Abstract
The aim of this study was to investigate an association between the development of cholangiocarcinoma (CCA) and the ABO variant rs505922 (known to increase pancreatic cancer risk) in a large cohort of European individuals with CCA. In total, 180 individuals with CCA and 350 CCA-free controls were included. The ABO variant rs505922 was genotyped using a polymerase chain reaction-based assay. Association between this single nucleotide polymorphism (SNP) and CCA was tested in contingency tables. Neither allele distributions nor association tests and regression analysis provided evidence for an increased risk of CCA among carriers of the ABO variant (all P > 0.05). Nevertheless, we documented a deviation from Hardy-Weinberg equilibrium in the entire CCA cohort (P = 0.028) and for patients with intrahepatic (P = 0.037) but not extrahepatic tumor localization (P > 0.05). The association tests did not provide evidence for a prominent role of the investigated SNP in the genetic risk of CCA. However, Hardy-Weinberg disequilibrium in the entire cohort and the intrahepatic CCA subgroup warrants future studies investigating a potential CCA risk modulation by individual blood groups.
Keywords: ABO, Biliary tract cancer, Blood groups, Genetic risk, Single nucleotide polymorphism
TO THE EDITOR
We were very interested to read the recent report by Greer et al[1], which further substantiates the association between an individual’s blood group and the risk of pancreatic cancer. In line with previous data, Greer et al[2] demonstrate that individuals with blood group O have a lower risk of pancreatic cancer relative to blood groups A or B. These serological data are consistent with results from a large genome-wide association study comprising 2457 patients with pancreatic cancer, whereby the common variant rs505922 (C > T) in the ABO locus was identified as a genetic risk factor for this malignancy. Interestingly, the [TT] genotype, which proved to be protective against pancreatic malignancy, is in complete linkage disequilibrium (r2 = 1.0) with blood group O. Conversely, the [C] allele is present in individuals with blood groups A, B or AB.
Cholangiocarcinoma (CCA) albeit uncommon, represents the second most prevalent primary liver cancer, and is globally increasing in incidence[3]. As with pancreatic cancer, CCA is usually diagnosed in the late stages with locally advanced or metastatic disease, and is therefore characterized by poor prognosis. Hence, the identification of genetic variants contributing to CCA development is warranted, to further elucidate the pathobiological mechanisms modulating disease risk, and to assist with the development of novel screening strategies for detecting patients at risk of biliary malignancy. Many low-risk variants have been postulated to confer an increased risk for cancers, including CCA[4]. Indeed, our previous study demonstrated the genetic risk of CCA to be modulated by heterozygosity for the alpha1-antitrypsin Z allele[5].
In the current study, we therefore specifically assessed the potential role of blood groups in CCA risk using a single nucleotide polymorphism (SNP)-based approach in a large European CCA cohort consisting of 180 individuals with CCA and 350 CCA-free controls. The details of this cohort are described in our previous study[5]. With regards to our methodology, the intronic variant rs505922 was genotyped with a 99% success rate, using a polymerase chain reaction-based assay with 5’-nuclease and fluorescence detection (Taqman, Applied Biosystems, Foster City, CA, United States). The consistency of our genotyping results with the Hardy-Weinberg equilibrium (HEW) was verified by exact tests (http://ihg2.helmholtz-muenchen.de/cgi-bin/hw/hwa1.pl). An association between the ABO variant and biliary cancer was tested in contingency tables (genotypes, Armitrage’s trend test; alleles, χ2 test) and by regression analysis using SPSS software (version 18.0).
Table 1 summarises the genotyping results. The frequency of [TT] individuals (known to carry blood group O) is consistent with the distributions reported in European populations (http://www.bloodbook.com/world-abo.html). As shown in Table 1, allele distributions did not differ significantly between cases and controls (P > 0.05). The association tests [common odds ratio (OR) = 1.01, P = 0.83)] and regression analysis (OR for the [TT] variant = 1.11, P = 0.56) did not provide evidence for the involvement of the ABO variant in CCA. Similarly, subsequent exploratory data analysis stratifying cases according to gender and intra- vs extrahepatic tumour localisation yielded no significant association between rs505922 and CCA (all P > 0.05). Interestingly, we detected a departure from HWE in patients with CCA (P = 0.028) and in the subgroup of cases with intrahepatic CCA (P = 0.037), but not in cases with extrahepatic disease (P > 0.05). Although the SNP was not associated with CCA in the above statistical tests, the presence of Hardy-Weinberg disequilibrium might however, be indicative of a possible association, since the assumption of no selection implies that the gene is not associated with the disease[6]. Confirmed consistency with HWE in the much larger control cohort and 100% consistent results in re-genotyped cancer individuals (n = 20) argue against genotyping errors as a reason for departure from HWE in the cancer group. However, the exploratory data analysis in the current study may be underpowered due to the limited sample size of the intrahepatic CCA subgroup (n = 40) (Figure 1). Of note, recent data from an Italian hospital-based cancer registry report a non-significant underrepresentation of serological blood group 0 in a chimeric cancer subgroup referred to as “liver and intrahepatic bile ducts” (36% vs 46 %; P = 0.015; n = 78)[7].
Table 1.
Allele and genotype distribution and association tests
| ABO rs505922 allele/genotype |
Counts of alleles/genotypes |
|
| Controls(2N = 700) | Cases(2N = 360) | |
| C | 246 (0.35) | 124 (0.34) |
| T | 454 (0.65) | 236 (0.66) |
| CC | 50 (0.14) | 28 (0.16) |
| CT | 146 (0.42) | 68 (0.38) |
| TT | 154 (0.44) | 84 (0.46) |
| Association test | χ2 | P |
| Allele frequency difference test | 0.05 | 0.82 |
| Armitrage’s trend test | 0.05 | 0.83 |
| OR statistics | OR | 95% CI |
| [T][C] | 1.03 | 0.79-1.35 |
| [TT][CC] | 0.94 | 0.57-1.66 |
| [TT][CT + CC] | 1.11 | 0.78-1.60 |
Patients with cholangiocarcinoma are defined as cases, the [C] allele represents the assumed cholangiocarcinoma risk allele. CI: Confidence interval; OR: Odds ratio.
Figure 1.
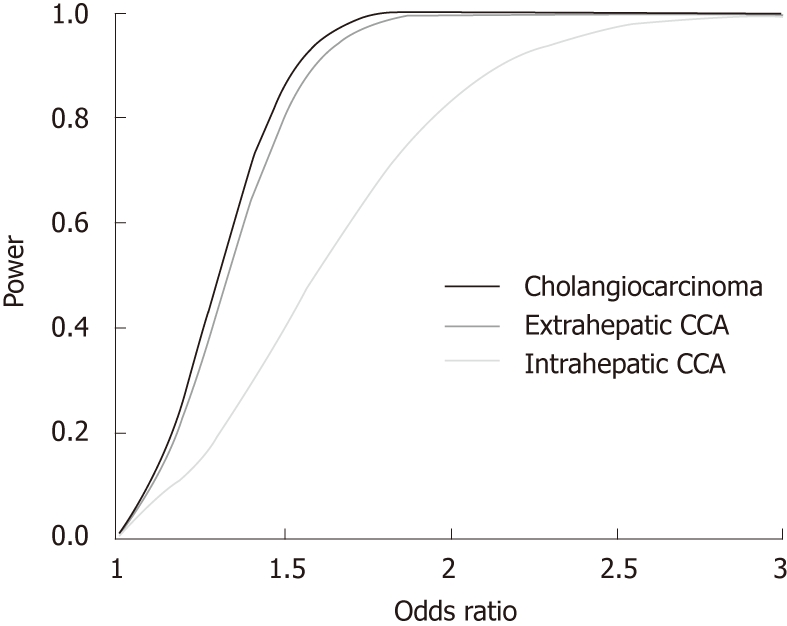
Statistical power as a function of the effect size (odds ratio) in the cholangiocarcinoma cohort (black) and in extra- and intrahepatic cholangiocarcinoma subgroups (dark grey and grey, respectively) (α set at 0.05). CCA: Cholangiocarcinoma.
In conclusion, the blood group polymorphism investigated in this study does not appear to alter the general risk of developing CCA. Furthermore, due to the relatively small number of patients with intrahepatic CCA, departure from HWE should be interpreted with caution. Nevertheless, further dedicated studies exploring the possible functional role of ABO blood types in cholangiocarcinogenesis in selected groups of patients (i.e., with intrahepatic CCA) may provide further insight into the pathobiological mechanisms that enhance the risk of this malignancy.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Caroline S Stokes (Saarland University Hospital, Homburg) for her expertise and help during the preparation of this manuscript.
Footnotes
Peer reviewers: Florencia Georgina Que, MD, Department of Surgery, Mayo Clinic, 200 First Street Southwest, Rochester, MN 55905, United States
S- Editor Sun H L- Editor Cant MR E- Editor Xiong L
References
- 1.Greer JB, Yazer MH, Raval JS, Barmada MM, Brand RE, Whitcomb DC. Significant association between ABO blood group and pancreatic cancer. World J Gastroenterol. 2010;16:5588–5591. doi: 10.3748/wjg.v16.i44.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Jacobs EJ, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;41:986–990. doi: 10.1038/ng.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis. 2008;12:131–150, ix. doi: 10.1016/j.cld.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Houlston RS, Peto J. The search for low-penetrance cancer susceptibility alleles. Oncogene. 2004;23:6471–6476. doi: 10.1038/sj.onc.1207951. [DOI] [PubMed] [Google Scholar]
- 5.Mihalache F, Höblinger A, Grünhage F, Krawczyk M, Gärtner BC, Acalovschi M, Sauerbruch T, Lammert F, Zimmer V. Heterozygosity for the alpha1-antitrypsin Z allele may confer genetic risk of cholangiocarcinoma. Aliment Pharmacol Ther. 2011;33:389–394. doi: 10.1111/j.1365-2036.2010.04534.x. [DOI] [PubMed] [Google Scholar]
- 6.Grover VK, Cole DE, Hamilton DC. Attributing Hardy-Weinberg disequilibrium to population stratification and genetic association in case-control studies. Ann Hum Genet. 2010;74:77–87. doi: 10.1111/j.1469-1809.2009.00552.x. [DOI] [PubMed] [Google Scholar]
- 7.Iodice S, Maisonneuve P, Botteri E, Sandri MT, Lowenfels AB. ABO blood group and cancer. Eur J Cancer. 2010;46:3345–3350. doi: 10.1016/j.ejca.2010.08.009. [DOI] [PubMed] [Google Scholar]


