Abstract
Background
Generalized Anxiety Disorder (GAD) is the most prevalent anxiety disorder among the elderly and has high functional and cognitive morbidity. However, late-life GAD is relatively understudied, and its functional neuroanatomy is uncharted. Several imaging studies have suggested abnormalities in the cognitive control systems of emotion regulation in anxiety disorders in young adults. The aim of this study was to examine the neural correlates of emotion regulation in late-life GAD.
Method
We compared seven elderly GAD subjects and ten elderly non-anxious comparison subjects using functional MRI. Regional cerebral blood flow (rCBF) was measured using Pulsed Arterial Spin Labeling (ASL) perfusion MRI at rest and during an emotion regulation paradigm.
Results
Relative to the rest condition, elderly non-anxious comparison subjects had increased rCBF during worry induction in the right insula, bilateral amygdala, and associative temporo-occipital areas. Elderly GAD subjects had increased rCBF during worry induction in the associative temporo-occipital areas, but not in the insula or the amygdala. During worry suppression, elderly non-anxious comparison subjects had increased rCBF in the prefrontal cortex (PFC) and dorsal ACC. Elderly GAD subjects had no changes in rCBF during worry suppression in the prefrontal cortex.
Conclusions
When attempting to regulate their emotional responses, elderly anxious subjects failed to activate prefrontal regions involved in the down-regulation of negative emotions. These results, showing that elderly anxious subjects are not effectively engaging the PFC in suppressing worry, may be clinically relevant for developing personalized therapeutic strategies for the treatment of late-life GAD.
Keywords: pathologic worry, generalized anxiety, elderly, functional MRI, arterial spin labeling
INTRODUCTION
Generalized Anxiety Disorder (GAD) is centrally defined by chronic, uncontrollable worry that causes significant distress and impairment in functioning (1, 2). With an estimated community prevalence of 7.3%, late-life GAD is the most common anxiety disorder among the elderly (3, 4). Late-life GAD is associated with decreased quality of life (5, 6), cognitive impairment (7, 8), increased health care utilization (5), and poorer recovery after disabling medical events (5, 9). Elderly GAD subjects have a three-fold increase in risk of health-related activity limitation, compared to non-anxious elderly (5), which is on par with the disability associated with late-life major depressive disorder (3).
Despite its prevalence and clinical impact, GAD is the least studied, the least understood and arguably least treated mental disorder in the elderly (4, 10). Due in part to our lack of understanding of GAD in the elderly, treatment response in late-life GAD is highly variable (11) and established treatments for midlife GAD, such as CBT, have failed to prove advantageous in late-life GAD (12, 13).
Emotion regulation facilitates adaptive behavior and decision-making in response to salient events. Poor emotion regulation strategies such as abnormalities in the capacity to down-regulate negative affect are crucial in defining the vulnerability to anxiety disorders (14). Data from anxiety-prone adolescents and young adults suggest an excessive engagement of the lateral prefrontal cortex (PFC) during down-regulation of negative emotions(15, 16). In midlife GAD subjects, Etkin et al recently reported abnormal amygdala-lateral PFC coupling (17). As the lateral PFC-amygdala connectivity was strongest in the least anxious subjects, the authors suggested a compensatory role for the lateral prefrontal cortex in the cognitive regulation of emotion in GAD (17). Other data reported in young adults show that GAD is associated with deficits in emotion regulation through failure to engage the ACC (18), exaggerated amygdala reactivity to warning cues preceding aversive/neutral pictures (19), disrupted connectivity patterns of the amygdala subregions (basolateral and centromedial nuclear groups) (17), treatment response correlated with greater pretreatment reactivity of the rACC and lesser pretreatment reactivity of the amygdala (20), or increased activation in the medial PFC and ACC during mood-induction sentences/faces (21). Furthermore, alterations have been described in other structures such as insula (24) and the bed nucleus of stria terminalis (22, 23). Overall though, the brain circuits altered in GAD and anticipatory anxiety are less well known at this time as the ones involved in fear and panic.
However, the neural network abnormalities described in younger adults might not be entirely translatable into the elderly, given the anatomical and pathophysiological changes observed in the aging brain (25). One particular gap in our understanding of late-life GAD concerns the underlying structural and functional neuroanatomy of worry regulation. Uncontrollable worry [= future-oriented anxious apprehension accompanied by a feeling of uncontrollability (2)] represents the cardinal feature of GAD, contributing to the clinical differentiation between GAD and depression (26, 27). Thus, defining the neural basis of worry modulation in the elderly would facilitate further insights into the treatment-response particularities of late-life GAD.
In the present study, we used arterial spin labeling (ASL) perfusion fMRI to study the neural basis of worry modulation of late-life GAD. ASL measures regional cerebral blood flow (rCBF) by magnetically labeling arterial blood water as an endogenous tracer (28). Since rCBF is a measure of changes at capillaries level, ASL offers a more accurate reflection of the neuronal activities than the gradient echo BOLD, which is biased toward venous blood flow. By labeling inflowing blood water proximal to the imaging location, the perfusion signal is subsequently obtained by subtracting the labeled image from the control image. Due to this pairwise subtraction of temporally adjacent images, ASL data demonstrate a reduction of the low-frequency signal drift that degrades fMRI based on BOLD contrast over time (29). This reduction renders ASL perfusion fMRI a useful tool for the investigation of slow changes in neural activity (e.g. changes sustained over several minutes), such as the changes evoked by mood states such as worry (29). This choice is further supported by a recent fMRI study that described the persistent nature of worry in young adult GAD subjects undergoing a worry-induction paradigm (30).
We examined changes in rCBF patterns following worry modulation (induction and suppression) in elderly GAD subjects, compared to elderly non-anxious subjects. Given the variable response reported in late-life GAD to top-down psychotherapeutic approaches (12), we hypothesized that compared with non-anxious elderly, elderly GAD would have abnormal rCBF in the lateral PFC areas associated with top-down cognitive control of negative emotions (31).
METHODS
Subjects
Seventeen participants, age 60 and older, were recruited: 7 GAD subjects and 10 elderly comparison subjects. Participants were recruited using the registry from the Advanced Center for Intervention and Services Research in Late-Life Mood disorders from the University of Pittsburgh as well as subjects previously enrolled in a study of pharmacotherapy for late-life GAD (11).
The study was approved by the University of Pittsburgh Institutional Review Board.
Inclusion and Exclusion Criteria
Anxious subjects had a principal diagnosis of GAD for at least six months according to the Structured Clinical Interview for DSM-IV (SCID)(32) and a score of 17 or higher on the Hamilton Anxiety Rating Scale (HARS)(33). Patients with other anxiety disorders were included if GAD was the principal diagnosis (based on severity and duration), as were patients with a past history of alcohol or substance abuse that was in full remission for at least 3 months. Although lifetime comorbid unipolar depression was allowed if GAD was the primary diagnosis (based on duration), none of the subjects included in this study had current Major Depressive Disorder at the time of scanning.
Exclusion criteria were lifetime psychosis or bipolar disorder, dementia, increased suicide risk (e.g. current ideation), medical instability according to review of medical chart data, ongoing psychotherapy, and current antidepressant or anxiolytic use. All subjects were psychotropic-free at the time of scanning.
Measures
Participants were further assessed using the self-report Penn State Worry Questionnaire (34) and the GAD Severity Scale (GADSS) (35). Cognitive status was evaluated with a comprehensive battery of neuropsychological measures widely used for older adults. The neuropsychological measures included the Repeatable Battery for the Assessment of Neuropsychological Status [RBANS; (36)]. The RBANS provides index scores measuring Attention (forwards digit span and coding task), Immediate Memory (list and story recall), Visuospatial Constructional Skills (figure copy and line orientation tasks), and Delayed Memory (delayed list, story, and figure recall). Because the RBANS does not assess executive functioning and other higher level abilities such as working memory, we also administered the Delis-Kaplan Executive Function System [D-KEFS; (37)] Sorting Test, Inhibition Test and Inhibition/Switching Tests to measure problem-solving, conceptual ability, and mental flexibility.
Experimental design
The fMRI block design involved an initial 5-min resting state phase followed by five blocks of the worry modulation task. During the resting state patients were asked to lie still in the scanner, eyes closed and not to think of anything in particular.
To examine the functional neurobiology of worry modulation, we used a personalized worry script. The worry script consists of five individualized worry statements alternating with instructions to suppress worry. During subjects’ initial evaluation, we elicited five specific worry themes. These themes were used to create sentences that instructed the subject to worry “as hard as s/he can, as s/he usually does it” about that specific theme (e.g., “worry about your back pain”). In order to standardize the paradigm, subjects rehearsed the worry modulation task prior to the experiment and they offered feedback regarding the accuracy of each worry sentence. During the experiment, each worry induction statement remained on the screen for 1 minute. The periods of worry induction alternated with five periods of worry suppression. During the worry suppression the subject read on the screen a sentence instructing him to stop worrying and to think about a personalized pleasant memory that had been elicited during initial evaluation (e.g., “please stop worrying and think about a beautiful sunset at the beach”). After each of the worry modulation block (worry induction or worry suppression) subjects were asked to self-report the severity of anxiety [1=no anxiety, 4=severe anxiety].
Data acquisition and analysis
We used the FAIREST pulsed ASL (PASL) sequence (29, 38-40). The water saturation pulse was applied at 800 msec after the global (corresponding to the control) or slice selective inversion (corresponding to the label). The delay after the saturation pulse was chosen as 1200 msec. 22 slices were acquired sequentially from inferior to superior using a gradient-echo EPI sequence. Imaging parameters: matrix size 64X64, TR/TE = 4000/28 msec, slice thickness 4mm, gap = 2 mm. The field of view (FOV) was set to 24 cm to insure full coverage of the head. We obtained 80 acquisitions, followed by a standard T1 map sequence. T1-weighted anatomical images were acquired parallel to the plane connecting the anterior and posterior commissures (TR/TE = 500/11 ms, FOV = 24×24cm, slice thickness = 3.8mm, matrix = 256×256).
Functional MRI data were preprocessed using the SPM5 software package (http:/www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (MathWorks, Inc., Natick, Mass.). After motion correction, the functional images from each subject were normalized into the standard Montreal Neurological Institute (MNI) template space (Colin27) (41). A Gaussian smoothing filter (10 mm full width at half maximum) was then used on the normalized functional images to reduce spatial noise.
The ASL data was analyzed with the SPM toolkit provided by Wang et al. (http://cfn.upenn.edu/perfusion/software.htm). This method generates quantitative cerebral perfusion maps estimates by subtracting the tagged and untagged EPI images. These quantitative maps, 1 for each task block, were then used for the first- and second-level analysis.
In first-level analysis the following conditions were modeled for each subject: resting state (RS), worry induction (WI), and worry suppression (WS).
In second-level analysis, in order to locate the regions with increased rCBF during worry induction we subtracted the RS perfusion map from the WI perfusion map (WI-RS). Similarly, in order to locate the regions with increased rCBF during worry suppresion, we subtracted the RS perfusion map from the WS perfusion map (WS-RS). Next, we used a group-level one-sample two-tailed t-test to identify within-group differences: non-anxious comparison subjects WI-RS, non-anxious comparison subjects WS-RS, GAD subjects WI-RS, GAD subjects WS-RS.
Between groups differences were identified using a two-sample two-tailed t-test (non-anxious comparison group versus GAD group).
For both within-group and between-groups analyses, the resulting t-maps were then thresholded at a corrected p < 0.05 using False Discovery Rate (FDR).
RESULTS
Demographic and clinical data are presented in Table 1. Late-life GAD subjects were significantly younger than elderly non-anxious comparison subjects, therefore, between-group differences were confirmed while controlling for age. Other Anxiety Disorders diagnosed in the late-life GAD group were Social Phobia (3/7), Specific Phobia (2/7) and Obsessive-Compulsive Disorder (1/7). There were no significant differences between the two groups with respect to the multiple cognitive domains analyzed. There were no differences in self-reported worry between elderly GAD and non-anxious subjects after worry induction (mean worry severity 2.92 elderly GAD vs. 2.87 non-anxious subjects; p=0.89) or after worry suppression (mean worry severity 1.73 elderly GAD vs. 1.46 non-anxious subjects; p=0.44).
Table 1.
Demographic and clinical characteristics of the subjects.
| Late-life GAD Subjects (N=7) | Elderly Comparison Subjects (N=10) | T-test, DF, p (2-tailed) | |
|---|---|---|---|
| Age (mean, SD) | 63.3 [3.9] | 76.3 [4.0] | 6.5, 15, p<0.001 |
| Gender (female) | 5/7 | 6/10 | |
| Race (white) | 6/7 | 8/10 | |
| HRSA (mean, SD) | 18.7 [2.8] | 3.2 [1.3] | 15.1, 15, p<0.001 |
| PSWQ (mean, SD) | 57.2 [11.5] | 33 [8.2] | 5.06, 15, p<0.001 |
| GADSS (mean, SD) | 9.5 [1.6] | 2.3 [1.1] | 7.3, 15, p<0.001 |
| DKEFS inhibition (mean, SD) | 11.8 [2.2] | 12.1 [2.18] | 0.2, 15, p=0.8 |
| DKEFS sorting (mean, SD) | 12.8 [3.8] | 11.5 [3.13] | 0.8, 15, p=0.4 |
| DKEFS inhibition/switching (mean, SD) | 11.4 [2.9] | 11.5 [1.77] | 0.06, 15, p=0.9 |
| RBANS Immediate Memory Index score (mean, SD) | 98.4 [8.6] | 105.7 [11.5] | 1.04, 15, p=0.2 |
| RBANS Visuospatial construction Index score (mean, SD) | 82.7 [12.9] | 91.0 [20.03] | 0.9, 15, p =0.3 |
| RBANS Attention Index Score (mean, SD) | 99.2 [14.5] | 109.8 [10.4] | 1.7, 15, p = 0.1 |
| RBANS Delayed Memory Index Score(mean, SD) | 101.1 [10] | 96.4 [10.6] | 0.9, 15, p = 0.4 |
HRSA= Hamilton Rating Scale for Anxiety; PSWQ= Penn State Worry Questionnaire; GADSS= Generalized Anxiety Disorder Severity Scale, DKEFS=Delis Kaplan executive function system; RBANS=Repeatable Battery for the Assessment of Neuropsychological Status
Effects of Worry Induction on rCBF
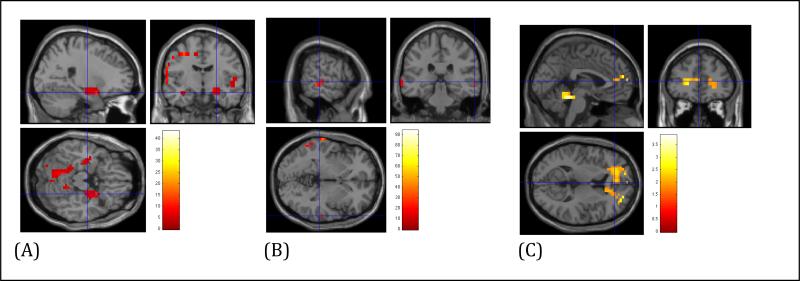
1. Non-anxious comparison subjects: worry induction - resting state (Fig 1A)
Figure 1.
Regional Cerebral Blood Flow (rCBF) differences during Worry Induction (A. Non-anxious elderly subjects; B. Elderly GAD; C. Between-group comparison: Elderly GAD vs. Elderly non-anxious subjects)
Compared with resting state, elderly controls showed increased rCBF during worry induction in the superior temporal gyrus [Brodmann area (BA) 42], ventrolateral PFC (vlPFC, BA 10) [t-test 4.29, df=9, p<0.001 (FDR corrected)], right insula and right amygdala [t-test=4.41, df=9, p<0.05 (FDR corrected)].
2. Elderly GAD subjects worry induction - resting state (Fig 1B)
Compared with resting state, elderly GAD subjects showed increased rCBF during worry induction in left posterior temporo-occipital associative areas (BA 21; BA 37) [t=13.4, df=6, p<0.001 (FDR corrected)].
3. Between groups comparison worry induction - resting state (Fig 1C)
Compared with non-anxious elderly, GAD subjects had increased rCBF in the rostral ACC bilaterally [t=1.75, df=15, p=0.05 uncorrected, x=-1.70, y=45.26, z=11.54, (BA32)]. We repeated the analysis adding age as covariate and the results were similar (results available upon request).
Effects of Worry Suppression on rCBF
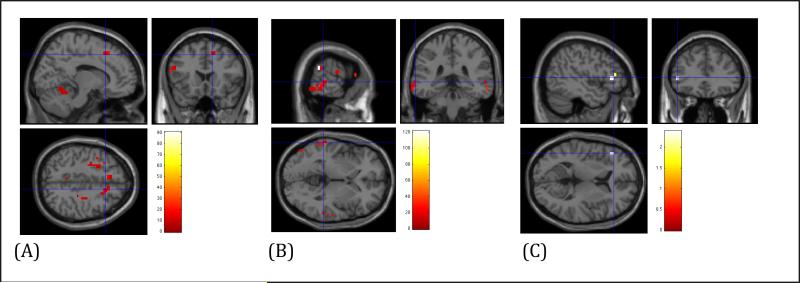
1. Non-anxious comparison subjects: worry suppression - resting state (Fig 2A)
Figure 2.
Regional Cerebral Blood Flow (rCBF) differences during Worry Suppression (A. Non-anxious elderly subjects; B. Elderly GAD; C. Between-group comparison: Elderly non-anxious subjects vs. Elderly GAD)
Compared with resting state, elderly controls showed increased rCBF during worry suppression in the left dlPFC (BA 46), left vlPFC (BA 10) and right dmPFC (BA 8) [t=9.7, df=9, p<0.001 (FDR corrected)].
2. Elderly GAD subjects worry suppression - resting state (Fig 2B)
Compared with resting state, elderly GAD subjects showed increased rCBF during worry suppression in posterior temporo-parieto-occipital associative areas (left>right) (BA 21, BA 37) [t=15.5, df=6, p<0.001 (FDR corrected)] and left dorsal ACC (BA 24)[t=4.8, df=6, p<0.05 (FDR corrected)], while having no suprathreshold rCBF in the prefrontal areas.
3. Between groups comparison worry suppression – resting state (Fig 2C)
Compared with non-anxious elderly, GAD subjects had increased rCBF in the left fusiform gyrus [t=3.7, df=15, p=0.001 uncorrected, x=-34.5, y=-56.7, z=-11.6]. Compared with GAD subjects, non-anxious elderly had increased rCBF in the left lateral prefrontal area [t=1.75, df=15, p=0.05 uncorrected, x=-48.1, y=30.3, z=4.7]. We repeated the analysis adding age as covariate and the results were similar (results available upon request).
None of the between-groups comparison analyses survived FDR multiple comparison corrections.
CONCLUSION
Our results indicate that during worry induction elderly GAD subjects have increased rCBF in the posterior associative temporo-occipital areas, most likely due to the role of these areas in the required mental visual imagery of the worry modulation task (42, 43). Non-anxious elderly also engage the associative temporo-occipital areas but, in addition, they have increased rCBF in the insula and the amygdala. These two latter areas have been cited frequently in the literature in connection with emotion processing (24, 44, 45). It is unclear why elderly GAD subjects engage less the insula/amygdala during worry induction. One explanation may be that these subjects have a baseline hyperactive limbic system and thus the differences in rCBF between resting state and worry induction would be minimal. To test this, we analyzed the between-groups rCBF differences during RS; we found no differences between elderly GAD and non-anxious subjects with regard to either amygdala or prefrontal areas, even when we relaxed the significance criteria to p<0.01, uncorrected (results available upon request). A more fitting interpretation places the “silent amygdala” in the context of the Borkovec model of GAD (46). The model considers worry a cognitive process designed to avoid the anxiogenic images that induce somatic activation through increased noradrenergic discharge (2, 46). Thus, as worry represents an avoidance process of anxious experiences, activation of amygdala during worry induction is unlikely in pathological worriers (47). Recent data support a possible malfunction of the amygdalar complex, reporting disrupted amygdalar subregions connectivity in midlife GAD (17). Our preliminary data extend the support of the model of a “silent” amygdala in GAD into late-life.
The between-group comparison showed that elderly GAD had increased rostral ACC rCBF during worry induction. The rostral ACC has been frequently involved in the neurobiology of emotional regulation as a key region in assessing the salience of emotional information and the regulation of emotional response (48-50). It is possible that the observed increased rCBF in rostral ACC during worry induction represents a first attempt of elderly GAD to automatically regulate overwhelming negative emotions (15, 51). We can speculate that worry induction triggers unconscious appraisal of threat and spontaneous response of areas such as the ACC, involved in automatic emotion regulation (52), while worry suppression (a prototypical top-down, conscious instruction) increases rCBF to areas involved in instructed emotion regulation (52, 53).
During worry suppression, elderly non-anxious subjects engaged predominantly the lateral prefrontal areas and the dorsal ACC, areas involved in cognitive control of negative emotions (31, 45, 54-57). Elderly GAD subjects did not present any increased lateral prefrontal rCBF during worry suppression.
Our results diverge from findings in young adults and adolescents, which described an excessive engagement of the lateral PFC during down-regulation of negative emotions in anxiety-prone participants (15, 16). There are several factors that might contribute to these divergent results in older age.
First, our project included only elderly with GAD. The disruption of the cognitive-control network in the elderly due to white matter tracts age-induced damage [e.g. demyelinations, gliosis or axonal degeneration, induced by inflammation or ischemic changes (58)] might contribute to the different results (31, 51). Our results suggest a rather distinct dysfunctional point in the emotion regulation process in elderly GAD: the prefrontal cognitive control regions fail to engage during attempts to suppress worry. We may speculate that this feature is a central part of the neurobiological signature underlying the persistent and uncontrollable quality of worry experienced by these subjects. While therapies such as CBT are efficacious in midlife GAD, several clinical trials and meta-analyses failed to prove an advantage of CBT over supportive therapy/waiting list in late-life GAD(13, 59, 60). The failure of elderly GAD to engage the prefrontal areas would offer a neurobiological basis for the decreased efficacy of top-down regulation psychotherapies (e.g. CBT) in elderly with GAD (13, 60). This distinctive feature may be relevant for novel treatment development (i.e., treatments that correct this deficit), prior to initiating psychotherapeutic strategies for the treatment of late-life GAD (61).
Second, the variability of tasks used to probe the emotion modulation may explain some of the differences in results. Thus, in a study exploring emotion regulation in Social Anxiety Disorder (SAD), subjects with Social Anxiety Disorder engaged cognitive control regions (DLPFC, dACC) to a larger degree than controls when exposed to physical threat stimuli but to lesser degree during regulation of responses to social threat stimuli (the latter being most relevant for SAD psychopathology)(62). We used a task that specifically targeted the suppression of personally relevant worry themes, which may explain the relative decrease in cerebral blood flow in the lateral prefrontal cortex.
Third, differences in the subject samples across studies influence the aspect of emotion regulation that is highlighted in the anxious subjects. The inclusion criteria of various studies exploring the neurobiology of anxiety encompass a vast array of symptoms from panic or simple phobia to social anxiety or pathological worry. Thus, the subjects’ heterogeneity across studies might also contribute to different results.
Although, relative to the non-anxious comparison subjects, the elderly GAD had significantly higher anxiety scores on all three scales (HARS, PSWQ, GADSS), they did not report significantly higher anxiety during either worry induction or worry suppression. The trend of higher self-reported anxiety in elderly GAD vs. controls (1.73 vs. 1.46) during worry suppression was not significant, but given the small sample we cannot make inferences regarding any correlation between persistent perceived anxiety and failure to engage the lateral PFC.
Our study has several strengths. We used an imaging technique (ASL perfusion fMRI) that reduces the low-frequency signal drift that usually degrades BOLD fMRI contrast over time, allowing us to investigate slower changes in neural activity such as those evoked by worry induction and suppression. Also, we applied an emotion regulation paradigm designed to specifically probe the core of GAD clinical presentation, namely the persistent and incontrollable quality of worry. As GAD may be particularly detrimental to cognition in older adults (63), subjects received a comprehensive cognitive evaluation that did not find any significant differences between the two groups. We had a control group and the GAD subjects were scanned medication-free, thus eliminating interferences related to treatment response.
Limitations and future directions
The small sample size makes the results unstable and increases the risk for false negatives when using high significance thresholds including multiple comparison corrections. While the within-group results survived stringent multiple comparison correction, due to the modest sample size and the variance components in brain perfusion, we obtained a weaker signal during the between-group comparison (64). The comparison group was significantly older than the GAD group; however, our results did not change when we controlled for the age difference. Moreover, there were no significant differences in cognitive performance between the two groups. One other limitation was the lack of behavioral data (heart rate, blood pressure, respiration rate, skin conductance) to correlate autonomic response with the phases of emotional regulation. However, behavioral data is more difficult to interpret in elderly samples, given the age-related changes in autonomic response and the modifications to heart rate and blood pressure induced by medications such as beta-blockers, which are widely used by the elderly. Our self-reported anxiety index, measured after worry induction and worry suppression, would have been more informative if compared with a baseline self-reported anxiety index. While the GAD subjects were free of psychotropics at the time of scanning, we do not know the duration of the medication-free prescanning interval. Further analysis exploring the correlation between worry severity (as measured by HARS, PSWQ and GADSS) and rCBF changes during worry modulation would require a larger sample.
In conclusion, our results provide evidence of the altered top-down regulation of worry modulation in elderly with GAD. Our results point toward several dysfunctional nodes in the extended network of emotion regulation that include sACC, amygdala and the hippocampus, insula, lateral and orbital PFC (31, 51). The recruitment sequence of each of these nodes, the feedforward/feedback interplay in the network during various stages of emotion regulation as well as the role of white matter structural lesions in altering the network's architecture are subject for future research.
Table 2.
Regions of significantly increased regional cortical blood flow (rCBF) in elderly GAD and elderly non-anxious comparison subjects.
| Emotional Paradigm | Subjects | Brain Region | |||||
|---|---|---|---|---|---|---|---|
| Brain region | Brodmann Area (BA) | Cluster size | T score | FDR p value | MNI (x,y,z) | ||
| WI-RS | GAD | Temp-occipital (L) | BA 21 BA 37 |
>20 >20 |
13.4 | <0.001 | x = -65.6, y=-44.5, z=-4.8 x = -52.05, y=-69.02, z=-4.8 |
| Non GAD | Sup Temp (L) vlPFC (L) Insula (R) Amygdala (R) |
BA 42 BA 10 |
>20 >5 >5 >20 |
4.29 4.41 |
<0.001 <0.001 <0.05 <0.05 |
x=-62.3, y=-22.7, z=10.8 x=-39.8, y=59.5, z=10.8 x=46.8, y=-5.6, z=0.0 x=23.4, y=-5.08, z=-18.0 |
|
| WS-RS | GAD | Temp-occipital (L) dACC (L) |
BA 21 BA 37 BA 24 |
>20 >20 >5 |
15.5 4.8 |
<0.001 <0.05 |
x=-63.6, y=-37.7, z=-6 x=-56.8, y=-63.5, z=-6 x=-13.5, y=9.3, z=48 |
| nonGAD | dlPFC (L) vlPFC (L) dmPFC (R) |
BA 46 BA 10 BA 8 |
>20 >20 >5 |
9.7 | <0.001 | x=-54.3, y=31.8, z=18 x=-40.4, y=57.5, z=18 x=22.1, y=17.3, z=48.2 |
|
WI= worry induction, WS= worry suppression, RS= resting state, GAD=generalized anxiety disorder, FDR=false discovery rate, MNI=Montreal Neurologic Institute, L=left, R=right, vlPFC=ventrolateral prefrontal cortex, dACC=dorsal anterior cingulate cortex, dlPFC= dorsolateral prefrontal cortex, dmPFC= dorsomedial prefrontal cortex.
Acknowledgments
Supported by NIH MH 086680, MH076079, MH070547, NARSAD Young Investigator Award, the John A. Hartford Foundation Center of Excellence in Geriatric Psychiatry from the University of Pittsburgh.
Footnotes
Conflict of Interest: Carmen Andreescu, James J. Gross, Costin Tanase, Kathreen Dunfee Edelman and Sara Snyder have no conflict of interest to report. Eric J. Lenze has received research support from OrthoMcNeill, Novartis and Forest Pharmaceuticals. Howard Aizenstein has received research support from Novartis.
REFERENCES
- 1.Diefenbach GJ, Stanley MA, Beck JG. Worry content reported by older adults with and without generalized anxiety disorder. Aging Ment Health. 2001;5(3):269–74. doi: 10.1080/13607860120065069. [DOI] [PubMed] [Google Scholar]
- 2.Borkovec TD, Shadick RN, Hopkins M. Rappe RM, Barlow DH, editors. The nature of normal and pathological worry. Chronic anxiety: Generalized anxiety disorder and mixed anxiety-depression. 1991. pp. 29–51.
- 3.Beekman AT, Bremmer MA, Deeg DJ, van Balkom AJ, Smit JH, de Beurs E, et al. Anxiety disorders in later life: a report from the Longitudinal Aging Study Amsterdam. Int J Geriatr Psychiatry. 1998;13(10):717–26. doi: 10.1002/(sici)1099-1166(1998100)13:10<717::aid-gps857>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Wittchen HU, Hoyer J. Generalized anxiety disorder: nature and course. J Clin Psychiatry. 2001;62(Suppl 11):15–9. discussion 20-1. [PubMed] [Google Scholar]
- 5.de Beurs E, Beekman AT, van Balkom AJ, Deeg DJ, van Dyck R, van Tilburg W. Consequences of anxiety in older persons: its effect on disability, well-being and use of health services. Psychol Med. 1999;29(3):583–93. doi: 10.1017/s0033291799008351. [DOI] [PubMed] [Google Scholar]
- 6.Wetherell JL, Gatz M, Pedersen NL. A longitudinal analysis of anxiety and depressive symptoms. Psychol Aging. 2001;16(2):187–95. doi: 10.1037//0882-7974.16.2.187. [DOI] [PubMed] [Google Scholar]
- 7.Mantella RC, Butters MA, Dew MA, Mulsant BH, Begley AE, Tracey B, et al. Cognitive impairment in late-life generalized anxiety disorder. Am J Geriatr Psychiatry. 2007;15(8):673–9. doi: 10.1097/JGP.0b013e31803111f2. [DOI] [PubMed] [Google Scholar]
- 8.Caudle DD, Senior AC, Wetherell JL, Rhoades HM, Beck JG, Kunik ME, et al. Cognitive errors, symptom severity, and response to cognitive behavior therapy in older adults with generalized anxiety disorder. Am J Geriatr Psychiatry. 2007;15(8):680–9. doi: 10.1097/JGP.0b013e31803c550d. [DOI] [PubMed] [Google Scholar]
- 9.Astrom M. Generalized anxiety disorder in stroke patients. A 3-year longitudinal study. Stroke. 1996;27(2):270–5. doi: 10.1161/01.str.27.2.270. [DOI] [PubMed] [Google Scholar]
- 10.Lenze EJ, Wetherell JL. Bringing the bedside to the bench, and then to the community: a prospectus for intervention research in late-life anxiety disorders. Int J Geriatr Psychiatry. 2009;24(1):1–14. doi: 10.1002/gps.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenze EJ, Rollman BL, Shear MK, Dew MA, Pollock BG, Ciliberti C, et al. Escitalopram for older adults with generalized anxiety disorder: a randomized controlled trial. JAMA. 2009;301(3):295–303. doi: 10.1001/jama.2008.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorp SR, Ayers CR, Nuevo R, Stoddard JA, Sorell JT, Wetherell JL. Meta-analysis Comparing Different Behavioral Treatments for Late-Life Anxiety. Am J Geriatr Psychiatry. 2008 doi: 10.1097/JGP.0b013e31818b3f7e. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wetherell JL, Gatz M, Craske MG. Treatment of generalized anxiety disorder in older adults. J Consult Clin Psychol. 2003;71(1):31–40. doi: 10.1037//0022-006x.71.1.31. [DOI] [PubMed] [Google Scholar]
- 14.Gross JJ, Thompson RA. In: Emotion Regulation: Conceptual Foundations. Gross JJ, editor. Guilford Press; 2007. [Google Scholar]
- 15.Campbell-Sills L, Simmons AN, Lovero KL, Rochlin AA, Paulus MP, Stein MB. Functioning of neural systems supporting emotion regulation in anxiety-prone individuals. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):568–76. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66(12):1361–72. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167(5):545–54. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166(3):302–10. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander AL, Davidson RJ, Kalin NH. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 2008;63:858–63. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulesu E, Sambugaro E, Torti T, Danelli L, Ferri F, Scialfa G, et al. Neural correlates of worry in generalized anxiety disorder and in normal controls: a functional MRI study. Psychol Med. 2010;40(1):117–24. doi: 10.1017/S0033291709005649. [DOI] [PubMed] [Google Scholar]
- 22.Davis M, Young Lim Lee. Fear and Anxiety: Possible Roles of the Amygdala and Bed Nucleus of Stria Terminalis. Cognition and Emotion. 1998;12(3):277–305. [Google Scholar]
- 23.Davis M, Shi Changjun. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Annals of the New York Academy of Sciences. 1999;877:281–91. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- 24.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164(2):318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 25.Smith GS, Gunning-Dixon FM, Lotrich FE, Taylor WD, Evans JD. Translational research in late-life mood disorders: implications for future intervention and prevention research. Neuropsychopharmacology. 2007;32(9):1857–75. doi: 10.1038/sj.npp.1301333. [DOI] [PubMed] [Google Scholar]
- 26.Chelminski I, Zimmerman M. Pathological worry in depressed and anxious patients. J Anxiety Disord. 2003;17(5):533–46. doi: 10.1016/s0887-6185(02)00246-3. [DOI] [PubMed] [Google Scholar]
- 27.Mennin DS, Heimberg RG, Fresco DM, Ritter MR. Is generalized anxiety disorder an anxiety or mood disorder? Considering multiple factors as we ponder the fate of GAD. Depress Anxiety. 2008;25(4):289–99. doi: 10.1002/da.20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89(1):212–6. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Aguirre GK, Rao H, Wang J, Fernandez-Seara MA, Childress AR, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26(2):261–9. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulesu E, Sambugaro T, Torti L, Danelli F, Scialfa G, Sberna M, Ruggiero GM, Bottini G, Sassaroli S. Neural correlates of worry in generalized anxiety disorder and in normal controls: a functional MRI study. Psychol Med. 2009 doi: 10.1017/S0033291709005649. epub ahead of print doi: 10.1017/S00332917090005649. [DOI] [PubMed] [Google Scholar]
- 31.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up- regulation of negative emotion. Neuroimage. 2004;23(2):483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 32.First MSR, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P) 2.0 ed. 1995.
- 33.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 34.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28(6):487–95. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 35.Andreescu C, Belnap BH, Rollman BL, Houck P, Ciliberti C, Mazumdar S, et al. Generalized anxiety disorder severity scale validation in older adults. Am J Geriatr Psychiatry. 2008;16(10):813–8. doi: 10.1097/JGP.0b013e31817c6aab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–9. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 37.Delis DC, Kaplan E, Kramer JH. Delis Kaplan Executive Funciton System Examiner's Manual: The Psychological Corporation. 2001.
- 38.Wang J, Licht DJ, Jahng GH, Liu CS, Rubin JT, Haselgrove J, et al. Pediatric perfusion imaging using pulsed arterial spin labeling. J Magn Reson Imaging. 2003;18(4):404–13. doi: 10.1002/jmri.10372. [DOI] [PubMed] [Google Scholar]
- 39.Koziak AM, Winter J, Lee TY, Thompson RT, St Lawrence KS. Validation study of a pulsed arterial spin labeling technique by comparison to perfusion computed tomography. Magn Reson Imaging. 2008;26(4):543–53. doi: 10.1016/j.mri.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Ewing JR, Cao Y, Knight RA, Fenstermacher JD. Arterial spin labeling: validity testing and comparison studies. J Magn Reson Imaging. 2005;22(6):737–40. doi: 10.1002/jmri.20451. [DOI] [PubMed] [Google Scholar]
- 41.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22(2):324–33. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 42.Ishai A, Ungerleider LG, Haxby JV. Distributed neural systems for the generation of visual images. Neuron. 2000;28(3):979–90. doi: 10.1016/s0896-6273(00)00168-9. [DOI] [PubMed] [Google Scholar]
- 43.Knauff M, Kassubek J, Mulack T, Greenlee MW. Cortical activation evoked by visual mental imagery as measured by fMRI. Neuroreport. 2000;11(18):3957–62. doi: 10.1097/00001756-200012180-00011. [DOI] [PubMed] [Google Scholar]
- 44.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(3):210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 46.Borkovec TD, Inz J. The nature of worry in generalized anxiety disorder: a predominance of thought activity. Behav Res Ther. 1990;28(2):153–8. doi: 10.1016/0005-7967(90)90027-g. [DOI] [PubMed] [Google Scholar]
- 47.Hoehn-Saric R, Schlund MW, Wong SH. Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Res. 2004;131(1):11–21. doi: 10.1016/j.pscychresns.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 48.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 49.Bissiere S, PLachta N, Hoyer D, McAllister KH, Olpe HK, Grace AA, Cryan JF. The rostral Anterior Cingulate Cortex modulates the efficiency of amygdala-dependent fear learning. Biol Psychiatry. 2008;63(9):821–31. doi: 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 51.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13(9):829, 33–57. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biol Psychiatry. 2009;65(5):367–73. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mauss E, Bunge SA, Gross JJ. Automatic Emotion Regulation. Social and Personality Psychology Compass. 2007;1:146–67. [Google Scholar]
- 54.Berkowitz RL, Coplan JD, Reddy DP, Gorman JM. The human dimension: how the prefrontal cortex modulates the subcortical fear response. Rev Neurosci. 2007;18(3-4):191–207. doi: 10.1515/revneuro.2007.18.3-4.191. [DOI] [PubMed] [Google Scholar]
- 55.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11(7):307–16. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97(4):1944–8. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63(6):577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ovbiagele B, Saver JL. Cerebral white matter hyperintensities on MRI: Current concepts and therapeutic implications. Cerebrovasc Dis. 2006;22(2-3):83–90. doi: 10.1159/000093235. [DOI] [PubMed] [Google Scholar]
- 59.Thorp SR, Ayers CR, Nuevo R, Stoddard JA, Sorrell JT, Wetherell JL. Meta-analysis comparing different behavioral treatments for late-life anxiety. Am J Geriatr Psychiatry. 2009;17(2):105–15. doi: 10.1097/JGP.0b013e31818b3f7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohlman J. Psychosocial treatment of late-life generalized anxiety disorder: current status and future directions. Clin Psychol Rev. 2004;24(2):149–69. doi: 10.1016/j.cpr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Wetherell JL, Stoddard JA, White KS, Kornblith S, Nguyen H, Andreescu C, et al. Augmenting antidepressant medication with modular CBT for geriatric generalized anxiety disorder: a pilot study. Int J Geriatr Psychiatry. 2010 doi: 10.1002/gps.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66(2):170–80. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beaudreau SA, O'Hara R. Late-life anxiety and cognitive impairment: a review. Am J Geriatr Psychiatry. 2008;16(10):790–803. doi: 10.1097/JGP.0b013e31817945c3. [DOI] [PubMed] [Google Scholar]
- 64.Viviani R, Sim EJ, Lo H, Richter S, Haffer S, Osterfeld N, et al. Components of variance in brain perfusion and the design of studies of individual differences: the baseline study. Neuroimage. 2009;46(1):12–22. doi: 10.1016/j.neuroimage.2009.01.041. [DOI] [PubMed] [Google Scholar]