Beijing used. In general, the data would suggest that BCG vaccination is less effective against Beijing strains, the current data serves to highlight the fact that the animal models can provide us with an indicator to suggest that certain strains of BCG can be effective against more virulent strains of M. tuberculosis. In regards to the mouse model, others have shown that BALB/c mice have a lower type 1 during mycobacterial infection [21] and thus may contribute to a reduced efficacy of BCG. Indeed, in the guinea pig model, in a head-to-head survival study using H37Rv and HN878, the latter caused death at a significantly earlier time than H37Rv, and that BCG vaccination was able to significantly extend the life of HN878 infected animals, although not to the same degree as BCG in H37Rv infected guinea pigs.
Overall, the data suggest that BCG can induce long-term protective immunity in a mouse model of infection, and that immunity and the capacity to reduce the mycobacterial did not depend entirely on the genomic makeup of the sub-strain. These data would suggest that BCG provides good backbone for the development of recombinant BCGs that enhance immunity and thus provide a greater advantage against virulent strains of M. tuberculosis.
Acknowledgements
Funding for this research was provided by NIH, NIAID NO1-AI-40091.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Egen JG, et al. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28(2):271–84. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fine PE, Vynnycky E. The effect of heterologous immunity upon the apparent efficacy of (e.g. BCG) vaccines. Vaccine. 1998;16(20):1923–8. doi: 10.1016/s0264-410x(98)00124-8. [DOI] [PubMed] [Google Scholar]
- 3.Behr MA. Comparative genomics of BCG vaccines. Tuberculosis (Edinb) 2001;81(1-2):165–8. doi: 10.1054/tube.2000.0253. [DOI] [PubMed] [Google Scholar]
- 4.Behr MA. Correlation between BCG genomics and protective efficacy. Scand J Infect Dis. 2001;33(4):249–52. doi: 10.1080/003655401300077180. [DOI] [PubMed] [Google Scholar]
- 5.Parwati I, van Crevel R, van Soolingen D. Possible underlying mechanisms for successful emergence of the Mycobacterium tuberculosis Beijing genotype strains. Lancet Infect Dis. 2010;10(2):103–11. doi: 10.1016/S1473-3099(09)70330-5. [DOI] [PubMed] [Google Scholar]
- 6.Irwin SM, et al. Immune response induced by three Mycobacterium bovis BCG substrains with diverse regions of deletion in a C57BU6 mouse model. Clin Vaccine Immunol. 2008;15(5):750–6. doi: 10.1128/CVI.00018-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huygen K, et al. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992;60(7):2880–6. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mostowy S, et al. The in vitro evolution of BCG vaccines. Vaccine. 2003;21(27-30):4270–4. doi: 10.1016/s0264-410x(03)00484-5. [DOI] [PubMed] [Google Scholar]
- 9.Garcia Pelayo MC, et al. A comprehensive survey of single nucleotide polymorphisms (SNPs) across Mycobacterium bovis strains and M. bovis BCG vaccine strains refines the genealogy and defines a minimal set of SNPs that separate virulent M. bovis strains and M. bovis BCG strains. Infect Immun. 2009;77(5):2230–8. doi: 10.1128/IAI.01099-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsenova L, et al. BCG vaccination confers poor protection against M. tuberculosis HN878-induced central nervous system disease. Vaccine. 2007;25(28):5126–32. doi: 10.1016/j.vaccine.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon BY, et al. Mycobacterium bovis BCG immunization induces protective immunity against nine different Mycobacterium tuberculosis strains in mice. Infect Immun. 2008;76(11):5173–80. doi: 10.1128/IAI.00019-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva CL, et al. Characterization of the memory/activated T cells that mediate the long-lived host response against tuberculosis after bacillus Calmette-Guerin orDNA vaccination. Immunology. 1999;97(4):573–81. doi: 10.1046/j.1365-2567.1999.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grover A, et al. Kinetics of the immune response profile in guinea pigs after vaccination with Mycobacterium bovis BCG and infection with Mycobacterium tuberculosis. Infect Immun. 2009;77(11):4837–46. doi: 10.1128/IAI.00704-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palanisamy GS, et al. Disseminated disease severity as a measure of virulence of Mycobacterium tuberculosis in the guinea pig model. Tuberculosis (Edinb) 2008;88(4):295–306. doi: 10.1016/j.tube.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palanisamy GS, et al. Clinical strains of Mycobacterium tuberculosis display a wide range of virulence in guinea pigs. Tuberculosis (Edinb) 2009;89(3):203–9. doi: 10.1016/j.tube.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Ioerger TR, et al. Variation among genome sequences of H37Rv strains of Mycobacterium tuberculosis from multiple laboratories. J Bacteriol. 2010;192(14):3645–53. doi: 10.1128/JB.00166-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castanon-Arreola M, et al. A new vaccine against tuberculosis shows greater protection in a mouse model with progressive pulmonary tuberculosis. Tuberculosis (Edinb) 2005;85(1-2):115–26. doi: 10.1016/j.tube.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Lopez B, et al. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol. 2003;133(1):30–7. doi: 10.1046/j.1365-2249.2003.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grode L, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest. 2005;115(9):2472–9. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsenova L, et al. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J Infect Dis. 2005;192(1):98–106. doi: 10.1086/430614. [DOI] [PubMed] [Google Scholar]
- 21.Wakeham J, Wang J, Xing Z. Genetically determined disparate innate and adaptive cell-mediated immune responses to pulmonary Mycobacterium bovis BCG infection in C57BU6 and BALB/c mice. Infect Immun. 2000;68(12):6946–53. doi: 10.1128/iai.68.12.6946-6953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]