Abstract
Because of recent declining malaria transmission in Latin America, some authorities have recommended against chemoprophylaxis for most travelers to this region. However, the predominant parasite species in Latin America, Plasmodium vivax, can form hypnozoites sequestered in the liver, causing malaria relapses. Additionally, new evidence shows the potential severity of vivax infections, warranting continued consideration of prophylaxis for travel to Latin America. Individualized travel risk assessments are recommended and should consider travel locations, type, length, and season, as well as probability of itinerary changes. Travel recommendations might include no precautions, mosquito avoidance only, or mosquito avoidance and chemoprophylaxis. There are a range of good options for chemoprophylaxis in Latin America, including atovaquone-proguanil, doxycycline, mefloquine, and—in selected areas—chloroquine. Primaquine should be strongly considered for nonpregnant, G6PD-nondeficient patients traveling to vivax-endemic areas of Latin America, and it has the added benefit of being the only drug to protect against malaria relapses.
Background
Although most malaria clinical cases and deaths globally are caused by the Plasmodium falciparum parasite, Plasmodium vivax is the most geographically widespread malaria species, with an estimated 2.85 billion population at risk1 and 80–300 million clinical cases each year.2 The absolute burden of vivax malaria is highest in Asia,1,3 but P. vivax is also the predominant malaria species in Latin America, accounting for 412,788 of 553,839 reported cases (74.5%) in which a species diagnosis was made in 2009.3 The estimated relative risk of contracting malaria among travelers to Latin America is low,4 but evidence has recently emerged highlighting the potential for P. vivax to cause severe and even fatal malaria.4,5 This article intends to explore the current debate surrounding malaria chemoprophylaxis for travelers to Latin America, in light of current evidence on malaria risk, chemoprophylaxis effectiveness, risk of adverse events, and other relevant factors.
Plasmodium vivax in Latin America and among travelers.
The burden of malaria among residents in Latin America has been declining in recent years. Reported malaria cases in 2008 numbered 560,221 in Latin America, down 30% from the previous year, and significantly decreased from earlier in the decade.6 Malaria remains endemic in 21 countries in Latin America (all countries except Chile and Uruguay), 12 of which experience predominantly P. vivax malaria transmission (see Table 1 for further details).
Table 1.
Proportion of malaria cases caused by Plasmodium vivax in endemic countries of Latin America
| Country | % P. vivax cases |
|---|---|
| Argentina | 100% |
| Belize | 100% |
| Bolivia | 91% |
| Brazil | 75% |
| Colombia | 60–65% |
| Costa Rica | 100% |
| Dominican Republic | 0% |
| Ecuador | 90% |
| El Salvador | 99% |
| French Guiana | ∼50% |
| Guatemala | 97% |
| Guyana | 50% |
| Haiti | 0% |
| Honduras | 93% |
| Mexico | 100% |
| Nicaragua | 95% |
| Panama | 99% |
| Paraguay | 95% |
| Peru | 70% |
| Suriname | 70% |
| Venezuela | 83% |
Source: Numbers taken from the CDC's travel statistics, available at: http://www.cdc.gov/malaria/travelers/country_table/a.html.
Note: Shaded rows represent countries where CDC recommends primaquine as one option for malaria chemoprophylaxis.
For more information on malaria endemicity in specific countries of Latin America, please refer to CDC's interactive malaria map, available at: http://www.cdc.gov/malaria/map/.
The proportion of vivax malaria reported among travelers varies by surveillance system, reflecting differing travel destination patterns for those whose illnesses are captured by the system, but it is generally much lower than for P. falciparum malaria. Of the 1,484 cases of malaria reported in the United States in 2009, 927 (62.5%) had a species reported; of these 927 cases, 166 (17.9%) were caused by P. vivax, and 20 (12.2%) of these cases originated from travel to Latin America.7 This has declined from 28.3% of United States cases caused by vivax from 2002 to 2008,4,7–12 with 24.0% originating from travel to Latin America.4,7–13 In Europe, P. vivax accounted for a lower proportion of malaria cases among travelers (618 of 4,801 (12.9%) between 1999 and 2003), 17.0% of which originated in Latin America.14 In Canada, the proportion of malaria cases among travelers that are P. vivax has been declining, from 70% in the early 1980s to 20% of cases between 1989 and 2001.15 Plasmodium vivax malaria is the cause of the majority of malaria cases among Australian travelers, although most of these cases originate from travel to South Asia and Papua New Guinea.16 Among sites reporting into the GeoSentinel database between 1997 and 2002, which includes global travelers from Europe, North America, Australia, and other countries, P. vivax accounted for 26.9% of cases with a confirmed malaria species, and 16% of vivax cases were imported from Latin America.17
Despite declining malaria cases in the region and among travelers to Latin America, two notable features of P. vivax malaria make considerations for malaria prevention, including chemoprophylaxis, especially important for travelers going to specific places within the region where malaria transmission still occurs. First, new evidence of the potential severity of P. vivax malaria makes it a more serious threat than previously thought; and second, the presence of a dormant parasite form, the hypnozoite, in the liver that can lead to a clinical episode of malaria months or years after the initial infection. The relapsing potential of vivax malaria warrants special consideration among health care workers providing travel advice.
Special considerations for P. vivax malaria.
Although P. falciparum is the predominant species causing severe malaria, new evidence has recently emerged that P. vivax can cause severe and even fatal malaria.5,18–20 Recent reports have indicated a growing awareness of the potential severity of vivax malaria, including complications of seizures, shock, jaundice, renal failure, and severe anemia,21–24 including acute respiratory distress syndrome in an Italian traveler to Venezuela.25 Recent evidence from two large cohort studies in Indonesia and Papua New Guinea indicated that rates of severe malaria in P. vivax infections can be as high or higher than rates of severe malaria among patients with P. falciparum infections and can cause severe anemia, respiratory distress and, in some instances, coma and even death.26,27 Of the two reported malaria deaths in the United States in 2008, one was caused by infection with P. vivax.4 Among the 190 vivax malaria cases reported in 2008 in the United States, 100 were hospitalized and nine had severe malaria,† including two with acute respiratory distress syndrome, three with renal failure, and one with severe anemia.28
A fascinating biological feature of P. vivax is its ability to survive for prolonged periods in liver cells as dormant hypnozoites, which can become reactivated and lead to clinical malaria weeks, months, or years after the primary infection. Only P. vivax and Plasmodium ovale have this ability to cause malaria relapses, further complicating prophylaxis and treatment considerations. Among antimalarial drugs used for chemoprophylaxis, only primaquine can prevent relapse. With P. vivax malaria, the risk of relapse without primaquine therapy is estimated to be between 5% and 80%.29 Relapse risk and the interval between the primary infection and the first relapse is P. vivax strain dependent and varies with geographic area. Latin American strains have quite variable relapse risks from 7.5% to 70%30,31 and relapse intervals between 1 and 6 months.32,33 Typically, vivax malaria can relapse 1–3 months post return, but relapses can occur up to a year or two after travel. Of 618 cases of P. vivax malaria reported between January 1999 and September 2003 among travelers returning to Europe, 118 (19.1%) had taken chemoprophylaxis. However, their symptom onset was significantly delayed compared with travelers not taking chemoprophylaxis, indicating that prophylaxis was probably successful against primary infection, but their symptoms were most likely caused by reactivated hypnozoites in the liver.14 The GeoSentinel database does not capture information on prophylaxis among travelers. However, among confirmed malaria cases between 1997 and 2002 in the GeoSentinel database, those with a pre-travel encounter presented with vivax malaria after a median of 58 days, compared with 15.5 days for those without a pre-travel encounter, presumably because those with a pre-travel encounter were more likely to have taken prophylaxis that prevented primary infection.17 Late illness onset (more than 2 months post return) was found in 44.7% of travelers with malaria returning to Israel and 35.0% of those returning to the United States during the 1990s, and the majority of these cases were caused by P. vivax; 80.6% of travelers with late-onset malaria in Israel and 62.2% of those in the United States had used a chemoprophylaxis regimen effective for the prevention of P. falciparum malaria but not effective at preventing a P. vivax relapse (i.e., did not contain primaquine).34
Chemoprophylaxis Recommendations For Travelers
The decision to use chemoprophylaxis for travelers depends on individual risk-benefit analyses weighing the risk of contracting malaria against the possible adverse effects from prophylaxis, yet high quality and timely data on both risks of contracting malaria in particular destinations and risks of adverse events may not be easily available. According to one estimate, the annual incidence per 100,000 travelers to Mexico and some parts of South America is 1.9 malaria cases per week of travel.35 Another recent estimate of malaria risk among travelers to Latin America found that it was 0.3 and 0.8 cases per 10,000 visits in the United States and United Kingdom travelers, respectively.36 Leder and others,17 using data from the GeoSentinel global surveillance network database, estimated that travelers to the Caribbean, South America, and Central America had risks for malaria of 1.8, 3.9, and 17.8 malaria cases, respectively, per 10 million travelers; in comparison, the authors estimated the risks for travelers to South Asia and sub-Saharan Africa to be 25.3 and 97.5 per 10 million travelers. Some malaria researchers have set their threshold for not recommending chemoprophylaxis for short-term travelers (for example, travel for 2 weeks or less) to areas where endemicity in the local population is below 10 cases per 1,000 populations, which includes most of South America.35
Location and travel details are key.
However, high-quality data for estimating malaria risk for travelers by country or region are lacking (e.g., because of underreporting of malaria cases, missing information on region where travelers were infected, and the chemoprophylaxis taken, and other factors), and methods of estimating risk are not consistent across studies. It is clear that there is substantial variation in malaria transmission within the Latin American region and within countries in Latin America. For example, many Latin American countries experience little to no malaria transmission in their capital cities or in coastal areas, but moderate transmission of malaria inland or in forested/jungle areas. In addition, malaria transmission in most locations is seasonal with periods of relatively high transmission during and shortly after the rainy seasons and periods of virtually no transmission during the dry season. Although trips to Latin America (including Mexico, Central America, South America, and the Caribbean) comprised 41.5% of all United States passenger travel internationally in 2008,37 malaria cases in the United States imported from Latin America comprised only 6.2% of all cases with a known origin of acquisition.4 However, this may be explained not only by lower malaria transmission risk for travelers to Latin America, but by the tendency of United States travelers to visit urban and coastal areas of Latin America, as opposed to the rural interior with highly endemic malaria.38 Box 1 provides examples of several travel destinations in Latin America, and chemoprophylaxis recommendations for each.
Box 1.

Examples of chemoprophylaxis recommendations for a hypothetical traveler: selected destinations in Latin America
References such as the interactive malaria map maintained by the United States-based Centers for Disease Control and Prevention (CDC)39 and the malaria chapter in the Traveler's Health (Yellow) book, available online at http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3-infectious-diseases-related-to-travel/malaria.htm, or the World Health Organization's (WHO) International and Travel Health book, available online at http://www.who.int/ith/ITH2010.pdf, can help clinicians and others giving travel advice provide more accurate risk assessments and recommendations. Individualized pre-travel advice based on travel route, season, length and type of travel is recommended.40,41 Travel advice may also vary based on the traveler's country of origin, as licensing and use of chemoprophylaxis regimens varies across countries.40 Although individual recommended regimens may vary, most countries' travel guidelines, as well as the WHO's, recommend chemoprophylaxis for travelers to endemic areas of most countries in Latin America. One notable exception is the Swiss public health agency, which recommends standby emergency treatment in endemic areas and chemoprophylaxis only in four more highly endemic countries in Latin America (Brazil, French Guiana, Guyana, and Suriname—see also Table 2). The French public health agency specifies that chemoprophylaxis for trips of < 7 days duration is optional for travelers to chloroquine-sensitive areas of Latin America.
Table 2.
Recommendations by various countries/organizations on chemoprophylaxis for travel to Latin America
| Country/organization | Type(s) of chemoprophylaxis recommended for Latin America | Website/references* |
|---|---|---|
| Australia, Department of Foreign Affairs and Trade |
|
Travel advisories, available at: http://www.smarttraveller.gov.au/zw-cgi/view/Advice/ |
| Canada, Public Health Agency of Canada |
|
Canadian recommendations for the prevention and treatment of malaria among international travelers 2009. Available at: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/09pdf/35s1-eng.pdf |
| France |
|
Available at: http://www.sante.gouv.fr/IMG/pdf/beh_21_22_2010.pdf |
| Switzerland, Confédération Suisse (Office Federal de la Santé Publique) |
|
General malaria advice for short stays Available at: http://www.bag.admin.ch/themen/medizin/00682/00684/02535/index.html?lang=fr Country-specific recommendations: Available at: http://www.safetravel.ch |
| UK, Health Protection Agency |
|
Chiodini P, Hill D, Lalloo D, Lea G, Walker E, Whitty C and Bannister B. Guidelines for malaria prevention in travelers from the United Kingdom. London, Health Protection Agency, January 2007. Available at: http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1203496943523 |
| USA, Centers for Disease Prevention and Control |
|
CDC Health Information for International Travel 2010 (“Yellow Book”) Available at: http://www.cdc.gov/malaria/travelers/country_table/a.html |
| World Health Organization |
|
International Travel and Health book 2010 (“Green book”) Available at: http://www.who.int/ith/ITH2010.pdf |
Note: Please refer to specific publications for further information on areas with malaria transmission. This table is not meant to serve as a comprehensive guide for destination-specific chemoprophylaxis recommendations.
Mosquito bite prevention is analogous to mosquito avoidance.
In most areas of Latin America, especially in the Amazon Basin, where malaria is endemic, the primary malaria mosquito vector is Anopheles darlingi, which has early peak feeding times between dusk and about 9:00 or 10:00 pm.42,43 However, there is regional variability and some feeding peaks occur later in the evening. Travelers should be advised that limiting their time outdoors during the biting hours of the Anopheles mosquito from dusk to dawn will help limit their exposure. Other measures, including the use of an effective repellant such as 50% N,N-diethyl-m-toluamide, which has been shown to be both safe and effective,44 and wearing clothing that covers as much skin as possible after dusk for any time spent outdoors may be especially beneficial in Latin America with the early peak biting times of An. darlingi. Staying in accommodations with air conditioning or screens in the windows, and use of permethrin-treated bednets when accommodations are not well screened or air-conditioned should be encouraged.
Depending on a traveler's overall risk assessment, travel recommendations might include mosquito avoidance measures only, mosquito avoidance measures and chemoprophylaxis, or no precautions.45 Standby emergency treatment, defined as self-treatment with an approved full course of malaria treatment of malaria symptoms when traveling, has been a controversial topic,38 because of misuse and associated potentially serious errors,40 and the potential for malaria to lead to severe complications even if treated with an effective medication. There is limited evidence in support of standby treatment. One previous study of airline personnel who were issued standby emergency treatment as an alternative to optional chemoprophylaxis indicated that only 1% of crew members used their emergency treatment and that malaria cases did not rise during a 10-year-period following the new policy.46 However, the United States-based CDC recommends that rather than standby emergency treatment, persons at high risk of developing malaria can be provided with a reliable supply of malaria medication. The term “reliable supply” refers to malaria medicines that are acquired in the home country, which are not likely to be counterfeit; are not likely to interact with concomitant medicines, including malaria chemoprophylaxis; will not deplete local medicine resources at the destination; and will be appropriate for potential drug resistance in the area. In the event that a person develops symptoms of malaria, they should seek medical attention and if diagnosed with malaria, that supply of medication can be used.45 If the medical practitioner providing travel advice assesses malaria to be a significant risk for a traveler, then chemoprophylaxis is always preferred over self-treatment. Appropriate chemoprophylactic regimens, discussed below in more detail, include atovaquone-proguanil (Malarone), doxycycline, mefloquine, and, in some areas, primaquine or chloroquine. Box 2 summarizes the relevant recommendations for health care providers to consider when providing advice on malaria prophylaxis to travelers going to Latin America.
Box 2.
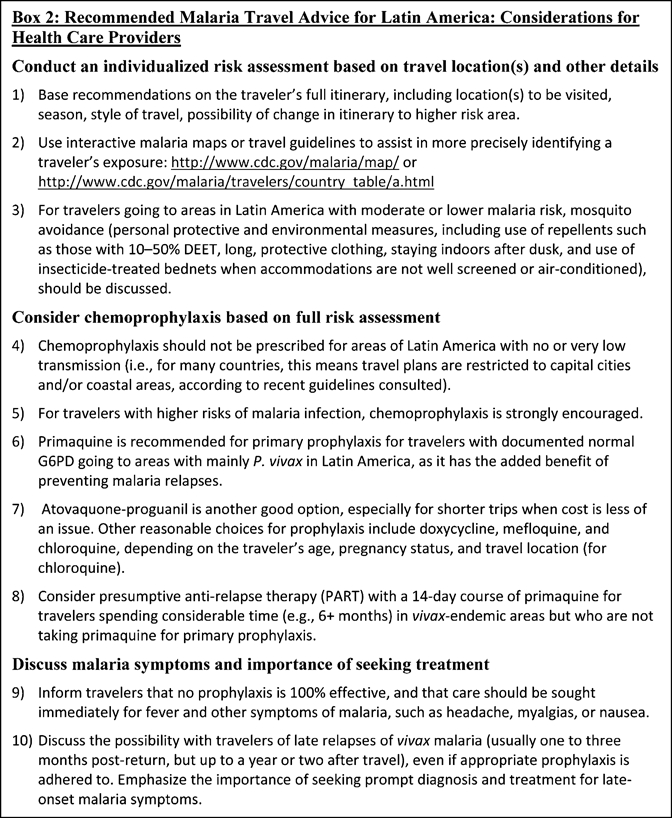
Recommended malaria travel advice for Latin America: considerations for health care providers
Atovaquone-proguanil—an expensive but solid choice for most.
The addition of atovaquone-proguanil, known commercially as Malarone (GlaxoSmithKline, Middlesex, UK), is an important development in malaria prophylaxis options for travelers, because of its high efficacy, low rates of side effects, and more convenient dosing schedule compared with other chemoprophylactic agents.47 Atovaquone-proguanil is currently approved to be taken 1–2 days before travel, daily while traveling, and for 7 days after return. Most studies of atovaquone-proguanil chemoprophylaxis have evaluated its efficacy against P. falciparum malaria, for which rates of protective efficacy as high as 100% have been reported.48,49 Studies evaluating the protective efficacy of atovaquone-proguanil against P. vivax malaria found a range from 84% among nonimmune residents in Papua, Indonesia50 to 100% efficacy in nonimmune soldiers in Colombia (compared with placebo).51 At least one case report has demonstrated that atovaquone-proguanil is not effective in preventing the establishment and survival of hypnozoites that can lead to relapses.52
The risk of mild to moderate, as well as more serious adverse events has been shown to be lower among travelers on a prophylactic regimen of either atovaquone-proguanil or doxycycline, compared with regimens of either mefloquine or chloroquine and proguanil.48,53 One placebo-controlled study found that 20% of those randomized to atovaquone-proguanil reported neuro-psychiatric adverse events; however, this rate was comparable to that in the placebo group, and much lower than the 37% in the group who received mefloquine.53 Because the placebo does not actually cause neuropsychiatric side effects, these symptoms are likely common and sometimes reported as drug adverse events. The rate of neuropsychiatric side effects reported for mefloquine was significantly higher than for placebo, supporting the fact that these types of side effects are a recognized phenomenon for this drug, although the actual rate is probably around 10% due to the very large placebo effect in this trial.
Another advantage of atovaquone-proguanil is that it does not require advanced dosing or prolonged dosing after travel, making it a more convenient option for travelers—especially short-term and last-minute travelers—and potentially leading to higher likelihood that the regimen will be taken completely and appropriately.54 Atovaquone-proguanil is safe for use in children > 5 kg, but adequate safety data are not yet available to recommend its use in pregnant women. Atovaquone-proguanil is also much more expensive than other prophylaxis regimens, making it less attractive for longer trips or for travelers whose insurance does not help defray the costs.
Other chemoprophylaxis agents.
Alternative antimalarial chemoprophylaxis agents recommended for travel to Latin America include doxycycline, mefloquine, chloroquine, and primaquine. Studies have shown high efficacy of doxycycline against P. vivax infection (98–99% according to randomized, placebo-controlled trials),55,56 and relatively low rates of adverse events comparable to atovaquone-proguanil.53 Despite its daily dosing schedule and longer regimen—up to 4 weeks post travel—doxycycline still remains the least expensive chemoprophylaxis option.
Mefloquine has been approved for malaria prophylaxis by the U.S. Food and Drug Administration since 1989 and has been shown to have high protective efficacy (100% against P. falciparum and P. vivax malaria, according to one placebo-controlled randomized trial).56 Although some travelers may consider the weekly dosing schedule of mefloquine to be more convenient compared with daily atovaquone-proguanil and doxycycline, studies have raised concerns about higher rates of adverse events, particularly neuropsychiatric events, and study withdrawals caused by lower drug tolerability.53,57 Chloroquine can be considered as a malaria prophylaxis for select countries in Latin America, some of the few areas where malaria remains sensitive to chloroquine. These countries include Mexico, all countries in Central America, Haiti, the Dominican Republic, Paraguay, Argentina, and parts of Panama. Chloroquine dosing is weekly, and it is safe for use by pregnant women and children of all ages. Table 3 provides more information on these chemoprophylaxis options for travel to Latin America.
Table 3.
Considerations for chemoprophylaxis in areas with Plasmodium vivax transmission
| Drug | Efficacy | Adverse events (AEs)/side effects | Dosing | Pregnant women | Children | Cost | Other consider-ations | References | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Common AEs | Prevalence (%)* | ||||||||||
| Mild-Moderate AEs | Severe AEs | Neuro-psychiatric AEs | |||||||||
| Atovaquone-proguanil | 96–100% against P. falciparum; 84–100% against P. vivax | Abdominal pain, nausea, vomiting, headache | 32 | 7 | 20 | Daily; 1–2 days before, during, and 7 days after travel | Not recommended; not enough evidence | Safe for children > 5 kg | High | Contraindicated in patients with severe renal impairment | 48,49,50,75 |
| Doxycycline | 96.3–99% against P. falciparum; 98–99% against P. vivax | Sun sensitivity, vaginal yeast infections, gastrointestinal upset (nausea, vomiting) reduced by taking with food. | 33 | 6 | 24 | Daily; 1–2 days before, during, and 4 weeks after travel | Not recommended | Safe for children > 8 years | Very low | 55 | |
| Mefloquine | 100% against P. falciparum; 100% against P. vivax | Gastrointestinal upset, headache, insomnia, abnormal dreams, visual disturbances, depression, anxiety, dizziness, motor neuropathies | 42 | 12 | 37 | Weekly; 2 weeks before, during, and 4 weeks after travel | Safe for pregnant women in 2nd and 3rd trimester | Safe for infants and children | Moderate | Contraindicated in travelers with history of depression, anxiety, psychosis, schizophrenia, and other psychiatric disorders | 56,76 |
| Chloroquine (chloroquine phosphate or hydroxyl-chloroquine sulfate) | Only effective in Central and South American countries West of Panama Canal | Gastrointestinal upset, headache, dizziness, blurred vision, insomnia, pruritus | 45 | 11 | 30 | Weekly; 1–2 weeks before travel, during, and 4 weeks after travel | Safe for pregnant women | Safe for infants and children | Low- moderate | Resistance to chloroquine in many areas | 45,76 |
| Primaquine | 88–95% against P. falciparum; 85–92% against P. vivax | Gastrointestinal upset, mild and self-limiting methemoglobinemia (6%) | Can cause fatal hemolysis in G6PD-deficient patients; patients must be tested for G6PD deficiency before primaquine use | Daily; 1–2 days before, during, and 7 days after travel | Not recommended because of unknown G6PD status of fetus | Safe for G6PD-normal children | Moderate, if include G6PD testing | Only drug that eliminates liver hypnozoites and prevents relapse; preferred for short-duration travel to areas with mainly P. vivax malaria | 60,59,58,61,77 | ||
Data on AE prevalence in first four drugs from Schlagenhauf and others53; data not available for primaquine. In pre-travel period, rates of AEs for placebo were comparable to rates for atovaquone-proguanil and doxycycline (no placebo used during travel period).
Table includes studies from travelers and nonimmune residents only. Cost data taken from Reference 78.
The benefits of primaquine in vivax areas.
Primaquine is currently the only drug that can destroy hypnozoites in the liver and prevent relapses of P. vivax infections. Three placebo-controlled randomized studies58–60 in residents of endemic areas and one observational study in nonimmune western travelers61 have indicated that primaquine is effective against vivax malaria, showing protective efficacy of 85% to more than 92% against P. vivax and 88% against P. falciparum malaria, although these studies had small sample sizes. A study in nonimmune travelers showed that primaquine was much more effective than either doxycycline or mefloquine in preventing the late relapse of vivax malaria acquired in a highly endemic area of Ethiopia, although there were five cases of P. falciparum malaria that occurred within 1 month of return in those on primaquine.61 Primaquine has insufficient activity against asexual blood-stage parasites of P. falciparum62,63 and is therefore not a recommended choice of prophylaxis against P. falciparum malaria.29,35
Primaquine has not been used extensively for primary prophylaxis, and it is not currently licensed in any country for primary prophylaxis.2 The exact mechanisms of primaquine activity against hypnozoites are still not known after more than 50 years of use, and questions remain about its safety, tolerability, and dosing.24,64 In addition to primary prophylaxis, other uses of primaquine include presumptive anti-relapse therapy (PART) to prevent possible relapses in travelers returning from P. vivax- or P. ovale-endemic areas, and radical cure, to prevent vivax or ovale relapse after confirmed infection. Primaquine is estimated to be highly effective (> 95%) for PART when given to returning travelers for 14 days at 30 mg daily, along with a blood schizonticide such as chloroquine or mefloquine, and very effective (> 90%) at the same dosing for radical cure.65 Malariologists agree that people with “intense” or “significant” exposure to P. vivax malaria should undergo PART, but there is no consensus on what constitutes a significant risk; some researchers believe that PART should only be considered for people who have spent considerable time (e.g., 6 or more months) in high-transmission vivax areas.65 Returning Peace Corps volunteers and United States military personnel who have spent considerable time in vivax- or ovale-endemic areas typically receive a course of primaquine for PART upon their departure from the endemic area. PART requires a 14-day regimen post travel that is prone to non-adherence, and studies have shown much higher rates of relapses when primaquine therapy is not supervised.29
Primaquine can only be used in patients who have been tested and have normal glucose-6-phosphate dehydrogenase (G6PD) activity, because it can cause potentially fatal hemolysis in G6PD-deficient individuals. Primaquine's side effects include primarily mild, asymptomatic, and self-limited methemoglobinemia35,59,65 and possible gastrointestinal upset.65 Common anecdotal experience, and limited evidence from clinical trials,66 suggests that gastrointestinal upset is lessened if primaquine is taken with food, although high-quality evidence from modern randomized, placebo-controlled clinical trials is lacking. Primaquine cannot be used in pregnant women. Primaquine is metabolized by Cytochrome p450 (CYP) isoforms that are polymorphic67 and can therefore result in a wide range of enzyme activity, which may lead to adverse effects or lack of effect at standard doses.67,68 In addition, gender and food have been found to significantly affect the pharmacokinetics of primaquine, leading to a high level of individual variability in drug levels.69,70 Weight may also play a role, yet primaquine prophylaxis dosing is a standard 30 mg daily, and the three randomized, controlled trials of daily prophylaxis with 30 mg primaquine were conducted in populations with significantly lower body weights than typical Western adults.
Despite its drawbacks, notably the need for G6PD testing before use, primaquine should be considered a recommended option for primary prophylaxis for travelers to vivax-endemic areas of Latin America.45 If G6PD testing results indicate normal activity, primaquine can be prescribed off-label in the United States for prophylaxis in travelers going to areas with mainly vivax malaria (see Table 1 for areas where CDC recommends primaquine).40,64 Primaquine's primary advantage includes protection against P. vivax relapses, in addition to protection against primary infection. It is generally lower cost than atovaquone-proguanil, and its dosing regimen is equally convenient—daily during travel and for only 7 days post return, making it ideal for last-minute trips or short-term travel. For travelers going to areas with low levels of vivax transmission (i.e., where P. falciparum predominates), a blood schizonticide may be sufficient prophylaxis, along with counseling about the potential for successfully prevented clinical malaria to relapse months after return from travel and about the importance of seeking prompt diagnosis and treatment of symptoms of malaria.35,40
Finally, there is a clear need to conduct further research to improve our understanding of primaquine and its dosing and modes of action. Despite 60 years of use, there is much we still do not understand.2,24 There has been much discussion regarding possible primaquine-resistant or primaquine-tolerant vivax malaria,5,29 but this issue is unclear because of confusion between therapeutic failure and actual drug resistance. Therapeutic failure can result from underdosing, missed dosing, or malabsorption of a drug, whereas actual resistance means that the target organism is no longer susceptible to the drug. There are numerous causes of therapeutic failure in addition to resistance.71 In addition, in the case of primaquine and vivax malaria, there are recognized differences in the susceptibility of different strains of P. vivax to primaquine that were observed at the time primaquine was introduced in the early 1950s.66,72
It has been difficult to characterize recurrences of vivax malaria after receiving a course of primaquine as either therapeutic failure (such as non-compliance and sub-therapeutic dosing) or possible resistance to primaquine, as directly observed treatment is not commonly performed and drug levels are not obtained. We have little understanding of the relationship between dose and pharmacokinetic parameters to therapeutic failure either in the radical cure or the prophylaxis indication. However, therapeutic failures in the radical cure indication are commonly documented at the currently recommended dose regimen of 30 mg for 14 days and seem to be more common when infection is acquired in Papua New Guinea,16,73 but have also been described in vivax infections acquired in Latin America as well.74 Whether these therapeutic failures represent occasional instances of true drug resistance or sub-therapeutic dosing, including noncompliance and rapid metabolism, remains unclear.
Conclusions And Recommendations
In summary, despite the declining incidence of malaria in Latin America, mainly P. vivax but also P. falciparum remain endemic in many parts of the region. Both species cause clinical malaria and, in some cases, severe disease in travelers. Travel advice should be tailored for individual travelers based on their itinerary, style of travel, season, and their clinical history and drug availability in their country of origin. Depending on these factors, travel advice might include no precautions, mosquito avoidance measures only, or mosquito avoidance measures and chemoprophylaxis. When the risk of malaria for travelers to Latin America warrants the use of chemoprophylaxis, atovaquone-proguanil is a good choice for most travelers, with few adverse effects, no resistance, and a convenient dosing schedule—started only 1–2 days before travel and for 7 days after return. Atovaquone-proguanil, however, cannot prevent vivax relapses, is more expensive than other drugs, and cannot be used during pregnancy or in children weighing < 5 kg. Because of its ability to prevent malaria relapses, primaquine can be considered an excellent choice for prophylaxis to areas with mainly P. vivax transmission in Latin America, although it is contraindicated in travelers with G6PD deficiency and pregnant women. Familiarity with other effective chemoprophylactic agents, such as those listed in Table 3, can help health professionals provide travelers with effective chemoprophylaxis regimens that meet their individual needs.
Disclaimer: The opinions and recommendations expressed in this article are the personal views of the authors and do not represent the official views of the Department of Health and Human Services, the Centers for Disease Control and Prevention, the Department of Defense, the US Army, or the Walter Reed Army Institute of Research.
Footnotes
Authors' addresses: Laura C. Steinhardt and Paul M. Arguin, Centers for Disease Control and Prevention, Malaria Branch, Division of Parasitic Diseases and Malaria, Atlanta, GA, E-mails: LSteinhardt@cdc.gov and PArguin@cdc.gov. Alan J. Magill, Kensington, MD, E-mail: alan.magill@us.army.mil.
Criteria for severe malaria include any one of the following clinical conditions: impaired consciousness/coma, severe normocytic anemia, renal failure, pulmonary edema, acute respiratory distress syndrome, circulatory shock, disseminated intravascular coagulation, spontaneous bleeding, acidosis, hemoglobinuria, jaundice, repeated generalized convulsions, and/or parasitemia of ≥ 5%.
References
- 1.Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, del Portillo HA. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 3.The World Health Organization . The World Malaria Report 2010. Geneva: WHO; 2010. [Google Scholar]
- 4.Mali S, Steele S, Slutsker L, Arguin PM. Malaria surveillance–United States, 2008. MMWR Surveill Summ. 2010;59:1–15. [PubMed] [Google Scholar]
- 5.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 6.Pan American Health Organization . Malaria situation in the Americas. 2010. [Google Scholar]
- 7.Mali S, Steele S, Slutsker L, Arguin PM. Malaria surveillance–United States, 2007. MMWR Surveill Summ. 2009;58:1–16. [PubMed] [Google Scholar]
- 8.Mali S, Steele S, Slutsker L, Arguin PM. Malaria surveillance–United States, 2006. MMWR Surveill Summ. 2008;57:24–39. [PubMed] [Google Scholar]
- 9.Thwing J, Skarbinski J, Newman RD, Barber AM, Mali S, Roberts JM, Slutsker L, Arguin PM. Malaria surveillance–United States, 2005. MMWR Surveill Summ. 2007;56:23–40. Centers for Disease Control and Prevention. [PubMed] [Google Scholar]
- 10.Skarbinski J, James EM, Causer LM, Barber AM, Mali S, Nguyen-Dinh P, Roberts JM, Parise ME, Slutsker L, Newman RD. Malaria surveillance–United States, 2004. MMWR Surveill Summ. 2006;55:23–37. [PubMed] [Google Scholar]
- 11.Eliades MJ, Shah S, Nguyen-Dinh P, Newman RD, Barber AM, Roberts JM, Mali S, Parise ME, Steketee R. Malaria surveillance–United States, 2003. MMWR Surveill Summ. 2005;54:25–40. [PubMed] [Google Scholar]
- 12.Shah S, Filler S, Causer LM, Rowe AK, Bloland PB, Barber AM, Roberts JM, Desai MR, Parise ME, Steketee RW. Malaria surveillance–United States, 2002. MMWR Surveill Summ. 2004;53:21–34. [PubMed] [Google Scholar]
- 13.Mali S, Tan KR, Arguin PM. Malaria surveillance-United States, 2009. MMWR Surveill Summ. 2011;60:1–15. [PubMed] [Google Scholar]
- 14.Muhlberger N, Jelinek T, Gascon J, Probst M, Zoller T, Schunk M, Beran J, Gjørup I, Behrens RH, Clerinx J, Björkman A, McWhinney P, Matteelli A, Lopoez-Velez R, Bisoffi Z, Hellgren U, Puente S, Schmid ML, Myrvang B, Holthoff-Stich ML, Luferl H, Hatz C, Kollaritsch H, Kapaun A, Knobloch J, Iversen J, Kotlowski A, Malvy DJ, Kern P, Fry G, Siikamaki H, Schulze MA, Soula G, Paul M, Gómez i Prat J, Lehmann V, Bouchaud O, da Cunha S, Atouguia J, Boecken G. Epidemiology and clinical features of vivax malaria imported to Europe: sentinel surveillance data from TropNetEurop. Malar J. 2004;3:5. doi: 10.1186/1475-2875-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacLean JD, Demers AM, Ndao M, Kokoskin E, Ward BJ, Gyorkos TW. Malaria epidemics and surveillance systems in Canada. Emerg Infect Dis. 2004;10:1195–1201. doi: 10.3201/eid1007.030826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott JH, O'Brien D, Leder K, Kitchener S, Schwartz E, Weld L, Brown GV, Kain KC, Torresi J. Imported Plasmodium vivax malaria: demographic and clinical features in nonimmune travelers. J Travel Med. 2004;11:213–217. doi: 10.2310/7060.2004.19004. GeoSentinel Surveillance Network. [DOI] [PubMed] [Google Scholar]
- 17.Leder K, Black J, O'Brien D, Greenwood Z, Kain KC, Schwartz E, Brown G, Torresi J. Malaria in travelers: a review of the GeoSentinel surveillance network. Clin Infect Dis. 2004;39:1104–1112. doi: 10.1086/424510. [DOI] [PubMed] [Google Scholar]
- 18.Anstey NM, Russell B, Yeo TW, Price RN. The pathophysiology of vivax malaria. Trends Parasitol. 2009;25:220–227. doi: 10.1016/j.pt.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Morales AJ, Benitez JA, Arria M. Malaria mortality in Venezuela: focus on deaths due to Plasmodium vivax in children. J Trop Pediatr. 2008;54:94–101. doi: 10.1093/tropej/fmm074. [DOI] [PubMed] [Google Scholar]
- 20.Alexandre MA, Ferreira CO, Siqueira AM, Magalhães BL, Mourão MP, Lacerda MV, Alecrim MG. Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis. 2010;16:1611–1614. doi: 10.3201/eid1610.100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochar DK, Saxena V, Singh N, Kochar SK, Kumar SV, Das A. Plasmodium vivax malaria. Emerg Infect Dis. 2005;11:132–134. doi: 10.3201/eid1101.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochar DK, Pakalapati D, Kochar SK, Sirohi P, Khatri MP, Kochar A, Das A. An unexpected cause of fever and seizures. Lancet. 2007;370:908. doi: 10.1016/S0140-6736(07)61417-2. [DOI] [PubMed] [Google Scholar]
- 23.Kochar DK, Das A, Kochar SK, Saxina V, Sirohi P, Garg S, Kochar A, Khatri MP, Gupta V. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. 2009;80:194–198. [PubMed] [Google Scholar]
- 24.Baird JK. Neglect of Plasmodium vivax malaria. Trends Parasitol. 2007;23:533–539. doi: 10.1016/j.pt.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Saleri N, Gulletta M, Matteelli A, Caligaris S, Tomasoni LR, Antonini B, Perandin F, Castelli F. Acute respiratory distress syndrome in Plasmodium vivax malaria in traveler returning from Venezuela. J Travel Med. 2006;13:112–113. doi: 10.1111/j.1708-8305.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- 26.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 2008;5:e128. doi: 10.1371/journal.pmed.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genton B, D'Acremont V, Rare L, Baea K, Reeder JC, Alpers MP, Müller I. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 2008;5:e127. doi: 10.1371/journal.pmed.0050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arguin P. Is malaria chemoprophylaxis appropriate for travel to endemic areas in the Americas? 14th International Congress on Infectious Diseases (ICID) Miami, FL: 2010. March 9–12, 2010. [Google Scholar]
- 29.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 30.Boulos M, Amato Neto V, Dutra AP, Di Santi SM, Shiroma M. Frequency of malaria relapse due to Plasmodium vivax in a non-endemic region (Sao Paulo, Brazil) Rev Inst Med Trop Sao Paulo. 1991;33:143–146. [PubMed] [Google Scholar]
- 31.Hanf M, Stephani A, Basurko C, Nacher M, Carme B. Determination of the Plasmodium vivax relapse pattern in Camopi, French Guiana. Malar J. 2009;8:278. doi: 10.1186/1475-2875-8-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Contacos PG, Collins WE, Jeffery GM, Krotoski WA, Howard WA. Studies on the characterization of Plasmodium vivax strains from Central America. Am J Trop Med Hyg. 1972;21:707–712. doi: 10.4269/ajtmh.1972.21.707. [DOI] [PubMed] [Google Scholar]
- 33.Mason J. Patterns of Plasmodium vivax recurrence in a high-incidence coastal area of El Salvador, C. A. Am J Trop Med Hyg. 1975;24:581–585. doi: 10.4269/ajtmh.1975.24.581. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz E, Parise M, Kozarsky P, Cetron M. Delayed onset of malaria–implications for chemoprophylaxis in travelers. N Engl J Med. 2003;349:1510–1516. doi: 10.1056/NEJMoa021592. [DOI] [PubMed] [Google Scholar]
- 35.Schlagenhauf P, Petersen E. Malaria chemoprophylaxis: strategies for risk groups. Clin Microbiol Rev. 2008;21:466–472. doi: 10.1128/CMR.00059-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behrens RH, Carroll B, Beran J, Bouchaud O, Hellgren U, Hatz C, Jelinek T, Legros F, Mühlberger N, Myrvang B, Siikamäki H, Visser L. The low and declining risk of malaria in travelers to Latin America: is there still an indication for chemoprophylaxis? Malar J. 2007;6:114. doi: 10.1186/1475-2875-6-114. TropNetEurop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Office of Travel and Tourism Industries U.S. Citizen Air Traffic to Overseas Regions, Mexico, and Canada in 2008. 2010. http://www.tinet.ita.doc.gov/view/m-2008-O-001/index.html Available at. Accessed December 21, 2010.
- 38.Freedman DO. Clinical practice. Malaria prevention in short-term travelers. N Engl J Med. 2008;359:603–612. doi: 10.1056/NEJMcp0803572. [DOI] [PubMed] [Google Scholar]
- 39.CDC CDC Malaria Map Application. 2010. http://www.cdc.gov/malaria/map/ Available at. Accessed January 12, 2011.
- 40.Chen LH, Wilson ME, Schlagenhauf P. Controversies and misconceptions in malaria chemoprophylaxis for travelers. JAMA. 2007;297:2251–2263. doi: 10.1001/jama.297.20.2251. [DOI] [PubMed] [Google Scholar]
- 41.Askling HH, Nilsson J, Tegnell A, Janzon R, Ekdahl K. Malaria risk in travelers. Emerg Infect Dis. 2005;11:436–441. doi: 10.3201/eid1103.040677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brochero HL, Buitrago LS, Olano VA. Biting activity and breeding sites of Anopheles species in the municipality Villavicencio, Meta, Colombia. J Am Mosq Control Assoc. 2005;21:182–186. doi: 10.2987/8756-971X(2005)21[182:BAABSO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 43.Harris AF, Matias-Arnez A, Hill N. Biting time of Anopheles darlingi in the Bolivian Amazon and implications for control of malaria. Trans R Soc Trop Med Hyg. 2006;100:45–47. doi: 10.1016/j.trstmh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Zielinski-Gutierrez E, Wirtz RA, Nasci RS. Traveler's Health—Yellow Book. Atlanta, GA: CDC Health Information for International Travel 2010; 2009. (Protection against mosquitoes, ticks, and other insects and arthropods. Centers for Disease Control and Prevention). [Google Scholar]
- 45.Arguin PM, Steele S. Traveler's Health—Yellow Book. Atlanta, GA: CDC Health Information for International Travel 2012; 2011. (Malaria. Centers for Disease Control and Prevention). [Google Scholar]
- 46.Steffen R, Holdener F, Wyss R, Nurminen L. Malaria prophylaxis and self-therapy in airline crew. Aviat Space Environ Med. 1990;11:942–945. [PubMed] [Google Scholar]
- 47.Chen LH, Keystone JS. New strategies for the prevention of malaria in travelers. Infect Dis Clin North Am. 2005;19:185–210. doi: 10.1016/j.idc.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Høgh B, Clarke PD, Camus D, Nothdurft HD, Overbosch D, Günther M, Joubert I, Kain KC, Shaw D, Roskell NS, Chulay JD. Atovaquone-proguanil versus chloroquine-proguanil for malaria prophylaxis in non-immune travelers: a randomized, double-blind study. Malarone International Study Team. Lancet. 2000;356:1888–1894. doi: 10.1016/s0140-6736(00)03260-8. [DOI] [PubMed] [Google Scholar]
- 49.Overbosch D, Schilthuis H, Bienzle U, Behrens RH, Kain KC, Clarke PD, Toovey S, Knobloch J, Nothdraft HD, Shaw D, Roskell NS, Chulay JD. Atovaquone-proguanil versus mefloquine for malaria prophylaxis in nonimmune travelers: results from a randomized, double-blind study. Clin Infect Dis. 2001;33:1015–1021. doi: 10.1086/322694. Malarone International Study Team. [DOI] [PubMed] [Google Scholar]
- 50.Ling J, Baird JK, Fryauff DJ, Sismadi P, Bangs MJ, Lacy M, Barcus MJ, Gramzinski R, Maguire JD, Kumusumangsih M, Miller GB, Jones TR, Chulay JD, Hoffman SL. Randomized, placebo-controlled trial of atovaquone/proguanil for the prevention of Plasmodium falciparum or Plasmodium vivax malaria among migrants to Papua, Indonesia. Clin Infect Dis. 2002;35:825–833. doi: 10.1086/342578. Naval Medical Research Unit 2 Clinical Trial Team. [DOI] [PubMed] [Google Scholar]
- 51.Soto J, Toledo J, Luzz M, Gutierrez P, Berman J, Duparc S. Randomized, double-blind, placebo-controlled study of Malarone for malaria prophylaxis in non-immune Colombian soldiers. Am J Trop Med Hyg. 2006;75:430–433. [PubMed] [Google Scholar]
- 52.Povinelli L, Monson TA, Fox BC, Parise ME, Morrisey JM, Vaidya AB. Plasmodium vivax malaria in spite of atovaquone/proguanil (malarone) prophylaxis. J Travel Med. 2003;10:353–355. doi: 10.2310/7060.2003.9318. [DOI] [PubMed] [Google Scholar]
- 53.Schlagenhauf P, Tschopp A, Johnson R, Nothdurft HD, Beck B, Schwartz E, Herold M, Krebs B, Veit O, Allwinn R, Steffen R. Tolerability of malaria chemoprophylaxis in non-immune travelers to sub-Saharan Africa: multicentre, randomized, double blind, four arm study. BMJ. 2003;327:1078. doi: 10.1136/bmj.327.7423.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alvarado GE, Soto GJ. Volcanoes in the pre-Columbian life, legend, and archaeology of Costa Rica (Central America) J Volcanol Geotherm Res. 2008;176:356–362. [Google Scholar]
- 55.Taylor WR, Richie TL, Fryauff DJ, Picarima H, Ohrt C, Tang D, Braitman D, Murphy GS, Widjaja H, Tjitra E, Ganjar A, Jones TR, Basri H, Berman J. Malaria prophylaxis using azithromycin: a double-blind, placebo-controlled trial in Irian Jaya, Indonesia. Clin Infect Dis. 1999;28:74–81. doi: 10.1086/515071. [DOI] [PubMed] [Google Scholar]
- 56.Ohrt C, Richie TL, Widjaja H, Shanks GD, Fitriadi J, Fryauff DJ, Handschin J, Tang D, Sandjaja B, Tjitra E, Hadiarso L, Watt G, Wignall FS. Mefloquine compared with doxycycline for the prophylaxis of malaria in Indonesian soldiers. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1997;126:963–972. doi: 10.7326/0003-4819-126-12-199706150-00006. [DOI] [PubMed] [Google Scholar]
- 57.Croft A, Garner P. Mefloquine to prevent malaria: a systematic review of trials. BMJ. 1997;315:1412–1416. doi: 10.1136/bmj.315.7120.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soto J, Toledo J, Rodriquez M, Sanchez J, Herrera R, Padilla J, Berman J. Primaquine prophylaxis against malaria in nonimmune Colombian soldiers: efficacy and toxicity. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;129:241–244. doi: 10.7326/0003-4819-129-3-199808010-00013. [DOI] [PubMed] [Google Scholar]
- 59.Baird JK, Lacy MD, Basri H, Barcus MJ, Maguire JD, Bangs MJ, Gramzinski R, Sismadi P, Krisin , Ling J, Wiady I, Kusumaningsih M, Jones TR, Fryauff DJ, Hoffman SL. Randomized, parallel placebo-controlled trial of primaquine for malaria prophylaxis in Papua, Indonesia. Clin Infect Dis. 2001;33:1990–1997. doi: 10.1086/324085. United States Naval Medical Research Unit 2 Clinical Trials Team. [DOI] [PubMed] [Google Scholar]
- 60.Fryauff DJ, Baird JK, Basri H, Sumawinata I, Purnomo , Richie TL, Ohrt CK, Mouzin E, Church CJ, Richards AL, Subianto B, Sandjaja B, Wignall FS, Hoffman SL. Randomized placebo-controlled trial of primaquine for prophylaxis of falciparum and vivax malaria. Lancet. 1995;346:1190–1193. doi: 10.1016/s0140-6736(95)92898-7. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz E, Regev-Yochay G. Primaquine as prophylaxis for malaria for nonimmune travelers: a comparison with mefloquine and doxycycline. Clin Infect Dis. 1999;29:1502–1506. doi: 10.1086/313527. [DOI] [PubMed] [Google Scholar]
- 62.Arnold J, Alving AS, Hockwald RS, Clayman CB, Dern RJ, Beutler E, Flanagan CL, Jeffery GM. The antimalarial action of primaquine against the blood and tissue stages of falciparum malaria (Panama, P-F-6 strain) J Lab Clin Med. 1955;46:391–397. [PubMed] [Google Scholar]
- 63.Basco LK, Bickii J, Ringwald P. In-vitro activity of primaquine against the asexual blood stages of Plasmodium falciparum. Ann Trop Med Parasitol. 1999;93:179–182. doi: 10.1080/00034989958663. [DOI] [PubMed] [Google Scholar]
- 64.Baird JK, Schwartz E, Hoffman SL. Prevention and treatment of vivax malaria. Curr Infect Dis Rep. 2007;9:39–46. doi: 10.1007/s11908-007-0021-4. [DOI] [PubMed] [Google Scholar]
- 65.Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg. 2006;75:402–415. [PubMed] [Google Scholar]
- 66.Clayman CB, Arnold J, Hockwald RS, Yount EH, Jr, Edgcomb JH, Alving AS. Toxicity of primaquine in Caucasians. J Am Med Assoc. 1952;149:1563–1568. doi: 10.1001/jama.1952.72930340022010b. [DOI] [PubMed] [Google Scholar]
- 67.Ganesan S, Tekwani BL, Sahu R, Tripathi LM, Walker LA. Cytochrome P(450)-dependent toxic effects of primaquine on human erythrocytes. Toxicol Appl Pharmacol. 2009;241:14–22. doi: 10.1016/j.taap.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 68.Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I. Clin Pharmacokinet. 2009;48:689–723. doi: 10.2165/11318030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 69.Binh VQ, Chinh NT, Thanh NX, Cuong BT, Quang NN, Dai B, Travers T, Edstein MD. Sex affects the steady-state pharmacokinetics of primaquine but not doxycycline in healthy subjects. Am J Trop Med Hyg. 2009;81:747–753. doi: 10.4269/ajtmh.2009.09-0214. [DOI] [PubMed] [Google Scholar]
- 70.Cuong BT, Binh VQ, Dai B, Duy DN, Lovell CM, Rieckmann KH, Edstein MD. Does gender, food or grapefruit juice alter the pharmacokinetics of primaquine in healthy subjects? Br J Clin Pharmacol. 2006;61:682–689. doi: 10.1111/j.1365-2125.2006.02601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White NJ. Why is it that antimalarial drug treatments do not always work? Ann Trop Med Parasitol. 1998;92:449–458. doi: 10.1080/00034989859429. [DOI] [PubMed] [Google Scholar]
- 72.Edgcomb JH, Arnold J, Yount EH, Jr, Alving AS, Eichelberger L, Jeffery GM, Eyles D, Young MD. Primaquine, SN 13272, a new curative agent in vivax malaria; a preliminary report. J Natl Malar Soc. 1950;9:285–292. [PubMed] [Google Scholar]
- 73.Jelinek T, Nothdurft HD, Von Sonnenburg F, Loscher T. Long-term efficacy of primaquine in the treatment of vivax malaria in nonimmune travelers. Am J Trop Med Hyg. 1995;52:322–324. doi: 10.4269/ajtmh.1995.52.322. [DOI] [PubMed] [Google Scholar]
- 74.Reddy P, Flaherty JP. Plasmodium vivax malaria relapses after primaquine prophylaxis. Emerg Infect Dis. 2006;12:1795–1796. doi: 10.3201/eid1211.060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boggild AK, Parise ME, Lewis LS, Kain KC. Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II) Am J Trop Med Hyg. 2007;76:208–223. [PubMed] [Google Scholar]
- 76.Lobel HO, Miani M, Eng T, Bernard KW, Hightower AW, Campbell CC. Long-term malaria prophylaxis with weekly mefloquine. Lancet. 1993;341:848–851. doi: 10.1016/0140-6736(93)93058-9. [DOI] [PubMed] [Google Scholar]
- 77.Baird JK, Fryauff DJ, Hoffman SL. Primaquine for prevention of malaria in travelers. Clin Infect Dis. 2003;37:1659–1667. doi: 10.1086/379714. [DOI] [PubMed] [Google Scholar]
- 78.Bryan JP. Cost considerations of malaria chemoprophylaxis including use of primaquine for primary or terminal chemoprophylaxis. Am J Trop Med Hyg. 2006;75:416–420. [PubMed] [Google Scholar]


