Abstract
Acute inflammatory reactions benefit the host by supporting the effective clearance of pathogens and fostering wound healing, in addition to other self-preservative processes. However, when the inflammatory program is not resolved, becoming chronic in nature, it creates an environment conducive to cancer development and progression. Therefore, minimizing exposure to risk factors that contribute to chronic inflammation and reconditioning the host towards a state of (at least locoregional) acute inflammation would meaningfully impact cancer incidence and its treatment, respectively. Regarding cancer therapy, combinational treatments that both disrupt chronic inflammation and install specific adaptive type I immunity are predicted to enhance quality of life and extend the overall survival of patients.
Keywords: cancer, chronic inflammation infection, immunotherapy, metastatic renal cell carcinoma, sunitinib, wound healing
Inflammation serves as the host’s natural response to alleviate infection and promote tissue healing, among other processes in the body. However, in cases where acute inflammation turns into a state of chronic persistence, consequences such as cancer can result. Chronic inflammation plays an instrumental role in promoting all phases of tumorigenesis, from initiation to metastasis, as this article will discuss. The article will focus primarily on inflammation-driven phenomena associated with solid malignancies, although chronic inflammatory responses may similarly advance the severity and worsen the prognosis of hematological cancers.
Developing a state of chronic inflammation
The most significant numbers of deaths associated with cancer worldwide involve the lung, stomach, liver, colon/rectum and breast, in decreasing order of magnitude [101]. Although the incidence of cancer continues to rise, particularly in low-/medium-income countries [1], a majority of these malignancies are now considered as preventable owing to the so called ‘avoidable’ risk factors that comprise environmental exposure (e.g., arsenic and tobacco smoke), infectious organisms and dietary behavior (e.g., obesity and alcohol consumption) [2].
In the case of lung, stomach, liver, colorectal and breast cancers, a variety of dietary and/or environmental agents have been reported as cofactors that increase the risk of tumorigenesis [1]. Notably, chronic infections with microbial pathogens are second only to tobacco usage and exposure, with regard to risk factors to develop cancer, accounting for 15–20% of the global cancer burden [3]. The bacterium Helicobacter pylori is typically implicated as a causative agent in stomach cancer cases [4], while approximately 80% of hepatocellular carcinomas can be linked to chronic infections with hepatitis B and C viruses (HBV and HCV), respectively [1,3]. In the case of cervical cancer, the fourth leading cause of cancer death in women worldwide, human papillomavirus-16 and -18 infections determine the onset of 70% of total cases [1]. Other notable malignancies such as Burkitt’s lymphoma, Hodgkin’s disease [5] and adult T-cell leukemia/lymphoma [6] can also be directly attributed to viral infections.
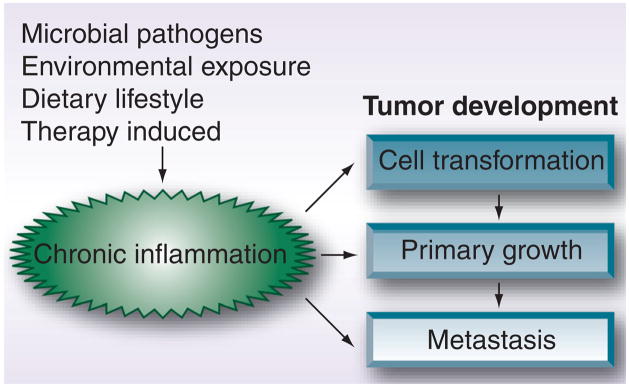
Aside from chemical carcinogens and some viruses (i.e., human papillomavirus, human T-lymphotropic virus-1, HBV, HCV and Epstein–Barr virus), there is little convincing evidence that the major risk factors directly initiate cell transformation. It appears that prolonged exposure eventually helps to contribute to the onset of the malignant cell phenotype, through a number of proposed mechanisms that include inflammatory-mediated effects [7–9]. In the case of viral and bacterial agents, chronic inflammation owing to infection is indicated as a predominant but indirect carcinogenic mechanism [3,10]. In an attempt to rid the body of pathogenic organisms, host cells synthesize and release a number of antimicrobial factors, which include reactive oxygen and nitrogen species, cytokines (e.g., IL-1, IL-6 and TNF-α) and chemokines (e.g., CCL2 and CXCL8), which foster the recruitment and activation of protective immune effector cells such as macrophages and neutrophils. The inflammatory response is intended to quickly resolve infection with minimal harm evolved against the host. However, in cases of unresolved infections such as those observed for H. pylori or HBV/HCV, the sustained assault of infected tissues by host immune cells results in an overwhelmingly negative condition, in which beneficial immunologic effects are limited and cancer promotion may ensue [11,12]. A similar program may also be initiated by extrinsic factors that contribute to procancerous inflammation, such as processes involved in normal tissue repair [13]. For example, surgical resection of primary tumors in patients leads to increased levels of systemic growth and angiogenic factors such as VEGF and cytokines such as TGF-β, which underlie the normal tissue repair process; however, such factors may favor the outgrowth of residual/occult tumor cells, as will be discussed [14,15]. Chronic inflammation therefore plays a decisive role as a rheostat in all stages of tumor development; initiation, progression and metastatic spread (particularly when combined with intrinsic cell factors driven by oncogenes) (Figure 1) [14,16].
Figure 1. Role of chronic inflammation in cancer development.
Chronic inflammation initiates and impacts all major stages of tumor progression, from cell transformation to widespread metastasis. The cause of chronic inflammation varies by individual and includes exposure to infectious microorganisms and harmful chemicals.
Chronic inflammation enforces malignancy
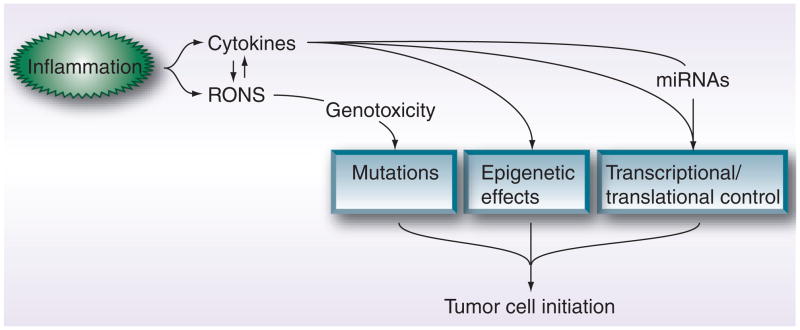
Regardless of the host’s underlying condition that contributes to chronic inflammatory reactions, the continued onslaught of the affected cells/tissues by inflammatory mediators has been demonstrated to enhance neoplastic cell growth and proliferation. On the whole, these inflammatory-based mechanisms either directly impact cell mutational rates or indirectly induce molecular effects that favor tumorigenesis, as summarized in Figure 2.
Figure 2. Chronic inflammation drives tumor cell initiation through direct mutational and indirect molecular effects.
Cytokines such as IL-6 promote cell transformation by modifying gene expression profiles, through mechanisms that include epigenetic effects and transcriptional factor control, as well as inducing protumorigenic miRNAs. RONS, on the other hand, serve to directly instigate gene mutations by damaging DNA and RNA. Interestingly, a positive feedback loop appears to exist between the ability of inflammatory cytokines to induce the synthesis of RONS and vice versa.
RONS: Reactive oxygen/nitrogen species.
High levels of host-derived reactive oxygen and nitrogen compounds damage DNA and RNA through nucleotide modifications that include deamination, oxygenation and adduct formation, with oxidized products such as lipids, carbohydrates and DNA [17]. These major effects instigate carcinogenesis by modulating the downstream functions of proto-oncogenes and tumor suppressor genes via a number of pathways [18]. For example, the direct oxidation by reactive oxygen species in the cell can lead to the reduced expression and enzymatic activity of DNA mismatch repair genes, resulting in increased cell mutagenesis and probability of tumor initiation [14,18]. The induction and direct effects of reactive oxygen and nitrogen species by inflammatory stimuli, however, do not follow a linear fashion; reactive oxygen and nitrogen species can also be regulated upon oncogene activation, as observed in the case of increased RAS-mediated signaling [18]. Recently, mitochondrial-derived reactive oxygen species have been reported to enhance the intracellular expression of proinflammatory cytokines such as IL-1 and IL-6 although the precise molecular mechanisms of this mode of control are presently unclear [19].
Host-derived proinflammatory cytokines induce the release of reactive oxygen and nitrogen species, which further the development of mutational effects and cooperate to induce epigenetic changes that promote tumorigenesis at the cell level [14]. IL-6 is capable of mediating modifications to the methylation status of genes associated with (tumor) cell growth and migration [20–22]. For example, by utilizing primary patient and established colonic cell lines in vitro, IL-6 was observed to negatively impact the expression of genes associated with tumor suppression and anchorage dependence [21]. These epigenetic silencing events were a result of IL-6 upregulating DNA methyltransferase levels in the cell. Inflammatory cytokines can also shape a cell’s self-sufficiency, largely through transcriptional factor control. A classic function of the proinflammatory cytokine, TNF-α, is to activate the transcriptional factors NF-κB and AP-1, which serve as regulators of premalignant cell proliferation and survival under conditions of chronic inflammation [23]. IL-6, among other proinflammatory cytokines, is also capable of driving transcription factors that control oncogene expression [24]. In a mouse model of colitis-associated cancer, myeloid expression of IL-6 stimulated the survival and proliferation of pre-malignant intestinal epithelial cells [25]. These protumorigenic effects were largely the result of IL-6-activated STAT3 signaling in intestinal epithelial cells.
Inflammatory stimuli also differentially modulate miRNAs associated with disease pathogenesis. miRNAs are single stranded RNA molecules that regulate gene translation and can induce a malignant phenotype at the cell level [26]. For example, the miRNA miR-21 is upregulated under conditions of chronic inflammation and in the case of a diverse array of solid and hematological human cancers [18,26]. IL-6 can induce the expression of miR-21 via STAT3 signaling. This induces downstream effects that enhance cell proliferation and prevent cellular apoptosis, by regulating oncogene and tumor suppressor gene (e.g., programd cell death 4, tropomyosin 1, phosphatase and tensin homolog and BTG family member 2) expression [18,27]. In settings where miR-21 has been functionally blocked, tumor cells have increased sensitivity to therapeutic assaults such as chemotherapy while tumor growth is inhibited in xenograft animal models [27]. However, the detailed carcinogenic role of miRNAs in cancer patients has been more difficult to assess since patient specimens are typically obtained from well-established tumor lesions. Such samples are less likely to yield information related to the early cell events that ultimately lead to tumorigenesis [26]. In the case of miR-21, non-small-cell lung cancer and colorectal patients with increased miR-21 sample expression have a worse prognosis and disease progression status compared with control cohorts [27,28]. Therefore, owing to the relative molecular ease of miRNA detection; miRNA status may serve as a preferred mode of cancer detection and prognosis (i.e., likelihood of response to an indicated therapy) in affected patients [29–31].
Chronic inflammation facilitates & sustains primary tumor growth
Progressive tumor cell proliferation eventually exceeds the capacity of the local tissue micro-environment to provide/exchange proper nutrients and oxygenation, necessitating the process of neoangiogenesis and/or neovascularization [32]. Tumor-associated blood vessels, however, are commonly observed to be functionally impaired, based on their inefficient structure (i.e., being composed of poorly-organized and interconnected, leaky vascular endothelial networks that are only loosely decorated with supportive pericytes) that fails to develop hierarchical transitions from arterioles-to-capillaries-to-venules. Such blood vessel deficiencies lead to increased interstitial fluid pressure, hypoxia and low pH within the tumor microenvironment (TME) [32–34], which negatively impacts protective lymphocyte homing, extravasation and function into/within the TME [33].
Cancer cell hypoxic signals induce the expression and release of VEGF and PDGF (rendered through HIFα signaling), among other factors, and cause a number of immediate effects that contribute to the instillation of a chronically inflamed microenvironment, which potentiates tumor growth and progression [35]. VEGF and PDGF bind their cognate receptors, expressed by endothelial cells and pericytes respectively [36,37], and initiate angiogenesis by recruiting endothelial precursor/pericyte cells, promoting endothelial/pericyte cell proliferation and generating capillaries [37]. Tumor cell hypoxia can also promote the immigration of inflammatory cells, such as tumor-associated macrophages (TAMs) into cancer lesions [38,39]. TAMs, in turn, encourage angiogenesis further by secreting proangiogenic factors such as VEGF [40]. This is consistent with the correlation between high TAM content and poor clinical prognosis in cancer patients [14]. Nevertheless, there is considerable cell plasticity in TAMs, regarding exposure to factors derived from tumor and infiltrating cells. Most TAMs isolated from human cancers display a M2-like phenotype, which drives tumorigenesis by promoting Th2-based reactions and dampening cells mediating cytolytic functions against tumor cells [16,40]. For instance, macrophages exposed to type 2 cytokines such as IL-4 or IL-10 can be polarized to an M2 phenotype, and in this functional state, contribute to support the development of regulatory T cell (Treg) function(s), which serve to limit protective immunity. M2-polarized TAMs may also promote tumor infiltration of Treg and Th2 cells via locoregional production of chemokines, such as CCL17, CCL22 and CCL24. Likewise, Tregs and Th2 cells in the TME may drive or sustain M2-like macrophage activity by elaborating IL-10 and IL-4, respectively [40].
Cancer cells are also adept at secreting chemokines and expressing chemokine receptors that function to support tumor-derived blood networks and to recruit tumor-promoting cells [41]. For example, endothelial cell production of chemokines such as CXCL1 and CXCL8 promotes angiogenesis through ligation with CXCR2 expressed by tumor cells. Interestingly, a variety of human carcinomas overexpress CXCL8, which further drives cancer cell establishment and spread. In the case of non-small-cell lung cancer, tumor secretion of CXCL8 contributes to such protumorigenic effects as angiogenesis, progression, and neutrophil infiltration [42,43]. TAMs and myeloid-derived suppressor cells (MDSCs) may also infiltrate tumor lesions, based on cancer cell-derived gradients of CCL2, which conditions functional M2 TAMs [40,41].
In addition to TAMs, both neutrophils and MDSCs recruited into the primary mass appear to contribute to and maintain an overall ‘suppressive’ TME. In turn, local inflammatory conditions sustain these infiltrating cell types in a reinforced feedback loop. Typically, poor prognosis in cancer patients correlates with high neutrophil volume, although the specific mechanism of action for how these cells promote malignant growth remains incomplete [44,45]. It is known that neutrophils induce the accumulation of inflammatory cells within the TME via production of IL-1, IL-6 and TNF-α, and that neutrophils may modulate cell mutational frequencies as a consequence of elaborating reactive oxygen species [45]. However, MDSCs are composed of a heterogeneous population of cells that express both monocytic and granulocytic markers [46]. Cancer patients typically present with elevated levels of MDSCs (in tumors and in blood), and the main apparent function of MDSC subsets in the setting of established tumor growth is to negatively regulate type 1 T and natural killer cell responses, via mechanisms that involve reactive oxygen and nitrogen species, arginase (i.e., depleting T cells of L-arginine and promoting their apoptosis), and immunosuppressive cytokines such as IL-10, which notably promotes Treg function [47,48].
In summary, the physical constraints and immunosuppressive properties of the TME serve to limit concurrent protective (proinflammatory) immune reactions (i.e., a concept broadly defined as immunosurveillance), whereby, immune effector cells such as CD8+ T and NK cells provide systemic protection against host cells exhibiting aberrant phenotypes (e.g., pre-malignant and cancerous cells). What emerges then is the concept that chronic inflammation helps to drive the creation of an early primary tumor lesion that is less receptive/responsive to spontaneous and therapeutically-induced type 1 immunity, allowing the lesion to progress and develop into an advanced mass containing cells with increased propensity to metastasize.
Cancer metastasis is impacted by inflammation
There exists much debate about how precisely metastasis occurs. Considering that over 90% of patients die from systemic disease to organs such as the brain, lung and liver, as opposed to a primary lesion, understanding the steps of metastasis more clearly allows the opportunity to develop interventional treatment strategies [49]. Overall, successful cancer metastasis is believed to involve a two-stage process: tumor cell emigration from the primary lesion into the circulation (intravasation) and corollary colonization of a distant tissue site (extravasation).
In terms of the first phase, there are competing hypotheses that describe precisely which types of tumor cells are involved in traversing the extracellular matrix (ECM) of the TME in order to access blood vessels. The cancer stem cell (CSC) hypothesis is a newly appreciated observation stipulating that only a few tumor cells maintain the self-renewal and growth potential of the solid cancer mass [49]. At some point during the development of the primary lesion (possibly even at a stage of occult disease), a CSC reaches and enters the local blood supply. To further support this metastatic potential, CSCs maintain inherent qualities that would predispose them to successful intravasation and extravasation processes, namely a perivascular location, in conjunction with enhanced properties of motility, invasiveness and resistance to apoptosis [49]. As an alternatively described phenomenon, the CSC phenotype may be ‘plastic’ in nature and rely upon environmental cues within the TME to induce ‘non-CSC’ tumor cells to evolve CSC characteristics. Although both hypotheses have been experimentally supported to certain degrees, neither has been fully described within the clinical setting, and the models are also not mutually exclusive. Both scenarios may exist at any given time in vivo, and there may be variance in the importance of the type of CSC (i.e., intrinsic vs induced) in founding distant metastatic niches, which would be based on the type of cancer involved [49,50]. For example, tumor cells induced to become CSC-like may play a more decisive role in the metastatic spread of cancers via the vasculature.
In addition to inflammatory infiltrates and a deviant vasculature network, the TME contains an altered ECM and an assortment of other cancer-associated stromal cell types, including fibroblasts that serve to further potentiate angiogenesis and metastasis [38]. The tumor-derived ECM is significantly remodeled (relative to its architecture among normal cells) during tumorigenesis, in order to allow for processes such as invasion and metastasis [38,39]. Structural alterations in the ECM are largely carried out by stromal-elaborated matrix metalloproteinases, which degrade ECM substrates such as collagen. However, inflammatory cells such as TAMs and neutrophils are also important contributor sof matrix metalloproteinases within the TME [16,40,45]. ECM expression of integrins and other cell surface receptors also provide tumor cells with survival/proliferative signals, along with the impetus for increased migratory capacity. In the end, such TME properties foster successful epithelial-to-mesenchymal transition in tumor cells, a proposed process that would hold key for the induced CSC hypothesis [49]. In this scenario, which could help explain intravasation, nonmotile epithelial tumor cells take on a morphological invasive switch to motile mesenchymal cells, owing to a variety of epithelial-to-mesenchymal transition-inducing signals that include TGF-β and FGF [39,49]. Although local sources for these molecules vary, inflammatory infiltrates such as TAMs, MDSCs, and cancer-associated fibroblasts could provide significant levels of TGF-β. Tumor cell hypoxic signaling in the TME would also induce the expression of and supply FGF [38,47].
We are still deficient in having a detailed understanding of the steps involving cancer cell entry into the blood circulation in order to colonize a distant site [49]. Mechanisms could certainly include cancer cells becoming trapped in capillary beds based on size (i.e., emboli), as well as being recruited into distant tissue sites owing to chemokine gradients originating at such sites. In the case of the latter, systemic or locoregional chronic inflammation may cause an upregulation of adhesion ligands, specific to cancer cell integrins, expressed by the blood vessel endothelia within target organs [14,51,52]. What follows upon homing is extravasation into the tissue, and the ability of malignant cells to quickly adapt to a foreign environment that is likely to be very dissimilar from the primary tumor site. A state of chronic inflammation may provide a hospitable environment to founder cancer cells; by preventing apoptosis and inducing epigenetic and mutational effects that would favor cancer progression within the distal tissue location (as detailed in Table 1). In addition, the aforementioned factors (see Table 1) secreted by locally recruited inflammatory cells, such as TAMs, could provide the protumorigenic support of neoangiogenesis essential to tumor growth of macrometastases.
Table 1.
General inflammatory-associated factors involved in cancer development.
| Inflammatory-induced agents | Representative protumorigenic effects |
|---|---|
| Cytokines | |
| IL-1 | Mediates inflammatory cell accumulation in the TME |
| IL-6 | Modifies gene expression through epigenetic effects, transcription factor control and miRNA induction; involved in promoting inflammatory cell migration into the TME |
| TNF-α | Activates transcription factors that mediate tumor cell proliferation/survival; promotes immune cell infiltration into the TME |
| TGF-β | Helps promote EMT in cancer cells |
| IL-4 | Polarizes macrophages to an M2 phenotype |
| IL-10 | Supports immunosuppression by sustaining M2-driven macrophages and Tregs |
| Chemokines | |
| CCL2 | Tumor-derived chemotactic factor for infiltrating TAMs and MDSCs; Helps promote the M2 phenotype in TAMs |
| CXCL8 | Instigates angiogenesis, tumor cell progression, and neutrophil migration |
| CCL17, CCL22, CCL24 | Migratory factors for Tregs and Th2 cells in the TME |
| CXCL1 | Promotes tumor angiogenesis through ligation with CXCR2 |
| Other factors | |
| RONS | Damages DNA/RNA resulting in cell mutagenesis |
| miRNAs | Regulates key genes involved in cancer cell proliferation/apoptosis |
| VEGF & PDGF | Angiogenic factors that promote tumor vascularization |
| FGF | Induces EMT in tumor cells |
EMT: Epithelial-to-mesenchymal transition; MDSC: Myeloid-derived suppressor cell; RONS: Reactive oxygen/nitrogen species; TAM: Tumor-associated macrophage; TME: Tumor-associated microenvironment; Tregs: Regulatory T cells.
Clinical intervention
Controlling chronic inflammation remains a logical step toward preventing many types of malignant disease (i.e., circumventing the ‘avoidable’ factors). For example, in the case of H. pylori infections, improvements to hygiene and the use of antibiotics are thought to have contributed towards the 80% lower incidence rates of stomach cancer in the USA since 1950 [1]. Reduced rates of liver cancer have also been reported in countries that have established infant vaccine campaigns against HBV compared with high incidence areas in the world (e.g., sub-Saharan Africa and many parts of Asia) where such programs do not exist [1,2]. As an additional proof-of-principle, individuals who prophylactically take NSAIDs such as aspirin have reduced incidence of breast cancer and decreased risks of prostate and colon cancer [14,53]. Although there are risks of side effects from long-term administration of such agents, the potential benefits far outweigh the risks for most individuals, except those with a genetic or environmental predisposition to develop cancer [14].
In cases of established malignant disease, rigorous therapeutic strategies will be required to combat the effects of inflammatory-based reactions that sustain and potentiate tumorigenesis. A number of single modality agents targeting various aspects of chronic inflammation (with specific focus on the TME) have entered clinical trials and have been recently reviewed [38,54]. Examples of these targets include inhibitors of angiogenesis, cytokines, ECM degradation and hypoxia. More generally, however, these experimental therapies alone have not resulted in significant long-term improvements in the quality of life and survival of patients with cancer. It appears likely that patient-specific combinational strategies will have to be developed and implemented, in order to mediate therapeutic efficacy in the clinic. A multipronged attack would be required to first abrogate the downstream effects of chronic inflammation, allowing cancer cells to become more effectively targeted by alternate methods. One such secondary approach that holds considerable promise involves immunotherapeutic strategies, with its ability to specifically target malignant growth and generate long-lasting immunity through memory recall responses. What follows is an example from our laboratory where the use of a pharmacological drug, targeting chronic inflammatory-induced angiogenesis, and a tumor cell-specific vaccine are utilized concurrently to mediate more effective inhibition of cancer progression than either agent can provide alone.
Administration of the US FDA approved receptor tyrosine kinase (RTK) inhibitor sunitinib in individuals with metastatic renal cell carcinoma (mRCC) mediates clinical responses by improving overall survival and time to progression, compared with standard protocols [55,56]. Sunitinib, therefore, remains a first-line treatment for good and intermediate-risk mRCC patients, as recommended by the National Comprehensive Cancer Network [57,58]. However, most patients will develop resistance to the drug with a median time to progression of 6–15 months post-treatment [59]. Sunitinib works by primarily disrupting the ATP binding site within the kinase domain of the VEGF and PDGF receptors, in addition to negatively regulating other RTKs, blocking the ensuing downstream signaling pathways [36,60,61]. Although there is no convincing evidence that sunitinib destroys renal cell carcinoma cells directly, much of the drug’s actions appear to mediate tumor lysis indirectly, by affecting the maturation of the tumor stroma as a result of locoregional chronic inflammation [62].
Normalization of the tumor vasculature through antiangiogenic strategies is hypothesized to help restore proper blood flow and vessel integrity within the tumor mass, and aid in improving the delivery and efficacy of co-applied cancer therapies [33,63,64]. In the case of sunitinib, the RTK inhibitor is observed to be adept at impacting immature endothelial cells [65], which serves to normalize the renal cell carcinoma vasculature by ‘pruning’ vessels that have not yet been fully stabilized by pericytes [65–68]. Indeed, several reports have documented the increased uptake of chemotherapeutic drugs, following sunitinib treatment in mouse models of cancer [66,68].
As mRCC patients are administered sunitinib, Treg levels diminish via an as-yet-undetermined mechanism of action [69,70]. The RTK inhibitor also normalizes the progeny of the myeloid lineage, effectively reducing MDSCs, while promoting the differentiation of mature immunostimulatory DCs [70,71]. In addition, T cells from mRCC patients treated with sunitinib exhibit a preferential capacity to secrete Th1 (over Th2) cytokines after mitogenic stimulation in vitro [69,70]. These drug functions appear to ultimately restore the beneficial properties of acute inflammation in the host, from the suppressive aspects of chronic inflammation that have rendered traditional treatment approaches such as surgery and cytokine therapy to mRCC unsuccessful. Sunitinib may, therefore, represent a suitable sensitizing therapy in patients to improve, for example, TME targeting/delivery of immunotherapeutic moieties (as similarly observed with other combination regimens [63,72]), which target associated antigens of the renal cell carcinoma stroma, based on the drug’s propensity to lessen chronic inflammation by normalizing the tumor vasculature and restoring type 1 immunity. To support this hypothesis, we have recently reported on the ability of sunitinib to work in concert with a specific immunotherapeutic approach to targeting malignant growth [73]. Combination therapy employing sunitinib plus specific vaccination resulted in a significant reduction in mean tumor size and increased long-term survival, when compared with either monotherapy. Tumor infiltrating lymphocytes harvested from animals treated with sunitinib/vaccine cotherapy also produced superior levels of IFN-γ in vitro in response to vaccine-associated peptide epitopes. Such responses appear to be facilitated by the coordinate loss of immunosuppressive MDSCs and Tregs from the TME in mice receiving the combined therapy. Overall, these studies highlight the ability of sunitinib to work effectively in concert with a specific immunotherapy, in order to initiate and sustain heightened Th1 reactions in the TME (at the expense of suppressive mechanisms), and these data support the translational evaluation of sunitinib/vaccine combinational strategies against solid vascularized lesions such as mRCC in pilot clinical trials.
Conclusion
Under conditions of pathogenic infection or tissue regeneration, the host immune system may productively mediate a state of acute inflammation. In the absence of event closure (i.e., episodes of chronic infection or wound healing), the benefits of such immune responses may become deviated towards a protumorigenic state, in which inflammatory mediators promote all stages of the malignant process, from the initiation of cancerous cells to their systemic dissemination. Although our understanding of the molecular/mechanistic underpinnings of cancer progression has advanced significantly over the last decade, the complexity and redundant biology of the TME presents a formidable challenge to therapeutic intervention for patients with established disease. It appears all but certain that combinational strategies will have to be incorporated clinically, in order to combat the multiple levels at which inflammation contributes to the malignant process. Considering that chronic inflammation plays such a vital role in tumorigenesis, particular attention should be devoted to first alleviating inflammatory-based constraints, so that additional strategic efforts can become increasingly efficacious in vivo.
Future perspective
Our understanding of the basic tenets governing inflammation-driven tumorigenesis has grown considerably over the last 10 years, and will continue to do so over the next decade. Our major challenge remains the translation of laboratory findings into the clinic (and corollary reinvestment in translational studies to better understand mechanisms of action and to refine systems-biology approaches applied in the clinic) where more effective treatments may be developed for cancer patients in randomized clinical trials. To date, single modality strategies targeting various aspects of chronic inflammation have failed to advance the long-term quality of life and survival of patients. We hypothesize that combinational therapies, incorporating targeted anti-(chronic)inflammatory strategies, may improve the objective clinical response rate of treated patients by attacking regulatory aspects that currently serve to limit the effectiveness of combined therapeutics such as chemo-/radio-/immuno-therapies.
Executive summary.
Chronic inflammation favors cancer initiation, progression and dissemination.
Dysregulated host inflammatory reactions induce the elaboration of reactive oxygen/nitrogen species and cytokines, which contribute to carcinogenesis through mutational and epigenetic effects.
Primary tumor growth and neoangiogenesis is supported by tumor microenvironment (TME) infiltrating immune cells, including tumor-associated macrophages, myeloid-derived suppressor cells, neutrophils and regulatory T cells.
Chronically activated proinflammatory cells in the TME contribute to the metastatic potential of cancer.
In order to decrease cancer incidence, known risk factors that induce chronic inflammation should be avoided (i.e., exposure to environmental hazards, infectious organisms and diet).
Targeted disruption of chronic inflammation should be addressed in the setting of established cancers – particularly as part of a combined therapeutic strategy – in order to yield more effective treatment options for patients; improving their quality of life and increasing overall survival. For example, the abrogation of inflammation in the TME may allow for additional successful clinical interventions that include chemotherapy, immunotherapy and/or radiotherapy.
Acknowledgments
The authors wish to thank Jennifer L Taylor and Nina Chi for their careful review and helpful comments during the preparation of this manuscript.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported by NIH grants P01 CA100327, R01 CA114071, R01 CA140375 and P50 CA121973 (to Walter J Storkus) and the University of Pittsburgh Cancer Center Support Grant (CCSG; P30 CA047904). Devin B Lowe is supported by a Postdoctoral Fellowship (PF-11–151–01-LIB) from the American Cancer Society. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1▪.Jemal A, Center MM, Desantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. Thorough epidemiological analysis that reports on global cancer incidence rates, with special attention given to major risk factors that also induce chronic inflammation. [DOI] [PubMed] [Google Scholar]
- 2.Brawley OW. Avoidable cancer deaths globally. CA Cancer J Clin. 2011;61(2):67–68. doi: 10.3322/caac.20108. [DOI] [PubMed] [Google Scholar]
- 3.Pisani P, Parkin DM, Munoz N, Ferlay J. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomarkers Prev. 1997;6(6):387–400. [PubMed] [Google Scholar]
- 4.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7(11):629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10(12):878–889. doi: 10.1038/nrc2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goncalves DU, Proietti FA, Ribas JG, et al. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev. 2010;23(3):577–589. doi: 10.1128/CMR.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKβ- and JNK1-dependent inflammation. Cancer Cell. 2010;17(1):89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29(36):4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 10.Davis BK, Wen H, Ting JP. The Inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aebischer T, Meyer TF, Andersen LP. Inflammation, immunity, and vaccines for Helicobacter. Helicobacter. 2010;15(Suppl 1):21–28. doi: 10.1111/j.1523-5378.2010.00777.x. [DOI] [PubMed] [Google Scholar]
- 12.Bouchard MJ, Navas-Martin S. Hepatitis B and C virus hepatocarcinogenesis: lessons learned and future challenges. Cancer Lett. 2010;305(2):123–143. doi: 10.1016/j.canlet.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Licitra L, Perrone F, Tamborini E, et al. Role of EGFR family receptors in proliferation of squamous carcinoma cells induced by wound healing fluids of head and neck cancer patients. Ann Oncol. 2011;22(8):1886–18893. doi: 10.1093/annonc/mdq756. [DOI] [PubMed] [Google Scholar]
- 16.Solinas G, Marchesi F, Garlanda C, Mantovani A, Allavena P. Inflammation-mediated promotion of invasion and metastasis. Cancer Metastasis Rev. 2010;29(2):243–248. doi: 10.1007/s10555-010-9227-2. [DOI] [PubMed] [Google Scholar]
- 17.Lonkar P, Dedon PC. Reactive species and DNA damage in chronic inflammation: reconciling chemical mechanisms and biological fates. Int J Cancer. 2011;128(9):1999–2009. doi: 10.1002/ijc.25815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31(1):37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208(3):417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Anello L, Sansone P, Storci G, et al. Epigenetic control of the basal-like gene expression profile via Interleukin-6 in breast cancer cells. Mol Cancer. 2010;9:300. doi: 10.1186/1476-4598-9-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foran E, Garrity-Park MM, Mureau C, et al. Upregulation of DNA methyltransferase-mediated gene silencing, anchorage-independent growth, and migration of colon cancer cells by interleukin-6. Mol Cancer Res. 2010;8(4):471–481. doi: 10.1158/1541-7786.MCR-09-0496. [DOI] [PubMed] [Google Scholar]
- 22.Wehbe H, Henson R, Meng F, Mize-Berge J, Patel T. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res. 2006;66(21):10517–10524. doi: 10.1158/0008-5472.CAN-06-2130. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Zhou BP. TNF-α/NF-κB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102(4):639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22(2):83–89. doi: 10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223(2):102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27▪.Saito M, Schetter AJ, Mollerup S, et al. The association of microRNA expression with prognosis and progression in early-stage, non-small cell lung adenocarcinoma: a retrospective analysis of three cohorts. Clin Cancer Res. 2011;17(7):1875–1882. doi: 10.1158/1078-0432.CCR-10-2961. Recent study in lung cancer patients providing mounting evidence that select miRNAs can be utilized as biomarkers to determine prognosis and treatment options for individuals with cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibuya H, Iinuma H, Shimada R, Horiuchi A, Watanabe T. Clinicopathological and prognostic value of microRNA-21 and microRNA-155 in colorectal cancer. Oncology. 2010;79(3–4):313–320. doi: 10.1159/000323283. [DOI] [PubMed] [Google Scholar]
- 29.Gabriely G, Yi M, Narayan RS, et al. Human glioma growth is controlled by microRNA-10b. Cancer Res. 2011;71(10):3563–3572. doi: 10.1158/0008-5472.CAN-10-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moussay E, Wang K, Cho JH, et al. MicroRNA as biomarkers and regulators in B-cell chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2011;108(16):6573–6578. doi: 10.1073/pnas.1019557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson EE, Holloway AK, Weng J, Fojo T, Kebebew E. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer. 2011;117(8):1630–1639. doi: 10.1002/cncr.25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heath Vl, Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol. 2009;6(7):395–404. doi: 10.1038/nrclinonc.2009.52. [DOI] [PubMed] [Google Scholar]
- 33▪.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. Reviews the hypothesis that strategies targeting the tumor vasculature will (at least temporarily) alleviate obstacles for the delivery of therapeutic strategies to the chronically inflamed tumor microenvironment. [DOI] [PubMed] [Google Scholar]
- 34.Qian CN, Huang D, Wondergem B, Teh BT. Complexity of tumor vasculature in clear cell renal cell carcinoma. Cancer. 2009;115(10 Suppl):2282–2289. doi: 10.1002/cncr.24238. [DOI] [PubMed] [Google Scholar]
- 35.Rini BI. Metastatic renal cell carcinoma: many treatment options, one patient. J Clin Oncol. 2009;27(19):3225–3234. doi: 10.1200/JCO.2008.19.9836. [DOI] [PubMed] [Google Scholar]
- 36.Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009;6(10):569–579. doi: 10.1038/nrclinonc.2009.130. [DOI] [PubMed] [Google Scholar]
- 37.Mena AC, Pulido EG, Guillen-Ponce C. Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: sunitinib. Anticancer Drugs. 2010;21(Suppl 1):S3–S11. doi: 10.1097/01.cad.0000361534.44052.c5. [DOI] [PubMed] [Google Scholar]
- 38.Allen M, Louise Jones J. Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J Pathol. 2011;223(2):162–176. doi: 10.1002/path.2803. [DOI] [PubMed] [Google Scholar]
- 39.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010;29(2):285–293. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 41.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16(3):133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luppi F, Longo AM, De Boer WI, Rabe KF, Hiemstra PS. Interleukin-8 stimulates cell proliferation in non-small-cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer. 2007;56(1):25–33. doi: 10.1016/j.lungcan.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8(8):618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 44.Dumitru CA, Gholaman H, Trellakis S, et al. Tumor-derived macrophage migration inhibitory factor modulates the biology of head and neck cancer cells via neutrophil activation. Int J Cancer. 2011;129(4):859–869. doi: 10.1002/ijc.25991. [DOI] [PubMed] [Google Scholar]
- 45.Gregory AD, McGarry Houghton A. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71(7):2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 46.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40(11):2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59(10):1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49▪.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi: 10.1126/science.1203543. Review explores details of cancer cell metastasis and provides new mechanistic perspectives, supporting a continuing role for chronic inflammation in promoting/maintaining systemic disease. [DOI] [PubMed] [Google Scholar]
- 50.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324(5935):1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grzesiak JJ, Cao HS, Burton DW, et al. Knockdown of the β(1) integrin subunit reduces primary tumor growth and inhibits pancreatic cancer metastasis. Int J Cancer. 2011 doi: 10.1002/ijc.25942. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kusuma N, Denoyer D, Eble JA, et al. Integrin-dependent response to laminin-511 regulates breast tumor cell invasion and metastasis. Int J Cancer. 2011 doi: 10.1002/ijc.26018. (in press) [DOI] [PubMed] [Google Scholar]
- 53.Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 54.Hayes DF. Bevacizumab treatment for solid tumors: boon or bust? JAMA. 2011;305(5):506–508. doi: 10.1001/jama.2011.57. [DOI] [PubMed] [Google Scholar]
- 55.Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol. 2010;66(2):357–371. doi: 10.1007/s00280-009-1170-y. [DOI] [PubMed] [Google Scholar]
- 56.Shablak A, Hawkins RE, Rothwell DG, Elkord E. T cell-based immunotherapy of metastatic renal cell carcinoma: modest success and future perspective. Clin Cancer Res. 2009;15(21):6503–6510. doi: 10.1158/1078-0432.CCR-09-1605. [DOI] [PubMed] [Google Scholar]
- 57.Faris JE, Michaelson MD. Targeted therapies: sunitinib versus interferon-α in metastatic RCC. Nat Rev Clin Oncol. 2010;7(1):7–8. doi: 10.1038/nrclinonc.2009.173. [DOI] [PubMed] [Google Scholar]
- 58.Pal SK, Figlin RA. Renal cell carcinoma therapy in 2010: many options with little comparative data. Clin Adv Hematol Oncol. 2010;8(3):191–200. [PubMed] [Google Scholar]
- 59.Rini BI, Atkins MB. Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol. 2009;10(10):992–1000. doi: 10.1016/S1470-2045(09)70240-2. [DOI] [PubMed] [Google Scholar]
- 60.Fabian MA, Biggs WH, 3rd, Treiber DK, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23(3):329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 61.Furge KA, Mackeigan JP, Teh BT. Kinase targets in renal-cell carcinomas: reassessing the old and discovering the new. Lancet Oncol. 2010;11(6):571–578. doi: 10.1016/S1470-2045(09)70380-8. [DOI] [PubMed] [Google Scholar]
- 62.Huang D, Ding Y, Li Y, et al. Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer Res. 2010;70(3):1053–1062. doi: 10.1158/0008-5472.CAN-09-3722. [DOI] [PubMed] [Google Scholar]
- 63▪.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70(15):6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. Murine melanoma model highlighting the ability of antivasculature agents to effectively promote the redistribution and antitumor efficacy of immune-based therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao X, Bose A, Komita H, et al. Intratumoral IL-12 gene therapy results in the crosspriming of Tc1 cells reactive against tumor-associated stromal antigens. Mol Ther. 2011;19(4):805–814. doi: 10.1038/mt.2010.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vroling L, van der Veldt AA, De Haas RR, et al. Increased numbers of small circulating endothelial cells in renal cell cancer patients treated with sunitinib. Angiogenesis. 2009;12(1):69–79. doi: 10.1007/s10456-009-9133-9. [DOI] [PubMed] [Google Scholar]
- 66.Czabanka M, Vinci M, Heppner F, Ullrich A, Vajkoczy P. Effects of sunitinib on tumor hemodynamics and delivery of chemotherapy. Int J Cancer. 2009;124(6):1293–1300. doi: 10.1002/ijc.24019. [DOI] [PubMed] [Google Scholar]
- 67.Helfrich I, Scheffrahn I, Bartling S, et al. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J Exp Med. 2010;207(3):491–503. doi: 10.1084/jem.20091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Q, Guo P, Gallo JM. Impact of angiogenesis inhibition by sunitinib on tumor distribution of temozolomide. Clin Cancer Res. 2008;14(5):1540–1549. doi: 10.1158/1078-0432.CCR-07-4544. [DOI] [PubMed] [Google Scholar]
- 69.Finke JH, Rini B, Ireland J, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14(20):6674–6682. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 70.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 71.Van Cruijsen H, van der Veldt AA, Vroling L, et al. Sunitinib-induced myeloid lineage redistribution in renal cell cancer patients: CD1c+ dendritic cell frequency predicts progression-free survival. Clin Cancer Res. 2008;14(18):5884–5892. doi: 10.1158/1078-0432.CCR-08-0656. [DOI] [PubMed] [Google Scholar]
- 72.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73▪.Bose A, Taylor JL, Alber S, et al. Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int J Cancer. 2011 doi: 10.1002/ijc.25863. (In Press). First study in mice to document the utilization of the US FDA-approved drug sunitinib to condition the tumor microenvironment for delivery and therapeutic action of vaccine-specific T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 101.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer; http://globocan.iarc.fr. [Google Scholar]