Abstract
Objective To evaluate current risk models and scores for type 2 diabetes and inform selection and implementation of these in practice.
Design Systematic review using standard (quantitative) and realist (mainly qualitative) methodology.
Inclusion criteria Papers in any language describing the development or external validation, or both, of models and scores to predict the risk of an adult developing type 2 diabetes.
Data sources Medline, PreMedline, Embase, and Cochrane databases were searched. Included studies were citation tracked in Google Scholar to identify follow-on studies of usability or impact.
Data extraction Data were extracted on statistical properties of models, details of internal or external validation, and use of risk scores beyond the studies that developed them. Quantitative data were tabulated to compare model components and statistical properties. Qualitative data were analysed thematically to identify mechanisms by which use of the risk model or score might improve patient outcomes.
Results 8864 titles were scanned, 115 full text papers considered, and 43 papers included in the final sample. These described the prospective development or validation, or both, of 145 risk prediction models and scores, 94 of which were studied in detail here. They had been tested on 6.88 million participants followed for up to 28 years. Heterogeneity of primary studies precluded meta-analysis. Some but not all risk models or scores had robust statistical properties (for example, good discrimination and calibration) and had been externally validated on a different population. Genetic markers added nothing to models over clinical and sociodemographic factors. Most authors described their score as “simple” or “easily implemented,” although few were specific about the intended users and under what circumstances. Ten mechanisms were identified by which measuring diabetes risk might improve outcomes. Follow-on studies that applied a risk score as part of an intervention aimed at reducing actual risk in people were sparse.
Conclusion Much work has been done to develop diabetes risk models and scores, but most are rarely used because they require tests not routinely available or they were developed without a specific user or clear use in mind. Encouragingly, recent research has begun to tackle usability and the impact of diabetes risk scores. Two promising areas for further research are interventions that prompt lay people to check their own diabetes risk and use of risk scores on population datasets to identify high risk “hotspots” for targeted public health interventions.
Introduction
The prevalence of diabetes is rising rapidly throughout the world.1 By 2010 its prevalence in the adult populations of the United Kingdom, the United States, mainland China, and the United Arab Emirates had exceeded 7%,2 11%,3 15%,4 and 17%,5 respectively. Americans born in 2000 or later have a lifetime risk of more than one in three of developing diabetes.6 Type 2 diabetes (which accounts for over 95% of diabetes worldwide) results from a complex gene-environment interaction for which several risk factors, such as age, sex, ethnicity, family history, obesity, and hypertension, are well documented. The precise interaction of these and other risk factors with one another is, however, a complex process that varies both within and across populations.7 8 9 10 11 Epidemiologists and statisticians are striving to produce weighted models that can be presented as scores to reflect this complexity but which at the same time are perceived as sufficiently simple, plausible, affordable, and widely implementable in clinical practice.12 13
Cohort studies have shown that early detection of established diabetes improves outcome, although the evidence base for screening the entire population is weak.14 15 The proportion of cases of incident type 2 diabetes in people with impaired glucose tolerance or impaired fasting glucose levels was reduced in landmark trials from China,16 Finland,17 and the United States18 by up to 33%, 50%, and 58%, respectively, through lifestyle changes (increased exercise, weight loss) or pharmacotherapy, or both, although changes may be more modest in a non-trial population. Some have argued that because combining known risk factors predicts incident diabetes at least as effectively as impaired glucose metabolism, a diabetes risk score may be a better and more practical means of identifying people for preventive interventions than either a glucose tolerance test or a fasting blood glucose level.19 Others favour targeting the assessment of diabetes risk in those with established impaired glucose metabolism on the basis that interventions in this group are particularly effective.20
Risk models and scores first emerged for cardiovascular disease, and these are widely used in clinical and public health practice. In the United Kingdom, for example, all electronic patient record systems in general practice offer the facility to calculate the Framingham score, a patient’s risk of a cardiovascular event within 10 years. This risk score features in many guidelines and decision pathways (such as the cut-off for statin therapy21), and general practitioners receive financial rewards for calculating it.22 In contrast, although numerous models and scores have been developed for diabetes risk, we found limited evidence for use of these as part of a formal health policy, guideline, or incentive scheme for practitioners in any country (one Australian scheme incentivises general practitioners’ measurement of the risk of diabetes in adults aged 40-4923). This is perhaps surprising, given that morbidity and mortality from cardiovascular disease has been decreasing in many countries since the 1970s,24 whereas those from diabetes continue to increase.3
A diabetes risk score is an example of a prognostic model.25 Such scores should ideally be developed by taking a large, age defined population cohort of people without diabetes, measuring baseline risk factors, and following the cohort for a sufficiently long time to see who develops diabetes.26 Although prospective longitudinal designs in specially assembled cohorts are expensive, difficult, and time consuming to execute, cross sectional designs in which risk factors are measured in a population including people both with and without diabetes are methodologically inferior. They use prevalence as a proxy for incidence and conflate characteristics of people with diabetes with risk factors in those without diabetes, and thus are incapable of showing that a putative risk factor predated the development of diabetes. In practice, researchers tend to take one of two approaches: they either study a cohort of people without diabetes, which was assembled some years previously with relevant baseline metrics for some other purpose (for example, the British Regional Heart Study27), or they analyse routinely available data, such as electronic patient records.8 Both approaches are potentially susceptible to bias.
Some diabetes risk scores are intended to be self administered using questions such as “have you ever been told you have high blood pressure?” Scores that rely entirely on such questions may be hosted on the internet (see for example www.diabetes.org.uk/riskscore). Some researchers have used self completion postal questionnaires as the first part of a stepwise detection programme.28 To the extent that these instruments are valid, they can identify two types of people: those who already have diabetes whether or not they know it (hence the questionnaire may serve as a self administered screening tool for undiagnosed diabetes) and those at high risk of developing diabetes—that is, it may also serve as a prediction tool for future diabetes. Prevalence rates for diabetes derived from self assessment studies thus cannot be compared directly with the rate of incident diabetes in a prospective longitudinal sample from which those testing positive for diabetes at baseline have been excluded.
A good risk score is usually defined as one that accurately estimates individuals’ risk—that is, predictions based on the score closely match what is observed (calibration); the score distinguishes reliably between high risk people, who are likely to go on to develop the condition, and low risk people, who are less likely to develop the condition (discrimination); and it performs well in new populations (generalisability). Validating a risk model or score means testing its calibration and discrimination either internally (by splitting the original sample, developing the score on one part and testing it on another), temporally (re-running the score on the same or a similar sample after a time period), or, preferably, externally (running the score on a new population with similar but not identical characteristics from the one on which it was developed).26 29 Caution is needed when extrapolating a risk model or score developed in one population or setting to a different one—for example, secondary to primary care, adults to children, or one ethnic group to another.30
Risk scores and other prognostic models should be subject to “impact studies”—that is, studies of the extent to which the score is actually used and leads to improved outcomes. Although most authors emphasise quantitative evaluation of impact such as through cluster randomised controlled trials,30 much might also be learnt from qualitative studies of the process of using the score, either alone or as an adjunct to experimental trials. One such methodology is realist evaluation, which considers the interplay between context, mechanism (how the intervention is perceived and taken up by practitioners), and outcome.31 In practice, however, neither quantitative nor qualitative studies of impact are common in the assessment of risk scores.30
We sought to identify, classify, and evaluate risk models and scores for diabetes and inform their selection and implementation in practice. We wanted to determine the key statistical properties of published scores for predicting type 2 diabetes in adults and how they perform in practice. Hence we were particularly interested in highlighting those characteristics of a risk score that would make it fit for purpose in different situations and settings. To that end we reviewed the literature on development, validation, and use of such scores, using both quantitative data on demographics of populations and statistical properties of models and qualitative data on how risk scores were perceived and used by practitioners, policy makers, and others in a range of contexts and systems.
Methods
Theoretical and methodological approach
We followed standard methodology for systematic reviews, summarised in guidance from a previous study and the York Centre for Reviews and Dissemination.32 33 The process was later extended by drawing on the principles of realist review, an established form of systematic literature review that uses mainly qualitative methods to produce insights into the interaction between context, mechanism, and outcome, hence explaining instances of both success and failure.34 Our team is leading an international collaborative study, the Realist and Meta-narrative Evidence Synthesis: Evolving Standards (RAMESES) to develop methodological guidance and publication standards for realist review.35
Search strategy
We identified all peer reviewed cohort studies in adults over age 18 that had derived or validated, or both, a statistically weighted risk model for type 2 diabetes in a population not preselected for known risk factors or disease, and which could be applied to another population. Studies were included that had developed a new risk model based on risk factors and that used regression techniques to weight risk factors appropriately, or validated an existing model on a new population, or did both. Exclusion criteria were cross sectional designs, studies that had not finished recruiting, studies on populations preselected for risk factors or disease, studies that did not link multiple risk factors to form a scoring system or weighted model, screening or early detection studies, genetic studies, case series, studies on under 18s, animal studies, and studies that applied a known risk model or score to a population but did not evaluate its statistical potential.
In January 2011 we undertook a scoping search, beginning with sources known to the research team and those recommended by colleagues. We used the 29 papers from this search to develop the definitive protocol, including search terms and inclusion and exclusion criteria. In February 2011 a specialist librarian designed a search strategy (see web extra) with assistance from the research team. Key words were predict, screen, risk, score, [type two] diabetes, model, regression, risk assessment, risk factor, calculator, analysis, sensitivity and specificity, ROC and odds ratio. Both MESH terms and text words were used. Titles and abstracts were searched in Medline, PreMedline, Embase, and relevant databases in the Cochrane Library from inception to February 2011, with no language restrictions.
Search results from the different databases were combined in an endnote file and duplicates removed electronically and manually. In February and March 2011 two researchers independently scanned titles and abstracts and flagged potentially relevant papers for full text analysis.
Two researchers independently read the interim dataset of full text papers and reduced this to a final dataset of studies, resolving disagreements by discussion. Bilingual academic colleagues translated non-English papers and extracted data in collaboration with one of the research team. To identify recently published papers two researchers independently citation tracked the final dataset of studies in Google Scholar. Reference lists of the final dataset and other key references were also scanned.
Quantitative data extraction and analysis
Properties of included studies were tabulated on an Excel spreadsheet. A second researcher independently double checked the extraction of primary data from every study. Discrepancies were resolved by discussion. Where studies trialled multiple models with minimal difference in the number of risk factors, a judgment was made to extract data from the authors’ preferred models or (if no preferences were stated in the paper) the ones judged by two researchers to be the most complete in presentation of data or statistical robustness. Data extraction covered characteristics of the population (age, sex, ethnicity, etc), size and duration of study, completeness of follow-up, method of diagnosing diabetes, details of internal or external validation, or both, and the components and metrics used by authors of these studies to express the properties of the score, especially their calibration and discrimination—for example, observed to predicted ratios, sensitivity and specificity, area under the receiver operating characteristic curve. We aimed to use statistical meta-analysis where appropriate and presented heterogeneous data in disaggregated form.
Qualitative data extraction and analysis
For the realist component of the review we extracted data and entered these on a spreadsheet under seven headings (box 1).
Box 1: Categories for data entry
Intended users
Authors’ assumptions (if any) about who would use the risk score, on which subgroups or populations
Proposed action based on the score result
Authors’ assumptions (if any) on what would be offered to people who score above the designated cut-off for high risk
Mechanism
Authors’ hypothesised (or implied) mechanism by which use of the score might improve outcomes for patients
Descriptor
Authors’ adjectives to describe their risk model or score
Relative advantage
Authors’ claims for how and in what circumstances their model or score outperforms previous ones
Concerns
Authors’ stated concerns about their model or score
Real world use, including citation tracking
Actual data in this paper or papers citing it on use of the score in the real world
One researcher extracted these data from our final sample of papers and another checked a one third sample of these. Our research team discussed context-mechanism-outcome interactions hypothesised or implied by authors and reread the full sample of papers with all emerging mechanisms in mind to explore these further.
Impact analysis
We assessed the impact of each risk score in our final sample using three criteria: any description in the paper of use of the score beyond the population for whom it was developed and validated; number of citations of the paper in Google Scholar and number of these that described use of the score in an impact study; and critical appraisal of any impact studies identified on this citation track. In this phase we were guided by the question: what is the evidence that this risk score has been used in an intervention which improved (or sought to improve) outcomes for individuals at high risk of diabetes?
Prioritising papers for reporting
Given the large number of papers, statistical models, and risk scores in our final sample, we decided for clarity to highlight a small number of scores that might be useful to practising clinicians, public health specialists, or lay people. Adapting previous quality criteria for risk scores,26 we favoured those that had external validation by a separate research team on a different population (generalisability), statistically significant calibration, a discrimination greater than 0.70, and 10 or fewer components (usability).
Results
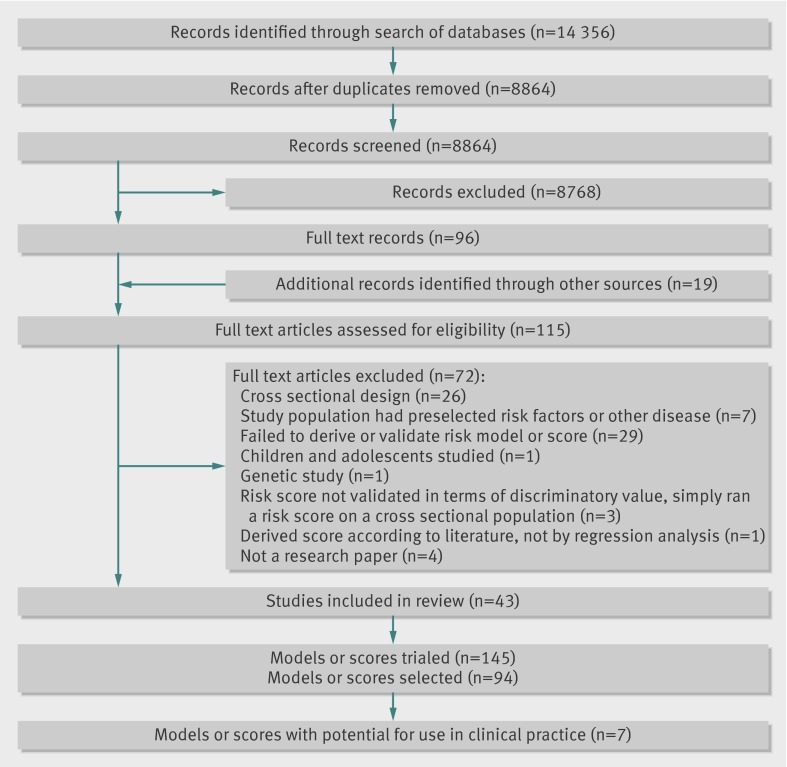
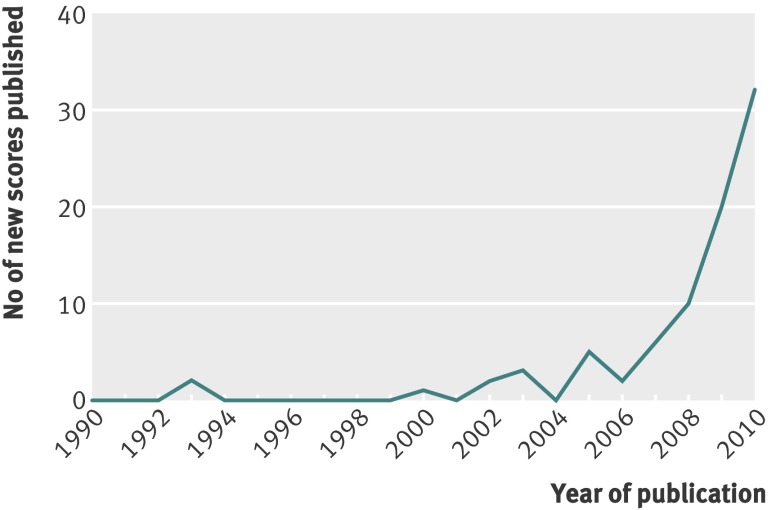
Figure 1 shows the flow of studies through the review. One hundred and fifteen papers were analysed in detail to produce a final sample of 43. Of these 43 papers, 18 described the development of one or more risk models or scores,8 27 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 17 described external validation of one or more models or scores on new populations,9 10 19 52 53 54 55 56 57 58 59 60 61 62 63 64 65 and eight did both.7 66 67 68 69 70 71 72 In all, the 43 papers described 145 risk models and scores, of which 94 were selected for extraction of full data (the other 51 were minimally different, were not the authors’ preferred model, or lacked detail or statistical robustness). Of the final sample of 94 risk models, 55 were derivations of risk models on a base population and 39 were external validations (of 14 different models) on new populations. Studies were published between 1993 and 2011, but most appeared in 2008-11 (fig 2). Indeed, even given that weaker cross sectional designs had been excluded, the findings suggest that new risk models and scores for diabetes are currently being published at a rate of about one every three weeks.
Fig 1 Flow of studies through review
Fig 2 Publication of diabetes risk models and scores 1990-2010. Eleven new risk models and scores had been published in the first five months of 2011
Table 1 gives full details of the studies in the sample, including the origin of the study, setting, population, methodological approach, duration, and how diabetes was diagnosed. The studies were highly heterogeneous. Models were developed and validated in 17 countries representing six continents (30 in Europe, 25 in North America, 21 in Asia, 8 in Australasia, 8 in the Middle East, 1 in South America, and 1 in Africa).
Table 1.
Summary of 43 papers from which 94 diabetes risk models or scores were identified for systematic review
| Study* | Country | Name of study | Name of risk score | Study design and sampling frame | Why inception cohort was assembled | Sample size | Duration: mean (SD), range (years), or as reported | Age: mean (SD) or range | How diabetes was excluded at inception | How incident diabetes was diagnosed |
|---|---|---|---|---|---|---|---|---|---|---|
| Aekplakorn 20067 (two of six models reported) | Thailand | Electric Generating Authority of Thailand Study | NS | Power plant workers: cohort derivation study; and cohort external validation study | Study of vascular risk; implicitly, study of diabetes risk | 3254; 2420 | 12, 1985-97; 5, 1998-2003 | 35-54 | History of diabetes, fasting plasma glucose, oral glucose tolerance test; and not stated | Diagnosis of diabetes, fasting plasma glucose, oral glucose tolerance test, diabetes drugs; and fasting plasma glucose |
| Alssema 200852 (two of three models reported) | Netherlands | Hoorn study, PREVEND study | Modified FINDRISC for Dutch population | Cohort external validation study, sample NS | Studies of glucose tolerance; cardiovascular disease and renal disease | 2439; 3345 | 6.4 (0.5), 1989-98; 4.2 (0.4), 1997-2003 | ≥45; 28-75 | Oral glucose tolerance test; fasting plasma glucose | NS |
| Alssema 201153 (two of three models reported) | Netherlands, Denmark, Sweden, UK, Australia, Mauritius | DETECT-2 (includes Ausdiab, Hoorn, Inter99, MONICA, Whitehall-II) | Based on FINDRISC | Cohort external validation study of FINDRISC in combined samples from five studies | NS | 18 301 | 4.8-5, 1986-2001 | Ranged from 46.3 (7.8) to 60.3 (6.9) in five studies | Oral glucose tolerance test | Oral glucose tolerance test |
| Balkau 200836 (both models reported) | France | DESIR | NS | Cohort derivation study in volunteers for free health examinations | Study of insulin resistance syndrome | 1863 and 1954 | 9 (<1996) | 47 (10) | NS | Fasting plasma glucose, diabetes drugs |
| Bozorgmanesh 201054 | Iran | Tehran Lipid and Glucose Study | Modified ARIC (Atherosclerosis Risk In Communities) | Cohort external validation study in general population | Study of lipid and glucose risk factors | 5018 | 6, 1999-2008 | Men 42.8 (14.8); women 40.7 (12.5) | Oral glucose tolerance test, fasting plasma glucose, diabetes drugs | Oral glucose tolerance test, fasting plasma glucose, diabetes drugs |
| Bozorgmanesh 201166 (all five models reported) | Iran | Tehran Lipid and Glucose Study | NS | Cohort derivation study, and cohort external validation study, in general population | Study of lipid and glucose risk factors | 5018 | 6, 1999-2008 | 41.6 (13.2) | Oral glucose tolerance test, fasting plasma glucose, diabetes drugs | Oral glucose tolerance test, fasting plasma glucose, diabetes drugs |
| Bozorgmanesh 201055 (one of six models reported) | Iran | Tehran Lipid and Glucose Study | San Antonio diabetes prediction model | Cohort external validation study in general population | Study of lipid and glucose risk factors | 5018 | 6.3, 1999-2008 | Men 42.8 (14.8); women 40.7 (12.5) | Oral glucose tolerance test, fasting plasma glucose, diabetes drugs | Oral glucose tolerance test, fasting plasma glucose, diabetes drugs |
| Cameron 200856 (both models reported) | Australia | AusDiab | Diabetes prediction model; and Finnish diabetes risk score | Cohort external validation study in general population | Diabetes incidence/prevalence study | 11 247 | 5, 2000 | 50.9 (50.6-51.2) | WHO criteria | WHO criteria |
| Chen 201037 (all six models reported) | Australia | Ausdiab | Ausdrisk | Cohort derivation study in general population | Diabetes incidence/prevalence study | 11 247 | 5, 1999-2005 | ≥25 | NS | Fasting plasma glucose, oral glucose tolerance test, diabetes drugs |
| Chien 200967 (seven of eight models reported) | Taiwan | Chin-Shan Community Cardiovascular Cohort | Cambridge risk score as well as several unnamed | Cohort derivation study in general population | NS | 2960 | 10, 1990 | 54 | Fasting plasma glucose, diabetes drugs | Fasting plasma glucose, diabetes drugs |
| Chuang 201138 (all six models reported) | Taiwan | MJ Health Screen | NS | Cohort derivation study in private health clinic patients | Data from routine health checks | 19 919 (3 scores), 6111 (3 scores) | 5.61 (3.33), 1994-2006 | 49.2 (10.4) | Fasting plasma glucose, diabetes drugs | Fasting plasma glucose, diabetes drugs |
| Collins 201157 | UK | THIN database | QDScore | Cohort external validation study in UK general practice population | Data from primary care database | 2 396 392 | 15, 1993-2008 | Median (interquartile range) men 44 (34-57), women 43 (34-56) | Read code C10 (diagnosis of diabetes) | Read code C10 (diagnosis of diabetes) |
| Gao 200939 (one of three models reported) | Mauritius | NS | NS | Cohort derivation study in random sample of entire island population | Study of non-communicable diseases | 1544 | 11, 1987-98 | <65 | History of diabetes, fasting plasma glucose, oral glucose tolerance test | Diagnosis of diabetes, fasting plasma glucose, oral glucose tolerance test, diabetes drugs |
| Guerrero-Romero 201058 (one of two models reported) | Mexico | NS | ITD (Instrumento Para El Tamizaje de la diabetes tipo 2) | Cohort external validation study, sample NS | NS | 525 | 7 (range 4.5-10), 1996-2006 | 20-65 | NS | NS |
| Hippisley-Cox 20098 (two of four models reported) | UK | QResearch database | QDScore | Cohort derivation study in general practice electronic record database | Data from primary care database | 2 samples 2 540 753 and 1 232 832 | 15, 1993-2008 | 25-79 (median 41) | Read code C10 (diagnosis of diabetes) less those receiving insulin <age 35 | Read code C10 (diagnosis of diabetes) less those receiving insulin <age 35 |
| Joseph 201040 | Norway | Tromsø Study | NS | Cohort derivation study in single academic health centre (Tromsø) | NS | 26 168 | 10.8 (median), 1994-2005 | 25-98 | Self report, haemoglobin A1c, ICD-10, plasma glucose, diabetes drugs | “T2DM event” |
| Kahn 200941 (all three models reported) | USA | ARIC (Atherosclerosis Risk in Communities) | NS | Cohort derivation study in four US communities | Study of atherosclerosis risk | 9587; 3142; 3142 | 14.9, 1987-2003 | 45-64 | NS | Varied over study period. Fasting plasma glucose, oral glucose tolerance test, self report, record, survey |
| Kanaya 200559 | USA | Health, Aging, and Body Composition Study (Validation) | NS | Cohort external validation study in two clinics (Memphis and Pittsburgh) | NS | 2503 | 6, 1997-2003 | 70-79 | Self report, diabetes drugs, fasting plasma glucose | Fasting plasma glucose |
| Kolberg 200942 | USA | Inter99 | NS | Cohort derivation study, sample from Danish civil register | Lifestyle intervention trial for cardiovascular disease | 632 | 5, NS | 30-60 | Fasting plasma glucose, oral glucose tolerance test | Fasting plasma glucose, oral glucose tolerance test |
| Lindstrom 200368 (both models reported) | Finland | FINRISK Studies | Diabetes risk score | Cohort derivation study, national population register; and cohort external validation study, FINRISK | NS | 4746; 4615 | 10, 1987-97; 5, 1992-7 | 45-64 | Fasting plasma glucose, oral glucose tolerance test, diabetes drugs | Fasting plasma glucose, oral glucose tolerance test, diabetes drugs |
| Liu 201143(all three models reported) | China | NS | Chinese diabetes risk score | Cohort derivation study in hospital screening centre for military officers | Analysis of routine data from health checks | 1457 | 10, 1996-2006 | 48-87 | Fasting plasma glucose, oral glucose tolerance test | Self report, fasting plasma glucose, oral glucose tolerance test, diabetes drugs |
| Mainous 200760 | USA | Coronary Artery Risk Development in Young Adults (CARDIA) | NS | Cohort external validation study in young adults recruited to CARDIA study | Study of coronary heart disease risk | 2543 | 10, 1985-95 | 18-30 | Self report, fasting plasma glucose | Self report, fasting plasma glucose |
| Mann 201019 (all three models reported) | USA | Multi-ethnic Study of Atherosclerosis (MESA) | NS | Cohort external validation study in adults without cardiovascular disease in six diverse US communities | Study of atherosclerosis risk | 5329 | 4.75, 2000-6 | 61.6 (45-84) | Fasting plasma glucose, diabetes drugs | Fasting plasma glucose, diabetes drugs |
| McNeely 200361 (one of two models reported) | USA | Japanese American Community Diabetes Study | NS | Cohort external validation study, sample NS | Community diabetes study | 518 | 5-10, NS | 52.1 (34-75) | Fasting plasma glucose, oral glucose tolerance test, diabetes drugs | Oral glucose tolerance test |
| Mehrabi 201044 (one of four models reported) | Iran | Tehran Lipid and Glucose Study | NS | Cohort derivation study, sample NS | Study of lipid and glucose risk factors | 5114 | 9, 1998-2007 | Men 43.4 (14.1), women 40.4 (12.6) | Fasting plasma glucose, oral glucose tolerance test, diabetes drugs | NS |
| Meigs 20089 | USA | Framingham Offspring Study | Genotype score | Cohort external validation study, sample NS | Study of children of Framingham Heart Study participants | 2377 | 28, 1971-2001 | 28-62 | Fasting plasma glucose, diabetes drugs | Fasting plasma glucose, diabetes drugs |
| Nichols 200862 (all three models reported) | USA | Kaiser Permanente Northwest electronic records | Framingham Offspring Study score | Cohort external validation study in health maintenance organisation registered population | Analysis of health maintenance organisation electronic records | 20, 644 | 7, 1999-2007 | 57.4 | NS | Diagnosis of diabetes (ICD-9 codes), fasting plasma glucose, diabetes drugs |
| Rahman 200863 | UK | European Prospective Investigation of Cancer (EPIC)-Norfolk | Cambridge risk score | Cohort external validation study in UK general practice | Study of causes of cancer | 24, 495 | 4.8 (1.3), 1993-2000 | 58.9 (40-79) | Self report, diabetes drugs, clinic registers, death certificates | As inception |
| Rathmann 201085 (all three models reported) | Germany | KORA S4/F4 study | NS | Cohort derivation study, sample NS | NS | 1202 | Implicitly, 7, 1999-2008 | 55-74 | Oral glucose tolerance test | Diagnosis of diabetes, oral glucose tolerance test |
| Rosella 201069 (all three models reported) | Canada | National Population Health Survey—Ontario | Dport (Diabetes population at risk tool) | Cohort derivation study, sample NS | Health survey | 19 795; 9899; 26 465 | 9, 1996-7; 9, 1996-2005; 5, 2000-5 | Men 44, women 46; men 44, women 47; men 44, women 46 | NS | Hospital diagnosis of diabetes (ICD code), physician claims |
| Schmidt 200546 (all three models reported) | USA | ARIC (Atherosclerosis Risk in Communities) | NS | Cohort derivation study in four US communities | Study of atherosclerosis risk | 7915 | 9, 1987-98 | Median 54 (45-64) | Diagnosis of diabetes (including self report), fasting plasma glucose, diabetes drugs | Diagnosis of diabetes, fasting plasma glucose, oral glucose tolerance test, diabetes drugs |
| Schulze 200770 (both models reported) | Germany | EPIC-Potsdam; and EPIC-Heidelberg | German diabetes risk score | Cohort derivation study (Potsdam); cohort external validation study (Heidelberg) | Study of causes of cancer | 27 548; 25 540 | 7, NS; 5, NS | Men 40-65, women 35-65; NS | NS | Self report, verified by ICD-10; self report, record, death certificate |
| Schulze 200947 | Germany | EPIC-Potsdam | Adaptation of German diabetes risk score | Cohort derivation study in general population (Potsdam) | Study of causes of cancer | 1962 | 7.1, 1994 | 35-65 | Self report verified by physician | Self report verified by physician |
| Simmons 200771 (both models reported) | UK | EPIC-Norfolk | NS; Cambridge risk score | Cohort derivation study; cohort external validation study, sample NS | Study of causes of cancer | 12 591 | 4.6, 1993-2000 | 40-79 | Self report | Health check, clinic registers, diabetes drugs, haemoglobin A1C |
| Stern 199348 (two of six models reported) | USA | San Antonio Heart Study | NS | Cohort derivation study, sample NS | Population based study of diabetes and cardiovascular disease | 2217 | 8, 1979-87 | 25-64 | Fasting plasma glucose, oral glucose tolerance test, diabetes drugs | Fasting plasma glucose, oral glucose tolerance test, diabetes drugs |
| Stern 200286 (both models reported) | USA | San Antonio Heart Study | NS | Cohort derivation study, sample NS | Population based study of diabetes and cardiovascular disease | 5158 | 7-8, 1979-88 | 25-64 | Fasting plasma glucose, oral glucose tolerance test, diabetes drugs | Fasting plasma glucose, oral glucose tolerance test, diabetes drugs |
| Sun 200972 (three of six models reported) | Taiwan | Taiwan health-check-up database (MJLPD) | Atherosclerosis Risk in Communities (ARIC) score | Cohort derivation study in private patient sample | NS | 10 294 | Median 3.15, 1997-2006 | 47.5 (35-74) | Fasting plasma glucose, diabetes drugs | NS |
| Talmud 201010 (two of three models reported) | UK | Whitehall II | Cambridge Risk Score; and Framingham Offspring Study score | Cohort external validation study in civil servant sample | Study of health in civil servants | 8713 | 11.7 (median), NS | 49 (35-55) | Oral glucose tolerance test | Oral glucose tolerance test, diabetes drugs, self report of doctor diagnosis |
| Urdea 200964 (one score, two studies, both reported) | Denmark | Inter99 | PreDx diabetes risk score training set; PreDx diabetes risk score validation set | Cohort external validation study, sample not stated | Primary prevention study of cardiovascular disease | 399; 400 | 5, NS | 40-55 | NS | NS |
| Von Eckardstein 200050 | Germany | PROCAM (Prospective Cardiovascular Münster Study) | Multiple logistic function model | Cohort derivation study in employees of 52 companies and authorities in Münster | To examine cardiovascular risk factors, events, and mortality | 3737 | 4-10, 1979-95 | 30-60 | Self report, fasting plasma glucose, diabetes drugs | Self report, diabetes drugs, fasting plasma glucose |
| Wannamethee 201127 (all three models reported) | UK | British Regional Heart Study and British Women’s Heart and Health Study | NS | Cohort derivation study, sample not stated | Study of cardiovascular risk | 6927 | 7, 1998-2007 | 60-79 | Doctor diagnosis of diabetes, fasting plasma glucose | Record review, self report |
| Wannamethee 200565 | UK | British Regional Heart Study | Framingham risk score | Cohort external validation study in sample of mostly manual social class | Heart study | 5128 | 21.3, 1978-2000 | 50.3 (5.7), 40-59 | Recall of doctor diagnosis, high blood glucose | NS |
| Wilson 200751 (one of seven models reported) | USA | Framingham Offspring Study | NS | Cohort derivation study, sample not stated | Population based study of health outcomes | 3140 | 7, mid-1990-2001 | 54 | History of diabetes, oral glucose tolerance test, fasting plasma glucose, diabetes drugs | Fasting plasma glucose, diabetes drugs |
NS=not stated; WHO=World Health Organization; ICD-10=International Classification of Disease, 10th revision; ICD-9=International Classification of Diseases, ninth revision.
Some studies tested multiple models, with minimal difference in number of risk factors; in such cases authors’ preferred models were selected or, if no preference stated, we made our own judgment.
*Bracketed information shows how many scores tested by the original authors were included in this systematic review.
Comparisons across studies were problematic owing to heterogeneity of data and highly variable methodology, presentation techniques, and missing data. Cohorts ranged in size from 399 to 2.54 million. The same data and participants were often included in several different models in the same paper. Ten research populations were used more than once in different papers.9 10 27 37 41 42 44 46 47 48 49 51 52 53 54 55 56 63 64 65 66 70 71 In total, risk models were tested on 6.88 million participants, although this figure includes duplicate tests on the same dataset. Participants aged 18 to 98 were studied for periods ranging from 3.15 to 28 years. Completeness of follow-up ranged from 54% to 99% and incidence of diabetes across the time periods studied ranged from 1.3% to 20.9%.
None of the models in the sample was developed on a cohort recruited prospectively for the express purpose of devising it. Rather, all authors used the more pragmatic approach of retrospectively studying a research dataset that had been assembled some years previously for a different purpose. Forty two studies excluded known diabetes in the inception cohort. Diagnosis of diabetes in a cohort at inception and completion of the study was done in different ways, including self report, patient questionnaires, clinician diagnosis, electronic code, codes from the International Classification of Diseases, disease or drug registers, diabetes drugs, dietary treatment, fasting plasma glucose levels, oral glucose tolerance test, and measurement of haemoglobin A1c. In some studies the method was not stated. Half the studies used different diagnostic tests at inception and completion of the study.
One third of the papers focused almost exclusively on the statistical properties of the models. Many of the remainder had a clinician (diabetologist or general practitioner) as coauthor and included an (often short and speculative) discussion on how the findings might be applied in clinical practice. Three described their score as a “clinical prediction rule.”45 51 59
Quantitative findings
Table 2 gives details of the components of the 94 risk models included in the final sample and their statistical properties—including (where reported) their discrimination, calibration, sensitivity, specificity, positive and negative predictive value, and area under the receiver operating characteristic curve. Many papers offered additional sophisticated statistical analysis, although there was no consistency in the approach used or statistical tests. Heterogeneity of data (especially demographic and ethnic diversity of validation cohorts and different score components) in the primary studies precluded formal meta-analysis.
Table 2.
Key characteristics of 94 diabetes risk models or scores included in systematic review
| Study | Diabetes incidence (%)* | Components of score | Sensitivity/specificity† % | AUROC (95% CI) | Positive/negative predictive value (%) | Calibration | % needing further tests |
|---|---|---|---|---|---|---|---|
| Aekplakorn 20067 | 11.1 | Age, BMI, waist circumference, hypertension, family history of diabetes in first degree relative | 77/60 | 0.74 (0.71 to 0.78) | NS/NS | Hosmer-Lemeshow P=0.8 | NS |
| Aekplakorn 20067 | 5.2 | Age, BMI, waist circumference, hypertension, family history of diabetes in first degree relative | 84.4/52.5 | 0.75 (0.71 to 0.80) | NS/NS | NS | NS |
| Alssema 200852 | 22.3 per 1000 person years | Age, BMI, waist circumference, use of antihypertensive drugs, parental history of diabetes, family history of diabetes in first degree relative | 84/42 (cut-off ≥7); 52/76 (cut-off ≥10) | 0.71 (0.68 to 0.75) | 19/94 (cut-off ≥7); 26/91 (cut-off ≥10) | NS | 28 |
| Alssema 200852 | 10.7 per 1000 person years | Age, BMI, waist circumference, use of antihypertensive drugs, parental history of diabetes, family history of diabetes in first degree relative | 78/64 (cut-off ≥7); 43/85 (cut-off ≥10) | 0.77 (0.73 to 0.80) | 9/98 (cut-off ≥7); 12/97 (cut-off ≥10) | NS | 16 |
| Alssema 201153 | Range 2.3-9.9 across five substudies | Age, BMI, waist circumference, use of antihypertensive drugs, history of gestational diabetes | NS/NS | 0.77 (0.75 to 0.78) | NS/NS | NS | NS |
| Alssema 201153 | Range 2.3-9.9 across five substudies | Age, BMI, waist circumference, use of antihypertensive drugs, history of gestational diabetes, sex, smoking, family history of diabetes | 76/63 | 0.76 (0.75 to 0.78) | 11/NS | Hosmer-Lemeshow P=0.27 | 40 |
| Balkau 200836 | 7.5 | Waist circumference, smoking, hypertension | NS/NS | 0.71 (NS) | NS/NS | Hosmer-Lemeshow P=0.8 | NS |
| Balkau 200836 | 3.2 | Waist circumference, family history of diabetes, hypertension | NS/NS | 0.83 | NS/NS | Hosmer-Lemeshow P=0.9 | NS |
| Bozorgmanesh 201154 | 4.6 | Age, family history of diabetes, hypertension, waist circumference, fasting plasma glucose level, height, pulse, triglyceride-high density lipoprotein ratio | Men 71.6/75.3, women 67.1/85.0 | Men 0.79, women 0.829 | NS/NS | Hosmer-Lemeshow P=0.129 | NS |
| Bozorgmanesh 201166 | 4.6 | Age, family history of diabetes, systolic blood pressure, waist-hip ratio, waist-height ratio | NS/NS | 0.75 (0.72 to 0.78) | NS/NS | NS | NS |
| Bozorgmanesh 201166 | 4.6 | Family history of diabetes, systolic blood pressure, waist-height ratio, triglyceride-high density lipoprotein ratio, fasting plasma glucose level | NS/NS | 0.85 (0.82 to 0.87) | NS/NS | NS | NS |
| Bozorgmanesh 201166 | 4.6 | Family history of diabetes, systolic blood pressure, waist-height ratio, triglyceride-high density lipoprotein ratio, fasting plasma glucose level, two hour postprandial plasma glucose level | NS/NS | 0.86 (0.83 to 0.89) | NS/NS | NS | NS |
| Bozorgmanesh 201166 | 4.6 | Systolic blood pressure, waist-height ratio, fasting plasma glucose level, triglyceride-high density lipoprotein ratio, family history of diabetes | 75/77 | 0.83 (0.80 to 0.86) | NS/NS | Hosmer-Lemeshow P=0.631 | NS |
| Bozorgmanesh 201166 | 4.6 | NS | NS/NS | 0.78 (0.75 to 0.81) | NS/NS | Hosmer-Lemeshow P=0.264 | NS |
| Bozorgmanesh 201055 | 4.6 | “San Antonio diabetes prediction model” | NS/NS | 0.83 (0.80 to 0.86) | NS/NS | Hosmer-Lemeshow P<0.001, when recalibrated P=0.131 | NS |
| Cameron 200856 | 2.0 | Age, sex, ethnicity, fasting plasma glucose level, systolic blood pressure, high density lipoprotein cholesterol level, BMI, family history of diabetes | 62.4/82.3 | NS | 11.9/98.3 | NS | 19.3 |
| Cameron 200856 | 2.0 | NS | 62.3/70.5 | NS | 6.8/98.2 | NS | 30.6 |
| Chen 201037 | 3.2 | Age, sex, ethnicity, parental history of diabetes, history of high blood glucose levels, use of antihypertensive drugs, lipid lowering drugs, smoking, physical inactivity, waist circumference, BMI, education, occupation | NS/NS | 0.79 (0.76 to 0.81) | NS/NS | Hosmer-Lemeshow P=0.06 | NS |
| Chen 201037 | 3.2 | Age, sex, ethnicity, parental history of diabetes, history of high blood glucose levels, use of antihypertensive drugs, lipid lowering drugs, smoking, physical inactivity, waist circumference, BMI, education | NS/NS | 0.79 (0.76 to 0.81) | NS/NS | Hosmer-Lemeshow P=0.02 | NS |
| Chen 201037 | 3.2 | Age, sex, ethnicity, parental history of diabetes, history of high blood glucose levels, use of antihypertensive drugs, lipid lowering drugs, smoking, physical inactivity, waist circumference, BMI | NS/NS | 0.79 (0.76 to 0.81) | NS/NS | Hosmer-Lemeshow P=0.06 | NS |
| Chen 201037 | 3.2 | Age, sex, ethnicity, parental history of diabetes, history of high blood glucose levels, antihypertensive drugs, smoking, physical inactivity, waist circumference, BMI | NS/NS | 0.79 (0.76 to 0.81) | NS/NS | Hosmer-Lemeshow P=0.02 | NS |
| Chen 201037 | 3.2 | Age, sex, ethnicity, parental history of diabetes, history of high blood glucose levels, use of antihypertensive drugs, smoking, physical inactivity, waist circumference | NS/NS | 0.78 (0.76 to 0.81) | NS/NS | Hosmer-Lemeshow P=0.85 | NS |
| Chen 201037 | 3.2 | Age, sex, ethnicity, parental history of diabetes, history of high blood glucose levels, use of antihypertensive drugs, smoking, physical inactivity, BMI | NS/NS | 0.78 (0.75 to 0.80) | NS/NS | Hosmer-Lemeshow P=0.66 | NS |
| Chien 200967 | 18.5 | Age, BMI, white blood cell count, triglyceride level, high density lipoprotein cholesterol level, fasting plasma glucose level | 52/78 | 0.70 (0.68 to 0.73) | NS/NS | Hosmer-Lemeshow P=0.874 | NS |
| Chien 200967 | 18.5 | Age, BMI, white blood cell count, triglyceride level, high density lipoprotein cholesterol level, fasting plasma glucose level, family history of diabetes, systolic blood pressure | 69/62 | 0.70 (0.68 to 0.73) | NS/NS | NS | NS |
| Chien 200967 | 18.5 | Age, sex, BMI, family history of diabetes, use of antihypertensive drugs | NS/NS | 0.65 (0.62 to 0.67) | NS/NS | NS | NS |
| Chien 200967 | 18.5 | NS | 66/56 | NS | NS/NS | Hosmer-Lemeshow P=0.008 | NS |
| Chien 200967 | 18.5 | NS | 72/40 | NS | NS/NS | Hosmer-Lemeshow P=0.001 | NS |
| Chien 200967 | 18.5 | NS | 55/72 | NS | NS/NS | Hosmer-Lemeshow P=0.002 | NS |
| Chien 200967 | 18.5 | NS | 48/78 | NS | NS/NS | Hosmer-Lemeshow P=0.032 | NS |
| Chuang 201138 | 6.4 | Age, sex, education, alcohol, BMI, waist circumference | NS/NS | 0.71 (0.70 to 0.73) | NS/NS | NS | NS |
| Chuang 201138 | 6.4 | Age, sex, education, alcohol, BMI, waist circumference, blood pressure, hypertension | NS/NS | 0.720 (0.71 to 0.74) | NS/NS | NS | NS |
| Chuang 201138 | 6.4 | Age, sex, education, alcohol, BMI, waist circumference, triglyceride level, blood pressure, hypertension, fasting plasma glucose level | NS/NS | 0.82 (0.81 to 0.83) | NS/NS | NS | NS |
| Chuang 201138 | 6.4 | Age, sex, education, alcohol, BMI, waist circumference, family history of diabetes | NS/NS | 0.75 (0.73 - 0.78) | NS/NS | NS | NS |
| Chuang 201138 | 6.4 | Age, sex, education, family history of diabetes, alcohol, BMI, waist circumference, blood pressure, hypertension | NS/NS | 0.76 (0.73 to 0.79) | NS/NS | NS | NS |
| Chuang 201138 | 6.4 | Age, sex, education, alcohol consumption, BMI, waist circumference, blood pressure, hypertension, fasting plasma glucose level, triglyceride level, family history of diabetes | NS/NS | 0.84 (0.81 to 0.86) | NS/NS | NS | NS |
| Collins 201157 | 3.0 | Age, sex, ethnicity, BMI, smoking, family history of diabetes, cardiovascular disease, Townsend score, treated high blood pressure, current use of corticosteroids | NS/NS | Women 0.81, men 0.80 | NS/NS | Brier score: men 0.053 (0.051-0.054), women 0.041 (0.040-0.043) | NS |
| Gao 200939 | 16.5 | BMI, waist circumference, family history of diabetes | Men 72 (71-74)/0.47 (0.45-0.49), women 77 (75-78)/0.50 (0.48-0.52) | Men 0.62 (0.56 to 0.68), women 0.64 (0.59 to 0.69) | NS/NS | NS | NS |
| Guerrero-Romero 201058 | 11.8 | Age, sex, family history of diabetes, family history of hypertension, family history of obesity, history of gestational diabetes or macrosomia, fasting plasma glucose level, physical inactivity, triglyceride level, systolic or diastolic blood pressure, BMI | 92/71 | 0.91 | 35/97.5 | NS | NS |
| Hippisley-Cox 20098 | 3.1 | Age, sex, ethnicity, BMI, smoking, family history of diabetes, Townsend score, treated hypertension, cardiovascular disease, current use of corticosteroids | NS/NS | NS | NS/NS | NS | NS |
| Hippisley-Cox 20098 | 3.0 | Age, sex, ethnicity, BMI, smoking, family history of diabetes, Townsend score, treated hypertension, cardiovascular disease, current use of corticosteroids | NS/NS | Women 0.85 (0.85 to 0.86), men 0.83 (0.83 to 0.84) | NS/NS | Brier score: men 0.078 (0.075-0.080), women 0.058 (0.055-0.060) | NS |
| Joseph 201040 | Men 2.5, women 1.5 | Age, BMI, total cholesterol, triglyceride level, high density lipoprotein cholesterol level, hypertension, family history of diabetes, education, physical inactivity, smoking | NS/NS | Men 0.87, women 0.88 | NS/NS | NS | NS |
| Kahn 200941 | Men 19.4, women 18.6 | See next two rows for description of both models | NS/NS | NS | NS/NS | NS | NS |
| Kahn 200941 | 17.7 at 10 years | Waist circumference, parental history of diabetes, hypertension, short stature, black race, age >55, weight, pulse, smoking | 69/64 | 0.71 (0.69 to 0.73) | NS/NS | NS | NS |
| Kahn 200941 | 17.7 at 10 years | Glucose, waist circumference, parental history of diabetes, hypertension, triglyceride level, black race, high density lipoprotein cholesterol level, short stature, high uric acid level, age >55, pulse, alcohol consumption | 74/71 | 0.79 (0.77 to 0.81) | NS/NS | NS | NS |
| Kanaya 200559 | 5.7 | Age, sex, triglyceride level, fasting plasma glucose level | NS/NS | 0.71 (NS) | NS/NS | NS | NS |
| Kolberg 200942 | 2.7 | Six biomarkers: adiponectin, C reactive protein, ferritin, glucose, interleukin 2 receptor A, insulin | NS/NS | 0.78 (NS) | NS/NS | NS | 10% classified as high risk |
| Lindstrom 200368 | 4.1 | Age, BMI, waist circumference, use of antihypertensive drugs, history of hypertension, physical inactivity, diet (vegetables, fruits or berries) | 78 (71-84)/77 (76-79) | 0.85 (NS) | 0.13 (0.11-0.15)/0.99 (0.98-0.99) | NS | 25% in two highest risk categories |
| Lindstrom 200368 | 1.5 | Age, BMI, waist circumference, use of antihypertensive drugs, history of hypertension, physical inactivity, diet (vegetables, fruit or berries) | 81 (69-89)/76 (74-77) | 0.87 (NS) | 0.05 (0.04-0.06)/0.996 (0.993-0.998) | NS | 26% of men and 24% of women in two highest risk categories |
| Liu 201143 | 20.9 | Age, hypertension, history of high blood glucose level, BMI | NS/NS | 0.68 (0.65 to 0.72) | NS/NS | NS | NS |
| Liu 201143 | 20.9 | Age, hypertension, history of high blood glucose level, BMI, fasting plasma glucose level | NS/NS | 0.71 (0.68 to 0.74) | NS/NS | NS | NS |
| Liu 201143 | 20.9 | Age, hypertension, history of high blood glucose level, BMI, fasting plasma glucose level, triglyceride level, high density lipoprotein cholesterol level | 64.5/71.6 | 0.72 (0.69 to 0.76) | 37.70/88.60 | NS | NS |
| Mainous 200760 | 3.9 | Waist circumference, hypertension or use of antihypertensive drugs, low density lipoprotein cholesterol level, triglyceride level, BMI, hyperglycaemia | 15/98 | 0.70 | NS/NS | NS | NS |
| Mann 201019 | 8.4 | Overweight or obese, impaired fasting glucose, high density lipoprotein cholesterol level, triglyceride level, hypertension, parental history of diabetes | NS/NS | 0.78 (0.74 to 0.82) | NS/NS | Hosmer-Lemeshow P<0.001 before calibration, P>0.10 after recalibration | 27.7 in highest risk fifth |
| Mann 201019 | 8.4 | Height, waist circumference, black ethnicity, systolic blood pressure, fasting plasma glucose level, high density lipoprotein cholesterol level, triglyceride level, parental history of diabetes, age | NS/NS | 0.84 (0.82 to 0.86) | NS/NS | Hosmer-Lemeshow P<0.001 before calibration, P>0.10 after recalibration | 27.6 in highest risk fifth |
| Mann 201019 | 8.4 | Age, sex, Mexican-American ethnicity, fasting plasma glucose level, systolic blood pressure, high density lipoprotein cholesterol level, BMI, family history of diabetes | NS/NS | 0.83 (0.81 to 0.85) | NS/NS | Hosmer-Lemeshow P<0.001 before calibration, P>0.10 after recalibration | 27.6 in highest risk fifth |
| McNeely 200361 | 9.7 at 5 years 14.3 at 10 years | Age, sex, ethnicity, BMI, systolic blood pressure, fasting plasma glucose level, high density lipoprotein cholesterol level, family history of diabetes in first degree relative | 60 and 73.3 at 5-6 years/64.9 and 78.4 at 10 years | 0.76 (0.70 to 0.81) at 5-6 years, 0.79 (0.74 to 0.85) at 10 years | NS/NS | NS | NS |
| Mehrabi 201044 | 4.2 | Impaired fasting glucose, family history of diabetes, impaired glucose tolerance, waist circumference, triglyceride level | NS/NS | 0.843 (0.813 to 0.874) | NS/NS | NS | NS |
| Meigs9 | 9.2 | Age, sex, family history of diabetes, BMI, triglyceride level, fasting plasma glucose level, systolic blood pressure, high density lipoprotein cholesterol level (Framingham simple clinical model) | NS/NS | 0.90 (0.88 to 0.92) | NS/NS | NS | NS |
| Nichols 200862 | 16.5 | Age, sex, parental history of diabetes, BMI | NS/NS | 0.68 (NS) | NS/NS | NS | NS |
| Nichols 200862 | 16.5 | Age, sex, parental history of diabetes, BMI, hypertension or antihypertensive drugs, high density lipoprotein cholesterol level, triglyceride level, fasting plasma glucose level | NS/NS | 0.82 (NS) | NS/NS | Hosmer-Lemeshow P<0.001 | NS |
| Nichols 200862 | 16.5 | Age, sex, parental history of diabetes, BMI, systolic blood pressure, high density lipoprotein cholesterol level, triglyceride level, fasting plasma glucose level, waist circumference | NS/NS | 0.84 (NS) | NS/NS | NS | NS |
| Rahman 200863 | 1.3 | Age, sex, current use of corticosteroids, use of antihypertensive drugs, family history of diabetes, BMI, smoking | 54.5/80 | 0.74 (NS) | NS/NS | NS | 20 |
| Rathmann 201085 | 7.6 | Age, sex, BMI, parental history of diabetes, smoking, hypertension | 69.2/74 | 0.76 (0.71 to 0.81) | 23.7/95.4 | Hosmer-Lemeshow P=0.66, Brier score 0.0848 | NS |
| Rathmann 201085 | 7.6 | Age, sex, BMI, parental history of diabetes, smoking, hypertension, fasting plasma glucose level, haemoglobin A1c concentration, uric acid level | 82.4/72.9 | 0.84 (0.80 to 0.89) | 26.1/97.3 | Hosmer-Lemeshow P=0.45, Brier score 0.0716 | NS |
| Rathmann 201085 | 7.6 | Age, sex, BMI, parental history of diabetes, smoking, hypertension, fasting plasma glucose level, haemoglobin A1c concentration, uric acid level, oral glucose tolerance test | 81.3/84.1 | 0.89 (0.85 to 0.92) | 37.4/97.5 | Hosmer-Lemeshow P=0.70, Brier score 0.0652 | NS |
| Rosella 201069 | 7.1 | Age, ethnicity, BMI, hypertension, immigrant status, smoking, education, cardiovascular disease | NS/NS | Men 0.77 (0.76 to 0.79), women 0.78 (0.76 to 0.79) | NS/NS | Hosmer-Lemeshow | NS |
| Rosella 201069 | 5.3 | Age, ethnicity, BMI, hypertension, immigrant status, smoking, education, cardiovascular disease | NS/NS | Men 0.77 (0.76 to 0.79), women 0.76 (0.74 to 0.77) | NS/NS | Hosmer-Lemeshow | NS |
| Rosella 201069 | 4.2 | Age, ethnicity, BMI, hypertension, immigrant status, smoking, education, cardiovascular disease | NS/NS | Men 0.79 (0.77 to 0.82), women 0.80 (0.77 to 0.82) | NS/NS | Hosmer-Lemeshow | NS |
| Schmidt 200546 | 16.3 | Age, waist circumference, height, systolic blood pressure, family history of diabetes, ethnicity | Range 40-77/55-84 (at different cut-offs) | 0.71 | Range 25-32/range 88-93 (at different cut-offs) | NS | 50 |
| Schmidt 200546 | 16.3 | Age, waist circumference, height, systolic blood pressure, family history of diabetes, ethnicity, fasting plasma glucose level | Range 51-83/56-86 (at different cut-offs) | 0.78 | Range 27-41/90-94 (at different cut-offs) | NS | 50 |
| Schmidt 200546 | 16.3 | Age, ethnicity, waist circumference, height, systolic blood pressure, family history of diabetes, fasting plasma glucose level, triglyceride level, high density lipoprotein cholesterol level | Range 52-85/57-86 (at different cut-offs) | 0.80 | Range 27-42/range 90-95 (at different cut-offs) | NS | 50 |
| Schulze 2007 70 | 3.1 | Age, waist circumference, height, history of hypertension, physical inactivity, smoking, consumption of red meat, whole grain bread, coffee, and alcohol | 83.1, 67.5, 50.3/68.3, 80.6, 89.9 (at different cut-offs) | 0.84 | 5.9, 7.7, 10.7 at different cut-offs/NS | Observed to predicted incidence | 23.20 |
| Schulze 200770 | 2.6 | Age, waist circumference, height, history of hypertension, physical inactivity, smoking, consumption of red meat, whole grain bread, coffee, and alcohol | 94.4 ≥500 points, 79.7 ≥550 points/66.7 ≥500 points, 79.3 ≥550 points | 0.82 | NS/NS | Observed to predicted incidence | NS |
| Schulze 200947 | 3 | Diabetes risk score plus haemoglobin A1c concentration, glucose level, triglyceride level, high density lipoprotein cholesterol level, γ-glutamyltransferase level, alanine aminotransferase level | NS/NS | 0.90 (0.89 to 0.91) | NS/NS | Hosmer-Lemeshow tests showed better calibration with haemoglobin A1c or glucose included | NS |
| Simmons 200771 | 1.7 | Age, sex, use of antihypertensive drugs, BMI, family history of diabetes, physical inactivity, diet (green leafy vegetables, fresh fruit, wholemeal bread) | NS/NS | 0.76 (0.73 to 0.79) | NS/NS | NS | NS |
| Simmons 200771 | 1.7 | Age, sex, current use of corticosteroids, use of antihypertensive drugs, family history of diabetes, BMI, smoking | NS/NS | 0.76 (0.73 to 0.79) | NS/NS | NS | NS |
| Stern 199348 | 3.7 | Fasting plasma glucose level, two hour postprandial plasma glucose level, BMI, high density lipoprotein cholesterol level, pulse pressure | 75/88.5 | NS | 26.80/98.40 | NS | 12.8 |
| Stern 199348 | 3.7 | Sex, fasting plasma glucose level, BMI, high density lipoprotein cholesterol level, pulse pressure | 69.6/88.1 | NS | 25.20/98.10 | NS | 14.7 |
| Stern 200286 | 6.0 | Age, sex, ethnicity, triglyceride level, total cholesterol level, low and high density lipoprotein cholesterol levels, fasting plasma glucose level, family history of diabetes in first degree relative, two hour postprandial plasma glucose level, systolic and diastolic blood pressure, BMI | NS/NS | 0.86 (0.84 to 0.88) | NS/NS | Hosmer-Lemeshow P>0.2 | NS |
| Stern 200286 | 6/0 | Age, sex, ethnicity, fasting plasma glucose level, systolic blood pressure, high density lipoprotein cholesterol level, BMI, family history of diabetes in first degree relative | NS/NS | 0.84 (0.82 to 0.87) | NS/NS | Hosmer-Lemeshow P>0.2 | NS |
| Sun 200972 | 4.7 | Age, sex, education, family history of diabetes, smoker, sport time, high blood pressure, BMI, waist circumference, fasting plasma glucose level | 72.3/82.8 | 0.85 (0.83 to 0.87) | 17.18/98.38 | Observed to predicted incidence P=0.410 | 31.2 |
| Sun 200972 | 4.7 | Age, ethnicity, waist circumference, height, systolic blood pressure, family history of diabetes, fasting plasma glucose level | 75.2/79.0 | 0.84 | 13.54/98.47 | NS | 23.5 |
| Sun 200972 | 4.7 | Age, ethnicity, waist circumference, height, systolic blood pressure, family history of diabetes, fasting plasma glucose level, triglyceride level, high density lipoprotein cholesterol level | 75.0/79.7 | 0.84 | 15.39/98.47 | NS | 22.7 |
| Talmud 2010 10 | 3.5 | NS | NS/NS | 0.72 (0.69 to 0.76) | NS/NS | Hosmer-Lemeshow P=0.77 | 19.2 |
| Talmud 201010 | 3.5 | NS | NS/NS | 0.78 (0.75 to 0.82) | NS/NS | Hosmer-Lemeshow P=0.42 | 26.6 |
| Urdea 200964 | 3.2 | Levels of adiponectin, C reactive protein, ferritin, glucose, haemoglobin A1c, interleukin 2, insulin | NS/NS | 0.84 (NS) | NS/NS | Observed to predicted risk | NS |
| Urdea 200964 | 3.2 | Levels of adiponectin, C reactive protein, ferritin, glucose, haemoglobin A1c, interleukin 2, insulin | NS/NS | 0.84 (NS) | NS/NS | Observed to predicted risk | NS |
| Von Eckardstein 200050 | 5.4 | Age, BMI, hypertension, glucose, family history of diabetes, high density lipoprotein cholesterol level | 69.5 (62.6-73.9) at 80% specificity, 57.0 (49.8-64.0) at 90% specificity/set at 80% and 90% | 0.79 (0.78 to 0.81) | 16.7 at 80% specificity, 24.6 at 90% specificity/NS | NS | NS |
| Wannamethee 201127 | 4.3 | Age, sex, family history of diabetes, smoking status, BMI, waist circumference, hypertension, recall of doctor diagnosed coronary heart disease | 79.2 (top 40%) 50.3 (top 20%)/61.8 (top 40%) 81.4 (top 20%) | 0.77 (0.74 to 0.79) | NS/NS | Hosmer-Lemeshow P=0.006 | 47 |
| Wannamethee 201127 | 4.3 | Age, sex, family history of diabetes, fasting plasma glucose level, smoking status, BMI, waist circumference, hypertension, recall of doctor diagnosed coronary heart disease, high density lipoprotein cholesterol level, triglyceride level | 84.2 (top 40%), 63.8 (top 20%)/62% (top 40%) 82 (top 20%) | 0.82 (0.79 to 0.84) | NS/NS | Hosmer-Lemeshow P=0.43 | NS |
| Wannamethee 201127 | 4.3 | Age, sex, family history of diabetes, smoking, BMI, waist circumference, hypertension, recall of doctor diagnosed coronary heart disease, high density lipoprotein cholesterol level, γ-glutamyltransferase level,, haemoglobin A1c concentration | 85.1 (top 40%), 62% (top 20%)/62.1 (top 40%), 82% (top 20%) | 0.81 (0.79 to 0.83) | NS/NS | Hosmer-Lemeshow P=0.61 | NS |
| Wannamethee 200565 | 5.8 | NS | 35.6/75.7 (both at 20 years) | 0.60 (0.56 to 0.64) at 20 years | NS/NS | NS | 10.8 |
| Wilson 200751 | 5.1 | Fasting plasma glucose level, BMI, high density lipoprotein cholesterol level, parental history of diabetes, triglyceride level, blood pressure | NS/NS | 0.85 (NS) | NS/NS | NS | 15.6 |
NS=not stated; BMI=body mass index.
*Incidence of diabetes was measured differently by different authors, such as annually, every five years, every 10 years, or per 1000 patient years.
†Sensitivity and specificity are based on authors’ preferred cut-off score.
All 94 models presented a combination of risk factors as significant in the final model, and different models weighted different components differently. The number of components in a single risk score varied from 3 to 14 (n=84, mean 7.8, SD 2.6). The seven risk scores that were classified as having high potential for use in practice offered broadly similar components and had similar discriminatory properties (area under receiver operating characteristic curve 0.74-0.85, table 4). Overall, the areas under the receiver operating characteristic curve ranged from 0.60 to 0.91. Certain components used in some models (for example, biomarkers) are rarely available in some pathology laboratories and potentially too expensive for routine use. Some models that exhibited good calibration and discrimination on the internal validation cohort performed much less well when tested on an external cohort,62 67 suggesting that the initial model may have been over-fitted by inclusion of too many variables that had only minor contributions to the total risk.73 Although this study did not seek out genetic components, those studies that had included genetic markers alongside sociodemographic and clinical data all found that the genetic markers added little or nothing to the overall model.9 10 36 50
Reporting of statistical data in some studies was incomplete—for example, only 40 of the 94 models quantified any form of calibration statistic. Forty three presented sensitivity and specificity, 27 justified the rationale for cut-off points, 22 presented a positive predictive value, 19 presented a negative predictive value, and 26 made some attempt to indicate the percentage of the population that would need clinical follow-up or testing if they scored as “high risk.” Some models performed poorly—for example, there was a substantial gap between expected and observed numbers of participants who developed diabetes over the follow-up period. The false positive and false negative rates in many risk scores raised questions about their utility in clinical practice (for example, positive predictive values ranged from 5% to 42%, negative predictive values from 88% to 99%). However, some scores were designed as non-invasive preliminary instruments, with a recommended second phase involving a blood test.7 43 52 53 55 58 65
Risk models and scores tended to “morph” when they were externally validated because research teams dropped components from the original (for example, if data on these were not available), added additional components (for example, to compensate for missing categories), or modified what counted in a particular category (for example, changing how ethnicity was classified); in some cases these modifications were not clarified. A key dimension of implementation is appropriate adaptation to a new context. It was considered that this did not negate the external validation.
Qualitative findings
Table 3 provides the qualitative findings from the risk scores. Of the 43 papers in the full sample, three did not recommend use of the model tested because the authors believed it had no advantage over existing ones.50 56 60 Authors of the other 40 papers considered that at least one of their scores should be adopted and used, and to justify this made various claims. The commonest adjective used by authors to describe their score was “simple” (26 of 43); others mentioned “low cost,” “easily implemented,” “feasible,” and “convenient.”
Table 3.
Summary of authors’ assumptions and claims about their diabetes risk models or scores
| Study | Authors’ assumptions | Mechanism by which use of risk score may improve outcome | Authors’ adjectives to describe their risk score | Authors’ claims for risk score over others | Authors’ stated concerns about their risk score | Data in paper on use of risk score in real world | Citation tracking (Google Scholar) for studies of real world use | |
|---|---|---|---|---|---|---|---|---|
| Who will use risk score, on which subgroups or populations | What will be offered to people who score above cut-off for “caseness” | |||||||
| Aekplakorn 20067 | “Primary health care” will use score on “individuals who are likely to develop diabetes” | Fasting plasma glucose test, “health education and the opportunity to engage in healthy lifestyles” | Clinical | Simple, “a practical tool,” low tech, no lab tests, non-invasive | “Almost as good as” and less expensive than models that rely on blood tests | Generalisability has not been shown beyond Thai population | Validated on another cohort in same factory | 64 citations, not relevant |
| Alssema 200852 | General practitioners, for use on high risk patients. Public health clinicians, for use on high risk populations | Blood test, preventive management according to protocol | Clinical, public health | “Pretty good” | NS | Only predicts getting diabetes, does not predict complications | None | 0 |
| Alssema 201153 | Intended users not stated. Refined previous risk score | Blood test, “integrated strategies” (addressing risk of cardiovascular disease as well) | Clinical, public health | Updated, refined, simple | Better discrimination | Some missing data in dataset | None | 1 citation, not relevant |
| Balkau 200836 | Implicit target audience epidemiologists and population geneticists | Focuses on population level, not clinical care of high risk people | None specifically hypothesised | Simple | Better area under receiver operating characteristic curve, simple (requires 3 variables for men, 4 for women) | 2 hour glucose level rarely used in practice | None | 34 citations, not relevant |
| Bozorgmanesh 201054 | Clinical (“targeted interventions”) and public health (“efficient allocation of resources”) | “Intensive diabetes prevention interventions” | Clinical | Simple, parsimonious | Better discrimination capacity, developed on large cohort | Sample may not be representative (too “urban”) | None | 1 citation, not relevant |
| Bozorgmanesh 201166 | Clinicians in Iran and other Middle Eastern countries; unselected Middle Eastern population | NS | Clinical | Simple, superior, pragmatic, parsimonious, comprehensive | Better discrimination capacity, developed on large cohort | NS | None | 2 citations, not relevant |
| Bozorgmanesh 201055 | Clinical practice (“to be ordinarily available in a routine clinical setting”), Middle Eastern countries | Formal test for diabetes, for example, oral glucose tolerance test, plus “Individualised primary prevention” | Clinical | Simple, clinical, parsimonious | Likely to be acceptable to patients and doctors | Response 65%; short follow-up, predictive value reduces with time | NA | 0 |
| Cameron 200856 | Intended users not stated. Does not consider how scores will be used | Implicitly, general population (Australians). “Lifestyle measures” | Clinical | No better at predicting diabetes than random blood glucose level | NA | Authors unconvinced that it adds value | NA | 22 citations, not relevant |
| Chen 201037 | Not stated but score has been converted to an online tool for self assessment of risk by lay people | “Interventions to prevent or delay [diabetes] onset” | Lay people | Simple, non-invasive | Better discrimination, easier to measure (for example, waist circumference more practicable than BMI for lay people) | Developed on narrow age band hence age not very significant in final model | Validated on second population as part of this study | 6 citations, of which one was an impact study |
| Chien 200967 | “Clinical practice” (Chinese population) | “Preventive and treatment strategies” | Clinical | Simple | First to be validated in Chinese (but others claim this too) | AUROC only 70%, diabetes not excluded at baseline | None | 24 citations, not relevant |
| Chuang 201138 | “Clinical professionals and general subjects,” for use in “middle aged Chinese adults living in Taiwan” | NS | Clinical | Simple | Menu of scores (some simple, some more complex with better discrimination); large validation cohort | None | None | 0 |
| Collins 201157 | Implicitly, epidemiologists and public health clinicians, for use in UK population | NS | Public health | Useful | Validated by an independent team on an independent cohort (unlike most others) | None | NA (not their risk score) | 0 |
| Gao 200939 | “To be used by laypersons” to detect diabetes and raise awareness, “particularly in low- income countries” | NS | Lay people | Simple | Simple, uses absolute risk, based on prospective cohort | Only moderately good predictive power (AUROC 71%) | None | 0 |
| Guerrero-Romero 201058 | Intended users not stated. For use on unselected Latin American population | Blood test, monitoring of risk, preventive intervention targeting particular risk factors | Implicitly, clinical | Quick and easy to use, few laboratory investigations, cheap | Statistically better than other scores for use on a Latin American population | Not shown to be cost effective or to improve quality of life, needs external validation | None | 0 |
| Hippisley-Cox 20098 | General practice and public health in areas of high socioeconomic and ethnic diversity; use in “clinical settings” and by lay public through a “simple web calculator” | “To identify and proactively intervene” | Clinical | Simple, good discrimination, well calibrated, readily implementable in primary care, cost effective | Includes deprivation and ethnicity, based on data from general practice record, good statistical properties, well validated, “likely to reduce . . . health inequalities” | Missing values (for example, smoking, ethnicity); internal validation on EMIS only; better design would be a prospective study of inception cohort | None, but authors emphasise that it could be used easily | 46 citations, not relevant |
| Joseph 201040 | Implicitly, epidemiologists (focus of paper is identification and refinement of risk factors in a population) | “Lifestyle advice advocating physical activity, healthy low fat diet, and weight reduction” | None specifically hypothesised | NS | More comprehensive, AUROC 0.85, longer follow-up, less bias (for example, in how incident diabetes was diagnosed) | None mentioned | None | 0 |
| Kahn 200941 | “Insurers or public health agencies . . . to optimise allocation of preventive medicine resources” | “Preventive interventions” | Clinical, public health | Low cost, clinical, simple | Prospectively validated, may illuminate cause of diabetes by demonstrating new associations | Limited to age 45-65 and to white or black ethnic groups | None | 29 citations, not relevant |
| Kanaya 200559 | To identify “older persons who should receive intensive lifestyle intervention” | “Lifestyle modification” | Clinical | Simple | Very simple, validated in several samples | Needs validating in a longitudinal study | None | 0 |
| Kolberg 200942 | For use on “individuals at highest risk of developing type 2 diabetes” | “for whom the most comprehensive prevention strategies should be considered” | None specifically hypothesised | Objective, quantitative | Biologically plausible (“multi-biomarker”), convenient, fewer logistical challenges to implementation, better discrimination | Developed in overweight middle aged white people, hence transferability may be limited | None | 29 citations, not relevant |
| Lindstrom 200368 | Intended users not stated. Implicitly, those who (like the authors) seek to undertake intervention studies of diabetes prevention. For use with “the general public” | “Direct attention to modifiable risk factors.” Also, doing one’s own risk score might prompt people to modify their lifestyle and prompt them to get their blood glucose level checked | Clinical, lay people | Simple, practical, informative, fast, non-invasive, inexpensive, reliable, safe | Prospective, large cohort. “The public health implications of the Diabetes Risk Score are considerable” | Possible circular argument—identifying people based on same risk factors that would have prompted their clinician to measure random blood glucose level in the first place | Not in this paper, but see citation track | 343 citations, of which eight described impact studies |
| Liu 201143 | Clinicians. “initial instrument for opportunistic screening in general population”, “could enhance people’s awareness” | Oral glucose tolerance test, education, “opportunity to engage in healthy lifestyles at an early stage” | Clinical | Practical, effective, simple, easily used in clinical practice | Validated on a mainland Chinese population, large cohort, prospective, stable prediction model | Validated in middle aged to older cohort so unproved benefit in younger people. Did not include family history of diabetes, as not on database | None | 0 |
| Mainous 200760 | Implicitly, clinicians. Paper describes validation of a previous risk score in a younger cohort | “Early recognition and treatment” | Clinical | NA (they don’t recommend it in this group) | NA | Poor discriminatory ability | None | 8 citations, not relevant |
| Mann 201019 | “Clinicians . . . to stratify their patient populations” | NS | None specifically hypothesised | High discriminative ability | Recalibration and revalidation of Framingham based score in large ethnically diverse population | Inability to isolate Mexicans | None | 3 citations, not relevant |
| McNeely 200361 | “Clinical practice.” To predict diabetes risk in Japanese Americans | NS | None specifically hypothesised | None, all data expressed in numbers | Better in short term than fasting blood glucose test but not in long term (younger people). Not as good as oral glucose tolerance test (older people) | “Further refinements that take into account the differential effects of age are needed” | None | 29 citations, not relevant |
| Mehrabi 201044 | NS | NS | Not specifically hypothesised | Useful, novel | Higher predictability rate than use of single risk factors alone | New and relatively untested, some missing data | None | 0 |
| Meigs 20089 | NA—negative study showing that genetic factors add nothing to clinical scores | NA | NA (authors suggest further research on key subgroups) | Less useful than data collected at a routine clinical examination | NA | Did not help to refine the prediction of diabetes risk | NA | 163 citations, but not relevant as paper cited for its negative findings |
| Nichols 200862 | Health maintenance organisations. Based on analysis of electronic record data, to identify members at high risk of developing diabetes | “Interventions” and targeting of healthcare resources | Clinical, public health, technology | “Extremely accurate,” simple | Better AUROC | If health maintenance organisation population has different incidence of type 2 diabetes from validation cohort, score will be inaccurate | None | 1 citation, not relevant |
| Rahman 200863 | Primary care and public health clinicians. Use for “defining individuals and populations for testing, treatment and prevention” | Not explicitly stated but authors suggest potential avenues for impact studies | Clinical, public health | Simple, effective | Based on data routinely available on general practice records | Will need to be validated in other prospective cohorts | None | 29 citations, not relevant |
| Rathmann 201085 | Intended users not stated. Use “to identify high-risk populations for preventive strategies” | “Preventive strategies” | Public health | Simple | Validated in older population | No external validation yet | None | 1 citation, not relevant |
| Rosella 201069 | Public health clinicians and health planners “to estimate diabetes incidence, to stratify the population by risk, and quantify the effect of interventions” | “New intervention strategies” | Public health, clinical | Simple | Uses data available on population registries | Could be further tested on other populations. Family history and poor diet not collected, relies on self reports | None | 1 citation, not relevant |
| Schmidt 200546 | Use “in clinical encounters,” “by managed care organizations . . . to identify high-risk individuals,” and to enrol to clinical trials | “Preventive actions of appropriate intensity” | Clinical, public health, research | Simple, based on readily available clinical information and simple laboratory tests | Good predictor for white and African-American men and women; may apply also to other ethnic groups in United States | High losses to follow-up, oral glucose tolerance test not done at baseline | None | 111 citations, not relevant |
| Schulze 200770 | Intended users not stated. “Identifying individuals at high risk of developing T2D [type 2 diabetes] in the general population” | Not explicitly stated | The public | Precise, non-invasive, accurate, useful | Good AUROC (0.84), used absolute values for age rather than broad categories | Self reports may have been biased | None | 114 citations, not relevant |
| Schulze 200947 | NS | NS | None specifically hypothesised | Improved discrimination | “A comprehensive basic model,” significantly improved by routine blood tests but not chemical or genetic biomarkers | Predictive for onset of diabetes in middle age but not from birth, since diabetes was excluded from inception cohort | None | 17 citations, not relevant |
| Simmons 200771 | Primary care: “could inform . . . health behaviour information . . . routinely collected in GP consultations or by administrative staff,” identify groups for targeted prevention | “Could be incorporated into new patient health checks and may provide a more feasible means of identifying those at risk than OGTT [oral glucose tolerance test], or select those suitable for OGTT” | Clinical, administrative | Simple, feasible | Relies only on simple questions about lifestyle, which would be asked in a routine health check. AUROC (0.76) is as good as many complex risk scores | No better than standard clinical dataset routinely collected in UK general practice (but may be feasible in other health settings) | Feasible to collect | 21 citations, not relevant |
| Stern 199348 | Implicitly, epidemiological researchers | “Identifying high-risk cohorts for prevention trials” | Research, clinical | Predictive, multivariate | Uses commonly measured clinical variables | NS | None | 45 citations, not relevant |
| Stern 200286 | “Could be incorporated as it stands into clinical practice and public health practice with the aid of a calculator or personal computer” | Clinical: “patient counselling.” Public health: “to identify target populations for preventive interventions” | Clinical, public health, technological, research | Simple | Less expensive and more convenient than oral glucose tolerance testing | Possible missing data | None | 245 citations, not relevant |
| Sun 200972 | Use in clinical encounter, by managed care organisations to identify high risk people, and to enrol to clinical trials | Further research | Clinical, technological, research | Simple, effective, accurate | Simple, uses readily available clinical information | Losses to follow-up, oral glucose tolerance test not done at baseline so some cases detected, especially early on, may be prevalent ones | None | 3 citations, not relevant |
| Talmud 201010 | Intended users not stated (but study used an existing risk score as a “control” for testing a genetic profile) | NS | Not specifically hypothesised | NA (revalidation) | Simple clinical risk scores performed much better than assessment of genetic risk from 40 polymorphisms | NA | None | 21 citations, not relevant |
| Urdea 200964 | “Current clinical practice”; for “identifying individuals at highest risk of developing T2DM [type 2 diabetes mellitus]” | “so that clinicians can implement an effective diabetes prevention program” | Clinical | Simple, accurate, convenient | “Better than any other clinical measure”, not over-fit, based on multiple biomarkers hence highly plausible | NS | None | 6 citations, not relevant |
| Von Eckardstein 200050 | NA (negative study, no better than fasting blood glucose test alone in this cohort) | NA (negative study) | NA | NA (negative study) | NA | Negative study | NA | 56 citations, not relevant |
| Wannamethee 201127 | Intended users not stated | Not stated, unit of analysis is the population | Not specifically hypothesised | NA (less effective than Framingham risk score) | “Useful predictor” (but not as good as Framingham score) | NS | None | 273 citations, not relevant |
| Wannamethee 200565 | Intended users not stated | Blood tests | Not specifically hypothesised | Simple, routine | Stepwise | Diabetes diagnosed by self reports | None | 0 |
| Wilson 200751 | Implicitly, clinicians | Implicitly, lifestyle advice and metformin | Clinical | Simple, effective, easy | Very good AUROC (85%) | NS | None | 143 citations, not relevant |
NS=not stated; NA=not applicable; BMI=body mass index; AUROC=area under receiver operating characteristic curve.
Sixteen of the 43 studies that recommended use of a particular risk model or score did not designate an intended user for it. Some authors assigned agency to a risk score—that is, they stated, perhaps inadvertently, that the score itself had the potential to prevent diabetes, change behaviour, or reduce health inequalities. Although most authors did state an intended target group, this was usually given in vague terms, such as “the general population” or “individuals who are likely to develop diabetes.” Eleven of the 43 papers gave a clear statement of what intervention might be offered, by whom, to people who scored above the cut-off for high risk; the other papers made no comment on this or used vague terms such as “preventive measures,” without specifying by whom these would be delivered.
In all, authors of the papers in the full sample either explicitly identified or appeared to presume 10 mechanisms (box 2) by which, singly or in combination, use of the diabetes risk score might lead to improved patient outcomes (see table 3).
Box 2: 10 suggested mechanisms by which diabetes risk scores could help improve patient outcomes
Clinical
Direct impact—clinicians will pick up high risk patients during consultations and offer advice that leads to change in patients’ behaviour and lifestyle
Indirect impact—routine use of the score increases clinicians’ awareness of risk for diabetes and motivation to manage it
Self assessment
Direct impact—people are alerted by assessing their own risk (for example, using an online tool), directly leading to change in lifestyle
Indirect impact—people, having assessed their own risk, are prompted to consult a clinician to seek further tests or advice on prevention
Technological
Individual impact—a risk model programmed into the electronic patient record generates a point of care prompt in the clinical encounter
Population impact—a risk model programmed into the electronic patient record generates aggregated data on risk groups, which will inform a public health intervention
Public health
Planners and commissioners use patterns of risk to direct resources into preventive healthcare for certain subgroups
Administrative
An administrator or healthcare assistant collects data on risk and enters these onto the patients’ records, which subsequently triggers the technological, clinical, or public health mechanisms
Research into practice
Use of the risk score leads to improved understanding of risk for diabetes or its management by academics, leading indirectly to changes in clinical practice and hence to benefits for patients
Future research
Use of the risk score identifies focused subpopulations for further research (with the possibility of benefit to patients in later years)
Risk models and scores had been developed in a range of health systems. Differences in components could be explained partly in terms of their intended context of use. For example, the QDScore, intended for use by general practitioners, was developed using a database of electronic records of a nationally representative sample of the UK general practice population comprising 2.5 million people. The QDScore is composed entirely of data items that are routinely recorded on general practice electronic records (including self assigned ethnicity, a deprivation score derived from the patient’s postcode, and clinical and laboratory values).8 Another score, also intended to be derived from electronic records but in a US health maintenance organisation (covering people of working age who are in work), has similar components to the QDScore except that ethnicity and socioeconomic deprivation are not included. In contrast, the FINDRISC score was developed as a population screening tool intended for use directly by lay people; it consists of questions on sociodemographic factors and personal history along with waist circumference but does not include clinical or laboratory data; high scorers are prompted to seek further advice from a clinician.52 Such a score makes sense in many parts of Finland and also in the Netherlands where health and information literacy rates are high, but would be less fit for purpose in a setting where these were low.
Prioritising scores for practising clinicians
Table 4 summarises the properties of seven validated diabetes risk scores which we judged to be the most promising for use in clinical or public health practice. The judgments on which this selection was based were pragmatic; other scores not listed in table 4 (also see tables 1 and 2) will prove more fit for purpose in certain situations and settings. One score that has not yet been externally validated was included in table 4 because it is the only score already being incentivised in a national diabetes prevention policy.23
Table 4.
Components of seven diabetes risk models or scores with potential for adaptation for use in routine clinical practice
| Score/study name, country, reference | Risk factors included in score | AUROC | Calibration | External validation | ||
|---|---|---|---|---|---|---|
| Year, country | AUROC | Calibration | ||||
| ARIC (Atherosclerosis Risk in Communities), Germany, Schmidt 200546 | Age, ethnicity, waist circumference, height, systolic blood pressure, family history of diabetes, fasting plasma glucose levels, triglyceride levels, high density lipoprotein cholesterol levels | 0.80 | NS | 2010,19 USA | 0.84 | Hosmer-Lemeshow P<0.001, after recalibration P>0.10 |
| Ausdrisk, Australia, Chen 201037 | Age, sex, ethnicity, parental history of diabetes, history of high blood glucose, use of antihypertensive drugs, smoking, physical inactivity, waist circumference | 0.78 | Hosmer-Lemeshow P=0.85 | Not externally validated but has been studied as part of an intervention to improve outcomes87 | ||
| Cambridge risk score, UK, Rahman 200863 | Age, sex, use of current corticosteroids, use of antihypertensive drugs, family history of diabetes, body mass index, smoking | 0.74 with threshold of 0.38 | NS | 2010,10 UK* | 0.72 | Hosmer-Lemeshow P=0.77 |
| FINDRISC, Finland, Lindstrom 200368 | Age, body mass index, waist circumference, use of antihypertensive drugs, history of high blood glucose, physical inactivity, daily consumption of vegetables, fruits, and berries | 0.85 | NS | 2010,53 Holland, Denmark, Sweden, UK, Australia* | 0.76 | Hosmer-Lemeshow P=0.27 |
| Framingham Offspring Study, USA, Wilson 200751 | Fasting plasma glucose levels, body mass index, high density lipoprotein cholesterol levels, parental history of diabetes, triglyceride levels, blood pressure | 0.85 | NS | 2010,19 USA | 0.78 | Hosmer-Lemeshow P<0.001, after recalibration P>0.10 |
| San Antonio risk score, clinical model, USA, Stern 200249 | Age, sex, ethnicity, fasting plasma glucose levels, systolic blood pressure, high density lipoprotein cholesterol levels, body mass index, family history of diabetes in first degree relative | 0.84 | Hosmer-Lemeshow P>0.2 | 2010,19 USA; 2010,55 Iran*; 2010,10 UK*; 2010,66 Iran* | 0.83; 0.83; 0.78; 0.78 | Hosmer-Lemeshow P<0.001, after recalibration P>0.10; Hosmer-Lemeshow P≤0.001, after recalibration P=0.131; Hosmer-Lemeshow P=0.42; Hosmer-Lemeshow P=0.264 |
| QDScore, UK, Hippisley-Cox 20098 | Age, sex, ethnicity, body mass index, smoking, family history of diabetes, Townsend deprivation score, treated hypertension, cardiovascular disease, current use of corticosteroids | 0.83 men, 0.85 women | Brier score: 0.078 men, 0.058 women | 2011,57 UK | 0.80 men, 0.81 women | Brier score: 0.053 men, 0.041 women |
AUROC=area under receiver operating characteristic curve; NS=not stated.
*Validation used more, less, or substituted risk factors from original risk score or did not state the exact factors it used. See table 2 for further details.
Studies of impact of risk scores on patient outcomes
None of the 43 papers that validated one or more risk scores described the actual use of that score in an intervention phase. Furthermore, although these papers had been cited by a total of 1883 (range 0-343, median 12) subsequent papers, only nine of those 1883 papers (table 5) described application and use of the risk score as part of an impact study aimed at changing patient outcomes. These covered seven studies, of which (to date) three have reported definitive results. All three reported positive changes in individual risk factors, but surprisingly none recalculated participants’ risk scores after the intervention period to see if they had changed. While one report on the ongoing FIN-D2D study suggests that incident diabetes has been reduced in “real world” (non-trial) participants who were picked up using a diabetes risk score and offered a package of preventive care,74 this is a preliminary and indirect finding based on drug reimbursement claims, and no actual data are given in the paper. With that exception, no published impact study on a diabetes risk score has yet shown a reduction in incident diabetes.
Table 5.
Results of impact citation search (studies using diabetes risk models or scores as part of an intervention to improve outcomes)
| Study (acronym) | Score used | Research question | Setting and sample | Study design, intervention | Main findings or expected reporting date | Comment |
|---|---|---|---|---|---|---|
| Absetz 2009 (GOAL study)88 | FINDRISC68 | Can diabetes risk be reduced by lifestyle counselling? | Australia, 352 high risk adults | Real world feasibility study: eight lifestyle counselling sessions | 271/352 completed study. Showed statistically significant reduction in weight, body mass index, and total cholesterol level, maintained at 36 months | Changes only reported on “completers”; those lost to follow-up were not included in analysis. Absolute changes were small and probably not clinically significant—for example, mean 1 kg weight loss. Change in FINDRISC score was not reported |
| Jallinoja 2008 (GOAL study)89 | FINDRISC68 | What is the experience of lifestyle change in people recruited into diabetes prevention studies? | Australia, 30 weight losers and 30 weight gainers from GOAL study | Focus groups with weight losers and weight gainers studied separately | Many found dietary change difficult and stressful; some who did not achieve weight loss felt despondent | Some but not all people encouraged to change lifestyle will achieve it, but most will struggle |
| Colaguiri 2010 (Sydney DPP)87 | AUSD-RISK37 | Can diabetes risk be reduced by a programme of intensive behaviour change? | Australia, 1550 high risk adults (100 indigenous people) | Real world feasibility study: individual assessment followed by group sessions | Results expected 2013. Main outcomes will be change in weight, physical activity, diet, fasting glucose levels, blood pressure, lipid levels, quality of life, and health service utilisation | Participants will be recruited in primary care, but intervention will be delivered as a public health/community based programme |
| Kulzer 2009 (PREDIAS)90 | FINDRISC68 | Can diabetes risk be reduced by lessons in lifestyle modification? | Germany, 182 high risk adults | Randomised trial. Intervention group received 12 group lessons in lifestyle modification, controls had leaflet | Statistically significant changes in weight, physical activity, diet, and fasting glucose levels at 12 months compared with controls | Weight loss in intervention group was clinically significant (3.8 kg); fasting glucose in the control group increased, whereas that in the intervention group decreased. However, follow-up was short |
| Laatikainen 2007 (GGTDPP)91 | FINDRISC68 | Can risk factor reduction be achieved in a high risk non-trial population? | Australia, 237 high risk adults | Real world feasibility study: six sessions of nurse led group education | Statistically significant improvements in weight, fasting and two hour glucose levels, and lipid levels at 12 months | Mean weight loss 2.52 kg. Authors view findings as “convincing evidence that a type 2 diabetes prevention programme using lifestyle intervention is feasible in Australian primary health care with reductions in risk factors approaching those observed in randomised controlled trials” |
| Saaristo 2007 (FIN-D2D)80 and Lindstrom 2010 (FIN-D2D)74 | FINDRISC68 | Can a population approach detect high risk people, modify their risk through educational intervention, and thereby reduce the incidence of new diabetes? | Finland, high risk adults (part of a national diabetes prevention programme that also included population component) | High scorers on FINDRISC had oral glucose tolerance and lipid levels tested; those without diabetes were offered nurse led community based individual or group sessions, or both, based on stages of change and tailored to individual profile | Preliminary results only. Numbers and detailed findings not given. “Desirable changes” at 12 months in risk factors and glucose tolerance in high risk cohort. Incident diabetes reduced (as measured by drug reimbursement registration data). Full results expected 2012-13 | Authors report that “certain problems and challenges were encountered, especially in relation to the limited resources allotted to preventive health-care.”74 A smaller ongoing prevention programme using FINDRISC along with occupational health screening on an occupational cohort in an airline company (FINNAIR diabetes prevention study) is also briefly outlined in Lindstrom paper74 |
| Schwarz 2007 (TUMANI)59 | FINDRISC68 | Can an intensive, multifaceted public health intervention prevent incident diabetes in high risk people? | Germany, high risk adults (part of a national prevention programme) | High scorers on FINDRISC had oral glucose tolerance test before being assigned a “prevention manager” for education, support, and telephone counselling | Results expected 2012-13 | Authors recognise that prevention on a large scale sits oddly within the existing treatment oriented health system. Key features of TUMANI are prevention managers working within the existing infrastructure, a structured quality control programme, and a population component—for example, website and links to mass media |
| Vermunt 2010 (APHRODITE)92 | FINDRISC68 | Can a mailed questionnaire from general practice identify high risk people to participate in a preventive intervention? | Netherlands, 48 general practices | General practitioners mailed questionnaires to their adult patients. High scorers were offered oral glucose tolerance test | 16 032 people were mailed; response rate to questionnaire 54.6%, of which 17.5% were classified as high risk. Of these, 73.1% booked a consultation with their general practitioner. Full results expected 2014 | Findings to date suggest that half of high risk patients were willing to fill out the FINDRISC questionnaire and follow-up with their general practitioner. Response rates to questionnaire varied significantly among practices |
Discussion
Numerous diabetes risk scores now exist based on readily available data and provide a good but not perfect estimate of the chance of an adult developing diabetes in the medium term future. A few research teams have undertaken exemplary development and validation of a robust model, reported its statistical properties thoroughly, and followed through with studies of impact in the real world.
Limitations of included studies
We excluded less robust designs (especially cross sectional studies). Nevertheless, included studies were not entirely free from bias and confounding. This is because the “pragmatic” use of a previously assembled database or cohort brings an inherent selection bias (for example, the British Regional Heart Study cohort was selected to meet the inclusion criteria for age and comorbidity defined by its original research team and oriented to research questions around cardiovascular disease; the population for the QDScore is drawn from general practice records and hence excludes those not registered with a general practitioner).
Most papers in our sample had one or more additional limitations. They reported models or scores that required collection of data not routinely available in the relevant health system; omitted key statistical properties such as calibration and positive and negative predictive values that would allow a clinician or public health commissioner to judge the practical value of the score; or omitted to consider who would use the score, on whom, and in what circumstances. We identified a mismatch between the common assumption of authors who develop a risk model (that their “simple” model can now be taken up and used) and the actual uptake and use of such models (which seems to happen very rarely). However, there has recently been an encouraging—if limited—shift in emphasis from the exclusive pursuit of statistical elegance (for example, maximising area under the receiver operating curve) to undertaking applied research on the practicalities and outcomes of using diabetes risk scores in real world prevention programmes.
Strengths and limitations of the review
The strengths of this review are our use of mixed methodology, orientation to patient relevant outcomes, extraction and double checking of data by five researchers, and inclusion of a citation track to identify recently published studies and studies of impact. We applied both standard systematic review methods (to undertake a systematic and comprehensive search, translate all non-English texts, and extract and analyse quantitative data) and realist methods (to consider the relation between the components of the risk score, the context in which it was intended to be used, and the mechanism by which it might improve outcomes for patients).
The main limitation of this review is that data techniques and presentation in the primary studies varied so much that it was problematic to determine reasonable numerators and denominators for many of the calculations. This required us to make pragmatic decisions to collate and present data as fairly and robustly as possible while also seeking to make sense of the vast array of available risk scores to the general medical reader. We recognise that the final judgment on which risk scores are, in reality, easy to use will lie with the end user in any particular setting. Secondly, authors of some of the primary studies included in this review were developing a local tool for local use and made few or no claims that their score should be generalised elsewhere. Yet, the pioneers of early well known risk scores49 68 have occasionally found their score being applied to other populations (perhaps ethnically and demographically different from the original validation cohort), their selection of risk factors being altered to fit the available categories in other datasets, and their models being recalibrated to provide better goodness of fit. All this revision and recalibration to produce “new” scores makes the systematic review of such scores at best an inexact science.
Why did we not recommend a “best” risk score?
We have deliberately not selected a single, preferred diabetes risk score. There is no universal ideal risk score, as the utility of any score depends not merely on its statistical properties but also on its context of use, which will also determine which types of data are available to be included.75 76 Even when a risk model has excellent discrimination (and especially when it does not) the trade-off between sensitivity and specificity plays out differently depending on context. Box 3 provides some questions to ask when selecting a diabetes risk score.
Box 3: Questions to ask when selecting a diabetes risk score, and examples of intended use
What is the intended use case for the score?
-
If intended for use:
In clinical consultations, score should be based on data on the medical record
For self assessment by lay people, score should be based on things a layperson would know or be able to measure
In prevention planning, score should be based on public health data
What is the target population?
If intended for use in high ethnic and social diversity, a score that includes these variables may be more discriminatory
What is expected of the user of the score?
If for opportunistic use in clinical encounters, the score must align with the structure and timeframe of such encounters and competencies of the clinician, and (ideally) be linked to an appropriate point of care prompt. Work expected from the intended user of the score may need to be incentivised or remunerated, or both
What is expected of the participants?
If to be completed by laypeople, the score must reflect the functional health literacy of the target population
What are the consequences of false positive and false negative classifications?
In self completion scores, low sensitivity may falsely reassure large numbers of people at risk and deter them from seeking further advice
What is the completeness and accuracy of the data from which the score will be derived?
A score based on automated analysis of electronic patient records may include multiple components but must be composed entirely of data that are routinely and reliably entered on the record in coded form, and readily searchable (thus, such scores are only likely to be useful in areas where data quality in general practice records is high)
What resource implications are there?
If the budget for implementing the score and analysing data is fixed, the cost of use must fall within this budget
Given the above, what would be the ideal statistical and other properties of the score in this context of use?
What trade-offs should be made (sensitivity v specificity, brevity v comprehensiveness, one stage v two stage process)?
Risk scores as complex interventions
Our finding that diabetes risk scores seem to be used rarely can be considered in the light of the theoretical literature on diffusion of innovation. As well as being a statistical model, a risk score can be thought of as a complex, technology based innovation, the incorporation of which into business as usual (or not) is influenced by multiple contextual factors including the attributes of the risk score in the eyes of potential adopters (relative advantage, simplicity, and ease of use); adopters’ concerns (including implications for personal workload and how to manage a positive score); their skills (ability to use and interpret the technology); communication and influence (for example, whether key opinion leaders endorse it); system antecedents (including a healthcare organisation’s capacity to embrace new technologies, workflows, and ways of working); and external influences (including policy drivers, incentive structures, and competing priorities).77 78
Challenges associated with risk scores in use
While the developers of most diabetes risk scores are in little doubt about their score’s positive attributes, this confidence seems not to be shared by practitioners, who may doubt the accuracy of the score or the efficacy of risk modification strategies, or both. Measuring diabetes risk competes for practitioners’ attention with a host of other tasks, some of which bring financial and other rewards. At the time of writing, few opinion leaders in diabetes seem to be promoting particular scores or the estimation of diabetes risk generally—perhaps because, cognisant of the limited impacts shown to date (summarised in table 5), they are waiting for further evidence of whether and how use of the risk score improves outcomes. Indeed, the utility of measuring diabetes risk in addition to cardiovascular risk is contested within the diabetes research community.79 In the United Kingdom, the imminent inclusion of an application for calculating QDScore on EMIS, the country’s most widely used general practice computer system, may encourage its use in the clinical encounter. But unless the assessment of diabetes risk becomes part of the UK Quality and Outcomes Framework, this task may continue to be perceived as low priority by most general practitioners. Given current evidence, perhaps this judgment is correct. Furthermore, the low positive predictive values may spell trouble for commissioners. Identifying someone as “[possibly] high risk” will inevitably entail a significant cost in clinical review, blood tests, and (possibly) intervention and follow-up. Pending the results of ongoing impact studies, this may not be the best use of scarce resources.
Delivering diabetes prevention in people without any disease requires skills that traditionally trained clinicians may not possess.80 We know almost nothing about the reach, uptake, practical challenges, acceptability, and cost of preventive interventions in high risk groups in different settings.12 The relative benefit of detecting and targeting high risk people rather than implementing population-wide diabetes prevention strategies is unknown.13 Effective prevention and early detection of diabetes are likely to require strengthening of health systems and development of new partnerships among the clinicians, community based lifestyle programmes, and healthcare funders.81
Mechanisms by which risk scores might have impact
Although most authors of papers describing diabetes risk scores have hypothesised (or seem to have assumed) a clinical mechanism of action (that the score would be used by the individual’s clinician to target individual assessment and advice), the limited data available on impact studies (see table 5) suggest that a particularly promising area for further research is interventions that prompt self assessment—that is, laypeople measuring their own risk of diabetes. The preliminary findings from the impact studies covered in this review also suggest that not everyone at high risk is interested in coming forward for individual preventive input, nor will they necessarily stay the course of such input. It follows that in areas where aggregated data from electronic patient records are available, the diabetes risk scores may be used as a population prediction tool—for example, to produce small area statistics (perhaps as pictorial maps) of diabetes risk across a population, thereby allowing targeted design and implementation of community level public health interventions.82 Small area mapping of diabetes risk may be a way of operationalising the recently published guidance on diabetes prevention from the National Institute for Health and Clinical Excellence, which recommends the use of “local and national tools . . . to identify local communities at high risk of developing diabetes to assess their specific needs.”83
Towards an impact oriented research agenda for risk scores
We recommend that funding bodies and journal editors help take this agenda forward by viewing the risk score in use as a complex intervention and encouraging more applied research studies in which real people identified as at “high risk” using a particular risk score are offered real interventions; success in risk score development is measured in terms of patient relevant intermediate outcomes (for example, change in risk score) and final outcomes (incident diabetes and related morbidity) rather than in terms of the statistical properties of the tool; a qualitative component (for example, process evaluation, organisational case study, patient’s experience of lifestyle modification) explores both facilitators and barriers of using the score in a real world setting; and an economic component evaluates cost and cost effectiveness.
Conclusion
Millions of participants across the world have already participated in epidemiological studies aimed at developing a diabetes risk score. An extensive menu of possible scores are now available to those who seek to use them clinically or to validate them in new populations, none of which is perfect but all of which have strengths. Nevertheless, despite the growing public health importance of type 2 diabetes and the enticing possibility of prevention for those at high risk of developing it, questions remain about how best to undertake risk prediction and what to do with the results. Appropriately, the balance of research effort is now shifting from devising new risk scores to exploring how best to use those we already have.
What is already known on this topic
The many known risk factors for type 2 diabetes can be combined in statistical models to produce risk scores
What this study adds
Dozens of risk models and scores for diabetes have been developed and validated in different settings
Sociodemographic and clinical data were much better predictors of diabetes risk than genetic markers
Research on this topic is beginning to shift from developing new statistical risk models to considering the use and impact of risk scores in the real world
We thank Helen Elwell, librarian at the British Medical Association Library, for help with the literature search; Samuel Rigby for manually removing duplicates; and Sietse Wieringa, Kaveh Memarzadeh, and Nicholas Swetenham for help with translation of non-English papers. BMJ reviewers Wendy Hu and John Furler provided helpful comments on an earlier draft.
Contributors: DN conceptualised the study, managed the project, briefed and supported all researchers, assisted with developing the search strategy and ran the search, scanned all titles and abstracts, extracted quantitative data on half the papers, citation tracked all papers, checked a one third sample of the qualitative data extraction, and cowrote the paper. TG conceptualised the qualitative component of the study, extracted qualitative data on all papers, independently citation tracked all papers, and led on writing the paper. RM independently scanned all titles and abstracts of the electronic search, extracted quantitative data from some papers, assisted with other double checking, and helped revise drafts of the paper. TD helped revise and refine the study aims, independently double checked quantitative data extraction from all papers, and helped revise drafts of the paper. CM advised on systematic review methodology, helped develop the search strategy, extracted quantitative data from some papers, and helped revise drafts of the paper. TG acts as guarantor.
Funding: This study was funded by grants from Tower Hamlets, Newham, and City and Hackney primary care trusts, by a National Institute of Health Research senior investigator award for TG, and by internal funding for staff time from Barts and the London School of Medicine and Dentistry. The funders had no input into the selection or analysis of data or the content of the final manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
Cite this as: BMJ 2011;343:d7163
Web Extra. Extra material supplied by the author
Details of search strategy
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4-14. [DOI] [PubMed] [Google Scholar]
- 2.Holman N, Forouhi NG, Goyder E, Wild SH. The Association of Public Health Observatories (APHO) Diabetes Prevalence Model: estimates of total diabetes prevalence for England, 2010-2030. Diabetic Med 2011;28:575-82. [DOI] [PubMed] [Google Scholar]
- 3.National Diabetes Clearinghouse. National Diabetes Statistics 2011. National Institute of Health, USA. http://diabetes.niddk.nih.gov/dm/pubs/statistics/#Diagnosed20.
- 4.Yang SH, Dou KF, Song WJ. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:2425-6. [PubMed] [Google Scholar]
- 5.Saadi H, Carruthers SG, Nagelkerke N, Al-Maskari F, Afandi B, Reed R, et al. Prevalence of diabetes mellitus and its complications in a population-based sample in Al Ain, United Arab Emirates. Diabetes Res Clin Pract 2007;78:369-77. [DOI] [PubMed] [Google Scholar]
- 6.Williamson DF, Vinicor F, Bowman BA. Primary prevention of type 2 diabetes mellitus by lifestyle intervention: implications for health policy. Ann Intern Med 2004;140:951-7. [DOI] [PubMed] [Google Scholar]
- 7.Aekplakorn W, Bunnag P, Woodward M, Sritara P, Cheepudomwit S, Yamwong S, et al. A risk score for predicting incident diabetes in the Thai population. Diabetes Care 2006;29:1872-7. [DOI] [PubMed] [Google Scholar]
- 8.Hippisley-Cox J, Coupland C, Robson J, Sheikh A, Brindle P. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ 2009;338:b880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meigs JB, Shrader P, Sullivan LM, McAteer JB, Fox CS, Dupuis J, et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med 2008;359:2208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talmud PJ, Hingorani AD, Cooper JA, Marmot MG, Brunner EJ, Kumari M, et al. Utility of genetic and non-genetic risk factors in prediction of type 2 diabetes: Whitehall II prospective cohort study. BMJ 2010;340:b4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park KS. The search for genetic risk factors of type 2 diabetes mellitus. Diabetes Metab J 2011;35:12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons RK, Unwin N, Griffin SJ. International Diabetes Federation: an update of the evidence concerning the prevention of type 2 diabetes. Diabetes Res Clin Pract 2010;87:143-9. [DOI] [PubMed] [Google Scholar]
- 13.Yates T, Davies M, Khunti K. Preventing type 2 diabetes: can we make the evidence work? Postgrad Med J 2009;85:475-80. [DOI] [PubMed] [Google Scholar]
- 14.Simmons RK, Echouffo-Tcheugui JB, Griffin SJ. Screening for type 2 diabetes: an update of the evidence. Diabetes Obes Metab 2010;12:838-44. [DOI] [PubMed] [Google Scholar]
- 15.Gillies CL, Lambert PC, Abrams KR, Sutton AJ, Cooper NJ, Hsu RT, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ 2008;336:1180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537-44. [DOI] [PubMed] [Google Scholar]
- 17.Tuomilehto J, Lindstrom J. The major diabetes prevention trials. Curr Diab Rep 2003;3:115-22. [DOI] [PubMed] [Google Scholar]
- 18.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann DM, Bertoni AG, Shimbo D, Carnethon MR, Chen H, Jenny NS, et al. Comparative validity of 3 diabetes mellitus risk prediction scoring models in a multiethnic US cohort: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2010;171:980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuomilehto J, Lindstrom J, Hellmich M, Lehmacher W, Westermeier T, Evers T, et al. Development and validation of a risk-score model for subjects with impaired glucose tolerance for the assessment of the risk of type 2 diabetes mellitus-The STOP-NIDDM risk-score. Diabetes Res Clin Pract 2010;87:267-74. [DOI] [PubMed] [Google Scholar]
- 21.National Institute for Health and Clinical Excellence. Lipid modification. 2011. www.nice.org.uk/nicemedia/live/11982/40675/40675.pdf.
- 22.McElduff P, Lyratzopoulos G, Edwards R, Heller RF, Shekelle P, Roland M. Will changes in primary care improve health outcomes? Modelling the impact of financial incentives introduced to improve quality of care in the UK. Qual Saf Health Care 2004;13:191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anon. Type 2 diabetes risk evaluation for men and women aged 40-49 years at high risk of type 2 diabetes. MBS item 713. 2011. www.agpn.com.au/__data/assets/pdf_file/0015/15009/20081126_brf_practice-detailing-card-item-713.pdf.
- 24.Sytkowski PA, Kannel WB, D’Agostino RB. Changes in risk factors and the decline in mortality from cardiovascular disease. The Framingham Heart Study. N Engl J Med 1990;322:1635-41. [DOI] [PubMed] [Google Scholar]
- 25.Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how? BMJ 2009;338:b375. [DOI] [PubMed] [Google Scholar]
- 26.Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ 2009;338:b605. [DOI] [PubMed] [Google Scholar]
- 27.Wannamethee SG, Papacosta O, Whincup PH, Thomas MC, Carson C, Lawlor DA, et al. The potential for a two-stage diabetes risk algorithm combining non-laboratory-based scores with subsequent routine non-fasting blood tests: results from prospective studies in older men and women. Diabet Med 2011;28:23-30. [DOI] [PubMed] [Google Scholar]
- 28.Wareham NJ, Griffin SJ. Risk scores for predicting type 2 diabetes: comparing axes and spades. Diabetologia 2011;54:994-5. [DOI] [PubMed] [Google Scholar]
- 29.Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ 2009;338:b604. [DOI] [PubMed] [Google Scholar]
- 30.Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ 2009;338:b606. [DOI] [PubMed] [Google Scholar]
- 31.Pawson R, Tilley N. Realistic evaluation. Sage, 1997.
- 32.Kahn KS, Kunz R, Kleijnen J, Antes G. Systematic reviews to support evidence-based medicine, 2nd ed. Royal Society of Medicine, 2011.
- 33.Centre for Reviews and Dissemination. Systematic Reviews: centre for reviews and dissemination guidance for undertaking reviews in health care. York Publishing Services. 2011. www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf.
- 34.Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review—a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10(suppl 1):21-34. [DOI] [PubMed] [Google Scholar]
- 35.Greenhalgh T, Wong G, Westhorp G, Pawson R, on behalf of the RAMESES study group. Protocol-realist and meta-narrative evidence synthesis: evolving standards (RAMESES). BMC Med Res Methodol 2011;11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balkau B, Lange C, Fezeu L, Tichet J, de Lauzon-Guillain B, Czernichow S, et al. Predicting diabetes: clinical, biological, and genetic approaches: data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2008;31:2056-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L, Magliano DJ, Balkau B, Colagiuri S, Zimmet PZ, Tonkin AM, et al. AUSDRISK: an Australian type 2 diabetes risk assessment tool based on demographic, lifestyle and simple anthropometric measures. Med J Aust 2010;192:197-202. [DOI] [PubMed] [Google Scholar]
- 38.Chuang SY, Yeh WT, Wu YL, Chang HY, Pan WH, Tsao CK. Prediction equations and point system derived from large-scale health check-up data for estimating diabetic risk in the Chinese population of Taiwan. Diabetes Res Clin Pract 2011;92:128-36. [DOI] [PubMed] [Google Scholar]
- 39.Gao WG, Qiao Q, Pitkaniemi J, Wild S, Magliano D, Shaw J, et al. Risk prediction models for the development of diabetes in Mauritian Indians. Diabet Med 2009;26:996-1002. [DOI] [PubMed] [Google Scholar]
- 40.Joseph J, Svartberg J, Njolstad I, Schirmer H. Incidence of and risk factors for type-2 diabetes in a general population: the Tromso Study. Scand J Public Health 2010;38:768-75. [DOI] [PubMed] [Google Scholar]
- 41.Kahn HS, Cheng YJ, Thompson TJ, Imperatore G, Gregg EW. Two risk-scoring systems for predicting incident diabetes mellitus in US adults age 45 to 64 years. Ann Intern Med 2009;150:741-51. [DOI] [PubMed] [Google Scholar]
- 42.Kolberg JA, Jorgensen T, Gerwien RW, Hamren S, McKenna MP, Moler E, et al. Development of a type 2 diabetes risk model from a panel of serum biomarkers from the Inter99 cohort. Diabetes Care 2009;32:1207-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu M, Pan C, Jin M. A Chinese diabetes risk score for screening of undiagnosed diabetes and abnormal glucose tolerance. Diabetes Technol Ther 2011;13:501-7. [DOI] [PubMed] [Google Scholar]
- 44.Mehrabi Y, Sarbakhsh P, Hadaegh F, Khadem-Maboudi A. Prediction of diabetes using logic regression. Iran J Endocrinol Metab 2010;12:16-24. [Google Scholar]
- 45.Rathmann W, Kowall B, Heier M, Herder C, Holle R, Thorand B, et al. Prediction models for incident type 2 diabetes mellitus in the older population: KORA S4/F4 cohort study. Diabet Med 2010;27:1116-23. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt MI, Duncan BB, Bang H, Pankow JS, Ballantyne CM, Golden SH, et al. Identifying individuals at high risk for diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care 2005;28:2013-8. [DOI] [PubMed] [Google Scholar]
- 47.Schulze MB, Weikert C, Pischon T, Bergmann MM, Al-Hasani H, Schleicher E, et al. Use of multiple metabolic and genetic markers to improve the prediction of type 2 diabetes: the EPIC-Potsdam Study. Diabetes Care 2009;32:2116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stern MP, Morales PA, Valdez RA, Monterrosa A, Haffner SM, Mitchell BD, et al. Predicting diabetes. Moving beyond impaired glucose tolerance. Diabetes 1993;42:706-14. [DOI] [PubMed] [Google Scholar]
- 49.Stern MP, Williams K, Haffner SM. Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med 2002;136:575-81. [DOI] [PubMed] [Google Scholar]
- 50.von Eckardstein A, Schulte H, Assmann G. Risk for diabetes mellitus in middle-aged Caucasian male participants of the PROCAM study: implications for the definition of impaired fasting glucose by the American Diabetes Association. Prospective Cardiovascular Munster. J Clin Endocrinol Metab 2000;85:3101-8. [DOI] [PubMed] [Google Scholar]
- 51.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB Sr. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med 2007;167:1068-74. [DOI] [PubMed] [Google Scholar]
- 52.Alssema M, Feskens EJ, Bakker SJ, Gansevoort RT, Boer JM, Heine RJ, et al. [Finnish questionnaire reasonably good predictor of the incidence of diabetes in The Netherlands]. Ned Tijdschr Geneeskd 2008;152:2418-24. [PubMed] [Google Scholar]
- 53.Alssema M, Vistisen D, Heymans MW, Nijpels G, Glumer C, Zimmet PZ, et al. The Evaluation of Screening and Early Detection Strategies for Type 2 Diabetes and Impaired Glucose Tolerance (DETECT-2) update of the Finnish diabetes risk score for prediction of incident type 2 diabetes. Diabetologia 2011;54:1004-12. [DOI] [PubMed] [Google Scholar]
- 54.Bozorgmanesh M, Hadaegh F, Azizi F. Transportability of the updated diabetes prediction model from Atherosclerosis Risk in Communities Study to a Middle Eastern adult population: community-based cohort study. Acta Diabetol 2010; 10.1007/s00592-010-0241-1. [DOI] [PubMed] [Google Scholar]
- 55.Bozorgmanesh M, Hadaegh F, Zabetian A, Azizi F. San Antonio heart study diabetes prediction model applicable to a Middle Eastern population? Tehran glucose and lipid study. Int J Public Health 2010;55:315-23. [DOI] [PubMed] [Google Scholar]
- 56.Cameron AJ, Magliano DJ, Zimmet PZ, Welborn TA, Colagiuri S, Tonkin AM, et al. The metabolic syndrome as a tool for predicting future diabetes: the AusDiab study. J Intern Med 2008;264:177-86. [DOI] [PubMed] [Google Scholar]
- 57.Collins GS, Altman DG. External validation of QDSCORE((R)) for predicting the 10-year risk of developing type 2 diabetes. Diabet Med 2011;28:599-607. [DOI] [PubMed] [Google Scholar]
- 58.Guerrero-Romero F, Rodriguez-Moran M. [Validation of an instrument for screening cases of type 2 diabetes and monitoring at-risk individuals in Mexico]. Rev Panam Salud Publica 2010;27:181-6. [DOI] [PubMed] [Google Scholar]
- 59.Kanaya AM, Wassel Fyr CL, de Rekeneire N, Shorr RI, Schwartz AV, Goodpaster BH, et al. Predicting the development of diabetes in older adults: the derivation and validation of a prediction rule. Diabetes Care 2005;28:404-8. [DOI] [PubMed] [Google Scholar]
- 60.Mainous AG, III, Diaz VA, Everett CJ. Assessing risk for development of diabetes in young adults. Ann Fam Med 2007;5:425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNeely MJ, Boyko EJ, Leonetti DL, Kahn SE, Fujimoto WY. Comparison of a clinical model, the oral glucose tolerance test, and fasting glucose for prediction of type 2 diabetes risk in Japanese Americans. Diabetes Care 2003;26:758-63. [DOI] [PubMed] [Google Scholar]
- 62.Nichols GA, Brown JB. Validating the Framingham Offspring Study equations for predicting incident diabetes mellitus. Am J Manag Care 2008;14:574-80. [PubMed] [Google Scholar]
- 63.Rahman M, Simmons RK, Harding AH, Wareham NJ, Griffin SJ. A simple risk score identifies individuals at high risk of developing type 2 diabetes: a prospective cohort study. Fam Pract 2008;25:191-6. [DOI] [PubMed] [Google Scholar]
- 64.Urdea M, Kolberg J, Wilber J, Gerwien R, Moler E, Rowe M, et al. Validation of a multimarker model for assessing risk of type 2 diabetes from a five-year prospective study of 6784 Danish people (Inter99). J Diabetes Sci Technol 2009;3:748-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med 2005;165:2644-50. [DOI] [PubMed] [Google Scholar]
- 66.Bozorgmanesh M, Hadaegh F, Ghaffari S, Harati H, Azizi F. A simple risk score effectively predicted type 2 diabetes in Iranian adult population: population-based cohort study. Eur J Public Health 2011;21:554-9. [DOI] [PubMed] [Google Scholar]
- 67.Chien K, Cai T, Hsu H, Su T, Chang W, Chen M, et al. A prediction model for type 2 diabetes risk among Chinese people. Diabetologia 2009;52:443-50. [DOI] [PubMed] [Google Scholar]
- 68.Lindstrom J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725-31. [DOI] [PubMed] [Google Scholar]
- 69.Rosella LC, Manuel DG, Burchill C, Stukel TA. A population-based risk algorithm for the development of diabetes: development and validation of the Diabetes Population Risk Tool (DPoRT). J Epidemiol Community Health 2011;65:613-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schulze MB, Hoffmann K, Boeing H, Linseisen J, Rohrmann S, Mohlig M, et al. An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care 2007;30:510-5. [DOI] [PubMed] [Google Scholar]
- 71.Simmons RK, Harding AH, Wareham NJ, Griffin SJ. Do simple questions about diet and physical activity help to identify those at risk of type 2 diabetes? Diabet Med 2007;24:830-5. [DOI] [PubMed] [Google Scholar]
- 72.Sun F, Tao Q, Zhan S. An accurate risk score for estimation 5-year risk of type 2 diabetes based on a health screening population in Taiwan. Diabetes Res Clin Pract 2009;85:228-34. [DOI] [PubMed] [Google Scholar]
- 73.Harrell F. Regression modelling strategies with applications to linear models, logistic regression and survival analysis. Springer-Verlag, 2001.
- 74.Lindstrom J, Absetz P, Hemio K, Peltomaki P, Peltonen M. Reducing the risk of type 2 diabetes with nutrition and physical activity—efficacy and implementation of lifestyle interventions in Finland. Public Health Nutr 2010;13(6A):993-9. [DOI] [PubMed] [Google Scholar]
- 75.Herman WH. Predicting risk for diabetes: choosing (or building) the right model. Ann Intern Med 2009;150:812-4. [DOI] [PubMed] [Google Scholar]
- 76.Wright C, Dent T. Quality standards in risk prediction: summary of an expert meeting. PHG Foundation, 2011.www.phgfoundation.org.
- 77.Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O, Peacock R. Diffusion of innovations in service organisations: systematic literature review and recommendations for future research. Milbank Q 2004;82:581-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Quarterly 1989;13:319-40. [Google Scholar]
- 79.Chamnan P, Simmons RK, Jackson R, Khaw KT, Wareham NJ, Griffin SJ. Non-diabetic hyperglycaemia and cardiovascular risk: moving beyond categorisation to individual interpretation of absolute risk. Diabetologia 2011;54:291-9. [DOI] [PubMed] [Google Scholar]
- 80.Saaristo T, Peltonen M, Keinanen-Kiukaanniemi S, Vanhala M, Saltevo J, Niskanen L, et al. National type 2 diabetes prevention programme in Finland: FIN-D2D. Int J Circumpolar Health 2007;66:101-12. [DOI] [PubMed] [Google Scholar]
- 81.Narayan KM, Williamson DF. Prevention of type 2 diabetes: risk status, clinic, and community. J Gen Intern Med 2010;25:154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stewart JE, Battersby SE, Lopez-De FA, Remington KC, Hardin JW, Mayfield-Smith K. Diabetes and the socioeconomic and built environment: geovisualization of disease prevalence and potential contextual associations using ring maps. Int J Health Geogr 2011;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.National Institute for Health and Clinical Excellence. Preventing type 2 diabetes—population and community interventions. 2011. www.nice.org.uk/guidance/PH35.
- 84.Stone MA, Camosso-Stefinovic J, Wilkinson J, de Lusignan S, Hattersley AT, Khunti K. Incorrect and incomplete coding and classification of diabetes: a systematic review. Diabet Med 2010;27:491-7. [DOI] [PubMed] [Google Scholar]
- 85.Rathmann W, Kowall B, Schulze MB. Development of a type 2 diabetes risk model from a panel of serum biomarkers from the Inter99 cohort: response to Kolberg et al. Diabetes Care 2010;33:e28. [DOI] [PubMed] [Google Scholar]
- 86.Stern M, Williams K, Eddy D, Kahn R. Validation of prediction of diabetes by the Archimedes model and comparison with other predicting models. Diabetes Care 2008;31:1670-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colagiuri S, Vita P, Cardona-Morrell M, Singh MF, Farrell L, Milat A, et al. The Sydney Diabetes Prevention Program: a community-based translational study. BMC Public Health 2010;10:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Absetz P, Oldenburg B, Hankonen N, Valve R, Heinonen H, Nissinen A, et al. Type 2 diabetes prevention in the real world: three-year results of the GOAL lifestyle implementation trial. Diabetes Care 2009;32:1418-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jallinoja P, Pajari P, Absetz P. Repertoires of lifestyle change and self-responsibility among participants in an intervention to prevent type 2 diabetes. Scand J Caring Sci 2008;22:455-62. [DOI] [PubMed] [Google Scholar]
- 90.Kulzer B, Hermanns N, Gorges D, Schwarz P, Haak T. Prevention of diabetes self-management program (PREDIAS): effects on weight, metabolic risk factors, and behavioral outcomes. Diabetes Care 2009;32:1143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laatikainen T, Dunbar JA, Chapman A, Kilkkinen A, Vartiainen E, Heistaro S, et al. Prevention of type 2 diabetes by lifestyle intervention in an Australian primary health care setting: Greater Green Triangle (GGT) Diabetes Prevention Project. BMC Public Health 2007;7:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vermunt PW, Milder IE, Wielaard F, van Oers JA, Westert GP. An active strategy to identify individuals eligible for type 2 diabetes prevention by lifestyle intervention in Dutch primary care: the APHRODITE study. Fam Pract 2010;27:312-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of search strategy