Abstract
Objective
Cancer incidence and mortality statistics provide limited insight regarding the cancer survivor population and its needs. Cancer prevalence statistics enumerate cancer survivors—those currently living with cancer. Commonly used limited-duration prevalence (LDP) methods yield biased estimates of the number of survivors. National estimates may not allow sufficient granularity to inform local survivorship programs. In this study, complete prevalence (CP) methods are applied to actual North Carolina Central Cancer Registry (NCCCR) data to generate better, more informative prevalence estimates than previous methods.
Methods
Data included all incident cases for 1995–2007 from the NCCCR and US Census population data. SEER*Stat software was used to calculate 13-year LDP. ComPrev software was used to estimate CP for each cancer site, gender, and race combination.
Results
CP methods estimated 362,810 survivors in North Carolina on January 1, 2008, 40% more than LDP estimates of 258,556, with substantial racial, regional, and gender differences in prevalence rankings of several cancers.
Conclusion
CP estimates are substantially higher than previous prevalence estimates. This study found previously unrecognized racial, regional, and gender differences. State and local programs may apply these methods using their own data to develop better, more detailed estimates to improve planning for their specific survivor populations' needs.
Keywords: Cancer survivorship, Prevalence, Epidemiology, Methods, Program planning
Introduction
With advances in early detection, treatment, and knowledge and management of late effects, cancer is evolving from an acute to a chronic disease that can be successfully managed for many years [1, 2]. Over the past 15 years, an aging United States population and declining cancer mortality rates have contributed to a simultaneous increase in the number of cancer survivors to more than 11.7 million as of January 1, 2007 [3]. The trends in North Carolina are similar [4]. Accordingly, addressing the needs of specific survivor populations is of growing importance among cancer centers, cancer care providers, and patient support programs; however, the traditional cancer surveillance metrics used by cancer programs and registries—incidence and mortality—provide limited insight to inform programmatic and population needs.
Understanding cancer prevalence—defined as “the number or percent of people alive on a certain date in a population who previously had a diagnosis of the disease” [5]—helps address this need for better guidance and provides additional information relevant to the specific services that will be needed. Conceptually, obtaining this measure appears straightforward—counting the number of survivors alive at any point in time. However, unlike incidence and mortality, which can be counted in this way and are currently captured by registries through population-based surveillance, prevalence must be estimated. Obtaining accurate estimates of prevalence requires either broad sampling by survey-based surveillance or calculations based on available registry data, neither of which is straightforward. For example, with survey-based reporting, recall bias may lead to under- or over-reporting of cancers, with certain subgroups of patients more likely to misreport their cancer history, and accuracy of self-reporting varying by cancer site with sensitivity varying from 54 to 96% when compared to registry data [6, 7]. With registry data, limited-duration prevalence (LDP) has been a convenient and commonly used method, calculated based on available data in the registry (e.g., 10-year LDP based on 10 years of available registry data). However, this simple measure often grossly underestimates actual prevalence for registries that have been operational for fewer years, registries covering older populations, and cancers that have longer survival periods. In these instances, the shorter-lived registries have captured data over a shorter surveillance period than longer-lived ones. As a result, there is a greater likelihood of individuals having had a cancer diagnosis prior to the registry opening for older populations or cancers with long survival periods, thus contributing to biased estimates when calculating limited-duration prevalence [8].
Developments in cancer surveillance methodology provide guidance for states and programs to use their own data and calculate complete prevalence (CP) statistics, which help correct for these sources of bias when estimating cancer prevalence in a population [8, 9]. For state and local cancer registries and programs, calculating CP based on their own data can provide important metrics to inform state and local programs to help them prioritize goals, coordinate efforts, and allocate resources for cancer control and survivorship planning. Calculating CP is an alternative to relying on prevalence estimates derived from other sources of data such as the Surveillance, Epidemiology, and End Results (SEER) program [10], which can be problematic due to both limited granularity and reporting details that may not address local programs' specific needs and infrequency of updating. This study uses North Carolina Central Cancer Registry (NCCCR) data to estimate and compare LDP and CP for North Carolina and provides a detailed model of how other states and programs may do the same using their own central cancer registry data.
Methods
CP estimation uses three software programs developed by the National Cancer Institute—SEER*Prep, SEER*Stat, and ComPrev [11, 12]. The methods for estimating CP are explained in detail elsewhere [8, 9, 13–15]. Briefly, the method first uses SEER*Prep software to format cancer registry data and then uses SEER*Stat software to estimate LDP. The CompPrev software is then used to estimate the number of cases diagnosed prior to (and thus not explicitly captured by) the initiation of surveillance by the population-based registry. Based on incidence and survival models originally developed using SEER registry data, it calculates a completeness index reflecting the degree to which these registries' LDP is complete for specific combinations of cancer sites, races, and genders. The actual LDP is then divided by the appropriate completeness index to estimate CP. [16] This study applies CP and LDP methods to actual incidence and mortality/survival data from the NCCCR, which is not part of the SEER program. (Note to Editor: This IRB information was moved to the Acknowledgments section of this manuscript.)
Data
A limited dataset was obtained from the NCCCR, including the following variables: case unique identifier, cancer type (primary site, histology, and behavior), cancer sequence number, year of birth, month of birth, year of diagnosis, month of diagnosis, vital status at last follow-up, year of last follow-up, month of last follow-up, race, ethnicity, gender, and county. These data reflect all incident cases of malignant cancer diagnosed from 1995 through 2007, currently the most complete year of NCCCR data, including current vital status, which was obtained by linking the case information to the North Carolina Death Files, Social Security Death Index, and the National Death Index. The NCCCR data were formatted to SEER*Stat standards using SEER*Prep software [16]. The data from the NCCCR were merged with North Carolina total population estimates for the same years from the US Census Bureau's Population Estimates Program, obtained through the SEER program [17, 18]. State population estimates used four expanded races (White, Black, American Indian/Alaska Native, Asian/Pacific Islander) and single ages (0–85+).
Calculating limited-duration prevalence
We first used the SEER*Stat software to calculate 13-year LDP, the most extended period possible for the NCCCR, as of January 1, 2008, for each cancer site, gender, and race combination [11]. In the SEER*Stat program selection window, we used the count method of prevalence estimation and selected the “First Primary in Database Only” for multiple primary selection. In the table window, we selected “Age at Prevalence Date” as the row display variable. An LDP matrix was generated and exported to a text file. “Crude prevalence” was selected in the statistic window, and the options of “Remove All Thousands Separators (Commas)” and “Remove Flags (Footnote), Prefix, and Suffix Characters” were selected when creating the text file.
Calculating complete prevalence
We used ComPrev software to convert LDP into CP for each cancer site, gender, and race combination [12]. We selected the mode of “Single Group | Compute Completeness from Imported Limited-Duration Prevalence | Default Parameters Only.” The prevalence date was set to be January 1, 2008 with duration of 13 years. “Single ages (0, 1, 2, 3,…, 85 +)” was selected for the age group output. After choosing a combination of site, gender, and race, we imported the corresponding text file from the LDP matrix as generated by SEER*Stat. Because the race-specific CP index has not yet been developed for American Indians/Alaska Natives, and Asian/Pacific Islanders, race-specific CP could not be estimated for these populations that comprise approximately 1.1% of the state's cancer population. ComPrev then calculated the CP for single ages and all White or Black patients, regardless of ethnicity.
Examination of regional variation and gender and race-specific estimates
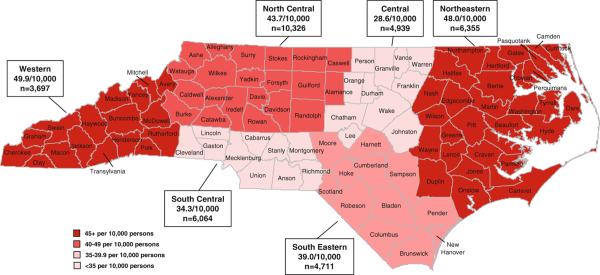
In regional analysis, North Carolina's one hundred counties were categorized into six geographic regions corresponding to the North Carolina Comprehensive Cancer Program's (NCCCP) Cancer Partnership Regions (See Fig. 2). We added an additional restriction in the selection window of SEER*Stat to calculate region-specific LDP. Regional prevalence rates for January 1, 2008 were calculated using the average of census population estimates for July 1, 2007 and July 1, 2008, as done previously [3]. We then ranked the most prevalent cancer sites by the sum of prevalence counts of men and women.
Fig. 2.
Estimated complete prevalence rates of colorectal cancer, by North Carolina Cancer partnership region, 1 January 2008
Sensitivity analysis
Estimates were calculated and examined as 10-year LDP and 10-year CP, and 13-year LDP and 13-year CP, using both NCCCR data and SEER-13 registry data. North Carolina estimates were compared to published North Carolina-specific and national prevalence estimates [3, 19, 20]. Race- and gender-specific estimates were estimated for 23 cancer types, all other cancer types combined, and all cancers overall, and compared among all sources for variation and stability of estimates among samples, focusing on consistency and predictability of changes in estimates among methods, time-periods, and data sources for all cancer types and sub-populations.
Results
Using NCCCR data, CP methods estimated 362,810 cancer survivors were living in North Carolina as of January 1, 2008. This estimate is 40% greater than the 13-year LDP estimate of 258,556 and is consistent with national prevalence rate estimates of 4%. [3, 21] LDP estimates are comparable to extrapolated values from prior North Carolina estimates [19, 20]. Table 1 presents the count estimates of LDP and CP for the most common cancers in North Carolina, listed according to common cancer registry reporting standards. The five cancers of greatest prevalence were female breast, prostate, colon/rectum, melanoma, and lung/bronchus, which comprise 62% of North Carolina cancer survivors. The rank order of the 5 most prevalent cancers did not differ between CP and LDP estimates; however, CP estimates were 32% greater than LDP estimates. The top ten cancers reflect 79% of cancer survivors, and they vary slightly in rank order between CP and LDP estimates.
Table 1.
Comparison of 13-year limited-duration and complete prevalence estimates in North Carolina, January 1, 2008
| Site | Male | Female | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LDP | CP | % Gained* | LDP | CP | % Gained* | LDP | CP | % Gained* | |
| Oral cavity | 3,656 | 5,205 | 42.4 | 1,701 | 2,610 | 53.4 | 5,357 | 7,815 | 45.9 |
| Esophagus | 694 | 771 | 11.1 | 198 | 231 | 16.7 | 892 | 1,002 | 12.3 |
| Stomach | 715 | 912 | 27.6 | 631 | 820 | 30.0 | 1,346 | 1,732 | 28.7 |
| Colon/Rectum | 13,300 | 17,460 | 31.3 | 13,345 | 18,711 | 40.2 | 26,645 | 36,171 | 35.8 |
| Liver | 409 | 438 | 7.1 | 183 | 238 | 30.1 | 592 | 676 | 14.2 |
| Pancreas | 402 | 440 | 9.5 | 487 | 552 | 13.3 | 889 | 992 | 11.6 |
| Larynx | 2,080 | 3,046 | 46.4 | 563 | 775 | 37.7 | 2,643 | 3,821 | 44.6 |
| Lung/Bronchus | 5,942 | 7,555 | 27.1 | 5,951 | 7,357 | 23.6 | 11,893 | 14,912 | 25.4 |
| Melanoma (skin) | 9,594 | 14,363 | 49.7 | 8,588 | 14,367 | 67.3 | 18,182 | 28,730 | 58.0 |
| Female breast | - | - | - | 61,814 | 88,085 | 42.5 | 61,814 | 88,085 | 42.5 |
| Cervix uteri | - | - | - | 3,555 | 7,452 | 109.6 | 3,555 | 7,452 | 109.6 |
| Corpus uteri | - | - | - | 7,958 | 14,528 | 82.6 | 7,958 | 14,528 | 82.6 |
| Ovary | - | - | - | 3,268 | 5,728 | 75.3 | 3,268 | 5,728 | 75.3 |
| Prostate | 50,860 | 56,479 | 11.0 | - | - | - | 50,860 | 56,479 | 11.0 |
| Testes | 2,237 | 4,477 | 100.1 | - | - | - | 2,237 | 4,477 | 100.1 |
| Bladder | 7,749 | 10,728 | 38.4 | 2,657 | 3,877 | 45.9 | 10,406 | 14,605 | 40.4 |
| Kidney | 4,637 | 6,176 | 33.2 | 3,057 | 4,240 | 38.7 | 7,694 | 10,416 | 35.4 |
| Thyroid | 1,275 | 2,323 | 82.2 | 4,730 | 8,454 | 78.7 | 6,005 | 10,777 | 79.5 |
| Multiple Myeloma | 908 | 962 | 5.9 | 837 | 906 | 8.2 | 1,745 | 1,868 | 7.0 |
| Leukemia | 2,486 | 3,237 | 30.2 | 1,839 | 2,515 | 36.8 | 4,325 | 5,752 | 33.0 |
| Brain/other CNS | 2,054 | 3,343 | 62.8 | 2,842 | 5,127 | 80.4 | 4,896 | 8,470 | 73.0 |
| Hodgkin's lymphoma | 1,097 | 2,196 | 100.2 | 1010 | 2,133 | 111.2 | 2,107 | 4,329 | 105.5 |
| NH lymphoma | 4,468 | 5,668 | 26.9 | 4,325 | 5,620 | 29.9 | 8,793 | 11,288 | 28.4 |
| Other cancers | 6,410 | 6,942 | 8.3 | 8,044 | 15,763 | 96.0 | 14,454 | 22,705 | 57.1 |
| All cancers | 120,973 | 152,721 | 26.2 | 137,583 | 210,089 | 52.7 | 258,556 | 362,810 | 40.3 |
% Gained reflects the percentage of cases added by CP to the LDP estimates
LDP 13-year limited-duration prevalence, CP complete prevalence, CNS central nervous system, NH non-Hodgkin's
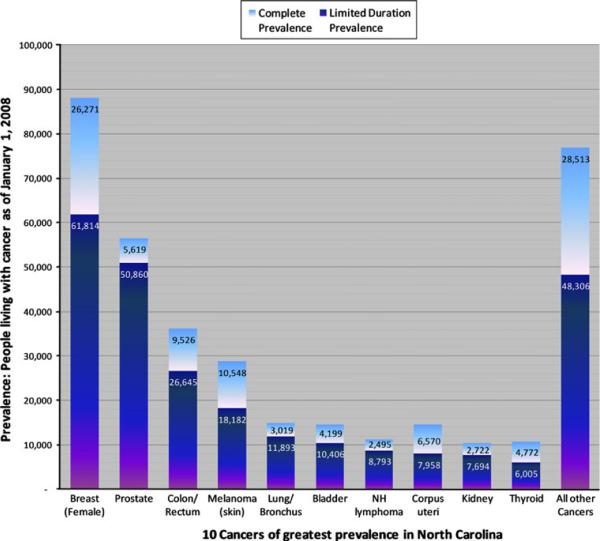
Figure 1 illustrates the differences in LDP (dark bars) and CP (light bars—additional cases added by CP) for the ten most prevalent cancers in North Carolina, ranked in order of descending LDP. Compared to cancers with typically short survival duration, cancers of greater survival duration tended to have greater difference between LDP and CP estimates. For example, pancreatic, liver, and lung cancers are associated with poor survival, and in these data CP estimates exceed those of LDP by 12, 14, and 25%, respectively. By comparison, cervical, testicular, and uterine cancers typically have very good survival, and CP estimates exceed those of LDP in these data by 110, 100, and 83%, respectively.
Fig. 1.
Top ten most prevalent cancers in North Carolina: 13-year Limited-Duration Prevalence estimates, and additional cases estimated using Complete Prevalence, as of January 1, 2008. Data include first cancer diagnosis only, and not multiple primaries. LDP limited-duration prevalence; estimated number of cancer-specific prevalent cases as of January 1, 2008, CP complete prevalence; estimated number of cases added by CP methods to overall prevalence estimate. Top 10 cancers are presented in order of descending LDP
The most prevalent cancers were examined by race and gender (see Table 2). Variation in rank order was notable by race and gender for cancers ranked three through five. Cervical cancer was ranked fifth for Black women, though fourteenth among all women. Notably, LDP estimates rank cervical cancer fifth among Black women, though CP estimates rank it third. For Whites, melanoma ranked third and lung/bronchus cancers ranked fifth for both men and women. Lung/bronchus cancer was ranked higher for Blacks than Whites. Prostate and breast cancers were by far the most prevalent cancers for men and women, respectively, and colorectal cancer ranked second regardless of race or gender.
Table 2.
Comparison of 13-year limited-duration and complete prevalence of top 5 cancers by race and gender
| Rank | Black | White | ||
|---|---|---|---|---|
| Site | Site | |||
| LDP | CP | LDP | CP | |
| Men | ||||
| 1 | Prostate | Prostate | ||
| 11,433 | 12,592 | 38,331 | 43,301 | |
| 2 | Colon/rectum | Colon/rectum | ||
| 2,220 | 2,815 | 10,863 | 14,341 | |
| 3 | Lung/bronchus | Melanoma | ||
| 982 | 1,158 | 9,405 | 14,052 | |
| 4 | Kidney | Urinary bladder | ||
| 807 | 1,064 | 7,172 | 9,753 | |
| 5 | Oral cavity | Lung/bronchus | ||
| 588 | 718 | 4,880 | 5,931 | |
| Top 5 total | 16,030 | 18,347 | 70,651 | 87,378 |
| All Cancer | 20,291 | 23,808 | 98,197 | 125,192 |
| Top 5% | 79.0 | 77.1 | 71.9 | 69.8 |
| Women | ||||
| 1 | Breast | Breast | ||
| 10,287 | 14,097 | 50,381 | 72,785 | |
| 2 | Colon/rectum | Colon/rectum | ||
| 2,795 | 3,694 | 10,273 | 14,514 | |
| 3 | Corpus uteri | Melanoma | ||
| 1,019 | 1,335 | 8,375 | 13,908 | |
| 4 | Lung/bronchus | Corpus uteri | ||
| 797 | 931 | 6,777 | 12,289 | |
| 5 | Cervix uteri | Lung/bronchus | ||
| 730 | 2,130 | 5,064 | 6,040 | |
| Top 5 total | 15,628 | 22,187 | 80,870 | 119,536 |
| All Cancer | 21,864 | 31,564 | 112,841 | 172,556 |
| Top 5% | 71.5 | 70.3 | 71.7 | 69.3 |
Numbers are in count prevalence; Top 5 cancer sites are ranked by their LDP
LDP limited-duration prevalence, CP complete prevalence, NH non-Hodgkin's
The most prevalent cancers were also examined by North Carolina Cancer Partnership Region (See Table 3, Fig. 2). Differences in the rank order for the regions were similar to those of the state overall for the four most prevalent cancers, though there were some variations among those ranked five through ten (Table 1). Differences between LDP and CP estimates for uterine cancer consistently pushed it up in rank; for example, it was elevated from rank 8 (LDP) to rank 5 (CP) in the central region. There was greater variation among regional estimated prevalence rates, for which colorectal cancer is presented in Fig. 2. The western and northeastern regions were estimated to have greater prevalence rates than elsewhere in the state.
Table 3.
Comparison of 13-year limited-duration and complete prevalence of top 10 cancers by NCCCP region
| Rank | Central | North Central | Northeastern | South Central | Southeastern | Western | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Site | Site | Site | Site | Site | |||||||
| LDP | CP | LDP | CP | LDP | CP | LDP | CP | LDP | CP | LDP | CP | |
| 1 | Breast | Breast | Breast | Breast | Breast | Breast | ||||||
| 10,469 | 14,566 | 17,126 | 24,558 | 9,522 | 13,674 | 10,810 | 15,214 | 7,563 | 10,754 | 6,213 | 9,154 | |
| 2 | Prostate | Prostate | Prostate | Prostate | Prostate | Prostate | ||||||
| 8,242 | 9,113 | 14,118 | 15,707 | 8,140 | 9,051 | 8,317 | 9,206 | 6,585 | 7,306 | 5,298 | 5,914 | |
| 3 | Colorectal | Colorectal | Colorectal | Colorectal | Colorectal | Colorectal | ||||||
| 3,666 | 4,939 | 7,592 | 10,326 | 4,681 | 6,355 | 4,496 | 6,064 | 3,475 | 4,711 | 2,693 | 3,697 | |
| 4 | Melanoma | Melanoma | Melanoma | Melanoma | Melanoma | Melanoma | ||||||
| 3,153 | 4,826 | 6,476 | 10,301 | 2,150 | 3,387 | 2,375 | 3,715 | 1,883 | 3,033 | 2,071 | 3,337 | |
| 5 | Corpus uteri | Urinary bladder | Lung | Urinary bladder | Lung | Urinary bladder | ||||||
| 1,282 | 2,278 | 3,236 | 4,528 | 1,798 | 2,250 | 1,744 | 2,436 | 1,688 | 2,094 | 1,361 | 1,926 | |
| 6 | Lung | Lung | Corpus uteri | Lung | Corpus uteri | Corpus uteri | ||||||
| 1,671 | 2,068 | 3,533 | 4,399 | 1,232 | 2,244 | 1,932 | 2,407 | 983 | 1,777 | 917 | 1,761 | |
| 7 | NH lymphoma | Corpus uteri | Kidney | Corpus uteri | Urinary bladder | Lung | ||||||
| 1,511 | 1,934 | 2,278 | 4,206 | 1,218 | 1,634 | 1,249 | 2,235 | 1,200 | 1,689 | 1,246 | 1,549 | |
| 8 | Urinary bladder | NH lymphoma | Urinary bladder | NH lymphoma | Thyroid | NH lymphoma | ||||||
| 1,376 | 1,922 | 2,480 | 3,188 | 1,474 | 1,622 | 1,524 | 1,951 | 880 | 1,568 | 937 | 1,205 | |
| 9 | Thyroid | Kidney | NH lymphoma | Thyroid | NH lymphoma | Oral Cavity | ||||||
| 1,005 | 1,746 | 2,286 | 3,106 | 1,236 | 1,586 | 1,084 | 1,947 | 1,085 | 1,390 | 598 | 895 | |
| 10 | Kidney | Thyroid | Oral cavity | Kidney | Kidney | Kidney | ||||||
| 1,197 | 1,632 | 1,709 | 3,088 | 861 | 1,256 | 1,353 | 1,831 | 990 | 1,320 | 635 | 870 | |
| Others | 7,937 | 12,766 | 14,350 | 22,324 | 6,780 | 11,890 | 8,257 | 13,312 | 5,918 | 9,505 | 4,772 | 7,685 |
| Total | 41,509 | 57,790 | 75,184 | 105,731 | 39,092 | 54,949 | 43,136 | 60,318 | 32,250 | 45,147 | 26,741 | 37,993 |
| Top 10% | 80.90 | 77.90 | 80.90 | 080.90 | 82.70 | 78.40 | 80.90 | 77.90 | 81.60 | 78.90 | 82.20 | 79.80 |
Numbers are in count prevalence; Top 10 cancer sites are ranked by complete prevalence
LDP limited-duration prevalence, CP complete prevalence, NH non-Hodgkin' s
Discussion
The Institute of Medicine (IOM) Report, Lost in Transition: From Cancer Patient to Cancer Survivor, and the Centers for Disease Control and Prevention's (CDC) Comprehensive Cancer Control Program recommends that states should consider survivorship care in their cancer control plans, including developing accurate estimates of cancer survivors, and focusing on improving the quality of life for people who survive cancer [22–25]. To inform local and statewide cancer survivorship research, coordination, and planning efforts, we used data from the NCCCR and applied recently developed statistical methods to generate cancer prevalence estimates that are more accurate than previous methods. Understanding prevalence of cancer at the state, regional, and local level will help provide survivorship programs with a better understanding of the size and composition of the survivor population, so that may tailor their resources and activities to meet the needs of specific populations [2, 19, 22, 26]. This study provides specific guidance for how other Comprehensive Cancer programs and states not included in the SEER Registries may use their own data to develop similar estimates and statistics to inform their specific cancer control and survivorship programs.
CP methods using NCCCR data provided an estimate of cancer prevalence that is 40% greater than LDP, the previously standard method, demonstrating significant underestimation of cancer prevalence using the prior methods. CP methods estimate that 4% of North Carolinians are living with a prior diagnosis of cancer, an estimate that is consistent with national estimates [3]. The relative burden presented by each cancer type parallels that of prior North Carolina estimates using LDP methods [19, 20], though the estimates calculated here reflect more current population characteristics and extend prior work in as much as these methods may yield a more accurate measure of the true prevalence of cancer both among individual cancer sites and overall. Breast, prostate, colorectal, melanoma, and lung/bronchus were found to comprise 62% of prevalent cancer in North Carolina, suggesting priority cancers for survivorship programs.
Consistent with our expectation, the greatest differences between CP and LDP estimates were typically seen in less fatal cancers that are commonly diagnosed at a younger age. Not following this pattern, the minimal differences between CP and LDP estimates for prostate cancer were notable and are likely in part influenced by the unique nature of prostate cancer and Prostate Specific Antigen (PSA) testing since the early 1990s. Prior to widespread adoption of PSA testing in the early 1990s, prostate cancer was more commonly diagnosed at a more advanced stage and at an older age. In the mid-1990s, observed prostate cancer incidence increased substantially. Over time, age at diagnosis has dropped by approximately 5 years (from approximately 72 in the mid-1980s to 67, recently), diagnoses typically occur at an earlier stage, and there has been a contemporaneous decline in mortality [27, 28]. In this study, surveillance data began in 1995, and so all surveillance is in the modern PSA testing era. Given that trends in these data follow the age and survival characteristics experienced nationally, it is reasonable to expect that relatively few men diagnosed in the pre-PSA testing era (i.e., at older age, with more advanced disease, and shorter survival) were alive at the time of these prevalence estimates, compared to those diagnosed and actually observed in the data. Thus, LDP is accounting for the majority of prevalent cases, which were actually observed during the surveillance period, and CP's adjustment for those who were not actually observed in the NCCCR data is limited. By contrast, testicular cancer is diagnosed among men who are much younger (average age 24), and has very high survival rates that have changed little in the past 20 years [29]. Accordingly, as expected, CP accounts for a greater proportion of the prevalence estimate, reflecting a larger proportion of the population diagnosed prior to the observation period and still living.
Important for this discussion, the granularity and accuracy of these estimates are relevant for survivorship planning in the context that survivorship is more than simply “survival.” That is, survivorship is complex and specific, and entails much more than a measure of the percentage of the population still alive following a diagnosis of cancer, frequently represented as a single statistic reflecting the proportion of the population alive 5-year post-diagnosis. Rather, the National Cancer Institute defines survivorship as “the physical, psychosocial, and economic issues of cancer, from diagnosis until the end of life…. Survivorship includes issues related to the ability to get health care and follow-up treatment, late effects of treatment, second cancers, and quality of life.” [30] To this end, these methods and the statistics they produce may help inform Comprehensive Cancer programs and other state policies aimed at addressing these goals, ranging from facilitating basic healthful behaviors, to ensuring insurance coverage for survivor-specific needs, to more informed advance-planning for long-term and end-of-life care. The goal of this examination is to develop better estimates and a more accurate and complete characterization of the survivor population and their burden, allowing state and local policies and programs to better meet the population's needs all along the survivorship spectrum. The regional, gender-based, and racial differences presented in this study's estimates demonstrate the importance of understanding the details of each local/regional community's prevalent cancer population when prioritizing local survivorship services for different cancer sites.
For example, this analysis helped identify racial and regional variations that have received limited attention to date. It demonstrates that relying on LDP alone would result in a significant underestimation of the relative burden of lung/bronchus cancer among Blacks, and identifies cervical and uterine cancers ranked as the 3rd and 4th most prevalent among Black women in North Carolina, whereas overall analysis in these data would only present them as 8th and 14th. Many public health and health-care systems may prioritize or allocate resources to the top 5 or top 10 priority cancers and, relying on other data sources alone, these cancers for this population may otherwise have gone overlooked. North Carolina health systems may look to the data in Table 3 and Fig. 2 to provide not only a ranking and corresponding prioritization of the cancers of greatest prevalence in their service areas, but also an estimate of the number of individuals with a history of each cancer and regional differences at a cancer-specific level. These formats allow a better understanding of regional differences with sufficient granularity to inform planning for cancer-specific survivorship, cancer control, and resource allocation. For example, Fig. 2 focuses on colorectal cancer—one of the cancers of greatest incidence and prevalence for both men and women—and may suggest an evaluation of colorectal cancer screening resource adequacy, both for primary prevention and to meet the needs for greater post-treatment surveillance screening in the colorectal cancer population. In the longer-term, it may also provide a measure against which to assess the effectiveness of local cancer control efforts. Moreover, other states may reference the methods described here to use their own data to develop their own estimates, systems for characterizing their state's needs and goals, and means of tracking progress toward them.
The NCCCP has embraced the IOM's and CDC's guidance [22–24] and set corresponding goals in support of its survivorship vision [31]. It can use these data as the basis for understanding the survivor populations in North Carolina and coordinating efforts to support them. The NCCCP and other programs can use these estimates not only to understand, monitor, and improve services for the survivor populations, but also as a basis for further understanding pre-diagnosis and ongoing health-care needs. For example, the regional variation in colorectal cancer prevalence rates may be partly explained by regional differences in cancer aggressiveness, as well as the availability of colonoscopy or other colorectal cancer screening services. It may be that in Central North Carolina, there is a significantly higher use of colonoscopy, commonly less-aggressive disease, better general health behaviors or greater use of general health-care services, and pre-cancerous lesions are more likely to be detected and removed. This may contrast to the northeastern and western regions of the state, which may use good health behaviors or general health services less, have greater prevalence of aggressive disease, and are comparatively under-resourced, historically.
There is no gold standard for measuring or estimating cancer prevalence; accordingly, it is not possible to validate these estimates against the actual proportion of the population living with the cancer. The CP methods used here have been validated and used as the basis of several studies, though the CP index was developed using SEER registry data for White and Black patients, and not applied in this context on fewer than 15 years of surveillance. While the performance of these indices on non-SEER data of fewer than 15 years of observation remains unknown, the stability, consistency, and parity of data with national estimates demonstrated during the sensitivity analyses suggest the methods are valid and at the very least substantially more accurate than previously used LDP estimates. Due to development and availability of race-specific CP indices for only Whites and Blacks, CP for those of other races (American Indian/Alaska Native, Asian/Pacific Islander) was not calculable, and thus, overall CP estimates are systematically very slightly lower than actual prevalence because of their exclusion. These groups reflect approximately 1.1% of the state's cancer population, and local programs should carefully examine their population to ascertain the effect that this under-reporting may have on their programs and populations, while ongoing research develops a more comprehensive set of race-specific indices for these other groups. While these findings were generally consistent with SEER regions, variation in sub-population characteristics preclude direct comparability, and no nearby states data were available to compare a “nearest neighbor.” Accounting for migration into and out of North Carolina also presents a challenge. It is likely that these North Carolina estimates are conservative, given the net population growth during the observation period and North Carolina's popularity as a retirement state [32].
As we continue to gain more information about survivor populations, one future direction is the use of CP estimates to measure and predict survivor burden by cancer type over time. Understanding how resource needs might differ depending on cancer type and diagnosis date would further inform our ability to provide necessary services for survivors. Pairing CP estimates with data from other sources, such as the Behavioral Risk Factor Surveillance System (BRFSS), may provide additional insight regarding the health status, health needs, and specific opportunities for intervention to improve the quality and duration of life among specific groups within the survivor population [25, 33]. As the NCCCR continues to add new cases, increased duration of cancer registration will allow continued examination of the validity of these methods and stability of these estimates at 15 years (LDP and CP), and either independently or linked to other data resources, will extend their utility.
While additional research is necessary to continue improving prevalence measures and validating them, this study demonstrates the value of understanding CP as it allows more informed planning of health-related survivorship services and resource allocation at the state, regional, and local levels. It illustrates the use of recent cancer surveillance methods for converting LDP to CP using data from the NCCCR and provides a model for states and programs to develop prevalence estimates with their own data to inform and tailor interventions and services to meet the needs of their populations, rather than relying on estimates based on other registries and their populations, and the vagaries of their level of reporting or reporting frequency.
Acknowledgments
Work on this study was supported by the UNC Lineberger Comprehensive Cancer Center with funding provided by the University Cancer Research Fund (UCRF) via the State of North Carolina (Yeh, Carpenter); the North Carolina Comprehensive Cancer Program (Carpenter); the Carolina Community Network (Carpenter, Godley; 1U01CA114629); and the Cancer Control Education Program (Carpenter, Wobker; R25CA057726). This study was reviewed and approved by the University of North Carolina Institutional Review Board (IRB #06-0853). We acknowledge the extraordinary documentation and tutorials that guided this work, developed by the NCI and IMS (http://seer.cancer.gov/resources/), and are grateful for the support they provided to this project, including especially Angela Mariotto (NCI) and Steve Scoppa (IMS). We also thank Karen Knight, Chandrika Rao, and Seth Tyree of the North Carolina Central Cancer Registry (CCR) for their assistance with CCR data. We thank Walter Shepherd, Executive Director of the North Carolina Comprehensive Cancer Program (NCCCP), for his guidance regarding the utility of these estimates for application in NCCCP, the North Carolina Cancer Partnership Regions, and other statewide cancer support and coordination programs. We thank Lisa Richardson from the Centers for Disease Control and Prevention for her valuable feedback on this manuscript, including the utility of these measures.
References
- 1.Aziz NM, Rowland JH. Trends and advances in cancer survivorship research: challenge and opportunity. Semin Radiat Oncol. 2003;13(3):248–266. doi: 10.1016/S1053-4296(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 2.Ganz P. Cancer survivorship: today and tomorrow. Springer; New York: 2007. [Google Scholar]
- 3.Horner M, et al. SEER cancer statistics review, 1975–2006. National Cancer Institute; Bethesda: 2009. [Google Scholar]
- 4.American Cancer Society . North Carolina, in South Atlantic cancer facts and figures 2008. ACS; Atlanta: 2008. pp. 50–57. [Google Scholar]
- 5.National Cancer Institute Surveillance Epidemiology and End Results Program Cancer Prevalence. 2011 Available from: http:// seer.cancer.gov/statistics/types/prevalence.html. Cited 3 Jan 2011.
- 6.Freedman DM, et al. Comparison between cancers identified by state cancer registry, self-report, and death certificate in a prospective cohort study of US radiologic technologists. Int J Epidemiol. 2006;35(2):495–497. doi: 10.1093/ije/dyi286. [DOI] [PubMed] [Google Scholar]
- 7.Parikh-Patel A, Allen M, Wright WE. Validation of self-reported cancers in the California teachers study. Am J Epidemiol. 2003;157(6):539–545. doi: 10.1093/aje/kwg006. [DOI] [PubMed] [Google Scholar]
- 8.Corazziari I, Mariotto A, Capocaccia R. Correcting the completeness bias of observed prevalence. Tumori. 1999;85(5):370–381. doi: 10.1177/030089169908500503. [DOI] [PubMed] [Google Scholar]
- 9.Capocaccia R, De Angelis R. Estimating the completeness of prevalence based on cancer registry data. Stat Med. 1997;16(4):425–440. doi: 10.1002/(sici)1097-0258(19970228)16:4<425::aid-sim414>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute [Accessed 15 Sept 2010];Surveillance, epidemiology, and end results. 2010 Available from: http://seer.cancer.gov/.
- 11.Surveillance Research Program and National Cancer Institute SEER*Stat Software 6.5.2. 2009 Available from: http://seer. cancer.gov/seerstat.
- 12.Surveillance Research Program and National Cancer Institute ComPrev software version 1.2—Beta 1. 2009 Available from: http://srab.cancer.gov/comprev/
- 13.Clegg LX, Gail MH, Feuer EJ. Estimating the variance of disease-prevalence estimates from population-based registries. Biometrics. 2002;58(3):684–688. doi: 10.1111/j.0006-341x.2002.00684.x. [DOI] [PubMed] [Google Scholar]
- 14.Gigli A, et al. Estimating the variance of cancer prevalence from population-based registries. Stat Methods Med Res. 2006;15(3):235–253. doi: 10.1191/0962280206sm427oa. [DOI] [PubMed] [Google Scholar]
- 15.Merrill RM, et al. Cancer prevalence estimates based on tumour registry data in the surveillance, epidemiology, and end results (SEER) program. Int J Epidemiol. 2000;29(2):197–207. doi: 10.1093/ije/29.2.197. [DOI] [PubMed] [Google Scholar]
- 16.Surveillance Research Program and National Cancer Institute SEER*Prep software 2.4.3. 2009 Available from: http://seer. cancer.gov/seerprep.
- 17.Bureau, U.S.C. Population estimates. 2010 Cited 2010; Available from: http://www.census.gov/popest/counties/asrh/
- 18.Surveillance Research Program and National Cancer Insitute Standard Population Data. 2009 Available from: http://seer.cancer.gov/stdpopulations/index.html.
- 19.Carpenter WR, et al. Towards a more comprehensive understanding of cancer burden in North Carolina: priorities for intervention. N C Med J. 2008;69(4):275–282. [PMC free article] [PubMed] [Google Scholar]
- 20.Grogan DM, et al. The prevalence of cancer in North Carolina. N C Med J. 1997;58(3):168–172. [PubMed] [Google Scholar]
- 21.National Cancer Institute Estimated US cancer prevalence counts: who are our cancer survivors in the U.S.? 2009 Available from: http://dccps.nci.nih.gov/ocs/prevalence/. Cited 2010 January.
- 22.Hewitt M, Greenfield S, Stovall E. From cancer patient to cancer survivor: lost in transition. The National Academies Press; Washington: 2006. [Google Scholar]
- 23.Centers for Disease Control and Prevention and Lance Armstrong Foundation . In: A national action plan for cancer survivorship: advancing public health strategies. U.S. Department of Health and Human Services; Centers for Disease Control and Prevention, editor. Atlanta: 2004. [Google Scholar]
- 24.Centers for Disease Control and Prevention What is comprehensive cancer control? 2011 Available from: http://www.cdc. gov/cancer/ncccp/what_is_cccp.htm. Cited 2011 January 3.
- 25.Richardson L, et al. Use of 2001–2002 behavioral risk factor surveillance system (BRFSS) data to characterize cancer survivors in North Carolina. NC Med J. 2010 November/December; in press. [PubMed] [Google Scholar]
- 26.Campbell MK, et al. Cancer survivorship. NC Med J. 2008;69(4):322–324. [PubMed] [Google Scholar]
- 27.National Cancer Institute Surveillance Epidemiology and End Results Program SEER Stat Fact Sheets: Prostate. 2011 Available from: http://seer.cancer.gov/statfacts/html/prost.html. Cited 2011 January 4.
- 28.National Cancer Insitute Surveillance Epidemiology and End Results . Incidence and mortality, prostate cancer trends. 1973-1995. [Google Scholar]
- 29.National Cancer Institute Surveillance Epidemiology and End Results Program SEER Stat Fact Sheets: Testis. Available from: http://seer.cancer.gov/statfacts/html/testis.html. Cited 2011 January 4.
- 30.National Cancer Institute Follow-up care after cancer treatment: fact sheet—survivorship. 2010 April 20; Available from: http://www.cancer.gov/cancertopics/factsheet/Therapy/followup/print.
- 31.North Carolina Comprehensive Cancer Program . North Carolina's plan for comprehensive cancer control: a living plan by the people of North Carolina. [Google Scholar]
- 32.Serow WJ. Retirement migration counties in the southeastern United States: geographic, demographic, and economic correlates. Gerontologist. 2001;41(2):220–227. doi: 10.1093/geront/41.2.220. [DOI] [PubMed] [Google Scholar]
- 33.Fairley TL, Hawk H, Pierre S. Health behaviors and quality of life of cancer survivors in Massachusetts, 2006: data use for comprehensive cancer control. Prev Chronic Dis. 2010;7(1):A09. [PMC free article] [PubMed] [Google Scholar]